Abstract
Objectives
Cognitive reactivity (CR) to sad mood is a risk factor for major depressive disorder (MDD). CR is usually measured by assessing change on the Dysfunctional Attitudes Scale (DAS‐change) after sad mood‐induction. It has, however, been suggested that the versions of the DAS (A/B) are not interchangeable, impacting the reliability and validity of the change score. The Leiden Index of Depression Sensitivity‐Revised (LEIDS‐R) is an alternative self‐report measure of CR. Studies examining the relationship between LEIDS‐R and DAS‐change have shown mixed results. We examined whether scores of these CR measures differed between remitted MDD and controls, the relationship between these CR measures, and the effect of order of DAS administration on DAS‐change.
Design
Cross‐sectional design with two groups (remitted MDD and controls).
Methods
Sixty‐eight MDD patients remitted from ≥2 previous episodes, not taking antidepressants, and 43 never‐depressed controls participated in a mood‐induction and filled in the DAS‐A/B in randomized order before and after mood‐induction, and LEIDS‐R separately.
Results
LEIDS‐R scores and pre‐mood‐induction DAS scores were significantly higher in remitted MDD than controls (p < .001, Cohen's d = 1.48; p = .001, Cohen's d = 0.66, respectively). DAS‐change did not differ between these groups (p = .67, Cohen’s d = 0.08). LEIDS‐R correlated with DAS‐change (r = .30, p = .042), but only in the group that filled in DAS‐B before DAS‐A. In remitted MDD, DAS‐change was dependent on the order of DAS versions before and after mood‐induction (10.6 ± 19.0 vs. −1.2 ± 10.5, for order B‐A and A‐B, respectively), with a significant group × order interaction (p = .012).
Conclusions
Existing DAS versions are not interchangeable, which compromises the usefulness of mood‐inductions in clinical practice. The LEIDS‐R seems a valid measure of cognitive vulnerability to depression.
Practitioner points
Clinical implications:
Cognitive reactivity (CR) is a risk factor of depressive recurrence. The current measurement of CR, by assessing change on the Dysfunctional Attitudes Scale (DAS) after mood‐induction, is not reliable.
The Leiden Index Depression Sensitivity‐Revised (LEIDS‐R) is an alternative CR measure. In contrast to mood‐induction, it reliably assesses depression vulnerability.
The use of mood‐inductions for clinical/research purposes is unnecessary.
Limitations of the study:
We were not able to examine the effect of previous treatment, which could have affected results as psychological treatments probably have differential effects on CR.
Examining un‐medicated patients may have led to selection of a sample not completely representative for the general MDD population.
We did not administer both parallel versions of the DAS (A/B) before and after mood‐induction. This might have provided better understanding of their differential sensitivity to change.
Keywords: depression, stress, cognitive reactivity, vulnerability, cognition
Background
One of the disabling aspects of major depressive disorder (MDD) is its recurrent nature (Kruijshaar et al., 2005). In about a third of patients, MDD becomes recurrent (Eaton et al., 2008) and the risk of recurrences increases after each episode (Solomon et al., 2000). Although percentages vary considerably across studies, recurrence rates of 60% have been reported in patients with two previous episodes, and these rates can rise up to 90% in patients with three previous episodes (Beshai, Dobson, Bockting, & Quigley, 2011; Bockting, Hollon, Jarrett, Kuyken, & Dobson, 2015). Understanding the underlying vulnerability of MDD patients at high risk of recurrence may help to decrease the personal and economic burden of MDD (Mathers & Loncar, 2006).
Vulnerability during MDD remission can be measured at various levels, including at the level of thinking patterns or so‐called dysfunctional attitudes. Dysfunctional attitudes about the world, the self, and the future develop in early life and are strengthened during depressive episodes. During remission, dysfunctional attitudes tend to decrease (though not always reported consistently) but can be reactivated by dysphoric mood and/or stressors (Lau, Segal, & Williams, 2004). The ease with which these attitudes can be reactivated is termed ‘cognitive reactivity’ (CR). Patients remitted from depression are thought to have higher CR scores than never‐depressed individuals (Lau et al., 2004).
Cognitive reactivity is typically measured in laboratory settings by experimentally inducing a sad mood and administering the Dysfunctional Attitudes Scale (DAS; Weissman, 1979) before and after the procedure. DAS scores tended to increase more in individuals with a history of depression than in never‐depressed individuals (Gemar, Segal, Sagrati, & Kennedy, 2001; Jeanne, Gross, Persons, & Hahn, 1998; Miranda & Persons, 1988; Van der Does, 2002). More interestingly, high CR (DAS‐change) scores predicted a shorter time to relapse or recurrence (Segal, Gemar, & Williams, 1999; Segal et al., 2006). In several studies, however, CR as measured by DAS‐change did not predict recurrence (Jarrett et al., 2012; Lethbridge & Allen, 2008; van Rijsbergen et al., 2013). These conflicting results may indicate that CR is not a stable vulnerability factor. Alternatively, the problem may lie with the validity of the instrument. In support of the latter option, several methodological issues have been raised with mood‐inductions. The procedure requires the 40‐item DAS to be administered twice within ±10 min. Most often, two parallel versions (DAS‐A/B) are used in varying order (Weissman & Beck, 1978). However, the factor structure of these two versions appears to be different (Power et al., 1994) and the mean scores of the A/B versions and DAS‐change scores may be dependent on the order of administration (Gemar et al., 2001). In research settings, such systematic differences can be corrected statistically at a group level (Segal et al., 1999; Van der Does, 2002), but this is impossible at the individual level in clinical settings. This limits the usefulness of DAS‐change scores in clinical practice. Finally, application of mood‐induction procedures may also not be feasible outside of research settings.
