Abstract
Myocardial contractile dysfunction is associated with an increase in mitochondrial fission in patients with diabetes. However, whether mitochondrial fission directly promotes diabetes‐induced cardiac dysfunction is still unknown. Melatonin exerts a substantial influence on the regulation of mitochondrial fission/fusion. This study investigated whether melatonin protects against diabetes‐induced cardiac dysfunction via regulation of mitochondrial fission/fusion and explored its underlying mechanisms. Here, we show that melatonin prevented diabetes‐induced cardiac dysfunction by inhibiting dynamin‐related protein 1 (Drp1)‐mediated mitochondrial fission. Melatonin treatment decreased Drp1 expression, inhibited mitochondrial fragmentation, suppressed oxidative stress, reduced cardiomyocyte apoptosis, improved mitochondrial function and cardiac function in streptozotocin (STZ)‐induced diabetic mice, but not in SIRT1−/− diabetic mice. In high glucose‐exposed H9c2 cells, melatonin treatment increased the expression of SIRT1 and PGC‐1α and inhibited Drp1‐mediated mitochondrial fission and mitochondria‐derived superoxide production. In contrast, SIRT1 or PGC‐1α siRNA knockdown blunted the inhibitory effects of melatonin on Drp1 expression and mitochondrial fission. These data indicated that melatonin exerted its cardioprotective effects by reducing Drp1‐mediated mitochondrial fission in a SIRT1/PGC‐1α‐dependent manner. Moreover, chromatin immunoprecipitation analysis revealed that PGC‐1α directly regulated the expression of Drp1 by binding to its promoter. Inhibition of mitochondrial fission with Drp1 inhibitor mdivi‐1 suppressed oxidative stress, alleviated mitochondrial dysfunction and cardiac dysfunction in diabetic mice. These findings show that melatonin attenuates the development of diabetes‐induced cardiac dysfunction by preventing mitochondrial fission through SIRT1‐PGC1α pathway, which negatively regulates the expression of Drp1 directly. Inhibition of mitochondrial fission may be a potential target for delaying cardiac complications in patients with diabetes.
Keywords: diabetes, Drp1, melatonin, mitochondrial fission, PGC‐1α, silent information regulator 1
1. INTRODUCTION
The prevalence of diabetes mellitus appears to increase rapidly during recent years in the world, currently estimated at 11.6% in China1 and 8.3% in America.2 Diabetic cardiomyopathy (DCM) is one major complication in diabetics, which impairs myocardial performance even without coronary artery disease or hypertension.3 Multiple factors are responsible for the pathogenesis of DCM. Mounting evidence from current studies has indicated that the generation of reactive oxygen species (ROS) contributes to the progression of diabetes‐induced cardiac dysfunction.4, 5 Mitochondria are critical organelles for ROS generation and energy production in cardiomyocytes. Recent work has highlighted the important role of mitochondrial fusion/fission dynamics in mitochondrial homeostasis.6 Mitochondrial fusion is considered to be beneficial because it is associated with an increase in the mitochondrial function and ATP production. In contrast, excessive mitochondrial fission seems to be detrimental because it is associated with decreased mitochondrial function and increased ROS.7, 8 Increased mitochondrial fission was observed in several types of cultured cells from the cardiovascular system under prolonged hyperglycemic conditions.9 Moreover, myocardial contractile dysfunction is associated with an increase in mitochondrial fission among diabetic patients.10 However, whether inhibition of mitochondrial fission is efficient to protect against diabetes‐induced cardiac dysfunction is still largely unknown.
Melatonin, chemically N‐acetyl‐5‐methoxytryptamine, has been proved to exert its protective properties in a variety of cardiovascular diseases.11, 12, 13, 14, 15 Recent studies have reported that melatonin has the ability to protect against diabetes‐induced cardiac dysfunction.16, 17 Our previous study demonstrated that melatonin alleviated posttraumatic myocardial injury and cardiac dysfunction. The mechanisms were associated with improved performance of mitochondrial dynamics and function.18 Several recent studies from others also demonstrated that melatonin‐inhibited mitochondrial fission under some pathological conditions,19, 20, 21, 22 indicating that melatonin exerts a substantial influence on the regulation of mitochondrial dynamics. Given the potentially important role of mitochondrial dynamics in diabetic hearts, we speculated that melatonin may alleviate diabetes‐induced cardiac dysfunction via inhibition of mitochondrial fission. Moreover, the exact underlying mechanisms that how melatonin protects against mitochondrial fission remain poorly understood.
Silent information regulator 1 (SIRT1) is an NAD+‐dependent protein deacetylase involved in the cardioprotective effects of melatonin. Melatonin reduced myocardial ischemia‐reperfusion injury in both nondiabetic and diabetic animals via activation of SIRT1.23, 24 SIRT1 exerts its beneficial effects via the reduction of oxidative stress and endoplasmic reticulum stress.25, 26 Decreased SIRT1 expression was found in diabetic hearts while normalizing or activating SIRT1 signaling alleviated diabetes‐induced cardiac dysfunction.27, 28 It has been reported that activation of SIRT1 improves mitochondrial function and attenuates cardiac dysfunction in diabetic animals.29, 30 However, whether SIRT1 signaling is involved in the regulatory effect of melatonin on mitochondrial fission is unknown.
Therefore, the aims of this study were (i) to determine whether melatonin alleviates diabetes‐induced cardiac dysfunction via inhibition of mitochondrial fission; (ii) if so, to investigate whether melatonin prevents mitochondrial fission via activation of SIRT1 signaling. Using in vivo diabetic mice and in vitro hyperglycemia‐treated cells, our results demonstrate for the first time that melatonin prevents Drp1‐mediated mitochondrial fission in diabetes in a SIRT1/PGC‐1α‐dependent manner.
2. MATERIALS AND METHODS
2.1. Reagents
Melatonin (Mel) and streptozocin (STZ) were obtained from Sigma‐Aldrich (St. Louis, MO, USA). Mdivi‐1 was obtained from MedChem Express (MCE, NJ, USA). Dihydroethidium (DHE) probe, mitochondria isolation kits, malondialdehyde (MDA), and manganese superoxide dismutase (MnSOD) activity assay kits were purchased from Beyotime Biotechnology (Jiangsu, China). Mitochondrial complex activity assay kits were purchased from GENMED Scientific Inc (Arlington, MA, USA). PGC‐1α siRNA, Drp1 siRNA, and control siRNA were obtained from Santa Cruz Biotechnology (Catalog Number: sc‐72151, sc‐270298, and sc‐37007, Dallas, TX, USA). MitoTracker Red CMXRos probe, MitoSOX probe, and Lipofectamine RNAiMAX reagent were obtained from Invitrogen (Carlsbad, CA, USA). Caspase‐3 and ATP assay kits were obtained from Biovision (CA, USA). Terminal deoxynucleotidyl nick‐end labeling (TUNEL) assay kits were purchased from Roche Molecular Biochemicals (Mannheim, Germany). Adenoviruses expressing SIRT1, PGC‐1α or GFP were purchased from Hanbio Technology Ltd (Shanghai, China). The primary antibodies against Drp1, Opa‐1, Mfn2, Nox4, and COX IV were obtained from Abcam Biotechnology (Cambridge, MA, USA). PGC‐1α antibody was obtained from Novus Biologicals (Littleton, CO, USA). The primary antibody against Fis1 was purchased from GeneTex (Irvine, CA, USA). The goat anti‐rabbit and goat anti‐mouse secondary antibodies were purchased from Beyotime (Jiangsu, China). SIRT1 siRNA (Product Number: 12241S), SIRT1 antibody, β‐actin antibody, and SimpleChIP Plus Enzymatic Chromatin IP kits were obtained from Cell Signaling Technology (Beverly, MA, USA).
2.2. Animal experiments
All animal experiments were performed in compliance with the NIH guidelines and were approved by the Institutional Animal Care and Use Committee of the Fourth Military Medical University. Mice homozygous for the floxed sirt1 alleles (>99% C57BL/6 genetic background, SIRT1fl/fl) were obtained from National Resource Center for Mutant Mice (NRCMM), initially from The Jackson Laboratory (stock number: 008041). Mutated estrogen receptor (Mer)‐Cre‐Mer Tg mice under the α‐myosin heavy chain promoter (Myh6‐MerCreMer) were obtained from NRCMM (>99% C57BL/6 genetic background). Cardiac‐specific SIRT1‐knockout (SIRT1−/−) mice were generated by crossing SIRTfl/fl mice with Myh6‐MerCreMer Tg mice, followed by 2‐week oral administration of tamoxifen (30 mg/kg/d in chow) as described previously.31 Male cardiac‐specific SIRT1‐knockout (SIRT1−/−) aged 8‐10 weeks were applied to the study at 2 weeks after the last administration of tamoxifen. Age‐matched male littermates (SIRT1fl/fl with tamoxifen) were served as controls.
