Abstract
Essentials.
Risk stratification for venous thromboembolism (VTE) in patients with brain tumors is challenging.
Patients with IDH1 wildtype and high podoplanin expression have a 6‐month VTE risk of 18.2%.
Patients with IDH1 mutation and no podoplanin expression have a 6‐month VTE risk of 0%.
IDH1 mutation and podoplanin overexpression in primary brain tumors appear to be exclusive.
Summary
Background
Venous thromboembolism (VTE) is a frequent complication in primary brain tumor patients. Independent studies revealed that podoplanin expression in brain tumors is associated with increased VTE risk, whereas the isocitrate dehydrogenase 1 (IDH1) mutation is associated with very low VTE risk.
Objectives
To investigate the interrelation between intratumoral podoplanin expression and IDH1 mutation, and their mutual impact on VTE development.
Patients/Methods
In a prospective cohort study, intratumoral IDH1 R132H mutation and podoplanin were determined in brain tumor specimens (mainly glioma) by immunohistochemistry. The primary endpoint of the study was symptomatic VTE during a 2‐year follow‐up.
Results
All brain tumors that expressed podoplanin to a medium‐high extent showed also an IDH1 wild‐type status. A score based on IDH1 status and podoplanin expression levels allowed prediction of the risk of VTE. Patients with wild‐type IDH1 brain tumors and high podoplanin expression had a significantly increased VTE risk compared with those with mutant IDH1 tumors and no podoplanin expression (6‐month risk 18.2% vs. 0%).
Conclusions
IDH1 mutation and podoplanin overexpression seem to be exclusive. Although brain tumor patients with IDH1 mutation are at very low risk of VTE, the risk of VTE in patients with IDH1 wild‐type tumors is strongly linked to podoplanin expression levels.
Keywords: brain neoplasms, cancer, glioma, thromboembolism, thrombosis
Introduction
Venous thromboembolism (VTE) is a frequent complication in brain tumor patients 1. Recently, novel insights into the pathophysiology of brain tumor‐associated VTE have been generated. We reported in a previous study that the expression of podoplanin by tumor cells led to platelet aggregation and was associated with increased risk of VTE 2. Podoplanin is a sialomucin‐like glycoprotein and has the ability to induce platelet aggregation via interacting with the C‐type lectin‐like receptor (CLEC)‐2 on platelets 3. Recent experimental studies using mouse models confirmed the important role of podoplanin in the pathophysiology of VTE 4, 5, 6. Based on these observations, podoplanin‐induced platelet aggregation might be a major driver of brain tumor‐associated VTE. Moreover, a study by Unruh et al. reported that a subgroup of glioma patients with tumors harboring the isocitrate dehydrogase 1 (IDH1) mutation, which leads to the production of D‐2‐hydroxyglutarate (D‐2‐HG), are at very low risk of VTE 7. The authors suggested that the risk of VTE in IDH1 mutant glioma patients is reduced by the inhibitory effect of D‐2‐HG on platelet aggregation. Interestingly, D‐2‐HG is an oncometabolite that is associated with DNA hypermethylation of several genes, including coagulation‐associated genes such as tissue factor (TF) 7 8.
Currently, risk stratification for VTE in patients with primary brain tumors still remains challenging. However, the interrelation between podoplanin and IDH1 mutation and their combined effect on risk of VTE has not been explored up to date. Here, we evaluated both molecular markers in a clinical prospective cohort study of patients with primary brain tumors and their association with prediction and risk stratification of VTE.
Methods
Study design and population
The study was performed within the framework of the Vienna Cancer and Thrombosis Study (CATS), a prospective observational cohort study. The aim of CATS is to identify risk factors for VTE in cancer patients. The detailed study design and methods have been published previously 9, 10. Briefly, patients with newly diagnosed cancer or progressive disease after cancer remission are enrolled and followed for a period of 2 years. The primary endpoint of CATS is defined as occurrence of symptomatic and objectively diagnosed VTE. The secondary endpoint is defined as death from any cause.
Immunohistochemistry
IDH1 R132H mutation in formalin‐fixed and paraffin‐embedded (FFPE) brain tumor samples was assessed by immunohistochemistry (IHC) via the monoclonal anti‐IDH1 R132H antibody (Dianova, Hamburg, Germany). Data on intratumoral podoplanin were available from our previous study 2. The intensity of podoplanin was semi‐quantitatively classified into four degrees as follows: negative (−); low expression (+), 50% of tumor cells express podoplanin and/or mild staining intensity; moderate expression (++), 50% to 70% of tumor cells express podoplanin at a moderate to strong intensity; and high expression (+++), 70% of tumor cells express podoplanin at a strong intensity level.
