Abstract
International variations in the prevalence of HPV infection derive from differences in sexual behaviors, which are also a key factor of the basic reproductive number (R0) of HPV infection in different populations. R 0 affects the strength of herd protection and hence the impact of a vaccination program. Similar vaccination programs may therefore generate different levels of impact depending upon the population's pre‐vaccination HPV prevalence. We used IARC's transmission model to estimate (i) the overall effectiveness of vaccination versus no vaccination in women aged 15–34 years measured as percent prevalence reduction (%PR) of HPV16 and (ii) the corresponding herd protection in populations with gender‐equal or traditional sexual behavior and with different levels of sexual activity, corresponding to pre‐vaccination HPV16 prevalence from 1 to 8% as observed worldwide. Between populations with different levels of gender‐equal sexual activity, the highest difference in %PR under girls‐only vaccination is observed at 40% coverage (91%PR vs. 48%PR for 1% and 8% pre‐vaccination prevalence, respectively). HPV16 elimination is obtained with 55 and 97% coverage, respectively. To achieve desirable levels of HPV16 prevalence after vaccination, different levels of coverage are required in populations with different levels of pre‐vaccination HPV16 prevalence, for example, in populations with gender‐equal sexual behavior a decrease to 1/1000 HPV16 from pre‐vaccination prevalence of 1 and 8% would require coverages of 37 and 96%, respectively. In traditional populations, corresponding coverages would need to be 28 and 93%, respectively. In conclusion, pre‐vaccination HPV prevalence strongly influences herd immunity and helps predict the overall effectiveness of HPV vaccination.
Keywords: herd effect, HPV vaccination, HPV prevalence, coverage threshold
Short abstract
What's new?
Differences in sexual activity from one population to another account for up to a ten‐fold difference in HPV prevalence, which in turn affects cervical‐cancer risk. How do these factors impact the effectiveness of HPV‐vaccination programs? In this epidemiological study, the authors found that, in countries where sexual behavior is based on traditional norms and HPV prevalence remains low, an early introduction of HPV vaccination will anticipate any increase of HPV prevalence among young women due to the liberalization of social attitudes.
Abbreviations
- HIC
high income countries
- HR HPV
high‐risk human papillomavirus
- HIV
human immunodeficiency virus
- IARC
International Agency for Research on Cancer
- LMIC
low/middle‐income countries
- %PR
percent prevalence reduction
- R0
average number of secondary infections resulting from one case of HPV infection in a totally susceptible population.
Introduction
Approximately 630,000 new cancer cases per year are attributable to high‐risk (HR) human papillomavirus (HPV) worldwide, 530,000 (83%) of which are cervical cancer.1, 2 The bivalent and quadrivalent vaccines target the types HPV16/18, which account for ∼70% of all cervical cancers worldwide,2 whereas the newer ninevalent vaccine also targets HR HPV31/33/45/52/58,3 raising the proportion of preventable cervical cancers to ∼90%.2 All three vaccines are nearly 100% efficacious in the prevention of infection from vaccine‐targeted HPV types.3, 4
Two‐thirds of cervical cancers occur in less developed countries, due to lack of effective cervical cancer screening programs and high HPV infection prevalence.2 Worldwide, more than 10‐fold differences are being reported in HPV prevalence5 mainly related to differences in sexual activity patterns and, in some regions, to the prevalence of human immunodeficiency virus (HIV), which is associated with increased acquisition6 and persistence7 of HPV infection.
The basic reproductive number (R 0) of HPV infection, defined as the average number of secondary infections resulting from one case of HPV infection in a totally susceptible population,8 is also regulated by sexual activity patterns, as well as infection acquisition rates and persistence. R 0 governs, along with vaccine efficacy and coverage, the strength of herd protection, that is, the indirect protection against infection offered by vaccinated to unvaccinated individuals. Herd protection, in turn, is a key determinant of the overall effectiveness (i.e., population‐level impact) of a vaccination program.9, 10 Similar vaccination programs may therefore generate different levels of overall effectiveness depending upon a population's pre‐vaccination HPV prevalence.