The mice were injected with STZ (50 mg/kg/d) in citrate buffer (pH 4.3) intraperitoneally for 5 consecutive days. Hyperglycemic mice with fasting blood glucose >11.1 mmol/L from three samplings 2 weeks after the first injection of STZ were considered to have diabetes. Nondiabetic animals were administrated with an equivalent volume of citrate buffer. Diabetic mice were treated with the vehicle, melatonin (10 mg/kg, once daily, intraperitoneally) or mdivi‐1 (10 mg/kg, twice per week, intraperitoneally), respectively for another 10 weeks. Melatonin was injected at 9:00 am every morning when serum melatonin level was relatively low. The dosage of melatonin or mdivi‐1 treatment was chosen based on previous studies about the effect of melatonin or mdivi‐1 on diabetic complications.16, 32, 33 At the end of the experiments (12 weeks after the first injection of STZ), hearts were collected for mitochondrial dynamics, apoptosis, ROS generation, and Western blot analyses.
2.3. Echocardiography measurements
Echocardiography was performed in M‐mode with a VEVO 2100 echocardiography system (Visual Sonics, Toronto, ON, Canada) as described previously.34 Left ventricular end‐systolic volume (LVESV), Left ventricular fractional shortening (LVFS), and ejection fraction (LVEF) were measured in M‐mode images using computer algorithms.
2.4. Transmission electron microscopy
Heart samples from the ventricular anterior wall were obtained and fixed in 2.5% glutaraldehyde (pH = 7.2) and 1% osmium tetroxide, and processed as described previously.35 The slices were viewed with one transmission electron microscope (JEM‐1230, JEOL Ltd., Tokyo, Japan). Images were obtained by a technician blinded to the treatment. Mitochondrial size and the number of mitochondria were analyzed with Image‐Pro Plus software. The percentage of mitochondria that classified into three size categories in a given field (<0.6 μm2, within 0.6‐1.0 μm2, >1.0 μm2) was counted as described previously.34, 36
2.5. Mitochondrial electron transport chain (ETC) complex activities and ATP content
Cardiac mitochondria were isolated from fresh cardiac tissues using the Mitochondria Isolation Kit (Beyotime, Jiangsu, China). Mitochondrial ETC complex (I‐V) activities and ATP content were determined according to manufacturer's instructions (GENMED, MA, USA).
2.6. Determination of myocardial apoptosis and ROS production
Myocardial apoptosis was determined by caspase‐3 activity measurement and TUNEL staining as described previously.34 Dihydroethidium (DHE) staining was utilized for detection of intracellular superoxide anion levels in fresh heart tissues. MitoSOX staining was utilized for detection of mitochondrial ROS levels. Measurements of malondialdehyde (MDA) level and manganese superoxide dismutase (MnSOD) activity were performed with commercial assay kits according to manufacturer's protocols.
2.7. Cell culture
H9c2 cells (obtained from Tiancheng Biotechnology, Shanghai, China) were grown in Dulbecco's modified Eagle's medium containing normal glucose (5.5 mmol/L, NG) with 10% fetal bovine serum supplement. Cells were incubated in humidified air (5% CO2) at 37°C. The media were changed every two days, and the cells were used in the experiments once they reached 70‐80% confluence. The cells were incubated in the vehicle or melatonin (100 μmol/L) for 4 hours17, 18 and subjected to high glucose culture (33 mmol/L, HG) for 48 hours.
2.8. siRNA transfection
H9C2 cells were transfected with SIRT1 siRNA, PGC‐1α siRNA, Drp1 siRNA, or control siRNA by employing Lipofectamine RNAiMAX reagent (Invitrogen). After siRNA transfection, the cells were treated with vehicle or melatonin (100 μmol/L) for 4 hours and subjected to HG for 48 hours.
2.9. Adenoviral transfection
H9C2 cells were transfected with adenoviruses harboring SIRT1, PGC‐1α, or GFP as described previously.37 The titers of adenoviruses employed in this study were 1.2 × 1010 PFU/mL, and the multiplicity of infection (MOI) was 100:1. After adenoviral transfection, the cells were subjected to NG or HG for 48 hours.
2.10. Assessment of mitochondrial morphology in the cells
Mitochondrial morphology was evaluated in H9c2 cells that were stained with MitoTracker Red CMXRos probe (100 nmol/L, 30 minutes at 37°C). Images were acquired by means of a confocal laser scanning microscope (Olympus FV 1000, Japan) as described previously.18 Mitochondrial volume and the number of mitochondria were analyzed and quantified. Percentage of cells with fragmented mitochondria (small and round) was determined.
2.11. Western blotting analysis
Mice hearts and H9c2 cells were lysed with lysis buffer containing protease inhibitor cocktail. Western blotting was carried out using the standard method as described previously.26 The primary antibodies used were as follows: SIRT1, Drp1, Fis1, Opa1, Mfn2, Nox4, COX IV, PGC‐1α, and β‐actin.
2.12. Real‐time quantitative PCR
RNA extraction and Real‐time quantitative PCR were performed as described previously.26 The primer sequences were as follows: Drp1 forward GGTGGAATTGGAGATGGTGGTCGA, reverse TTCGTGCAACTGGAACTGG CACA; Actin forward GTCCCTCACCCTCCCAAAAG, reverse GCTGCCTCAACACCTCAACCC. Data were normalized relative to actin and expressed as a relative ratio.
2.13. Chromatin immunoprecipitation (ChIP)
Chromatin immunoprecipitation was carried out using the SimpleChIP Plus Enzymatic Chromatin IP kit (Cell Signaling) as described previously.38 In brief, H9c2 cells were fixed with formaldehyde and then quenched with glycine. The sheared chromatins were incubated with PGC‐1α antibody and protein G magnetic beads. DNA released from the precipitation was detected by PCR analysis. The primers specific to the PGC‐1α binding region within Drp1 promoter region were as follows: Drp1 promoter forward: 5′‐CAGATTCACGGACCCAGCTT‐3′, reverse: 5′‐ CAAAGGCTGTCGGGAGATGT‐3′. IgG was used as the negative control.
2.14. Statistical analysis
All data were presented as mean ± standard error (SEM). All measured data were subjected to One‐way ANOVA followed by Bonferroni post hoc test with the utilization of GraphPad Prism software version 5.0. P < .05 was taken as statistically significant.
3. RESULTS
3.1. Melatonin reduced cardiomyocyte apoptosis and improved cardiac function in diabetic mice but not in SIRT1−/− diabetic mice
Cardiac‐specific SIRT1‐knockout (SIRT1−/−) mice were generated by crossing SIRTfl/fl mice with Myh6‐MerCreMer Tg mice (Figure S1A,B), followed by 2‐week tamoxifen administration. There was almost no SIRT1 protein expression in cardiac tissues of SIRT1−/− mice after tamoxifen treatment. In other tissues such as the brain, liver, and muscle, the protein levels of SIRT1 were comparable between wild‐type (WT), SIRTfl/fl and SIRT1−/− mice (Figure S1C). Cardiac function assessed at 2 weeks after tamoxifen treatment showed that there were no significant changes in LVEF and LVESV between SIRTfl/fl and SIRT1−/− mice (Figure S1D‐F).
Compared with nondiabetic control mice, diabetic mice developed significantly cardiac dysfunction as evidenced by decreased LVEF and LVFS and increased LVESV at 12 weeks after STZ injection (Figure 1A‐D). Concomitantly, myocardial caspase‐3 activity and apoptosis index were significantly increased in diabetic hearts (Figure 1E‐G). Although melatonin treatment did not significantly affect blood glucose and body weight in diabetic animals (Table S1), administration of melatonin alleviated cardiac dysfunction and reduced cardiomyocyte apoptosis in diabetic hearts (Figure 1A‐G). Moreover, melatonin administration restored the decreased expression of SIRT1 in diabetic hearts (Figure 1H). Nevertheless, when cardiac SIRT1 was knocked out, melatonin treatment failed to improve cardiac dysfunction and inhibit myocardial apoptosis in diabetic mice (Figure 1A‐H).
Figure 1.
