Abstract
Aims
To assess the pharmacokinetic and pharmacodynamic profile of a single dose of empagliflozin in young people with Type 2 diabetes to identify the appropriate doses for further paediatric development.
Methods
We conducted a single‐dose, open‐label, randomized, parallel‐group study with empagliflozin 5 mg, 10 mg and 25 mg in young people with Type 2 diabetes aged 10–17 years.
Results
Of 39 participants screened, 27 were randomized and completed the study; their mean (± sd) age was 14.1±2.0 years and body weight was 96.7±23.5 kg. Compared with similar studies in adults with Type 2 diabetes, the maximum observed plasma concentrations were slightly lower with the 10‐mg and 25‐mg doses, and the area under the plasma concentration–time curve was slightly lower with the 10‐mg but slightly higher with the 25‐mg dose. The adjusted mean increases in urinary glucose excretion were 53 g/24 h (95% CI 32,74), 73 g/24 h (95% CI 52,94) and 87 g/24 h (95% CI 68,107), and the adjusted mean decreases in fasting plasma glucose were 0.9 mmol/l (95% CI –1.6,–0.1), 0.9 mmol/l (95% CI –1.7,–0.2) and 1.1 mmol/l (95% CI –1.8,–0.5) for the 5‐ 10‐ and 25‐mg doses, respectively. There were no serious adverse events and one investigator‐reported drug‐related event (dehydration).
Conclusions
After a single oral dose of empagliflozin, adults and young people with Type 2 diabetes had similar exposure–response relationships after adjusting for significant covariates. These data support testing 10‐mg and/or 25‐mg doses of empagliflozin in an upcoming paediatric phase III Type 2 diabetes trial.
(ClinicalTrials.gov registration no.: NCT02121483).
What's new?
Metformin and insulin remain the only drugs approved for the treatment of young people with Type 2 diabetes, highlighting the need to perform and complete studies with newer glucose‐lowering drugs in the paediatric population.
In this paper we report the results of the pharmacokinetics and pharmacodynamics of the sodium‐glucose co‐transporter‐2 inhibitor empagliflozin in young people with Type 2 diabetes, an important first step in identifying the doses of empagliflozin to be used in a future pivotal trial.
The results indicate that the 10‐mg and/or 25‐mg doses of empagliflozin may be further investigated in an upcoming paediatric phase III study.
What's new?
Metformin and insulin remain the only drugs approved for the treatment of young people with Type 2 diabetes, highlighting the need to perform and complete studies with newer glucose‐lowering drugs in the paediatric population.
In this paper we report the results of the pharmacokinetics and pharmacodynamics of the sodium‐glucose co‐transporter‐2 inhibitor empagliflozin in young people with Type 2 diabetes, an important first step in identifying the doses of empagliflozin to be used in a future pivotal trial.
The results indicate that the 10‐mg and/or 25‐mg doses of empagliflozin may be further investigated in an upcoming paediatric phase III study.
Introduction
Recent data confirm the increased occurrence of Type 2 diabetes in young people, with incidence rates increasing by almost 5% annually since the beginning of the 21st century 1. Despite the burgeoning numbers of young people with Type 2 diabetes, metformin and insulin remain the only approved pharmacological therapies, while the armamentarium of therapies for adults with Type 2 diabetes is expansive. Furthermore, the TODAY study showed the rapid decline of glycaemic control in people with youth‐onset, short‐duration Type 2 diabetes, as well as the importance of dual pharmacotherapy to prevent glycaemic deterioration 2.
One of the newest classes of Type 2 diabetes agents, sodium‐glucose co‐transporter‐2 (SGLT2) inhibitors, of which empagliflozin is an example, offers the potential to improve glycaemic management of Type 2 diabetes in young people. In placebo‐controlled phase III trials in adults with Type 2 diabetes, treatment with empagliflozin (10 or 25 mg) as monotherapy or add‐on therapy improved glycaemic control and led to reductions in weight and blood pressure 3, 4, 5, 6, 7, 8, 9. In the EMPA‐REG OUTCOME trial, empagliflozin, given in addition to standard of care, reduced the risk of cardiovascular and renal events; it reduced cardiovascular mortality by 38% and incident or worsening nephropathy by 39% in people with Type 2 diabetes who had established cardiovascular disease 10, 11.
