Abstract
Background and purpose
The aim of this pooled patient‐level data analysis was to test if multidomain interventions, addressing several modifiable vascular risk factors simultaneously, are more effective than usual post‐stroke care for the prevention of cognitive decline after stroke.
Methods
This pooled patient‐level data analysis included two randomized controlled trials using a multidomain approach to target vascular risk factors in stroke patients and cognition as primary outcome. Changes from baseline to 12 months in the trail making test (TMT)‐A, TMT‐B and 10‐words test were analysed using stepwise backward linear mixed models with study as random factor. Two analyses were based on the intention‐to‐treat (ITT) principle using different imputation approaches and one was based on complete cases.
Results
Data from 322 patients (157 assigned to multidomain intervention and 165 to standard care) were analysed. Differences between randomization groups for TMT‐A scores were found in one ITT model (P = 0.014) and approached significance in the second ITT model (P = 0.087) and for complete cases (P = 0.091). No significant intervention effects were found for any of the other cognitive variables.
Conclusion
We found indications that multidomain interventions compared with standard care can improve the scores in TMT‐A at 1 year after stroke but not those for TMT‐B or the 10‐words test. These results have to be interpreted with caution due to the small number of patients.
Keywords: cognition, cognitive decline, lifestyle, multidomain intervention, pooled data analysis, post‐stroke dementia, prevention, stroke
Introduction
Post‐stroke dementia occurs in between 7% (in population‐based studies of first‐ever strokes) and 41% (in hospital‐based studies that included recurrent strokes) of patients 1. Up to 78% of patients show some cognitive deficits within 1 month after the stroke 2, 3. Although cognitive function improves or remains stable in the majority of patients during the months following the event, it declines in a delayed fashion in approximately one‐third of patients 4. The mechanisms of this delayed progression remain unclear, but both vascular and neurodegenerative mechanisms are involved when the stroke seems to accelerate subclinical gradual processes leading to cognitive decline 5.
Thus far, no therapeutic strategy has shown convincing clinical evidence for preventing cognitive decline after stroke 6, 7. Multidomain interventions, with rigorous control of vascular risk factors including pharmacological treatment and lifestyle modification, addressing neuropsychiatric symptoms and provision of a cognitive and social stimulating environment, may potentially prevent the delayed cognitive impairment. Such a multidomain intervention in people at risk of dementia has shown improvement or maintenance of cognitive functions after a period of 2 years 8. However, a nurse‐led vascular multidomain intervention in elderly people did not find any effect on cognition after 6 years 9. In stroke survivors, only two small randomized controlled trials have explored the effects of multidomain interventions on cognition 10, 11. Both studies failed to show significant improvement in cognitive function. As both studies were probably underpowered, the aim of this pooled data analysis was to retest the effect of multidomain interventions compared with standard care on cognition at 1 year after stroke combining the data of both randomized controlled trials.
Methods
In an update of a previous systematic literature search 12, we identified two published prospective randomized open‐label blinded‐endpoint trials using a multidomain approach to target lifestyle and vascular risk factors in stroke patients with cognition as primary outcome 10, 11, 13. Study designs and quality are shown in Table S1 and Fig. S1.
The authors agreed to perform a combined analysis of the individual level data from the two trials. Studies were approved by the Regional Committees for Medical Research Ethics ‐ South East Norway and the Ethics Committee for Lower Austria. All patients gave written consent at the time of inclusion in the study. All data used in the present analysis were anonymous. The regional Norwegian ethics committee approved the pooled data analysis and no additional approval was necessary from the Austrian local ethics committee.
