Abstract
Adapalene 0.1% (ADA) with clindamycin phosphate 1.2% (CLNP; ADA + CLNP) and the fixed‐dose combination containing CLNP and benzoyl peroxide 3% (CLNP/BPO 3%) are strongly recommended for the early treatment of acne vulgaris in Japan. Here, we compare the early efficacy and safety of CLNP/BPO 3% with Japanese standard topical use of ADA + CLNP in the treatment of acne vulgaris. In this phase IV, multicenter study, 351 patients were randomized to receive CLNP/BPO 3% or ADA + CLNP for 12 weeks. The primary end‐point was percentage change from baseline in total lesion (TL) counts at week 2. Secondary end‐points included the percentage change from baseline in TL, inflammatory and non‐inflammatory lesion (IL and non‐IL) counts, Investigator's Static Global Assessment (ISGA), quality of life (QoL [Skindex‐16]) and patient preference. Local tolerability scores and adverse events were also recorded. CLNP/BPO 3% provided a significantly greater percentage reduction from baseline in TL compared with ADA + CLNP at week 2, and week 4. Compared with ADA + CLNP, CLNP/BPO 3% was superior at reducing IL (but not non‐IL) over weeks 2–12, was more effective at improving patient QoL and ISGA, and scored higher in patient‐preference assessments. Both treatments were well tolerated; adverse drug reactions occurred more frequently in patients receiving ADA + CLNP (37%) than in those receiving CLNP/BPO 3% (17%). In conclusion, CLNP/BPO 3% showed greater efficacy for the early treatment of acne vulgaris in Japan, with a more favorable safety profile compared with ADA + CLNP.
Keywords: acne vulgaris, adapalene, benzoyl peroxide, clindamycin, drug combinations
Introduction
Acne vulgaris is a common skin condition that affects males and females of various ages worldwide, with a global prevalence of 9.4%.1 Acne vulgaris occurs when excess sebum (androgen‐dependent) and keratinocytes combine to form a microcomedo, which blocks excretion of sebum from the hair follicle within the pilosebaceous unit.2 Proliferation of Propionibacterium acnes in the microcomedo leads to local inflammation. These events cause both non‐inflammatory open and closed comedones to become inflammatory papules, pustules and nodules.2 Acne vulgaris is generally accepted as a chronic and relapsing inflammatory condition of varying degrees of severity, which may require long‐term treatment.2 Acne therapies can be classified as either intervention treatments, which target the characteristic inflammatory and non‐inflammatory lesions (IL and non‐IL); maintenance treatments to help prevent disease relapse and adjunctive therapies for disease sequelae, such as scars and post‐inflammatory hyperpigmentation.2
The first edition of the Japanese acne treatment guidelines was published in 2008 after the introduction of the topical retinoid adapalene (ADA) 0.1% in Japan for the treatment of acne vulgaris.3 As treatment options in 2008 were limited to ADA and antibacterial drugs, the use of ADA with a topical antibacterial drug was recommended as the standard treatment regimen (note: benzoyl peroxide [BPO] has not been approved at this time). However, the use of ADA with clindamycin phosphate (CLNP) 1.2% requires patients apply two separate topical products, which may result in lower adherence to therapy compared with a single‐application product.
In 2015, topical BPO as monotherapy and a combination gel containing CLNP and BPO 3% (CLNP/BPO 3%) were approved in Japan. With the increasing availability of acne treatment options, particularly BPO‐containing products, treatment guidelines were revised in 2016. These guidelines provided treatment recommendations for the “acute inflammatory phase” (up to 3 months, in principle) for decreasing acne lesions (particularly IL) and the “maintenance phase” for prevention of new lesions and emergence of antibiotic‐resistant P. acnes.4
The current standard regimen for first‐line treatment of mild to moderate acne in Japan is the use of topical ADA with a topical antibacterial drug (excluding BPO) (recommendation level A in the Japanese acne treatment guidelines).4 CLNP/BPO 3% fixed‐dose combination gel also has recommendation level A and both are strongly recommended in these guidelines.4 As CLNP/BPO 3% has shown to be efficacious at reducing total lesions (TL), IL and non‐IL compared with twice‐daily CLNP monotherapy (evaluated an investigational dosing regimen as an application over the entire face),5 it may address the unmet medical need of achieving fast resolution of lesions due to the slow onset of action of topical acne therapies, which is often disappointing for patients and may lead to decreased adherence, in turn diminishing the likelihood of achieving optimal outcomes. To date, there have been no studies comparing CLNP/BPO 3% with the standard treatment, namely the use of ADA with a topical antibacterial drug.
This study aims to evaluate the early efficacy (at week 2) and the safety (throughout the study) of CLNP/BPO 3% in comparison with ADA + CLNP for the treatment of acne vulgaris in Japanese patients.
Methods
Study design
This study was an interventional phase IV, multicenter (15 centers in Japan), randomized, investigator‐blinded, active‐controlled, parallel‐group study in Japanese patients with facial acne vulgaris. Patients who provided written informed consent were randomized (using validated internal software) in a 1:1 ratio to receive either CLNP/BPO 3% or ADA + CLNP for 12 weeks. Patients were assessed at screening (baseline) and at weeks 1, 2, 4, 8 and 12 of treatment.
This study (ClinicalTrials.gov identifier NCT02557399, GSK study no. 201884) was conducted from October 2015 to February 2016 in accordance with the Ministerial Ordinance on the Standards for the Conduct of Clinical Trials of Medicinal Products (MHW notification no. 18, dated 27 March 1997) and Articles 14‐3 and 80‐2 of the Pharmaceutical Affairs Law as well as the guidelines laid out in the International Conference on Harmonisation of Technical Requirements for Registration of Pharmaceuticals for Human Use Good Clinical Practice and the Declaration of Helsinki of 2008.
Patients
Eligible participants were male or female, were 12–45 years old and had facial acne vulgaris (defined as 17–60 IL [papules and pustules] and 20–150 non‐IL [open/closed comedones] on the face, including nasal lesions), with an Investigator's Static Global Assessment (ISGA) score of 2 or more (mild to severe) at baseline. Women of child‐bearing age and less than 2 years from their last menstruation had to agree to avoid becoming pregnant.
Key exclusion criteria included nodulocystic lesions at baseline, a medical history suggestive of an immunocompromised status and receipt of certain medications within 2 weeks before baseline (e.g. topical facial antibiotics and systemic antibiotics, topical anti‐acne medications, non‐mild facial cleansers or products containing glycolic or other acids). Patients were also excluded if they had used topical corticosteroids or had a facial procedure, used systemic retinoids or received treatment with estrogens, androgens or anti‐androgenic agents (all within specified periods before baseline). Pregnant or breast‐feeding patients were also excluded.