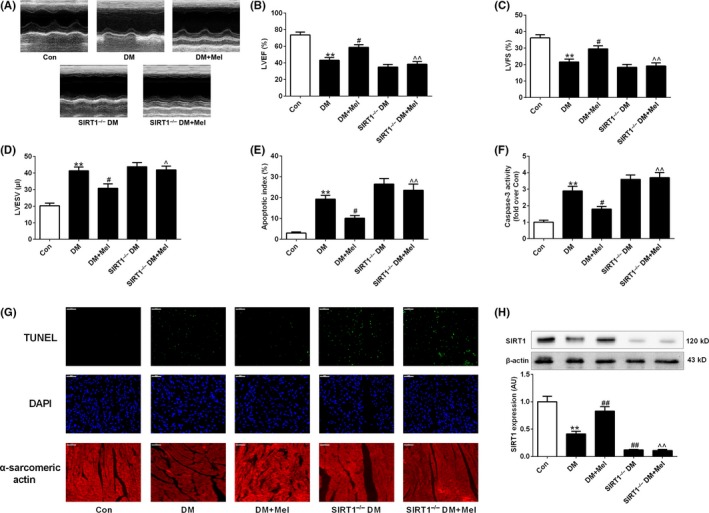
Melatonin reduced cardiomyocyte apoptosis and improved cardiac function in diabetic mice but not in SIRT1−/− diabetic mice. (A) Representative echocardiography images. (B) Left ventricular ejection fraction (LVEF). (C) Left ventricular fractional shortening (LVFS). (D) Left ventricular end‐systolic volume (LVESV). (E) Apoptosis index. (F) Myocardial caspase‐3 activity (fold over Con). (G) Representative photomicrographs of TUNEL‐stained and DAPI‐stained heart sections. Original magnification ×400. (H) Protein expression of SIRT1. Presented values are means ± SEM. DM, diabetes mellitus; Mel, melatonin. n = 8 in each group. **P < .01 vs Con. # P < .05, ## P < .01 vs DM. ^ P < .05, ^^ P < .01 vs DM+Mel
3.2. Melatonin prevented Drp1‐mediated mitochondrial fission and improved mitochondrial function in diabetic mice but not in SIRT1−/− diabetic mice
There was no significant difference in the number of mitochondria per μm2 among all the groups (Figure 2A,B). Mean mitochondrial size was smaller in diabetic hearts compared to control hearts (Figure 2A,C). Moreover, the percentage of mitochondria smaller than 0.6 μm2 was significantly increased in diabetic hearts compared to control hearts. The percentage of mitochondria bigger than 1 μm2 was decreased in diabetic hearts compared to control hearts (Figure 2D). Meanwhile, the expression pattern of the main mitochondrial fission‐related proteins (Drp1 and Fis1) and fusion‐related proteins (Opa1 and Mfn2) were assessed. Compared with nondiabetic control hearts, diabetic hearts exhibited higher levels of Drp1, while there were no significant changes in the protein expressions of Fis1, Opa1, and Mfn2 between diabetic and control hearts (Figure 2E,F). These results suggested that Drp1‐mediated mitochondrial fission was enhanced in diabetic hearts. In addition, mitochondrial complex I, IV, V activities, and ATP levels were lower in diabetic hearts compared with control hearts (Figure 2G,H).
Figure 2.
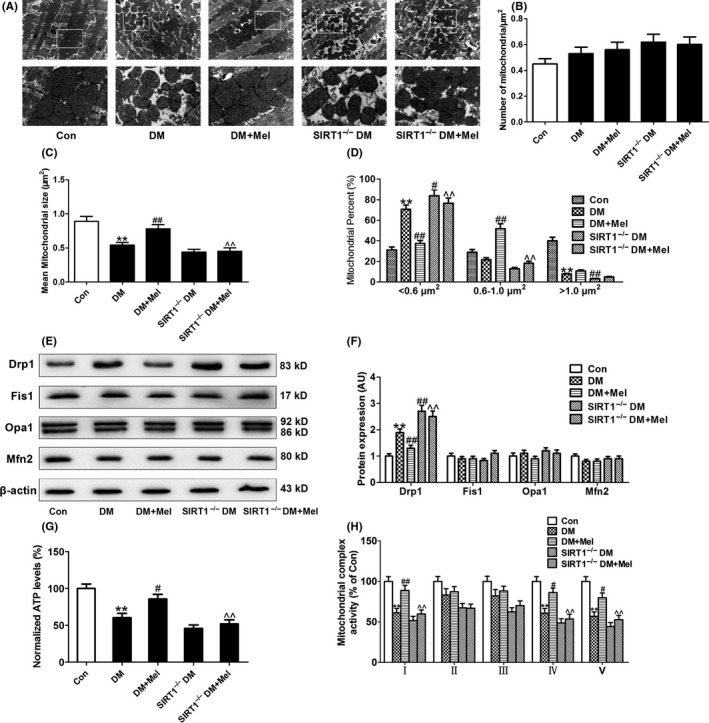
Melatonin prevented Drp1‐mediated mitochondrial fission and improved mitochondrial function in diabetic mice but not in SIRT1−/− diabetic mice. (A) Representative transmission electron microscopic images of the myocardium (major finding is B‐D). (B) The number of mitochondria per μm2. (C) Mean size of mitochondria. (D) Percentage of mitochondria that sorted into three size categories based on size. (E,F) Protein expressions of mitochondrial fission‐related proteins (Drp1 and Fis1) and fusion‐related proteins (Opa1 and Mfn2). (G) Normalized ATP levels. (H) Mitochondrial complex activity (percentage of Con). Presented values are means ± SEM. DM, diabetes mellitus; Mel, melatonin. n = 4‐8 in each group. **P < .01 vs Con. # P < .05, ## P < .01 vs DM. ^ P < .05, ^^ P < .01 vs DM+Mel
Diabetic mice treated with melatonin displayed reduced levels of mitochondrial fission compared to vehicle‐treated diabetic mice as evidenced by increased mean size of mitochondria, decreased percentage of mitochondria smaller than 0.6 μm2 and increased percentage of mitochondria between 0.6 μm2 and 1 μm2 (Figure 2A,C,D). Melatonin treatment reduced diabetes‐enhanced Drp1 expression but had no significant effect on the expression of Fis1, Opa1, and Mfn2 in mouse hearts (Figure 2E,F). Mitochondrial complex I, IV, V activities, and ATP levels were increased in melatonin‐treated diabetic hearts (Figure 2G,H). Compared with diabetic hearts, SIRT1−/− diabetic hearts exhibited further enhanced Drp1‐mediated mitochondrial fission evidenced by increased Drp1 expression, elevated percentage of mitochondria smaller than 0.6 μm2 and lowered percentage of mitochondria bigger than 1 μm2 (Figure 2A,D,E,F). Treatment with melatonin did not affect mitochondrial dysfunction and Drp1‐mediated mitochondrial fission in SIRT1−/− diabetic mice (Figure 2A‐H).
3.3. Melatonin inhibited oxidative stress in diabetic hearts but not in SIRT1−/− diabetic hearts
Diabetic hearts has been reported to exhibit enhanced ROS generation and oxidative stress. Nox4‐derived ROS is the major source of oxidative stress in diabetic hearts and contributes to myocardial cell injury at early stages.39 As expected, myocardial superoxide anion production (stained by DHE) and Nox4 expression and MDA levels were significantly higher in diabetic mice compared with control animals (Figure 3A‐D), while MnSOD activity of diabetic hearts was lowered (Figure 3E). There was a small trend toward enhanced myocardial oxidative stress (elevated superoxide anion production, increased MDA levels, and decreased MnSOD activity) in SIRT1−/− diabetic hearts compared to diabetic hearts while the difference did not reach a significant level. Melatonin treatment inhibited the increase in myocardial oxidative stress in diabetic mice, but not in SIRT1−/− diabetic mice (Figure 3A‐E).
Figure 3.