Because of these methodological and practical issues, the Leiden Index of Depression Sensitivity‐Revised (LEIDS‐R) was developed (Solis, Antypa, Conijn, Kelderman, & Van der Does, 2016; Van der Does, 2002). The LEIDS‐R is a self‐report questionnaire, which asks participants to respond to statements when in an imagined sad mood. LEIDS‐R scores distinguished previously depressed individuals from never‐depressed controls in at least six studies (Elgersma et al., 2015; Merens, Booij, & Van Der Does, 2008; Moulds et al., 2008; Raes, Dewulf, Van Heeringen, & Williams, 2009; Van der Does, 2002, 2005). LEIDS‐R scores also differentiated between recurrently depressed patients and those with a single prior episode in two studies (Elgersma et al., 2015; Yamamoto, Yamano, Shimada, Ichikawa, & Nakaya, 2014). Furthermore, total LEIDS‐R scores predicted depression vulnerability above and beyond rumination scores as measured by the Ruminative Response Scale (Moulds et al., 2008). LEIDS‐R scores are also associated with biological vulnerability markers, such as response to tryptophan depletion and the polymorphism in the serotonin transporter gene (5‐HTTLPR; Antypa, Van der Does, & Penninx, 2010; Booij & Van der Does, 2007). Finally, LEIDS‐R scores predicted the first onset of depression in never‐depressed individuals (Kruijt et al., 2013) and recurrence during a 3.5‐year period in remitted patients (Figueroa et al., 2015). The reliability and construct and ecological validity of the LEIDS‐R have also been demonstrated (Solis et al., 2016; Takano, Raes, & Van der Does, manuscript submitted for publication).
The two CR measures (LEIDS‐R and DAS‐change) have only been compared in two studies. In the first study (Van der Does, 2002), 48 non‐depressed students, eight of whom had a history of depression, were randomly assigned to fill in the DAS‐A or the DAS‐B before mood‐induction followed by the other version after the procedure (further referred to as DAS‐A/B in randomized order). In this study, LEIDS scores correlated with DAS‐change (Van der Does, 2002). In the second study, 24 remitted MDD and 24 controls participated in a mood‐induction and filled in DAS‐A/B in randomized order. In this study, DAS‐change did not correlate with LEIDS scores (Van der Does, 2005). Further, LEIDS scores were significantly higher in remitted MDD than in controls, but CR scores determined by DAS‐change were not. Given that mood‐induction‐based CR scores are less practical and seem to be less reliable than LEIDS‐R scores (Van der Does, 2005), the LEIDS‐R may be the preferable index of CR, in particular to assess depression vulnerability or recurrence risk in clinical settings (Figueroa et al., 2015).
The aim of this study was to investigate whether the DAS‐change and LEIDS‐R scores differed between remitted recurrent MDD (rrMDD), who are at high risk of depressive recurrence and controls without personal or familial MDD history, who are relatively resilient to developing a depressive episode (Ruhe, Mason, & Schene, 2007). Further, we examined whether the two indices are correlated, and whether DAS version‐order impacts DAS‐change scores. We hypothesized that (1) the scores on both LEIDS‐R and DAS‐change would differ between rrMDD and controls; (2) LEIDS‐R and DAS‐change scores would be correlated; and (3) the DAS‐change score differs between the A‐B and B‐A administration order.
Methods
Participants
Participants were recruited from several psychiatric institutions across the Netherlands, via general practitioners, advertisements, patient organizations, and other research projects, in the context of a larger study. Informed consent for participation was obtained from all participants. rrMDD patients had experienced ≥2 depressive episodes and were in stable remission for at least 8 weeks (according to the Structured Clinical Interview for DSM‐IV Axis I Disorders Patient Edition [SCID‐I/P] and a Hamilton Depression Rating Scale score [HDRS] ≤7). Patients did not use psychotropic medication. Only controls without personal (SCID‐I/P) or first‐degree familial psychiatric history were included. Exclusion criteria were as follows: alcohol/drug dependency; psychotic/bipolar disorder; primary anxiety disorder; personality disorder; electroconvulsive therapy within 2 months before the experiment; history of severe head trauma; neurological disease; severe general physical illness; no Dutch/English proficiency.
Measures
Leiden Index of Depression Sensitivity‐Revised
The LEIDS‐R assesses CR by instructing participants to think about the last time they felt ‘somewhat sad’ and to indicate the degree to which a list of statements describe their typical cognitions and behaviours in response to sad mood. The LEIDS‐R contains 34 items on six subscales (Hopelessness/Suicidality, Acceptance/Coping, Aggression, Control/Perfectionism, Risk Aversion, and Rumination) and has good psychometric properties (Solis et al., 2016). In a previous study, the LEIDS‐R had internal consistencies (Cronbach's α) ranging from .87 to .95 (Solis et al., 2016) measured over four time‐points, and Cronbach's α = .93 in the current study.
Dysfunctional Attitudes Scale
Dysfunctional attitudes were assessed with the 40‐item self‐report DAS (Van der Gucht, Takano, Van Broeck, & Raes, 2014). DAS items are rated on a seven‐point Likert scale (‘totally agree’ to ‘totally disagree’). The two versions (A and B) were used in this study, which have shown good internal consistencies; Cronbach's α = .86 and .87, respectively, in a previous study (Weissman & Beck, 1978), and Cronbach's α = .81 and .83, respectively, in the current study, and showed correlations of .8 in a previous study (Dozois, Covin, & Brinker, 2003). In the Dutch version of the DAS (Douma, 1991), five items overlap in the A and B versions; see Supporting Information for more information on the Dutch DAS‐A and DAS‐B.
Visual analogue scale
Patients rated their current mood on a visual analogue scale (VAS), a 10‐cm straight line with the descriptor ‘sad’ located to the left of the centre, and ‘happy’ located on the right.
Hamilton Depression Rating Scale (HDRS‐17)
The HDRS‐17 is an observer‐rated scale to assess the severity of depression. Its internal consistency is high, with previously reported Cronbach's α = .80 (Hamilton, 1960).
Procedure
Before the baseline measurement, participants completed the HDRS and, as preparation of the mood‐induction procedure, picked one of five sad music fragments. They were asked to describe in detail an autobiographical memory, which they rated as one of the saddest in their life. The memory was scripted by the researchers and read to the participants during the first (baseline) visit while participants listened to the selected fragment of sad music. Combination of the sad autobiographical memory to sad music is a slight modification of the procedure used by Segal et al. (2006). Like Segal et al. (2006), before and immediately after the mood‐induction procedure, participants filled in the DAS‐A or the DAS‐B and rated their mood with the VAS (one version). Patients were a priori randomized to receive a different order of administration of the DAS (A‐B or B‐A). The mood‐induction was defined as effective if the subject's mood score decreased by 10% or more (Rush, Gullion, Basco, Jarrett, & Trivedi, 1996). We expected lower mood after mood‐induction, which would be comparable between groups (Segal et al., 1999). After the baseline visit, all participants received a booklet with the LEIDS‐R to fill out before the second visit, which was an MRI session in context of a larger study (mean time between baseline visit (visit 1) and MRI visit (visit 2) was 17.7 ± 17.2 days). The LEIDS‐R was only filled in once (Figure S2).