Statistical and TCGA analysis
All statistical analyses were performed using Stata 14.0 (Stata Corp., Houston, TX, USA). Continuous variables are summarized as medians (25th–75th percentile) and categorical variables are reported as absolute counts (%). The association between variables was assessed with rank‐sum tests and χ2 tests, and the correlation between continuous variables using Spearman's rank‐based correlation coefficient. The cumulative incidence of VTE was estimated with competing risk cumulative incidence estimators, treating death from any cause other than fatal VTE as the competing event of interest. VTE incidences between groups were compared with log‐rank tests. Cause‐specific hazards of VTE in relation to predictor variables were modelled with univariable and multivariable Cox proportional hazards models, yielding hazard ratios (HRs) with 95% confidence intervals. Two datasets with available IDH1 (R132H) mutation data (from whole exome sequencing), podoplanin methylation levels (from HM450 array, expressed as beta‐values) and mRNA expression levels of podoplanin (from RNA‐Seq V2, expressed on a log2‐scale) from patients with lower‐grade glioma (LGG ‘TCGA, provisional’, n = 280) and glioblastoma (GBM ‘TCGA, provisional’, n = 135) were extracted from The Cancer Genome Atlas (TCGA) 11.
Results and discussion
Baseline analysis
In total, 213 patients with primary brain tumors, mostly glioma, were included (Table 1). The exact tumor subtypes have been reported in our previous publication 2. Patients with podoplanin‐positive tumors (IHC score: +, ++, and +++; n = 151, 71%) were older, had a higher probability of having glioblastoma, and had lower platelet counts and higher D‐dimer (all P ≤ 0.001). An IDH1 mutation was found in 42 tumor specimens (20%). On average, patients with IDH1 mutant tumors were younger, suffered from diffuse or anaplastic glioma or from secondary glioblastoma, and had higher platelet counts and lower D‐dimer.
Table 1.
Baseline characteristics including podoplanin expression, IDH1 mutation status and peripheral blood parameters in our patient cohort
| Demographics | Patients studied n (% miss.) | Podoplanin +, ++ and +++ (n = 151) | Podoplanin – (n = 62) | P‐value | IDH1 mutant (n = 42) | IDH1 wild‐type (n = 171) | P‐value | |
|---|---|---|---|---|---|---|---|---|
| Median age at study entry | 213 (0%) | 55 [44–66] | 60 [48–68] | 46 [37–56] | <0.0001 | 42 [36–50] | 60 [48–67] | <0.0001 |
| Female sex | 213 (0%) | 79 (37%) | 54 (36%) | 25 (40%) | 0.53 | 17 (40%) | 62 (36%) | 0.61 |
| Body mass index (BMI, kg/m²) | 213 (0%) | 25 [23–28] | 26 [23–28] | 25 [23–27] | 0.53 | 24 [23–27] | 26 [23–28] | 0.25 |
| Newly‐diagnosed malignancy | 213 (0%) | 185 (87%) | 136 (90%) | 49 (79%) | 0.03 | 35 (83%) | 150 (88%) | 0.45 |
