Abstract
We report on a rotaxane‐like architecture secured by the in situ tying of an overhand knot in the tris(2,6‐pyridyldicarboxamide) region of the axle through complexation with a lanthanide ion (Lu3+). The increase in steric bulk caused by the knotting locks a crown ether onto the thread. Removal of the lutetium ion unties the knot, and when the axle binding site for the ring is deactivated, the macrocycle spontaneously dethreads. When the binding interaction is switched on again, the crown ether rethreads over the 10 nm length of the untangled strand. The overhand knot can be retied, relocking the threaded structure, by once again adding lutetium ions.
Keywords: chemical topology, molecular knots, rotaxanes, supramolecular chemistry
Macroscopic knots persist and have distinctive properties owing to intra‐strand mechanics and inertia (parts of the strand move only when a force is applied, and in directions determined by the knot structure). In contrast, at the nanoscale, every part of a knotted strand undergoes random thermal motion in all directions. Accordingly, extrapolating knot properties between such disparate length scales, as with machine mechanisms,1 will not always be valid.2 Although a number of small‐molecule knots have been synthesized,3, 4 to date the knotting of a strand has only been exploited in functions that have no macroscopic counterpart (anion binding5a,5b and allosterically regulated5c and asymmetric5d catalysis).1, 5, 6 In our everyday world, “stopper knots”7 are tied to prevent unreeving (that is, to prevent a strand from passing through a narrow aperture or slipping through another knot8). Stopper knots are routinely used to secure ropes for sailing and rock climbing, and threads when sewing. While considering which macroscopic properties of knots might be transferable to the molecular level (and how), we wondered whether the increase in steric bulk that accompanies the tying of a knot could be used to prevent the dethreading of a ring from an axle in a rotaxane‐like architecture.9, 10, 11 In addition to demonstrating that an everyday use of knots can be extrapolated to molecules, the concept is appealing because the locking of the ring on the axle would be accomplished solely through strand entanglement, induced by metal ion coordination, rather than by any functional‐group alterations12 (Scheme 1).
Scheme 1.
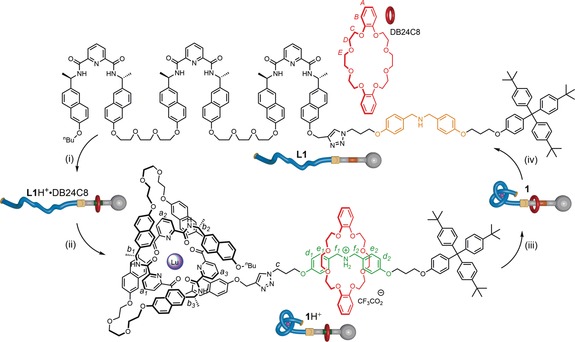
Threading, securing, and releasing a macrocycle on an axle by tying and untying a molecular stopper knot. Reagents and conditions: (i) CF3CO2H (10 equiv), CD2Cl2, RT, 48 h, quantitative; (ii) Lu(SO3CF3)3 (1.1 equiv), MeCN‐d 3, 80 °C, 16 h, 90 % ; (iii) Et3N (10 equiv), CD2Cl2, RT, 1 h, quantitative; (iv) Et4NF (10 equiv), CD2Cl2, RT, 5 min, quantitative.
We designed a rotaxane system to explore this idea by using an axle bearing an ammonium group, an excellent binding site for dibenzo‐24‐crown‐8 (DB24C8),13 terminated at one end by a bulky trityl derivative and at the other by a tris(2,6‐pyridinedicarboxamide)14 region (1H+, Scheme 1). Tris(2,6‐pyridinedicarboxamide) ligands can be tied5d into overhand knots15 through coordination to lanthanide(III) ions.14, 16, 17 We found it convenient to assemble the complete axle as a rotaxane (Scheme 2; see also the Supporting Information). Ligand building block L2 was treated with Lu(CF3SO3)3 in MeCN at 80 °C to generate overhand knot Λ‐L2⋅Lu (Λ refers to the handedness of the knot;18 for steric reasons, only the Λ knot can form from the R,R,R,R,R,R enantiomer of L1).5d, 19 After 16 h, the knotted lanthanide complex was the only species evident by electrospray ionization mass spectrometry (ESI‐MS) and 1H NMR spectroscopy (Scheme 2, step (i)). Treatment of azide L3H+ with DB24C8 in the presence of trifluoroacetic acid generated pseudorotaxane L3H+⋅DB24C8 (Scheme 2, step (ii)). A CuAAC reaction of threaded complex L3H+⋅DB24C8 with Λ‐L2⋅Lu generated rotaxane architecture 1H+ in 41 % yield, following purification by size exclusion chromatography (Scheme 2, step (iii)).