Treatments
Patients received instructions on topical application of: (i) CLNP/BPO 3% gel (Duac® Combination Gel; Stiefel Laboratories, a GlaxoSmithKline company); or (ii) ADA (Differin™ Gel 0.1%; Galderma, Tokyo, Japan) with CLNP (clindamycin phosphate gel 1% Sawai; Sawai Pharmaceutical, Osaka, Japan). CLNP/BPO 3% or ADA gel was applied once daily in the evening before bedtime, in a quantity sufficient to cover the entire face. CLNP was only applied to IL in the morning and after applying ADA in the evening. Study products were applied according to package insert instructions. Investigators responsible for end‐point assessments did not have access to or administrate the study products and were prohibited from collecting information regarding the investigational products or compliance records.
End‐points and assessments
The primary end‐point was percentage change from baseline in TL at week 2. This time point was chosen to assess the early efficacy of the treatments, corresponding to the usual first treatment review visit for patients with acne in Japan. Key secondary efficacy end‐points included the percentage changes in TL at subsequent visits, change in IL and non‐IL counts from baseline and disease severity. Additional end‐points included safety, local tolerability, treatment compliance and quality of life (QoL) and patient preference assessments.
All facial IL (erythematous papules, pustules and cystic/nodular lesions) and non‐IL (open and closed comedones; confirmed by palpation) were counted by investigators at baseline and at each visit on weeks 1, 2, 4, 8 and 12 or study end.
Disease severity was assessed at weeks 1, 2, 4, 8 and 12 by investigators using the ISGA, a 6‐point scale to assess the severity of acne vulgaris, from clear (0) to very severe.5 The ISGA scale includes one additional value (score 5, very severe) to the IGA scale recommended in the US Food and Drug Administration guidelines (refer to footnote in Table 1)6 and its use is consistent with previous studies.5, 7 The facial area was used to evaluate lesion counts and ISGA scores throughout the study. Patient QoL was assessed at baseline and at weeks 2, 4, 8 and 12 using the Skindex‐16 questionnaire, a dermatological‐specific questionnaire that evaluates a patient's symptoms, emotions and functioning over the previous week of treatment that has been validated for use in Japanese subjects.8, 9
Table 1.
Patient demographics and disease characteristics at baseline (ITT)
| CLNP/BPO 3% (n = 172) | ADA + CLNP (n = 177) | |
|---|---|---|
| Age, mean (SD) | 20.3 (5.9) | 19.8 (4.9) |
| <16 years, n (%) | 41 (23.8) | 36 (20.3) |
| 16–20 years, n (%) | 61 (35.5) | 68 (38.4) |
| >20 years, n (%) | 70 (40.7) | 73 (41.2) |
| Sex, n (%) | ||
| Female | 97 (56.4) | 110 (62.1) |
| Male | 75 (43.6) | 67 (37.9) |
| Weight (kg), mean (SD) | 57.0 (9.6) | 54.8 (9.0) |
| Lesion counts at baseline, mean (SD) | ||
| TL | 102.7 (35.7) | 101.9 (36.6) |
| IL | 32.4 (11.8) | 31.8 (12.5) |
| Non‐IL | 70.4 (31.0) | 70.1 (31.8) |
| Total lesion count at baseline, n (%) | ||
| <71 | 37 (21.5) | 41 (23.2) |
| 71–140 | 111 (64.5) | 107 (60.4) |
| <140 | 24 (14.0) | 29 (16.4) |
| ISGA at baseline,† n (%) | ||
| 2 (mild) | 31 (18.0) | 43 (24.3) |
| 3 (moderate) | 114 (66.3) | 113 (63.8) |
| 4 (severe) | 27 (15.7) | 21 (11.9) |
†ISGA is a 6‐point scale ranging from 0 (clear) to 5 (very severe), defined as follows: 0 (clear), clear skin with no IL or non‐IL; 1 (almost clear), rare non‐IL with no more than rare papules; 2 (mild), greater than grade 1, some non‐IL with no more than a few IL (papules/pustules only, no nodular lesions); 3 (moderate), greater than grade 2, up to many non‐IL and may have some IL, but no more than one small nodular lesion; 4 (severe), greater than grade 3, up to many non‐IL and IL, but no more than a few nodular lesions; and 5 (very severe), many non‐IL and IL, and more than a few nodular lesions. May have cystic lesions. ADA, adapalene; BPO, benzoyl peroxide; CLNP, clindamycin phosphate; IL, inflammatory lesion; ISGA, Investigator's Static Global Assessments; ITT, intent‐to‐treat; SD, standard deviation; TL, total lesion.
Patient preference was assessed at weeks 1, 2, 4, 8 and 12 using a questionnaire that evaluated ease of application, comfort, satisfaction in comparison with prior therapies and willingness to continue using the product. Each index was scored on a 5‐point scale where 1 indicated “not at all” and 5 “very much”.
Treatment compliance was assessed at weeks 1, 2, 4, 8 and 12. Patients were asked to record their compliance in the study log at each visit. Compliance was calculated as the percentage of treatment applications of CLNP/BPO 3% and ADA (but not CLNP) logged by each patient against the planned number of applications as specified per protocol.
Local tolerability was assessed using a local tolerability assessment scale, from absent (0) to severe (4). At each visit, patients assessed their itching and burning/stinging symptoms, and skin dryness, peeling and erythema were assessed by the investigators.
The safety of both treatments was assessed by recording all adverse events (AE) that occurred during the study, which were classified using the current Medical Dictionary for Regulatory Activities. As moisturizers are often used to help prevent local adverse skin reactions and improve treatment adherence, patients were asked to confirm their use of moisturizer throughout the study. Non‐comedogenic, powder‐based (liquid or solid) cosmetics or make‐up were allowed, if removed at least 30 min before each study visit. Cosmetic lotions were not considered to be moisturizers in subgroup analyses, as the moisturizing effect of these products is negligible.
Statistical analysis and patient populations
A total of 350 patients were randomized with a 1:1 ratio to achieve more than 90% power to detect a treatment difference of 10% in the percentage change from baseline in TL at week 2 (estimated at 45% vs 35% for CLNP/BPO 3% and ADA + CLNP groups, respectively, with a common standard deviation of 28%) using a Student's t‐test (two‐sided significance level of 5%).
All efficacy and safety analyses were performed on the intent‐to‐treat (ITT) population, which comprised all randomized patients who received at least one application of study product. Subgroup analyses (age, sex, baseline TL, baseline ISGA, use of moisturizing agent during study period) were performed for change from baseline in TL and safety end‐points; “use of moisturizing agent at baseline” subgroup was considered for safety end‐points only. The primary end‐point, the percentage change from baseline in TL counts at week 2, was analyzed using a mixed model for repeated measures (MMRM). The fitted model included treatment, center, visit and treatment‐by‐visit interaction as fixed categorical effects and baseline TL counts and baseline‐by‐visit interaction as fixed continuous effects. An unstructured variance structure was used to model the within‐subject errors. The treatment difference was tested based on the fitted model at the two‐sided significance level of 5%. The least squares means difference and the corresponding 95% confidence interval (CI) were estimated.