| Brain tumor types | 213 (0%) | / | / | / | <0.0001 | / | / | <0.0001 |
| Glioblastoma/sarcoma (WHO IV) | / | 152 (71%) | 124 (82%) | 28 (18%) | / | 18 (12%) | 134 (88%) | / |
| Anaplastic glioma (WHO III) | / | 38 (18%) | 14 (37%) | 24 (63%) | / | 18 (47%) | 20 (53%) | / |
| Low‐grade glioma (WHO II) | / | 10 (5%) | 3 (30%) | 7 (70%) | / | 4 (40%) | 6 (60%) | / |
| Other | 13 (6%) | 10 (77%) | 3 (23%) | / | 2 (15%) | 11 (85%) | / | |
| Laboratory parameters | ||||||||
| Platelet count (G L−1) | 213 (0%) | 240 [194–311] | 227 [186–285] | 286 [241–355] | <0.0001 | 297 [244–338] | 232 [190–289] | 0.0004 |
| D‐dimer μg mL−1 | 190 (11%) | 0.7 [0.3–1.4] | 0.8 [0.5–1.9] | 0.4 [0.2–0.8] | <0.0001 | 0.4 [0.3–0.8] | 0.7 [0.4–1.7] | 0.008 |
| Soluble P‐selectin, ng mL−1 | 197 (8%) | 37 [29–49] | 37 [29–48] | 39 [29–51] | 0.51 | 37 [29–49] | 38 [29–50] | 0.91 |
| Peak thrombin generation, nm | 212 (0%) | 347 [184–543] | 374 [198–552] | 284 [167–493] | 0.12 | 280 [163–490] | 363 [190–551] | 0.23 |
| Serum calcium (total), mmol L−1 | 124 (42%) | 2.37 [2.27–2.46] | 2.36 [2.25–2.44] | 2.37 [2.31–2.50] | 0.05 | 2.40 [2.31–2.50] | 2.36 [2.26–2.44] | 0.09 |
| Serum calcium (albumin corrected), mmol L−1 | 124 (42%) | 2.31 [2.27–2.38] | 2.32 [2.27–2.39] | 2.30 [2.23–2.36] | 0.15 | 2.28 [2.22–2.37] | 2.32 [2.27–2.39] | 0.06 |
Median levels [25th–75th percentile] are given for continuous data and absolute frequencies (%) for count data. P‐values for the comparison between podoplanin‐positive and podoplanin‐negative tumors, as well as between IDH1 wild‐type and IDH1 mutation, are reported.
IDH1 mutation and podoplanin are inversely correlated in primary brain tumors
Immunohistochemical analysis revealed a strong inverse association between podoplanin expression levels and IDH1 mutation (P < 0.0001), with 34 (55%) of the 62 podoplanin‐negative tumors, but only eight (5%) of the 151 podoplanin‐positive tumors, having an IDH1 mutation (Fig. 1). To externally validate these findings, data from the TCGA were analyzed. Here, mean podoplanin mRNA levels were 6‐fold lower in the 194 LGG patients with an IDH1 mutation than in the 86 LGG patients with an IDH1 wild‐type tumor (log2‐ratios: 7.1 vs. 9.6, P < 0.0001), and 8‐fold lower in the six GBM patients with an IDH1 mutation than in the 129 GBM patients with an IDH1 wild‐type tumor (log2‐ratios: 9.1 vs. 12.1, P = 0.0002), respectively. These results are also in concordance with previous findings by Birner et al., suggesting an inverse relationship of IDH1 mutation with podoplanin expression in glioma patients 12.
Figure 1.
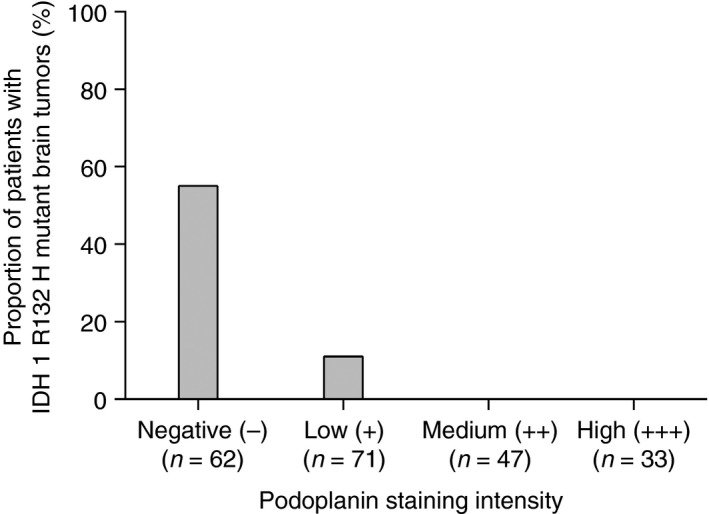
Proportion of primary brain tumors with IDH1 mutation and different levels of podoplanin expression. IDH1 R132H mutation is only found in tumors with no or low podoplanin expression.
