Abstract
Objective
To assess effectiveness and tolerability of first‐line and conversion to lacosamide monotherapy for focal seizures.
Materials and Methods
Retrospective, non‐interventional chart review of lacosamide monotherapy patients aged ≥16 years in Europe. Outcomes included retention rate at observational point (OP) 3 (12 ± 3 months), seizure freedom rates at OP2 (6 ± 3 months) and OP3 and adverse drug reactions (ADRs).
Results
A total of 439 patients were included (98 first‐line and 341 conversion to monotherapy; 128 aged ≥65 years [25 first‐line and 103 conversion to monotherapy]). First‐line and conversion to monotherapy retention rates were 60.2% (59/98; 95% confidence interval [CI] 49.8%‐70.0%) and 62.5% (213/341; 57.1%‐67.6%), respectively. Kaplan‐Meier estimates of 12‐month retention rates were 81.2% and 91.4% for first‐line and conversion to monotherapy, respectively. First‐line and conversion to monotherapy retention rates in patients aged ≥65 years were 60.0% (38.7%‐78.9%) and 68.9% (59.1%‐77.7%), respectively. At OP2, 66.3% of first‐line and 63.0% of conversion to monotherapy patients were seizure free. At OP3, 60.2% of first‐line and 52.5% of conversion to monotherapy patients were seizure free. In the ≥65 years subgroup, seizure freedom rates at OP2 were 72.0% and 68.0% for first‐line and converted to monotherapy, respectively, and at OP3, 68.0% and 56.3%, respectively. Overall, 52 of 439 (11.8%) patients reported ADRs (16.4% in ≥65 years subgroup), most commonly dizziness (5.0%), headache (2.1%) and somnolence (1.6%).
Conclusions
Lacosamide was effective and well tolerated as first‐line or conversion to monotherapy in a clinical setting in adult and elderly patients with focal seizures.
Keywords: antiepileptic drug, clinical practice, epilepsy, focal seizures, monotherapy, partial, partial‐onset
1. INTRODUCTION
The aim of epilepsy treatment is to achieve seizure freedom without clinically significant adverse effects.1 The majority of patients with epilepsy attain seizure freedom with a single antiepileptic drug (AED), either as initial monotherapy or as a subsequent monotherapy substitution.2 Theoretically, monotherapy for the treatment of epilepsy has a number of advantages over polytherapy including a reduced likelihood of adverse events (AEs) and drug‐drug interactions, improved compliance and reduced likelihood of pregnancy complications.3, 4
Lacosamide is approved for the treatment of focal (partial‐onset) seizures with or without secondary generalization as monotherapy and adjunctive therapy (≥16 years of age) in the European Union (EU)5 and in the United States (US) (≥17 years of age).6 Its efficacy as monotherapy in patients with focal seizures has been demonstrated in two double‐blind randomized trials; a head‐to‐head non‐inferiority trial of lacosamide vs the continuous‐release (CR) formulation of carbamazepine used for EU approval (SP993)7; and a historical‐controlled, conversion to monotherapy trial (SP902) for US approval.8 In the SP993 non‐inferiority trial in patients with newly diagnosed epilepsy, 73.6% of lacosamide‐treated and 69.7% of carbamazepine‐CR‐treated patients (Full Analysis Set) completed 6 months on last evaluated dose without a seizure.
Randomized controlled trials are considered the gold standard in assessing efficacy, but many of these trials can be restrictive in design with fixed titration schedules, and doses that are not reflective of clinical practice.9 As per trial design, patients with certain clinical characteristics and comorbidities may be excluded, further limiting the applicability of the trial results to a wider population of patients. Observational studies may complement findings from randomized controlled trials by assessing the effectiveness of an intervention in the patients encountered in daily clinical practice. In observational studies, physicians are free to individualize titration and maintenance dosages to optimize efficacy and tolerability.9 Additionally, observational studies often have a longer duration and more diverse patient populations, including patients with comorbidities commonly excluded from randomized controlled trials, which means that they may be more likely to detect rare or late‐onset adverse events (AEs) compared with short‐term, randomized controlled trials.9
Although the long‐term use of lacosamide monotherapy has been assessed in an open‐label extension to SP902 after conversion to monotherapy—where, of the 151 patients who received lacosamide monotherapy for the duration of their participation in the study, 107 had a longest monotherapy duration of ≥24 months—there is limited published clinical practice experience with lacosamide monotherapy. A number of smaller open‐label and retrospective studies in Europe have included patients receiving lacosamide as monotherapy,10, 11, 12, 13, 14 usually after conversion to monotherapy. In a prospective study, 58 patients converted to lacosamide monotherapy and the majority (63.8%) remained on lacosamide monotherapy after 1 year; 55% were seizure free for the treatment period. Giráldez et al11 included 48 patients who converted to lacosamide monotherapy and 18 AED‐naïve patients. One‐year retention rates were not statistically different between groups. In a retrospective study including 199 patients treated with adjunctive lacosamide after 1‐2 prior AEDs, 22 patients withdrew concomitant AEDs and remained on lacosamide monotherapy for ≥6 months.12
Here we report results from a retrospective chart review of patients treated with lacosamide monotherapy at specialized epilepsy centres in Italy, Spain, and the Netherlands. Patients could be either initiated on lacosamide monotherapy (“first‐line monotherapy” subgroup) or converted to lacosamide monotherapy from another AED.