Scheme 2.
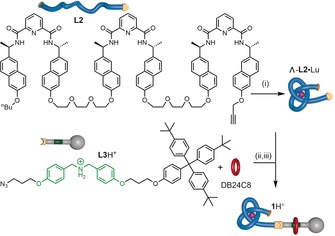
Synthesis of rotaxane architecture 1H+. Reagents and conditions: (i) Lu(CF3SO3)3 (1 equiv), MeCN, 80 °C, 16 h, 75 %; (ii) L3H+, DB24C8 (4 equiv), CF3COOH (10 equiv), CH2Cl2, RT, 10 min; then (iii) Λ‐L2⋅Lu, (CH3CN)4Cu(CF3SO3) (2 equiv), MeCN/MeOH (1:1), RT, 20 h, 41 %.
1H NMR spectroscopy (600 MHz, 298 K, MeCN‐d 3) confirmed that both the knotted conformation of the strand and the threaded structure are maintained in 1H+ (Figure 1). The overhand knot is evident from the upfield shift of the pyridine Ha1/Ha2 protons (see the Supporting Information, Spectrum S11), which is due to π‐stacking in the knotted conformation,5d and the diastereotopic splitting of various axle signals caused by the asymmetry of the knot (Spectrum S18). The benzylic protons Hf1 and Hf2 appear at δ=4.55 ppm in 1H+ (Figure 1 b), which corresponds to a downfield shift of 0.40 ppm compared to the macrocycle‐free knotted axle Λ‐L1H+⋅Lu (Figure 1 a). ESI‐MS analysis of 1H+ was consistent with the rotaxane structure (m/z 830.1 [1H+]4+, 1156.0 [1H+][CF3SO3]3+, 1808.6 [1H+][CF3SO3]2 2+; see Figure S1).
Figure 1.
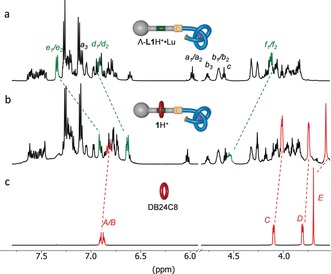
Partial 1H NMR spectra (600 MHz, MeCN‐d 3, 298 K) showing the threaded structure of 1H+. a) Λ‐L1H+⋅Lu. b) 1H+. c) DB24C8.
To confirm that the overhand knot blocks dethreading of the macrocycle, the dibenzylammonium binding site of 1H+ was “switched off” by deprotonation with triethylamine, forming 1 (Scheme 1, step (iii); Et3N, CD2Cl2, RT, 1 h; see also Figures S2 and S3).20 In CD2Cl2, the 1H NMR spectrum of 1 is broad for internal regions of the axle (Figure 2 a), suggesting slow dynamics of ring movement between various sites and conformations.21 In MeCN‐d 3, the macrocycle samples much of the axle as evidenced by modest shifts in the 1H NMR resonances of protons all along the length of the thread (Figure S3). X‐ray crystal structures of knotted tris(2,6‐pyridinedicarboxamide) lanthanide complexes5d, 16 indicate that the tied knot has a diameter of approximately 2 nm, whereas the aperture of DB24C8 is <1 nm. Accordingly, with the binding site for the crown ether deactivated, the rotaxane architecture of 1 is still kinetically stable; at room temperature, 1 showed no signs of dethreading over several weeks in CD2Cl2 or MeCN‐d3 solution.
Figure 2.
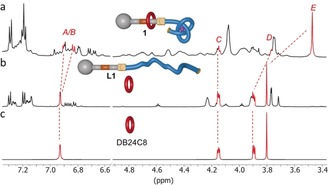
Triggered dethreading of 1. a) Partial 1H NMR spectrum (600 MHz, CD2Cl2, 298 K) of 1. b) Partial 1H NMR spectrum (600 MHz, CD2Cl2, 298 K) recorded 5 min after addition of Et4NF (10 equiv) to 1. c) Partial 1H NMR spectrum (600 MHz, CD2Cl2, 298 K) of DB24C8.