To explore whether decreased podoplanin expression in IDH1 mutant tumors may be a result of the impact of mutant IDH1‐dependent genome hypermethylation, we further compared podoplanin methylation between IDH1 mutant and wild‐type tumors in the TCGA subset with available HM450 methylation data (n = 321). Indeed, podoplanin methylation was strongly increased in the IDH1 mutant compared with IDH1 wild‐type tumors, and this applied to both the LGG cohort (median beta‐value for IDH1 mutant vs. wild‐type: 0.48 vs. 0.05, P < 0.0001) and the GBM cohort (0.52 vs. 0.03, P = 0.03). However, higher podoplanin methylation resulted in lower podoplanin mRNA expression only in LGG patients (Spearman's rho = −0.64, P < 0.0001) but not GBM patients (rho = −0.15, P = 0.35). Interestingly, Noushmehr et al. demonstrated that IDH1 mutation is tightly associated with a glioma‐CpG island methylator phenotype (G‐CIMP), and podoplanin is one of the top‐ranked hypermethylated genes in G‐CIMP‐positive tumors 13.
Previously, it was reported that intravascular thrombosis occurs more frequently in glioblastoma than in other brain tumors, pointing to an intrinsic coagulant phenotype 14. However, four molecular subgroups in glioblastoma have been recently described that also differ in expression levels of coagulation‐associated genes (e.g. TF) that might be regulated by oncogenes 15, 16, 17. The so‐called ‘proneural’ subtype is highly associated with IDH1 mutation, platelet‐derived growth factor receptor A (PDGFRA) alterations and very low TF expression 15, 16. In contrast, the ‘mesenchymal’ subtype is linked to higher TF and podoplanin expression as well as to neurofibromatosis type 1 (NF1) mutation and loss of the phosphatase and tensin homolog (PTEN) 15, 16, 18. Of note, PTEN is an inhibitor of the phosphatidylinositol 3‐kinase (PI3K)/Akt signaling pathway, which is also involved in the upregulation of TF and podoplanin 19, 20, 21. However, in previous studies, we demonstrated that podoplanin expression was associated with occurrence of VTE in patients with primary brain tumors and no association between TF expression and VTE risk could be found 2, 22.
IDH1 mutation and VTE risk in primary brain tumor patients
During a median follow‐up time of 731 days (range, 3–731 days), VTE occurred in 29 (14%) brain tumor patients. The cumulative 6‐, 12‐ and 24‐month risks of VTE were 10.1%, 13.3% and 14.8%, respectively. The presence of an IDH1 mutation was associated with a lower risk of VTE. Only one out of 42 brain tumor patients with an IDH1 mutation developed VTE during follow‐up in our study, and this patient had several VTE events in the medical history (already years before cancer diagnosis), suggesting that in this patient other thrombotic risk factors existed independently from the brain tumor. The cumulative 6‐, 12‐ and 24‐month VTE risks were 13.0%, 15.4% and 17.0% in patients with IDH1 wild‐type tumors, and 0%, 2.4% and 2.4% in patients with IDH1 mutant tumors, respectively (log‐rank P = 0.008). In univariable time‐to‐VTE regression, the IDH1 mutation predicted a lower risk of VTE (hazard ratio [HR] = 0.11; 95% CI, 0.01–0.80; P = 0.029), and this prevailed after multivariable adjustment for glioblastoma and age (Models #1 and #2, Table 2). Thus, these clinical data externally validate previous findings demonstrating that mutant IDH1 is a predictor of low VTE risk in patients with primary brain tumors 7. Based on a mouse model, Unruh et al. proposed that IDH1 mutation leads to decreased serum calcium levels with subsequent inhibition of platelet activation, thereby preventing VTE 7. However, we were not able to confirm a statistically significant association between IDH1 status and serum calcium concentrations in primary brain tumor patients (Table 1).
Table 2.
