Abstract
Objectives
To validate the enhanced therapeutic effect of Salvia miltiorrhiza Bunge (SM) for brain ischemic stroke through the anti‐apoptotic and survival ability of mesenchymal stem cells (MSCs).
Methods
The viability and the expression level of cell apoptotic and survival‐related proteins in MSCs by treatment of SM were assessed in vitro. In addition, the infarcted brain region and the behavioural changes after treatment of MSCs with SM were confirmed in rat middle cerebral artery occlusion (MCAo) models.
Key findings
We demonstrated that SM attenuates apoptosis and improves the cell viability of MSCs. In the rat MCAo model, the recovery of the infarcted region and positive changes of behaviour are observed after treatment of MSCs with SM.
Conclusions
The therapy using SM enhances the therapeutic effect for brain ischemic stroke by promoting the survival of MSCs. This synergetic effect thereby proposes a new experimental approach of traditional Chinese medicine and stem cell‐based therapies for patients suffering from a variety of diseases.
Keywords: brain ischemic stroke, cell survival, mesenchymal stem cells, Salvia miltiorrhiza extract
Introduction
A major destructive disease, ischemic stroke is the leading cause of serious and complex changes in the brain function, neurological disability and high mortality rates of patients.1, 2 Despite numerous advances in medical technology, the treatments available for patients have their limitations, such as a narrow pharmacological window for treatment involving anti‐thrombosis and thrombolysis (within 3–4.5 h after the outbreak of the ischemic attack), and focus on suppressing the extent of brain injury.3, 4, 5 Recently, many researchers have investigated alternative strategies for brain stroke treatment, including application of various cytokines and growth factors, cells and herbal components.4, 6, 7, 8
For thousands of years, Eastern cultures have used traditional Chinese medicine (TCM) for treating stroke.4, 9 Salvia miltiorrhiza Bunge (SM), commonly known as Danshen, is a widely known herb used for the treatment of various diseases, such as cardiovascular disease, Alzheimer's, hyperlipidemia and acute cerebrovascular disease.10, 11, 14 SM is a well‐known herbal medicine, effective in revitalizing blood circulation and alleviating blood stasis.12, 13 It is commonly found in many prescriptions of TCM, and is effective for treating ischemic stroke.11 SM reduces cellular damage in ischemia by promoting blood flow, and attenuates the formation of brain oedema by enhancing neuroprotection and blood‐brain barrier (BBB) protection.15, 16, 17 Additionally, it prevents cerebral infarction through its anti‐atherosclerosis and anti‐inflammatory effects, and treats cerebral infarction through its anti‐platelet aggregation and anti‐oxidative effects.5
Stem cell‐based therapy is a promising therapeutic approach for treating ischemic stroke, suppressing the inflammatory response, remodelling the BBB and steering neurorestoration.18, 19, 20 Mesenchymal stem cells (MSCs) have the therapeutic ability of self‐renewal, proliferation and multi‐lineage differentiation into cells, such as myocytes, hepatocytes, osteoblast, chondrocytes and adipocytes. These abilities make MSCs an attractive candidate for cellular replacement therapies.18, 21 Transplanted MSCs can migrate into the ischemic stroke region, and differentiate into astrocytes or neuron‐like cells.22, 23 Further, they are able to indirectly stimulate tissue repairing and restructuring, and functional recovery of the infarcted brain by secreting trophic factors into the injured brain region.24 MSCs are stimulated to produce several factors, including vascular endothelial growth factor (VEGF), basic fibroblast growth factor (bFGF), brain‐derived neurotrophic factor (BDNF) and nerve growth factor (NGF), on addition of damaged brain tissue extracts into the culture medium. These factors affect anti‐apoptosis and angiogenesis processes in the infarcted area.25, 26, 27, 28 However, in another in vivo study, MSCs were ineffective for the treatment of ischemic stroke because the transplanted cells were found to be insufficient in the infarcted region.19 To compensate this limitation, several studies suggested MSCs therapy combined with TCM, including Tongxinluo, Naomai Yihao and Buyang Huanwu Tang, for ischemic stroke.29, 30, 31
Based on previous mentions, the present study demonstrated that the treatment of SM enhanced the anti‐apoptotic and survival ability of MSCs under hypoxic conditions. We confirmed if apoptosis‐related components existed in SM extracts through ultra‐high‐performance liquid chromatography (UHPLC). And then, we evaluated the viability of MSCs by treatment of SM and assessed the expression level of cell apoptotic and survival‐related proteins of MSCs by treatment of SM under hypoxic conditions. The recovery of the infarcted region and the behavioural changes after treatment of MSCs with SM were studied in a rat middle cerebral artery occlusion (MCAo) model. Based on our results, we propose that the administration of SM promotes the therapeutic effects of MSCs by enhancing cell survival. In conclusion, to alleviate the symptoms of brain ischemic stroke, we suggest a synergistic therapeutic approach of herbal medicine and stem cell‐based therapies.
Materials and methods
Preparation of SM extract
Roots of SM were purchased from a medicinal materials company (Onggihanyakguk, Daegu, Korea). Once the roots passed the sensory test based on the National Standard of Traditional Medicinal (Herbal and Botanical) Materials, the extract was prepared by the following process. The dried roots (500 g) were boiled in ×10 distilled water for 2 h, filtered, concentrated under vacuum and stored at −20 °C. The total yield of the extract was 11.1% of the dried powder. The extract was dissolved in phosphate‐buffered saline (PBS; Hyclone, Logan, UT, USA) before use.
UHPLC‐UV/Q‐TOF‐MS conditions
For the performance of ultra‐high‐performance liquid chromatography hybrid quadrupole time‐of‐flight mass spectrometry (UHPLC‐UV/Q‐TOF‐MS) method, 1 g of extract powder was dissolved in 5 ml of autoclaved distilled water and then filtered with 0.22‐μm syringe filter (Millipore, Bedford, MA, USA). The sample was separated on a BEH C18 (2.1 × 100 mm, 1.7 μm; Waters, Dublin, Ireland) column at 40 °C and injection volume was 5 and 10 μl (for positive and negative ion modes). The mobile phase consisted of (A) 0.1% formic acid in distilled water and (B) 0.1% formic acid in acetonitrile for positive ion mode; (A) 0.1% formic acid in distilled water with 5 mm ammonium formate and (B) 0.1% formic acid in acetonitrile for negative ion mode using a gradient elution of 0–1 min, 2% B; 1–21 min, 2–100% B; 21–25 min, 100% B; 25.1–30 min, 2% B. The flow rate was 0.3 ml/min and absorbance was measured at 280 nm. Electrospray ionization‐mass spectrometry (ESI‐MS) was also performed in positive and negative ion modes, respectively. And the conditions of ESI were as follows: heater temperature (TEM), 500 °C; curtain gas (CUR), 25 psi; nebulizing gas (GS1), 50 psi; heater gas (GS2), 50 psi; scan mode, 50–1000 m/z; ion‐spray voltage (ISVF), 5300 V and −4500 V respectively, for positive and negative polarity. The data of UHPLC were arranged with Peakview software version 2.2 (SCIEX, Foster City, CA, USA) based on the accurate mass of the components (Table 1).
Table 1.
The chromatograms and high‐resolution (HR) mass data of components of Salvia miltiorrhiza extract
| Peak no. | Found at RT (min) | HR‐mass (m/z) | Tolerance (ppm) | Formula | Components |
|---|---|---|---|---|---|
| 1 | 1.24 | 197.04555 [M − H]− | 1.1 | C9H10O5 | Danshensu |
| 2 | 4.67 | 493.11402 [M − H]− | 1.2 | C26H22O10 | Salvianolic acid A |
| 3 | 5.14 | 717.14611 [M − H]− | 2.3 | C36H30O16 | Salvianolic acid B |
| 4 | 5.22 | 359.07724 [M − H]− | 1 | C18H16O8 | Rosmarinic acid |
| 5 | 13.07 | 297.14852 [M + H]+ | 0.7 | C19H20O3 | Cryptotanshinone |
The analysis data were derived using an UHPLC‐UV/Q‐TOF‐MS method, performed on Agilent 1290 infinity II (Agilent Technologies, Palo Alto, CA, USA) coupled to TripleTOF 4600 mass spectrometer (SCIEX) with an ESI (DuoSpray; SCIEX) source. All of the solvents and reagents for analysis were HPLC grade. Acetonitrile, water, formic acid and ammonium formate were purchased from Sigma‐Aldrich (St. Louis, MO, USA).