In the present report, we used the International Agency for Research on Cancer's (IARC) deterministic transmission dynamic model8, 11 to estimate overall effectiveness and herd protection according to pre‐vaccination HPV prevalence, separately for girls‐only or gender‐neutral vaccination programs. The focus is on HPV16, the most frequent and most carcinogenic type,12, 13 which is also the most difficult to control by vaccination8 due to its special propensity to persist. In a previous study,14 using idealized sexual contact structures, we have shown how HPV control through vaccination becomes more challenging in a population transitioning from a traditional to a gender‐equal sexual behavior. In the present paper, using the same prototypical sexual contact structures, we aimed at describing how HPV vaccination impact differs between populations characterized by different sexual activity levels and pre‐vaccination prevalences.
Methods
Model parameterization and calibration
The probability of developing type‐specific immunity after infection clearance was assumed to be 30% in women and 0% in men,15 and the probability of transmission per sexual partnership was assumed to be 70% in both genders11, 16, 17 (Table 1). We then fitted our model to HPV16 age‐specific prevalence curves in Italian women (Supporting Information Fig. S1)11, 18, 19 by calibrating the rate of clearance of HPV16 infection. HPV16 clearance rate was found to decrease with time since infection (Supporting Information Table S1), in accordance with empirical evidence.20, 21 The corresponding average duration of HPV16 infection was 11 months.
Table 1.
Model parameters related to natural history of HPV16 infection, sexual behavior, and vaccine performance
| Parameter | Value |
|---|---|
| Assumed | |
| Transmission probability per sexual partnership (%) | 7011, 16, 17 |
| Immunity after infection clearance in women (%) | 3015 |
| Mixing between sexual activity classesa | 0.758 |
| Mixing between age groupsa | 0.308, 11 |
| Vaccine efficacy (%) | 954 |
| Duration of vaccine protection | Lifelong23, 24 |
| Calibrated | |
| Average infection duration (months) | 118, 11 |
Abbreviation: HPV, human papillomavirus.
This is a measure of the tendency for individuals with similar sexual activity to form sexual partnerships. It is measured on a scale where fully assortative (i.e., like‐with‐like) and randomly assortative mixing corresponds to value 0 and 1, respectively.
In a previous study, we reported the calibration and validation process of our model for 13 different HR HPV types including HPV16.11 Briefly, 100,000 sets of parameter values were generated by independently sampling, for each parameter, a uniform distribution within a pre‐specified range of values, using a Latin hypercube algorithm. Each set of values was used to generate a model based age‐specific curve of prevalence for each HPV type. The model outputs were calibrated by simulating the sexual behavior of the Italian population, as reported in a nationwide population based survey.22 Finally, each model's output was compared to the observed age‐specific prevalence of each HR HPV, using binomial log likelihood. For our analyses, we used the best‐fitting set of parameters corresponding to the pre‐specified assumptions (Table 1). The parameters governing HPV16 natural history were kept unchanged across all simulations. Further details about the model calibrating process are reported in Supporting Information Data S1.