Univariable and multivariable hazard ratios (HRs) for venous thromboembolism (VTE) and death in primary brain tumors patients based on IDH1 mutation and podoplanin
| Outcome | Model | Variable | HR | 95% CI | P |
|---|---|---|---|---|---|
| VTE | Model #1 | IDH1 R132H mutation (n = 42) | 0.11 | 0.01–0.80 | 0.029 |
| Model #2 | IDH1 R132H mutation (n = 42) | 0.11 | 0.01–0.83 | 0.032 | |
| Glioblastoma (n = 152) | 2.31 | 0.79–6.76 | 0.127 | ||
| Age (per 10 years increase) | 0.83 | 0.62–1.12 | 0.222 | ||
| Model #3* | 0 points (n = 34) | Ref. | Ref. | Ref. | |
| 1 point (n = 36) | 3.13 | 0.33–30.10 | 0.323 | ||
| 2 points (n = 63) | 6.65 | 0.84–52.64 | 0.073 | ||
| 3 points (n = 47) | 8.40 | 1.04–67.58 | 0.045 | ||
| 4 points (n = 33) | 13.28 | 1.65–106.97 | 0.015 | ||
| Death | Model #4 | IDH1 R132H mutation (n = 42) | 0.19 | 0.09–0.39 | <0.0001 |
| Model #5 | IDH1 R132H mutation (n = 42) | 0.30 | 0.14–0.64 | 0.002 | |
| Glioblastoma (n = 152) | 2.07 | 1.18–3.63 | 0.011 | ||
| Age (per 10 years increase) | 1.26 | 1.08–1.48 | 0.004 | ||
| Model #6* | 0 points (n = 34) | Ref. | Ref. | Ref. | |
| 1 point (n = 36) | 1.93 | 0.76–4.90 | 0.168 | ||
| 2 points (n = 63) | 4.89 | 2.18–10.96 | <0.0001 | ||
| 3 points (n = 47) | 7.21 | 3.17–16.40 | <0.0001 | ||
| 4 points (n = 33) | 6.79 | 2.89–15.97 | <0.0001 |
CI, confidence interval. *The score is based on the sum of IDH1 status (mutant = 0, wild‐type = 1) and podoplanin expression levels (− = 0, + = 1, ++ = 2 and +++ = 3).
IDH1 and podoplanin are joint prognostic markers for brain tumor‐associated VTE
To quantify a potential additive contribution of IDH1 and podoplanin towards the risk of VTE, we evaluated the sum of IDH1 status (mutant = 0, wild‐type = 1) and podoplanin staining intensity (− = 0, + = 1, ++ = 2 and +++ = 3) as a putative predictor of brain tumor‐associated VTE. The 6‐month risk of VTE was 0%, 5.6%, 9.6%, 17.2% and 18.2% in patients with 0 (n = 34), 1 (n = 36), 2 (n = 63), 3 (n = 47) and 4 (n = 33) points of this joint IDH1 and podoplanin sum, respectively (log‐rank P = 0.02, Fig. 2). Brain tumors with both wild‐type IDH1 and high podoplanin expression were associated with increased risk of VTE compared to those with mutant IDH1 and no podoplanin expression (follow‐up time of 2 years: HR = 13.28, 95% CI 1.65–106.97, P = 0.015) (Table 2). Based on our results, the risk of developing VTE in patients with wild‐type IDH1 is strongly linked to intratumoral podoplanin expression levels. Thus, the combination of IDH1 mutation and podoplanin expression might be a useful immunohistochemical marker for VTE risk assessment in patients with primary brain tumors. In addition, IDH1 and podoplanin are also joint prognostic markers for overall survival in patients with primary brain tumors (Table 2).
Figure 2.
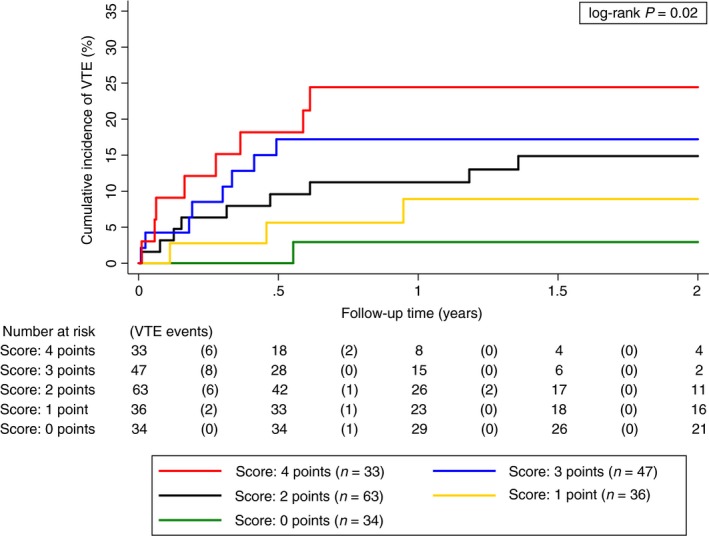
Cumulative incidence of venous thromboembolism accounting for competing mortality according to the IDH1 status and podoplanin expression in primary brain tumor patients. To predict the risk of brain cancer‐associated VTE, a score was established based on the sum of the IDH1 status (mut = 0, wt = 1) and podoplanin expression levels (no = 0, low = 1, medium = 2, high = 3). Brain tumor patients with the combination of IDH1wt and high podoplanin expression were at highest risk of VTE, whereas patients with IDH1 R132H mutation combined with no podoplanin expression showed the lowest risk of developing VTE during the follow‐up time. As IDH1 mutation and podoplanin overexpression (medium, high) appear to be exclusive, only the score of 1 included a heterogenous subgroup of tumors. All other scores (0, 2–4) included homogenous subgroups regarding IDH1 status and podoplanin expression levels: score 0 = IDH1mut with no podoplanin; score 1 = IDH1mut with low podoplanin and IDH1wt with no podoplanin; score 2 = IDH1wt with low podoplanin; score 3 = IDH1wt with medium podoplanin; score 4 = IDH1wt with high podoplanin (mut, mutant; wt, wild‐type). [Colour figure can be viewed at http://wileyonlinelibrary.com]