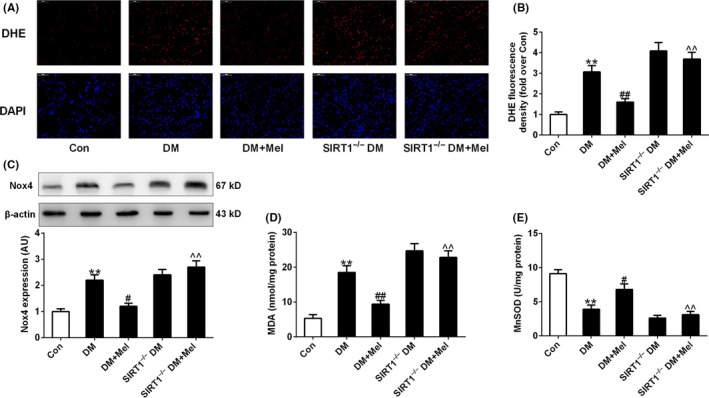
Melatonin inhibited oxidative stress in diabetic hearts but not in SIRT1−/− diabetic hearts. (A) Representative microphotographs of DHE staining in heart sections. Original magnification × 400. (B) Quantitative analysis of DHE fluorescence density (fold over Con). (C) Protein expression of Nox4. (D) Myocardial malondialdehyde (MDA) content. (E) Mitochondrial manganese superoxide dismutase (MnSOD) activity. Presented values are means ± SEM. DM, diabetes mellitus; Mel, melatonin. n = 8 in each group. **P < .01 vs Con. # P < .05, ## P < .01 vs DM. ^^ P < .01 vs DM+Mel
3.4. Melatonin inhibited Drp1‐mediated mitochondrial fission and mitochondria‐derived superoxide production in hyperglycemia‐treated cells
Very few reports have studied mitochondrial dynamics in cultured adult ventricular cardiomyocytes in vitro, as acute manipulation of fission and fusion proteins resulted in significant morphological changes in animal hearts and H9c2 cells but only mild changes in adult cardiomyocytes.35, 40 We then investigated whether melatonin inhibits high glucose‐induced mitochondrial fragmentation in H9c2 cells by visualizing mitochondrial morphology with MitoTracker Red probe. As shown in Figure S2, in H9c2 cells cultured in normal glucose medium (NG, 5.5 mmol/L) for 48 hours, mitochondrial morphology mainly appeared as elongated tubules with highly interconnecting networks. There were no significant changes in mitochondrial morphology in cells cultured in osmotic control (OC, 27.5 mmol/L mannitol plus 5.5 mmol/L glucose) medium for 48 hours compared with those cultured in NG. After stimulation with high glucose (HG 33 mmol/L) for 48 hours, mitochondria became spherical and shorter. The volume of mitochondria was decreased and the number of mitochondria was increased, indicating mitochondrial fragmentation. Compared with NG and OC treatments, HG increased Drp1 protein expression without affecting Fis1, Opa1, and Mfn2 expression (Figure S2E‐F). Notably, melatonin treatment attenuated HG‐induced mitochondrial fragmentation (Figure 4A,C‐E) and reduced the expression of Drp1 in both cytoplasmic fractions and mitochondrial fractions (Figure 4G,H). Moreover, melatonin treatment reduced mitochondria‐derived superoxide production (stained by MitoSOX) in the cells cultured in HG medium (Figure 4B,F).
Figure 4.
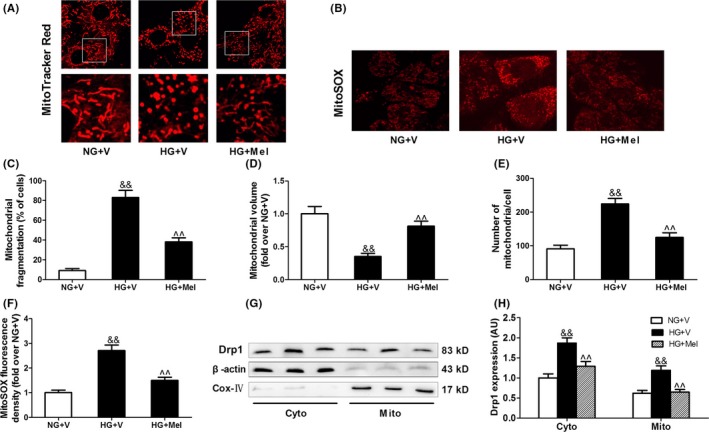
Melatonin inhibited Drp1‐mediated mitochondrial fission and mitochondrial‐derived superoxide production in hyperglycemia‐treated H9c2 cells. (A) Representative confocal microscope images showing mitochondrial morphology stained by MitoTracker Red (major finding is C‐E). Original magnification ×600. (B) Representative confocal microscope images showing mitochondria‐derived superoxide production stained by MitoSOX (major finding is F). Original magnification ×600. (C) The percentage of cells with fragmented mitochondria. (D) Mean volume of mitochondria (fold over NG+V). (E) The number of mitochondria per cell. (F) Quantitative analysis of MitoSOX fluorescence density (fold over NG+V). (G,H) Western blot analysis of Drp1 protein expression in cytoplasmic (Cyto) and mitochondrial (Mito) fractions. Presented values are means ± SEM. NG, normal glucose (5.5 mmol/L); HG, high glucose (33 mmol/L glucose); V, vehicle; Mel, melatonin. n = 6 in each group. && P < .01 vs NG+V. ^^ P < .01 vs HG+V
3.5. Melatonin reduces Drp1 expression and mitochondrial fission through SIRT1‐PGC1α signaling pathway
Whether SIRT1 is essential for the melatonin‐induced inhibition of mitochondrial fission was also tested in the cells. As shown in Figure 5, silencing SIRT1 expression with small interfering (si)RNA blocked the inhibitory effect of melatonin on Drp1 expression and mitochondrial fission in HG‐treated H9c2 cells. On the other hand, transfection with the adenovirus encoding SIRT1 (Ad SIRT1) increased the expression of SIRT1 and reduced Drp1 protein expression and mitochondrial fission in HG‐treated cells (Figure 6). These data suggest that melatonin prevents HG‐induced mitochondrial fission via the SIRT1‐dependent down‐regulation of Drp1 protein expression.
Figure 5.
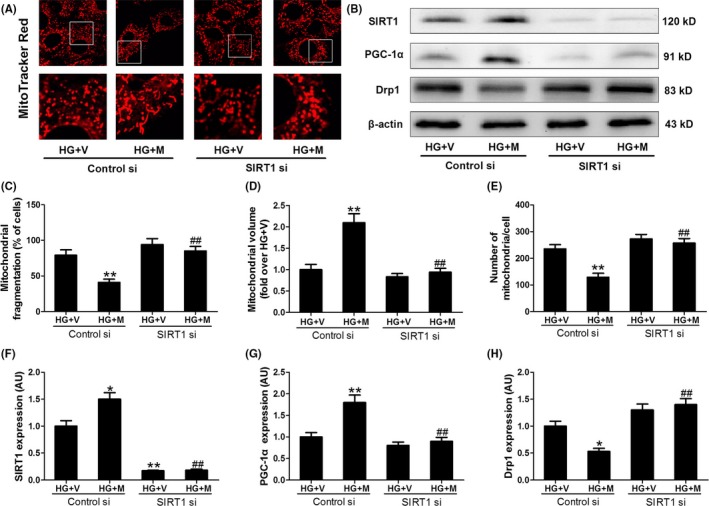
SIRT1 siRNA blunted the effect of melatonin on PGC‐1α, Drp1 and mitochondrial fission in hyperglycemia‐treated H9c2 cells. (A) Representative confocal microscope images showing mitochondrial morphology stained by MitoTracker Red (major finding is C‐E). Original magnification ×600. (B,F‐H) Protein expressions of SIRT1, PGC‐1α, and Drp1 were determined by Western blotting. (C) The percentage of cells with fragmented mitochondria. (D) Mean volume of mitochondria (fold over HG+V). (E) The number of mitochondria per cell. Presented values are means ± SEM. HG, high glucose (33 mmol/L glucose); V, vehicle; Mel, melatonin. n = 6 in each group. *P < .05, **P < .01 vs HG+V with Control si. ## P < .01 vs HG+M with Control si
Figure 6.
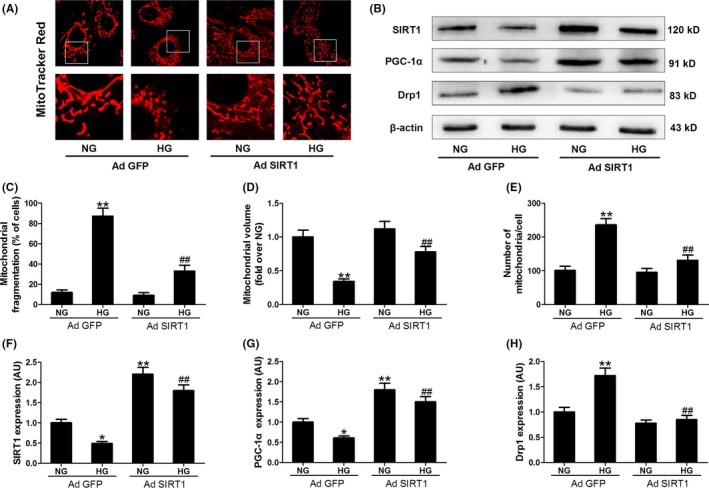
Adenoviral (Ad) over‐expression of SIRT1 increased PGC‐1α expression, reduced Drp1 expression and inhibited mitochondrial fission. (A) Representative confocal microscope images showing mitochondrial morphology stained by MitoTracker Red (major finding is C‐E). Original magnification ×600. (B,F‐H) Protein expressions of SIRT1, PGC‐1α, and Drp1 were determined by Western blotting. (C) The percentage of cells with fragmented mitochondria. (D) Mean volume of mitochondria (fold over NG with Ad GFP). (E) The number of mitochondria per cell. Presented values are means ± SEM. NG, normal glucose (5.5 mmol/L); HG, high glucose (33 mmol/L glucose). n = 6 in each group. *P < .05, **P < .01 vs NG with Ad GFP. ## P < .01 vs HG with Ad GFP
We further explored how the protein expression of Drp1 was down‐regulated by SIRT1. As shown in Figure S3, Drp1 mRNA expression was also increased in HG‐treated cells, while over‐expression of SIRT1 (Ad SIRT1) reduced Drp1 mRNA expression. It seemed that the expression of Drp1 was regulated at the level of transcription. PGC‐1α is one key transcription factor, which has been considered as a target of SIRT1. As expected, SIRT1 siRNA blocked the up‐regulation effect of melatonin on PGC‐1α expression in HG‐treated H9c2 cells (Figure 5B,G), and SIRT1 knockout blunted up‐regulation effect of melatonin on PGC‐1α expression in diabetic mice (Figure S4). Over‐expression of SIRT1 increased PGC‐1α expression in the cells cultured in NG or HG medium (Figure 6B,G). Subsequently, we determined whether PGC‐1α was responsible for the regulation of Drp1 expression and mitochondrial morphology. It was shown that PGC‐1α siRNA blocked the inhibitory effect of melatonin on Drp1‐mediated mitochondrial fission in HG‐treated H9c2 cells (Figure 7 and Figure S3C). Over‐expression of PGC‐1α (Ad PGC‐1α) reduced Drp1 expression in both protein and mRNA levels and prevented mitochondrial fission in the cells cultured in HG medium (Figure 8 and Figure S3D). Moreover, ChIP and PCR analysis revealed that PGC‐1α directly regulated the expression of Drp1 by binding to its promoter (Figure 8C). These data suggest that SIRT1‐PGC1α signaling is directly responsible for the protective effects of melatonin against Drp1‐mediated mitochondrial dynamics under hyperglycemia condition.