The overhand knot of 1 was untied, and the macrocycle released, by treatment of the deprotonated rotaxane with tetraethylammonium fluoride (10 equiv) in CD2Cl2 (Scheme 1, step (iv)). 1H NMR analysis indicated quantitative dethreading of the crown ether within 5 min of the addition of the fluoride salt (Figure 2 b). MALDI mass spectrometry confirmed the presence of L1 (m/z 2716.4, [M+Na+]; Figure S6), with no evidence of a demetalated pseudorotaxane. This indicates that with the binding site deactivated, ring dethreading occurs as soon as the knot is untied. The quantitative dethreading further confirms the complete deprotonation of 1 (the DB24C8–secondary ammonium equilibrium in CD2Cl2 strongly favours the pseudorotaxane).20b
The presence of the knot also prevents threading onto the axle: Treatment of the protonated knotted thread Λ‐L1H+⋅Lu with DB24C8 failed to generate any [2]rotaxane (Scheme S9).
Finally, we demonstrated that the ring could thread over the about 10 nm length of the untangled strand22 and the rotaxane architecture subsequently be secured by tying the stopper knot (Scheme 1, steps (i) and (ii)). Addition of trifluoroacetic acid to a solution of DB24C8 (10 equiv) and L1 in CD2Cl2 afforded the pseudorotaxane complex L1H+ over 48 h (Figures 3 a and S7). Excess trifluoroacetic acid was removed under reduced pressure, Lu(CF3SO3)3 (1.1 equiv in MeCN‐d 3) was added, and the solution heated at 80 °C. After 16 h, both 1H NMR and ESI‐MS analysis confirmed the formation of 1H+ in approximately 90 % yield (determined by 1H NMR analysis; Figures 3 b and S8).
Figure 3.
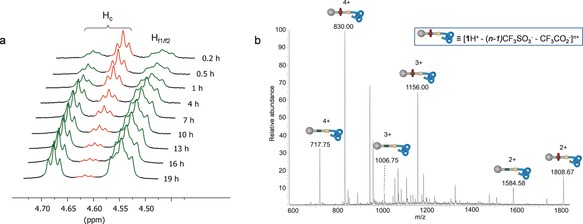
Securing a rotaxane architecture (1H+) by threading a ring (DB24C8) onto an axle (L1) and tying a molecular stopper knot. a) Partial 1H NMR spectrum (600 MHz, MeCN‐d 3, 298 K) stack plot of the threading process to form L1H+⋅DB24C8 (Scheme 1, step (i)), showing signals for Hc and Hf1/Hf2. b) Detection of knotted, threaded 1H+ by ESI‐MS (positive mode) following in situ tying of an overhand stopper knot in L1H+⋅DB24C8 through coordination to Lu3+ (Scheme 1, step (ii)).
In conclusion, we have demonstrated that despite the fundamental differences in mechanical behaviour across length scales, a synthetic molecular knot can perform a mechanical function analogous to a mechanical function performed by macroscopic knotting. Once a thermodynamic driving force has been used to thread the molecular ring onto the axle, addition of a lanthanide ion ties the molecular stopper knot, locking the ring on the axle even after the ring binding site has been deactivated. The knot can subsequently be untied, and the ring released from the axle, by removing the metal ion. Release of the macrocycle requires no change to the covalent structure of the molecule. The difference in the timescales required for tying (16 hours at 80 °C) and untying (<5 min at room temperature) the stopper knot is particularly striking. Such processes may prove useful in the development of functional knotted molecules and materials.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
We thank the Engineering and Physical Sciences Research Council (EPSRC; EP/P027067/1), the EU (Marie Curie Individual Postdoctoral Fellowship to F.S.; EC 746993), and the University of Manchester (President's Doctoral Scholarship to L.Z.) for funding, and the EPSRC National Mass Spectrometry Service Centre (Swansea, UK) for high‐resolution mass spectrometry. D.A.L. is a Royal Society Research Professor.
