Abstract
Atopic dermatitis (AD) requires long‐term management, mainly with topical anti‐inflammatory agents. Topical corticosteroids (TCS) and tacrolimus ointment (TAC‐O) are recommended as first‐line treatments for AD. However, the long‐term use of TCS is limited by cutaneous adverse events such as skin atrophy. For TAC‐O, Japanese and US labelings were updated in 2003 and 2006, respectively, to include a boxed warning about a theoretical risk of skin cancer and lymphoma in patients treated with topical calcineurin inhibitors. However, TAC‐O has been used worldwide for longer than 15 years to treat adult and pediatric patients with AD. Available data suggest that TAC‐O is effective and well tolerated, and can improve quality of life. TAC‐O has successfully been used in the proactive management of AD consisting of long‐term intermittent use to prevent, delay or reduce the occurrence of AD flares. Systemic drug absorption after TAC‐O application is negligible and unlikely to result in systemic immunosuppression. There is currently no strong evidence of an increased rate of malignancy in treated patients, and observational data from postmarketing surveillance studies have shown no safety concerns. In the absence of robust evidence, the warning about the carcinogenic potential in the Japanese labeling for TAC‐O does not appear justified and should be reconsidered. This mitigation of description would allow adult and pediatric patients with AD to receive the effective treatment more appropriately.
Keywords: adult, atopic dermatitis, child, drug labeling, tacrolimus
Introduction
Atopic dermatitis (AD) is a chronic relapsing–remitting skin disease characterized by pruritus and skin inflammation.1 Interactions among skin barrier dysfunction, immune abnormalities and infectious/environmental agents are thought to play roles in the development of AD, although the precise disease pathogenesis is not fully elucidated.1
Atopic dermatitis usually appears in infancy and childhood (~85% of cases), and more than two‐thirds of pediatric patients with AD outgrow the condition before adolescence.2 However, approximately 1–3% of adults are also affected.3 The chronic nature of AD and the requirement for the frequent application of emollients and topical medications mean that its burden is high for patients and the health‐care system.
The main goals of therapy in patients with AD include relieving pruritus, improving skin barrier function, reducing inflammation and preventing flares.1 Although preventing disease flares requires long‐term management, it has also been suggested that achieving remission with initial therapy may have a “disease‐modifying” effect and therefore play an important role in the long‐term management of AD.4 The effective treatment of AD is important not only for improving symptoms and quality of life but also for preventing the development of chronic or severe symptoms. The use of topical anti‐inflammatory agents in pediatric patients with AD has been described as an important, potentially disease‐modifying strategy.5 Therefore, the ideal first‐line pharmacological agents for the treatment of AD should have an excellent safety/tolerability profile as well as reliable anti‐inflammatory efficacy.
Currently recommended first‐line treatments include topical corticosteroids (TCS) and tacrolimus ointment (TAC‐O).6, 7, 8 Although TCS have potent anti‐inflammatory activity in the maintenance treatment as well as in the crisis intervention,9, 10 their long‐term use is known to be associated with cutaneous adverse events such as skin atrophy.11, 12, 13 The occurrence of these side‐effects can decrease patient adherence to topical treatments, sometimes leading to the cessation of TCS.14, 15
The mechanism of action of TAC‐O differs from those of TCS.16 Topical tacrolimus inhibits the activation of pro‐inflammatory cells such as T lymphocytes and mast cells17, 18 and prevents the progression of cytokine‐driven inflammation (Fig. 1).
Figure 1.
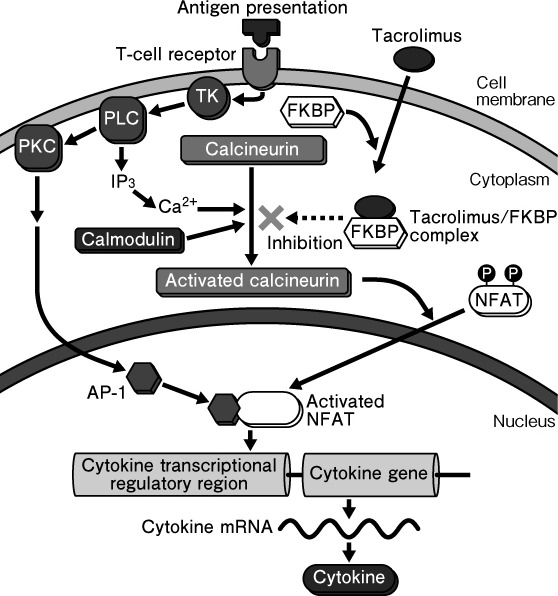
Mechanism of action for tacrolimus. FKBP, FK506‐binding protein; NFAT, nuclear factor of activated T cells; PKC, protein kinase C; PLC, phospholipase C; TK, tyrosine kinase.
In 2003, the Japanese labeling for TAC‐O was updated to include a warning about a possible risk of lymphoma and skin cancer. The US Food and Drug Administration (FDA) followed suit in 2006, issuing a boxed warning requirement for the labeling for two topical calcineurin inhibitors, namely tacrolimus and pimecrolimus, and highlighting a possible risk of lymphoma and skin cancer secondary to these agents’ effects on the immune system. The FDA's boxed warning and Japanese labeling warning emphasize this theoretical risk by stating that health‐care providers and patients should be aware of the long‐term risks of these products (i.e. their potential to cause cancer) and that the use of these agents in children under 2 years of age is not recommended.19, 20 Consequently, the European Medicines Agency and many other regulatory authorities took the same actions to increase health professionals’ awareness of these risks associated with topical calcineurin inhibitors.
Tacrolimus ointment has been available in Japan for more than 15 years, including the 10 years since the FDA approved the labeling update to include the boxed warning about its carcinogenic potential. Therefore, a review of the safety data of TAC‐O, including its carcinogenic potential, as well as its benefits in patients with AD would be useful at this point. This review of the available published work concludes with a consensus opinion from Japanese pediatric, dermatological and allergology experts.