Statistical analysis
General statistical principals
This study involved two between‐subjects factors: depression status (rrMDD or control) and order of DAS administration (A‐B or B‐A), and one within subjects factor (pre‐ and post‐mood induction).
To compute pre‐/post‐mood‐induction change scores, we used the standardized residual scores of DAS‐change and VAS change, because variability in pre‐scores can impede the comparison of pre‐/post‐change scores. Standardized residual scores are a measure of the strength of the difference between observed and expected values and, thus, represent change scores that are independent of variability among pre‐scores. These scores are more precise and less affected by higher scores which are expected to change more than lower scores (Cohen, Cohen, West, & Aiken, 2013). Several studies (Segal et al., 2006) have used residual DAS‐change rather than simple change scores. In line with Segal et al. (1999), standardized residual scores were constructed using a simple linear regression in which post‐DAS/VAS scores were predicted by pre‐DAS/VAS scores. Original scores are presented in the tables and in the text. For all analyses, unless reported otherwise, we used standardized residual DAS‐change and VAS change scores.
We compared the demographic characteristics of rrMDD and controls using t‐tests for normally distributed, continuous variables, χ2 tests for categorical variables, and a between‐groups analysis of variance (ANOVA) to determine whether the clinical variables (HDRS, pre‐mood‐induction DAS, and VAS change) differed between groups. Because HDRS scores differed between groups, we also report the observed difference following the inclusion of HDRS scores as a covariate in a between‐groups analysis of covariance (ANCOVA) when a clinical variable and HDRS scores were correlated. For pre‐mood‐induction DAS, we calculated effect size, using between‐groups Cohen's d. We performed all statistical analyses using SPSS version 21.0 (IBM United Kingdom Limited, Portsmouth, UK) and regarded two‐tailed p‐values <.05 as statistically significant.
Differences in DAS‐change and LEIDS‐R scores between rrMDD and controls
We used a between‐groups ANOVA to examine whether DAS‐change and LEIDS‐R scores differed between rrMDD and controls. Furthermore, we calculated effect sizes for LEIDS‐R and DAS‐change scores, using between‐groups Cohen's d.
Correlation between LEIDS‐R and DAS‐change
We present Pearson's correlation coefficient between LEIDS‐R and DAS‐change scores for all participants, for rrMDD and controls separately, and for participants presented with A‐B and B‐A ordering of the DAS, separately.
Effect of DAS version‐order (A‐B or B‐A) on DAS‐change
First, we examined whether DAS version‐order impacted pre‐mood‐induction DAS scores. Therefore, we used a 2 × 2 between‐groups ANOVA with DAS before mood‐induction as the dependent variable and included main effects of version‐order and patient group, and their interaction terms.
Second, we used a 2 × 2 between‐groups ANCOVA with DAS‐change as the dependent variable and included main effects of version‐order, patient group, and their interaction terms, while adding VAS change as a covariate.
Results
Participants
Forty‐six controls and 73 rrMDD were initially eligible, of whom two and four, respectively, declined further participation (Figure S1). Therefore, 69 rrMDD and 44 controls underwent the mood‐induction and filled in the LEIDS‐R. Of both groups, one participant was excluded due to evident psychiatric illness when visiting for the mood‐induction. Thereafter, one rrMDD participant and one control did not participate in the mood‐induction, and due to a procedural error, four rrMDD participants filled in the same DAS version (A) twice, leaving 63 rrMDD and 42 controls for the mood‐induction analyses. One outlier in the rrMDD group was excluded from analyses with DAS‐change scores only, based on a Z‐score of DAS‐change = 4.68.
Demographic and clinical characteristics
No significant differences were observed between rrMDD and controls for sex, age, education, IQ, living situation, and employment status. rrMDD had higher residual depression symptoms (HDRS scores; p < .001; Table 1). Pre‐mood‐induction DAS scores were higher in rrMDD than controls, with an effect size (Cohen's d) of 0.66 (p = .001), which remained significantly higher in rrMDD after correcting for residual symptoms (p = .046).
Table 1.
Clinical and demographic characteristics
| Between‐group statistics | ||||||
|---|---|---|---|---|---|---|
| rrMDD (n = 68) | HC (n = 43) | χ2 | T/F | p | ||
| Female | N (%) | 45 (66%) | 30 (70%) | 0.16 | .69 | |
| Age | Years; mean (SD) | 53.3 (7.7) | 51.5 (8.2) | −1.15 | .25 | |
| Education | Levelsa | 0/0/0/4/22/27/15 | 0/0/0/1/16/18/8 | 1.1 | .78 | |
| IQ | Mean (SD) | 108.9 (8.2) | 106.3 (9.6) | −1.42 | .16 | |
| Living situation | Levelsb | 29/0/19/17/2/2/0 | 11/0/16/11/4/0/0 | 5.23 | .26 | |
| Employment status | Levelsc | 26/27/15/0 | 21/17/5/0 | 2.29 | .318 | |
| Age of onset | Years; mean (SD) | 27.18 (11.18) | – | |||
| Episodes | Median (IQR) | 4.00 (2–7) | – | |||
| HDRS | Mean (SD) | 2.66 (2.4) | 1.02 (1.4) | 16.6 | <.001 | |
| DAS | Mean (SD) | 118.3 (28.1) | 101.7 (21.6) | 10.7 | .001 | |
| LEIDS‐R | Mean (SD) | 39.5 (15.2) | 16.5 (15.9) | 57.9 | <.001 | |
| LEIDS‐R subscales | ||||||
| Hopelessness | Mean (SD) | 6.14 (3.98) | 1.44 (2.12) | 50.5 | <.001 | |
| Acceptance | Mean (SD) | 1.60 (1.96) | 0.61 (1.61) | 7.8 | .006 | |
| Aggression | Mean (SD) | 4.58 (4.12) | 2.42 (2.59) | 9.4 | .003 | |
| Control | Mean (SD) | 6.23 (3.60) | 2.79 (3.04) | 27.5 | <.001 | |
| Risk avoidance | Mean (SD) | 9.80 (4.49) | 4.28 (4.56) | 39.1 | <.001 | |
| Rumination | Mean (SD) | 11.18 (4.60) | 5.0 (4.70) | 46.7 | <.001 | |
χ2 = chi‐square test statistic; DAS = Dysfunctional Attitudes Scale; HC = healthy controls; HDRS = Hamilton Depression Rating Scale; LEIDS‐R = Leiden Index of Depression Sensitivity‐Revised; p = p‐value; rrMDD = remitted recurrent major depressive disorder; T/F = T statistic from independent samples t‐test or F statistic from ANOVA (HDRS, LEIDS‐R and DAS).