2. METHODS
2.1. Design
This chart review had a retrospective observation period of 12 months (±3 months) with a historical baseline of 6 months prior to Day 1 (day of first administration of lacosamide monotherapy). Observation points (OP) were the first day of lacosamide monotherapy (OP1), and 6 (±3) and 12 (±3) months after initiating treatment (OP2 and OP3). The last documented OP had to take place prior to initiation of the chart review; therefore, data collection did not have any influence on treatment decisions. The choice of treatment was made independently by the physician, according to standard clinical practice.
2.2. Patients
Participating sites reviewed patient charts to identify individuals aged ≥16 years with focal seizures (with or without evolution to bilateral tonic‐clonic seizures; using the latest ILAE seizure classification15) who initiated lacosamide monotherapy (first‐line, second‐line or later) at least 12 months prior to the site initiation and who had at least 6 months’ retrospective follow‐up documentation after the start of lacosamide monotherapy. Two separate groups of patients were assessed in this analysis. First‐line monotherapy patients were required to have experienced at least one seizure during the last 6 months prior OP1 and to have received no more than 2 weeks’ prior AED treatment. Conversion to monotherapy patients (including second‐line or later lacosamide monotherapy) were required to have received other AEDs for longer than 2 weeks before starting lacosamide monotherapy. A minimum seizure frequency was not required for conversion to monotherapy patients, but seizure frequency during 6 months before the start of lacosamide monotherapy was recorded.
Cases were excluded if the patient had received lacosamide for non‐epilepsy indications, had a history of alcoholism or drug abuse, or had inaccurate or unreliable clinical records according to the treating physician (including patients who had not attended the clinic regularly or had not taken medication).
2.3. Outcome measures
The primary outcome was retention on lacosamide monotherapy after at least 12 months of treatment. Secondary outcomes included the proportion of patients who were seizure free at ~6 months (OP2) and at ~12 months (OP3), number of emergency room (ER) visits and number and duration of hospitalizations. Safety and tolerability variables were the reported frequency of adverse drug reactions (ADRs), discontinuation of lacosamide due to ADRs, and serious ADRs. An ADR was defined as any untoward medical occurrence in a patient or clinical investigation subject administered a pharmaceutical product that was temporally associated with the use of that pharmaceutical product and that was assessed by the reporter and/or the sponsor as “related” to the administration of the pharmaceutical product. A serious ADR was defined as an ADR that met one or more of the following criteria: death, life‐threatening, significant or persistent disability/incapacity, congenital anomaly/birth defect (including that occurring in a foetus), important medical event, initial inpatient hospitalization or prolongation of hospitalization.
2.4. Analysis
All analyses were exploratory in nature and missing values were not imputed. Safety variables were analysed using the Safety Set (all patients in the database who had been treated with at least one dose of lacosamide). Effectiveness and efficacy variables were analysed using the Full Analysis Set (FAS; all patients in the Safety Set [SS] who had post‐baseline data). Additional predefined subanalyses were performed for patients who received lacosamide as first‐line monotherapy vs those converting from another AED monotherapy, for those who were aged <65 years vs those aged ≥65 years, and for those patients converting to lacosamide monotherapy who had received ≤3 vs >3 lifetime AEDs before initiating lacosamide monotherapy. All seizure freedom rates were calculated on the FAS population implying that all‐cause discontinuations are considered as not seizure free.
2.4.1. Sample size
Assuming a conservative retention rate of 50% (based on the retention rates observed in a German retrospective study of adjunctive lacosamide (data on file) and an Italian prospective study of conversion to lacosamide monotherapy,10 63% and 63.8%, respectively) and a sample size of 400 patients, two‐sided 95% confidence interval (CI) covered the estimated retention rate with a precision of <5%.
3. RESULTS
A total of 439 patients were included in the chart review and were evaluable for both effectiveness (FAS) and safety (SS) analyses; 98 (22.3%) patients had initiated lacosamide as first‐line monotherapy; 341 (77.7%) had converted to lacosamide monotherapy from their previous AED treatment (Figure 1). The most commonly documented reasons for conversion to lacosamide monotherapy were lack of effectiveness of a prior monotherapy (151/439, 34.4%), intolerance of previous AED combination therapy (104/439, 23.7%), and successful combination therapy with lacosamide (71/439, 16.2%).
Figure 1.