Continuous variables in the secondary end‐points were also analyzed using MMRM and an unadjusted analysis (post‐hoc) that was performed to determine the unadjusted mean treatment difference in percentage change in TL count, and absolute change in TL, IL and non‐IL counts. The treatment difference by visit and the corresponding 95% CI were estimated based on the fitted model for each variable using the MMRM. For the unadjusted analysis, the mean treatment difference and corresponding 95% CI were calculated using observed data. For dichotomized variables in secondary end‐points, the treatment difference in proportion at each visit was tested using the Cochran–Mantel–Haenszel test stratified by center. The treatment difference in patient preference at each visit was tested using the Wilcoxon rank sum test. The multiplicity for secondary end‐points was not adjusted. The local tolerability score was summarized by treatment group. The statistical software package SAS® (SAS Institute, Cary, NC, USA; release 9.3 for UNIX/Linux platform) was used for all analyses in this study.
Results
Study population and patient disposition
Of the 351 patients enrolled, 174 were randomized to the CLNP/BPO 3% group and 177 were randomized to the ADA + CLNP group; 349 patients were included in the ITT population (Fig. 1). Overall, six (3%) and five (3%) patients withdrew from the CLNP/BPO 3% and ADA + CLNP groups, respectively, owing to AE. A total of 165 (96%) and 169 (95%) patients in the CLNP/BPO 3% and ADA + CLNP groups, respectively, completed this study. Patient demographics and baseline characteristics were similar between the groups; the majority of patients were female and approximately 40% were more than 20 years old (Table 1).
Figure 1.
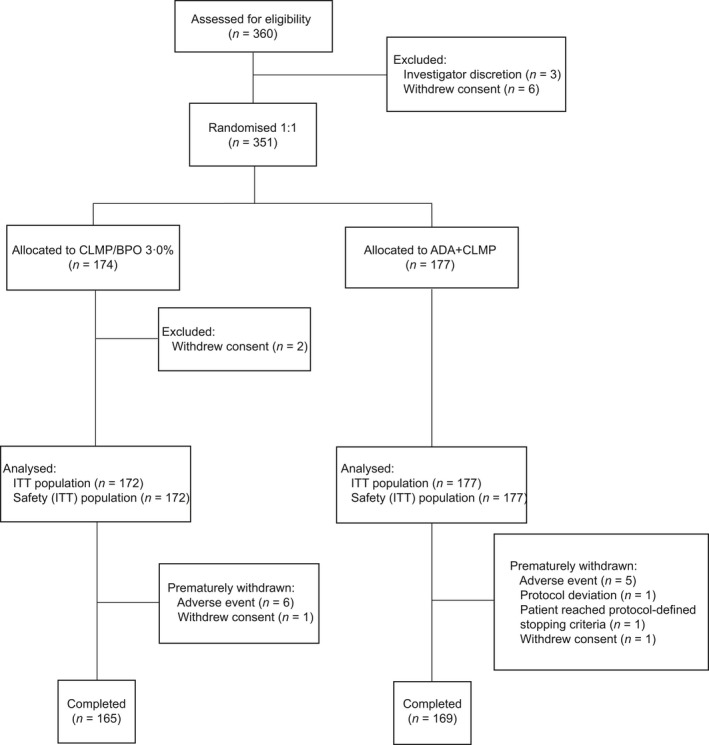
Disposition of patients and study population. ADA, adapalene; BPO, benzoyl peroxide; CLNP, clindamycin phosphate; ITT, intent‐to‐treat.
Efficacy
Percentage change in total lesion count at week 2 (primary end‐point)
After 2 weeks of treatment, there was evidence of greater efficacy in the CLNP/BPO 3% group compared with the ADA + CLNP group in reducing TL. Both treatments reduced TL from baseline, but there was a statistically significant difference in the adjusted mean percentage changes from baseline at week 2 in TL between the CLNP/BPO 3% (−42.2%) and ADA + CLNP (−35.3%) groups, in favor of CLNP/BPO 3% (treatment difference: −6.83% [95% CI, −11.88 to −1.78]; P = 0.008) (Fig. 2a, Table 2). A post‐hoc analysis of unadjusted mean treatment difference revealed that the difference in percentage change in TL between CLNP/BPO 3% and ADA + CLNP was −6.92 (95% CI, −12.64 to −1.20) at week 2.
Figure 2.
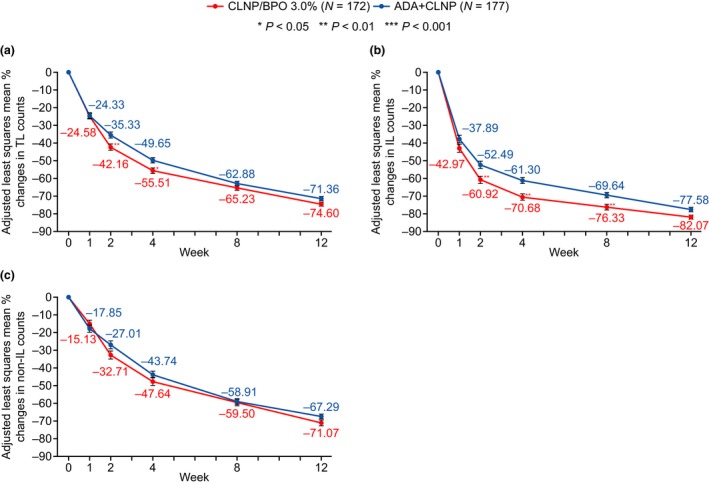
The adjusted mean percentage reduction from baseline over 12 weeks in (a) total lesion (TL), (b) inflammatory lesion (IL) and (c) non‐IL counts. Error bar represents standard error of the mean. Statistical analysis used a mixed model for repeated measures (MMRM) with treatment, center, visit and treatment‐by‐visit interaction as fixed categorical effects and baseline lesion counts and baseline‐by‐visit interaction as fixed continuous effects. The adjusted means and P‐values for treatment difference at each visit were calculated based on the fitted MMRM model. ADA, adapalene; BPO, benzoyl peroxide; CLNP, clindamycin phosphate.
Table 2.
Mean % reduction from baseline to week 2 in total lesion counts (primary end‐point) for clindamycin phosphate 1.2% (CLNP)/benzoyl peroxide (BPO) 3% fixed‐dose combination gel versus combination therapy of adapalene 0.1% (ADA) + CLNP (MMRM analysis [ITT population])
| CLNP/BPO 3% (n = 172) | ADA + CLNP (n = 177) | |
|---|---|---|
| n | 169 | 176 |
| Mean (SD) | −46.3 (24.4) | −39.3 (29.3) |
| Adjusted mean (SE) | −42.2 (1.9) | −35.3 (1.9) |
| Difference vs ADA + CLNP (MMRM) | −6.83 | |
| Difference vs ADA + CLNP (unadjusted; post‐hoc) | −6.92 | |
| 95% CI for treatment difference (MMRM) | −11.88 to −1.78 | |
| 95% CI for treatment difference (unadjusted; post‐hoc) | −12.64 to −1.20 | |
| P‐value (MMRM) | 0.008 | |
| P‐value (unadjusted; post‐hoc) | 0.018 | |
ADA, adapalene; BPO, benzoyl peroxide; CI, confidence interval; CLNP, clindamycin phosphate; ITT, intent‐to‐treat; MMRM, mixed model for repeated measures SD, standard deviation; SE, standard error.
