Abstract
Objective
To volumetrically compare peri‐implant mid‐facial soft tissue changes in immediately placed and provisionalized implants in the aesthetic zone, with or without a connective tissue graft.
Material and methods
Sixty patients were included. All implants were placed immediately after extraction. After randomization, in one group, a connective tissue graft (test group, n = 30) was inserted at the buccal aspect of the implant. The other group (control group, n = 30) received no connective tissue graft. Clinical parameters, digital photographs and conventional impressions were obtained before extraction (T pre) and at 12 months following definitive crown placement (T 12). The casts were digitized by a laboratory scanner, and a volumetric analysis was performed between T pre and T 12.
Results
Twenty‐five patients in each group were available for analysis at T 12. Volumetric change, transformed to a mean (±SD) change in thickness, was −0.68 ± 0.59 mm (test) and −0.49 ± 0.54 mm (control) with a non‐significant difference between groups (p = .189). The mid‐facial mucosa level was significantly different between both groups (p = .014), with a mean (±SD) change of +0.20 ± 0.70 mm (test) and −0.48 ± 1.13 mm (control). The Pink Esthetic Score was similar between both groups.
Conclusions
The use of a CTG in immediately placed and provisionalized implants in the aesthetic zone did not result in less mucosal volume loss after 12 months, leading to the assumption that a CTG cannot fully compensate for the underlying facial bone loss, although a significantly more coronally located mid‐facial mucosa level was found when a CTG was performed.
Keywords: aesthetic zone, immediate placement, soft tissue graft, volumetric changes
1. INTRODUCTION
Different clinical protocols exist to replace a failing tooth in the aesthetic zone by implant therapy (Hämmerle, Chen, & Wilson, 2004). In type 1, implants may be placed immediately after extraction of the failing tooth and be provisionalized within 24 hr. Apart from a reduced treatment time, immediate implant placement and provisionalization (IIPP) is considered a predictable treatment option in terms of survival (Slagter et al., 2014). However, recent systematic reviews have shown that immediate implant placement bears a significant risk for mid‐facial mucosal recession as a result of resorption of the facial bone wall (Chen & Buser, 2009, 2014). Furthermore, clinical studies showed that in 11% of low‐risk IIPP cases, advanced (≥1 mm) mid‐facial mucosal recession is taking place (Khzam et al., 2015). Moreover, this mucosal recession seems to continue for a long period, up to 5 years after implant placement (Cosyn, De Bruyn, & Cleymaet, 2013; Cosyn et al., 2016).
To reduce mid‐facial mucosa recession and volume loss of peri‐implant tissues, it has been proposed to use a connective tissue graft (Migliorati, Amorfini, Signori, Biavati, & Benedicenti, 2015). The connective tissue graft can either be harvested from the palate or the tuberosity region and is placed submucosally at the buccal aspect of the implant. Two randomized clinical studies concluded that placement of a connective tissue graft leads to less vertical loss of the mid‐facial mucosa level after 1 year, resulting in more stable peri‐implant mucosa levels (Migliorati et al., 2015; Yoshino, Kan, Rungcharassaeng, Roe, & Lozada, 2014). However, these studies showed limitations regarding the small number of patients (Yoshino et al., 2014) and possible selection bias (Migliorati et al., 2015). Other studies reporting on connective tissue grafts and immediately placed and provisionalized implants mostly consist of case series and have shown inconclusive results (Lee, Tao, & Stoupel, 2016).
Until recent, studies have focused mainly on stability of mid‐facial mucosa levels as a parameter for aesthetic success. However, the introduction of volumetric analysis (Windisch et al., 2007) enables us to objectively and volumetrically compare larger areas of preoperative and post‐operative peri‐implant soft tissue levels. Therefore, the aim of this randomized controlled clinical trial was to volumetrically compare the outcome of immediately placed and provisionalized implants, with or without a connective tissue graft. It was hypothesized that the use of a connective tissue graft leads to more volumetrically stable peri‐implant tissues.
2. MATERIAL AND METHODS
2.1. Study design
This randomized controlled clinical trial (RCT) included 60 patients who were enrolled and treated at the Department of Oral and Maxillofacial Surgery of the University Medical Center Groningen, University of Groningen, Groningen, the Netherlands. The RCT was approved by the Medical Ethical Committee (NL43085.042.13), registered in a trial register (http://www.trialregister.nl: TC3815), and the CONSORT 2010 checklist was used as a guideline to report on the outcomes. All eligible patients were informed about the features of the study and granted their informed consent before enrolment. Patients were included between December 2012 and July 2015. Randomization was carried out by an independent research assistant with a 1:1 allocation ratio using sealed envelopes, to be opened after implant placement, resulting in two study groups of immediately placed and provisionalized implants in the aesthetic zone (first bicuspid to first bicuspid in the maxilla) with:
a connective tissue graft (CTG) harvested from the tuberosity region (test group).
no soft tissue graft (control group).