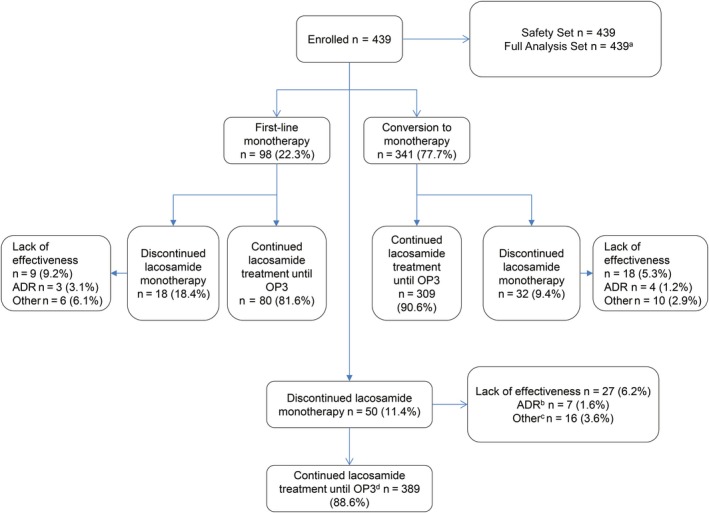
Patient disposition during the retrospective observation period. ADR, adverse drug reaction; OP, observational point. aFull Analysis Set includes seven patients with a daily lacosamide dose >400 mg/d. bSeven patients discontinued lacosamide monotherapy due to ADRs. Six patients stopped lacosamide completely and one patient remained on lacosamide adjunctive therapy. cIncludes lost to follow‐up (n = 11, 2.5%), non‐compliance (n = 3, 0.7%), withdrawal (n = 1, 0.2%) and one patient who did not have any seizures. dOP3: 12 months ± 3 months
Discontinuation of lacosamide monotherapy was documented for 18.4% of the patients receiving lacosamide as first‐line monotherapy. In the conversion to monotherapy patients, 9.4% of the patients discontinued lacosamide therapy. Lack of effectiveness was the most commonly recorded reason for discontinuation for both assessment patient groups.
Baseline demographics were generally comparable between patients who received lacosamide as first‐line therapy and those who converted to lacosamide monotherapy (Table 1). Patients who received lacosamide as first‐line monotherapy had a higher incidence of focal evolving to bilateral tonic‐clonic seizures (54.1% vs 35.5%) compared with patients who converted to monotherapy.
Table 1.
Baseline demographics and characteristicsa
| Variable | First‐line lacosamide monotherapy (n = 98) | Conversion to lacosamide monotherapy (n = 341) |
|---|---|---|
| Age, mean (SD), years | 48.8 (19.0) | 51.1 (18.8) |
| <65 y, n (%) | 73 (74.5) | 238 (69.8) |
| ≥65 y, n (%) | 25 (25.5) | 103 (30.2) |
| Gender | ||
| Male | 47 (48.0) | 163 (47.8) |
| Female | 51 (52.0) | 178 (52.2) |
| Time since diagnosis, median (min, max), years | 0.10 (0.0, 32.7) | 4.80 (0.0, 70.3) |
| Seizure frequency per 28 d, median (min, max) | 0.5 (0, 137) | 0.6 (0, 77) |
| Focal seizures (I, partial‐onset), n (%) | ||
| Aware (IA, simple partial) | 27 (27.6) | 131 (38.4) |
| Impaired awareness (IB, complex partial) | 56 (57.1) | 184 (54.0) |
| Evolving to bilateral tonic‐clonic seizure (IC, partial evolving to secondary generalized) | 53 (54.1) | 121 (35.5) |
| Generalized seizures (II),a n (%) | 0 | 6 (1.8) |
| History of seizure clusters and/or status epilepticus | 3 (3.1) | 17 (5.0) |
| Aetiology not known | 56 (57.1) | 168 (49.3) |
| Idiopathic | 6 (6.1) | 24 (7.0) |
| Cryptogenic | 50 (51.0) | 144 (42.2) |
| Aetiology knownb | 42 (42.9) | 173 (50.7) |
| Cerebrovascular | 18 (18.4) | 46 (13.5) |
| Progressive neurodegenerative | 0 | 36 (10.6) |
| Cranial trauma | 6 (6.1) | 25 (7.3) |
| Cerebral neoplasm | 5 (5.1) | 21 (6.2) |
| Congenital | 6 (6.1) | 13 (3.8) |
| Brain surgery | 4 (4.1) | 14 (4.1) |
| Mesial temporal sclerosis | 2 (2.0) | 16 (4.7) |
| Cerebral infection | 1 (1.0) | 8 (2.3) |
| Perinatal events | 1 (1.0) | 8 (2.3) |
| Otherc | 3 (3.1) | 2 (0.6) |
| Genetic origin | 1 (1.0) | 3 (0.9) |
| Any prior AED, n (%) (taken by >10% of patients in the SS) | 21 (21.4) | 340 (99.7) |
| Levetiracetam | 6 (6.1) | 199 (58.4) |
| Carbamazepine | 2 (2.0) | 102 (29.9) |
| Valproic acid | 4 (4.1) | 80 (23.5) |
| Lamotrigine | 3 (3.1) | 75 (22.0) |
| Oxcarbazepine | 1 (1.0) | 46 (13.5) |
AED, antiepileptic drug; SD, standard deviation; SS, Safety Set.
All patients were also experiencing focal seizures.
Cortical developmental malformations were not predefined and some of these patients may have been included in congenital or other categories.
Other aetiologies were specified in five patients (1.1%) and further described as autoimmune, cortical dysplasia, focal cortical dysplasia, nodular heterotopia and temporal cystic lesion.
The median lacosamide dose in first‐line monotherapy and conversion to monotherapy patients remained stable from baseline to each time point (200 mg/d [min, max: 50, 400] and 300 mg/d [min, max: 50, 600], respectively).