Level of educational attainment. Levels range from 1 to 7 (1 = primary school not finished, 7 = pre‐university/university degree).
Living situation: alone/living with parents/cohabiting/cohabiting with children/single living with children/other/unknown.
Employment status: low/middle/high/never worked.
Mood ratings
Table 2 shows VAS scores before and after mood‐induction and mean change scores. In 86.4% of participants, the mood‐induction resulted in a mood decrease of >10%. The change in mood induced by the mood‐induction (VAS change) did not differ between rrMDD and controls, F (1, 105) = 1.5, p = .22, also when correcting for residual symptoms (p = .67).
Table 2.
DAS and VAS scores before and after MIP and mean change scores for the whole group and for A‐B/B‐A version‐order separately
| Between‐group statistics | ||||||||
|---|---|---|---|---|---|---|---|---|
| rrMDD | HC | p‐change | ||||||
| Pre‐MIP | Post‐MIP | Change score | Pre‐MIP | Post‐MIP | Change score | |||
|
All (A‐B and B‐A) (n = 62 rrMDD; n = 42 HC) |
DAS Mean (SD) | 117.0 (27.6) | 121.2 (30.1) | 4.17 (16.0) | 101.7 (3.3) | 106.1 (21.0) | 4.40 (11.1) | .67 |
| VAS Mean (SD) | 6.54 (1.43) | 3.98 (2.00) | 2.56 (1.90) | 7.14 (1.32) | 4.71 (1.8) | 2.43 (1.73) | .22 | |
|
Order A‐B (n = 34 rrMDD; n = 22 HC) |
DAS Mean (SD) | 117.9 (32.5) | 116.8 (31.9) | −1.16 (10.5) | 97.2 (17.4) | 103.1 (19.7) | 5.96 (11.5) | .083 |
| VAS Mean (SD) | 6.48 (1.48) | 3.77 (1.83) | 2.71 (1.78) | 7.26 (1.30) | 4.97 (1.41) | 2.30 (1.48) | .087 | |
|
Order B‐A (n = 28 rrMDD; n = 20 HC) |
DAS Mean (SD) | 115.9 (20.7) | 126.6 (27.3) | 10.6 (19.0) | 106.7 (25.0) | 109.3 (22.4) | 2.68 (10.7) | .064 |
| VAS Mean (SD) | 6.68 (1.43) | 4.31 (2.20) | 2.38 (2.08) | 7.02 (1.36) | 4.43 (2.08) | 2.59 (2.00) | .90 | |
A‐B = participants who filled in the DAS‐A before MIP and BAS‐B after MIP; B‐A = participants who filled in the DAS‐B before MIP and BAS‐A after MIP; DAS = Dysfunctional Attitudes Scale; HC = healthy controls; MIP = mood‐induction procedure; rrMDD = remitted recurrent major depressive disorder; SD = standard deviation; p = p‐value.
Group differences in VAS change scores did not significantly change when correcting for HDRS scores. Bold: significant within‐group difference in remitted MDD for the A‐B sequence versus B‐A sequence (p = .003).
Differences in DAS‐change and LEIDS‐R scores for rrMDD and controls
The mean DAS‐change scores after mood‐induction did not differ between rrMDD and controls, F (1, 102) = 18, p = .67; Cohen's d = 0.08.
In rrMDD, LEIDS‐R scores (including all subscales) were higher, F (1, 109) = 57.9, p < .001, Table 1, with a between‐groups effect size (Cohen's d) of 1.48, which remained significantly different after correcting for residual symptoms (p < .001).
Correlation LEIDS‐R and DAS‐change
The correlation between the LEIDS‐R and DAS‐change was low and non‐significant for rrMDD and controls combined (r = .09; n = 104; p = .36) and for groups separately (controls r = −.11, p = .51, rrMDD r = .17, p = .91). However, the correlation between LEIDS‐R scores and DAS‐change was significant in participants who received DAS order B‐A (r = .30; n = 48; p = .042), but not in those who received order A‐B (r = −.19; n = 56; p = .16; Figure 1A/B).
Figure 1.
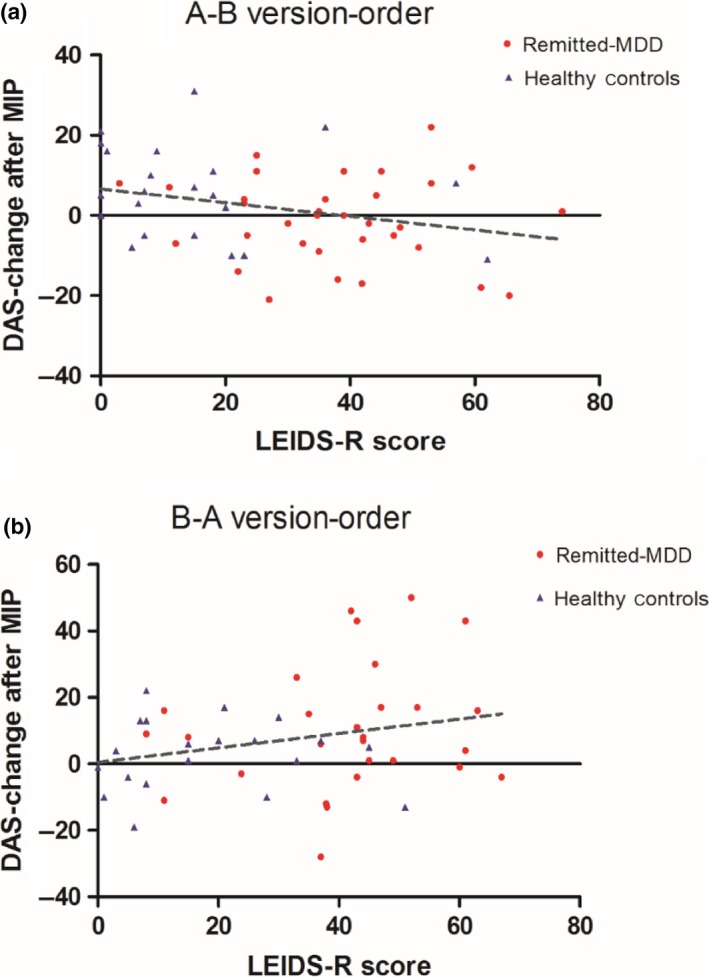
A/B, Correlation LEIDS‐R and DAS‐change (standardized residual) by form sequence (A‐B and B‐A). (A) Non‐significant negative correlation between LEIDS‐R and DAS‐change for the A‐B version‐order (n = 56; Pearson's r = −.19; p = .16). (B) Significant positive correlation between LEIDS‐R and DAS‐change for the B‐A version‐order (n = 48; Pearson's r = .30; p = .042). Abbreviations: LEIDS‐R; Leiden Index Sensitivity‐Revised; DAS; Dysfunctional Attitudes Scale; MIP: mood‐induction procedure. [Colour figure can be viewed at http://wileyonlinelibrary.com]
The effect of order (A‐B or B‐A) on DAS‐change scores
The administration of either the DAS‐A or DAS‐B before mood‐induction did not significantly influence pre‐mood‐induction DAS scores between rrMDD and controls (group × version‐order interaction; p = .26; Table S1A).