Clinical Efficacy of Tacrolimus in AD
Several systematic reviews and meta‐analyses have summarized the clinical data on TAC‐O in AD. Based on the combined results of 19 studies of calcineurin inhibitors (TAC‐O or pimecrolimus) in patients with AD, TAC‐O 0.03% and 0.1% were more effective than mild TCS and had similar efficacy to that of medium‐potency TCS.21 In a more recent analysis of calcineurin inhibitor studies, the overall efficacy of TAC‐O 0.1% was at least as good as that of medium‐potency TCS, although TAC‐O 0.03% was less effective.22 TAC‐O 0.1% was demonstrated to have similar efficacy to those of medium‐ and high‐potency TCS in other analyses.23, 24 In a meta‐analysis focusing specifically on studies in pediatric patients with AD, there were no statistically significant differences in efficacy between TAC‐O 0.03% and 0.1%, and response rates with TAC‐O were higher than those for patients with AD treated with hydrocortisone 1% or pimecrolimus 1% cream.25 The anti‐inflammatory activity of TAC‐O 0.03% has been defined as equipotent to medium‐ to high‐potency TCS.26, 27, 28
One of the important effects of TAC‐O is its ability to relieve pruritus.29, 30 This is thought to be attributed to desensitization of the cutaneous sensory neurons.31, 32 TAC‐O has also been shown to decrease neuropeptide levels in the lesional skin of patients with AD, suggesting that interference with neurogenic tissue inflammation is a possible mechanism for the beneficial effects of TAC‐O.33 Data from an in vitro study of human mast cells suggest that the subsets of chemokines inhibited by tacrolimus differ from those inhibited by corticosteroids,34 raising the possibility that combination treatment may be more effective than either individual agent alone and highlighting the importance of individualizing the choice of therapeutic agents in patients with AD.
The effective treatment of AD with TAC‐O has been associated with improved quality of life both in pediatric35, 36 and adult patients.37 In a study of 30 pediatric patients with AD, significant improvements in overall quality of life obtained in the first week of therapy maintained throughout the 4‐week study period, as well as the improvements in erythema and papulation scores, pruritus and sleeplessness.35 In adult patients with AD, data from a double‐blind randomized controlled trial (RCT) comparing 6‐month treatment with TAC‐O 0.1% and TCS showed that TAC‐O was associated with a clinically significant improvement of quality of life that was sustained throughout the treatment period and that quality of life improvements with TAC‐O were significantly greater than those with TCS.37
The conventional treatment approach to managing AD has consisted of a combination of daily application of emollients combined with the application of topical anti‐inflammatory agents (TCS or topical calcineurin inhibitors) as required to manage visible skin lesions. More recently, a proactive approach to the long‐term management of AD has been investigated. This approach includes intensive topical anti‐inflammatory treatment of visible lesions until clearance has been obtained followed by the intermittent application of low‐dose anti‐inflammatory agents to these areas to prevent disease flares.9, 10, 38 This approach is based on evidence that although skin may appear normal after the resolution of an AD episode, a number of defects persist, including impaired barrier function and subclinical inflammation.39, 40 Proactive treatment with TAC‐O has been shown to prevent, delay and reduce the occurrence of disease exacerbations in adults and children with AD.38, 41, 42, 43, 44 Long‐term TAC‐O treatment may also help attenuate the progression of AD to allergic rhinitis or bronchial asthma, the so‐called “atopic march”.45
Clinical Safety of Tacrolimus in AD
The overall tolerability profile of TAC‐O is favorable and serious adverse events are quite rare. The most common adverse events in adults and children are application site reactions including burning and pruritus.46, 47, 48, 49, 50, 51, 52 However, these adverse events are usually transient and decrease in severity after the first few days of treatment. Local irritation associated with TAC‐O is thought to occur through the activation of TRPV1 nociceptors distributed throughout sensory neuron terminals, which is a known mechanism of action of the drug.31 The attenuation of skin irritation over time has a physiological basis because repeated applications have been shown to desensitize subsequent sensory neuronal activation31 and eventually nullify the irritant response.24, 46
Tacrolimus ointment has a potential to increase the risk of cutaneous infections due to its immunosuppressive effect. However, several clinical trials have shown similar frequencies of cutaneous infections in patients treated with TAC‐O and control patients treated with vehicle.53 A retrospective study including 388 Japanese adult AD patients with total treatment periods ranging 5–17 years revealed that the overall incidence of herpes simplex virus infection during treatment with TAC‐O and TCS did not exceed the incidence in patients with AD.54 Moreover, it has also been reported that tacrolimus actually reduces the staphylococcal colonization of AD lesions, possibly as a result of reduced skin inflammation and improved skin barrier function.55 This restoration of skin barrier function has been documented in studies specifically focusing on this issue, showing recovery of the lamellar structure of the skin,56, 57 and is in contrast to the impairment of skin barrier function associated with long‐term TCS use.