Outcomes
Both studies used neuropsychological test batteries that included the trail making test (TMT)‐A (assessing attention), TMT‐B (executive functions) and a 10‐words list recall test (verbal memory). The number of words retrieved after the first presentation of the word list assesses the ability for immediate retention (0–10 words), whereas the sum of correctly remembered words after four repetitions (0–40 words) describes the memorization and learning ability 14. Changes in cognitive scores from baseline to 12 months were used as outcomes in the pooled analysis with TMT‐B as pre‐specified primary outcome and the other cognitive variables as secondary outcomes. Other secondary outcomes included depressive symptoms, stroke severity [National Institutes of Health Stroke Scale (NIHSS) score], functional outcome [modified Rankin Scale (mRS) score] and activity of daily living (Barthel index) at 12 months. Two different instruments that are valid in stroke patients were used to assess depressive symptoms, i.e. the Centre for Epidemiologic Studies Depression Scale 10, 15 and Hospital Anxiety and Depression Scale 11, 16. Data were combined using the cut‐offs for non‐depressed patients, i.e. 8 points for the Hospital Anxiety and Depression Scale depression subscore and 16 points for the Centre for Epidemiologic Studies Depression Scale. The mRS score was analysed as a binary variable with 0–1 points defined as a good outcome 17.
Statistical analysis
In a pre‐defined pooled data analysis, we included all patients with at least one of the four cognitive baseline variables. We restricted the analysis to ischemic strokes to increase homogeneity and because only one study included transitory ischemic attacks and haemorrhagic strokes. Patients who died (n = 8) or suffered a recurrent stroke (n = 6) were excluded.
Missing data due to drop‐out or inability to perform cognitive tests can bias the results 18 and therefore efficacy analyses were performed using the following three approaches. (i) Dataset 1 (complete cases): per protocol principle including only patients with four valid cognitive baseline assessments and four valid cognitive 12‐month assessments. (ii) Dataset 2: intention‐to‐treat (ITT) principle where missing data for drop‐outs were imputed using the hot‐deck imputation method (an algorithm using available data to provide values for records with missing data). (iii) Dataset 3: Dataset 2 where additional missing data for patients who were not able to perform a cognitive test were imputed by using the worst possible value (300 s for TMT‐B, 180 s for TMT‐A, 0 for 10‐words test). We pre‐specified the most conservative approach (the full ITT Dataset 3) as the primary analysis method.
Stepwise backward linear mixed models were performed for each of the four cognitive variables with the difference in the cognitive values between baseline and 12 months as the dependent variables (see Supporting Information for details). Non‐cognitive secondary outcomes were analysed in the full ITT dataset (Dataset 3) only, using stepwise backward linear mixed models for the continuous outcomes (NIHSS score, Barthel index) and logistic regression models for the binary outcomes (depressive symptoms, mRS score 0–1 vs. 2–5) (see Supporting Information for sensitivity analyses).
Unadjusted effect sizes are presented as mean differences between randomization groups. Differences in baseline variables between randomization groups were tested using chi‐square or Fisher exact test for categorical data and Mann–Whitney U‐test for continuous data. Statistical analyses were performed with R version 3.3.1 19.
Results
After the exclusion of 75 patients (Fig. 1), 185 patients from Austria and 137 from Norway with at least one cognitive baseline assessment were included in the analysis. Due to different inclusion criteria, the populations differed significantly in several aspects (Table S2), including poorer cognitive results at baseline in the Norwegian cohort.
Figure 1.