Inflammatory, non‐inflammatory and calculated TL (secondary end‐points)
Treatment with CLNP/BPO 3% and ADA + CLNP resulted in a progressive decrease in TL, IL and non‐IL across the study (Fig. 2). For TL, there was a statistically significant difference in percentage change from baseline between treatment groups at weeks 2 and 4 in favor of CLNP/BPO 3% treatment (P = 0.008 and 0.01, respectively; Fig. 2a). Absolute lesion count change from baseline is shown in Table 3. There was a statistically significant greater reduction in absolute TL count with CLNP/BPO 3% treatment at week 2 (treatment difference −6.2 [95% CI, −11.2 to −1.21; P = 0.015]) and week 4 (treatment difference −4.7 [95% CI, −9.2 to −0.1; P = 0.044]). The analysis using MMRM also demonstrated a significant treatment–visit interaction, indicating that both percentage reduction and absolute reduction in TL in the two treatment arms was different over time (P = 0.016 and 0.015, respectively).
Table 3.
Mean change from baseline from week 1 to week 12 in total lesion counts, inflammatory lesion counts, and non‐inflammatory lesion counts after CLNP/BPO 3% or ADA + CLNP treatment (ITT population)
| Total lesion count | Inflammatory lesion count | Non‐inflammatory lesion count | ||||
|---|---|---|---|---|---|---|
| CLNP/BPO 3% (n = 172) | ADA + CLNP (n = 177) | CLNP/BPO 3% (n = 172) | ADA + CLNP (n = 177) | CLNP/BPO 3% (n = 172) | ADA + CLNP (n = 177) | |
| Week 1 | ||||||
| n | 172 | 176 | 172 | 176 | 172 | 176 |
| Mean (SD) | −28.5 (25.64) | −28.0 (23.30) | −14.4 (11.12) | −12.4 (10.31) | −14.1 (20.35) | −15.5 (19.73) |
| Week 2 | ||||||
| n | 169 | 176 | 169 | 176 | 169 | 176 |
| Mean (SD) | −45.9 (27.08) | −39.3 (31.61) | −20.5 (11.20) | −17.3 (11.27) | −25.4 (22.18) | −22.0 (22.86) |
| Week 4 | ||||||
| n | 169 | 174 | 169 | 174 | 169 | 174 |
| Mean (SD) | −60.4 (31.52) | −54.7 (33.25) | −23.7 (10.85) | −20.5 (11.90) | −36.8 (26.99) | −34.2 (26.91) |
| Week 8 | ||||||
| n | 167 | 172 | 167 | 172 | 167 | 172 |
| Mean (SD) | −70.7 (33.88) | −68.8 (34.87) | −25.5 (11.11) | −23.1 (12.08) | −45.2 (28.43) | −45.7 (29.19) |
| Week 12 | ||||||
| n | 164 | 169 | 164 | 169 | 164 | 169 |
| Mean (SD) | −80.7 (34.03) | −78.1 (36.33) | −27.2 (11.02) | −25.6 (11.71) | −53.5 (28.40) | −52.5 (31.46) |
ADA, adapalene; BPO, benzoyl peroxide; CLNP, clindamycin phosphate; ITT, intent‐to‐treat; SD, standard deviation.
Clindamycin phosphate/BPO 3% also reduced IL (percentage change) to a statistically significant greater extent compared with ADA + CLNP treatment at week 2 through week 12 (P < 0.05; Fig. 2b). There was a significant treatment difference in absolute reduction in IL in favor of CLNP/BPO 3% at weeks 2, 4 and 8 (P = 0.002, 0.002 and 0.012, respectively).
No statistically significant difference was observed between treatment groups for reduction in non‐IL at any time point in terms of percentage change from baseline or absolute change from baseline (Fig. 2c, Table 3).
Post‐hoc analyses of the unadjusted mean lesion count at week 2 were performed for TL, IL and non‐IL. Mean unadjusted treatment difference between groups in absolute change in TL between CLNP/BPO 3% and ADA + CLNP was −6.6 (95% CI, −12.9 to −0.4). For IL, the mean unadjusted treatment difference in absolute change was −8.2 (95% CI, −14.9 to −1.5). For non‐IL, mean unadjusted treatment difference in absolute change was −3.4 (95% CI, −8.5 to 1.7).
There was no clear trend for the differences in percentage change from baseline to week 12 in TL across subgroups (age, sex, baseline TL, baseline ISGA, use of moisturizing agent during study period) compared with the overall population.
ISGA
The proportion of patients who showed a 2 or more grade improvement in ISGA score increased at each time point for both therapies throughout the study (Fig. 3a). The proportion of patients who achieved a 2 or more grade improvement in ISGA score from baseline was significantly higher with CLNP/BPO 3% compared with ADA + CLNP at weeks 8 (22% vs 12%, respectively [P = 0.006]) and 12 (37% vs 27%, respectively [P = 0.022]). Both treatments increased the proportion of patients with an ISGA score of 0 (clear) or 1 (almost clear) from week 1 to week 12 (Fig. 3b). At weeks 4, 8 and 12, CLNP/BPO 3% was associated with a significantly greater improvement in ISGA scores compared with ADA + CLNP (P = 0.016, 0.034 and 0.018, respectively).
Figure 3.
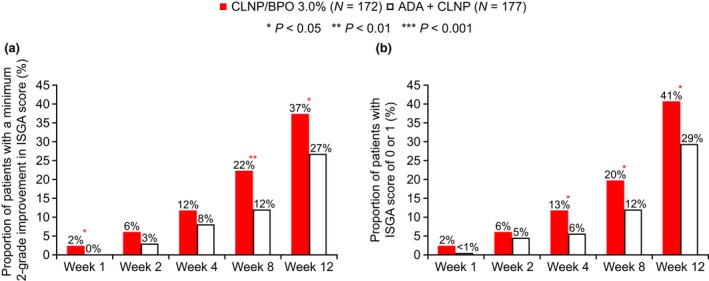
Proportion of patients with (a) at least a two‐grade improvement in Investigator's Static Global Assessment (ISGA) score or (b) with an Investigator's Static Global Assessment (ISGA) score of 0 or 1 at each study visit. Statistical analysis used the Cochran–Mantel–Haenszel test. ADA, adapalene; BPO, benzoyl peroxide; CLNP, clindamycin phosphate.