MSCs culture
Mesenchymal stem cells were isolated from the tibial and femoral bone marrow of 4‐week‐old male Sprague‐Dawley (SD) rats weighing approximately 100 g (KOATECH, Gyeonggi‐do, Korea). The bone marrow was aspirated with 10 ml of Dulbecco's modified Eagle's medium (DMEM; Hyclone) supplemented with 10% fetal bovine serum (FBS; Hyclone) and 1% antibiotic‐penicillin/streptomycin solution (Hyclone). After density gradient centrifugation using Ficoll (GE Healthcare, Piscataway, NJ, USA), the layer of cells was collected, washed and resuspended in 10% FBS‐DMEM. The cells were plated in flasks at a density of 1 × 106 cells per 100 cm2, and cultured in a humidified atmosphere at 37 °C and 5% CO2. After 72 h, the non‐adherent cells were discarded by washing with PBS. Fresh culture medium was replaced every 3–4 days.
Cell proliferation assay
MSCs were seeded into 6‐well cell culture plates at a density of 8 × 104 cells per well. Cells were exposed to varying concentration of SM (0.001, 0.01, 0.1, 1, 10, 100 μg/ml), and cultured in a humidified atmosphere at 37 °C and 5% CO2. After 24‐h incubation, the cells were washed and the adherent cells were trypsinized using trypsin‐ ethylenediaminetetraacetic acid (EDTA; Gibco, NY, USA), the cell number was microscopically assessed using a hemocytometer. The experiment was repeated three times.
Cell viability assessment in hypoxic and normoxic conditions
Mesenchymal stem cells were plated at a density of 8 × 104 cells per 60 mm petriplate, and cultured under normoxic condition (in a humidified atmosphere at 37 °C and 5% CO2) for 12 h. The non‐adherent cells were discarded by washing with PBS, and new culture medium was replaced in all palates. The plates were divided into 4 groups (n = 6 per a group): group I, cells were cultured in 10% FBS‐DMEM under normoxia (Normoxia‐control) for 12 h; group II, cells were treated with 10 μg/ml of SM extract, and incubated under normoxia (Normoxia‐SM) for 12 h; group III, cells were cultured with degassed DMEM under hypoxic condition (37 °C, 1% O2, 5% CO2 blended with N2) in an anaerobic system (Hypoxia‐control) for 12 h; group IV, cells were treated with SM extract, and incubated under hypoxia (Hypoxia‐SM) for 12 h. The hypoxic‐ischemic‐like conditions were prepared using an anaerobic system (Forma scientific, Cleveland, OH, USA). We evaluated cell viability by cell counting and 3‐(4,5‐dimethylthiazolyl‐2)‐2, 5‐diphenyltetrazolium bromide (MTT) assay, and performed western blotting. Cell viability was measured using the MTT assay. Briefly, the cells (2 × 103 cells/well) were seeded in 96‐well plates and cultured in each condition (as described above). After 0‐, 24‐, 48‐ and 72‐h incubation, 50 μl MTT (5 mg/ml; Sigma‐Aldrich) was added to each well and incubated for 4 h in a CO2 incubator. And then, the media was discarded gently, and 100 μl of dimethyl sulfoxide (DMSO; Sigma‐Aldrich) was added to each well. The data are expressed as the optical density (OD) value and measured at 570 nm in an automatic enzyme‐linked immunosorbent assay (ELISA) reader (Bio‐Rad, Hercules, CA, USA). The experiment was repeated three times.
Western blotting
Cells were subjected to lysis using lysis buffer (Cell Signaling Technology, Beverly, MA, USA). Lysates were centrifuged and protein concentrations were measured in the resultant supernatant using a Bradford Protein Assay Kit (Thermo Fisher Scientific, Waltham, MA, USA). The quantified proteins were fractionated on a 10% sodium dodecyl sulfate‐polyacrylamide gel electrophoresis (SDS‐PAGE), and transferred to polyvinylidene difluoride membranes. The membranes were then blocked with 5% non‐fat dried milk/Tris‐buffered saline‐0.1% Tween 20 (TBS‐T), for 1 h at room temperature. Following blocking, the membranes were washed in TBS, and incubated with primary antibodies in TBS‐T containing 1% bovine serum albumin (BSA), overnight at 4 °C. The primary antibodies were as follows: β‐actin, p‐Akt, Akt, p‐ERK and ERK (Cell Signaling), Bax (Abcam, Cambridge, UK), Bcl‐2 (Santa Cruz Biotechnology, Santa Cruz, CA, USA), Caspase‐3 (Millipore) were employed at dilutions of 1 : 1000. After washing with TBS‐T, the membranes were incubated with horseradish peroxidase‐conjugated secondary antibodies for 1 h at room temperature. The secondary antibodies were utilized at working concentrations of 1 : 4000, 1 : 2000 and 1 : 2000 for anti‐mouse, anti‐goat and anti‐rabbit IgG (Thermo Fisher Scientific), respectively. This was followed by six washes in TBS‐T. Bands were detected using a detection kit (western Bright™ ECL; Advansta, Menlo Park, CA, USA), and the imaging was performed using the ImageJ software version 1.40 g (NIH, https://www.nih.gov/). The experiment was repeated three times.
Animals
For animal studies, we used 9‐week‐old male Sprague‐Dawley rats weighing an average of 290 ± 10 g (KOATECH). The animals were housed separately in cages, at an ambient temperature 25 ± 1 °C and humidity of 50 ± 10% and 12‐h light/dark cycle, with free access to food and water. Handling of all animals was based on the animal welfare guidelines for the care and use of laboratory animals, formulated by the Korean Academy of Medical Sciences and the Korean National Institute of Health.
Preparation of the ischemic stroke rat model
Using the experimental procedure presented in our previous study, preparation of ischemic stroke rat model was performed by transient MCAo and reperfusion. Briefly, the rats were anesthetized using 1–4% isoflurane in as mixture of 70% N2O and 30% O2. The exposed left common carotid artery (CCA) was ligated with a 4–0 nylon suture, after which the external carotid artery (ECA) and occipital artery (OA) were ligated together. The tip of a rounded 3–0 nylon suture was used to block the internal carotid artery (ICA) through the ECA, after which it was ligated twice. Two hours later, reperfusion was started by eliminating the tip.
The rats were randomly divided into five groups (n = 10 per a group): group I, ECA was surgically prepared for insertion of the tip, but the tip was not inserted (sham); group II, reperfusion with PBS treatment (control); group III, 50 mg/kg SM‐treated group (SM); group IV, 1 × 106 MSC‐treated group (MSC); and group V, 50 mg/kg SM and 1 × 106 MSCs‐treated group (SM + MSC). All animals were euthanized after 2 weeks of reperfusion, and the brains were collected for subsequent measurements of the brain infarct volume (n = 5 per group), and water content (n = 5 per group).
Two more groups of randomly divided rats were treated to prepare for immunohistochemical study (n = 2 per a group): group I, ECA was surgically prepared for insertion of the tip and reperfusion with 1 × 107 PKH26‐labelled MSCs with PBS (PBS + MSC); group II, reperfusion with 1 × 107 PKH26‐labelled MSCs with 50 mg/kg SM (SM + MSC). All animals were euthanized after 4 weeks of reperfusion, and the brain tissues were collected for subsequent experiments.