Mechanistically, we suggest that podoplanin induces platelet aggregation via the platelet receptor CLEC‐2, which might subsequently trigger systemic thrombosis. Recent studies have shown that thrombosis is induced by podoplanin upregulation and its interaction with CLEC‐2: Shirai et al. demonstrated that CLEC‐2‐deficiency in mice was protective against thrombosis and hematogenous metastasis. Thrombus formation in tumor vessels was significantly inhibited in CLEC‐2‐depleted mice bearing podoplanin‐expressing B16F10 melanoma cells 4. Payne et al. again revealed that mice deficient in CLEC‐2 were protected against deep vein thrombosis (DVT) 6. Furthermore, they demonstrated that podoplanin was highly expressed in the inferior vena cava wall of wild‐type mice that developed thrombosis and inhibition of podoplanin reduced the size of the thrombus.
Our study has some limitations. Intratumoral podoplanin levels were determined only semi‐quantitatively. In practice, this might lead to subjective and laboratory‐specific variability. To address this issue, all tumor sections were reviewed by two experienced neuropathologists and no disagreement was observed. Moreover, IDH1 mutation was assessed immunohistochemically rather than on a genetic level in our clinical study. Finally, we were not able to provide in vivo evidence confirming the mechanistic pathways leading to thrombosis. However, we extracted mRNA and methylation data from a public database (TCGA) to look closer at the interrelation between the IDH1 mutation status and podoplanin. IDH1 mutation was associated with podoplanin hypermethylation, presumably via D‐2‐HG, and inversely linked to podoplanin overexpression. As discussed, we assume that in IDH1 wild‐type tumors the amount of podoplanin upregulation might depend on PTEN loss and PI3K/Akt signaling 15, 18, 19.
In summary, primary brain tumors, which harbor the IDH1 mutation, are associated with a very low risk of VTE, whereas a high risk of VTE is observed in patients with IDH1 wild‐type tumors. IDH1 wild‐type was associated with upregulation of podoplanin, and increasing podoplanin expression levels were associated with a gradual increase in the risk of VTE. We concluded that measurement of IDH1 mutation and podoplanin allows differentiating between subgroups of brain tumors that show distinct VTE risk profiles. Whether primary thromboprophylaxis in high‐risk patients based on IHC staining for IDH1 mutation and podoplanin expression is able to reduce the risk of VTE needs to be investigated in clinical trials. Our proposed combination marker panel of IDH1 mutation and podoplanin could be used to enrich these trials with patients who have the highest risk of VTE and might benefit most from primary thromboprophylaxis compared with the risk of intracerebral bleeding.
Addendum
P. M. S. Nazari, J. Riedl, I. Pabinger and C. Ay designed the study; P. M. S. Nazari, J. Riedl, M. Preusser, P. Birner, G. Ricken and J. A. Hainfellner designed and performed the experiments; J. Riedl, M. Preusser, C. Marosi, J. Thaler and C. Ay recruited patients; P. M. S. Nazari, J. Riedl, C. Ay and F. Posch performed statistical analyses; and P. M. S. Nazari, J. Riedl and C. Ay wrote the article, which was reviewed and edited by all the other authors.
Disclosure of Conflict of Interests
The authors state that they have no conflict of interest.
Acknowledgements
We would like to thank the Austrian Science Fund (FWF), Special Research Program (SFB) F 5405‐B21 and the Austrian National Bank (Anniversary Fund, project 14744) for funding this project.