D. A. Leigh, L. Pirvu, F. Schaufelberger, D. J. Tetlow, L. Zhang, Angew. Chem. Int. Ed. 2018, 57, 10484.
References
- 1. Zhang L., Marcos V., Leigh D. A., Proc. Natl. Acad. Sci. USA 2018, 10.1073/pnas.1712788115. [DOI] [Google Scholar]
- 2. Micheletti C., Marenduzzo D., Orlandini E., Phys. Rep. 2011, 504, 1–73. [Google Scholar]
- 3.
- 3a. Fielden S. D. P., Leigh D. A., Woltering S. L., Angew. Chem. Int. Ed. 2017, 56, 11166–11194; [DOI] [PMC free article] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 11318–11347; [Google Scholar]
- 3b. Fenlon E. E., Eur. J. Org. Chem. 2008, 5023–5035; [Google Scholar]
- 3c. Forgan R. S., Sauvage J.-P., Stoddart J. F., Chem. Rev. 2011, 111, 5434–5464; [DOI] [PubMed] [Google Scholar]
- 3d. Ayme J.-F., Beves J. E., Campbell C. J., Leigh D. A., Chem. Soc. Rev. 2013, 42, 1700–1712; [DOI] [PubMed] [Google Scholar]
- 3e. Horner K. E., Miller M. A., Steed J. W., Sutcliffe P. M., Chem. Soc. Rev. 2016, 45, 6432–6448; [DOI] [PubMed] [Google Scholar]
- 3f. Sauvage J.-P., Angew. Chem. Int. Ed. 2017, 56, 11080–11093; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2017, 129, 11228–11242. [Google Scholar]
- 4.For examples of small-molecule knots, see:
- 4a. Dietrich-Buchecker C. O., Sauvage J.-P., Angew. Chem. Int. Ed. Engl. 1989, 28, 189–192; [Google Scholar]; Angew. Chem. 1989, 101, 192–194; [Google Scholar]
- 4b. Ashton P. R., Matthews O. A., Menzer S., Raymo F. M., Spencer N., Stoddart J. F., Williams D. J., Liebigs Ann./Recl. 1997, 2485–2494; [Google Scholar]
- 4c. Safarowsky O., Nieger M., Fröhlich R., Vögtle F., Angew. Chem. Int. Ed. 2000, 39, 1616–1618; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2000, 112, 1699–1701; [Google Scholar]
- 4d. Feigel M., Ladberg R., Engels S., Herbst-Irmer R., Fröhlich R., Angew. Chem. Int. Ed. 2006, 45, 5698–5702; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2006, 118, 5827–5831; [Google Scholar]
- 4e. Guo J., Mayers P. C., Breault G. A., Hunter C. A., Nat. Chem. 2010, 2, 218–220; [DOI] [PubMed] [Google Scholar]
- 4f. Barran P. E., Cole H. L., Goldup S. M., Leigh D. A., McGonigal P. R., Symes M. D., Wu J. Y., Zengerle M., Angew. Chem. Int. Ed. 2011, 50, 12280–12284; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 12488–12492; [Google Scholar]
- 4g. Romuald C., Coutrot F., Angew. Chem. Int. Ed. 2012, 51, 2544–2545; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2012, 124, 2594–2595; [Google Scholar]
- 4h. Ponnuswamy N., Cougnon F. B. L., Clough J. M., Pantoş D. G., Sanders J. K. M., Science 2012, 338, 783–785; [DOI] [PubMed] [Google Scholar]
- 4i. Ayme J.-F., Beves J. E., Leigh D. A., McBurney R. T., Rissanen K., Schultz D., Nat. Chem. 2012, 4, 15–20; [DOI] [PubMed] [Google Scholar]
- 4j. Prakasam T., Lusi M., Elhabiri M., Platas-Iglesias C., Olsen J.-C., Asfari Z., Cianférani-Sanglier S., Debaene F., Charbonnière L. J., Trabolsi A., Angew. Chem. Int. Ed. 2013, 52, 9956–9960; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2013, 125, 10140–10144; [Google Scholar]
- 4k. Ponnuswamy N., Cougnon F. B. L., Pantoş D. G., Sanders J. K. M., J. Am. Chem. Soc. 2014, 136, 8243–8251; [DOI] [PubMed] [Google Scholar]
- 4l. Ayme J.-F., Gil-Ramírez G., Leigh D. A., Lemonnier J.-F., Markevicius A., Muryn C. A., Zhang G., J. Am. Chem. Soc. 2014, 136, 13142–13145; [DOI] [PubMed] [Google Scholar]
- 4m. Danon J. J., Krüger A., Leigh D. A., Lemonnier J.-F., Stephens A. J., Vitorica-Yrezabal I. J., Woltering S. L., Science 2017, 355, 159–162; [DOI] [PubMed] [Google Scholar]
- 4n. Zhang L., August D. P., Zhong J., Whitehead G. F. S., Vitorica-Yrezabal I. J., Leigh D. A., J. Am. Chem. Soc. 2018, 140, 4982–4985. [DOI] [PubMed] [Google Scholar]
- 5.