2.2. Patients
All referred patients with a failing tooth in the maxillary aesthetic zone were considered for inclusion. The fulfilment of the inclusion criteria was verified by clinicians at the screening session, including:
≥18 years of age;
the failing tooth is an incisor, canine or first bicuspid in the maxilla;
the failing tooth has adjacent and opposing natural teeth;
adequate oral hygiene and absence of active and uncontrolled periodontal disease;
sufficient mesial–distal and interocclusal space for placement of the implant and definitive restoration;
sufficient interocclusal space to design a non‐occluding provisional restoration;
an intact facial bone wall is present on the preoperative CBCT.
Exclusion criteria were as follows:
medical and general contraindications for the surgical procedure, expressed by ASA score ≥ III (Smeets, de Jong, & Abraham‐Inpijn, 1998);
presence of periodontal disease, expressed by pocket probing depths of ≥4 mm and bleeding on probing (modified sulcus bleeding index score ≥2);
smoking;
earlier treatment with radiotherapy to the head and neck region;
pregnancy;
A post‐extraction bony defect and a distance, measured in a vertical direction from the bony defect of the facial bone wall to the mucosa at the cement–enamel junction of the adjacent teeth, that exceeded 5 mm (example given: 3 mm bony defect and 2 mm mucosa). This distance was assessed with a periodontal probe (Williams Color‐Coded Probe; Hu‐Friedy, Chicago, IL, USA) to the nearest millimetre.
2.3. Surgical and prosthetic protocol
All implants were placed under a prophylactic antibiotic regime, starting one day prior to surgery (amoxicillin 500 mg, three times daily for 7 days or clindamycin 300 mg, four times daily for 7 days in case of amoxicillin allergy). Furthermore, patients used a 0.2% chlorhexidine mouthwash (two times daily for 7 days) for oral disinfection. All surgical procedures were performed by one experienced oral and maxillofacial surgeon (G.R.). First, a sulcular incision was made to separate the attached periodontal ligament from the failing tooth. Next, periotomes were used to atraumatically extract the tooth without raising a mucoperiosteal flap. After extraction, the implant bed was prepared on the palatal side of the extraction socket according to the manufacturer's instructions. Then, an implant drill was placed in the implant preparation to serve as a space maintainer. The gap between the implant drill and the facial bone wall was filled using a 1:1 mixture of autogenous bone, harvested from the flutes of the implant drill and anorganic bovine bone (Geistlich Bio‐Oss®; Geistlich Pharma AG, Wolhusen, Switzerland). Afterwards, the implant (NobelActive; Nobel Biocare AG, Gothenburg, Sweden) was placed 3 mm apical of the cement–enamel junction of the adjacent teeth. Primary implant stability was achieved by final insertion torque ≥45 Ncm. An implant‐level impression was taken for the fabrication of a screw‐retained provisional restoration. In the test group, a connective tissue graft (CTG) was harvested from the tuberosity region and placed submucosally on the labial bone plate through an envelope technique. In both groups, a provisional restoration free of occlusal and eccentric contacts was placed the same day. After 3 months, a definitive implant crown was fabricated. In case, the screw access hole was located palatally; a screw‐retained implant crown was fabricated by means of a veneered zirconia abutment (NobelProcera; NobelBiocare AB). If the location of the screw access hole did not allow a screw‐retained implant crown, a customized zirconia abutment (NobelProcera; NobelBiocare AB) was fabricated, and a veneered zirconia crown (NobelProcera; NobelBiocare AB) was cemented (Fuji Plus Cement; GC Europe, Leuven, Belgium). All prosthetic procedures were executed by two experienced prosthodontists, and all provisional and final implant restorations were fabricated by one experienced dental technician.
2.4. Outcome measures
The primary outcome measure of this study was volumetric change, transformed to a mean linear change in thickness (mm), from baseline (T pre) to 12 months after placement of the definitive implant crown (T 12). Secondary outcome measures were gingival biotype, plaque scores, bleeding scores, mucosal inflammation, mid‐facial mucosa level, Pink Esthetic Score (PES) and patient satisfaction. All clinical measurements were performed by one examiner (E.Z.). The photographic assessments and aesthetic assessment of soft tissues were performed by two calibrated examiners (E.Z. and L.d.H.). The volumetric measurements and analysis were performed by one examiner (W.v.N.). A software calibration session was conducted before the volumetric analysis to ensure reproducibility.