Study population and assumptions about sexual behavior and vaccination
To allow for heterogeneity in sexual activity patterns, we simulated two types of heterosexual behaviors: (i) gender‐equal behavior typical of many high‐income countries (HIC) and (ii) traditional behavior found in many low/middle‐income countries (LMIC).14 In populations with gender‐equal sexual behavior, genders have similar age‐specific sexual activity rates (Fig. 1, panel a) and preferentially choose partners in the same age five‐year age group (Supporting Information Table S2). Conversely, in traditional populations, genders have different age‐specific sexual activity rates (Fig. 1, panel b) and women are preferentially five years younger on average than their partners (Supporting Information Table S2). The simulated populations were stable and stratified by age (range 10–70 years) and according to three classes of sexual activity (high, intermediate, and low). For both gender‐equal (Supporting Information Fig. S2a) and traditional (Supporting Information Fig. S2b) populations, we simulated different background HPV16 prevalence, ranging from 1 to 8% (hereafter also referred to as “low” and “high” prevalence, respectively), as observed in HPV prevalence surveys carried out by the IARC from 1999 to 2012.5 To obtain different levels of simulated HPV prevalence we scaled the average annual number of new sexual partners, that is 1.1, estimated from the Italian population based survey,22 by a factor constant across age and sexual activity groups. Sexual mixing within age and sexual activity group, which is measured on a scale from 0 (fully assortative, i.e., like‐with‐like) to 1 (randomly assortative), was kept constant in all the simulated populations (Table 1), by assuming little assortative mixing (0.75) by sexual activity and high assortative mixing (0.3) by age preference. The assumed levels of assortativeness are consistent with estimates from the Italian population11 and have been used in previous modeling exercises.8 As sexual preferences may differ across populations, we have assessed the sensitivity of our findings to variations in sexual assortativeness. In particular, we separately assessed the combined effect of high assortative mixing (0.3) by both sexual activity and age and of low assortative mixing (0.75) by both sexual activity and age in a population with gender‐equal sexual behavior.
Figure 1.
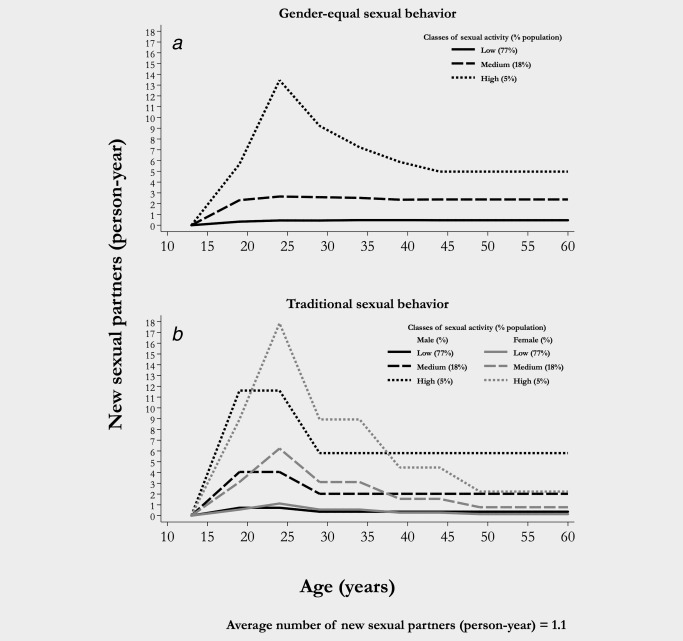
Assumed mean number of new sexual partners per year, by class of sexual activity for population with gender‐equal sexual behavior (a)11 and traditional sexual behavior (b).14
Vaccine efficacy against HPV16 was set to be 95%.4 We assumed that vaccine‐induced immunity for HPV16 was the same and lifelong in both genders,23, 24 and that vaccine coverage in gender‐neutral vaccination programs was the same in boys and girls.
Model based analyses
We calculated the R 0 corresponding to each level of simulated HPV16 pre‐vaccination prevalence separately for populations with gender‐equal and traditional sexual behavior, using the next generation matrix method.25 For model‐based predictions of the impact of vaccination, we assessed both absolute prevalence reduction and overall vaccine effectiveness, measured as percent prevalence reduction (%PR), i.e. the relative reduction in prevalence, of HPV16 compared to no vaccination in women aged 15–34 years at a new steady state (i.e., ∼70 years after the introduction of vaccination). Herd protection was calculated as the difference between overall effectiveness, estimated by the model, and direct vaccine efficacy, estimated by multiplying vaccination coverage by 95% efficacy. Girls‐only or gender‐neutral vaccination programs were separately assessed.