- 5a. Ayme J.-F., Beves J. E., Campbell C. J., Gil-Ramírez G., Leigh D. A., Stephens A. J., J. Am. Chem. Soc. 2015, 137, 9812–9815; [DOI] [PubMed] [Google Scholar]
- 5b. Bilbeisi R. A., Prakasam T., Lusi M., El-Khoury R., Platas-Iglesias C., Charbonnière L. J., Olsen J.-C., Elhabiri M., Trabolsi A., Chem. Sci. 2016, 7, 2524–2532; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5c. Marcos V., Stephens A. J., Jaramillo-Garcia J., Nussbaumer A. L., Woltering S. L., Valero A., Lemonnier J.-F., Vitorica-Yrezabal I. J., Leigh D. A., Science 2016, 352, 1555–1559; [DOI] [PubMed] [Google Scholar]
- 5d. Gil-Ramírez G., Hoekman S., Kitching M. O., Leigh D. A., Vitorica-Yrezabal I. J., Zhang G., J. Am. Chem. Soc. 2016, 138, 13159–13162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Lim N. C. H., Jackson S. E., J. Phys. Condens. Matter 2015, 27, 354101. [DOI] [PubMed] [Google Scholar]
- 7. Ashley C. W., The Ashley Book of Knots, Doubleday, Doran & Co, New York, 1944, pp. 83–92. [Google Scholar]
- 8. Trefz B., Siebert J., Virnau P., Proc. Natl. Acad. Sci. USA 2014, 111, 7948–7951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Technically, 1 is a kinetically stable pseudorotaxane, rather than a rotaxane proper, as it can be dethreaded without breaking a covalent bond (see footnote [194] in
- 9a. Kay E. R., Leigh D. A., Zerbetto F., Angew. Chem. Int. Ed. 2007, 46, 72–191; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2007, 119, 72–196). However, kinetically stable pseudorotaxanes, such as those formed by “slippage” [ [Google Scholar]
- 9b. Ashton P. R., Bělohradský M., Philp D., Stoddart J. F., J. Chem. Soc. Chem. Commun. 1993, 1269–1274], are often referred to as rotaxanes [ [Google Scholar]
- 9c. Bruns C., Stoddart J. F., The Nature of the Mechanical Bond: From Molecules to Machines, Wiley, Hoboken, 2017]. [Google Scholar]
- 10.Rotaxanes with stoppers made from closed-loop knots, which cannot be tied or untied, have previously been described; see:
- 10a. Lukin O., Kubota T., Okamoto Y., Schelhase F., Yoneva A., Müller W. M., Müller U., Vögtle F., Angew. Chem. Int. Ed. 2003, 42, 4542–4545; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2003, 115, 4681–4684; [Google Scholar]
- 10b. Lukin O., Vögtle F., Angew. Chem. Int. Ed. 2005, 44, 1456–1477; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2005, 117, 1480–1501. An assertion made in that paper and review that the macrocycle “…should exhibit a unidirectional rotation in the enantiomeric forms of the knotaxanes.” is incorrect; chirality (knot or otherwise) is neither a sufficient nor a necessary condition for directionally biasing Brownian motion; see Ref. [9a]. [Google Scholar]
- 11.Spontaneous knotting in DNA chains is believed to cause jamming during nanopore sequencing; see:
- 11a. Rosa A., Di Ventra M., Micheletti C., Phys. Rev. Lett. 2012, 109, 118301; [DOI] [PubMed] [Google Scholar]
- 11b. Plesa C., Verschueren D., Pud S., van der Torre J., Ruitenberg J. W., Witteveen M. J., Jonsson M. P., Grosberg A. Y., Rabin Y., Dekker C., Nat. Nanotechnol. 2016, 11, 1093–1097; [DOI] [PubMed] [Google Scholar]
- 11c. Suma A., Micheletti C., Proc. Natl. Acad. Sci. USA 2017, 114, E2991–E2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.The change in shape of a folded axle region has previously been observed to result in slow dethreading of a pseudorotaxane, see:
- 12a. Zhang K. D., Zhao X., Wang G.-T., Liu Y., Zhang Y., Lu H.-J., Jiang X.-K., Li Z.-T., Angew. Chem. Int. Ed. 2011, 50, 9866–9870; [DOI] [PubMed] [Google Scholar]; Angew. Chem. 2011, 123, 10040–10044; for a self-entangled [1]rotaxane featuring a crown ether and ammonium groups, see: [Google Scholar]
- 12b. Clavel C., Fournel-Marotte K., Coutrot F., Molecules 2013, 18, 11553–11575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Gibson H. W., Jones J. W., Zakharov L. M., Rheingold A. L., Slebodnick C., Chem. Eur. J. 2011, 17, 3192–3206. [DOI] [PubMed] [Google Scholar]
- 14.