However, for DAS‐change, the group × version‐order interaction term was significant (p = .012), indicating that the effect of order of DAS‐A/B on DAS‐change differed between rrMDD and controls (Table S1B). In detail, DAS‐change was greater in rrMDD who had filled in B‐A versus A‐B, mean change (±SD): 10.64 ± 19.0 versus −1.16 ± 10.6. In a stratified analysis of the 48 subjects who received the B‐A version‐order, the DAS‐change scores differed trendwise between rrMDD and controls, F (1, 46) = 3.6; p = .064.
Post hoc, this difference in DAS‐change in rrMDD for the different version‐orders can be further interpreted by examining the groupwise means of the DAS‐A and B in rrMDD patients when administered before or after the mood‐induction (thus illustrating the means of two different groups, without any formal statistical testing). Given the randomization of order at baseline, this approach could provide a proxy for what DAS‐change scores would hypothetically be when using the same version twice. For DAS‐A, there was a difference between pre‐ and post‐mood‐induction: mean (±SD) 117.9 ± 32.5) and 126.6 ± 27.3, respectively), whereas DAS‐B showed almost no difference (pre‐mood‐induction: 115.9 ± 20.7 vs. post‐mood‐induction: 116.8 ± 31.9; Table 2). This suggests that the DAS‐A is sensitive to a mood‐induction while DAS‐B is not.
Discussion
In this study, we compared two measures of CR: DAS‐change after mood‐induction and the LEIDS‐R. Based on our findings, the validity and reliability of measuring CR by DAS‐change after mood‐induction with the two DAS versions (A/B) can be questioned. Contrary to our hypothesis, overall DAS‐change scores were low and comparable between groups. Instead, LEIDS‐R scores were significantly higher in rrMDD than in controls. Unexpectedly, LEIDS‐R scores only correlated with DAS‐change scores for the B‐A version‐order of the DAS administration. In line with our hypothesis, DAS‐change scores depended on the order of the DAS administration (A‐B or B‐A), with a significant group × version‐order interaction. In rrMDD, DAS‐change was larger when subjects filled in the DAS‐B before A, compared to the group that filled in A before B, while this effect was opposite in controls. Our post‐hoc analyses indicated that the DAS‐B is insensitive to change by mood‐induction whereas the DAS‐A appears sensitive. Even after regressing out the effect of version‐order, DAS‐change only differed trendwise significantly between rrMDD and controls in the B‐A administration.
Our finding that DAS‐change scores were higher in rrMDD who had filled in DAS‐B before A is corroborative with previous reports that indicated that these versions may not be interchangeable (Gemar et al., 2001; Power et al., 1994). For instance, Gemar et al. (2001) reported that DAS‐change scores after mood‐induction were dependent on the version‐order in a sample of 23 remitted MDD and 27 controls. Both Gemar et al. (2001) and our study show that DAS‐A is more sensitive to change by mood‐induction than DAS‐B in remitted MDD. We speculate that non‐interchangeability of the two versions could partly explain why several studies that used counter‐balanced version‐order or only A‐B sequence, did not find differences in DAS‐change scores (Jarrett et al., 2012; Van der Does, 2005), or did not find that CR measured by DAS‐change predicted recurrence (Jarrett et al., 2012; Lethbridge & Allen, 2008; van Rijsbergen et al., 2013).
After regressing out the effect of version‐order, LEIDS‐R and DAS‐change, as expected, were correlated in the B‐A order. However, the association is still low (r = .30) for measures that intend to capture the same concept. These results, and the fact that only one study has found a moderately strong association between the LEIDS and DAS‐change (Segal et al., 2006), make it difficult to be certain that the LEIDS‐R measures CR. Further, a potential limitation of the questionnaire is that it asks participants to imagine how they would feel during a sad mood and thus does not measure actual rise in dysfunctional cognitions during sad mood or stress. A recent study has demonstrated, however, that LEIDS‐R scores correlate with actual fluctuations in negative thinking during daily stressful events, supporting the construct and ecological validity of the scale (Takano et al., manuscript submitted for publication). More studies using ecological momentary assessments are needed to confirm that the LEIDS‐R measures increases in negative thinking during sad mood or stress. Of note, as suggested by Raes (2015), a difference between the two measures might exist because the LEIDS‐R and DAS‐change may examine different aspects of CR. The LEIDS‐R measures reactivity of cognitive processes, including the form of thoughts, for example rumination or hopelessness, included in LEIDS‐R subscales, whereas the DAS measures plain cognition (dysfunctional attitudes; Raes, 2015).
As a post‐hoc analysis, we found that the rumination subscale correlates most strongly with the total LEIDS‐R score (r = .93, p < .001), followed by risk avoidance (r = .91, p < .001; Table S2), suggesting these might be the driving factors of CR. Previous studies have reported that individuals who are more likely to engage in behavioural avoidance are more likely to ruminate (Moulds, Kandris, Starr, & Wong, 2007) and that rumination may be a method of avoiding active problem solving (van Rijsbergen, Kok, Elgersma, Hollon, & Bockting, 2014). These findings are in line with the metacognitive model, which proposes that MDD is maintained by inflexible and maladaptive response patterns (e.g., persistent rumination), which further extend negative thinking and maintain low mood. We therefore suggest that CR processes (measured by the LEIDS‐R) are independent and possibly even more prominent risk factors for MDD vulnerability and MDD recurrence than an increase of dysfunctional attitudes (as measured by DAS‐change; Jarrett et al., 2012; Lethbridge & Allen, 2008; van Rijsbergen et al., 2013). Importantly, recent research has indicated that these (ruminative) processes might be susceptible to psychological interventions as mindfulness‐based therapy (Cladder‐Micus et al., 2017) and metacognitive therapy (Normann, van Emmerik, & Morina, 2014).