Results
HPV16 prevalence in IARC HPV surveys ranged from 0.4 to 10% (Fig. 2). Figure 3 a shows the relationship between mean number of new sexual partners per year and HPV16 prevalence separately in gender‐equal and traditional populations according to our simulations whereas Figure 3 b shows the steady increase in R 0 increased as pre‐vaccination HPV16 prevalence rises. R 0 increased from 1.6 for 1% to 6.6 for 8% HPV16 prevalence in populations with gender‐equal sexual behavior and from 1.2 to 3.8, respectively, in populations with traditional sexual behavior. Of note, the higher the HPV16 prevalence, the larger was R 0 in populations with gender‐equal compared to traditional sexual behavior.
Figure 2.
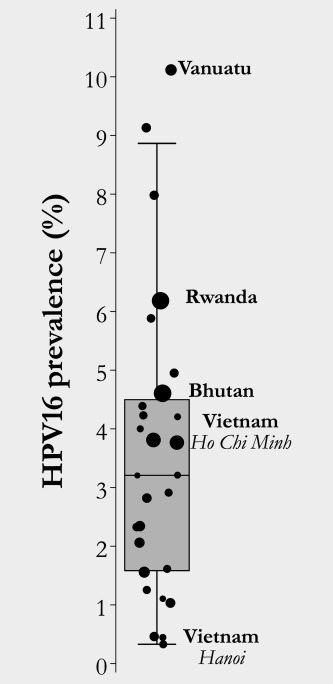
HPV16 prevalence (%) in sexually active women aged 15–35 years (Data are from IARC Prevalence Surveys, 1990–2016).5 The size of the dot is proportional to the number of women recruited in each survey.
Figure 3.
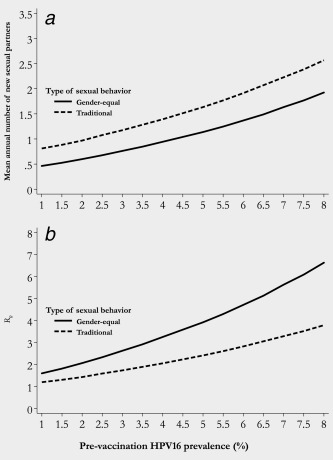
(a) Mean annual number of new sexual partners and (b) Basic reproductive number (R 0) by pre‐vaccination HPV16 prevalence and type of a population's sexual behavior.
Figure 4 focuses on populations with either gender‐equal or traditional sexual behavior after girls‐only vaccination and shows that for any given level of coverage the impact of HPV vaccination is higher in population with lower HPV16 pre‐vaccination prevalence. Figure 4 a shows the HPV16%PR that could be achieved by vaccination of 11 year‐old girls‐only, according to coverage and whether pre‐vaccination HPV16 prevalence is 1% (dashed line) or 8% (solid line). The areas between each of these curves and the straight curve of 95% vaccine efficacy (dash‐dotted line) represent herd protection. The difference in HPV16%PR between low‐ and high‐prevalence populations steadily increases up to ∼40% coverage (91% effectiveness for low vs. 48% for high pre‐vaccination prevalence). The difference is entirely accounted for by the larger contribution of herd protection in populations with low against high pre‐vaccination prevalence (53% vs. 10% of the total HPV16%PR, respectively). Elimination of the infection could be obtained with 55 and 97% coverage, respectively. Figure 4 b shows absolute levels of HPV16 prevalence (i.e., 5/1,000, 1/1,000, 1/10,000) that could be achieved at equilibrium after vaccination, according to coverage and pre‐vaccination HPV16 prevalence. For example, to lower HPV16 prevalence from 1% to 1/1000, 37% coverage would be sufficient whereas 96% coverage would be necessary if pre‐vaccination prevalence were 8%.
Figure 4.