Even prior to the approval of new drugs for use in adults, pharmaceutical companies are required to submit paediatric study plans to both the US Food and Drug Administration and the European Medicines Agency. Such study plans often consist of a stand‐alone pharmacokinetic and pharmacodynamic study, the results of which inform the choice of drug dose(s) to be used in the subsequent phase III pivotal trial on efficacy and safety of the experimental drug vs placebo. In the present paper, we report the results of a single‐dose pharmacokinetic and pharmacodynamic study of empagliflozin in young people with Type 2 diabetes.
Patients and Methods
Trial design
This was a single‐dose, phase I, open‐label, randomized, parallel‐group study, with three treatment arms of different empagliflozin doses (5, 10 and 25 mg), that was conducted at 11 sites in five countries (France, Israel, Mexico, USA and South Africa) between September 2014 and February 2016. Young people aged 10–17 years with Type 2 diabetes and HbA1c concentrations ≤ 91 mmol/mol (10.5%), who were treated with diet and exercise and/or metformin with or without insulin therapy, were eligible for participation. To be enrolled in the study, the young people also had to have negative islet cell antigen autoantibodies, negative glutamic acid decarboxylase autoantibodies, and fasting serum C‐peptide levels ≥0.28 nmol/l.
Exclusion criteria included uncontrolled hyperglycaemia with repeatedly elevated glucose levels (>13.3 mmol/l) after an overnight fast at screening, history of acute metabolic decompensation, such as diabetic ketoacidosis, within 3 months of screening and impaired renal function, defined as estimated GFR (eGFR) <90 ml/min/1.73 m2 (according to the Schwartz formula).
The protocol was approved by independent ethics committees or institutional review boards of all participating centres. The study was carried out according to the principles of the Declaration of Helsinki and the International Conference on Harmonization Guideline for Good Clinical Practice. Prior to participation, written informed consent was obtained from the parents/guardians of all participants, and the young people themselves provided assent.
The computer‐generated randomization sequence was produced by the study sponsor, and was concealed using a central interactive voice and web response system. Eligible participants were randomly allocated to one of the three treatment groups (empagliflozin 5, 10 and 25 mg). Randomization was stratified by sex (at least 33% and no more than 67% of the participants being female) and by age (at least six participants aged < 15 years). During the treatment period, each of the participants received a single dose of one of the three possible dose treatments in the morning prior to breakfast, and blood and urine samples were obtained for the following 48 h. An end‐of‐trial examination was performed in all participants 1 week after the end of the study (Fig. S1).
Endpoints and assessment
The pharmacokinetic endpoints were: area under the plasma concentration–time curve from time 0 extrapolated to infinity, area under the plasma concentration–time curve from time 0 to the time of the last quantifiable concentration, maximum observed plasma concentration, time from dosing until maximum observed concentration is reached, and terminal plasma half‐life. In addition to these pharmacokinetic endpoints, area under the plasma concentration–time curve from time 0 to 24 h was calculated for comparison with a similar endpoint measured in adults in a previous trial 13. Pharmacodynamic endpoints were: change from baseline in urinary glucose excretion over 24 h after study drug intake, change from baseline in fasting plasma glucose at 24 h after study drug intake and change from baseline in mean daily glucose over 24 h after study drug intake. Safety endpoints included the incidence and intensity of adverse events, including hypoglycaemia, clinically relevant new or worsening findings in electrocardiogram, and changes from baseline in vital signs, clinical laboratory values, kidney function tests and hydration status.
For the purpose of pharmacokinetic and pharmacodynamic measurements, blood samples were drawn at 0.5 h before and 0.5, 1, 1.5, 2, 4, 8, 12, 24, 34 and 48 h after empagliflozin administration. All urine voided was collected during time intervals –24 to 0 h (pre‐treatment and served as ‘urinary glucose excretion at baseline’) and 0–5, 5–12, 12–24 and 24–48 h after study drug administration. Empagliflozin concentrations in plasma and urine were determined using a validated high‐performance liquid chromatography tandem mass spectrometry assay. The analyses were performed at BASi (Bioanalytical Systems Incorporation), West Lafayette, IN, USA.