Measurement of infarct volume
After 2 weeks of reperfusion, all animals were euthanized and their brain tissues were obtained and cut into 2‐mm coronal slices. To measure the infarct volume, each slice was stained with 2,3,5‐triphenyltetrazolium chloride (TTC); we observed for unstained infarction areas and the red‐stained normal areas. Using a computerized image analysing system, the infarct volume was calculated as the infarct volume per brain. To compensate for oedema formation in the ipsilateral hemisphere, the infarct volume was expressed as a percentage of the contralateral hemisphere volume using the following formula: (area of the intact contralateral hemisphere‐area of the intact region of the ipsilateral hemisphere).32 The experiment was repeated five times.
Measurement of the water content in the brain
After 2 weeks of reperfusion, all animals were sacrificed and their brains were taken. The wet weight (ww) was measured after removing the pons and olfactory bulbs. The brains were dried for 24 h at 110 °C, following which we measured the dry weight (dw). Entire brain water content was calculated using the following formula: (ww − dw)/ww × 100, as an index for brain oedema.10 The experiment was repeated five times.
Behaviour test
Using the cylinder test and the forced swim test, the exercise capacities of the rats were evaluated. During the cylinder test, the rat was placed in an open transparent cylinder (height, 30 cm; diameter, 20 cm) for 6 min. After 2 min of habituation period, we checked the number of independent wall placements of the right or left forelimb. We also checked both the forelimbs simultaneously. In the forced swim test, the rat was placed in the same cylinder (height, 60 cm; diameter, 20 cm) with 30‐cm water at 25 °C; the rat was exposed to this environment for 6 min. Like the cylinder test, we measured the time of the immobile state in water for the last 4 min. The experiments were repeated 10 times.
Immunohistochemical analysis
After 4 weeks of PKH26‐labelled MSCs transplantation, the animals were sacrificed and their brains were collected, fixed with 4% paraformaldehyde (in PBS) for 24 h and stored in 20% sucrose (in PBS) at 4 °C. Prior to sectioning, the tissues were embedded in OCT compound (Tissue‐Tek, Sakura Finetek, Torrance, CA, USA) in cryomolds; 10‐μm‐thick sections of tissue were mounted on superfrost plus microscope slides (Fisher Scientific, Pittsburgh, PA, USA). The mounted slides were warmed at room temperature for 30 min and fixed with cold acetone for 6 min, washed thrice in PBS for 5 min and treated with DAPI (Sigma‐Aldrich) for nucleic acid staining (in dark) for 10 min. The slides were covered with coverslips and the infarcted areas were observed under Axio observer fluorescence microscopy (Zeiss, Oberkochen, Germany). The experiment was repeated five times.
Statistical analysis
All data are expressed as mean ± SEM. Comparisons between more than two groups were performed by one‐way ANOVA using Bonferroni's correction. A P‐value <0.05 was considered significant.
Results
Analysis of components in SM extract
Analysis of UHPLC was based on the accurate mass of each component. We investigated the presence of apoptosis‐related components such as danshensu, salvianolic acid A (Sal A), salvianolic acid B (Sal B), rosmarinic acid and cryptotanshinone (Table 1). Table 1 shows each component of the SM extract which was utilized for chromatography (formula, HR‐mass of components) and results of chromatography (found at RT, tolerance). The total ion current chromatogram (TIC) of the SM extract was obtained at 50–1000 m/z and the representative UV chromatogram was confirmed at 280 nm (Figure 1a and 1b). The extracted ion chromatograms (XIC) of each component are shown in Figure 1c. In a negative ion mode, we could propose the existence of danshensu (found at 197.04576 m/z), Sal A (493.11461 m/z), Sal B (717.14776 m/z) and rosmarinic acid (197.04576 m/z). On the other side, the cryptotanshinone (297.14874 m/z) appeared in the positive mode. These results indicated that SM extract contained apoptosis‐related components.
Figure 1.
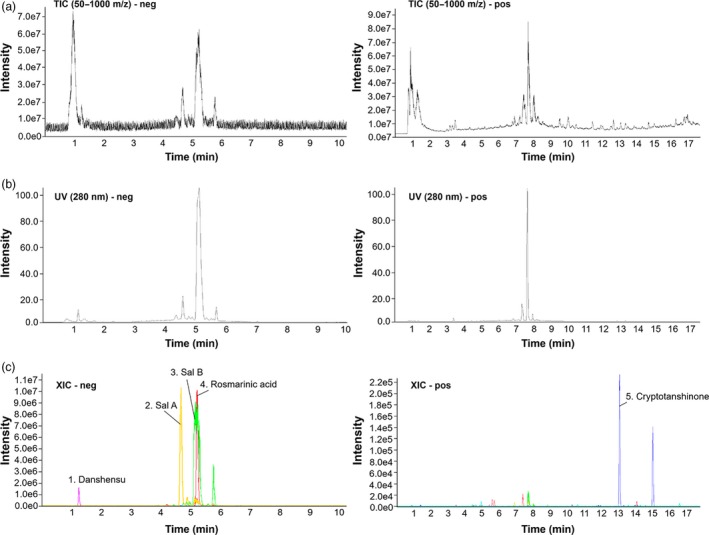
Ultra‐high‐performance liquid chromatography of Salvia miltiorrhiza Bunge extract in negative and positive modes, respectively (a) The total ion current chromatograms of S. miltiorrhiza Bunge extract at 50–1000 m/z. (b) UV chromatograms of S. miltiorrhiza Bunge extract at 280 nm. (c) The extracted ion chromatograms of danshensu, Sal A, Sal B, rosmarinic acid and cryptotanshinone. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Proliferation assay of MSCs by treatment of SM
To select the most effective concentration of SM extract for proliferation of MSCs, we performed a proliferation assay, wherein the cell count was evaluated for each concentration: 0.001, 0.01, 0.1, 1, 10, 100 μg/ml. Increasing the concentration of the extract, up to 100 μg/ml of diluted SM in PBS, enhanced the number of seeded MSCs as compared to control and water groups. However, a decrease in cell number of MSCs was observed at 100 μg/ml of diluted SM in PBS (Figure 2). We estimated that the concentration had cytotoxic effects for the proliferation of MSCs. These results suggest that the treatment of SM extract effectively enhances the proliferation of MSCs in a concentration‐dependent manner in vitro, with 10 μg/ml being the most effective concentration.
Figure 2.
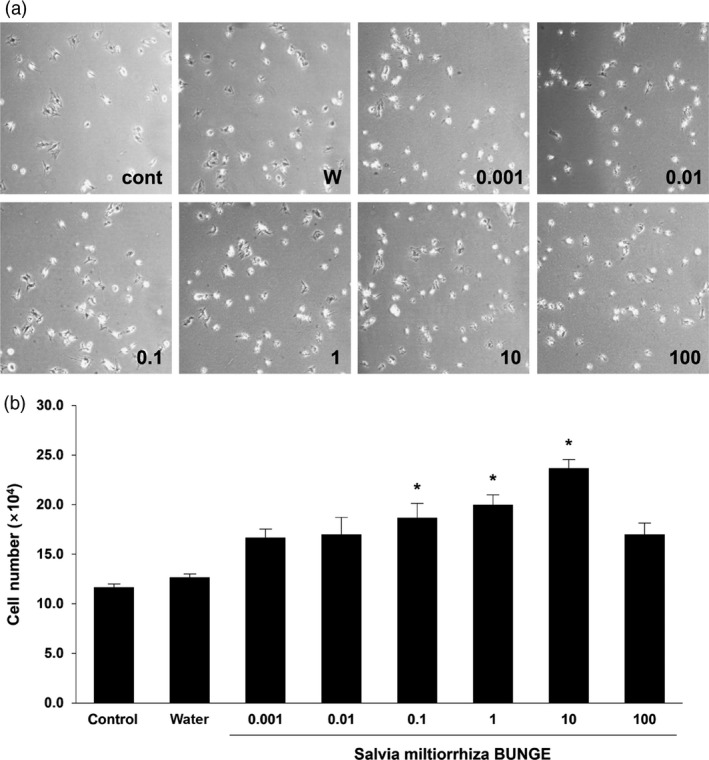
Proliferation of mesenchymal stem cells by treatment of Salvia miltiorrhiza Bunge (a) Representative proliferation of mesenchymal stem cells cultured with varying concentrations of S. miltiorrhiza Bunge extract for 24 h: 0.001, 0.01, 0.1, 1, 10, 100 μg/ml. (b) Statistical analysis demonstrated that mesenchymal stem cells cultured with S. miltiorrhiza Bunge extract enhanced their proliferation in a concentration‐dependent manner. The concentration of 10 μg/ml of S. miltiorrhiza Bunge is the most effective concentration for mesenchymal stem cells proliferation. Columns, mean; bars, SE. *P < 0.05.