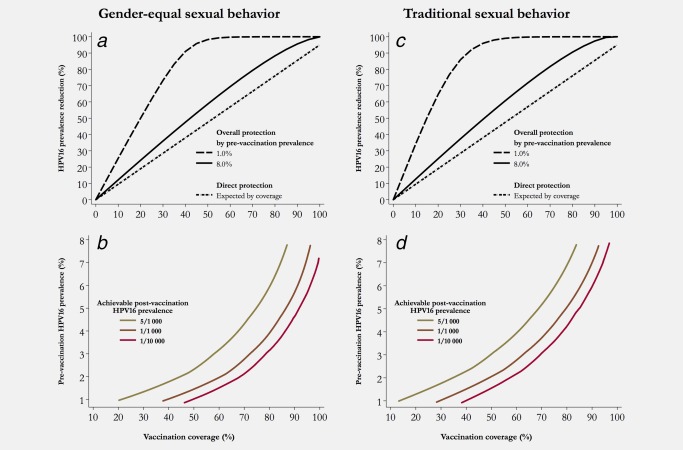
(a) Relative reduction in HPV16 prevalence and (b) Achievable post‐vaccination HPV16 prevalence, among women 15–34 years of age after vaccination of 11 year‐old girls in a population with gender‐equal sexual behavior, by coverage and pre‐vaccination prevalence. (c) Relative reduction in HPV16 prevalence and (d) Achievable post‐vaccination HPV16 prevalence, among women 15–34 years of age after vaccination of 11 year‐old girls in a population with traditional sexual behavior, by coverage and pre‐vaccination HPV16 prevalence.
Figure 4 c and 4 d show similar simulations in a population with traditional sexual behavior. The largest difference in HPV16%PR is already reached at ∼30% coverage (93% for low vs. 37% for high pre‐vaccination prevalence), whereas elimination of the infection could be obtained with 50 and 95% coverage, respectively (Fig. 4 c). Post‐vaccination absolute prevalence of 1/1000 would be achievable by 28% coverage in a population with pre‐vaccination HPV16 prevalence of 1% but would require 93% if pre‐vaccination HPV16 prevalence were 8%.
Supporting Information Figure S3 show the same simulations as in Figure 4 in populations with gender‐equal or traditional sexual behavior if 11 year‐old boys were vaccinated in addition to 11 year‐old girls. In both scenarios, lower coverage would be necessary to produce the same overall effectiveness and the same decreases in HPV16 absolute prevalence than after vaccination of girls only but the impact of pre‐vaccination prevalence would be attenuated. Also, Supporting Information Figure S4 and S5 show the effect of sexual assortativeness on R 0 and on the impact of HPV vaccination in populations with different levels of pre‐vaccination HPV16 prevalence.
Discussion
Our present report highlights the importance of pre‐vaccination HPV prevalence as a key driver of the overall effectiveness of a vaccination program through its influence on the strength of herd protection. Consequently, the higher the pre‐vaccination prevalence, the lower the relative reduction of HPV would be for a given vaccination coverage and accordingly the higher the coverage would have to be to achieve pre‐defined HPV control targets.