2.5. Volumetric assessment
Hydrocolloid impressions (Cavex; Cavex Holland BV, Haarlem, the Netherlands) were taken at T pre and T 12. Thereafter, the impressions were poured in dental stone type IV (Sherahard‐rock; Shera Werkstoff‐Technologie, Lemförde, Germany), and the stone casts were optically scanned with a laboratory optical scanner (IScan D301i; Imetric, Courgenay, Switzerland) resulting in digital STL files (Standard Tessellation Language). For each patient, the digital surface models representing the two study time points were imported into the volume analysis software (Swissmeda/SMOP, Zürich, Switzerland). The best‐fit algorithm was used to superimpose the digital surface models based on unchanged neighbouring tooth surfaces as reference. Thereafter, the study‐relevant area of interest was defined with anatomical reference structures using the border of the mesial and distal papilla adjacent to the implant crown, the apically located mucogingival line and the coronally located crown margin (Figure 1). The area of interest located at the crown margin was shifted 1–2 mm more apically in all patients to avoid an invalid superimposition as a result of mid‐facial mucosa recession. As a result, the area of interest was of variable size (mm2) between patients (Schneider, Grunder, Ender, Hammerle, & Jung, 2011; Thoma et al., 2010).
Figure 1.
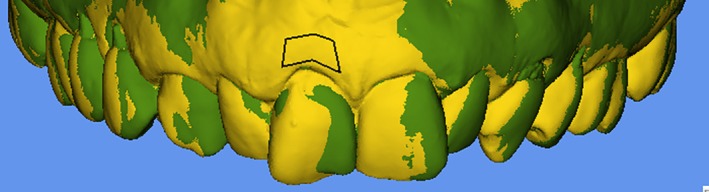
The stereolithographic files from baseline (yellow) and 1‐year follow‐up (green) were superimposed, and the region of interest was determined (black box)
After the area of interest was defined, the volumetric analysis software (Swissmeda/SMOP) calculated a mean dimensional change (mm3) for each patient. To allow for a direct comparison between patients and study groups, the mean dimensional change per area was transformed to a mean linear change in thickness in mm (Schneider et al., 2011; Thoma et al., 2010). After the volumetric analysis was completed, the volumetric analysis was run again for 10 randomly selected patients by an independent examiner (S.M.) to calculate interoperator reliability (Intraclass Correlation Coefficient).
2.6. Photographic assessment of mid‐facial gingival level
Standardized digital photographs (Meijndert, Meijer, Raghoebar, & Vissink, 2004) (Canon EOS 650 with ring flash; Canon Inc., Ota, Tokyo, Japan) were taken at T pre and T 12. A periodontal probe (Williams Color‐Coded probe) was used for calibration of the photographs. The change in mid‐facial mucosa level was measured by a full‐screen analysis using Adobe Photoshop (Adobe Photoshop CS5.1; Adobe Systems Inc., San Jose, USA).
2.7. Clinical assessments
The following clinical parameters were assessed the following:
gingival biotype at T pre, measured at the mid‐facial aspect of the marginal gingiva of the failing tooth, using a periodontal probe (Williams Color‐Coded Probe) (Kan, Morimoto, Rungcharassaeng, Roe, & Smith, 2010);
implant probing depths at T 12, measured to the nearest 1 mm using a periodontal probe (Williams Color‐Coded Probe) at the mid‐facial aspect of the implant;
plaque scores at T 12, using the modified plaque index (Mombelli, van Oosten, Schurch, & Land, 1987);
bleeding scores at T 12, using the modified sulcus bleeding index (Mombelli et al., 1987);
mucosal inflammation at T 12, using the Gingival Index (Loe, 1967).
2.8. Aesthetic assessment of mid‐facial soft tissues
Standardized digital photographs (Meijndert et al., 2004) (Canon EOS 650 with ring flash) of the aesthetic zone were taken at T 12 to assess the PES as described by Fürhauser et al. (2005). The PES consists of seven topics regarding mesial papilla fill (0–2 points), distal papilla fill (0–2 points), level of gingival margin (0–2 points), contour (0–2 points), alveolar process (0–2 points), colour (0–2 points) and texture (0–2 points), resulting in a total score (0–14 points) with 0 = lowest score and 14 = highest score.
2.9. Patient satisfaction
Assessment of patient satisfaction was performed at T 12 with a self‐administered patient questionnaire regarding overall satisfaction and satisfaction of colour and shape of the mucosa using a visual analogue scale (VAS, left = very dissatisfied [0], right = very satisfied [10]).
2.10. Statistical analysis
An a priori analysis was performed to determine the minimum sample size for both study groups (G*power version 3.1, Faul, Erdfelder, Buchner, & Lang, 2009). A mean linear change in thickness of 0.5 mm from T pre to T 12 was considered as a clinically relevant difference between both groups with an expected average standard deviation of 0.56 mm as derived from the literature (Schneider et al., 2011). A two‐sided test with an α error probability of 5% and a power of 80% was then carried out, resulting in a sample size of 21 patients per study group. To deal with the withdrawal of patients, the number of patients per study group was set at 30.