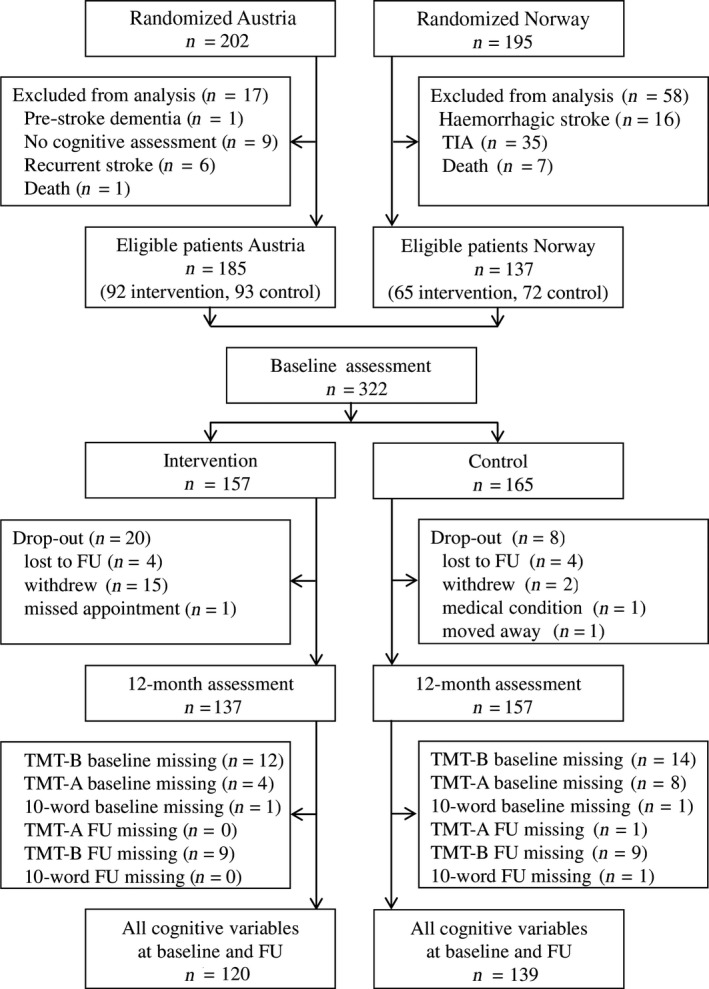
Flow chart of patients included in the pooled data analysis. FU, follow‐up; TIA, transient ischemic attack; TMT, trail making test.
Of the patients, 157 were assigned to the intervention and 165 to the control group. These two groups did not differ in baseline characteristics except for body mass index, which was higher in the intervention group (Table S3). There were 28 patients (20 intervention and 8 control) who did not complete the 12‐month visit. These drop‐outs did not differ significantly from patients who completed follow‐up in demographic, stroke‐related or cognitive characteristics at baseline (Table S4). Overall, 259 subjects (120 intervention, 139 control) had a full set of cognitive variables (Dataset 1, Fig. 1). TMT‐B was the most demanding test with 29/322 patients at baseline and 18/294 patients at follow‐up unable to complete it. Only two patients with complete TMT‐B data had missing data in other cognitive measures.
Outcomes
Randomization groups did not differ for TMT‐B, in either univariate comparisons or in any of the multivariable models (Tables 1 and 2, Tables S5–S7). The intervention group showed a significantly larger improvement in TMT‐A compared with controls in the univariate analysis after imputation of missing data and drop‐outs [Dataset 3 (mean ± SD): 10.9 ± 31.5 vs. 6.0 ± 25.8 s; P = 0.015] (Table 1), as well as in the complete case dataset (7.7 ± 18.3 vs. 3.6 ± 20.2 s; P = 0.044) (Table S5) and Dataset 2 (intervention, 8.6 ± 18.8 s vs. control, 3.0 ± 20.5 s; P = 0.006). In multivariable models, group differences for TMT‐A were found to be significant only in Dataset 2 (imputation of drop‐outs only; P = 0.014) (Table S6) and showed a non‐significant trend in Dataset 3 (full imputation; P = 0.087) (Table 2) and Dataset 1 (complete cases; P = 0.091) (Table S7). No significant group effect was found for the 10‐words test, in either univariate (Table 1) or multivariable (Table 2, Tables S6 and S7) models. The random factor ‘study’ did not contribute significantly to any of the multivariable models except for the model testing the variable 10‐words test first trial in Dataset 2 (Table S6). Sensitivity analyses showed similar effects of the randomization group for all cognitive variables. Significant improvements in TMT‐A (P = 0.022) and 10‐word sum (P = 0.041) were only found for the intervention group in Dataset 1 of subjects aged 40–80 years.
Table 1.