Sexual activity patterns, along with infection persistence, are known to determine population‐specific HPV16 prevalence in young adult women, which has been shown to vary by about one order of magnitude between and within countries.5 To reproduce the observed range of HPV16 prevalence, we varied the average number of new sexual partners in the simulated populations. The range of average number of new sexual partners assumed to obtain the expected HPV16 prevalence is consistent with the values reported in the literature.26 Furthermore, to account for differences in sexual activity patterns between populations, we simulated two types of patterns characterized by different age‐specific rates of sexual activity and age differences between sexual partners.14
As previously described, the circulation of HPV16 in a population with gender‐equal sexual behavior is more efficient than in a population with traditional behavior, due to overlapping periods of intense, mainly premarital, sexual activity at a young age.27 Accordingly, in the present study, for any given level of HPV16 prevalence, R 0 was higher in “gender‐equal” than traditional populations, making vaccination consistently more effective in the latter. Of note, Our R 0 estimates for HPV16, ranging between 1.6 and 6.6 in populations with gender‐equal and between 1.2 and 3.8 in populations with traditional sexual behavior, fall within the range of other sexually transmitted infections such as HIV,28 syphilis,29 Neisseria gonorrheae and Chlamydia trachomatis.30
We additionally predicted that HPV16 prevalence affected vaccination effectiveness, irrespective of the sexual activity pattern of a population. The overall effectiveness of girls‐only vaccination programs with coverage in the 30–50% range was at least two‐fold larger in low‐ prevalence than in high‐prevalence populations. Gender‐neutral vaccination further enhanced herd protection making elimination of HPV16 possible with 85 and 74% coverage in high‐prevalence populations with gender‐equal and traditional sexual behavior, respectively. In low‐prevalence populations, HPV16 vaccination of boys in addition to girls would further lower the minimum required coverage to below 40%. We also assessed the effect of sexual assortativeness on the impact on HPV vaccination. As expected,10 HPV vaccination is more effective in populations with a more homogeneous (low assortative) sexual mixing than in populations with a more heterogeneous (high assortative) sexual mixing. Nevertheless, for each sexual mixing pattern, the impact of HPV vaccination was lower in populations with higher pre‐vaccination HPV16 prevalence.
Strengths of our report include the use of a validated transmission model to represent changes in HPV prevalence. Transmission models can capture the dynamics of infection circulation10 in a population and have the distinct advantage of including the effect of herd immunity.31 We could also derive estimates of the parameters governing the natural history of HPV16 infections from the calibration to a large screening trial conducted in Italy18 in which we were able to predict accurately the incidence of HPV16 infection in HPV16 negative women.11 This allowed us to provide a range of uncertainty for each parameter estimate.11 The values used in our simulations were those which best reflected the findings of previous studies on HPV16 natural history (i.e., probability of transmission,11, 16, 17, 32 probability of developing type‐specific immunity after infection clearance,15 and duration of infection12, 33, 34).
The limitations of our present study mainly derive from the uncertainties that remain in some of the model assumptions. For example, we assumed that the variation of HPV16 prevalence across populations was attributable exclusively to differences in sexual activity. We did not include, for instance, the potential influence of HIV infection that affects HPV prevalence through increased acquisition and duration of the infection. We probably also oversimplified the sexual behavior of women and men on account of the lack of exhaustive information on sexual networks, for example, prevalence of sequential or concurrent sexual partnerships. On the other hand, since the main objective of the present paper was to describe how the impact of HPV vaccination differs between populations characterized by different sexual activity levels and HPV prevalence, accounting for HIV would have added unnecessary complexity to the modeling process and results interpretation. Finally, although cervical cancer reduction is the ultimate aim of vaccination, we chose HPV16 infection rather than cervical disease as endpoint to avoid the uncertainties that would be introduced by the parameters that regulate the progression or regression of HPV infection. We also chose to focus on HPV16 only because it is the best understood, most carcinogenic1 type and also the type that a recent modeling study has shown to be the greatest challenge in vaccination programs.8
In conclusion, pre‐vaccination HPV16 prevalence strongly influences herd immunity and hence determines population‐specific overall effectiveness of HPV vaccination and the levels of coverage that could be sufficient to control HPV‐infection and related cancers. HPV control is harder when pre‐vaccination HPV prevalence is high. Our findings are particularly relevant for those LMICS in which sexual behavior is based on traditional norms and HPV prevalence remains low.14 In these countries, it is advantageous to introduce vaccination while HPV prevalence in young women is low, anticipating any increase that may occur with liberalization of social attitudes.
Supporting information
Supporting Information
Acknowledgments
Dr Baussano is an Honorary Research Fellow at the School of Public Health, Imperial College, London, UK.
Conflicts of Interest: We declare that we have no conflicts of interest.