- 14a. Barry D. E., Caffrey D. F., Gunnlaugsson T., Chem. Soc. Rev. 2016, 45, 3244–3274; [DOI] [PubMed] [Google Scholar]
- 14b. Kitchen J. A., Coord. Chem. Rev. 2017, 340, 232–246. [Google Scholar]
- 15.
- 15a. Carina R. F., Dietrich-Buchecker C., Sauvage J.-P., J. Am. Chem. Soc. 1996, 118, 9110–9116; [Google Scholar]
- 15b. Adams H., Ashworth E., Breault G. A., Guo J., Hunter C. A., Mayers P. C., Nature 2001, 411, 763. [DOI] [PubMed] [Google Scholar]
- 16. Zhang G., Gil-Ramírez G., Markevicius A., Browne C., Vitorica-Yrezabal I. J., Leigh D. A., J. Am. Chem. Soc. 2015, 137, 10437–10442. [DOI] [PubMed] [Google Scholar]
- 17.For a lanthanide-coordinated rotaxane, see: Allain C., Beer P. D., Faulkner S., Jones M. W., Kenwright A. M., Kilah N. L., Knighton R. C., Sørensen T. J., Tropiano M., Chem. Sci. 2013, 4, 489–493. [Google Scholar]
- 18.The overhand knot formed is of single handedness (Λ), induced by the asymmetric carbon centres adjacent to the strand amide groups (see Ref. [5d]). The chirality has no affect per se in the present study, but we found the folding process to be quicker with methyl substituents at these positions (these restrict conformations of the strand, presumably facilitating folding to the knot) than with an achiral analogue lacking the methyl groups.
- 19. Kotova O., Kitchen J. A., Lincheneau C., Peacock R. D., Gunnlaugsson T., Chem. Eur. J. 2013, 19, 16181–16186. [DOI] [PubMed] [Google Scholar]
- 20.Triethylamine and bases of similar strength deprotonate DB24C8 ammonium rotaxanes as long as the crown ether can be sufficiently stabilized on other parts of the axle, or in the presence of particular anions; see:
- 20a. Nakazono K., Takata T., Chem. Eur. J. 2010, 16, 13783–13794; [DOI] [PubMed] [Google Scholar]
- 20b. Elizarov A. M., Chiu S.-H., Stoddart J. F., J. Org. Chem. 2002, 67, 9175–9181; [DOI] [PubMed] [Google Scholar]
- 20c. Busseron E., Romuald C., Coutrot F., Chem. Eur. J. 2010, 16, 10062–10073. [DOI] [PubMed] [Google Scholar]
- 21.
- 21a. Zheng H., Zhou W., Lv J., Yin X., Li Y., Liu H., Li Y., Chem. Eur. J. 2009, 15, 13253–13262; [DOI] [PubMed] [Google Scholar]
- 21b. Coutrot F., ChemistryOpen 2015, 4, 556–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.For an investigation into the mechanism of macrocycle threading onto polymer chains, see: Deutman A. B. C., Monnereau C., Elemans J. A. A. W., Ercolani G., Nolte R. J. M., Rowan A. E., Science 2008, 322, 1668–1671. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