Figure 7.
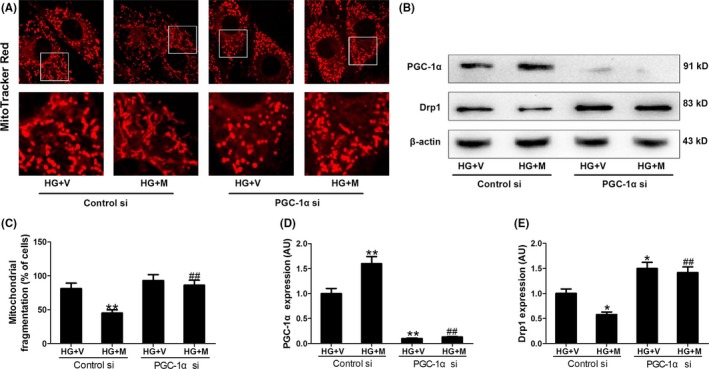
PGC‐1α siRNA blunted the inhibitory effect of melatonin on Drp1 expression and mitochondrial fission in hyperglycemia‐treated H9c2 cells. (A) Representative confocal microscope images showing mitochondrial morphology stained by MitoTracker Red (major finding is C). Original magnification ×600. (B,D,E) Protein expressions of PGC‐1α and Drp1 were determined by Western blotting. (C) The percentage of cells with fragmented mitochondria. Presented values are means ± SEM. HG, high glucose (33 mmol/L glucose); V, vehicle; Mel, melatonin. n = 6 in each group. *P < .05, **P < .01 vs HG+V with Control si. ## P < .01 vs HG+M with Control si
Figure 8.
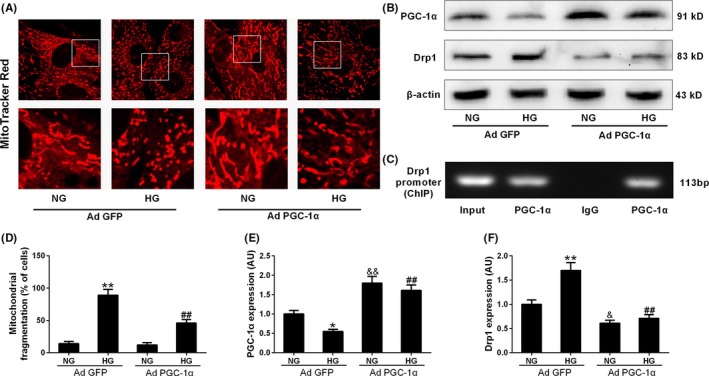
Adenoviral over‐expression of PGC‐1α reduced Drp1 expression and inhibited mitochondrial fission. (A) Representative confocal microscope images showing mitochondrial morphology stained by MitoTracker Red (major finding is D). Original magnification ×600. (B,E,F) Protein expressions of PGC‐1α and Drp1 were determined by Western blotting. (C) ChIP analysis for PGC‐1α binding to the Drp1 promoter in H9c2 cells. (D) The percentage of cells with fragmented mitochondria. Presented values are means ± SEM. NG, normal glucose (5.5 mmol/L); HG, high glucose (33 mmol/L glucose). n = 6 in each group. *P < .05, **P < .01 vs NG with Ad GFP. ## P < .01 vs HG with Ad GFP
3.6. Inhibition of mitochondrial fission suppressed oxidative stress and alleviated mitochondrial dysfunction in diabetes
Melatonin inhibited Drp1‐mediated mitochondrial fission and ROS production and alleviated mitochondrial dysfunction in diabetes; however, whether enhanced Drp1‐mediated mitochondrial fission causes oxidative stress and mitochondrial dysfunction in diabetes remains unclear. The Drp1 inhibitor, mdivi‐1, and Drp1 siRNA were therefore used to investigate whether the inhibition of Drp1‐mediated mitochondrial fission suppressed oxidative stress and alleviated mitochondrial dysfunction in diabetes. Although mdivi‐1 treatment did not affect blood glucose and body weight in diabetic animals (Table S2), administration of mdivi‐1 significantly attenuated mitochondrial fission (Figure 9A‐C) and increased mitochondrial complex I, IV, V activities, and ATP levels in diabetic hearts (Figure 9D,E). Importantly, administration of mdivi‐1 increased LVEF and reduced cardiomyocyte apoptosis in diabetic mice (Figure 9F‐I). In addition, mdivi‐1 markedly attenuated superoxide anion production (stained by DHE, Figure 10A) and Nox4 expression (Figure 10B) and increased MnSOD activity (Figure 10C) in diabetic hearts. Gene silencing of Drp1 with siRNA (Drp1 si) consistently prevented mitochondrial fission (Figure 10E) and reduced mitochondria‐derived superoxide production (stained by MitoSOX, Figure 10F) in HG‐treated H9c2 cells.
Figure 9.
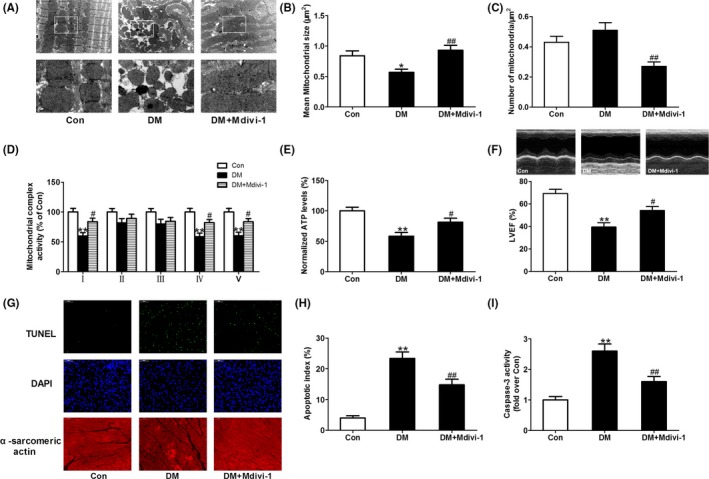
Inhibition of mitochondrial fission with mdivi‐1 improved mitochondrial function and cardiac function and reduced cardiomyocyte apoptosis in diabetic mice. (A) Representative transmission electron microscopic images of the myocardium (major finding is B,C). (B) Mean size of mitochondria. (C) The number of mitochondria per μm2. (D) Mitochondrial complex activity (percentage of Con). (E) Normalized ATP levels. (F) Left ventricular ejection fraction (LVEF). (G) Representative photomicrographs of TUNEL‐stained and DAPI‐stained heart sections. Original magnification ×400. (H) Apoptosis index. (I) Myocardial caspase‐3 activity (fold over Con). Presented values are means ± SEM. DM, diabetes mellitus. n = 8 in each group. *P < .05, **P < .01 vs Con. # P < .05, ## P < .01 vs DM
Figure 10.