Exposure–response analyses
In order to compare the relationship between pharmacokinetics and urinary glucose excretion in the paediatric participants with that in adults, an exposure–response model was developed based on data from three clinical trials 12, 13, 14 in adults with Type 2 diabetes to describe the change from baseline in 24‐h urinary glucose excretion. Population model development followed three steps: (1) identification of a structural model and a stochastic model to describe the central tendency; (2) residual variability and variability within the population; and (3) analysis of prespecified covariates together with numerical, graphical and simulation‐based model evaluation. The best model accounted for covariates such as a measure of glycaemic control, eGFR, demographics (age, weight, sex, race) and presence of metformin as background therapy. Based on the model, the exposure–response profile for a typical adult with Type 2 diabetes was simulated using fixed‐effect parameter estimates and their associated uncertainty (n=1000 simulated profiles). The simulated profile (median and 95% CI) of the typical adult patient was graphically compared with the geometric mean (95% CI) of the observed urinary glucose excretion change from baseline for each dose group in our paediatric population. Following the same model development steps, the data from this trial were evaluated together with the adult data to allow a direct comparison of the exposure–response in both populations to support dose selection for paediatric phase III trial development. Population pharmacokinetic and pharmacodynamic analyses for repeated‐measures endpoints were conducted via nonlinear mixed‐effects modelling with a qualified installation of the nonlinear mixed‐effects modelling (NONMEM) software, version 7, level 2.0 (ICON Development Solutions, Hanover, MD, USA). All simulations were performed with R version 2.14.2 or higher.
Statistical analysis
The 27 participants who were randomized into the study provided a sample size that was considered sufficient to achieve the required precision (ratio of the upper to lower limit of the 95% CI of twofold or less) for the pharmacokinetic variables with 90% probability. All endpoints were analysed using descriptive statistics. Safety and pharmacodynamic analyses were carried out in the treated set (i.e. all participants who received one dose of trial medication), and pharmacokinetic analyses were carried out in the pharmacokinetic set (i.e. all treated participants who provided at least one pharmacokinetic value for statistical assessment). Results from analyses of study endpoints are presented descriptively as arithmetic mean and sd or arithmetic coefficient of variation. Dose proportionality of empagliflozin was explored using a regression model on the double‐log scale. A 95% CI for the slope was computed. For the changes from baseline in urinary glucose excretion, adjusted means per treatment group were calculated based on an analysis of covariance, including ‘treatment’ as a fixed effect and ‘urinary glucose excretion at baseline’ and ‘fasting plasma glucose at baseline’ as continuous covariates. For the changes from baseline in mean daily glucose and fasting plasma glucose, adjusted means per treatment group were calculated based on an analysis of covariance, including ‘treatment’ as a fixed effect and ‘mean daily glucose at baseline’ or ‘fasting plasma glucose at baseline’, respectively, as a continuous covariate.
Results
Study sample, demographics and baseline characteristics
Of the 39 young people screened, 27 were randomized (nine participants to the 5‐mg dose group, eight participants to the 10‐mg dose group, and 10 participants to the 25‐mg dose group). All randomized participants received study medication and completed the trial.
As shown in Table 1, the demographic and clinical characteristics of the three treatment groups were generally similar; however, compared with the other two groups, weight and BMI were higher in the 10‐mg dose group, and baseline fasting plasma glucose and urinary glucose excretion were lowest in the 25‐mg dose group. The 10‐mg dose group included three American Indian/Alaska Native participants (37.5%) and only one white participant (12.5%), while both the 5‐mg dose group and the 25‐mg dose group contained no American Indian/Alaska Native and five (55.6%) and six (60.0%) white participants, respectively.
Table 1.