3.1. Retention rate
The retention rates in first‐line monotherapy and conversion to monotherapy patients after at least 12 months’ treatment were 60.2% (95% CI according to method of Clopper and Pearson16: 49.8%, 70.0%) and 62.5% (95% CI: 57.1%, 67.6%), respectively; Table 2. In sensitivity analyses of the primary variable, estimates of the retention rate after 12 months using Kaplan‐Meier methodology17 were 81.2% in first‐line patients and 91.4% in conversion to lacosamide monotherapy patients (Figure 2).
Table 2.
Retention rate at after ≥12 mo of lacosamide monotherapy (OP3) (FAS)
| First‐line monotherapy (n = 98) | Conversion to lacosamide monotherapy | |||
|---|---|---|---|---|
| n = 341 | ≤3 lifetime AEDs (n = 300) | >3 lifetime AEDs (n = 41) | ||
| Patients at OP3, n (%) | 85 (86.7) | 322 (94.4) | 284 (94.7) | 38 (92.7) |
| Patients continuing with lacosamide monotherapy, n (%) | 59 (60.2) | 213 (62.5) | 186 (62.0) | 27 (65.9) |
| Patients with discontinuation of lacosamide monotherapy at OP3, n (%) | 26 (26.5) | 109 (32.0) | 98 (32.7) | 11 (26.8) |
| 95% CI for continuation (Normal approximation) | 50.5; 69.9 | 57.3; 67.6 | 56.5; 67.5 | 51.3; 80.4 |
| 95% CI for continuation (Clopper‐Pearson) | 49.8; 70.0 | 57.1; 67.6 | 56.2; 67.5 | 49.4; 79.9 |
AEDs, antiepileptic drugs; CI, confidence interval; FAS, Full Analysis Set; OP, observational point.
Figure 2.
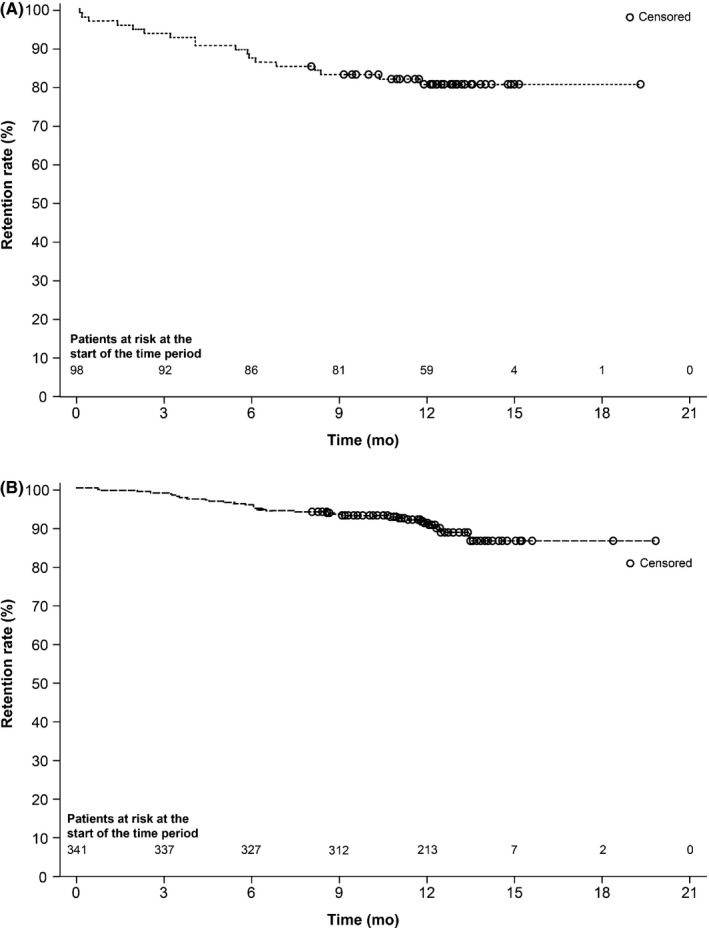
Kaplan‐Meier estimates for time to discontinuation from lacosamide monotherapy in subgroups of patients who (A) received lacosamide as first‐line lacosamide and (B) converted to lacosamide monotherapy
In first‐line monotherapy patients, the retention rate for patients aged ≥65 years and those aged <65 years was 60.0% (95% CI: 38.7%, 78.9%) and 60.3% (95% CI: 48.1%, 71.5%), respectively. Retention rates were higher in conversion to monotherapy patients aged ≥65 years (68.9%, 95% CI: 59.1%, 77.7%) compared with younger patients (59.7%, 95% CI: 53.1%, 66.0%). The retention rates in conversion to monotherapy patients stratified by number of previous AEDs were 62.0% (95% CI: 56.2%, 67.5%) in patients who had previously received ≤3 lifetime AEDs and 65.9% (95% CI: 49.4%, 79.9%) in patients who had previously received >3 lifetime AEDs.