An assessment of continuous variables was carried out using the Shapiro–Wilk test and normal Q–Q‐plots. Differences in means between groups were calculated using the independent t test or the Mann–Whitney test. Categorical variables were analysed using the chi‐square test or Fisher's exact test. For within‐group statistical comparison, the Wilcoxon test was used. The interobserver reliability of the volumetric measurements was calculated using the intraclass correlation coefficient (ICC, two‐way mixed, single measures). All analyses were carried out with SPSS using a p‐value of .05 to determine statistical significance (SPSS Statistics 23.0; SPSS Inc.; IBM Corporation, Chicago, IL, USA).
3. RESULTS
3.1. Patients
This study included a total of 60 patients, consisting of 30 patients in a test group and 30 in a control group. Details regarding patient characteristics at baseline are shown in Table 1. The allocation process and follow‐up are shown in Figure 2. All included patients received their assigned treatment. One implant was lost in both groups due to early failure of osseointegration, resulting in a one‐year implant survival rate of 96.7%. In both groups, four patients were excluded from final analysis due to irregularities in the stone casts, taken at T pre and/or T 12.
Table 1.
Patient characteristics at baseline (T pre)
| Test group | Control group | |
|---|---|---|
| Male/female ratio | 13/17 | 15/15 |
| Age in years—mean ± SD (range) | 45.5 ± 15.5 (19.5–67.8) | 47.8 ± 16.5 (20.9–82.2) |
| Gingival biotype thin/thick | 20/10 | 15/15 |
| Implant site location I1/I2/C/P1 | 16/9/3/2 | 12/10/7/1 |
Figure 2.
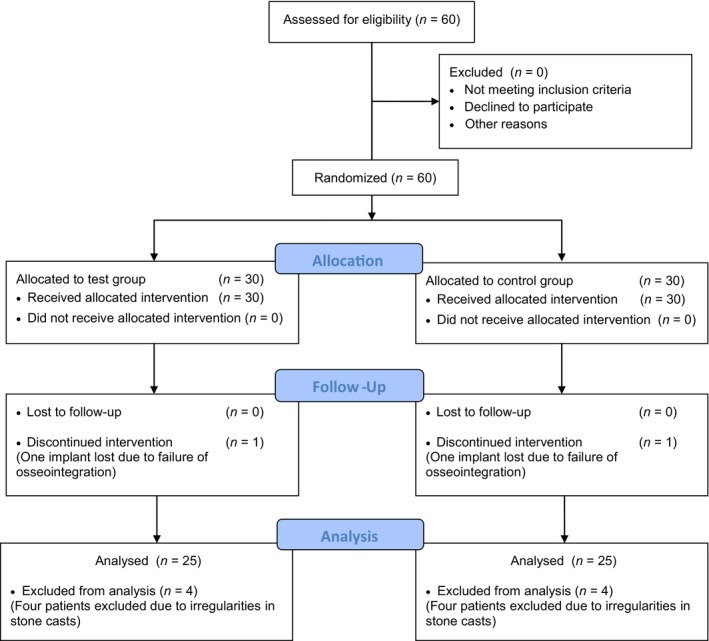
CONSORT flow diagram
3.2. Volumetric measurements
The mean (±SD) area of measurements for the evaluation of volume changes between T pre and T 12 was 11.97 ± 4.43 mm2 in the test group and 13.45 ± 3.56 mm2 in the control group, respectively. The mean volumetric changes in this time period were 9.32 ± 7.19 mm3 in the test group and 7.77 ± 7.26 mm3 in the control group. To allow for comparison between groups, the volumetric changes were transformed to mean linear measurements in mm. The resulting change in thickness between T pre and T 12 is shown in Table 2. Both groups displayed a loss of volume at T 12, being −0.68 ± 0.59 mm (test group) and −0.49 ± 0.54 mm (control group). Although the test group displayed slightly more mucosal volume loss with a mean (SE) difference of 0.19 (0.16) mm, no statistical significance was found (p = .24). The interoperator reliability (ICC), measured for 10 randomly selected patients, was 0.821 (p = .001).
Table 2.
Change in mucosal thickness in mm between baseline (T pre) and 1 year after definitive crown placement (T 12)
| Test groupN = 25 | Control groupN = 25 | p‐Value | |||
|---|---|---|---|---|---|
| Mean ± SD/Median | −0.68 ± 0.59 | −0.56 | −0.49 ± 0.54 | −0.27 | .24 |
3.3. Photographic measurements
The change in mid‐facial mucosa level is shown in Table 3. A mean (±SD) change of +0.20 ± 0.70 mm in the test group and a mean change of −0.48 ± 1.13 mm in the control group were reported between T pre and T 12. The change in mid‐facial mucosa levels was significantly different between both groups with a mean (SE) difference of 0.68 (0.27) mm (p = .014). In addition, two of 25 patients (8%) displayed advanced cases of mid‐facial mucosa recession (≥1 mm) in the test group against eight of 25 patients (32%) in the control group.