Comparison of cell survival in hypoxic and normoxic conditions
After evaluating the most effective concentration (10 μg/ml) for proliferation of MSCs, we compared the survival ability of the cells treated with SM extract under hypoxic and normoxic conditions, respectively. In the normoxic condition, the number of MSCs treated with SM extract was 1.3‐fold higher than in control. Representative data showed that survival and proliferation of MSCs decreased in hypoxia. Similar to the result in normoxic condition, the number of MSCs treated with SM extract was 1.2‐fold higher than in control under the hypoxic condition (Figure 3a and 3b). Additionally, the results of MTT assay also confirmed that treatment with SM extract enhanced MSCs viability (Figure 3c). Since the hypoxic condition in the anaerobic system was similar ischemic stroke environment, these results suggest treatment with SM would impart a better survival of these cells during an ischemic stroke.
Figure 3.
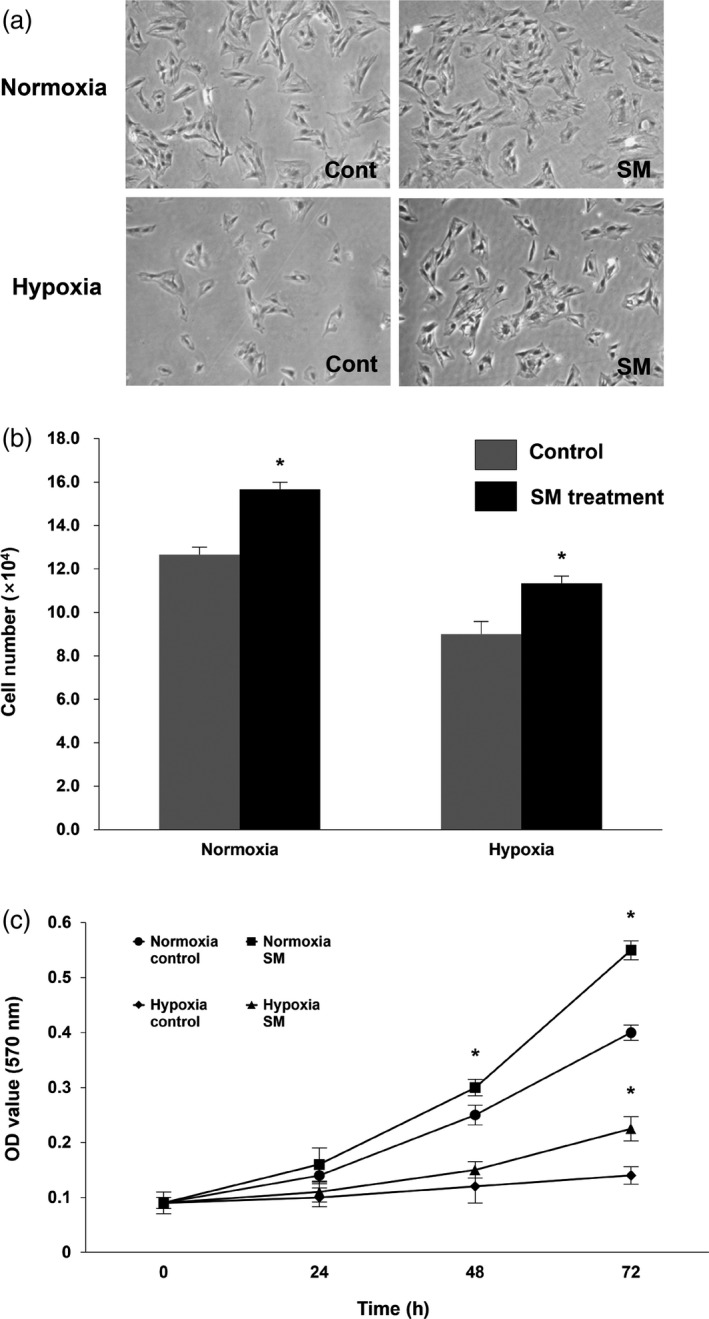
Cell survival of mesenchymal stem cells treated with Salvia miltiorrhiza Bunge under normoxic and hypoxic conditions (a) Quantification of mesenchymal stem cells treated with S, miltiorrhiza Bunge and cultured in normoxic and hypoxic conditions for 12 h: division into four groups (Normoxia‐control, Normoxia‐S. miltiorrhiza Bunge, Hypoxia‐control and Hypoxia‐S. miltiorrhiza Bunge; n = 6 per a group). (b) Statistical analysis demonstrated that the treatment of S. miltiorrhiza Bunge enhanced the survival of mesenchymal stem cells as compared to control, in normoxic conditions as well as hypoxic conditions. (c) The 3‐(4,5‐dimethylthiazolyl‐2)‐2, 5‐diphenyltetrazolium bromide assay (at 570 nm) of mesenchymal stem cells treated with S. miltiorrhiza Bunge in normoxic and hypoxic conditions. Columns, mean; bars, SE. *P < 0.05.
Effect of SM treatment on the expression of apoptosis‐related proteins in MSCs
To identify the effect of SM on apoptosis of MSCs, we examined the protein expression levels of the apoptosis‐related factors such as Bcl‐2, Caspase‐3 and Bax using the western blot technique. Bcl‐2 and Bax are implicated in the control of cell apoptosis, and Caspase‐3 is the key executioner of apoptosis.33, 34 Bcl‐2 is an inhibitor of apoptosis and a major player in the mechanism of anti‐apoptosis, while Bax and Caspase‐3 are pro‐apoptosis‐related proteins.35 The expression levels of pro‐apoptotic factors, Bax and Caspase‐3, in hypoxia control were 1.4‐fold and 2.4‐fold higher, respectively, than in normoxia control. However, they decreased 8.6‐ and 12‐fold in the expression levels of factors due to being treated with SM extract as compared to control under hypoxic conditions. On the other hand, the level of anti‐apoptotic factor Bcl‐2 in hypoxia control was 3.0‐fold lower than control in normoxic condition, whereas the level of the factor in the SM extract‐treated group was 2.0‐fold higher than the control group under hypoxia (Figure 4). In all experiments, we observed that fold difference between the control and SM‐treated groups in hypoxia clearly differed from the variance observed under normoxia. Taken together, these results clearly indicate that the treatment of SM downregulates the expression of pro‐apoptotic factors and upregulates the expression of anti‐apoptotic factors. Hence, we suggest that the treatment of SM inhibits apoptosis in ischemic stroke conditions.
Figure 4.