Regardless of conceptual differences between the LEIDS‐R and DAS‐change, it is important to note that the LEIDS‐R has satisfactory psychometric properties (Solis et al., 2016). It is associated with recurrence risk (Figueroa et al., 2015) and with risk of first episodes (Kruijt et al., 2013). Furthermore, it does not present with the methodological problems of DAS assessment after mood‐induction. In conclusion, the LEIDS‐R measures a clinically relevant construct and recent data indicate that it measures CR (Takano et al., manuscript submitted for publication).
Interestingly, rrMDD scored high on the acceptance (ACC) subscale, which relates to increased interpersonal sensitivity, creativity, and acceptance during sad mood. It has been hypothesized that this subscale might characterize recurrent depression, as after multiple episodes a depressive identity may become internalized, leading to an increased acceptance of sad mood (Solis et al., 2016). Different subtypes of depression might respond differently to the ACC subscale, which could be problematic when assessing its total score. Future research should investigate the clinical relevance and utility of the ACC subscale.
In addition to LEIDS‐R scores, pre‐mood‐induction DAS scores were higher in rrMDD than in controls. Thus, a high level of dysfunctional attitudes, independent of mood‐induction, might also be a marker of depressive vulnerability. However, many studies also found that remitted patients no longer exhibit the maladaptive cognitions as measured by the DAS (Haffel et al., 2005). In contrast, previous evidence has consistently indicated LEIDS‐R score as a marker of depression vulnerability. Further, in this study, the effect size (Cohen's d) of the difference in LEIDS‐R scores was higher than the effect size of the pre‐mood‐induction DAS, indicating that the LEIDS‐R is more precise in predicting depression vulnerability. More studies should compare LEIDS‐R scores and pre‐mood induction DAS scores as measures of depressive vulnerability and marker for recurrence.
Some limitations of this study must be taken into account. First, we were not able to examine the effect of previous treatment, which could have affected results as psychological treatments might have differential effects on CR (Raes et al., 2009; Segal et al., 1999). Nevertheless, previous treatments will likely not have influenced our examination of the order of DAS‐A/B, as this was randomized. A second limitation is that we did not administer both DAS‐A and DAS‐B before and after mood‐induction. This might have provided better understanding of their differential sensitivity to change. Finally, although examining patients free of antidepressants allows precluding confounding medication effects, this might have led to selection of a less vulnerable sample of participants not completely representative for the general rrMDD population.
Conclusion
We conclude that the DAS‐A/B versions are not interchangeable. This finding impacts reliability and validity of the CR measurement by DAS‐change. The LEIDS‐R distinguished rrMDD from controls, in contrast to DAS‐change, and was moderately associated with DAS‐change in the order B‐A. The LEIDS‐R likely assesses different aspects of CR than DAS‐change after mood‐induction. Importantly, the LEIDS‐R does not present with the methodological issues of DAS‐change and, in line with previous research, is a valid measure of cognitive vulnerability. We therefore propose that the use of mood‐inductions has become unnecessary as the LEIDS‐R has several advantages to assess CR in both research and clinical settings, and no known disadvantages.
Funding/support
This study was supported by unrestricted personal grants from the Academic Medical Centre to C.A. Figueroa (AMC MD‐PhD Scholarship) and R.J. Mocking (AMC PhD Scholarship) and a dedicated grant from the Dutch Brain Foundation (Hersenstichting Nederland: 2009(2)‐72). Dr. H.G. Ruhé was supported by a NWO/ZonMW VENI‐Grant #016.126.059. None of the supporting organizations had any role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; and preparation, review, or approval of the manuscript; and decision to submit the manuscript for submission.
Supporting information
Figure S1. Participant flowchart.
Figure S2. Study procedure.
Table S1. (A) 2 × 2 ANOVA for pre‐mood‐induction DAS scores. (B) 2 × 2 ANCOVA for DAS‐change scores.
Table S2. Correlation total LEIDS‐R with subscales.
Data S1. Dysfunctional Attitudes Scale A and B in Dutch.
Acknowledgements
First of all, we would like to thank the study subjects who participated in this research. Second, we acknowledge the thoughtful comments of Prof. Z. Segal (University of Toronto, Toronto, Canada) regarding the set‐up of our mood‐induction procedure. Third, we would like to thank the following persons who helped collect/process data: Eline Meijer (the Academic Medical Center/University of Amsterdam, Amsterdam, the Netherlands), Lisa Bouma, BSc (the Academic Medical Center/University of Amsterdam, Amsterdam, the Netherlands) helped with collection of data; Henk Hallie (University Medical Center Groningen, University of Groningen, Groningen, the Netherlands) helped with input and checking of questionnaire data. Last, we would like to thank Dr. R. Holman (the Academic Medical Center/University of Amsterdam, Amsterdam, the Netherlands) for advice regarding the statistical analysis. No one who helped collect/process data received financial compensation for their contributions.