Table 3.
Change in mid‐facial mucosa levels in mm between baseline (T pre) and 1 year after definitive crown placement (T 12)
| Test groupN = 25 | Control groupN = 25 | p‐Value | |||
|---|---|---|---|---|---|
| Mean ± SD/Median | +0.20 ± 0.70 | +0.24 | −0.48 ± 1.13 | −0.55 | 0.014 |
3.4. Clinical measurements
Implant probing depths at T 12 are shown in Table 4. Both groups displayed probing depths of ≤3 mm with a mean (SE) difference of 0.16 (0.29) mm between both groups (p = .813). Plaque scores at T 12 were very low (98%: no plaque). Bleeding on probing scores showed no peri‐implant bleeding in 50% of all patients, peri‐implant isolated bleeding spots in 38% of all patients and confluent lines of bleeding in 12% of all patients. A Gingival Index score of 0 was found in 96% of all patients at T 12. No significant differences in plaque scores, bleeding scores and Gingival Index scores were found between groups at T 12.
Table 4.
Implant probing depths in mm 1 year after definitive crown placement (T 12)
| Mid‐facial probing depth | Test groupN = 25 | Control groupN = 25 | p‐Value |
|---|---|---|---|
| Mean ± SD | 2.28 ± 0.79 | 2.44 ± 1.19 | .813 |
3.5. Aesthetic measurements
Pink Esthetic Score at T 12 is displayed in Table 5. The aesthetics of the gingival margin level were rated significantly higher in the test group than the control group (p = .034), although the texture of the peri‐implant soft tissues was scored significantly lower in the test group (p = .039). No significant difference was found in total score between both groups.
Table 5.
Pink esthetic scores at T 12
| Topics PES | Test groupMean ± SD (95% CI) | Control groupMean ± SD (95% CI) | p‐Value |
|---|---|---|---|
| Mesial papilla | 1.48 ± 0.51 (1.27–1.69) | 1.44 ± 0.51 (1.23–1.65) | .779 |
| Distal papilla | 1.48 ± 0.65 (1.21–1.75) | 1.68 ± 0.48 (1.48–1.88) | .299 |
| Level gingival margin | 1.80 ± 0.50 (1.59–2.01) | 1.44 ± 0.71 (1.15–1.73) | .034 |
| Contour | 1.40 ± 0.71 (1.11–1.69) | 1.60 ± 0.58 (1.36–1.84) | .318 |
| Alveolar process | 1.48 ± 0.65 (1.21–1.75) | 1.24 ± 0.72 (0.94–1.54) | .223 |
| Colour | 1.84 ± 0.37 (1.69–1.99) | 1.96 ± 0.20 (1.88–2.04) | .162 |
| Texture | 1.80 ± 0.50 (1.59–2.01) | 2.00 ± 0.00 | .039 |
| Total score | 11.28 ± 1.67 (10.59–11.97) | 11.36 ± 1.65 (10.68–12.04) | .866 |
3.6. Patient satisfaction
Patient satisfaction at T 12 regarding an overall score and soft tissue aesthetics is displayed in Table 6. Total satisfaction showed a mean (±SD) score of 8.38 ± 2.28 (range 0.8–10) in the test group and 8.84 ± 1.23 (range 5–10) in the control group with a mean (SE) non‐significant difference of 0.46 (0.52) between both groups (p = .861). Patient satisfaction regarding colour and shape of peri‐implant mucosa showed similar scores with no significant differences between test and control group (p = .711 and p = .892), respectively.
Table 6.
Patient satisfaction regarding overall score and soft tissue aesthetics at T 12
| Topics | Test groupMean ± SD (95% CI) | Control groupMean ± SD (95% CI) | p‐Value |
|---|---|---|---|
| Soft tissue aesthetics | |||
| Colour of the peri‐implant mucosa | 8.37 ± 2.20 (7.46–9.27) | 8.70 ± 1.76 (7.98–9.43) | .711 |
| Shape of the peri‐implant mucosa | 8.27 ± 2.25 (7.34–9.20) | 8.18 ± 2.38 (7.20–9.17) | .892 |
| Overall satisfaction | |||
| Total score | 8.38 ± 2.28 (7.44–9.32) | 8.84 ± 1.23 (8.33–9.34) | .861 |
4. DISCUSSION
The aim of this RCT was to volumetrically compare the outcome of immediately placed and provisionalized implants in the aesthetic zone, with or without a connective tissue graft. It was hypothesized that the use of a connective tissue graft leads to more stable peri‐implant mucosal soft tissues. Volume measurements at T 12 showed non‐significant differences between both groups, with the test group displaying the most mucosal volume loss. In contrast, mid‐facial mucosa levels were significantly more stable in the test group than the control group between T pre and T 12. These findings reject the hypothesis that the use of a CTG leads to less mucosal volume loss, although mid‐facial mucosa levels seem more stable after 1‐year follow‐up when a CTG was applied.