References
- 1. IARC . Biological agents. IARC Monogr Eval Carcinog Risks Hum. 2012;100B:1–475. Accessed September 12, 2017 at http://monographs.iarc.fr/ENG/Monographs/vol100B/index.php. [PMC free article] [PubMed] [Google Scholar]
- 2. de Martel C, Plummer M, Vignat J, et al. Worldwide burden of cancer attributable to HPV by site, country and HPV type. Int J Cancer 2017;141:664–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Joura EA, Giuliano AR, Iversen OE, et al. A 9‐valent HPV vaccine against infection and intraepithelial neoplasia in women. N Engl J Med. 2015;372:711–23. [DOI] [PubMed] [Google Scholar]
- 4. Lehtinen M, Dillner J. Clinical trials of human papillomavirus vaccines and beyond. Nat Rev Clin Oncol 2013;10:400–10. [DOI] [PubMed] [Google Scholar]
- 5. Crosbie EJ, Einstein MH, Franceschi S, et al. Human papillomavirus and cervical cancer. Lancet 2013;382:889–99. [DOI] [PubMed] [Google Scholar]
- 6. Massad LS, Xie X, Burk R, et al. Long‐term cumulative detection of human papillomavirus among HIV seropositive women. Aids 2014;28:2601–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Rowhani‐Rahbar A, Hawes SE, Sow PS, et al. The impact of HIV status and type on the clearance of human papillomavirus infection among Senegalese women. J Infect Dis 2007;196:887–94. [DOI] [PubMed] [Google Scholar]
- 8. Baussano I, Lazzarato F, Ronco G, et al. Different challenges in eliminating HPV16 compared to other types: A modeling study. J Infect Dis 2017;216:336–44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Halloran ME, Struchiner CJ, Longini IM Jr., Study designs for evaluating different efficacy and effectiveness aspects of vaccines. Am J Epidemiol 1997;146:789–803. [DOI] [PubMed] [Google Scholar]
- 10. Garnett GP. Role of herd immunity in determining the effect of vaccines against sexually transmitted disease. J Infect Dis 2005;191 Suppl 1:S97–106. [DOI] [PubMed] [Google Scholar]
- 11. Baussano I, Elfström KM, Lazzarato F, et al. Type‐specific human papillomavirus biological features: Validated model‐based estimates. Plos One 2013;8:e81171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Schiffman M, Doorbar J, Wentzensen N, et al. Carcinogenic human papillomavirus infection. Nat Rev Dis Primers 2016;2:16086 [DOI] [PubMed] [Google Scholar]
- 13. Guan P, Howell‐Jones R, Li N, et al. Human papillomavirus types in 115,789 HPV‐positive women: A meta‐analysis from cervical infection to cancer. Int J Cancer 2012;131:2349–59. [DOI] [PubMed] [Google Scholar]
- 14. Baussano I, Lazzarato F, Brisson M, et al. Human papillomavirus vaccination at a time of changing sexual behavior. Emerg Infect Dis 2016;22:18–23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Beachler DC, Jenkins G, Safaeian M, et al. Natural acquired immunity against subsequent genital human papillomavirus infection: A systematic review and meta‐analysis. J Infect Dis 2016;213:1444–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Bogaards JA, Xiridou M, Coupe VM, et al. Model‐based estimation of viral transmissibility and infection‐induced resistance from the age‐dependent prevalence of infection for 14 high‐risk types of human papillomavirus. Am J Epidemiol 2010;171:817–25. [DOI] [PubMed] [Google Scholar]
- 17. Vänskä S, Auranen K, Leino T, et al. Impact of vaccination on 14 high‐risk HPV type infections: A mathematical modelling approach. Plos One 2013;8:e72088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ronco G, Giorgi‐Rossi P, Carozzi F, et al. Efficacy of human papillomavirus testing for the detection of invasive cervical cancers and cervical intraepithelial neoplasia: A randomised controlled trial. Lancet Oncol 2010;11:249–57. [DOI] [PubMed] [Google Scholar]