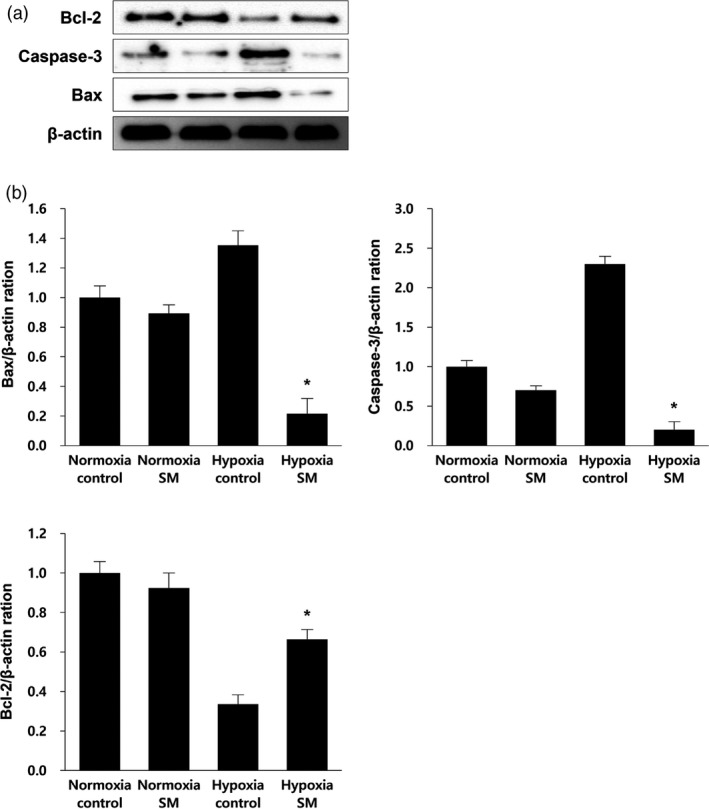
Effect of Salvia miltiorrhiza Bunge treatment on expression level of apoptosis‐related proteins in mesenchymal stem cells (a) Following treatment of S. miltiorrhiza Bunge and normoxic and hypoxic conditions, western blotting using antibodies Bcl‐2, Caspase‐3 and Bax was performed for detecting apoptosis‐related factors. (b) After treatment of S. miltiorrhiza Bunge in each condition, relative folds of Bcl‐2 (anti‐apoptosis related factor), Caspase‐3 and Bax (pro‐apoptosis related factors) were calculated by normalization to β‐actin. The expression level of pro‐apoptosis related factors in Hypoxia‐S. miltiorrhiza Bunge group was 6.5‐ and 7.6‐fold lower than in Hypoxia‐control group. On the other hand, the expression level of anti‐apoptosis‐related factor in Hypoxia‐S. miltiorrhiza Bunge group was 2.0‐fold higher than in Hypoxia‐control group. Columns, mean; bars, SE. *P < 0.05.
Effect of treatment of SM on the expression of cell survival‐related proteins in MSCs
The protein expression levels of survival‐related factors such as ERK and Akt were evaluated by western blot to help identify whether the treatment with SM has an effect on cell survival. The serine/threonine protein kinases are key regulatory proteins; ERK is associated with many cellular programmes such as cell death, motility and proliferation, and Akt is related to cell survival and growth.36, 37 Through phosphorylation of several targets, ERK and Akt promote cell survival.38 Figure 5b shows that the expression level of p‐Akt in SM‐treated MSCs under normoxic and hypoxic conditions was, respectively, 1.8‐ and 1.7‐fold higher than their controls, indicating a similar increasing trend in both conditions. The expression level of p‐ERK in SM‐treated MSCs was 2.3‐fold higher than in normoxia and 5.7‐fold higher than in hypoxia. Taken together, these results suggest that the treatment of SM enhances the phosphorylation of ERK and Akt, thereby promoting cell survival of MSCs in hypoxic conditions such as ischemic stroke, as well as in normoxic conditions.
Figure 5.
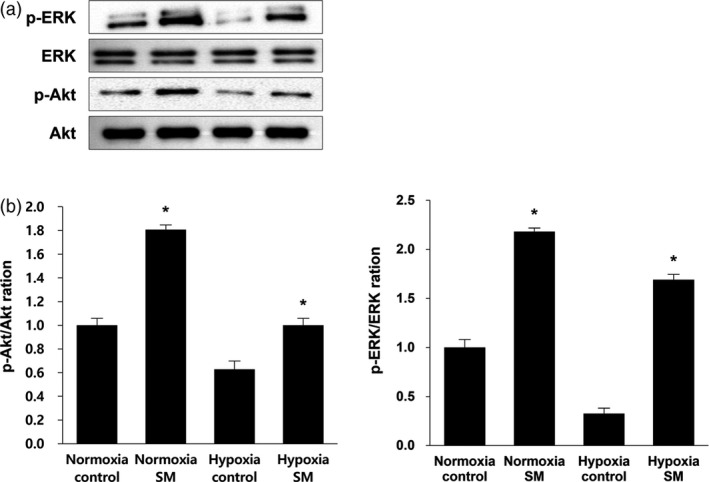
Effect of treatment of Salvia miltiorrhiza Bunge extract on expression of cell survival‐related proteins in mesenchymal stem cells (a) Western blotting indicated increase in mesenchymal stem cells’ viability by treatment of S. miltiorrhiza Bunge under normoxic and hypoxic conditions. (b) Following treatment of S. miltiorrhiza Bunge and normoxic and hypoxic conditions, the relative folds of p‐ERK and p‐Akt (cell survival‐related factors) were calculated by normalization to ERK and Akt. The expression of two survival factors increased in Hypoxia‐S. miltiorrhiza Bunge group by 1.6‐ and 4.5‐fold than in the Hypoxia‐control group. Columns, mean; bars, SE. *P < 0.05.
Effects of treatment of MSCs with SM on brain infarction
By measuring the brain infarct volumes of MCAo rat models stained by TTC, we evaluated the effect of SM treatment with MSCs on stroke. In Figure 6a and 6b, the infarct volume of control was 46 ± 2.2%, and the infarct volume of SM‐treatment alone was 41 ± 2.3%, thereby indicating no significant decrease in the infarct. However, the infarct volume of MSCs treatment (33 ± 1.7%) was 1.4‐fold lesser than the control, and treatment of MSCs with SM resulted in a 2.0‐fold decrease (23 ± 2.8%) as compared to the control. Additionally, measurement of brain water contents by the wet‐dry method showed a decrease in brain water content as 0.2%, 0.8% and 1.4% in the SM, MSCs and SM + MSCs groups, respectively, when compared to the control group (Figure 6c). Taken together, we construe that the combined treatment of SM and MSCs was apparently more effective than the treatment of SM or MSCs alone. Therefore, these data suggest that the SM extract supports the population of MSCs by increasing the survival rate of the cells, thereby affecting the recovery of the brain infarct.
Figure 6.