Physiological bone resorption of the facial bone wall, after extraction and immediate implant placement, has to be considered as an important factor to the volume loss in both groups (Chappuis et al., 2013). Another possible factor contributing to the higher mucosal volume loss in the test group is the surgical envelope technique used to place the CTG submucosally, inducing additional bone loss by cutting of vascularization from the mucosa to the facial bone wall. Also, it is unknown whether shrinkage or thickening of the CTG in the present study had any influence on the mucosal volume loss. De Bruyckere, Eghbali, Younes, De Bruyn, and Cosyn (2015) reported that mucosal thickness, measured with an ultrasound device, increased after 12‐month follow‐up when a CTG was placed 3 months after implant placement. This might imply that a CTG remains stable after placement and that the mucosal volume loss is mostly related to underlying facial bone loss. This leads to the assumption that the use of a CTG cannot fully compensate for alterations of the facial bone wall following immediate implant placement and provisionalization.
In recent years, post‐extraction facial bone plate thickness has been recognized as an important risk factor for facial bone loss and a risk factor for soft tissue alterations. The study of Chappuis et al. (2013) showed that in case of a post‐extraction thin wall phenotype (<1 mm), significantly higher bone alterations were found. Additionally, clinical studies have shown that the anterior maxilla is dominated by thin wall biotypes (Braut, Borstein, Belser, & Buser, 2011; Januário et al., 2011; Zekry, Wang, Chau, & Lang, 2014). Due to the fact that the present study was designed and commenced before publication of these studies, post‐extraction thickness of the facial bone wall has not been incorporated as a risk factor. Therefore, further research with cone beam computed technology (CBCT) data is needed to determine the exact role of facial bone alterations in relation to the use of a CTG and mucosal volume loss.
Mid‐facial mucosa levels displayed a significant difference between both groups between T pre and T 12, with a small mean gain in the test group and a loss in the control group. Furthermore, two of 25 patients (8%) displayed advanced cases of recession (≥1 mm) in the test group against eight of 25 patients (32%) in the control group. This confirms the findings of two RCT's which found significantly more stable mid‐facial mucosa levels when a CTG was applied, although a small recession of the mid‐facial mucosa was still reported in these studies when a CTG was applied (Migliorati et al., 2015; Yoshino et al., 2014).
The aesthetic assessment with PES showed significantly higher scores in the test group regarding the marginal gingival level, as a result of the applied CTG. In contrast, the texture of the peri‐implant soft tissues was scored significantly lower in the test group. A possible explanation is that the surgical envelope technique used to place the CTG resulted in more mucosal deformation and scarring of the peri‐implant soft tissues. Regarding the total PES score, no significant differences were found in between both groups. This contradicts the findings of Migliorati et al. (2015), who found significantly better PES scores when a CTG was applied.
In addition to the aesthetic outcome, patient satisfaction showed high overall scores, regardless of study group and topic. These findings are compared with other studies describing immediately placed and provisionalized implants in the aesthetic zone (Hartlev et al., 2014; Van Nimwegen et al., 2016).
In summary, a higher mucosal volume loss, a similar PES and significantly more coronally placed mid‐facial mucosa level were found in the test group. These findings lead to the statement that a connective tissue graft should only be considered concomitant with immediate implant placement in order to prevent asymmetry in facial mucosa levels between the peri‐implant mucosa and the gingival contour of the neighbouring teeth.
5. CONCLUSION
The use of a CTG in immediately placed and provisionalized implants in the aesthetic zone does not result in less mucosal volume loss after 12 months. A significantly more coronally located facial mucosa level is detected when a connective tissue graft was performed. Further research with CBCT data is needed to explore the role of facial bone loss in relation to mucosal volume loss.
CONFLICT OF INTEREST
The authors have stated explicitly that there is no conflict of interest in connection with this article.
Supporting information
ACKNOWLEDGEMENTS
The authors would like to thank Gerrit van Dijk (TMFL van Dijk, Groningen, Groningen, the Netherlands) for providing access to his 3D optical scanner. The authors would also like to thank Dr L. den Hartog for his help with the photographic and aesthetic assessments. Furthermore, the authors express their gratitude to Dr J.F. van Nimwegen for her statistical support of this study and Prof. Dr A. Vissink for his help editing the manuscript.
van Nimwegen WG, Raghoebar GM, Zuiderveld EG, Jung RE, Meijer HJA, Mühlemann S. Immediate placement and provisionalization of implants in the aesthetic zone with or without a connective tissue graft: A 1‐year randomized controlled trial and volumetric study. Clin Oral Impl Res. 2018;29:671–678. 10.1111/clr.13258
Funding informationThe study was supported by an unrestricted grant of Nobel Biocare Services AG (implant materials were provided, research grant: 2012‐1135).