Demographic data and baseline characteristics of participants
| 5 mg empagliflozin | 10 mg empagliflozin | 25 mg empagliflozin | Total | |
|---|---|---|---|---|
| Number of participants, n (%) | 9 (100) | 8 (100) | 10 (100) | 27 (100) |
| Sex, n (%) | ||||
| Male | 3 (33.3) | 3 (37.5) | 3 (30.0) | 9 (33.3) |
| Female | 6 (66.7) | 5 (62.5) | 7 (70.0) | 18 (66.7) |
| Race, n (%) | ||||
| American Indian/Alaska Native | 0 | 3 (37.5) | 0 | 3 (11.1) |
| Asian | 1 (11.1) | 0 | 0 | 1 (3.7) |
| Black/African American | 3 (33.3) | 4 (50.0) | 4 (40.0) | 11 (40.7) |
| Hawaiian/Pacific Isle | 0 | 0 | 0 | 0 |
| White | 5 (55.6) | 1 (12.5) | 6 (60.0) | 12 (44.4) |
| Mean (sd) age, years | 13.7 (2.0) | 14.5 (1.9) | 14.2 (2.1) | 14.1 (2.0) |
| Mean (sd) weight, kg | 90.0 (19.0) | 111.0 (21.3) | 91.1 (25.7) | 96.7 (23.5) |
| Mean (sd) BMI, kg/m2 | 33.9 (5.8) | 39.6 (6.2) | 33.7 (7.0) | 35.5 (6.7) |
| Mean (sd) BMI SDS | 2.9 (0.7) | 3.4 (0.5) | 2.8 (0.9) | 3.0 (0.8) |
| Smoking status, n (%) | ||||
| Never smoked | 9 (100) | 8 (100) | 9 (90.0) | 26 (96.3) |
| Ex‐smoker | 0 | 0 | 0 | 0 |
| Currently smokes | 0 | 0 | 1 (10.0) | 1 (3.7) |
| Follow diet/exercise recommendation, n (%) | ||||
| No | 2 (22.2) | 1 (12.5) | 1 (10.0) | 4 (14.8) |
| Yes | 7 (77.8) | 7 (87.5) | 9 (90.0) | 23 (85.2) |
| Background therapy, n (%) | ||||
| None | 1 (11.1) | 3 (37.5) | 2 (20.0) | 6 (22.2) |
| Metformin alone | 5 (55.6) | 3 (37.5) | 6 (60.0) | 14 (51.9) |
| Insulin alone | 0 | 0 | 0 | 0 |
| Metformin and insulin | 3 (33.3) | 2 (25.0) | 2 (20.0) | 7 (25.9) |
| Mean (sd) eGFR, ml/min/1.73m2 | 178.3 (17.4) | 162.3 (38.8) | 157.3 (15.2) | 165.8 (25.8) |
| Mean (sd) urinary glucose excretion, g/24 h | 16.2 (30.3)a | 12.3 (25.4) | 0.08 (0.05) | 8.8 (22.1) |
| Mean (sd) HbA1c, mmol/mol | 57 | 57 | 45 | 53 |
| Mean (sd) HbA1c, % | 7.4 (1.4) | 7.4 (1.1) | 6.3 (1.0) | 7.0 (1.2) |
| Mean (sd) FPG, mmol/mol | 8.5 (4.2) | 8.6 (3.1) | 6.4 (1.4) | 7.7 (3.1) |
| Tanner scale score, n (%) | ||||
| 1 | 0 | 0 | 0 | 0 |
| 2 | 0 | 1 (12.5) | 0 | 1 (3.7) |
| 3 | 1 (11.1) | 1 (12.5) | 1 (10.0) | 3 (11.1) |
| 4 | 2 (22.2) | 4 (50.0) | 1 (10.0) | 7 (25.9) |
| 5 | 6 (66.7) | 2 (25.0) | 8 (80.0) | 16 (59.3) |
SDS, standard deviation score; eGFR, estimated GFR; FPG, fasting plasma glucose.
*Mean baseline urinary glucose excretion in the 5‐mg dose group was based on the data of only eight participants, as the pre‐dose urine collection of one participant was incomplete.
Pharmacokinetic outcomes
After single‐dose administration, empagliflozin was rapidly absorbed in the participants of all three dose groups. Maximum peak plasma concentrations were reached after ~1.5 h (Table 2). Empagliflozin plasma concentrations increased with increasing dose (Fig. 1a,b) but not in a dose‐proportional manner. The mean values of the pharmacokinetic characteristics of empagliflozin are shown in Table 2. Terminal plasma half‐life values were in the range of 7.03 h to 8.09 h for all empagliflozin doses. One participant in the empagliflozin 5 mg group had two abnormally high empagliflozin plasma concentrations at 8 h and 48 h post dose (approximately eightfold and 32‐fold above the respective values of the others in this dose group), while the remainder of the plasma concentrations of this participant were within the expected range (Fig. S2). Although these two values were physiologically implausible, they were included in the final analysis because no error relating to the study conduct or the bioanalytical method was found. A sensitivity analysis excluding these two values was performed in addition to the final analyses. Overall, the difference in the results of the main analysis and sensitivity analysis in empagliflozin plasma exposure amounted to < 20%. The mean terminal half‐life was not different between the two analyses; however, the median observed time from dosing until maximum concentration was 1.50 h in the main analysis and 1.05 h in the sensitivity analysis (Table S1)
Table 2.