Correction added on 30 April, 2018, after first online publication: Author name was changed from Eric HG Ruhe to Henricus G. Ruhe
References
- Antypa, N. , Van der Does, A. J. W. , & Penninx, B. W. J. H. (2010). Cognitive reactivity: Investigation of a potentially treatable marker of suicide risk in depression. Journal of Affective Disorders, 122(1–2), 46–52. 10.1016/j.jad.2009.06.013 [DOI] [PubMed] [Google Scholar]
- Beshai, S. , Dobson, K. S. , Bockting, C. L. , & Quigley, L. (2011). Relapse and recurrence prevention in depression: Current research and future prospects. Clinical Psychology Review, 31, 1349–1360. 10.1016/j.cpr.2011.09.003 [DOI] [PubMed] [Google Scholar]
- Bockting, C. L. , Hollon, S. D. , Jarrett, R. B. , Kuyken, W. , & Dobson, K. (2015). A lifetime approach to major depressive disorder: The contributions of psychological interventions in preventing relapse and recurrence. Clinical Psychology Review, 41, 16–26. 10.1016/j.cpr.2015.02.003 [DOI] [PubMed] [Google Scholar]
- Booij, L. , & Van der Does, A. J. (2007). Cognitive and serotonergic vulnerability to depression: Convergent findings. Journal of Abnormal Psychology, 116(1), 86–94. 10.1037/0021-843X.116.1.86 [DOI] [PubMed] [Google Scholar]
- Cladder‐Micus, M. B. , van Aalderen, J. , Donders, A. R. , Spijker, J. , Vrijsen, J. N. , & Speckens, A. E. (2017). Cognitive reactivity as outcome and working mechanism of mindfulness‐based cognitive therapy for recurrently depressed patients in remission. Cognition and Emotion, 1–8. 10.1080/02699931.2017.1285753 [DOI] [PubMed] [Google Scholar]
- Cohen, J. , Cohen, P. , West, S. G. , & Aiken, L. S. (2013). Applied multiple regression/correlation analysis for the behavioral sciences. Mahwah, NJ: Routledge. [Google Scholar]
- Dozois, D. J. , Covin, R. , & Brinker, J. K. (2003). Normative data on cognitive measures of depression. Journal of Consulting and Clinical Psychology, 71(1), 71–80. 10.1037/0022-006X.71.1.71 [DOI] [PubMed] [Google Scholar]
- Douma, M. (1991). The measurement of trait depression. Construction of the Dutch Dysfunctional Attitude Scale (A Version) of Arlene Weissman. Meerssen, The Netherlands: St. Lois Marie Jamin. [Google Scholar]
- Eaton, W. W. , Shao, H. , Nestadt, G. , Lee, H. B. , Bienvenu, O. J. , & Zandi, P. (2008). Population‐based study of first onset and chronicity in major depressive disorder. Archives of General Psychiatry, 65, 513–520. 10.1001/archpsyc.65.5.513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgersma, H. J. , de Jong, P. J. , van Rijsbergen, G. D. , Kok, G. D. , Burger, H. , van der Does, W. , … Bockting, C. L. (2015). Cognitive reactivity, self‐depressed associations, and the recurrence of depression. Journal of Affective Disorders, 183, 300–309. 10.1016/j.jad.2015.05.018 [DOI] [PubMed] [Google Scholar]
- Figueroa, C. A. , Ruhe, H. G. , Koeter, M. W. , Spinhoven, P. , Van der Does, W. , Bockting, C. L. , & Schene, A. H. (2015). Cognitive reactivity versus dysfunctional cognitions and the prediction of relapse in recurrent major depressive disorder. Journal of Clinical Psychiatry, 76(10), e1306–e1312. 10.4088/JCP.14m09268 [DOI] [PubMed] [Google Scholar]
- Gemar, M. C. , Segal, Z. V. , Sagrati, S. , & Kennedy, S. J. (2001). Mood‐induced changes on the Implicit Association Test in recovered depressed patients. Journal of Abnormal Psychology, 110, 282–289. 10.1037/0021-843X.110.2.282 [DOI] [PubMed] [Google Scholar]
- Haffel, G. J. , Abramson, L. Y. , Voelz, Z. R. , Metalsky, G. I. , Halberstadt, L. , Dykman, B. M. , … Alloy, L. B. (2005). Negative cognitive styles, dysfunctional attitudes, and the remitted depression paradigm: A search for the elusive cognitive vulnerability to depression factor among remitted depressives. Emotion, 5, 343–348. 10.1037/1528-3542.5.3.343 [DOI] [PubMed] [Google Scholar]
- Hamilton, M. (1960). A rating scale for depression. Journal of Neurology, Neurosurgery and Psychiatry, 23, 56–62. 10.1136/jnnp.23.1.56 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarrett, R. B. , Minhajuddin, A. , Borman, P. D. , Dunlap, L. , Segal, Z. V. , Kidner, C. L. , … Thase, M. E. (2012). Cognitive reactivity, dysfunctional attitudes, and depressive relapse and recurrence in cognitive therapy responders. Behavior Research and Therapy, 50, 280–286. 10.1016/j.brat.2012.01.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeanne, M. , Gross, J. , Persons, J. , & Hahn, J. (1998). Mood matters: Negative mood induction activates dysfunctional attitudes in women vulnerable to depression. Cognitive Therapy and Research, 22, 363–376. 10.1023/A:1018709212986 [DOI] [Google Scholar]
- Kruijshaar, M. E. , Barendregt, J. , Vos, T. , de Graaf, R. , Spijker, J. , & Andrews, G. (2005). Lifetime prevalence estimates of major depression: An indirect estimation method and a quantification of recall bias. European Journal of Epidemiology, 20(1), 103–111. 10.1007/s10654-004-1009-0 [DOI] [PubMed] [Google Scholar]
- Kruijt, A.‐W. , Antypa, N. , Booij, L. , de Jong, P. J. , Glashouwer, K. , Penninx, B. W. J. H. , & Van der Does, W. (2013). Cognitive reactivity, implicit associations, and the incidence of depression: A two‐year prospective study. PLoS One, 8(7), e70245 10.1371/journal.pone.0070245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau, M. A. , Segal, Z. V. , & Williams, J. M. G. (2004). Teasdale's differential activation hypothesis: Implications for mechanisms of depressive relapse and suicidal behaviour. Behaviour Research and Therapy, 42, 1001–1017. 10.1016/j.brat.2004.03.003 [DOI] [PubMed] [Google Scholar]
- Lethbridge, R. , & Allen, N. B. (2008). Mood induced cognitive and emotional reactivity, life stress, and the prediction of depressive relapse. Behavior Research and Therapy, 46, 1142–1150. 10.1016/j.brat.2008.06.011 [DOI] [PubMed] [Google Scholar]
- Mathers, C. D. , & Loncar, D. (2006). Projections of global mortality and burden of disease from 2002 to 2030. PLoS Medicine, 3(11), e442 10.1371/journal.pmed.0030442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merens, W. , Booij, L. , & Van Der Does, A. J. W. (2008). Residual cognitive impairments in remitted depressed patients. Depression and Anxiety, 25(6), E27–E36. 10.1002/da.20391 [DOI] [PubMed] [Google Scholar]