Group differences for the changes in cognitive and secondary outcome variables from baseline to 12 months in the full intention‐to‐treat population [Dataset 3; n = 322 (157 intervention, 165 control)]
| Outcome | Change from baseline to 12 monthsa | Between‐group difference | |||
|---|---|---|---|---|---|
| Intervention–control (95% CI) | P‐value | ||||
| Intervention | Control | Univariate | Model 3* | ||
| Cognition | |||||
| TMT‐B | 10.4 ± 62.4 | 18.4 ± 62.3 | −8.0 (−21.7 to 5.7) | 0.075 | 0.880 |
| TMT‐A | 10.9 ± 31.5 | 6.0 ± 25.8 | 4.9 (−1.4 to 11.2) | 0.015 | 0.087 |
| 10‐words first trial | 0.5 ± 1.8 | 0.3 ± 1.5 | 0.2 (−0.2 to 0.5) | 0.402 | 0.065 |
| 10‐words sum | 2.5 ± 6.1 | 1.8 ± 5.0 | 0.7 (−0.5 to 2.0) | 0.305 | 0.076 |
| Secondary outcomes | |||||
| Depressive symptoms | 23 (14.6%) improved | 29 (17.6%) improved | OR 0.81 (0.44 to 1.46) | 0.476 | 0.409 |
| NIHSS score | 1.0 ± 1.6 | 0.8 ± 1.5 | 0.2 (−0.1 to 0.6) | 0.087 | 0.397 |
| mRS score 0–1 | 41 (26.1%) improved | 33 (20.0%) improved | OR 1.41 (0.84 to 2.38) | 0.192 | 0.224 |
| Barthel index | 3.0 ± 11.3 | 2.3 ± 9.2 | 0.7 (−1.6 to 2.9) | 0.667 | 0.473 |
Data are given as mean ± SD and n (%). CI, confidence intervals; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; OR, odds ratio; TMT, trail making test. aPositive values indicate improvement. *P‐value for group effect in the linear mixed model with study as random factor adjusted for age, sex, vascular risk factors, time from stroke onset to cognitive testing, cognitive scores at baseline and stroke severity.
Table 2.
Baseline variables entering the final stepwise backward linear mixed models with study as random factor testing for effects of the intervention on changes in the cognitive variables between baseline and 12 months in the full intention‐to‐treat dataset (Dataset 3, n = 322)
| Baseline variable | TMT‐Bb | TMT‐Ab | 10‐words first trialb | 10‐words totalb | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Coefficient | SE | P‐value | Coefficient | SE | P‐value | Coefficient | SE | P‐value | ||||
| Treatment groupa | −0.90 | 5.98 | 0.880 | 4.10 | 2.39 | 0.087 | 0.27 | 0.14 | 0.065 | 0.93 | 0.52 | 0.076 |
| Age | −0.68 | 0.31 | 0.028 | −3.02 | 0.13 | 0.018 | −0.01 | 0.01 | 0.110 | Not entered | ||
| Sex | Not entered | −4.42 | 2.62 | 0.093 | Not entered | Not entered | ||||||
| Diabetes | −31.18 | 7.43 | <0.001 | −6.65 | 2.92 | 0.024 | −0.33 | 0.18 | 0.068 | −1.03 | 0.66 | 0.122 |
| Hyperlipidemia | 15.09 | 7.57 | 0.047 | Not entered | 0.43 | 0.18 | 0.019 | 1.91 | 0.67 | 0.005 | ||
| Smoking status | Not entered | −4.35 | 2.84 | 0.127 | Not entered | Not entered | ||||||
| Onset to test time | −0.33 | 0.17 | 0.057 | −0.16 | 0.07 | 0.025 | Not entered | Not entered | ||||
| TMT‐B | 0.54 | 0.05 | <0.001 | −0.08 | 0.02 | <0.001 | Not entered | −0.01 | 0.00 | 0.005 | ||
| TMT‐A | −0.32 | 0.11 | 0.004 | 0.59 | 0.05 | <0.001 | Not entered | Not entered | ||||
| 10‐words first trial | −5.40 | 3.29 | 0.101 | Not entered | −1.02 | 0.08 | <0.001 | −0.51 | 0.29 | 0.078 | ||
| 10‐words sum | 2.23 | 0.81 | 0.006 | Not entered | 0.12 | 0.02 | <0.001 | −0.41 | 0.07 | <0.001 | ||
| MMSE | Not entered | 0.92 | 0.62 | 0.137 | 0.09 | 0.03 | 0.013 | 0.31 | 0.13 | 0.020 | ||
| Total cholesterol | Not entered | 0.14 | 0.05 | 0.008 | 0.01 | 0.00 | 0.024 | 0.02 | 0.01 | 0.157 | ||
| HDL cholesterol | −0.40 | 0.20 | 0.044 | −0.34 | 0.09 | <0.001 | −0.02 | 0.01 | 0.001 | −0.05 | 0.02 | 0.007 |