Black Box and Japanese Labeling Safety Risk of Skin Cancer or Lymphoma
There is currently no strong evidence supporting the boxed warning requirement issued by the US FDA and the warning of Japanese labeling regarding the use of TAC‐O in the treatment of AD. These warnings themselves actually state that a causal relationship between malignancy and topical calcineurin inhibitor use has not been established.20
The risk of malignancy in chronically immunosuppressed human organ transplant recipients has been well documented.58, 59 For liver transplantation, the p.o. or i.v. administration of immunosuppressants, such as calcineurin inhibitors including tacrolimus, has been reported to be associated with the development of malignancy and lymphoma.60, 61, 62, 63, 64 Systemic immunosuppression is thought to be a mechanism underlying the development of cancer in human organ transplant recipients receiving systemic treatment with a calcineurin inhibitor.65, 66 In both animals and humans receiving oral immunosuppressants, the extent of systemic drug exposure resulting in systemic immune suppression is significantly greater than that after use of TAC‐O.19 In fact, systemic drug exposure after TAC‐O administration is absent or minimal,47, 67, 68 and no immunosuppression was observed after 1–4 years’ intermittent treatment with TAC‐O.69
Skin malignancies and lymphoma have been reported in animals after topical calcineurin inhibitor treatment. The carcinogenic effect of topical tacrolimus was investigated using a mouse model and some tumor‐promoting effects were noted.70 Mice naturally have thinner skin and more fragile skin barriers than humans. In the carcinogenicity study using mice, TAC‐O was applied to a large area for 2 years. This resulted in much higher systemic drug concentrations than those in patients during routine topical treatment of AD at recommended drug doses.71 Data from a study using an initiation/promotion mouse model of cutaneous carcinogenesis designed to investigate the carcinogenic effects of TAC‐O showed that TAC‐O 0.03% or 0.1% actually had an inhibitory effect on skin neoplasms.72 This was thought to be secondary to the inhibition of 12‐O‐tetradecanoylphorbol‐13‐acetate promotion without any immunosuppressive effects. Furthermore, animal studies showing the development of lymphoma after the application of topical tacrolimus or pimecrolimus, another topical calcineurin inhibitor available in the world so far, used drugs dissolved in ethanol at concentrations 26 and 47 times the maximum recommended human dose, respectively.73
Cases of cutaneous cancer or lymphoma have been observed following use of topical calcineurin inhibitors. Several published studies have investigated the incidence of cancers, including lymphoma, in patients with AD treated with TAC‐O.22, 69, 74, 75, 76, 77, 78, 79, 80 Looking specifically at skin cancers, a case–control study was conducted using a questionnaire in 3074 evaluable adults with AD.74 In this study, topical calcineurin inhibitor exposure was not associated with an increased risk of developing non‐melanoma skin cancer; subjects who had used TAC‐O had the lowest rate of non‐melanoma skin cancer versus those treated with pimecrolimus or those who did not receive a topical calcineurin inhibitor. An evaluation of malignancy risk related to AD and the use of topical calcineurin inhibitors from published work found no evidence of association with topical calcineurin inhibitors in melanoma or non‐melanoma skin cancer, although the inference that calcineurin inhibitors do not cause malignancy was not derived for overall malignancies.75
A large case–control study investigated the relationship between topical calcineurin inhibitor use and lymphoma.76 On adjusted analysis, the odds ratio (OR) for lymphoma development was 0.8 (95% confidence interval [CI], 0.4–1.7) in patients treated with TAC‐O versus 2.4 (95% CI, 1.5–3.8) for those with severe AD regardless of topical treatments, suggesting that disease severity is a more potent risk factor for lymphoma than TAC‐O use. AD severity indicated by referral to a dermatologist was also associated with increased lymphoma risk in a similar study conducted in the UK (OR, 3.72; 95% CI, 1.40–9.87); lymphoma risk was also increased in users of high‐strength TCS (OR, 1.80; 95% CI, 1.54–2.11) but not in those of topical calcineurin inhibitors, in whom no cases of lymphoma occurred during the study period.77 The results of a meta‐analysis also support an association of both AD severity and high‐strength TCS with increased lymphoma risk, whereas the majority of included studies showed no relationship between topical calcineurin inhibitor use and lymphoma.78
A large retrospective cohort observational study based on an integrated health‐care insurance database (follow‐up duration, 2–2.5 years) has shown an increased risk for cutaneous T‐cell lymphoma in eczema patients with the use of TAC‐O, but not pimecrolimus, compared with patients who were not exposed to topical calcineurin inhibitors.79 There was an increase in the risk of T‐cell lymphoma in patients treated with TAC‐O at higher concentrations and cumulative doses, but the difference did not reach statistical significance. On the other hand, when compared with the general population, an increased risk for cutaneous lymphoma with the use of TAC‐O, pimecrolimus and TCS has been reported, but needed longer‐term studies for the conclusion.80
Based on the results of these investigations, the risk for malignancies associated with topical calcineurin inhibitors is best addressed by the systematic review on the efficacy, safety and cost‐effectiveness of topical calcineurin inhibitors in AD.22 It has been suggested that the lymphoma diagnosed after treatment with topical calcineurin inhibitor was pre‐existing cutaneous T‐cell lymphoma, which often initially presents as an eczematous rash and may be misdiagnosed as AD, leading to overestimation of the association between topical calcineurin inhibitors and lymphoma.71, 75, 79 In a Japanese postmarketing surveillance study of pediatric patients with AD being treated with TAC‐O 0.03%, no cases of treatment‐related malignancy have been identified over 7 years of a planned 10‐year follow up.81
Discussion
Atopic dermatitis has a relapsing–remitting course in which periods of relative remission alternate with periods of acute worsening, namely disease flares. The proactive management strategy includes scheduled regular intermittent use of topical anti‐inflammatory agents along with regular use of emollients to prevent AD flares. This requires long‐term use of TCS or topical calcineurin inhibitors. The use of TCS in this setting is limited by its unfavorable effects on skin barrier function.11, 12, 13 Therefore, TAC‐O may be a preferred option as maintenance treatment for AD in remission.
Although the US FDA issued a boxed warning requirement in 2006, cautioning about an increased risk of skin cancer and lymphoma with the use of topical calcineurin inhibitors including TAC‐O, there is no robust evidence to support this association from clinical trials and postmarketing surveillance studies. A report by the Topical Calcineurin Inhibitor Task Force of the American College of Allergy, Asthma and Immunology and the American Academy of Allergy, Asthma and Immunology stated that current data based on use in nearly 7 million individuals do not provide any evidence of an increased incidence of lymphoma with short‐term or intermittent application of topical TAC‐O or topical pimecrolimus.73 In addition, the American Academy of Dermatology Association Task Force conference failed to find any causal proof of a relationship between topical calcineurin inhibitor use and skin cancer or lymphoma, and it also suggested that systemic immunosuppression after short‐term or intermittent long‐term use of these agents was an unlikely mechanism for cancer risk.82
Furthermore, it has been reported that combination therapy of narrowband ultraviolet B (NBUVB) and topical calcineurin inhibitors including TAC‐O showed better responses in repigmentation of vitiligo affecting the face and the neck than NB‐UVB monotherapy,83 and there are a series of reports on double‐blind RCT regarding those combination therapies.84, 85, 86, 87, 88 Although the combination therapy is contraindicated in Japanese guidelines for the diagnosis and treatment of vitiligo,89 it is stated in the European Dermatology Forum consensus guidelines for the management of vitiligo, that there is good evidence for the efficacy of combination of NBUVB and topical calcineurin inhibitors and that it provides better results than the two treatments used alone.90 The European guidelines also mention that accumulated data suggest that the combination therapy may be effective and safe, although long‐term data on carcinogenicity are still lacking.