Mir Seyed Nazari P, Riedl J, Preusser M, Posch F, Thaler J, Marosi C, Birner P, Ricken G, Hainfellner JA, Pabinger I, Ay C. Combination of isocitrate dehydrogenase 1 (IDH1) mutation and podoplanin expression in brain tumors identifies patients at high or low risk of venous thromboembolism. J Thromb Haemost 2018; 16: 1121–1127.
Manuscript handled by: M. Carrier
Final decision: F. R. Rosendaal, 30 March 2018
References
- 1. Semrad TJ, O'Donnell R, Wun T, Chew H, Harvey D, Zhou H, White RH. Epidemiology of venous thromboembolism in 9489 patients with malignant glioma. J Neurosurg 2007; 106: 1121–1127. [DOI] [PubMed] [Google Scholar]
- 2. Riedl J, Preusser M, Nazari PMS, Posch F, Panzer S, Marosi C, Birner P, Thaler J, Brostjan C, Lötsch D, Berger W, Hainfellner JA, Pabinger I, Ay C. Podoplanin expression in primary brain tumors induces platelet aggregation and increases risk of venous thromboembolism. Blood 2017; 129: 1831–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Suzuki‐Inoue K, Fuller GLJ, García A, Eble JA, Pöhlmann S, Inoue O, Gartner TK, Hughan SC, Pearce AC, Laing GD, Theakston RDG, Schweighoffer E, Zitzmann N, Morita T, Tybulewicz VLJ, Ozaki Y, Watson SP. A novel Syk‐dependent mechanism of platelet activation by the C‐type lectin receptor CLEC‐2. Blood 2006; 107: 542–9. [DOI] [PubMed] [Google Scholar]
- 4. Shirai T, Inoue O, Tamura S, Tsukiji N, Sasaki T, Endo H, Satoh K, Osada M, Sato‐Uchida H, Fujii H, Ozaki Y, Suzuki‐Inoue K. C‐type lectin‐like receptor 2 promotes hematogenous tumor metastasis and prothrombotic state in tumor‐bearing mice. J Thromb Haemost JTH 2017; 15: 513–25. [DOI] [PubMed] [Google Scholar]
- 5. Hitchcock JR, Cook CN, Bobat S, Ross EA, Flores‐Langarica A, Lowe KL, Khan M, Dominguez‐Medina CC, Lax S, Carvalho‐Gaspar M, Hubscher S, Rainger GE, Cobbold M, Buckley CD, Mitchell TJ, Mitchell A, Jones ND, Van Rooijen N, Kirchhofer D, Henderson IR, et al Inflammation drives thrombosis after Salmonella infection via CLEC‐2 on platelets. J Clin Invest 2015; 125: 4429–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Payne H, Ponomaryov T, Watson SP, Brill A. Mice with a deficiency in CLEC‐2 are protected against deep vein thrombosis. Blood 2017; 129: 2013–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Unruh D, Schwarze SR, Khoury L, Thomas C, Wu M, Chen L, Chen R, Liu Y, Schwartz MA, Amidei C, Kumthekar P, Benjamin CG, Song K, Dawson C, Rispoli JM, Fatterpekar G, Golfinos JG, Kondziolka D, Karajannis M, Pacione D, et al Mutant IDH1 and thrombosis in gliomas. Acta Neuropathol (Berl) 2016; 132: 917–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Xu W, Yang H, Liu Y, Yang Y, Wang P, Kim S‐H, Ito S, Yang C, Wang P, Xiao M‐T, Liu L, Jiang W, Liu J, Zhang J, Wang B, Frye S, Zhang Y, Xu Y, Lei Q, Guan K‐L, et al Oncometabolite 2‐hydroxyglutarate is a competitive inhibitor of α‐ketoglutarate‐dependent dioxygenases. Cancer Cell 2011; 19: 17–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ay C, Simanek R, Vormittag R, Dunkler D, Alguel G, Koder S, Kornek G, Marosi C, Wagner O, Zielinski C, Pabinger I. High plasma levels of soluble P‐selectin are predictive of venous thromboembolism in cancer patients: results from the Vienna Cancer and Thrombosis Study (CATS). Blood 2008; 112: 2703–8. [DOI] [PubMed] [Google Scholar]