| LDL cholesterol | −0.14 | 0.09 | 0.104 | −0.19 | 0.06 | 0.001 | −0.01 | 0.00 | 0.003 | −0.03 | 0.01 | 0.020 |
| Systolic blood pressure | Not entered | Not entered | 0.01 | 0.003 | 0.030 | 0.03 | 0.01 | 0.038 | ||||
| Modified Rankin Scale score | 10.16 | 7.02 | 0.149 | 4.42 | 2.78 | 0.113 | Not entered | 1.31 | 0.60 | 0.030 | ||
| Barthel index | Not entered | −0.17 | 0.11 | 0.127 | Not entered | Not entered | ||||||
| Depressive symptoms | Not entered | −6.97 | 2.77 | 0.012 | Not entered | Not entered | ||||||
HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; MMSE, Mini‐Mental State Examination; not entered, variable did not enter the final model; SE, standard error. aReference is the control group. bDifferences between baseline and 12 months; positive values indicate improvement. Variances explained by the final model: R 2 = 0.49 for trail making test (TMT)‐A; R 2 = 0.31 for TMT‐B; R 2 = 0.44 for 10‐words test first trial; R 2 = 0.33 for 10‐words test sum. P‐values ≤ 0.05 are in bold.
The factors that significantly influenced cognition differed in the three datasets and for the four cognitive variables. However, worse baseline scores were associated with greater improvement in the cognitive variables in all datasets (Table 2, Tables S6 and S7). Age and the presence of diabetes at baseline had a negative influence on cognitive improvement in TMT‐A and TMT‐B in all models.
The randomization group showed no effect on the following secondary outcomes: depressive symptoms, mRS score, NIHSS score and Barthel index (Table 1).
Discussion
In this pooled patient data analysis of two trials, we found indications for better scores in the TMT‐A, but not the TMT‐B (the primary outcome) or 10‐words test following a multidomain risk factor intervention after stroke.
It has repeatedly been shown that stroke is more likely to affect executive functions and speed of mental processing than memory 2, 5. The FINGER study, which tested the effect of a multidomain intervention on cognition in people at risk of dementia, found group differences for executive functions and processing speed but not for memory 8. Performance on TMT‐B is thought to reflect complex planning and working memory, whereas TMT‐A is thought to reflect simple planning ability, speed of mental processing and attention. The 10‐words test assesses verbal memory. Therefore, we expected to find the largest positive effect of the intervention for TMTs and the weakest for the 10‐words test. Indeed, in both groups, the changes in cognitive performance during the 12‐month observation period were largest for the TMTs and small for the 10‐words test; statistically significant group differences were only found for TMT‐A in one of three multivariable models.
The small effect found on TMT‐A may indicate a possible effect on the progression of ongoing vascular processes. It has been suggested that TMT‐A has a stronger correlation with the NIHSS score than TMT‐B 20. The multidomain intervention may have supported patients during their recovery after stroke and this effect may be reflected by the test results in TMT‐A. It is also possible that, as suggested by the large SD, individual differences in cognitive abilities were more pronounced in the TMT‐B, making it more difficult to identify effects of interventions. In addition, the heterogeneity of the stroke patient population may contribute to the lack of group differences.