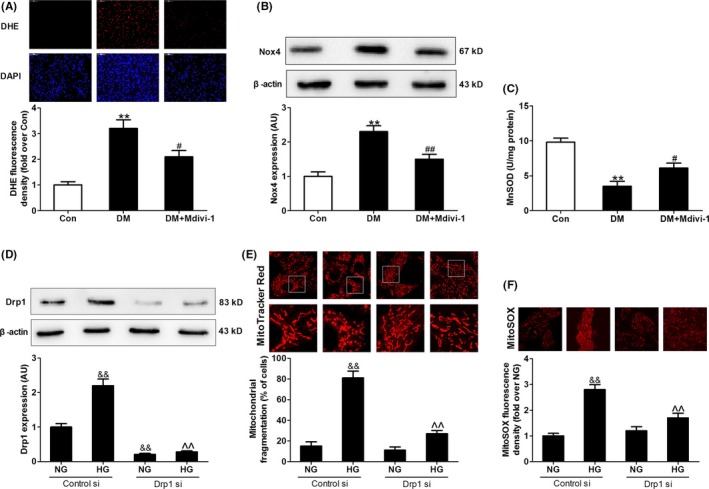
Inhibition of mitochondrial fission with mdivi‐1 or Drp1 siRNA suppressed hyperglycemia‐induced oxidative stress. (A) Quantitative analysis of DHE fluorescence density in heart sections (fold over Con). (B) Protein expression of Nox4. (C) Mitochondrial manganese superoxide dismutase (MnSOD) activity. (D) Protein expression of Drp1. (E) The percentage of cells with fragmented mitochondria. (F) Quantitative analysis of MitoSOX fluorescence density (fold over NG with Control si). Presented values are means ± SEM. NG, normal glucose (5.5 mmol/L); HG, high glucose (33 mmol/L glucose). n = 6‐8 in each group. **P < .01 vs Con. # P < .05, ## P < .01 vs DM. && P < .01 vs NG with Control si. ^^ P < .01 vs HG with Control si
4. DISCUSSION
In this study, we uncover a novel molecular mechanism that how melatonin prevents against hyperglycemia‐induced mitochondrial fission. We provide in vivo and in vitro evidence that melatonin reduced Drp1 expression and prevented mitochondrial fission in mice with diabetes via activating SIRT1‐PGC1α signaling. Inhibition of mitochondrial fission suppressed mitochondrial ROS production, alleviated mitochondrial dysfunction, reduced cell apoptosis, and consequently improved cardiac function (Figure 11). Our data demonstrate for the first time that melatonin prevents against Drp1‐mediated mitochondrial fission in mice with diabetes in a SIRT1/PGC‐1α‐dependent manner.
Figure 11.
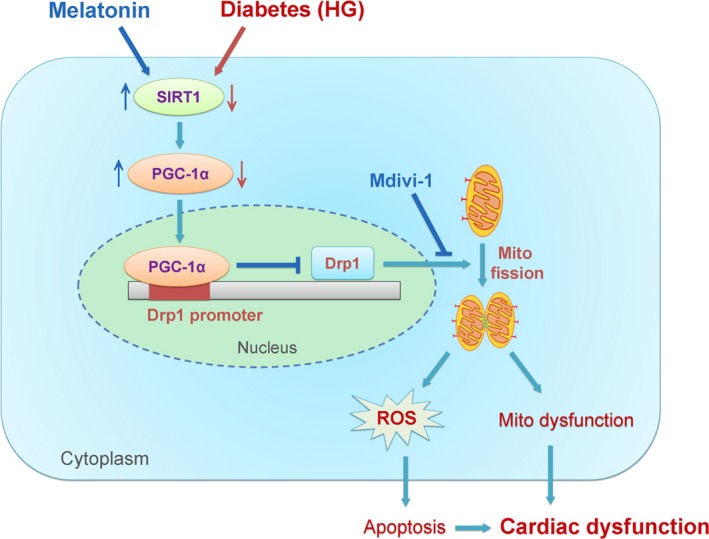
Schematic figure illustrating that melatonin prevents against diabetes‐induced mitochondrial fission and cardiac dysfunction through SIRT1‐PGC‐1α‐Drp1 pathway. SIRT1 positively regulates the expression of PGC‐1α, which negatively regulates the expression of Drp1 directly by binding to its promoter. Diabetes (hyperglycemia, HG) reduced the expression of SIRT1 and PGC‐1α (red arrows) and then increased the expression of Drp1. Drp1 triggers mitochondrial fission and subsequently leads to mitochondria‐derived ROS production and mitochondrial dysfunction, which results in the development of cardiac dysfunction. Treatment with melatonin increased the expression of SIRT1 and PGC‐1α (blue arrows) and then prevented Drp1‐mediated mitochondrial fission. Mdivi‐1 prevented diabetes‐induced mitochondrial fission by inhibiting Drp1 activity. As a result, melatonin or mdivi‐1 suppressed mitochondria‐derived ROS production, alleviated mitochondrial dysfunction, reduced cell apoptosis, and protected against diabetes‐induced cardiac dysfunction. Mito, mitochondrial; ROS, reactive oxygen species
Mitochondria are dynamic organelles that are intimately involved in the regulation of various cellular functions, including energy production, ROS generation, and cell death.41, 42 Accumulating evidence suggests that both mitochondrial oxidative damage and mitochondrial dysfunction contribute to the pathogenesis of diabetes‐induced cardiac dysfunction.43, 44 Previous studies have indicated that enhanced mitochondrial fragmentation is associated with increased mitochondrial ROS production in hyperglycemic conditions in vitro.9, 45 However, it is still unclear whether mitochondrial fission is involved in the pathogenesis of diabetes‐induced cardiac dysfunction, especially in vivo. Our study provided compelling evidence showing that diabetes (high glucose) increased Drp1 expression and caused mitochondrial fission both in vivo and in vitro. Inhibition of Drp1 with mdivi‐1 alleviated mitochondrial dysfunction and cardiac dysfunction in STZ‐induced diabetic mice. Meanwhile, mdivi‐1 inhibited mitochondrial ROS production and reduced cell apoptosis. These results suggest that increased mitochondrial fission may accelerate mitochondrial dysfunction and cardiac dysfunction in mice with diabetes. Overall, our study develops a new concept that hyperglycemia‐induced mitochondrial fission contributes to cardiac dysfunction in mice with diabetes, partly by enhancing mitochondrial oxidative stress.
Similar to the cardioprotective effects of mdivi‐1, we found that melatonin prevented Drp1‐mediated mitochondrial fission and alleviated cardiac dysfunction in diabetic mice. Previous studies have reported that melatonin alleviated cardiac dysfunction via activation of retinoic acid‐related orphan receptor‐α and regulation of autophagy in diabetic hearts.16, 17 The present work indicates that melatonin‐inhibited mitochondrial fission may be a novel mechanism underlying its cardioprotective effect in diabetes. Moreover, melatonin treatment increased SIRT1 expression in both diabetic hearts and hyperglycemia‐incubated cells. Previous findings have reported a rise in the expression of SIRT1 in cells and animal models after melatonin treatment.46, 47 The SIRT1 inhibitor was used to verify that SIRT1 plays a pivotal role in several functions of melatonin including protecting against kidney injury and myocardial ischemia‐reperfusion injury.23, 48 However, it is still largely unknown whether melatonin‐induced up‐regulation of SIRT1 is responsible for its inhibitory effect on mitochondrial fission. In the current study, we found that over‐expression of SIRT1 reduced Drp1 expression and mitochondrial fragmentations. In contrast, SIRT1 knockout or knockdown blocked the inhibitory effect of melatonin on mitochondrial fission in response to high glucose both in vivo and in vitro, suggesting that the regulated effect of melatonin on mitochondrial dynamics may be dependent on the action of SIRT1.
SIRT1 knockout also blocked the inhibitory effect of melatonin on oxidative stress in diabetic hearts, while it has been reported that melatonin has direct anti‐oxidative protection including directly scavenging free radicals and enhancing the activity of the antioxidant enzyme and indirect anti‐oxidative protection dependent on its interaction with RORα.16, 49 Interestingly, recent study has found that melatonin increases SIRT1‐dependent NF‐kB deacetylation through a RORα‐dependent mechanism,50 suggesting the existence of a melatonin‐RORα‐SIRT1 connection may be involved in the inhibition of ROS. Moreover, melatonin and SIRT1 have a long evolutionary history and exert cooperative biological effects in some conditions. For example, melatonin as well as some of its effects undergoes a circadian cycle, and SIRT1 is an accessory amplitude‐enhancing component of cellular circadian oscillators.51, 52 It is possible that melatonin and SIRT1 have some interaction in a certain way. SIRT1 deficiency may disrupt the interaction and blunt direct anti‐oxidative ability of melatonin. Then melatonin cannot exert its anti‐oxidative protection in SIRT1 deficient mice. Further study is needed to clarify this interesting issue.