Arithmetic mean values of pharmacokinetic characteristics of empagliflozin in plasma
| Characteristic | 5 mg empagliflozin (N=9) | 10 mg empagliflozin (N=8) | 25 mg empagliflozin (N=10) | |||
|---|---|---|---|---|---|---|
| Mean | %CV | Mean | %CV | Mean | %CV | |
| AUC0‐∞, nmol h/l | 1270 | 51.9 | 1450 | 17.2 | 5250 | 27.6 |
| AUC0‐tz, nmol h/l | 1240 | 54.2 | 1420 | 16.9 | 5150 | 27.6 |
| AUC0‐24, nmol h/l | 1110 | 42.7 | 1310 | 18.9 | 4720 | 27.4 |
| Cmax, nmol/l | 175 | 54.2 | 211 | 59.1 | 692 | 57.3 |
| tmax *, h | 1.50 | 0.95–7.92 | 1.25 | 0.97–4.17 | 1.78 | 0.50–4.00 |
| t1/2, h | 7.03 | 18.9 | 7.61 | 27.0 | 8.09 | 26.8 |
AUC0‐∞, area under the plasma concentration–time curve from time 0 extrapolated to infinity; AUC0‐tz, area under the plasma concentration–time curve from time 0 to the time of the last quantifiable concentration; AUC0–24, area under the plasma concentration‐time curve from time 0 to 24 h post dose; Cmax, maximum observed plasma concentration; CV, arithmetic coefficient of variation; tmax, time from dosing until maximum observed concentration is reached in plasma; t1/2, terminal half‐life in plasma.
*For tmax, the median and range are given (instead of mean and %CV).
Figure 1.
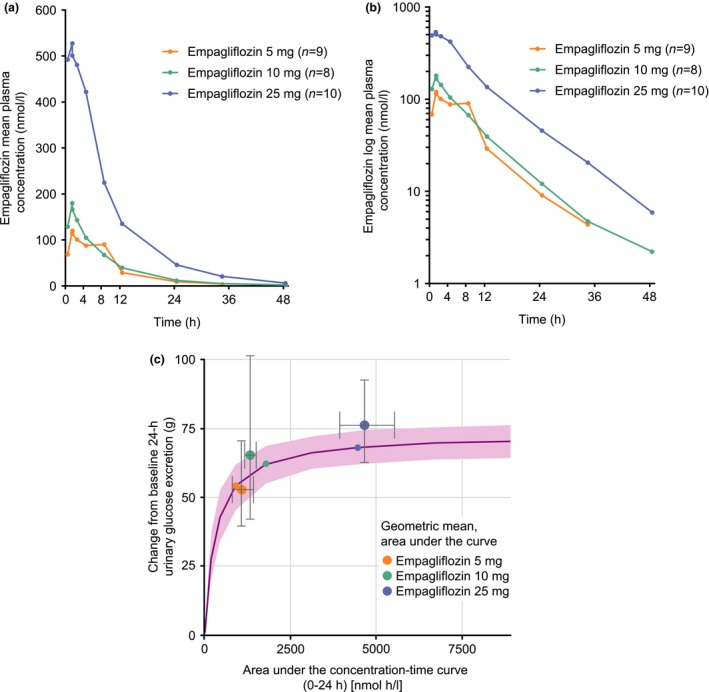
Arithmetic mean concentration–time profiles of empagliflozin in plasma, (a) linear scale and (b) log scale, and (c) simulated exposure–response profile. (a and b) The mean empagliflozin plasma concentration for the 5‐mg dose group at 48 h post dose was not calculated as only three individual values were available at this time point. As predefined, descriptive statistics of concentrations at specific time points were calculated only if at least two‐thirds of the participants had concentrations within the validated concentration range. (c) Pink line: median of simulations, pink shaded area: 95% CI of simulated median. Small circles: change from baseline in 24‐h urinary glucose excretion for an adult participant at the median 24‐h area under the concentration–time curve in the respective dose group of the simulations [typical adult person: 58‐year‐old male with Type 2 diabetes; baseline mean daily glucose: 6.9 mmol/l (adjusted to typical value for paediatric patient)]. Large circles: geometric mean (gMean) change from baseline in 24‐h urinary glucose excretion for paediatric participant at the gMean 24‐h area under the curve in the respective dose group. Error bars: 95% CI of the gMeans in each dose group in paediatric participants, calculated as gMean ± 1.96×se.