Many patients (9% at baseline) were not able to complete TMT‐B and were thus either excluded from analysis or their data were imputed. This methodological problem has been reported previously, but has not been solved 18. Patients with missing data on TMT‐B were probably the most affected patients and excluding their data may have led to a bias, whereas imputation of a large number of data may have diluted possible effects.
Both studies found significant improvements in risk factors targeted by the intervention programme 11, 13. However, because assessments for lifestyle habits differed between studies and because the multifactorial intervention was delivered as a ‘package’, we could not analyze the potential influence of the specific elements constituting the multimodal intervention on cognitive variables. The achieved lifestyle changes were modest (Table S8, 11, 13) and the observation period may have been too short to be reflected by group differences in cognitive variables. Small randomized controlled trials in patients with stroke have previously found that physical exercise can improve cognitive functions 21, 22. However, the multidomain intervention did not improve the level of physical activity compared with controls in both studies included 11, 13. A greater intensity of exercise training and supervision may be needed after stroke to achieve effects on cognition. Furthermore, the control group received various interventions as part of their ‘usual care’ after stroke.
Patients with the worst baseline scores had the largest changes in cognitive outcome, suggesting that the ceiling effect as well as recovery after stroke may mask effects on individuals at risk of cognitive decline. Although all patients probably profit from intensified lifestyle intervention with respect to secondary stroke prevention, it might be necessary to select specific patients to detect effects on the preservation of cognitive abilities. Interventions could, for example, focus on people with diabetes or elevated blood glucose at admission with acute stroke. In line with our finding that diabetic subjects improved less in TMT scores, diabetes has previously been associated with a higher risk of dementia 23 and with a worse outcome after stroke 24. We found no group differences for patients suffering from diabetes; however, the analysis was probably underpowered to test for this specific effect.
This pooled data analysis has a number of limitations: Only two trials with a small number of patients were included. Both trials mainly included patients with mild strokes, which may limit the generalizability of our results. Different inclusion criteria regarding stroke‐related impairment and age were applied. A limited number of cognitive assessments were analyzed. However, these assessments covered three important cognitive domains (executive functions, speed of mental processing, verbal memory). The number of missing data was high and, furthermore, drop‐out was higher in the intervention group leading to a potential bias due to selective drop‐out. Furthermore, we observed group differences only for one of the secondary outcomes (TMT‐A) in one of three datasets and it is unclear whether this observed difference is of clinical significance.
Conclusion
A statistically significant effect of multidomain lifestyle interventions compared with standard care was not found for the primary outcome (TMT‐B) measuring executive functions and only for one of the investigated secondary cognitive variables (TMT‐A) in one dataset. These results suggest that multidomain interventions may help in the prevention of post‐stroke cognitive impairment, but these results have to be interpreted with caution due to the small number of patients and the number of missing data for certain variables.
Disclosure of conflicts of interest
The authors declare no financial or other conflicts of interest.
Supporting information
Table S1. Comparison of study designs.
Table S2. Comparison of the baseline characteristics according to study (n = 322).
Table S3. Comparison of the baseline characteristics of the two randomization groups (n = 322).
Table S4. Baseline characteristics of drop‐outs (n = 28; this does not include deaths and recurrent strokes, which were excluded from the analysis) compared with patients with follow‐up (n = 294).
Table S5. Group differences for the changes in cognitive and secondary outcome variables from baseline to 12 months [complete cases, n = 259 (120 intervention, 139 control)].
Table S6. Baseline variables entering the final stepwise backward multivariable models testing for effects of the intervention on changes in the cognitive variables between baseline and 12 months in the intention‐to‐treat population with imputation of drop‐outs only (Dataset 2, n = 284).