Drp1 is a cytosolic protein that initiates mitochondrial fission by binding to Fis1 over the mitochondrial surface in mammalian cells. The regulation of Drp1 properties, such as mRNA transcription, protein expression, and mitochondrial translocation, is important for the modulation of Drp1 function. Previous studies have shown that high glucose increases Drp1 protein expression in several types of cells including cardiomyocytes,53 hippocampal neurons,54 and pancreatic β‐cells.55 In this study, we found that SIRT1 reduced HG‐induced Drp1 expression in both mRNA and protein levels, suggesting that SIRT1 modulated the expression of Drp1 at the level of transcription. As SIRT1 is not a transcription factor in the nucleus, it is possible that SIRT1 regulates the transcription of Drp1 through its downstream transcription factor. PGC‐1α is a well‐known transcription factor, which is considered as a major downstream target of SIRT1. In our study, over‐expression of SIRT1 increased PGC‐1α expression as expected, while SIRT1 knockout or knockdown blocked the up‐regulation effect of melatonin on PGC‐1α expression. Importantly, we have further found that PGC‐1α modulated Drp1‐mediated mitochondrial fission directly by binding to its transcription promoter. Interestingly, PGC‐1α also plays a pivotal role in the regulation of mitochondrial biogenesis. Previous studies have demonstrated that melatonin increased the expression of PGC‐1α and promoted mitochondrial biogenesis in pathological conditions.56, 57 Therefore, the effects of melatonin on mitochondria function might be multiple, well beyond its regulation on mitochondrial dynamics. Mitochondrial dynamics and mitochondrial biogenesis might both be involved in the melatonin‐mediated mitochondrial adaptations. Overall, our results provide the first clue for understanding the exact regulatory mechanisms of melatonin on mitochondrial fission in diabetes.
There are still some limitations in our study. First, our in vivo experiments were exclusively performed in STZ‐induced insulin‐deficient type 1 diabetic mice. Whether the research findings can be applied to insulin‐resistance type 2 diabetic models needs further investigation. Second, the conclusion that melatonin prevents Drp1‐mediated mitochondrial through SIRT1‐PGC‐1α pathway was verified using cardiac‐specific SIRT1‐knockout mice and SIRT1/PGC‐1α siRNA. The use of PGC‐1α‐knockout mice will be very helpful in clarifying the role of PGC‐1α in Drp1‐mediated mitochondrial fission. Despite these limitations, we believe that this study has provided important new information for the understanding of effects of melatonin on mitochondrial dynamics.
In summary, our study demonstrates that diabetes results in impaired cardiac function by triggering Drp1‐mediated mitochondrial fission. Melatonin prevents mitochondrial fission and improves cardiac function in mice with diabetes through SIRT1‐PGC1α pathway, which negatively regulates the expression of Drp1 directly. These findings identify melatonin‐modulated mitochondrial fission as a potential target for treating cardiac dysfunction and other cardiac complications in diabetes.
AUTHORS’ CONTRIBUTIONS
Feng Fu and Jianming Pei conceived and designed the study. Mingge Ding, Zeyang Li, Yuemin Wang, and Feng Fu performed the animal experiments. Mingge Ding, Daishi Tang, and Na Feng carried out the cell experiments. Na Feng, Daishi Tang, Jiahao Feng, Zhenhua Liu, and Min Jia performed the molecular biology experiments. Mingge Ding, Daishi Tang, and Feng Fu analyzed the data. Mingge Ding drafted the manuscript. Xiaoming Gu, Feng Fu, and Jianming Pei revised and edited the manuscript. All authors have read and approved the final version of this manuscript.
COMPETING INTERESTS
The authors declare that they do not have any competing interests.
Supporting information
ACKNOWLEDGEMENTS
This study was supported by the grants from National Natural Science Foundation of China (No. 81670354, No. 81600235, No. 81770243) and Key Project of Natural Science Foundation of Shaanxi, China (2016KTCL03‐11).
Ding M, Feng N, Tang D, et al. Melatonin prevents Drp1‐mediated mitochondrial fission in diabetic hearts through SIRT1‐PGC1α pathway. J Pineal Res. 2018;65:e12491 10.1111/jpi.12491
Mingge Ding, Na Feng, Daishi Tang contributed equally to this study.
Contributor Information
Feng Fu, Email: fufeng048@126.com.
Jianming Pei, Email: jmpei8@fmmu.edu.cn.
REFERENCES
- 1. Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948‐959. [DOI] [PubMed] [Google Scholar]
- 2. Bugger H, Abel ED. Molecular mechanisms of diabetic cardiomyopathy. Diabetologia. 2014;57:660‐671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shah MS, Brownlee M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ Res. 2016;118:1808‐1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Arkat S, Umbarkar P, Singh S, et al. Mitochondrial peroxiredoxin‐3 protects against hyperglycemia induced myocardial damage in diabetic cardiomyopathy. Free Radic Biol Med. 2016;97:489‐500. [DOI] [PubMed] [Google Scholar]
- 5. Ni R, Cao T, Xiong S, et al. Therapeutic inhibition of mitochondrial reactive oxygen species with mito‐TEMPO reduces diabetic cardiomyopathy. Free Radic Biol Med. 2016;90:12‐23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Archer SL. Mitochondrial dynamics–mitochondrial fission and fusion in human diseases. N Engl J Med. 2013;369:2236‐2251. [DOI] [PubMed] [Google Scholar]
- 7. Chiong M, Cartes‐Saavedra B, Norambuena‐Soto I, et al. Mitochondrial metabolism and the control of vascular smooth muscle cell proliferation. Front Cell Dev Biol. 2014;2:72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ong SB, Hall AR, Hausenloy DJ. Mitochondrial dynamics in cardiovascular health and disease. Antioxid Redox Signal. 2013;19:400‐414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Yu T, Sheu SS, Robotham JL, et al. Mitochondrial fission mediates high glucose‐induced cell death through elevated production of reactive oxygen species. Cardiovasc Res. 2008;79:341‐351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Montaigne D, Marechal X, Coisne A, et al. Myocardial contractile dysfunction is associated with impaired mitochondrial function and dynamics in type 2 diabetic but not in obese patients. Circulation. 2014;130:554‐564. [DOI] [PubMed] [Google Scholar]
- 11. Zhang Y, Liu X, Bai X, et al. Melatonin prevents endothelial cell pyroptosis via regulation of long noncoding RNA MEG3/miR‐223/NLRP3 axis. J Pineal Res. 2017;64:e12449. [DOI] [PubMed] [Google Scholar]
- 12. Zhou H, Li D, Zhu P, et al. Melatonin suppresses platelet activation and function against cardiac ischemia/reperfusion injury via PPARgamma/FUNDC1/mitophagy pathways. J Pineal Res. 2017;63:e12438. [DOI] [PubMed] [Google Scholar]
- 13. Zhai M, Liu Z, Zhang B, et al. Melatonin protects against the pathological cardiac hypertrophy induced by transverse aortic constriction through activating PGC‐1beta: in vivo and in vitro studies. J Pineal Res. 2017;63:e12433. [DOI] [PubMed] [Google Scholar]
- 14. Nduhirabandi F, Lamont K, Albertyn Z, et al. Role of toll‐like receptor 4 in melatonin‐induced cardioprotection. J Pineal Res. 2016;60:39‐47. [DOI] [PubMed] [Google Scholar]
- 15. Simko F, Baka T, Paulis L, et al. Elevated heart rate and nondipping heart rate as potential targets for melatonin: a review. J Pineal Res. 2016;61:127‐137. [DOI] [PubMed] [Google Scholar]
- 16. Zhao Y, Xu L, Ding S, et al. Novel protective role of the circadian nuclear receptor retinoic acid‐related orphan receptor‐alpha in diabetic cardiomyopathy. J Pineal Res. 2017;62:e12378. [DOI] [PubMed] [Google Scholar]
- 17. Zhang M, Lin J, Wang S, et al. Melatonin protects against diabetic cardiomyopathy through Mst1/Sirt3 signaling. J Pineal Res. 2017;63:e12418. [DOI] [PubMed] [Google Scholar]
- 18. Ding M, Ning J, Feng N, et al. Dynamin‐related protein 1‐mediated mitochondrial fission contributes to post‐traumatic cardiac dysfunction in rats and the protective effect of melatonin. J Pineal Res. 2018;64:e12447. [DOI] [PubMed] [Google Scholar]
- 19. Tan DX, Manchester LC, Qin L, et al. Melatonin: a mitochondrial targeting molecule involving mitochondrial protection and dynamics. Int J Mol Sci. 2016;17:E2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chuang JI, Pan IL, Hsieh CY, et al. Melatonin prevents the dynamin‐related protein 1‐dependent mitochondrial fission and oxidative insult in the cortical neurons after 1‐methyl‐4‐phenylpyridinium treatment. J Pineal Res. 2016;61:230‐240. [DOI] [PubMed] [Google Scholar]