3.2. Seizure freedom
At OP2, 66.3% of first‐line monotherapy patients and 63.0% of conversion to monotherapy patients were seizure free. At OP3, seizure freedom rates were 60.2% and 52.5% for first‐line and conversion to monotherapy patients, respectively (Figure 3). In conversion to monotherapy patients, the seizure freedom rates at OP2 in patients who had previously received ≤3 lifetime AEDs vs >3 lifetime AEDs were 65.7% vs 43.9% (OR 2.47; 95% CI 1.273, 4.781). By OP3, seizure freedom rates in these patients had reduced to 55.3% and 31.7%, respectively (OR 2.73; 95% CI 1.340, 5.554). Seizure freedom rates for first‐line monotherapy patients aged ≥65 years were 72.0% at OP2 and 68.0% at OP3. Corresponding values in first‐line monotherapy patients aged <65 years were 64.4% and 57.5%. In conversion to monotherapy patients aged ≥65 years, seizure freedom rates were 68.0% at OP2 and 56.3% at OP3. Seizure freedom rates at OP2 and OP3 in conversion to monotherapy patients aged <65 years were 60.9% and 50.8%, respectively.
Figure 3.
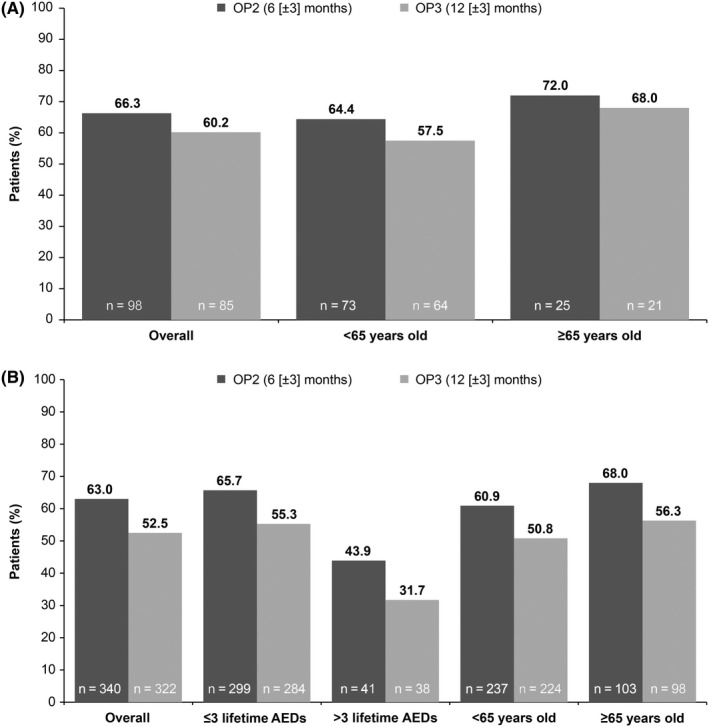
Seizure‐free status at OP2 and OP3 on lacosamide monotherapy in subgroups of (A) first‐line monotherapy and (B) conversion to monotherapy patients
3.3. Emergency room visits and hospitalizations
A total of seven patients (1.6%) visited an ER during the historical baseline period, all due to epilepsy. During the observation period, 11 patients (2.5%) had a documented ER visit while receiving lacosamide monotherapy. Of these, nine patients had one ER visit, one had two visits, and one had nine visits. Epilepsy was the documented reason for ER visits for four patients (0.9%). Duration of hospitalization was usually short both during the historical baseline period and during the study duration (median: 1 day). In patients aged ≥65 years, four patients visited the ER, all for reasons other than epilepsy. Three patients had one visit and one had two visits. Six patients aged <65 years had one ER visit and one patient had more than four ER visits. Four patients visited the ER due to epilepsy and four for reasons other than epilepsy, ADRs or elective procedures.
3.4. Safety and tolerability
ADRs were documented for 52 patients (11.8%) (Table 3), most commonly (≥1% of patients) dizziness (5.0%), headache (2.1%), and somnolence (1.6%). No serious or severe ADRs were documented, and no patients died during the observation period. ADRs of mild intensity were reported by 45 patients (10.3%) with seven (1.6%) patients reporting moderate ADRs. Six patients (1.4%) discontinued lacosamide monotherapy due to eight ADRs: dizziness (n = 2), asthenia, epigastric discomfort, gait disturbance, irritability, somnolence, and vomiting (each n = 1). All but one ADR resolved during the observation period following treatment discontinuation. One additional patient had a lacosamide dose reduction from 350 mg/d due to intermittent mild headache, and concomitant AED medication was administered. The patient recovered and remained on lacosamide as adjunctive therapy.
Table 3.