QoL and patient preference
Treatment with CLNP/BPO 3% resulted in improved total Skindex‐16 scores (symptom, emotion and functioning scores) across the study compared with ADA + CLNP (Fig. 4), owing mainly to patients in the CLNP/BPO 3% group scoring more favorably than the ADA + CLNP group on their symptoms (Fig. 4b). The mean change from baseline in Skindex‐16 total scores was statistically significantly greater in the CLNP/BPO 3% group compared with the ADA + CLNP group at weeks 2 (P = 0.017), 4 (P = 0.017) and 8 (P = 0.024), and was numerically greater at week 12 (P = 0.080). There was no significant difference between treatment groups for emotion and functioning scores.
Figure 4.
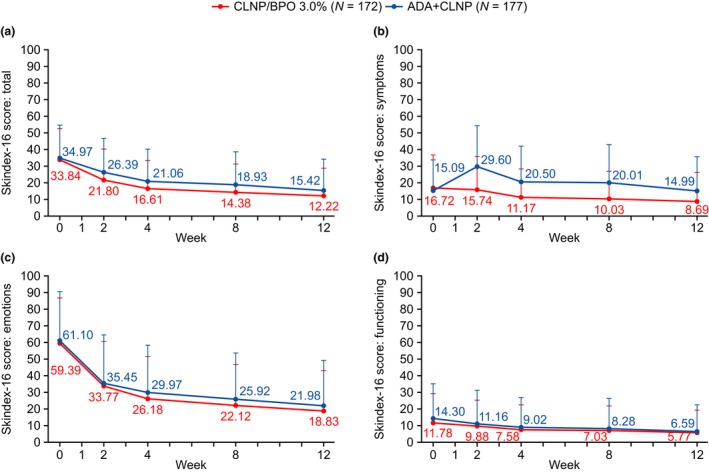
Skindex‐16 assessment scores (a) total, (b) symptom, (c) emotion and (d) functioning domains. Error bar represents standard error of the mean. Statistical analysis presented as summary statistics. ADA, adapalene; BPO, benzoyl peroxide; CLNP, clindamycin phosphate.
The proportion of patients recording a higher score for the various categories in the patient preference assessment was significantly greater in the CLNP/BPO 3% group than the ADA + CLNP group throughout the study (Fig. 5).
Figure 5.
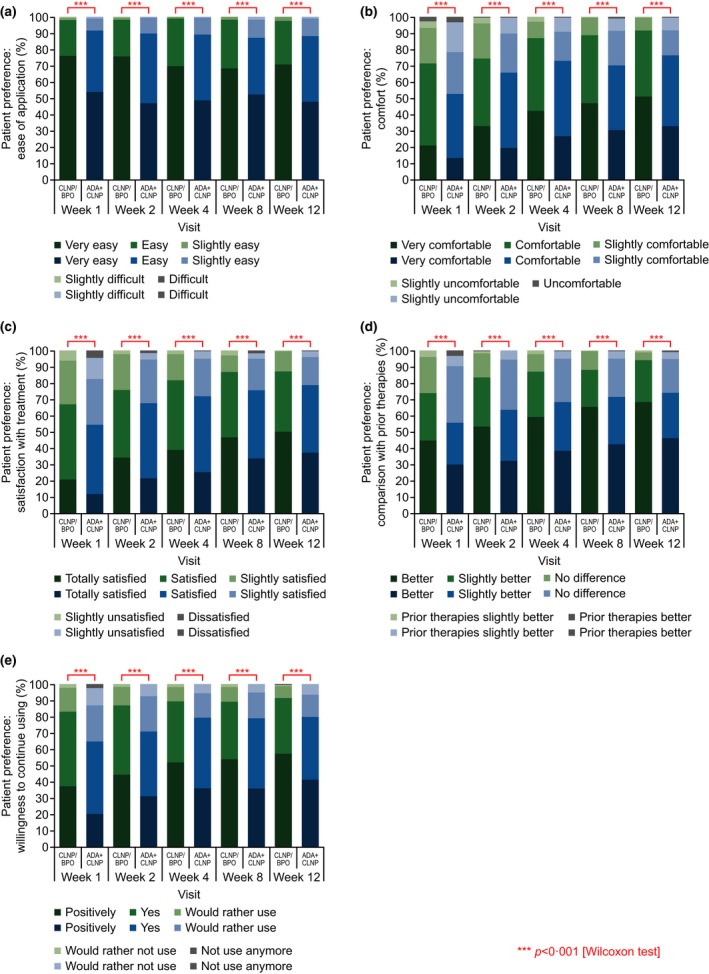
Patient preference assessment scores for (a) ease of application, (b) comfort, (c) satisfaction with treatment, (d) comparison with prior treatments and (e) willingness to continue using treatment (ITT population). ADA, adapalene; BPO, benzoyl peroxide; CLNP, clindamycin phosphate; ITT, intent‐to‐treat.
Tolerability and safety
Compliance for both treatments was similar, with a mean (standard deviation) of 95.04% (7.71%) and 94.11% (9.20%) for CLNP/BPO 3% and ADA + CLNP, respectively. In both groups, local tolerability scores for erythema, dryness, peeling, itching and burning/stinging were generally low at each study visit, except at week 1 in the ADA + CLNP group when the highest incidences of intolerability (except itching) were reported (Fig. 6).
Figure 6.
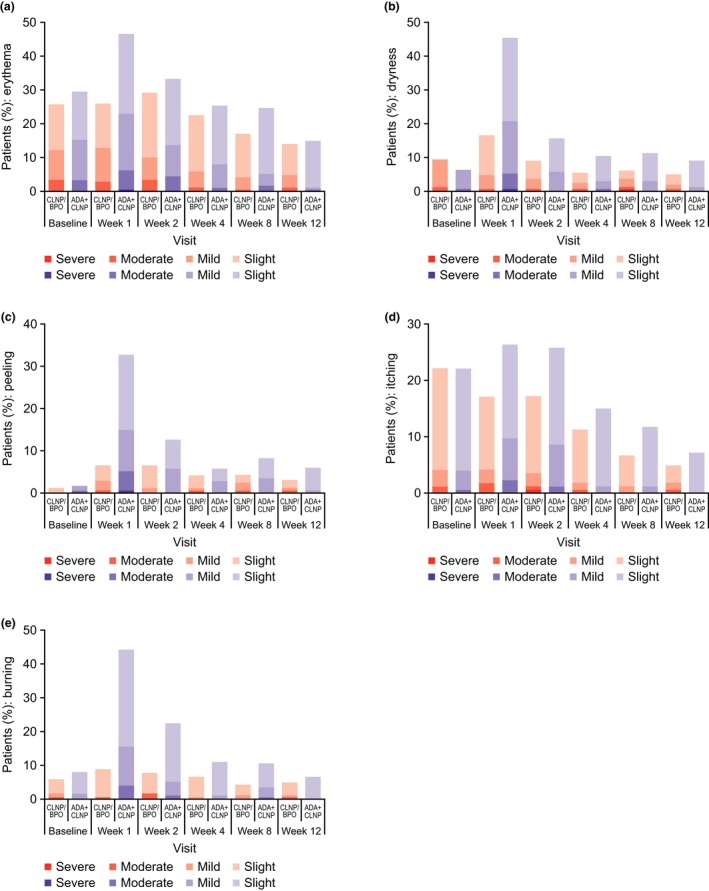
Distribution of local tolerability assessment scores among patients for (a) erythema, (b) dryness, (c) peeling, (d) itching and (e) burning/stinging (ITT population). ADA, adapalene; BPO, benzoyl peroxide; CLNP, clindamycin phosphate; ITT, intent‐to‐treat.