- 21. Xu S, Pi H, Zhang L, et al. Melatonin prevents abnormal mitochondrial dynamics resulting from the neurotoxicity of cadmium by blocking calcium‐dependent translocation of Drp1 to the mitochondria. J Pineal Res. 2016;60:291‐302. [DOI] [PubMed] [Google Scholar]
- 22. Zhou H, Zhang Y, Hu S, et al. Melatonin protects cardiac microvasculature against ischemia/reperfusion injury via suppression of mitochondrial fission‐VDAC1‐HK2‐mPTP‐mitophagy axis. J Pineal Res. 2017;63:e12413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Yu L, Sun Y, Cheng L, et al. Melatonin receptor‐mediated protection against myocardial ischemia/reperfusion injury: role of SIRT1. J Pineal Res. 2014;57:228‐238. [DOI] [PubMed] [Google Scholar]
- 24. Yu L, Liang H, Dong X, et al. Reduced silent information regulator 1 signaling exacerbates myocardial ischemia‐reperfusion injury in type 2 diabetic rats and the protective effect of melatonin. J Pineal Res. 2015;59:376‐390. [DOI] [PubMed] [Google Scholar]
- 25. Hsu YJ, Hsu SC, Hsu CP, et al. Sirtuin 1 protects the aging heart from contractile dysfunction mediated through the inhibition of endoplasmic reticulum stress‐mediated apoptosis in cardiac‐specific Sirtuin 1 knockout mouse model. Int J Cardiol. 2017;228:543‐552. [DOI] [PubMed] [Google Scholar]
- 26. Ding M, Lei J, Han H, et al. SIRT1 protects against myocardial ischemia‐reperfusion injury via activating eNOS in diabetic rats. Cardiovasc Diabetol. 2015;14:143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guo R, Liu W, Liu B, et al. SIRT1 suppresses cardiomyocyte apoptosis in diabetic cardiomyopathy: an insight into endoplasmic reticulum stress response mechanism. Int J Cardiol. 2015;191:36‐45. [DOI] [PubMed] [Google Scholar]
- 28. Karbasforooshan H, Karimi G. The role of SIRT1 in diabetic cardiomyopathy. Biomed Pharmacother. 2017;90:386‐392. [DOI] [PubMed] [Google Scholar]
- 29. Fang WJ, Wang CJ, He Y, et al. Resveratrol alleviates diabetic cardiomyopathy in rats by improving mitochondrial function through PGC‐1alpha deacetylation. Acta Pharmacol Sin. 2018;39:59‐73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ma S, Feng J, Zhang R, et al. SIRT1 activation by resveratrol alleviates cardiac dysfunction via mitochondrial regulation in diabetic cardiomyopathy mice. Oxid Med Cell Longev. 2017;2017:4602715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Wu R, Chang HC, Khechaduri A, et al. Cardiac‐specific ablation of ARNT leads to lipotoxicity and cardiomyopathy. J Clin Invest. 2014;124:4795‐4806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ozdemir G, Ergun Y, Bakaris S, et al. Melatonin prevents retinal oxidative stress and vascular changes in diabetic rats. Eye (Lond). 2014;28:1020‐1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Wang Q, Zhang M, Torres G, et al. Metformin suppresses diabetes‐accelerated atherosclerosis via the inhibition of Drp1‐mediated mitochondrial fission. Diabetes. 2017;66:193‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Ding M, Dong Q, Liu Z, et al. Inhibition of dynamin‐related protein 1 protects against myocardial ischemia‐reperfusion injury in diabetic mice. Cardiovasc Diabetol. 2017;16:19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Zepeda R, Kuzmicic J, Parra V, et al. Drp1 loss‐of‐function reduces cardiomyocyte oxygen dependence protecting the heart from ischemia‐reperfusion injury. J Cardiovasc Pharmacol. 2014;63:477‐487. [DOI] [PubMed] [Google Scholar]
- 36. Shimizu Y, Lambert JP, Nicholson CK, et al. DJ‐1 protects the heart against ischemia‐reperfusion injury by regulating mitochondrial fission. J Mol Cell Cardiol. 2016;97:56‐66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Xu X, Zhang Q, Hu JY, et al. Phosphorylation of DYNLT1 at serine 82 regulates microtubule stability and mitochondrial permeabilization in hypoxia. Mol Cells. 2013;36:322‐332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Chen WX, Zhang ZG, Ding ZY, et al. MicroRNA‐630 suppresses tumor metastasis through the TGF‐beta‐ miR‐630‐Slug signaling pathway and correlates inversely with poor prognosis in hepatocellular carcinoma. Oncotarget. 2016;7:22674‐22686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Maalouf RM, Eid AA, Gorin YC, et al. Nox4‐derived reactive oxygen species mediate cardiomyocyte injury in early type 1 diabetes. Am J Physiol Cell Physiol. 2012;302:C597‐C604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Zhang H, Wang P, Bisetto S, et al. A novel fission‐independent role of dynamin‐related protein 1 in cardiac mitochondrial respiration. Cardiovasc Res. 2017;113:160‐170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Willems PH, Rossignol R, Dieteren CE, et al. Redox homeostasis and mitochondrial dynamics. Cell Metab. 2015;22:207‐218. [DOI] [PubMed] [Google Scholar]
- 42. Nan J, Zhu W, Rahman MS, et al. Molecular regulation of mitochondrial dynamics in cardiac disease. Biochim Biophys Acta. 2017;1864:1260‐1273. [DOI] [PubMed] [Google Scholar]
- 43. Mariappan N, Elks CM, Sriramula S, et al. NF‐kappaB‐induced oxidative stress contributes to mitochondrial and cardiac dysfunction in type II diabetes. Cardiovasc Res. 2010;85:473‐483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Verma SK, Garikipati V, Kishore R. Mitochondrial dysfunction and its impact on diabetic heart. Biochim Biophys Acta. 2017;1863:1098‐1105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Yu T, Robotham JL, Yoon Y. Increased production of reactive oxygen species in hyperglycemic conditions requires dynamic change of mitochondrial morphology. Proc Natl Acad Sci USA. 2006;103:2653‐2658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Cristofol R, Porquet D, Corpas R, et al. Neurons from senescence‐accelerated SAMP8 mice are protected against frailty by the sirtuin 1 promoting agents melatonin and resveratrol. J Pineal Res. 2012;52:271‐281. [DOI] [PubMed] [Google Scholar]
- 47. Zhou L, Chen X, Liu T, et al. Melatonin reverses H2 O2 ‐induced premature senescence in mesenchymal stem cells via the SIRT1‐dependent pathway. J Pineal Res. 2015;59:190‐205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Bai XZ, He T, Gao JX, et al. Melatonin prevents acute kidney injury in severely burned rats via the activation of SIRT1. Sci Rep. 2016;6:32199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Galano A, Tan DX, Reiter RJ. Melatonin: a versatile protector against oxidative DNA damage. Molecules. 2018;23:E530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Garcia JA, Volt H, Venegas C, et al. Disruption of the NF‐kappaB/NLRP3 connection by melatonin requires retinoid‐related orphan receptor‐alpha and blocks the septic response in mice. FASEB J. 2015;29:3863‐3875. [DOI] [PubMed] [Google Scholar]
- 51. Claustrat B, Leston J. Melatonin: physiological effects in humans. Neurochirurgie. 2015;61:77‐84. [DOI] [PubMed] [Google Scholar]
- 52. Hardeland R. Melatonin and the theories of aging: a critical appraisal of melatonin's role in antiaging mechanisms. J Pineal Res. 2013;55:325‐356. [DOI] [PubMed] [Google Scholar]
- 53. Yu J, Maimaitili Y, Xie P, et al. High glucose concentration abrogates sevoflurane post‐conditioning cardioprotection by advancing mitochondrial fission but dynamin‐related protein 1 inhibitor restores these effects. Acta Physiol (Oxf). 2017;220:83‐98. [DOI] [PubMed] [Google Scholar]
- 54. Huang S, Wang Y, Gan X, et al. Drp1‐mediated mitochondrial abnormalities link to synaptic injury in diabetes model. Diabetes. 2015;64:1728‐1742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Men X, Wang H, Li M, et al. Dynamin‐related protein 1 mediates high glucose induced pancreatic beta cell apoptosis. Int J Biochem Cell Biol. 2009;41:879‐890. [DOI] [PubMed] [Google Scholar]
- 56. Kang JW, Hong JM, Lee SM. Melatonin enhances mitophagy and mitochondrial biogenesis in rats with carbon tetrachloride‐induced liver fibrosis. J Pineal Res. 2016;60:383‐393. [DOI] [PubMed] [Google Scholar]
- 57. Jin JX, Lee S, Taweechaipaisankul A, et al. Melatonin regulates lipid metabolism in porcine oocytes. J Pineal Res. 2017;62:e12388. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