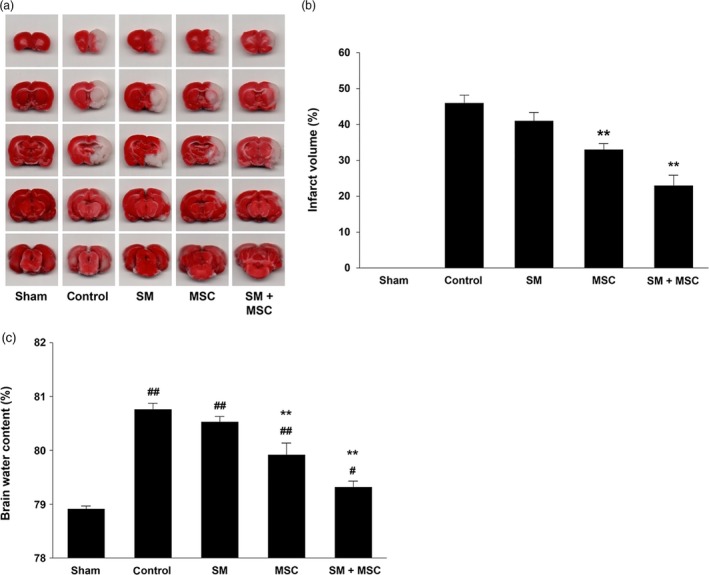
Effects on mesenchymal stem cells’ treatment with Salvia miltiorrhiza Bunge on infarcted volume and water content of ischemic brains (a,b) Representative images and statistical analysis of 2,3,5‐triphenyltetrazolium chloride staining in five different treatment groups at 2 weeks after middle cerebral artery occlusion/reperfusion. The 2,3,5‐triphenyltetrazolium chloride staining images indicated the unstained infarct area (white) and stained non‐infarct area (red). The 2,3,5‐triphenyltetrazolium chloride staining results demonstrated that the infarct volume in the group of mesenchymal stem cells treatment with S. miltiorrhiza Bunge significantly decreased compared to control group. (c) % water content of ischemic stroke brains was calculated by an equation: ×100 (wet weight − dry weight)/wet weight. The results indicated that treatment of mesenchymal stem cells with S. miltiorrhiza Bunge reduced the water content in injured brains than other treatment groups. Columns, mean; bars, SE. **: P < 0.01, compared with the values of the control group; #: P < 0.05, ##: P < 0.01, compared with the values of the sham operation group. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Enhancement of declined movement after ischemic stroke
The cylinder test was conducted to evaluate asymmetries in postural weight support and use of forelimbs during exploratory activity of the MCAo rat model.39 The control group exhibited increased asymmetry, and the use of the affected left forelimb was 4.1‐fold lower than the sham group during upright support. The SM‐treated group was also significantly not different from the control. However, in the group treated with MSCs only, the left forelimb use was 1.8‐fold greater than the control. Moreover, there was improved movement and more use of the left forelimb (3.0‐fold) compared with the control in the combined treatment of SM and MSCs (Figure 7a). In the forced swim test, we focused on a decrease in cumulative activity, composed of swimming, climbing and immobility times, as a result of stroke.40 The immobility time of the control group was 3.0‐fold higher than the sham group; however, all treatment groups showed a decreasing trend in their immobility times (Figure 7b). These results suggest that the combined treatment of SM and MSCs assists in the recovery of mobility.
Figure 7.
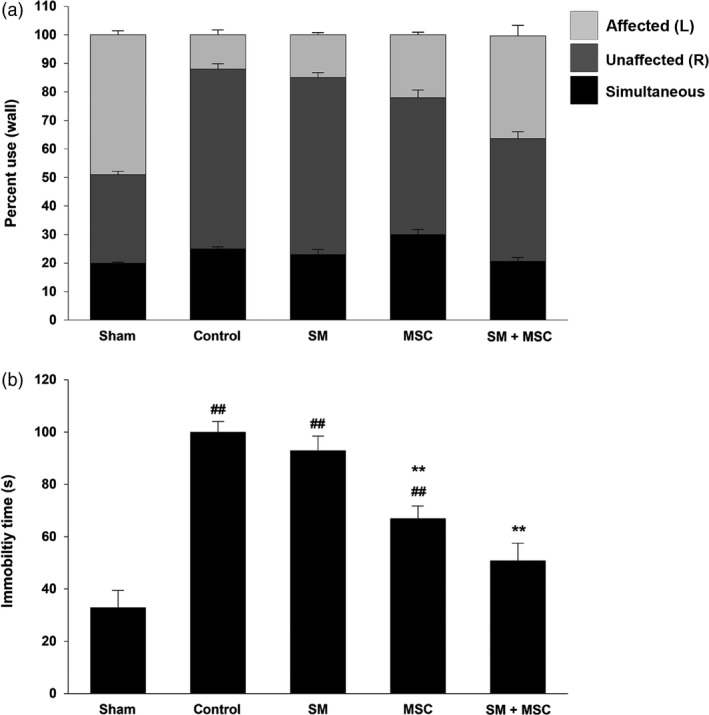
Restoration of attenuated movement in ischemic stroke following mesenchymal stem cells transplantation with Salvia miltiorrhiza Bunge (a) The percentage use of the affected (left), unaffected (right) and simultaneous limb was tested using cylinder test in the different treatment groups: Sham operation; control; S. miltiorrhiza Bunge only; mesenchymal stem cell only; mesenchymal stem cell treatment with S. miltiorrhiza Bunge. Treatment of mesenchymal stem cell with S. miltiorrhiza Bunge is the most effective method, showing the restoration of the percentage use of the affected limb. (b) The immobility time of animals in the group of treatment of mesenchymal stem cell with S. miltiorrhiza Bunge was reduced in a significant manner compared to other groups. Columns, mean; bars, SE. ##: P < 0.01, compared with the values of the sham operation group; **: P < 0.01, compared with the values of the control group.
Enhancement of transplantation of MSCs treated with SM on brain infarction
Immunohistochemical staining was performed to observe the survival of transplanted MSCs in the stroke region. The transplanted cells were stained red by PKH26 staining, and nuclei of whole cells stained blue by DAPI. Following the transplantation of stained MSCs with PBS or SM extract, we confirmed the increased survival of transplanted cells in the group of MSCs treatment with SM (Figure 8a). And then, the rate of labelled cells per whole cells was compared to a similar location as the stroke area of the two groups. The survival rate of transplanted MSCs with PBS group was approximately 4%, whereas survival in the SM‐treated group was about 2.5‐fold higher than the PBS group, at approximately 10% (Figure 8b). These results suggest that the treatment of SM extract enhances the survival rate of transplanted MSCs and may support their arrival and settlement in the ischemic stroke brain region.
Figure 8.
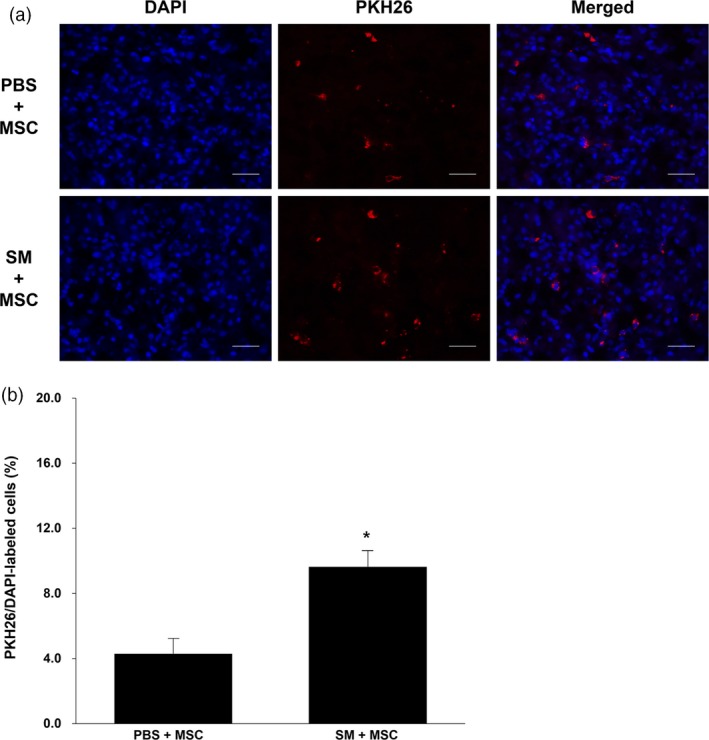
Cell survival of transplanted PKH26‐labelled mesenchymal stem cells in brain infarction (a) Representative images of localized PKH26‐labelled mesenchymal stem cells showed the ischemic areas of two different treatment groups (mesenchymal stem cells with phosphate‐buffered saline; mesenchymal stem cells with Salvia miltiorrhiza Bunge extract) by fluorescence microscope. Red = PKH26; blue = DAPI (b) Statistical analysis demonstrated that treatment of mesenchymal stem cells with S. miltiorrhiza Bunge extract enhanced the survival ratio of injected PKH26‐labelled mesenchymal stem cells compared to treatment of mesenchymal stem cells with phosphate‐buffered saline. Scale bars: 50 μm; Columns, mean; bars, SE. *P < 0.05. [Colour figure can be viewed at http://wileyonlinelibrary.com]
Discussion
In the present study, treatment of MSCs with SM was intravenously transplanted in the ischemic region, following rat MCAo. This study differs from other previous studies which confirmed the individual components at the site of injury. One study suggested that SM induced the differentiation of rat MSCs into neurons with neurophysiological functions.41 Since MSCs can differentiate into neuron‐like cells in vitro, subsequent studies suggested that the recovery of the infarcted area through MSCs transplantation is related to angiogenesis enhancing glial‐axonal remodelling and functional recovery on the region.23, 42 However, the degree of differentiation of transplanted MSCs was poor, and the effects of cell transplantation are unlikely to exert a strong beneficial effect for treatment of stroke due to ischemia.19 Therefore, we confirmed the synergetic effects of treatment of MSCs with SM for ischemic brain stroke therapy.