Overall incidence of AE in the CLNP/BPO 3% group (n = 53, 31%) was lower than the ADA + CLNP group (n = 100, 56%) and most were mild or moderate in severity. The most frequently reported AE was application‐site dryness across both treatment groups (n = 16, 9% in CLNP/BPO 3% group; n = 44, 25% in ADA + CLNP group). Only one serious AE, duodenal ulcers unrelated to the study treatment, occurred in the CLNP/BPO 3% group.
There was a higher incidence of facial adverse drug reactions (ADR) in the ADA + CLNP group (n = 65, 37%) compared with the CLNP/BPO 3% group (n = 29, 17%) (Table 4). The most commonly reported ADR were application‐site dryness (n = 42, 24%), pain (n = 16, 9%) and erythema (n = 11, 6%) in the ADA + CLNP group compared with application‐site dryness (n = 16, 9%) and pruritus (n = 5, 3%) in the CLNP/BPO 3% group. The majority of ADR were mild/moderate in severity. The proportion of ADR leading to permanent study discontinuation/withdrawal was similar in both groups (2%); all were due to application‐site events.
Table 4.
Summary of common ADR in 2% or more patients by with/without moisturizers at baseline (ITT population)
| CLNP/BPO 3% | ADA + CLNP | |||||
|---|---|---|---|---|---|---|
| With moisturizer (n = 82) | Without moisturizer (n = 90) | Total (n = 172) | With moisturizer (n = 90) | Without moisturizer (n = 87) | Total (n = 177) | |
| Any event | 13 (15.9) | 16 (17.8) | 29 (16.9) | 24 (26.7) | 41 (47.1) | 65 (36.7) |
| Application‐site dryness | 6 (7.3) | 10 (11.1) | 16 (9.3) | 12 (13.3) | 30 (34.5) | 42 (23.7) |
| Application‐site pain | 1 (1.2) | 2 (2.2) | 3 (1.7) | 8 (8.9) | 8 (9.2) | 16 (9.0) |
| Application‐site erythema | 3 (3.7) | 1 (1.1) | 4 (2.3) | 6 (6.7) | 5 (5.7) | 11 (6.2) |
| Application‐site pruritus | 4 (4.9) | 1 (1.1) | 5 (2.9) | 6 (6.7) | 2 (2.3) | 8 (4.5) |
| Application‐site exfoliation | 2 (2.4) | 1 (1.1) | 3 (1.7) | 3 (3.3) | 3 (3.4) | 6 (3.4) |
| Application‐site reaction | 0 | 2 (2.2) | 2 (1.2) | 2 (2.2) | 3 (3.4) | 5 (2.8) |
| Application‐site dermatitis | 0 | 0 | 0 | 2 (2.2) | 1 (1.1) | 3 (1.7) |
Application‐site reaction consists of “application site asteatosis”, “application site skin tightness” in verbatim text. ADA, adapalene; ADR, adverse drug reaction; BPO, benzoyl peroxide; CLNP, clindamycin phosphate; ITT, intent‐to‐treat. All values are n (%).
A subgroup analysis showed that there was no significant difference in the incidence of ADR in patients who did versus those who did not use moisturizers at baseline in the CLNP/BPO 3% group (Table 4). However, in the ADA + CLNP group, the proportion of ADR was greater in the subgroup without moisturizers compared with the subgroup with moisturizers at baseline.
Discussion
This study was designed to assess early treatment efficacy and demonstrated that once‐daily application of the CLNP/BPO 3% gel was more efficacious than topical combination uses of ADA + CLNP at reducing TL at week 2 and also IL from week 2 onward (but not non‐IL) in Japanese patients with acne vulgaris. Interestingly, it showed that there was no significant difference in TL at week 8 and 12 between the treatment groups. This may reflect the different onset of action with respect to IL between BPO (earlier efficacy onset of action) and ADA (slower efficacy onset of action), consistent with previous reports.10, 11 The primary end‐point of this study was to assess the early efficacy of CLNP/BPO 3% gel versus ADA + CLNP at week 2. High levels of compliance with ADA + CLNP resulted in patients continuing treatment to the end of the 12‐week period; a reduction in the treatment difference between the two groups was observed between weeks 2 and 12 due to the later onset treatment effect of ADA + CLNP compared with CLNP/BPO. Also, a key difference between the study treatments was the presence or absence of BPO or ADA. CLNP/BPO 3% combination gel contains two active ingredients that have antibacterial effects, and Burkhart et al. have previously reported synergistic activity of BPO and tertiary amines such as CLNP.12 Therefore, we expected that CLNP/BPO 3% would be more effective compared with ADA + CLNP, especially in the treatment of IL during the early stage. Patients receiving CLNP/BPO 3% experienced a significantly greater percentage reduction in acne lesions and improvement in acne severity within the 12‐week treatment period, compared with ADA + CLNP, with a difference between therapies being apparent from week 2 of treatment. The efficacy of CLNP/BPO 3%, particularly in terms of IL reduction, is consistent with a previous Japanese study showing a similar percentage reduction of IL from baseline at week 2 (~60%) in Japanese patients with mild to moderate acne vulgaris.5 This earlier‐efficacy onset of action of CLNP/BPO 3% may lead to a higher proportion of patients “clear” or “almost clear” in terms of acne severity in Japanese patients at weeks 4, 8 and 12, consistent with studies comparing CLNP/BPO 3% with CLNP or azelaic acid monotherapy.5, 13 A statistically significant greater proportion of patients receiving CLNP/BPO 3% therapy reported favorable scores in terms of ease of application, comfort, satisfaction, comparison with prior therapies and willingness to continue using the product compared with treatment with ADA + CLNP. Skindex‐16 assessments also demonstrated that treatment with CLNP/BPO 3% improved patient QoL to a greater extent than did ADA + CLNP. Although there is a conflicting result at week 2 between the decrease in acne lesions and increase in Skindex‐16 symptom scores in the ADA + CLNP group, this may be explained by the fact that the latter reflects the high score in some categories of local tolerability and/or the occurrence of application site‐related AE.