In Table 3, the pharmacokinetic characteristics of 10 mg and 25 mg empagliflozin in the present paediatric population with Type 2 diabetes were compared with the results from a previous trial 13 in adults with Type 2 diabetes after first‐dose administration of 10 mg and 25 mg empagliflozin (adults did not receive 5 mg dose). Pharmacokinetic exposure was generally similar in the paediatric population and the adults with Type 2 diabetes, with a slightly lower exposure (with respect to area under the plasma concentration–time curve and maximum observed plasma concentration) in the paediatric population in the 10‐mg dose group compared with the adult population. For the 25‐mg dose group, the maximum observed plasma concentration was slightly lower and the area under the plasma concentration–time curve was slightly higher in the young people compared with the adults. For the 10‐mg and 25‐mg doses, the median time to reach maximum observed plasma concentration and the mean terminal plasma half‐life were similar in the adult and paediatric sample populations (Table 3). The fraction of the dose eliminated in the urine as well as the renal clearance of empagliflozin was higher in the paediatric vs the adult population (Table 3).
Table 3.
Comparison of pharmacokinetic characteristics of empagliflozin in the paediatric population in this trial and adults 13 with Type 2 diabetes after single‐dose empagliflozin administration
| Population | Empagliflozin dose, mg | AUC0‐∞, nmol h/l | AUC0‐24, nmol h/l | Cmax, nmol/l | tmax *, h | t1/2, h | fe0‐24, % | CLR,0‐24, ml/min |
|---|---|---|---|---|---|---|---|---|
| Paediatric (n=8) | 10 | 1450 (17.2) | 1310 (18.9) | 211 (59.1) | 1.25 (0.97‐4.17) | 7.61 (27.0) | 18.4 (24.2) | 52.2 (23.8) |
| Adults 13 (n =16) | 10 | 1740 (16.4) | 1550 (16.2) | 309 (45.2) | 1.50 (1.00‐2.50) | 8.76 (13.0) | 12.5 (24.0) | 30.1 (25.1) |
| Paediatric (n =10) | 25 | 5250 (27.6) | 4720 (27.4) | 692 (57.3) | 1.78 (0.50‐4.00) | 8.09 (26.8) | 19.4 (15.4) | 40.0 (26.9) |
| Adults 13 (n =16) | 25 | 4340 (23.1) | 3930 (22.9) | 722 (20.0) | 1.50 (0.75‐2.00) | 8.24 (14.9) | 13.3 (24.5) | 32.4 (28.1) |
AUC0‐∞, area under the plasma concentration‐time curve from time 0 extrapolated to infinity; AUC0‐24, area under the plasma concentration‐time curve from time 0 to 24 h post dose; CLR,0‐24, renal clearance of the analyte from time 0 to 24 h post dose; Cmax, maximum observed plasma concentration; CV, arithmetic coefficient of variation; Fe0‐24, fraction of analyte eliminated in urine from time 0 to 24 h post dose; tmax, time from dosing until maximum observed concentration is reached in plasma; t1/2, terminal half‐life in plasma. For AUC0‐∞, AUC0‐24, Cmax, and t1/2, the arithmetic mean and %CV are given.
*For tmax, the median and range are given (instead of mean and %CV).
Pharmacodynamic outcomes
The mean baseline urinary glucose excretion was similar for the 5‐mg and 10‐mg dose groups but considerably lower for the 25‐mg dose group. Similarly, the mean baseline fasting plasma glucose level was similar for the 5‐mg and 10‐mg dose groups but somewhat lower for the 25‐mg dose group (Table 1).