- 10. Thaler J, Ay C, Kaider A, Reitter E‐M, Haselböck J, Mannhalter C, Zielinski C, Marosi C, Pabinger I. Biomarkers predictive of venous thromboembolism in patients with newly diagnosed high‐grade gliomas. Neuro‐Oncol 2014; 16: 1645–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Gao J, Aksoy BA, Dogrusoz U, Dresdner G, Gross B, Sumer SO, Sun Y, Jacobsen A, Sinha R, Larsson E, Cerami E, Sander C, Schultz N. Integrative analysis of complex cancer genomics and clinical profiles using the cBioPortal. Sci Signal 2013; 6: pl1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Birner P, Pusch S, Christov C, Mihaylova S, Toumangelova‐Uzeir K, Natchev S, Schoppmann SF, Tchorbanov A, Streubel B, Tuettenberg J, Guentchev M. Mutant IDH1 inhibits PI3K/Akt signaling in human glioma. Cancer 2014; 120: 2440–7. [DOI] [PubMed] [Google Scholar]
- 13. Noushmehr H, Weisenberger DJ, Diefes K, Phillips HS, Pujara K, Berman BP, Pan F, Pelloski CE, Sulman EP, Bhat KP, Verhaak RGW, Hoadley KA, Hayes DN, Perou CM, Schmidt HK, Ding L, Wilson RK, Van Den Berg D, Shen H, Bengtsson H, et al Identification of a CpG island methylator phenotype that defines a distinct subgroup of glioma. Cancer Cell 2010; 17: 510–22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tehrani M, Friedman TM, Olson JJ, Brat DJ. Intravascular thrombosis in central nervous system malignancies: a potential role in astrocytoma progression to glioblastoma. Brain Pathol Zurich Switz 2008; 18: 164–71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Verhaak RGW, Hoadley KA, Purdom E, Wang V, Qi Y, Wilkerson MD, Miller CR, Ding L, Golub T, Mesirov JP, Alexe G, Lawrence M, O'Kelly M, Tamayo P, Weir BA, Gabriel S, Winckler W, Gupta S, Jakkula L, Feiler HS, et al Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010; 17: 98–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Magnus N, Gerges N, Jabado N, Rak J. Coagulation‐related gene expression profile in glioblastoma is defined by molecular disease subtype. J Thromb Haemost JTH 2013; 11: 1197–200. [DOI] [PubMed] [Google Scholar]
- 17. Magnus N, Garnier D, Rak J. Oncogenic epidermal growth factor receptor up‐regulates multiple elements of the tissue factor signaling pathway in human glioma cells. Blood 2010; 116: 815–8. [DOI] [PubMed] [Google Scholar]
- 18. Shiina S, Ohno M, Ohka F, Kuramitsu S, Yamamichi A, Kato A, Motomura K, Tanahashi K, Yamamoto T, Watanabe R, Ito I, Senga T, Hamaguchi M, Wakabayashi T, Kaneko MK, Kato Y, Chandramohan V, Bigner DD, Natsume A. CAR T cells targeting podoplanin reduce orthotopic glioblastomas in mouse brains. Cancer Immunol Res 2016; 4: 259–68. [DOI] [PubMed] [Google Scholar]
- 19. Peterziel H, Müller J, Danner A, Barbus S, Liu H‐K, Radlwimmer B, Pietsch T, Lichter P, Schütz G, Hess J, Angel P. Expression of podoplanin in human astrocytic brain tumors is controlled by the PI3K‐AKT‐AP‐1 signaling pathway and promoter methylation. Neuro‐Oncol 2012; 14: 426–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Rong Y, Post DE, Pieper RO, Durden DL, Van Meir EG, Brat DJ. PTEN and hypoxia regulate tissue factor expression and plasma coagulation by glioblastoma. Cancer Res 2005; 65: 1406–13. [DOI] [PubMed] [Google Scholar]
- 21. Rong Y, Belozerov VE, Tucker‐Burden C, Chen G, Durden DL, Olson JJ, Van Meir EG, Mackman N, Brat DJ. Epidermal growth factor receptor and PTEN modulate tissue factor expression in glioblastoma through JunD/activator protein‐1 transcriptional activity. Cancer Res 2009; 69: 2540–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Thaler J, Preusser M, Ay C, Kaider A, Marosi C, Zielinski C, Pabinger I, Hainfellner JA. Intratumoral tissue factor expression and risk of venous thromboembolism in brain tumor patients. Thromb Res 2013; 131: 162–5. [DOI] [PubMed] [Google Scholar]