The presence of the boxed US FDA warning for topical calcineurin inhibitors and the warning in Japanese labeling for TAC‐O has had a number of negative consequences. Prescription rates decreased and some health‐care payers introduced additional hurdles for physicians and patients such as health‐care payment restrictions, making it more difficult to access these agents.19 The results of an anonymous survey of US dermatology conference attendees in 2007 (after the boxed warning was issued) showed that changes to therapies based on the warning were associated with a loss of disease control in some patients whose conditions were previously well controlled using topical calcineurin inhibitors.91 In addition, the therapies chosen to replace topical calcineurin inhibitors, including TCS, systemic corticosteroids and other systemic immunosuppressants, often had worse tolerability profiles and risk–benefit ratios than the treatment they replaced. Some physicians also felt that the warnings would be a deterrent to participation in future clinical trials, limiting the ability to accurately determine the real risk of malignancy during long‐term use of topical calcineurin inhibitors.92
Several investigators pointed out that “topical corticosteroid phobia” encompasses concerns, fears, worries, anxiety, sorrow and doubts about TCS use. The term “topical corticosteroid phobia” was suggested to be a misnomer because the concerns have a rational background of available evidence.93, 94, 95 Instead, it has been proposed to use the term “corticosteroid concerns” rather than “corticosteroid phobia” in future discussions.95 On the other hand, in the case of topical calcineurin inhibitors, we believe that we should use the term “topical calcineurin inhibitor phobia” rather than “topical calcineurin inhibitor concerns” for the warning on malignancies due to the lack of established scientific evidence.
Review Recommendations
Overall, the published data summarized above suggest that the warning included in the current Japanese labeling for TAC‐O is not based on the reports of malignancies whose causal relationship to the drug has been determined. As a result, the warning does not appear justified in the context of optimal clinical use at currently approved doses, as was stated by Topical Calcineurin Inhibitor Task Force of the American College of Allergy, Asthma and Immunology and the American Academy of Allergy, Asthma and Immunology.73 Therefore, we conclude that TAC‐O is a safe and effective option for the treatment of AD in both children and adults.
We join these other experts in stating that the current warning of the Japanese labeling for TAC‐O is not appropriate based on available scientific evidence and that the boxed US FDA warning should also be reconsidered.73, 82, 96 We believe that this would be the best approach to ensuring that all patients with AD receive the most appropriate and effective treatment.
Conflict of Interest
M. O. is a member of scientific advisory boards of Maruho Co., Ltd; received research grants from Mitsubishi Tanabe Pharma, and also received consultant and/or speaker fees from Mitsubishi Tanabe Pharma, Novartis Pharma and Janssen Pharma. H. M. is a full‐time employee of Maruho Co., Ltd. H. N. has received funds for research from Maruho Co., Ltd, and also received consultant and speaker fees from Maruho Co., Ltd.
Acknowledgments
Nicola Ryan, on behalf of Springer Healthcare Communications, provided medical writing assistance in preparing the first draft of this article. This assistance was funded by Maruho Co., Ltd, Japan. Author contributions are as follows: M. O. and H. M. contributed equally to the preparation of the manuscript.
References
- 1. Watson W, Kapur S. Atopic dermatitis. Allergy Asthma Clin Immunol 2011; 7(Suppl 1): S4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bieber T. Atopic dermatitis. N Engl J Med 2008; 358: 1483–1494. [DOI] [PubMed] [Google Scholar]
- 3. Larsen W, Nakayama H, Fischer T et al Fragrance contact dermatitis ‐ a worldwide multicenter investigation (Part III). Contact Dermatitis 2002; 46: 141–144. [DOI] [PubMed] [Google Scholar]
- 4. Tang TS, Bieber T, Williams HC. Are the concepts of induction of remission and treatment of subclinical inflammation in atopic dermatitis clinically useful? J Allergy Clin Immunol 2014; 133: 1615–1625. e1. [DOI] [PubMed] [Google Scholar]
- 5. Spergel JM, Paller AS. Atopic dermatitis and the atopic march. J Allergy Clin Immunol 2003; 112: S118–S127. [DOI] [PubMed] [Google Scholar]
- 6. Sidbury R, Tom WL, Bergman JN et al Guidelines of care for the management of atopic dermatitis: section 4. Prevention of disease flares and use of adjunctive therapies and approaches. J Am Acad Dermatol 2014; 71: 1218–1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Ring J, Alomar A, Bieber T et al Guidelines for treatment of atopic eczema (atopic dermatitis) part I. J Eur Acad Dermatol Venereol 2012; 26: 1045–1060. [DOI] [PubMed] [Google Scholar]
- 8. Saeki H, Nakahara T, Tanaka A et al Clinical practice guidelines for the management of atopic dermatitis 2016. J Dermatol 2016; 43: 1117–1145. [DOI] [PubMed] [Google Scholar]