A dose‐dependent increase in urinary glucose excretion was observed on day 1, with mean changes from baseline (adjusted for baseline urinary glucose excretion and baseline fasting plasma glucose) of 53.1 g/24 h (95% CI 31.8,74.4) in the 5‐mg dose group, 73.0 g/24 h (95% CI 51.9,94.1) in the 10‐mg dose group, and 87.4 g/24 h (95% CI 67.9,107.0) in the 25‐mg dose group (Fig. 2). Administration of a single dose of empagliflozin led to a mean decrease in fasting plasma glucose (adjusted for baseline fasting plasma glucose) of 0.9 mmol/l (95% CI –1.6 –0.1) in the 5‐mg dose group, 0.9 mmol/l (95% CI –1.7,–0.2) in the 10‐mg dose group, and 1.1 mmol/l (95% CI –1.8,–0.5) in the 25‐mg dose group at 24 h post dose (Fig. 3).
Figure 2.
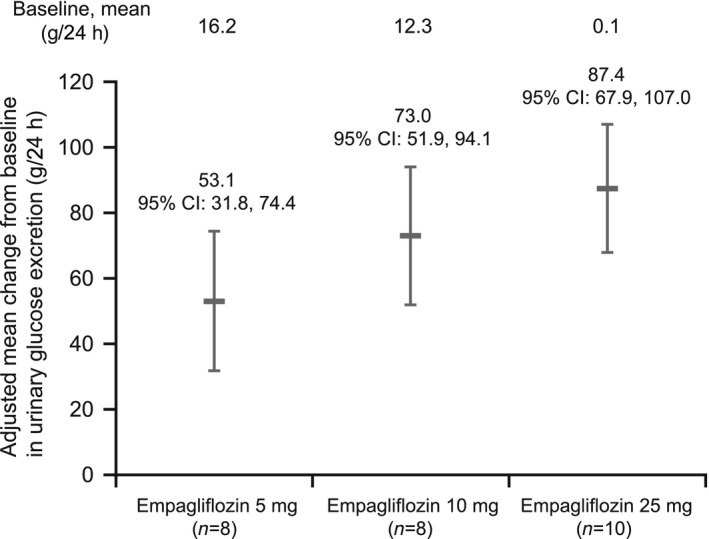
Mean changes from baseline in urinary glucose excretion (including 95% CIs) on day 1 (adjusted for baseline urinary glucose excretion and fasting plasma glucose).
Figure 3.
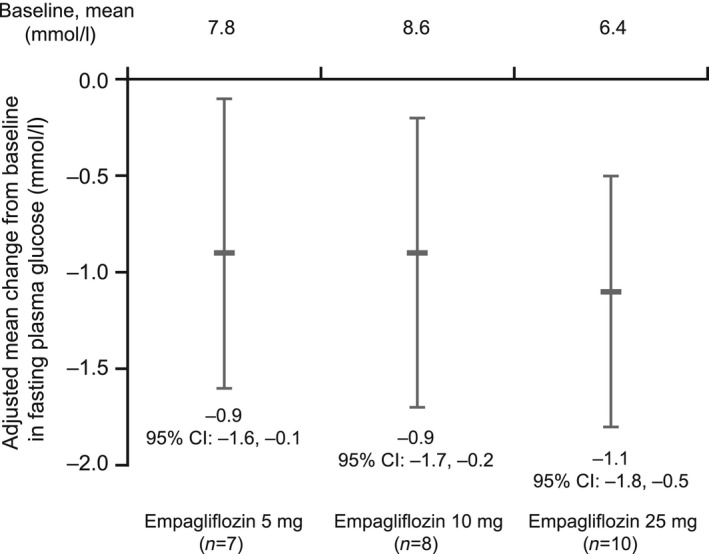
Mean changes from baseline in fasting plasma glucose (including 95% confidence intervals) after 24 h (adjusted for baseline fasting plasma glucose). Analysis includes only patients with both a baseline and on‐treatment fasting plasma glucose value.
The mean baseline daily glucose concentration differed among the three dose groups, with mean values of 7.6 mmol/l in the 5‐mg dose group, 8.4 mmol/l in the 10‐mg dose group, and 6.3 mmol/l in the 25‐mg group. For all three dose groups, mean daily glucose was lower in the 24‐h period after single‐dose empagliflozin administration compared with the baseline value.