REFERENCES
- Braut, V. , Borstein, M. M. , Belser, U. , & Buser, D. (2011). Thickness of the anterior maxillary facial bone wall – A retrospective radiographic study using cone beam computed tomography. International Journal of Periodontics and Restorative Dentistry, 2, 125–131. [PubMed] [Google Scholar]
- Chappuis, V. , Engel, O. , Reyes, M. , Shahim, K. , Nolte, L.‐P. , & Buser, D. (2013). Ridge alterations post‐extraction in the esthetic zone: A 3D analysis with CBCT. Journal of Dental Research, 12, 195–201. 10.1177/0022034513506713 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen, S. T. , & Buser, D. (2009). Clinical and esthetic outcomes of implants placed in postextraction sites. International Journal of Oral and Maxillofacial Implants, 24, 186–217. [PubMed] [Google Scholar]
- Chen, S. T. , & Buser, D. (2014). Esthetic outcomes following immediate and early implant placement in the anterior maxilla – A systematic review. International Journal of Oral and Maxillofacial Implants, 29, 186–215. 10.11607/jomi.2014suppl.g3.3 [DOI] [PubMed] [Google Scholar]
- Cosyn, J. , De Bruyn, H. , & Cleymaet, R. (2013). Soft tissue preservation and pink aesthetics around single immediate implant restorations: A 1‐year prospective study. Clinical Implant Dentistry Related Research, 6, 847–857. 10.1111/j.1708-8208.2012.00448.x [DOI] [PubMed] [Google Scholar]
- Cosyn, J. , Eghbali, A. , Hermans, A. , Vervaeke, S. , De Bruyn, H. , & Cleymaet, R. (2016). A 5‐year prospective study on single immediate implants in the aesthetic zone. Journal of Clinical Periodontology, 8, 702–709. 10.1111/jcpe.12571 [DOI] [PubMed] [Google Scholar]
- De Bruyckere, T. , Eghbali, A. , Younes, F. , De Bruyn, H. , & Cosyn, J. (2015). Horizontal stability of connective tissue grafts at the buccal aspect of single implants: A 1‐year prospective case series. Journal of Clinical Periodontology, 9, 876–882. 10.1111/jcpe.12448 [DOI] [PubMed] [Google Scholar]
- Faul, F. , Erdfelder, E. , Buchner, A. , & Lang, A. G. (2009). Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behavioral Research Methods, 4, 1149–1160. 10.3758/BRM.41.4.1149 [DOI] [PubMed] [Google Scholar]
- Fürhauser, R. , Florescu, D. , Benesch, T. , Haas, R. , Mailath, G. , & Watzek, G. (2005). Evaluation of soft tissue around single‐tooth implant crowns: The pink esthetic score. Clinical Oral Implant Research, 16, 639–644. 10.1111/j.1600-0501.2005.01193.x [DOI] [PubMed] [Google Scholar]
- Hämmerle, C. H. , Chen, S. T. , & Wilson Jr, T. G. (2004). Consensus statements and recommended clinical procedures regarding the placement of implants in extraction sockets. International Journal of Oral and Maxillofacial Implants, 19, 26–28. [PubMed] [Google Scholar]
- Hartlev, J. , Kohberg, P. , Ahlmann, S. , Andersen, N. T. , Schou, S. , & Isidor, F. (2014). Patient satisfaction and esthetic outcome after immediate placement and provisionalization of single‐tooth implants involving a definitive individual abutment. Clinical Oral Implants Research, 11, 1245–1250. 10.1111/clr.12260 [DOI] [PubMed] [Google Scholar]
- Januário, A. L. , Duarte, W. R. , Barriviera, M. , Mesti, J. C. , Araújo, M. G. , & Lindhe, J. (2011). Dimension of the facial bone wall in the anterior maxilla: A cone‐beam computed tomography study. Clinical Oral Implant Research, 10, 1168–1171. 10.1111/j.1600-0501.2010.02086.x [DOI] [PubMed] [Google Scholar]
- Kan, J. Y. , Morimoto, T. , Rungcharassaeng, K. , Roe, P. , & Smith, D. H. (2010). Gingival biotype assessment in the esthetic zone: Visual versus direct measurement. International Journal of Periodontics and Restorative Dentistry, 3, 237–243. [PubMed] [Google Scholar]
- Khzam, N. , Arora, H. , Kim, P. , Fisher, A. , Mattheos, N. , & Ivanovski, S. (2015). Systematic review of soft tissue alterations and esthetic outcomes following immediate implant placement and restoration of single implants in the anterior maxilla. Journal of Periodontology, 12, 1321–1330. 10.1902/jop.2015.150287 [DOI] [PubMed] [Google Scholar]