- 19. Carozzi F, De Marco L, Gillio‐Tos A, et al. Age and geographic variability of human papillomavirus high‐risk genotype distribution in a large unvaccinated population and of vaccination impact on HPV prevalence. J Clin Virol 2014;60:257–63. [DOI] [PubMed] [Google Scholar]
- 20. Plummer M, Schiffman M, Castle PE, et al. A 2‐year prospective study of human papillomavirus persistence among women with a cytological diagnosis of atypical squamous cells of undetermined significance or low‐grade squamous intraepithelial lesion. J Infect Dis 2007;195:1582–9. [DOI] [PubMed] [Google Scholar]
- 21. Rodriguez AC, Schiffman M, Herrero R, et al. Rapid clearance of human papillomavirus and implications for clinical focus on persistent infections. J Natl Cancer Inst 2008;100:513–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Signorelli C, Pasquarella C, Limina RM, et al. Third Italian national survey on knowledge, attitudes, and sexual behaviour in relation to HIV/AIDS risk and the role of health education campaigns. Eur J Publ Health 2006;16:498–504. [DOI] [PubMed] [Google Scholar]
- 23. Safaeian M, Porras C, Pan Y, et al. Durable antibody responses following one dose of the bivalent human papillomavirus L1 virus‐like particle vaccine in the Costa Rica Vaccine Trial. Cancer Prev Res (Phila) 2013;6:1242–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Kreimer AR, Struyf F, Del Rosario‐Raymundo MR, et al. Efficacy of fewer than three doses of an HPV‐16/18 AS04‐adjuvanted vaccine: Combined analysis of data from the Costa Rica Vaccine and PATRICIA Trials. Lancet Oncol 2015;16:775–86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vynnycky E, White RG. Sexually transmitted infections An Introduction to Infectious Disease Modellinged. Oxford: Oxford University Press, 2010. [Google Scholar]
- 26. Hertog S. Heterosexual behavior patterns and the spread of HIV/AIDS: The interacting effects of rate of partner change and sexual mixing. Sex Transm Dis 2007;34:820–8. [DOI] [PubMed] [Google Scholar]
- 27. Baussano I, Diaz M, Tully S, et al. Effect of age‐difference between heterosexual partners on risk of cervical cancer and human papillomavirus infection. Papillomavirus Res (Amsterdam, Netherlands) 2017;3:98–104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Nsubuga RN, White RG, Mayanja BN, et al. Estimation of the HIV basic reproduction number in rural south west Uganda: 1991–2008. PLoS One 2014;9:e83778 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Grassly NC, Fraser C, Garnett GP. Host immunity and synchronized epidemics of syphilis across the United States. Nature 2005;433:417–21. [DOI] [PubMed] [Google Scholar]
- 30. Brunham RC, Nagelkerke NJ, Plummer FA, et al. Estimating the basic reproductive rates of Neisseria gonorrhoeae and Chlamydia trachomatis: The implications of acquired immunity. Sex Transm Dis 1994;21:353–6. [DOI] [PubMed] [Google Scholar]
- 31. Brisson M, Bénard É, Drolet M, et al. Population‐level impact, herd immunity, and elimination after human papillomavirus vaccination: A systematic review and meta‐analysis of predictions from transmission‐dynamic models. Lancet Public Health 2016;1:e8–e17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Burchell AN, Coutlee F, Tellier PP, et al. Genital transmission of human papillomavirus in recently formed heterosexual couples. J Infect Dis 2011;204:1723–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Schiffman M, Herrero R, Desalle R, et al. The carcinogenicity of human papillomavirus types reflects viral evolution. Virology 2005;337:76–84. [DOI] [PubMed] [Google Scholar]
- 34. Rositch AF, Koshiol J, Hudgens MG, et al. Patterns of persistent genital human papillomavirus infection among women worldwide: A literature review and meta‐analysis. Int J Cancer 2013;133:1271–85. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information