Table S7. Baseline variables entering the final stepwise backward multivariable models testing for effects of the intervention on changes in the cognitive variables between baseline and 12 months in the complete cases (Dataset 1, n = 236).
Table S8. Group differences for the changes in vascular risk factors from baseline to 12 months in the full intention‐to‐treat population [Dataset 3, n = 322 (157 intervention, 165 control)].
Figure S1. Risk of bias assessed according to the Cochrane collaboration criteria.
Acknowledgements
ASPIS was supported by the NÖ Forschungs‐ und Bildungsges.m.b.H., Austria (Grant Agreement no. LS09‐002). The pooled data analysis was supported by a grant from Land Niederösterreich, Austria.
References
- 1. Pendlebury ST, Rothwell PM. Prevalence, incidence, and factors associated with pre‐stroke and post‐stroke dementia: a systematic review and meta‐analysis. Lancet Neurol 2009; 8: 1006–1018. [DOI] [PubMed] [Google Scholar]
- 2. Hurford R, Charidimou A, Fox Z, Cipolotti L, Werring DJ. Domain‐specific trends in cognitive impairment after acute ischaemic stroke. J Neurol 2013; 260: 237–241. [DOI] [PubMed] [Google Scholar]
- 3. Leśniak M, Bak T, Czepiel W, Seniów J, Członkowska A. Frequency and prognostic value of cognitive disorders in stroke patients. Dement Geriatr Cogn Disord 2008; 26: 356–363. [DOI] [PubMed] [Google Scholar]
- 4. Ballard C, Rowan E, Stephens S, Kalaria R, Kenny RA. Prospective follow‐up study between 3 and 15 months after stroke: improvements and decline in cognitive function among dementia‐free stroke survivors >75 years of age. Stroke 2003; 34: 2440–2444. [DOI] [PubMed] [Google Scholar]
- 5. Levine DA, Galecki AT, Langa KM, et al Trajectory of cognitive decline after incident stroke. JAMA 2015; 314: 41–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Brainin M, Tuomilehto J, Heiss W‐D, et al Post‐stroke cognitive decline: an update and perspectives for clinical research. Eur J Neurol 2015; 22: 229–238. [DOI] [PubMed] [Google Scholar]
- 7. Dichgans M, Zietemann V. Prevention of vascular cognitive impairment. Stroke 2012; 43: 3137–3146. [DOI] [PubMed] [Google Scholar]
- 8. Ngandu T, Lehtisalo J, Solomon A, et al A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at‐risk elderly people (FINGER): a randomised controlled trial. Lancet 2015; 385: 2255–2263. [DOI] [PubMed] [Google Scholar]
- 9. Moll van Charante EP, Richard E, Eurelings LS, et al Effectiveness of a 6‐year multidomain vascular care intervention to prevent dementia (preDIVA): a cluster‐randomised controlled trial. Lancet 2016; 388: 797–805. [DOI] [PubMed] [Google Scholar]
- 10. Matz K, Teuschl Y, Firlinger B, et al Multidomain lifestyle interventions for the prevention of cognitive decline after ischemic stroke: randomized trial. Stroke 2015; 46: 2874–2880. [DOI] [PubMed] [Google Scholar]
- 11. Ihle‐Hansen H, Thommessen B, Fagerland MW, et al Multifactorial vascular risk factor intervention to prevent cognitive impairment after stroke and TIA: a 12‐month randomized controlled trial. Int J Stroke 2014; 9: 932–938. [DOI] [PubMed] [Google Scholar]
- 12. Teuschl Y, Matz K, Brainin M. Prevention of post‐stroke cognitive decline: a review focusing on lifestyle interventions. Eur J Neurol 2013; 20: 35–49. [DOI] [PubMed] [Google Scholar]
- 13. Teuschl Y, Matz K, Firlinger B, et al Preventive effects of multiple domain interventions on lifestyle and risk factor changes in stroke survivors: evidence from a two‐year randomized trial. Int J Stroke 2017; 12: 976–984. [DOI] [PubMed] [Google Scholar]
- 14. Luria AR. The Neuropsychology of Memory. New York, NY: John Wiley, 1976. [Google Scholar]