In this study, localized application‐site reactions such as dryness, peeling and burning/stinging were the most frequently reported ADR in both groups. Both treatments had comparable tolerability but CLNP/BPO 3% was associated with fewer total ADR: 17% compared with 37% for ADA + CLNP. As topical acne treatments are known to cause drug‐related skin reactions,14, 15, 16, 17, 18 dermatologists should consider appropriate measures to improve patient tolerability, such as the co‐administration of non‐comedogenic moisturizers. In the ADA + CLNP group, the proportion of ADR was lower in the subgroup of participants who used moisturizers at baseline compared with those who had not. However, a similar difference in incidence of ADR in the two subgroups was not observed in the CLNP/BPO 3% group; this lack of difference is likely due to the presence of emollient (dimethylpolysiloxane) and humectant (glycerol) excipients in the CLNP/BPO 3% combination gel, which may provide beneficial moisturizing effects thereby promoting preservation of skin barrier integrity.19, 20
Our study showed that adherence was high and similar between the two groups, suggesting that CLNP/BPO 3% gel did not improve compliance compared with the use of ADA + CLNP over a 12‐week period in the clinical trial setting. Miyachi et al.21 reported that one of the factors associated with poor adherence was lack of satisfaction with treatment (odds ratio, 3.59). Therefore, one possible reason why CLNP/BPO combination gel did not improve compliance in this study, compared with topical combination use of ADA + CLNP, could be that there was high compliance (>90%) and high patient satisfaction in both groups. However, in a real‐world setting, it is likely that a once‐daily combination gel would be more convenient to apply than two separate treatments, and the earlier onset of action (as demonstrated by greater efficacy of the combination gel at week 2), would encourage patients to continue using the treatment, resulting in improved compliance, clinical outcomes and QoL.
A limiting factor of this study was the inability to blind participants to the study treatments as each therapy was administrated differently. However, this was not likely to have had a meaningful impact on the study results as the efficacy assessments were carried out by the investigators in a blinded fashion. Future studies could be designed to evaluate the mid‐ or long‐term outcomes beyond 12 weeks of treatment. The impact of non‐judicious and long‐term use of antibiotics on the development of antibiotic resistance is of growing interest to clinicians. Prudent use of antibiotics is of great importance and we acknowledge that microbiological assessments were not performed in this study because the 12‐week use of CLNP/BPO combination and CLNP monotherapy has already been investigated in a previous phase III study.5 On the other hand, several other studies have assessed the impact of CLNP/BPO on the skin of patients with acne through microbiological sampling.22, 23, 24 In two 16‐week studies, CLNP/BPO 5% combination gel suppressed the emergence of CLNP‐resistant P. acnes relative to CLNP monotherapy22 or CLNP with retinoids.23 Also, it has been demonstrated that BPO may have the potential to prevent bacterial resistance in other studies.24, 25, 26 CLNP should be combined with BPO to reduce the potential development of bacterial resistance. However, it is unclear whether CLNP/BPO combination therapy leads to the development of bacterial resistance when used for longer periods of time. We therefore recommend that CLNP/BPO should be used in line with current prescribing information, Japanese and international guidelines, and that dermatologists should avoid treating with CLNP/BPO for over 12 weeks.
In conclusion, CLNP/BPO 3% gel was shown to be more efficacious at week 2 for treating TL than ADA + CLNP, one of the current standard topical regimens in the treatment of acne vulgaris in Japan. CLNP/BPO 3% was well tolerated, and patients reported better QoL and patient‐preference scores compared with those using ADA + CLNP. These results support the strong recommendation of CLNP/BPO 3% as a first‐line treatment of patients with acne vulgaris in the current Japanese guidelines.4
Conflict of Interest
A. E., T. H. and M. Y. are employees of GSK, Tokyo, Japan. O. S. is an employee of GSK, London, UK. N. H., I. K. and M. K. served as coordinating investigators in the current study, for which they received compensation. N. H. has served as a consultant to GSK, Galderma, Shionogi Pharmaceutical, Maruho, Rohto Pharmaceutical, Sato Pharmaceutical, POLA Pharma and Otsuka Pharmaceutical. I. K .has served as a consultant to GSK, Galderma, Shionogi Pharmaceutical, Maruho and Rohto Pharmaceutical. M. K. has served as a consultant to GlaxoSmithKline, Galderma, Shionogi Pharmaceutical, Maruho, Nippon Zoki Pharmaceutical, Mochida Pharmaceutical, Rohto Pharmaceutical, Mitsubishi Tanabe Pharma, Allergan, Nippon Shinyaku and Sato Pharmaceutical.
Acknowledgments
We gratefully acknowledge the contributions of 15 principal investigators in the 201884 Study Group: A. Kume, Dermatology Ophthalmology Kume Clinic; K. Toyofuku, Yamate Dermatological Clinic; Y. Nomura, Nomura Dermatology; A. Yoshioka, Yoshioka Dermatology Clinic; N. Kaji, Hoten Clinic; Y. Kudo, Tampopo Dermatology Clinic; N. Fujita, Sumire Dermatology Clinic; Y. Yanagihara, Yanagihara Dermatology Clinic; K. Mori, Mori Dermatology Clinic; C. Watanabe, Chiharu Dermatology Clinic; T. Yoshikawa, Yoshikawa Derma Clinic; T. Funai, Funai Derma Clinic; T. Kono, Central Clinic; Y. Sai, Sai Urology and Dermatology Clinic; and S. Sai, Sai Dermatology Urology Clinic. We also gratefully acknowledge the contributions of the Contract Research Organization monitors: S. Iizuka (leader), K. Ichikawa, J. Igari, T. Kuribayashi, M. Noguchi and R. Mikami; and study contributors: Y. Suzuki, K. Hoyano, T. Onoue, H. Takagi, Y. Terui, M. Arai, E. Nakamura, R. Yaegashi, H. Tojo, T. Hara, T. Ebihara, H. Ohgaki, Y. Niwa, A. Tsutsui and M. Sato. We also acknowledge the advice and support of GlaxoSmithKline global members: C. Miller, C. Eksteen and Z. Lulic‐Burns and the editorial support of Fishawack Indicia, UK. Editorial support in the preparation of the initial draft based on input from all authors, and collation and incorporation of author feedback to develop subsequent drafts, assembling tables and figures, copyediting and referencing was provided by Karen Yee, Ph.D., and Rachael Baylie, Ph.D., of Fishawack Indicia, and was funded by GlaxoSmithKline (GSK). This work was funded by GSK. Editorial support was provided by Fishawack Indicia, funded by GSK. GSK was involved in study design, data collection and data analysis.
References
- 1. Tan JKL, Bhate K. A global perspective on the epidemiology of acne. Br J Dermatol 2015; 172: 3–12. [DOI] [PubMed] [Google Scholar]
- 2. Gollnick HPM. From new findings in acne pathogenesis to new approaches in treatment. J Eur Acad Dermatol Venereol 2015; 29: 1–7. [DOI] [PubMed] [Google Scholar]
- 3. Hayashi N, Akamatsu H, Iwatsuki K. Guideline for the treatment of acne vulgaris. (in Japanese). Jpn J Dermatol 2008; 118(10): 1893–1923. [Google Scholar]
- 4. Hayashi N, Akamatsu H, Iwatsuki K. Guideline for the treatment of acne vulgaris (in Japanese). Jpn J Dermatol 2016; 2016(126): 1045–1086. [Google Scholar]
- 5. Kawashima M, Hashimoto H, Alió Sáenz AB, Ono M, Yamada M. Clindamycin phosphate 1.2%–benzoyl peroxide 3.0% fixed‐dose combination gel has an effective and acceptable safety and tolerability profile for the treatment of acne vulgaris in Japanese patients: a phase III, multicentre, randomised, single‐blinded, active‐controlled, parallel‐group study. Br J Dermatol 2015; 172(2): 494–503. [DOI] [PubMed] [Google Scholar]
- 6. FDA , FADA . FDA Guidance for Industry: Acne Vulgaris – Developing Drugs for Treatment (Draft) 2005.