- 9. Peserico A, Städtler G, Sebastian M, Fernandez RS, Vick K, Bieber T. Reduction of relapses of atopic dermatitis with methylprednisolone aceponate cream twice weekly in addition to maintenance treatment with emollient: a multicentre, randomized, double‐blind, controlled study. Br J Dermatol 2008; 158: 801–807. [DOI] [PubMed] [Google Scholar]
- 10. Glazenburg EJ, Wolkerstorfer A, Gerretsen AL, Mulder PG, Oranje AP. Efficacy and safety of fluticasone propionate 0.005% ointment in the long‐term maintenance treatment of children with atopic dermatitis: differences between boys and girls? Pediatr Allergy Immunol 2009; 20: 59–66. [DOI] [PubMed] [Google Scholar]
- 11. Hengge UR, Ruzicka T, Schwartz RA, Cork MJ. Adverse effects of topical glucocorticosteroids. J Am Acad Dermatol 2006; 54: 1–15. [DOI] [PubMed] [Google Scholar]
- 12. Aschoff R, Schmitt J, Knuschke P, Koch E, Bräutigam M, Meurer M. Evaluation of the atrophogenic potential of hydrocortisone 1% cream and pimecrolimus 1% cream in uninvolved forehead skin of patients with atopic dermatitis using optical coherence tomography. Exp Dermatol 2011; 20: 832–836. [DOI] [PubMed] [Google Scholar]
- 13. Jensen JM, Scherer A, Wanke C et al Gene expression is differently affected by pimecrolimus and betamethasone in lesional skin of atopic dermatitis. Allergy 2012; 67: 413–423. [DOI] [PubMed] [Google Scholar]
- 14. Aubert‐Wastiaux H, Moret L, Le Rhun A et al Topical corticosteroid phobia in atopic dermatitis: a study of its nature, origins and frequency. Br J Dermatol 2011; 165: 808–814. [DOI] [PubMed] [Google Scholar]
- 15. Krejci‐Manwaring J, Tusa MG, Carroll C et al Stealth monitoring of adherence to topical medication: adherence is very poor in children with atopic dermatitis. J Am Acad Dermatol 2007; 56: 211–216. [DOI] [PubMed] [Google Scholar]
- 16. Cohan VL, Undem BJ, Fox CC, Adkinson NF Jr, Lichtenstein LM, Schleimer RP. Dexamethasone does not inhibit the release of mediators from human mast cells residing in airway, intestine, or skin. Am Rev Respir Dis 1989; 140: 951–954. [DOI] [PubMed] [Google Scholar]
- 17. de Paulis A, Stellato C, Cirillo R, Ciccarelli A, Oriente A, Marone G. Anti‐inflammatory effect of FK‐506 on human skin mast cells. J Invest Dermatol 1992; 99: 723–728. [DOI] [PubMed] [Google Scholar]
- 18. Sakuma S, Higashi Y, Sato N et al Tacrolimus suppressed the production of cytokines involved in atopic dermatitis by direct stimulation of human PBMC system. (Comparison with steroids). Int Immunopharmacol 2001; 1: 1219–1226. [DOI] [PubMed] [Google Scholar]
- 19. Siegfried EC, Jaworski JC, Hebert AA. Topical calcineurin inhibitors and lymphoma risk: evidence update with implications for daily practice. Am J Clin Dermatol 2013; 14: 163–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Astellas Pharma . PROTOPIC® (tacrolimus) ointment 0.03%, ointment 0.1%: prescribing information. Available from URL: https://astellas.us/docs/protopic.pdf [Accessed: 25 July 2016].
- 21. El‐Batawy MM, Bosseila MA, Mashaly HM, Hafez VS. Topical calcineurin inhibitors in atopic dermatitis: a systematic review and meta‐analysis. J Dermatol Sci 2009; 54: 76–87. [DOI] [PubMed] [Google Scholar]
- 22. Chia BKY, Tey HL. Systematic review on the efficacy, safety, and cost‐effectiveness of topical calcineurin inhibitors in atopic dermatitis. Dermatitis 2015; 26: 122–132. [DOI] [PubMed] [Google Scholar]
- 23. Svensson A, Chambers C, Gånemo A, Mitchell SA. A systematic review of tacrolimus ointment compared with corticosteroids in the treatment of atopic dermatitis. Curr Med Res Opin 2011; 27: 1395–1406. [DOI] [PubMed] [Google Scholar]
- 24. Reitamo S, Ortonne JP, Sand C et al A multicentre, randomized, double‐blind, controlled study of long‐term treatment with 0.1% tacrolimus ointment in adults with moderate to severe atopic dermatitis. Br J Dermatol 2005; 152: 1282–1289. [DOI] [PubMed] [Google Scholar]
- 25. Yan J, Chen SL, Wang XL, Zhou W, Wang FS. Meta‐analysis of tacrolimus ointment for atopic dermatitis in pediatric patients. Pediatr Dermatol 2008; 25: 117–120. [DOI] [PubMed] [Google Scholar]
- 26. Bieber T, Vick K, Fölster‐Holst R et al Efficacy and safety of methylprednisolone aceponate ointment 0.1% compared to tacrolimus 0.03% in children and adolescents with an acute flare of severe atopic dermatitis. Allergy 2007; 62: 184–189. [DOI] [PubMed] [Google Scholar]
- 27. Hofman T, Cranswick N, Kuna P et al Tacrolimus ointment does not affect the immediate response to vaccination, the generation of immune memory, or humoral and cell‐mediated immunity in children. Arch Dis Child 2006; 91: 905–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Reitamo S, Harper J, Bos JD et al 0.03% Tacrolimus ointment applied once or twice daily is more efficacious than 1% hydrocortisone acetate in children with moderate to severe atopic dermatitis: results of a randomized double‐blind controlled trial. Br J Dermatol 2004; 150: 554–562. [DOI] [PubMed] [Google Scholar]
- 29. Fleischer AB Jr, Abramovits W, Breneman D, Jaracz E. Tacrolimus ointment is more effective than pimecrolimus cream in adult patients with moderate to very severe atopic dermatitis. J Dermatolog Treat 2007; 18: 151–157. [DOI] [PubMed] [Google Scholar]