Incidence of adverse drug reactions (ADRs, SS)
| Overall (n = 439) | Aged <65 y (n = 311) | Aged ≥65 y (n = 128) | |
|---|---|---|---|
| n (%) | n (%) | n (%) | |
| Any ADR | 52 (11.8) | 31 (10.0) | 21 (16.4) |
| Serious ADR | 0 | 0 | 0 |
| Severe ADR | 0 | 0 | 0 |
| Discontinuation due to ADR | 6 (1.4) | 4 (1.3) | 2 (1.6) |
| Deaths | 0 | 0 | 0 |
| Incidence of individual ADRs | |||
| Dizziness | 22 (5.0) | 11 (3.5) | 11 (8.6) |
| Headache | 9 (2.1) | 7 (2.3) | 2 (1.6) |
| Somnolence | 7 (1.6) | 3 (1.0) | 4 (3.1) |
| Abdominal discomfort | 2 (0.5) | 2 (0.6) | 0 |
| Asthenia | 2 (0.5) | 0 | 2 (1.6) |
| Constipation | 2 (0.5) | 2 (0.6) | 0 |
| Gait disturbance | 2 (0.5) | 2 (0.6) | 0 |
| Irritability | 2 (0.5) | 2 (0.6) | 0 |
| Nausea | 2 (0.5) | 0 | 2 (1.6) |
| Anxiety | 1 (0.2) | 1 (0.3) | 0 |
| Depression | 1 (0.2) | 1 (0.3) | 0 |
| Diplopia | 1 (0.2) | 1 (0.3) | 0 |
| Dysuria | 1 (0.2) | 0 | 1 (0.8) |
| Epigastric discomfort | 1 (0.2) | 1 (0.3) | 0 |
| Fatigue | 1 (0.2) | 1 (0.3) | 0 |
| Flatulence | 1 (0.2) | 1 (0.3) | 0 |
| Increased appetite | 1 (0.2) | 1 (0.3) | 0 |
| Insomnia | 1 (0.2) | 1 (0.3) | 0 |
| Mood altered | 1 (0.2) | 0 | 1 (0.8) |
| Paraesthesia | 1 (0.2) | 1 (0.3) | 0 |
| Solar dermatitis | 1 (0.2) | 0 | 1 (0.8) |
| Vertigo | 1 (0.2) | 1 (0.3) | 0 |
| Vomiting | 1 (0.2) | 1 (0.3) | 0 |
ADR, adverse drug reaction; SS, Safety Set.
Of those patients who received lacosamide as first‐line monotherapy, 11 (11.2%) had a documented ADR compared with 41 (12.0%) patients who had converted to lacosamide monotherapy. Three patients in each subset (3.1% and 0.9%, respectively) discontinued lacosamide due to ADRs. In patients aged ≥65 years, 21 (16.4%) had an ADR and two (1.6%) discontinued lacosamide due to ADRs. Of the patients aged <65 years, 31 patients (10.0%) had an ADR and four (1.3%) discontinued treatment due to ADRs.
4. DISCUSSION
This was a retrospective chart review of patients with focal seizures who received lacosamide either as first‐line monotherapy or after converting to lacosamide monotherapy in specialist epilepsy centres.
Retention rates after approximately 12 months of treatment (OP3) were comparable in patients who had converted to lacosamide monotherapy vs those who received first‐line lacosamide monotherapy. However, using Kaplan‐Meier methodology to censor for missing data, the estimated retention rate among patients converting to lacosamide monotherapy was higher than that for first‐line monotherapy patients. Retention rates were higher in conversion to monotherapy patients aged ≥65 years compared with younger patients. There was no difference in retention rates between age groups in first‐line monotherapy patients. Retention rates in the current trial were comparable with those observed in a single‐centre, open‐label study in 58 patients who converted to monotherapy after they had experienced 1‐year seizure freedom with adjunctive lacosamide.10 Moreover, these retention rates are also consistent with the 59.3% retention rate at 1 year described in a study of 59 adults receiving lacosamide in adjunctive use.18 When comparing to Kaplan‐Meier estimates the 1‐year retention rates in our study are higher than those described in another long‐term follow‐up study with lacosamide in adjunctive use (74.5% Kaplan‐Meier estimates).19 However, this may be due to the less severe patient population included into our study.
In general, seizure freedom rates were lower at ~12 months (OP3) than at ~6 months (OP2) but were higher in patients who received lacosamide as first‐line monotherapy compared with those who converted to monotherapy (60.2% and 52.5%, respectively, at ~12 months [OP3] and 66.3% and 63.0% at ~6 months [OP2]). As may be expected, seizure freedom rates were higher in conversion to monotherapy patients who had received ≤3 lifetime AEDs prior to lacosamide monotherapy vs those with >3 lifetime AEDs and who are more treatment‐resistant (55.3% vs 31.7%, respectively, at ~12 months [OP3]). Notably, in this study, seizure freedom rates were calculated using the full FAS not just patients completing treatment. These results are consistent with those from SP993, where 73.6% of newly diagnosed epilepsy patients treated with lacosamide monotherapy completed 6 months’ and 59.5% completed 12 months’ treatment without seizures.7 Smaller studies have also shown comparable seizure freedom rates.10, 11 A retrospective chart review performed at six Spanish centres showed higher, but not significantly different, seizure freedom rates in drug‐naïve patients (n = 18) vs those with prior AED treatment (n = 48) (72.2% vs 60.4%; P = .375).11 A history of treatment with fewer than three lifetime AEDs was predictive of seizure freedom (unadjusted OR 6.30, 95% CI 1.85‐21.48, P = .003; adjusted OR 6.38, 95% CI 1.85‐21.98, P = .003) in a prospective, 1‐year, open‐label study in a clinical practice setting.10 In the current study, in both first‐line and conversion to monotherapy patients a larger proportion of patients aged ≥65 years were seizure free at OP2 (and OP3) compared with younger patients.
This chart review recorded ER visits and hospitalizations, but as the amount of missing values cannot be sufficiently derived from the received retrospective study data, reasonable conclusions are not possible for these variables. It was noted, however, that few patients required hospitalization or visited an ER during the observation period.