Proliferation assay revealed the most effective concentration of SM for MSCs’ proliferation (Figure 2). Using this concentration of SM for proliferating the MSCs, we compared the survival rates of MSCs in normoxic and hypoxic conditions. Additionally, we confirmed changes in MSCs’ viability by treatment with SM extract using MTT assay. Our results indicate the effects of SM extract on MSCs’ viability under hypoxic condition like the environment of ischemia area (Figure 3). Based on these results, we anticipated that the treatment of SM might affect the expression of apoptosis‐related proteins including caspase‐3, Bcl‐2 and Bax in MSCs. Previous studies demonstrated that the root of SM contains lipophilic and hydrophilic compounds such as Sal A, Sal B, salvianic borneol ester (SBE), tanshinone IIA (Tan IIA), rosmarinic acid, cryptotanshinone and danshensu, which decrease cell apoptosis and promote neuroprotective effects against oxidative injury through multiple pathways.43, 44 Using UHPLC‐UV/Q‐TOF‐MS method, we confirmed that the apoptosis‐related components were included in SM extract (Figure 1 and Table 1). Especially, Sal B presents anti‐apoptotic mechanisms such as inhibition of the caspase‐3 and Bax expression and enhancement of the Bcl‐2 expression. Sal A and rosmarinic acid are also known as components that reduce apoptosis via the decrease in the caspase‐3 expression against cytotoxicity and neurotoxicity.45, 46, 47 The data of western blotting assay are proposed that SM regulates the apoptosis of MSCs under the hypoxic condition (Figure 4). Furthermore, treatment with SM enhanced the expression of phosphorylated survival factors such as ERK and Akt in MSCs under hypoxia (Figure 5). The previous study also noted that danshensu amplified the expression level of p‐Akt promoting cell survival by inactivation and phosphorylation of pro‐apoptotic proteins in cerebral ischemic injury.48 These data indicate that the components of SM regulate the expression of cell survival‐related protein in MSCs under hypoxic conditions.
On the basis of our data, we confirmed the combinatory effects of SM and MSCs treatment in vivo by TTC staining, behaviour test and immunohistochemistry. Using TTC staining for measuring the brain infarct volume in the MCAo rat model, our results indicate that the combination of SM and MSCs is the most effective in recovery of brain infarct (Figure 6). In a previous study, SM promoted the survival of transplanted induced pluripotent stem cells (iPSCs)‐derived neural stem cells (NSCs) in vivo, and enhanced the recovery of rat ischemic brain region after transplantation.49 Furthermore, we confirmed that treatment of MSCs with SM derived positive effect for behavioural changes in result of two different behavioural tests (Figure 7). PKH26 immunohistochemistry further confirmed the effect of SM on the survival rate of MSCs in the ischemic region (Figure 8). Many studies have previously reported the effect of SM components on MSCs. Bi et al.50 established that Sal B protected spinal cord injury and improved the survival rate of MSCs in vivo and in vitro. Hu et al. (2011) suggested that the transplantation of MSCs with SM may be an efficient way in the treatment of neurodegenerative diseases.41 These results proposed a combination treatment of MSCs with SM could help increase the establishment of transplanted MSCs, leading to the restoration of the infarcted brain region by decreasing apoptosis and enhancing the survival of MSCs.
Conclusion
In conclusion, treatment of SM regulates the apoptotic and survival factors of MSCs, enhancing transplantation probability and viability of MSCs to significantly affect the recovery of ischemic stroke. This study overcomes the limitation of previous studies that applied only MSCs, and proposes synergetic therapeutic effects by combining herbal medicine and stem cell‐based therapies. The approach in the current study proposes a new experimental method of herbal medicine with stem cell‐based therapies for patients suffering from various diseases, including stroke as well as inflammation, liver cirrhosis, spinal cord injury and acute myocardial infarction.
Declarations
Conflict of interest
The authors report no conflicts of interest
Acknowledgements
This study was supported by the Traditional Korean Medicine R&D program funded by the Ministry of Health & Welfare through the Korea Health Industry Development Institute (KHIDI) (HI15C0163) and the Bio & Medical Technology Development Program of the National Research Foundation (NRF) funded by the Ministry of Science & ICT (NRF‐2017M3A9G7072568).
Ran Kim and Seokyeon Lee equally contributed to this work as joint first authors.
[Correction added on 10 July 2018, after first online publication: The author's name “Jee‐Young” is incorrect and has been corrected to “Jee‐Yeong” in this version.]
References
- 1. Ölmestig JNE et al Phosphodiesterase 5 inhibition as a therapeutic target for ischemic stroke: a systematic review of preclinical studies. Cell Signal 2017; 38: 39–48. [DOI] [PubMed] [Google Scholar]
- 2. Balkaya MG et al Behavioral outcome measures to improve experimental stroke research. Behav Brain Res 2017; S0166–4328: 30732–30735. [DOI] [PubMed] [Google Scholar]
- 3. Goldstein LB. Modern medical management of acute ischemic stroke. Methodist Debakey Cardiovasc J 2014; 10: 99–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Ip FC et al Neuroprotective effect of a novel Chinese herbal decoction on cultured neurons and cerebral ischemic rats. BMC Complement Altern Med 2016; 16: 437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Lin TH, Hsieh CL. Pharmacological effects of Salvia miltiorrhiza (Danshen) on cerebral infarction. Chin Med 2010; 5: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Wang L et al Treatment of stroke with erythropoietin enhances neurogenesis and angiogenesis and improves neurological function in rats. Stroke 2004; 35: 1732–1737. [DOI] [PubMed] [Google Scholar]
- 7. Rabinovich SS et al Cell therapy of brain stroke. Bull Exp Biol Med 2005; 139: 126–128. [DOI] [PubMed] [Google Scholar]
- 8. Schäbitz WR et al Effect of brain‐derived neurotrophic factor treatment and forced arm use on functional motor recovery after small cortical ischemia. Stroke 2004; 35: 992–997. [DOI] [PubMed] [Google Scholar]
- 9. Hung YC et al Adjuvant Chinese herbal products for preventing ischemic stroke in patients with atrial fibrillation. PLoS ONE 2016; 11: e0159333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Vakili A et al Role of arginine vasopressin V1 and V2 receptors for brain damage after transient focal cerebral ischemia. J Cereb Blood Flow Metab 2005; 25: 1012–1019. [DOI] [PubMed] [Google Scholar]
- 11. Lam BY et al Neuroprotective effects of tanshinones in transient focal cerebral ischemia in mice. Phytomedicine 2003; 10: 286–291. [DOI] [PubMed] [Google Scholar]
- 12. Qiao Z et al Evaluation of the antioxidant potential of Salvia miltiorrhiza ethanol extract in a rat model of ischemia‐reperfusion injury. Molecules 2011; 16: 10002–10012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Lo C et al Effect of salvia miltiorrhiza bunge on cerebral infarct in ischemia‐reperfusion injured rats. Am J Chin Med 2003; 31: 191–200. [DOI] [PubMed] [Google Scholar]