- 30. Takeuchi S, Saeki H, Tokunaga S et al A randomized, open‐label, multicenter trial of topical tacrolimus for the treatment of pruritis in patients with atopic dermatitis. Ann Dermatol 2012; 24: 144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Pereira U, Boulais N, Lebonvallet N, Pennec JP, Dorange G, Misery L. Mechanisms of the sensory effects of tacrolimus on the skin. Br J Dermatol 2010; 163: 70–77. [DOI] [PubMed] [Google Scholar]
- 32. Kido M, Takeuchi S, Esaki H, Hayashida S, Furue M. Scratching behavior does not necessarily correlate with epidermal nerve fiber sprouting or inflammatory cell infiltration. J Dermatol Sci 2010; 58: 130–135. [DOI] [PubMed] [Google Scholar]
- 33. Kim HO, Lee CH, Ahn HK, Park CW. Effects of tacrolimus ointment on the expression of substance P, nerve growth factor, and neurotrophin‐3 in atopic dermatitis. Int J Dermatol 2009; 48: 431–438. [DOI] [PubMed] [Google Scholar]
- 34. Kato A, Chustz RT, Ogasawara T et al Dexamethasone and FK506 inhibit expression of distinct subsets of chemokines in human mast cells. J Immunol 2009; 182: 7233–7243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Kondo Y, Nakajima Y, Komatsubara R et al Short‐term efficacy of tacrolimus ointment and impact on quality of life. Pediatr Int 2009; 51: 385–389. [DOI] [PubMed] [Google Scholar]
- 36. Kubota Y, Yoneda K, Nakai K et al Effect of sequential applications of topical tacrolimus and topical corticosteroids in the treatment of pediatric atopic dermatitis: an open‐label pilot study. J Am Acad Dermatol 2009; 60: 212–217. [DOI] [PubMed] [Google Scholar]
- 37. Poole CD, Chambers C, Allsopp R, Currie CJ. Quality of life and health‐related utility analysis of adults with moderate and severe atopic dermatitis treated with tacrolimus ointment vs. topical corticosteroids. J Eur Acad Dermatol Venereol 2010; 24: 674–678. [DOI] [PubMed] [Google Scholar]
- 38. Wollenberg A, Reitamo S, Girolomoni G et al Proactive treatment of atopic dermatitis in adults with 0.1% tacrolimus ointment. Allergy 2008; 63: 742–750. [PubMed] [Google Scholar]
- 39. Mihm MC Jr, Soter NA, Dvorak HF, Austen KF. The structure of normal skin and the morphology of atopic eczema. J Invest Dermatol 1976; 67: 305–312. [DOI] [PubMed] [Google Scholar]
- 40. Proksch E, Fölster‐Holst R, Jensen JM. Skin barrier function, epidermal proliferation and differentiation in eczema. J Dermatol Sci 2006; 43: 159–169. [DOI] [PubMed] [Google Scholar]
- 41. Breneman D, Fleischer AB Jr, Abramovits W et al Intermittent therapy for flare prevention and long‐term disease control in stabilized atopic dermatitis: a randomized comparison of 3‐times‐weekly applications of tacrolimus ointment versus vehicle. J Am Acad Dermatol 2008; 58: 990–999. [DOI] [PubMed] [Google Scholar]
- 42. Thaçi D, Reitamo S, Gonzalez Ensenat MA et al Proactive disease management with 0.03% tacrolimus ointment for children with atopic dermatitis: results of a randomized, multicentre, comparative study. Br J Dermatol 2008; 159: 1348–1356. [DOI] [PubMed] [Google Scholar]
- 43. Paller AS, Eichenfield LF, Kirsner RS, Shull T, Jaracz E, Simpson EL. Three times weekly tacrolimus ointment reduces relapse in stabilized atopic dermatitis: a new paradigm for use. Pediatrics 2008; 122: e1210–e1218. [DOI] [PubMed] [Google Scholar]
- 44. Chung BY, Kim HO, Kim JH, Cho SI, Lee CH, Park CW. The proactive treatment of atopic dermatitis with tacrolimus ointment in Korean patients: a comparative study between once‐weekly and thrice‐weekly applications. Br J Dermatol 2013; 168: 908–910. [DOI] [PubMed] [Google Scholar]
- 45. Mandelin JM, Remitz A, Virtanen HM, Malmberg LP, Haahtela T, Reitamo S. A 10‐year open follow‐up of eczema and respiratory symptoms in patients with atopic dermatitis treated with topical tacrolimus for the first 4 years. J Dermatolog Treat 2010; 21: 167–170. [DOI] [PubMed] [Google Scholar]
- 46. Reitamo S, Rustin M, Ruzicka T et al Efficacy and safety of tacrolimus ointment compared with that of hydrocortisone butyrate ointment in adult patients with atopic dermatitis. J Allergy Clin Immunol 2002; 109: 547–555. [DOI] [PubMed] [Google Scholar]
- 47. Soter NA, Fleischer AB Jr, Webster GF, Monroe E, Lawrence I. Tacrolimus ointment for the treatment of atopic dermatitis in adult patients: part II, safety. J Am Acad Dermatol 2001; 44: S39–S46. [DOI] [PubMed] [Google Scholar]
- 48. Nakagawa H. Comparison of the efficacy and safety of 0.1% tacrolimus ointment with topical corticosteroids in adult patients with atopic dermatitis: review of randomised, double‐blind clinical studies conducted in Japan. Clin Drug Investig 2006; 26: 235–246. [DOI] [PubMed] [Google Scholar]
- 49. Baldo A, Cafiero M, Di Caterino P, Di Costanzo L. Tacrolimus ointment in the management of atopic dermatitis. Clin Cosmet Investig Dermatol 2009; 2: 1–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Mandelin JM, Rubins A, Remitz A et al Long‐term efficacy and tolerability of tacrolimus 0.03% ointment in infants:* a two‐year open‐label study. Int J Dermatol 2012; 51: 104–110. [DOI] [PubMed] [Google Scholar]
- 51. Chen SL, Yan J, Wang FS. Two topical calcineurin inhibitors for the treatment of atopic dermatitis in pediatric patients: a meta‐analysis of randomized clinical trials. J Dermatolog Treat 2010; 21: 144–156. [DOI] [PubMed] [Google Scholar]