- 7. Eichenfield LF, Alio Saenz AB. Safety and efficacy of clindamycin phosphate 1.2%‐benzoyl peroxide 3% fixed‐dose combination gel for the treatment of acne vulgaris: A phase 3, multicenter, randomized, double‐blind, active‐ and vehicle‐controlled study. J Drugs Dermatol 2011; 10: 1382–1396. [PubMed] [Google Scholar]
- 8. Chren M, Lasek R, Sahay A, Sands L. Measurement properties of Skindex‐16: a brief quality‐of‐life measure for patients with skin diseases. J Cutan Med Surg 2001; 5(2): 105–110. [DOI] [PubMed] [Google Scholar]
- 9. Higaki Y, Kawamoto K, Kamo T, Horikawa N, Kawashima M, Chren M‐M. The Japanese version of Skindex‐16: a brief quality‐of‐life measure for patients with skin diseases. J Dermatol 2002; 29(11): 693–698. [DOI] [PubMed] [Google Scholar]
- 10. Babaeinejad SH, Fouladi RF. The efficacy, safety and tolerability of adapalene versus benzoyl peroxide in the treatment of mild acne vulgaris; a randomized trial. J Drugs Dermatol 2013; 12(9): 1033–1038. [PubMed] [Google Scholar]
- 11. Gollnick HP, Draelos Z, Glenn MJ, Rosoph LA, Kaszuba A, Cornelison R, et al Adapalene‐benzoyl peroxide, a unique fixed‐dose combination topical gel for the treatment of acne vulgaris: a transatlantic, randomized, double‐blind, controlled study in 1670 patients. Br J Dermatol 2009; 161(5): 1180–1189. [DOI] [PubMed] [Google Scholar]
- 12. Burkhart CN, Specht K, Neckers D. Synergistic activity of benzoyl peroxide and erythromycin. Skin Pharmacol Appl Skin Physiol 2000; 13(5): 292–296. [DOI] [PubMed] [Google Scholar]
- 13. Schaller M, Sebastian M, Ress C, Seidel D, Hennig M. A multicentre, randomized, single‐blind, parallel‐group study comparing the efficacy and tolerability of benzoyl peroxide 3%/clindamycin 1% with azelaic acid 20% in the topical treatment of mild‐to‐moderate acne vulgaris. J Eur Acad Dermatol Venereol 2016; 30(6): 966–973. [DOI] [PubMed] [Google Scholar]
- 14. Dosik JS, Vamvakias G. Comparative irritation potential of two combination acne products. Am J Clin Dermatol 2008; 9(5): 313–317. [DOI] [PubMed] [Google Scholar]
- 15. Faqundes DS, Fraser JM, Klauda HC. New therapy update – a unique combination formulation in the treatment of inflammatory acne. Cutis 2003; 72(1 Suppl): 16–19. [PubMed] [Google Scholar]
- 16. Green L, Cirigliano M, Gwazdauskas JA, Gonzalez P. The tolerability profile of clindamycin 1%/benzoyl peroxide 5% gel vs. adapalene 0.1%/benzoyl peroxide 2.5% gel for facial acne: results of two randomized, single‐blind, split‐face studies. J Clin Aesthet Dermatol 2012; 5(5): 16–24. [PMC free article] [PubMed] [Google Scholar]
- 17. Hayashi N, Kawashima M. Study of the usefulness of moisturizers on adherence of acne patients treated with adapalene. J Dermatol 2014; 41(7): 592–597. [DOI] [PubMed] [Google Scholar]
- 18. Kwon HH, Park SY, Yoon JY, Min S, Suh DH. Do tutorials on application method enhance adapalene–benzoyl peroxide combination gel tolerability in the treatment of acne? J Dermatol 2015; 42(11): 1058–1065. [DOI] [PubMed] [Google Scholar]
- 19. Draelos ZD, Callender V, Young C, Dhawan SS. The effect of vehicle formulation on acne medication tolerability. Cutis 2008; 82(4): 281–284. [PubMed] [Google Scholar]
- 20. Jones TM, Jasper S, Alio Saenz AB. Bioavailability of clindamycin from a new clindamycin phosphate 1.2%‐benzoyl peroxide 3% combination gel. Clinical Pharmacol Drug Dev 2013; 2(1): 33–47. [DOI] [PubMed] [Google Scholar]
- 21. Miyachi Y, Hayashi N, Furukawa F, Akamatsu H, Matsunaga K, Watanabe S, et al Acne management in Japan: study of patient adherence. Dermatology 2011; 223(2): 174–181. [DOI] [PubMed] [Google Scholar]
- 22. Cunliffe WJ, Holland KT, Bojar R, Levy SF. A randomized, double‐blind comparison of a clindamycin phosphate/benzoyl peroxide gel formulation and a matching clindamycin gel with respect to microbiologic activity and clinical efficacy in the topical treatment of acne vulgaris. Clin Ther 2002; 24(7): 1117–1133. [DOI] [PubMed] [Google Scholar]
- 23. Jackson JM, Fu JJ, Almekinder JL. A randomized, investigator‐blinded trial to assess the antimicrobial efficacy of a benzoyl peroxide 5%/ clindamycin phosphate 1% gel compared with a clindamycin phosphate 1.2%/tretinoin 0.025% gel in the topical treatment of acne vulgaris. J Drugs Dermatol 2010; 9(2): 131–136. [PubMed] [Google Scholar]
- 24. Kawashima M, Nagare T, Katsuramaki T. An Open‐label, randomized, multi‐center, phase III study to evaluate the safety and efficacy of benzoyl peroxide gel in long‐term use in patients with acne vulgaris. Rinshoiyaku 2014; 30: 669–689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Eady EA, Farmery MR, Ross JI, Cove JH, Cunliffe WJ. Effects of benzoyl peroxide and erythromycin alone and in combination against antibiotic‐sensitive and ‐resistant skin bacteria from acne patients. Br J Dermatol 1994; 131(3): 331–336. [DOI] [PubMed] [Google Scholar]
- 26. Kawashima M, Nagare T, Katsuramaki T. Open‐label, randomized, multicenter, phase III study to evaluate the safety and efficacy of benzoyl peroxide gel in long‐term use in patients with acne vulgaris: A secondary publication. J Dermatol 2017; 44(6): 635–643. [DOI] [PMC free article] [PubMed] [Google Scholar]