- Miranda, J. , & Persons, J. B. (1988). Dysfunctional attitudes are mood‐state dependent. Journal of Abnormal Psychology, 97(1), 76–79. 10.1037/0021-843X.97.1.76 [DOI] [PubMed] [Google Scholar]
- Moulds, M. L. , Kandris, E. , Starr, S. , & Wong, A. C. M. (2007). The relationship between rumination, avoidance and depression in a non‐clinical sample. Behaviour Research and Therapy, 45, 251–261. 10.1016/j.brat.2006.03.003 [DOI] [PubMed] [Google Scholar]
- Moulds, M. L. , Kandris, E. , Williams, A. D. , Lang, T. , Yap, C. , & Hoffmeister, K. (2008). An investigation of the relationship between cognitive reactivity and rumination. Behavior Therapy, 39(1), 65–71. 10.1016/j.beth.2007.05.001 [DOI] [PubMed] [Google Scholar]
- Normann, N. , van Emmerik, A. A. , & Morina, N. (2014). The efficacy of metacognitive therapy for anxiety and depression: A meta‐analytic review. Depression and Anxiety, 31, 402–411. 10.1002/da.22273 [DOI] [PubMed] [Google Scholar]
- Power, M. J. , Katz, R. , McGuffin, P. , Duggan, C. F. , Lam, D. , & Beck, A. T. (1994). The Dysfunctional Attitude Scale (DAS): A comparison of forms A and B and proposals for a new subscaled version. Journal of Research in Personality, 28, 263–276. 10.1006/jrpe.1994.1019 [DOI] [Google Scholar]
- Raes, F. (2015). Reactivity at different levels of cognitive analysis: Products versus operations. Journal of Clinical Psychiatry, 76(10), e1318–e1319. 10.4088/JCP.14com09709 [DOI] [PubMed] [Google Scholar]
- Raes, F. , Dewulf, D. , Van Heeringen, C. , & Williams, J. M. (2009). Mindfulness and reduced cognitive reactivity to sad mood: Evidence from a correlational study and a non‐randomized waiting list controlled study. Behavior Research and Therapy, 47, 623–627. 10.1016/j.brat.2009.03.007 [DOI] [PubMed] [Google Scholar]
- Ruhe, H. G. , Mason, N. S. , & Schene, A. H. (2007). Mood is indirectly related to serotonin, norepinephrine and dopamine levels in humans: A meta‐analysis of monoamine depletion studies. Molecular Psychiatry, 12, 331–359. 10.1038/sj.mp.4001949 [DOI] [PubMed] [Google Scholar]
- Rush, A. J. , Gullion, C. M. , Basco, M. R. , Jarrett, R. B. , & Trivedi, M. H. (1996). The Inventory of Depressive Symptomatology (IDS): Psychometric properties. Psychological Medicine, 26, 477–486. 10.1017/S0033291700035558 [DOI] [PubMed] [Google Scholar]
- Segal, Z. V. , Gemar, M. , & Williams, S. (1999). Differential cognitive response to a mood challenge following successful cognitive therapy or pharmacotherapy for unipolar depression. Journal of Abnormal Psychology, 108(1), 3–10. 10.1037/0021-843X.108.1.3 [DOI] [PubMed] [Google Scholar]
- Segal, Z. V. , Kennedy, S. , Gemar, M. , Hood, K. , Pedersen, R. , & Buis, T. (2006). Cognitive reactivity to sad mood provocation and the prediction of depressive relapse. Archives of General Psychiatry, 63, 749–755. 10.1001/archpsyc.63.7.749 [DOI] [PubMed] [Google Scholar]
- Solis, E. , Antypa, N. , Conijn, J. M. , Kelderman, H. , & Van der Does, W. (2016). Psychometric properties of the Leiden Index of Depression Sensitivity (LEIDS). Psychological Assessment, 29, 158 10.1037/pas0000326 [DOI] [PubMed] [Google Scholar]
- Solomon, D. A. , Keller, M. B. , Leon, A. C. , Mueller, T. I. , Lavori, P. W. , Shea, M. T. , … Endicott, J. (2000). Multiple recurrences of major depressive disorder. American Journal of Psychiatry, 157, 229–233. 10.1176/appi.ajp.157.2.229 [DOI] [PubMed] [Google Scholar]
- Takano, K. , Raes, F. , & Van der Does, W. (2017). Conceptual, predictive and ecological validity of the Leiden Index of Depression Sensitivity. Manuscript submitted for publication.
- Van der Does, W. (2002). Cognitive reactivity to sad mood: Structure and validity of a new measure. Behavior Research and Therapy, 40(1), 105–120. 10.1016/S0005-7967(00)00111-X [DOI] [PubMed] [Google Scholar]
- Van der Does, W. (2005). Thought suppression and cognitive vulnerability to depression. British Journal of Clinical Psychology, 44(Pt 1), 1–14. 10.1348/014466504x19442 [DOI] [PubMed] [Google Scholar]
- Van der Gucht, K. , Takano, K. , Van Broeck, N. , & Raes, F. (2014). A mindfulness‐based intervention for economically disadvantaged people: Effects on symptoms of stress, anxiety, and depression and on cognitive reactivity and overgeneralization. Mindfulness, 6, 1042–1052. 10.1007/s12671-014-0353-8 [DOI] [Google Scholar]
- van Rijsbergen, G. D. , Bockting, C. L. , Burger, H. , Spinhoven, P. , Koeter, M. W. , Ruhe, H. G. , … Schene, A. H. (2013). Mood reactivity rather than cognitive reactivity is predictive of depressive relapse: A randomized study with 5.5‐year follow‐up. Journal of Consulting and Clinical Psychology, 81, 508–517. 10.1037/a0032223 [DOI] [PubMed] [Google Scholar]
- van Rijsbergen, G. D. , Kok, G. D. , Elgersma, H. J. , Hollon, S. D. , & Bockting, C. L. H. (2014). Personality and cognitive vulnerability in remitted recurrently depressed patients. Journal of Affective Disorders, 173C, 97–104. 10.1016/j.jad.2014.10.042 [DOI] [PubMed] [Google Scholar]
- Weissman, A. N. (1979). Assessing depressogenic attitudes: A validation study. Unpublished thesis. University of Pennsylvania, Philadelphia, PA. [Google Scholar]
- Weissman, A. N. , & Beck, A. T. (1978). Development and validation of the Dysfunctional Attitude Scale: A preliminary investigation. Paper presented at the meeting of the Association for the Advancement of Behavior Therapy, Chicago. [Google Scholar]
- Yamamoto, T. , Yamano, M. , Shimada, H. , Ichikawa, K. , & Nakaya, M. (2014). The specificity of cognitive reactivity in recurrent major depressive episodes. Shinrigaku Kenkyu, 85(1), 29–39. 10.4992/jjpsy.85.29 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Participant flowchart.
Figure S2. Study procedure.
Table S1. (A) 2 × 2 ANOVA for pre‐mood‐induction DAS scores. (B) 2 × 2 ANCOVA for DAS‐change scores.
Table S2. Correlation total LEIDS‐R with subscales.
Data S1. Dysfunctional Attitudes Scale A and B in Dutch.