- 52. Siegfried EC, Jaworski JC, Kaiser JD, Hebert AA. Systematic review of published trials: long‐term safety of topical corticosteroids and topical calcineurin inhibitors in pediatric patients with atopic dermatitis. BMC Pediatr 2016; 16: 75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Fleischer AB Jr, Ling M, Eichenfield L et al Tacrolimus ointment for the treatment of atopic dermatitis is not associated with an increase in cutaneous infections. J Am Acad Dermatol 2002; 47: 562–570. [DOI] [PubMed] [Google Scholar]
- 54. Hashizume H, Yagi H, Ohshima A et al Comparable risk of herpes simplex virus infection between topical treatments with tacrolimus and corticosteroids in adults with atopic dermatitis. Br J Dermatol 2006; 154: 1204–1206. [DOI] [PubMed] [Google Scholar]
- 55. Remitz A, Kyllönen H, Granlund H, Reitamo S. Tacrolimus ointment reduces staphylococcal colonization of atopic dermatitis lesions. J Allergy Clin Immunol 2001; 107: 196–197. [DOI] [PubMed] [Google Scholar]
- 56. Dähnhardt‐Pfeiffer S, Dähnhardt D, Buchner M, Walter K, Proksch E, Fölster‐Holst R. Comparison of effects of tacrolimus ointment and mometasone furoate cream on the epidermal barrier of patients with atopic dermatitis. J Dtsch Dermatol Ges 2013; 11: 437–443. [DOI] [PubMed] [Google Scholar]
- 57. Danby SG, Chittock J, Brown K, Albenali LH, Cork MJ. The effect of tacrolimus compared with betamethasone valerate on the skin barrier in volunteers with quiescent atopic dermatitis. Br J Dermatol 2014; 170: 914–921. [DOI] [PubMed] [Google Scholar]
- 58. Chapman JR, Webster AC, Wong G. Cancer in the transplant recipient. Cold Spring Harb Perspect Med 2013; 3: a015677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Jensen P, Hansen S, Mφller B et al Skin cancer in kidney and heart transplant recipients and different long‐term immunosuppressive therapy regimens. J Am Acad Dermatol 1999; 40: 177–186. [DOI] [PubMed] [Google Scholar]
- 60. Jain AB, Yee LD, Nalesnik MA et al Comparative incidence of de novo nonlymphoid malignancies after liver transplantation under tacrolimus using surveillance epidemiologic end result data. Transplantation 1998; 66: 1193–1200. [DOI] [PubMed] [Google Scholar]
- 61. Jonas S, Rayes N, Neumann U et al De novo malignancies after liver transplantation using tacrolimus‐based protocols or cyclosporine‐based quadruple immunosuppression with an interleukin‐2 receptor antibody or antithymocyte globulin. Cancer 1997; 80: 1141–1150. [DOI] [PubMed] [Google Scholar]
- 62. Kaufman DB, Leventhal JR, Stuart J, Abecassis MM, Fryer JP, Stuart FP. Mycophenolate mofetil and tacrolimus as primary maintenance immunosuppression in simultaneous pancreas‐kidney transplantation: initial experience in 50 consecutive cases. Transplantation 1999; 67: 586–593. [DOI] [PubMed] [Google Scholar]
- 63. Otley CC, Pittelkow MR. Skin cancer in liver transplant recipients. Liver Transpl 2000; 6: 253–262. [DOI] [PubMed] [Google Scholar]
- 64. Wiesner RH. A long‐term comparison of tacrolimus (FK506) versus cyclosporine in liver transplantation: a report of the United States FK506 Study Group. Transplantation 1998; 66: 493–499. [DOI] [PubMed] [Google Scholar]
- 65. Rangwala S, Tsai KY. Roles of the immune system in skin cancer. Br J Dermatol 2011; 165: 953–965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Gutierrez‐Dalmau A, Campistol JM. Immunosuppressive therapy and malignancy in organ transplant recipients: a systematic review. Drugs 2007; 67: 1167–1198. [DOI] [PubMed] [Google Scholar]
- 67. Harper J, Smith C, Rubins A et al A multicenter study of the pharmacokinetics of tacrolimus ointment after first and repeated application to children with atopic dermatitis. J Invest Dermatol 2005; 124: 695–699. [DOI] [PubMed] [Google Scholar]
- 68. Reitamo S, Mandelin J, Rubins A et al The pharmacokinetics of tacrolimus after first and repeated dosing with 0.03% ointment in infants with atopic dermatitis. Int J Dermatol 2009; 48: 348–355. [DOI] [PubMed] [Google Scholar]
- 69. Remitz A, Reitamo S. Long‐term safety of tacrolimus ointment in atopic dermatitis. Expert Opin Drug Saf 2009; 8: 501–506. [DOI] [PubMed] [Google Scholar]
- 70. Niwa Y, Terashima T, Sumi H. Topical application of the immunosuppressant tacrolimus accelerates carcinogenesis in mouse skin. Br J Dermatol 2003; 149: 960–967. [DOI] [PubMed] [Google Scholar]
- 71. Ormerod AD. Topical tacrolimus and pimecrolimus and the risk of cancer: how much cause for concern? Br J Dermatol 2005; 153: 701–705. [DOI] [PubMed] [Google Scholar]
- 72. Mitamura T, Doi Y, Kawabe M et al Inhibitory potency of tacrolimus ointment on skin tumor induction in a mouse model of an initiation‐promotion skin tumor. J Dermatol 2011; 38: 562–570. [DOI] [PubMed] [Google Scholar]
- 73. Fonacier L, Spergel J, Charlesworth EN et al Report of the Topical Calcineurin Inhibitor Task Force of the American College of Allergy, Asthma and Immunology and the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol 2005; 115: 1249–1253. [DOI] [PubMed] [Google Scholar]