Lacosamide was generally well tolerated with a low frequency of ADRs (11.8% in the overall population). The recorded ADRs were consistent with those observed in clinical trials of lacosamide as monotherapy and as adjunctive therapy,7, 8, 20 and the incidence of ADRs was comparable between first‐line monotherapy and conversion to monotherapy patients. Dizziness was the most commonly documented ADR in the overall and elderly population (5.0% and 8.6%, respectively); however, the incidence of dizziness was lower than that observed in a Phase III trial in newly diagnosed epilepsy (12%)7 and considerably lower than in a conversion to monotherapy trial (24.0%) using a fixed titration schedule for lacosamide before the withdrawal of the baseline drugs.8 While the low documented incidence of dizziness may indicate a level of underreporting of less severe AEs in retrospective studies, it may also be possible that the incidence of this ADR is lower in clinical practice due to individualized titration (usually slower than in clinical trials) and dosing. Indeed, cross‐titration and flexible titration have previously been suggested as feasible and practical approaches for dosing lacosamide while minimizing pharmacodynamic interactions with a sodium channel blocking AED.21 The incidence of ADRs in patients aged ≥65 years (16.4%) tended to be higher than that observed in those aged <65 years (10.0%).
Discontinuation of lacosamide due to ADRs was uncommon and occurred more frequently in first‐line patients compared with conversion to monotherapy patients (3.1% vs 0.9%, respectively). There was no difference in discontinuations due to ADRs between patients aged ≥65 years and those aged <65 years (1.6% vs 1.3%, respectively). No serious or severe ADRs (including adverse cardiac affects) or deaths were documented, and no new safety signals were identified.
The lacosamide doses received by first‐line monotherapy patients included in this study (median 200 mg [min, max: 50, 400]) were lower than those used as adjunctive therapy in a similar retrospective study in Spain22 and a prospective observational study in Germany.23 Lacosamide doses used by conversion to monotherapy patients (median 300 mg [min, max: 50, 600]) were comparable to those used in these two studies.
Interpretation of the results of this study is limited by the retrospective design of the study, potential inclusion bias, and the exploratory nature of the analyses. There was also the potential for underreporting of ADRs and possibly also of hospitalizations, ER visits and healthcare provider outpatient consultations. There are also likely to be substantial differences between treatment centres and countries in treatment and patients. Despite these limitations, this study benefitted from patients receiving expert‐based diagnosis, observation and treatment from tertiary epilepsy centres, and also provides valuable information on the safety and effectiveness of lacosamide monotherapy in a real‐world setting. A prospective study in clinical practice would help provide additional information on the clinical utility of lacosamide monotherapy including further monotherapy studies in special populations complementing the existing experience in adjunctive use (eg brain tumour‐related epilepsy).24
5. CONCLUSIONS
The results of this retrospective, non‐interventional chart review indicate that lacosamide monotherapy may be an effective treatment option for focal seizures in patients receiving their first AED monotherapy and for those converting to lacosamide monotherapy from other AEDs.
CONFLICT OF INTEREST
M. De Backer and L. Joeres are employees of UCB Pharma. M. Brunnert and P. Dedeken were employees of UCB Pharma at the time of the study. V. Villanueva has participated in advisory boards and pharmaceutical industry‐sponsored symposia for Eisai, UCB Pharma, Merck Sharp & Dohme, Bial, Pfizer, GSK, Esteve, Novartis, Shire, Medtronic and Cyberonics. M. Toledo has received grants from UCB Pharma, Bial, Eisai and honoraria from UCB Pharma, Bial, Eisai, GSK, Shire and Esteve. G.J. De Haan has participated in advisory boards for Eisai, UCB Pharma and GSK, and was moderator in workshops sponsored by UCB Pharma and GSK. E. Cumbo has participated in advisory boards and pharmaceutical industry‐sponsored symposia for Pfizer, UCB Pharma, Novartis Pharma and Lundbeck. J. Serratosa has received honoraria from UCB Pharma, Esteve, Eisai, Bial and Cyberonics for participating in advisory boards and pharmaceutical industry‐sponsored symposia.
ACKNOWLEDGEMENTS
The authors thank the patients and their caregivers in addition to the clinical project team and the investigators and their teams who contributed to this trial. The authors also acknowledge Suzannah Ryan, PhD (UCB Pharma, Dublin, Ireland) for publication coordination and Jonathon Gibbs, CMPP (Evidence Scientific Solutions, Horsham, UK) for writing support which was funded by UCB Pharma.
Villanueva V, Giráldez BG, Toledo M, et al. Lacosamide monotherapy in clinical practice: A retrospective chart review. Acta Neurol Scand. 2018;138:186–194. 10.1111/ane.12920
Funding information
This study was supported by UCB Pharma.