- Lee, C. T. , Tao, C. Y. , & Stoupel, J. (2016). The effect of subepithelial connective tissue graft placement on esthetic outcomes after immediate implant placement: Systematic review. Journal of Periodontology, 2, 156–167. 10.1902/jop.2015.150383 [DOI] [PubMed] [Google Scholar]
- Loe, H. (1967). The gingival index, the plaque index and the retention index systems. Journal of Periodontology, 6, 610–616. 10.1902/jop.1967.38.6_part2.610 [DOI] [PubMed] [Google Scholar]
- Meijndert, L. , Meijer, H. J. A. , Raghoebar, G. M. , & Vissink, A. (2004). A technique for standardized evaluation of soft and hard peri‐implant tissues in partially edentulous patients. Journal of Periodontology, 75, 646–651. 10.1902/jop.2004.75.5.646 [DOI] [PubMed] [Google Scholar]
- Migliorati, M. , Amorfini, L. , Signori, A. , Biavati, A. S. , & Benedicenti, S. (2015). Clinical and aesthetic outcome with post‐extractive implants with or without soft tissue augmentation: A 2‐year randomized clinical trial. Clinical Implant Dentistry Related Research, 5, 983–995. 10.1111/cid.12194 [DOI] [PubMed] [Google Scholar]
- Mombelli, A. , van Oosten, M. A. , Schurch Jr, E. , & Land, N. P. (1987). The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiology and Immunology, 4, 145–151. 10.1111/j.1399-302X.1987.tb00298.x [DOI] [PubMed] [Google Scholar]
- Schneider, D. , Grunder, U. , Ender, A. , Hammerle, C. H. , & Jung, R. E. (2011). Volume gain and stability of peri‐implant tissue following bone and soft tissue augmentation: 1‐year results from a prospective cohort study. Clinical Oral Implants Research, 1, 28–37. 10.1111/j.1600-0501.2010.01987.x [DOI] [PubMed] [Google Scholar]
- Slagter, K. W. , den Hartog, L. , Bakker, N. A. , Vissink, A. , Meijer, H. J. , & Raghoebar, G. M. (2014). Immediate placement of dental implants in the esthetic zone: A systematic review and pooled analysis. Journal of Periodontology, 7, 241–250. 10.1902/jop.2014.130632 [DOI] [PubMed] [Google Scholar]
- Smeets, E. C. , de Jong, K. J. , & Abraham‐Inpijn, L. (1998). Detecting the medically compromised patient in dentistry by means of the medical risk‐related history. A survey of 29,424 dental patients in The Netherlands. Journal of Preventive Medicine, 4, 530–535. 10.1006/pmed.1998.0285 [DOI] [PubMed] [Google Scholar]
- Thoma, D. S. , Jung, R. E. , Schneider, D. , Cochran, D. L. , Ender, A. , & Jones, A. A. (2010). Soft tissue volume augmentation by the use of collagen‐based matrices: A volumetric analysis. Journal of Clinical Periodontology, 7, 659–666. 10.1111/j.1600-051X.2010.01581.x [DOI] [PubMed] [Google Scholar]
- Van Nimwegen, W. G. , Goene, R. J. , Van Daelen, A. C. , Stellingsma, K. , Raghoebar, G. M. , & Meijer, H. J. (2016). Immediate implant placement and provisionalisation in the aesthetic zone. Journal of Oral Rehabilitation, 10, 745–752. 10.1111/joor.12420 [DOI] [PubMed] [Google Scholar]
- Windisch, S. I. , Jung, R. E. , Sailer, I. , Studer, S. P. , Ender, A. , & Hammerle, C. H. (2007). A new optical method to evaluate three‐dimensional volume changes of alveolar contours: A methodological in vitro study. Clinical Oral Implants Research, 5, 545–551. 10.1111/j.1600-0501.2007.01382.x [DOI] [PubMed] [Google Scholar]
- Yoshino, S. , Kan, J. Y. , Rungcharassaeng, K. , Roe, P. , & Lozada, J. L. (2014). Effects of connective tissue grafting on the facial gingival level following single immediate implant placement and provisionalization in the esthetic zone: A 1‐year randomized controlled prospective study. International Journal of Oral and Maxillofacial Implants, 2, 432–440. 10.11607/jomi.3379 [DOI] [PubMed] [Google Scholar]
- Zekry, A. , Wang, R. , Chau, A. C. , & Lang, N. P. (2014). Facial alveolar bone wall width – A cone beam computed tomography study in Asians. Clinical Oral Implant Research, 2, 194–206. 10.1111/clr.12096 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