- 14. Ji XY et al Salvia miltiorrhiza and ischemic diseases. Acta Pharmacol Sin 2000; 21: 1089–1094. [PubMed] [Google Scholar]
- 15. Zhang H et al Contents of four active components in different commercial crude drugs and preparations of danshen (Salvia miltiorrhiza). Acta Pharmacol Sin 2002; 23: 1163–1168. [PubMed] [Google Scholar]
- 16. Yu XY et al Tanshinone IIB, a primary active constituent from Salvia miltiorrhza, exhibits neuro‐protective activity in experimentally stroked rats. Neurosci Lett 2007; 417: 261–265. [DOI] [PubMed] [Google Scholar]
- 17. Tang C et al The effects of Tanshinone IIA on blood‐brain barrier and brain edema after transient middle cerebral artery occlusion in rats. Phytomedicine 2010; 17: 1145–1149. [DOI] [PubMed] [Google Scholar]
- 18. Hosseini SM et al Combination cell therapy with mesenchymal stem cells and neural stem cells for brain stroke in rats. Int J Stem Cells 2015; 8: 99–105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dharmasaroja P. Bone marrow‐derived mesenchymal stem cells for the treatment of ischemic stroke. J Clin Neurosci 2009; 16: 12–20. [DOI] [PubMed] [Google Scholar]
- 20. Chung TN et al Adipose‐derived mesenchymal stem cells reduce neuronal death after transient global cerebral ischemia through prevention of blood‐brain barrier disruption and endothelial damage. Stem Cells Transl Med 2015; 4: 178–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Azad TD et al Neurorestoration after stroke. Neurosurg Focus 2016; 40: E2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Kopen GC et al Marrow stromal cells migrate throughout forebrain and cerebellum, and they differentiate into astrocytes after injection into neonatal mouse brains. Proc Natl Acad Sci USA 1999; 96: 10711–10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Wislet‐Gendebien S et al Plasticity of cultured mesenchymal stem cells: switch from nestin‐positive to excitable neuron‐like phenotype. Stem Cells 2005; 23: 392–402. [DOI] [PubMed] [Google Scholar]
- 24. Zhu J et al Enhanced angiogenesis promoted by human umbilical mesenchymal stem cell transplantation in stroked mouse is Notch1 signaling associated. Neuroscience 2015; 290: 288–299. [DOI] [PubMed] [Google Scholar]
- 25. Chen J et al Intravenous administration of human bone marrow stromal cells induces angiogenesis in the ischemic boundary zone after stroke in rats. Circ Res 2003; 92: 692–699. [DOI] [PubMed] [Google Scholar]
- 26. Chen X et al Ischemic rat brain extracts induce human marrow stromal cell growth factor production. Neuropathology 2002; 22: 275–279. [DOI] [PubMed] [Google Scholar]
- 27. Chen J et al Intravenous bone marrow stromal cell therapy reduces apoptosis and promotes endogenous cell proliferation after stroke in female rat. J Neurosci Res 2003; 73: 778–786. [DOI] [PubMed] [Google Scholar]
- 28. Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem 2006; 98: 1076–1084. [DOI] [PubMed] [Google Scholar]
- 29. Hu XY et al Tongxinluo promotes mesenchymal stem cell tube formation in vitro . J Zhejiang Univ Sci B 2011; 12: 644–651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Guo JW et al Combinatorial effects of naomai yihao capsules and vascular endothelial growth factor gene‐transfected bone marrow mesenchymal stem cells on angiogenesis in cerebral ischemic tissues in rats. J Tradit Chin Med 2012; 32: 87–92. [DOI] [PubMed] [Google Scholar]
- 31. Zhang YK et al Effects of buyang huanwu tang combined with bone marrow mesenchymal stem cell transplantation on the expression of VEGF and Ki‐67 in the brain tissue of the cerebral ischemia‐reperfusion model rat. J Tradit Chin Med 2010; 30: 278–282. [DOI] [PubMed] [Google Scholar]
- 32. Oh TW et al Neuroprotective effect of the hairy root extract of Angelica gigas NAKAI on transient focal cerebral ischemia in rats through the regulation of angiogenesis. BMC Complement Altern Med 2015; 15: 101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Selvakumaran M et al Immediate early up‐regulation of bax expression by p53 but not TGF beta 1: a paradigm for distinct apoptotic pathways. Oncogene 1994; 9: 1791–1798. [PubMed] [Google Scholar]
- 34. Cohen GM. Caspases: the executioners of apoptosis. Biochem J 1997; 326: 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol 2007; 35: 495–516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Roux PP, Blenis J. ERK and p38 MAPK‐activated protein kinases: a family of protein kinases with diverse biological functions. Microbiol Mol Biol Rev 2004; 68: 320–344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pap M, Cooper GM. Role of glycogen synthase kinase‐3 in the phosphatidylinositol 3‐Kinase/Akt cell survival pathway. J Biol Chem 1998; 273: 19929–19932. [DOI] [PubMed] [Google Scholar]
- 38. Cardone MH et al Regulation of cell death protease caspase‐9 by phosphorylation. Science 1998; 282: 1318–1321. [DOI] [PubMed] [Google Scholar]
- 39. Schallert T et al CNS plasticity and assessment of forelimb sensorimotor outcome in unilateral rat models of stroke, cortical ablation, parkinsonism and spinal cord injury. Neuropharmacology 2000; 39: 777–787. [DOI] [PubMed] [Google Scholar]
- 40. Buga AM et al Up‐regulation of serotonin receptor 2B mRNA and protein in the peri‐infarcted area of aged rats and stroke patients. Oncotarget 2016; 7: 17415–17430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hu L et al Effects of Salvia miltorrhiza in neural differentiation of rat mesenchymal stem cells with optimized protocol. J Ethnopharmacol 2011; 136: 334–340. [DOI] [PubMed] [Google Scholar]
- 42. Zhang ZG et al Correlation of VEGF and angiopoietin expression with disruption of blood‐brain barrier and angiogenesis after focal cerebral ischemia. J Cereb Blood Flow Metab 2002; 22: 379–392. [DOI] [PubMed] [Google Scholar]
- 43. Bonaccini L et al Effects of Salvia miltiorrhiza on CNS Neuronal Injury and Degeneration: a Plausible Complementary Role of Tanshinones and Depsides. Planta Med 2015; 81: 1003–1016. [DOI] [PubMed] [Google Scholar]
- 44. Zhang XZ et al Salvia miltiorrhiza: a source for anti‐Alzheimer's disease drugs. Pharm Biol 2016; 54: 18–24. [DOI] [PubMed] [Google Scholar]
- 45. Tian LL et al Salvianolic acid B, an antioxidant from Salvia miltiorrhiza, prevents 6‐hydroxydopamine induced apoptosis in SH‐SY5Y cells. Int J Biochem Cell Biol 2008; 40: 409–422. [DOI] [PubMed] [Google Scholar]
- 46. Wang XJ, Xu JX. Salvianic acid A protects human neuroblastoma SH‐SY5Y cells against MPP+‐induced cytotoxicity. Neurosci Res 2005; 51: 129–138. [DOI] [PubMed] [Google Scholar]
- 47. Iuvone T et al The spice sage and its active ingredient rosmarinic acid protect PC12 cells from amyloid‐beta peptide‐induced neurotoxicity. J Pharmacol Exp Ther 2006; 317: 1143–1149. [DOI] [PubMed] [Google Scholar]
- 48. Guo Y et al Salvia miltiorrhiza: an ancient Chinese herbal medicine as a source for anti‐osteoporotic drugs. J Ethnopharmacol 2014; 155: 1401–1416. [DOI] [PubMed] [Google Scholar]
- 49. Shu T et al Effects of Salvia miltiorrhiza on neural differentiation of induced pluripotent stem cells. J Ethnopharmacol 2014; 153: 233–241. [DOI] [PubMed] [Google Scholar]
- 50. Bi XB et al Salvianolic acid B promotes survival of transplanted mesenchymal stem cells in spinal cord‐injured rats. Acta Pharmacol Sin 2008; 29: 169–176. [DOI] [PubMed] [Google Scholar]