REFERENCES
- 1. Panayiotopoulos CP. The Epilepsies: Seizures, Syndromes and Management. Oxfordshire, UK: Bladon Medical Publishing; 2005. [PubMed] [Google Scholar]
- 2. Kwan P, Brodie MJ. Epilepsy after the first drug fails: substitution or add‐on? Seizure. 2000;9:464‐468. [DOI] [PubMed] [Google Scholar]
- 3. St Louis EK, Rosenfeld WE, Bramley T. Antiepileptic drug monotherapy: the initial approach in epilepsy management. Curr Neuropharmacol. 2009;7:77‐82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tomson T, Battino D, Bonizzoni E, et al. Antiepileptic drugs and intrauterine death: a prospective observational study from EURAP. Neurology. 2015;85:580‐588. [DOI] [PubMed] [Google Scholar]
- 5. European Medicines Agency . Vimpat (Lacosamide). EMA Summary of Product Characteristics. Brussels, BE: UCB Pharma SA; 2017. [Google Scholar]
- 6. UCB Pharma Inc . Vimpat (Lacosamide) US Prescribing Information. Smyrna, GA: UCB Pharma Inc; 2017. [Google Scholar]
- 7. Baulac MJ, Rosenow F, Toledo M, et al. Efficacy and tolerability of lacosamide monotherapy versus controlled‐release carbamazepine in patients with newly‐diagnosed epilepsy: a phase 3 randomised double‐blind non‐inferiority trial. Lancet Neurol. 2016;16:43‐54. [DOI] [PubMed] [Google Scholar]
- 8. Wechsler RT, Li G, French J, et al. Conversion to lacosamide monotherapy in the treatment of focal epilepsy: results from a historical‐controlled, multicenter, double‐blind study. Epilepsia. 2014;55:1088‐1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Concato J. Observational versus experimental studies: what's the evidence for a hierarchy? NeuroRx. 2004;1:341‐347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Lattanzi S, Cagnetti C, Foschi N, Provinciali L, Silvestrini M. Lacosamide monotherapy for partial onset seizures. Seizure. 2015;27:71‐74. [DOI] [PubMed] [Google Scholar]
- 11. Giráldez BG, Toledano R, Garcia‐Morales I, et al. Long‐term efficacy and safety of lacosamide monotherapy in the treatment of partial‐onset seizures: a multicenter evaluation. Seizure. 2015;29:119‐122. [DOI] [PubMed] [Google Scholar]
- 12. Villanueva V, Garces M, Lopez‐Gomariz E, et al. Early add‐on lacosamide in a real‐life setting: results of the REALLY study. Clin Drug Invest. 2015;35:121‐131. [DOI] [PubMed] [Google Scholar]
- 13. Borzi G, di Gennaro G, Schmitt FC, et al. Lacosamide in patients with temporal lobe epilepsy: an observational multicentric open‐label study. Epilepsy Behav. 2016;58:111‐114. [DOI] [PubMed] [Google Scholar]
- 14. Stephen LJ, Kelly K, Parker P, Brodie MJ. Adjunctive lacosamide–5 years’ clinical experience. Epilepsy Res. 2014;108:1385‐1391. [DOI] [PubMed] [Google Scholar]
- 15. Fisher RS, Cross JH, French JA, et al. Operational classification of seizure types by the International League Against Epilepsy: position paper of the ILAE Commission for Classification and Terminology. Epilepsia. 2017;58:522‐530. [DOI] [PubMed] [Google Scholar]
- 16. Clopper CJ, Pearson ES. The use of confidence or fiducial limits illustrated in the case of the binomial. Biometrika. 1934;26:404‐413. [Google Scholar]
- 17. Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457‐481. [Google Scholar]
- 18. Verrotti A, Loiacono G, Pizzolorusso A, et al. Lacosamide in pediatric and adult patients: comparison of efficacy and safety. Seizure. 2013;22:210‐216. [DOI] [PubMed] [Google Scholar]
- 19. Rosenow F, Kelemen A, Ben‐Menachem E, McShea C, Isojarvi J, Doty P. Long‐term adjunctive lacosamide treatment in patients with partial‐onset seizures. Acta Neurol Scand. 2016;133:136‐144. [DOI] [PubMed] [Google Scholar]
- 20. Biton V, Gil‐Nagel A, Isojarvi J, Doty P, Hebert D, Fountain NB. Safety and tolerability of lacosamide as adjunctive therapy for adults with partial‐onset seizures: analysis of data pooled from three randomized, double‐blind, placebo‐controlled clinical trials. Epilepsy Behav. 2015;52:119‐127. [DOI] [PubMed] [Google Scholar]
- 21. Baulac M, Byrnes W, Williams P, et al. Lacosamide and sodium channel‐blocking antiepileptic drug cross‐titration against levetiracetam background therapy. Acta Neurol Scand. 2017;135:434‐441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Villanueva V, Lopez FJ, Serratosa JM, et al. Control of seizures in different stages of partial epilepsy: LACO‐EXP, a Spanish retrospective study of lacosamide. Epilepsy Behav. 2013;29:349‐356. [DOI] [PubMed] [Google Scholar]
- 23. Runge U, Arnold S, Brandt C, et al. A noninterventional study evaluating the effectiveness and safety of lacosamide added to monotherapy in patients with epilepsy with partial‐onset seizures in daily clinical practice: The VITOBA study. Epilepsia. 2015;56:1921‐1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Toledo M, Molins A, Quintana M, et al. Outcome of cancer‐related seizures in patients treated with lacosamide. Acta Neurol Scand. 2018;137:67‐75. [DOI] [PubMed] [Google Scholar]