- 74. Margolis DJ, Hoffstad O, Bilker W. Lack of association between exposure to topical calcineurin inhibitors and skin cancer in adults. Dermatology 2007; 214: 289–295. [DOI] [PubMed] [Google Scholar]
- 75. Tennis P, Gelfand JM, Rothman KJ. Evaluationof cancer risk related to atopic dermatitis and use of topical calcineurin inhibitors. Br J Dermatol 2011; 165: 465–473. [DOI] [PubMed] [Google Scholar]
- 76. Arellano FM, Wentworth CE, Arana A, Fernández C, Paul CF. Risk of lymphoma following exposure to calcineurin inhibitors and topical steroids in patients with atopic dermatitis. J Invest Dermatol 2007; 127: 808–816. [DOI] [PubMed] [Google Scholar]
- 77. Arellano FM, Arana A, Wentworth CE, Fernández‐Vidaurre C, Schlienger RG, Conde E. Lymphoma among patients with atopic dermatitis and/or treated with topical immunosuppressants in the United Kingdom. J Allergy Clin Immunol 2009; 123: 1111–1116. e1‐13. [DOI] [PubMed] [Google Scholar]
- 78. Legendre L, Barnetche T, Mazereeuw‐Hautier J, Meyer N, Murrell D, Paul C. Risk of lymphoma in patients with atopic dermatitis and the role of topical treatment: a systematic review and meta‐analysis. J Am Acad Dermatol 2015; 72: 992–1002. [DOI] [PubMed] [Google Scholar]
- 79. Hui RL, Lide W, Chan J, Schottinger J, Yoshinaga M, Millares M. Association between exposure to topical tacrolimus or pimecrolimus and cancers. Ann Pharmacother 2009; 43: 1956–1963. [DOI] [PubMed] [Google Scholar]
- 80. Schneeweiss S, Doherty M, Zhu S et al Topical treatments with pimecrolimus, tacrolimus and medium‐ to high‐potency corticosteroids, and risk of lymphoma. Dermatology 2009; 219: 7–21. [DOI] [PubMed] [Google Scholar]
- 81. Ohtsuki M, Shiragasawa C, So M, Nakagawa H. The long‐term safety and effectiveness of 0.03% tacrolimus ointment in the treatment of pediatric patients with atopic dermatitis ‐ interim report of a long‐term special drug use results survey. J Pediatr Dermatol 2013; 32: 127–137. [Google Scholar]
- 82. Berger TG, Duvic M, Van Voorhees AS, Van Beek MJ, Frieden IJ, American Academy of Dermatology Association Task Force . The use of topical calcineurin inhibitors in dermatology: safety concerns. Report of the American Academy of Dermatology Association Task Force. J Am Acad Dermatol 2006; 54: 818–823. [DOI] [PubMed] [Google Scholar]
- 83. Li R, Qiao M, Wang X, Zhao X, Sun Q. Effect of narrow band ultraviolet B phototherapy as monotherapy or combination therapy for vitiligo: a meta‐analysis. Photodermatol Photoimmunol Photomed 2017; 33: 22–31. [DOI] [PubMed] [Google Scholar]
- 84. Sokolova A, Lee A, Smith SD. The safety and efficacy of narrow band ultraviolet B treatment in dermatology: a review. Am J Clin Dermatol 2015; 16: 501–531. [DOI] [PubMed] [Google Scholar]
- 85. Nordal EJ, Guleng GE, Rönnevig JR. Treatment of vitiligo with narrowband‐UVB (TL01) combined with tacrolimus ointment (0.1%) vs. placebo ointment, a randomized right/left double‐blind comparative study. J Eur Acad Dermatol Venereol 2011; 25: 1440–1443. [DOI] [PubMed] [Google Scholar]
- 86. Majid I. Does topical tacrolimus ointment enhance the efficacy of narrowband ultraviolet B therapy in vitiligo? A left‐right comparison study. Photodermatol Photoimmunol Photomed 2010; 26: 230–234. [DOI] [PubMed] [Google Scholar]
- 87. Klahan S, Asawanonda P. Topical tacrolimus may enhance repigmentation with targeted narrowband ultraviolet B to treat vitiligo: a randomized, controlled study. Clin Exp Dermatol 2009; 34: 1029–1030. [DOI] [PubMed] [Google Scholar]
- 88. Fai D, Cassano N, Vena GA. Narrow‐band UVB phototherapy combined with tacrolimus ointment in vitiligo: a review of 110 patients. J Eur Acad Dermatol Venereol 2007; 21: 916–920. [DOI] [PubMed] [Google Scholar]
- 89. Oiso N, Suzuki T, Wataya‐Kaneda M et al Guidelines for the diagnosis and treatment of vitiligo in Japan. J Dermatol 2013; 40: 344–354. [DOI] [PubMed] [Google Scholar]
- 90. Taieb A, Alomar A, Böhm M et al Guidelines for the management of vitiligo: the European Dermatology Forum consensus. Br J Dermatol 2013; 168: 5–19. [DOI] [PubMed] [Google Scholar]
- 91. Ceilley R, Eisenthal A. The unintended effects of a boxed warning. J Clin Aesthet Dermatol 2009; 2: 33–39. [PMC free article] [PubMed] [Google Scholar]
- 92. Siegfried E, Silverman RA, Mancini AJ, Friedlander SF. ‘Black box’ warning ill‐advised for eczema drugs. Dermatol Times 2006; 27: 6. [Google Scholar]
- 93. Smith SD, Hong E, Fearns S, Blaszczynski A, Fischer G. Corticosteroid phobia and other confounders in the treatment of childhood atopic dermatitis explored using parent focus groups. Australas J Dermatol 2010; 51: 168–174. [DOI] [PubMed] [Google Scholar]
- 94. Gustavsen HE, Gjersvik P. Topical corticosteroid phobia among parents of children with atopic dermatitis in a semirural area of Norway. J Eur Acad Dermatol Venereol 2016; 30: 168. [DOI] [PubMed] [Google Scholar]
- 95. Müller SM, Tomaschett D, Euler S, Vogt DR, Herzog L, Itin P. Topical corticosteroid concerns in dermatological outpatients: a cross‐sectional and interventional study. Dermatology 2016; 232: 444–452. [DOI] [PubMed] [Google Scholar]
- 96. Bieber T, Cork MJ, Ellis C et al Consensus statement on the safety profile of topical calcineurin inhibitors. Dermatology 2005; 211: 77–78. [DOI] [PubMed] [Google Scholar]