- 15. Shinar D, Gross CR, Price TR, Banko M, Bolduc PL, Robinson RG. Screening for depression in stroke patients: the reliability and validity of the Center for Epidemiologic Studies Depression Scale. Stroke 1986; 17: 241–245. [DOI] [PubMed] [Google Scholar]
- 16. Johnson G, Burvill PW, Anderson CS, Jamrozik K, Stewart‐Wynne EG, Chakera TM. Screening instruments for depression and anxiety following stroke: experience in the Perth community stroke study. Acta Psychiatr Scand 1995; 91: 252–257. [DOI] [PubMed] [Google Scholar]
- 17. Weisscher N, Vermeulen M, Roos YB, de Haan RJ. What should be defined as good outcome in stroke trials; a modified Rankin score of 0‐1 or 0‐2? J Neurol 2008; 255: 867–874. [DOI] [PubMed] [Google Scholar]
- 18. Pendlebury ST, Klaus SP, Thomson RJ, et al Methodological factors in determining risk of dementia after transient ischemic attack and stroke: (III) applicability of cognitive tests. Stroke 2015; 46: 3067–3073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. R Development Core Team . A Language and Environment for Statistical Computing. Vienna, Austria: R Foundation for Statistical Computing; http://cran.r-project.org/ (accessed 11/05/2018). [Google Scholar]
- 20. Tamez E, Myerson J, Morris L, White DA, Baum C, Connor LT. Assessing executive abilities following acute stroke with the trail making test and digit span. Behav Neurol 2011; 24: 177–185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Moore SA, Hallsworth K, Jakovljevic DG, et al Effects of community exercise therapy on metabolic, brain, physical, and cognitive function following stroke: a randomized controlled pilot trial. Neurorehabil Neural Repair 2015; 29: 623–635. [DOI] [PubMed] [Google Scholar]
- 22. Liu‐Ambrose T, Eng JJ. Exercise training and recreational activities to promote executive functions in chronic stroke: a proof‐of‐concept study. J Stroke Cerebrovasc Dis 2015; 24: 130–137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Cheng G, Huang C, Deng H, Wang H. Diabetes as a risk factor for dementia and mild cognitive impairment: a meta‐analysis of longitudinal studies. Intern Med J 2012; 42: 484–491. [DOI] [PubMed] [Google Scholar]
- 24. Luitse MJ, Biessels GJ, Rutten GE, Kappelle LJ. Diabetes, hyperglycaemia, and acute ischaemic stroke. Lancet Neurol 2012; 11: 261–271. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Comparison of study designs.
Table S2. Comparison of the baseline characteristics according to study (n = 322).
Table S3. Comparison of the baseline characteristics of the two randomization groups (n = 322).
Table S4. Baseline characteristics of drop‐outs (n = 28; this does not include deaths and recurrent strokes, which were excluded from the analysis) compared with patients with follow‐up (n = 294).
Table S5. Group differences for the changes in cognitive and secondary outcome variables from baseline to 12 months [complete cases, n = 259 (120 intervention, 139 control)].
Table S6. Baseline variables entering the final stepwise backward multivariable models testing for effects of the intervention on changes in the cognitive variables between baseline and 12 months in the intention‐to‐treat population with imputation of drop‐outs only (Dataset 2, n = 284).
Table S7. Baseline variables entering the final stepwise backward multivariable models testing for effects of the intervention on changes in the cognitive variables between baseline and 12 months in the complete cases (Dataset 1, n = 236).
Table S8. Group differences for the changes in vascular risk factors from baseline to 12 months in the full intention‐to‐treat population [Dataset 3, n = 322 (157 intervention, 165 control)].
Figure S1. Risk of bias assessed according to the Cochrane collaboration criteria.