The micturition frequency was higher in the majority of participants (87.5% in the 5‐mg dose group, 83.3% in the 10‐mg dose group, and 80.0% in the 25‐mg dose group) in the 24‐h period after single‐dose empagliflozin administration than at baseline (in the 24 h before trial drug intake). Similarly, the mean micturition volume increased in the 24‐h period after single‐dose empagliflozin administration (Fig. S3).
Nearly all participants had a high baseline eGFR (based on the Schwartz formula) corresponding to the hyperfiltration range (Table 1). A small decrease in mean eGFR was seen in all dose groups 24 h after single‐dose empagliflozin administration [mean (sd) –14.8 (7.6), –7.9 (12.8) and –9.7 (11.0) ml/min/1.73 m2 with empagliflozin 5 mg, 10 mg and 25 mg, respectively).
Exposure–response analysis (data from adults) and comparison with observed data from young people
The comparison of the simulated exposure–response profile of a typical adult (male, 58 years of age) with observed changes in 24‐h urinary glucose excretion for the respective dose groups suggests a similar exposure–response relationship in the adult and paediatric cohorts when adjusting for differences in mean daily glucose (10.3 mmol/l in adults vs 6.9 mmol/l in young people) during simulations (Fig. 1c). This was confirmed by the joint analysis of data in adults and young people which showed that the exposure–response was similar in the two populations when accounting for differences in covariate distributions (baseline mean daily glucose, female sex, Asian race, body weight).
Safety
Out of 27 treated participants, seven (25.9%) reported at least one adverse event during the on‐treatment phase of the trial, including four of the nine participants (44.4%) in the 5‐mg dose group, one of eight participants (12.5%) in the 10‐mg dose group, and two of 10 participants (20.0%) in the 25‐mg dose group (Table S2). All adverse events were of mild or moderate intensity. There was one adverse event of ‘dehydration’ of mild intensity in one participant in the empagliflozin 10‐mg group during the on‐treatment phase of the trial, which was judged to be related to the trial medication. One case of hypoglycaemia was reported (plasma glucose value of 3.8 mmol/l at 32 h after the empagliflozin administration). This hypoglycaemic event was not considered as an adverse event. Protocol‐specified adverse events of special interest (hepatic injury, decreased renal function and diabetic ketoacidosis) were not reported. No clinically relevant findings regarding safety, laboratory measurements, electrocardiogram recordings, physical examinations or vital sign measurements were reported as adverse events. For systolic and diastolic blood pressure, no clinically meaningful changes were observed after single‐dose administration of empagliflozin (data not shown).
Discussion
There is an unmet need for pharmacological agents to manage hyperglycaemia in young people with Type 2 diabetes. The present study provides initial results regarding the dose response of the SGLT2 inhibitor empagliflozin, an approved drug with established efficacy and safety for management of Type 2 diabetes in adults which has also been shown to reduce the risk of cardiovascular death in adults with Type 2 diabetes who have established cardiovascular disease 10, 11, 15. Study plans for approval of new drugs in children and adolescents often include a dose‐finding study with the primary aim of ensuring that paediatric doses that have been extrapolated from approved drug doses in adults do not result in inappropriate drug exposures in the paediatric population. Thus, the most important finding of the present study was that the pharmacokinetic characteristics of the 10‐ and 25‐mg doses of empagliflozin approved for use in adults were generally similar to those in young people with Type 2 diabetes. Indeed, the area under the plasma concentration–time curve and other pharmacokinetic values indicated only modest differences in drug exposure in the paediatric population compared with the adult population. The slightly lower exposure of participants in the 10‐mg dose group compared with adults is likely to be attributable to the higher body weight observed in the young people in this group than in adults with Type 2 diabetes in previous pharmacokinetic and pharmacodynamic studies. Nevertheless, the mean body weight of 111 kg in the 10‐mg treatment group is typical of the weight often observed in adolescents with Type 2 diabetes 16.
As seen in adults, the pharmacodynamic actions of empagliflozin showed dose‐dependent increases in urinary glucose excretion and corresponding decreases in fasting plasma glucose and mean daily glucose levels. Indeed, findings for the 10‐mg and 25‐mg drug doses were similar in young people and adults. This was confirmed in a model‐based exposure–response analysis that included data from paediatric and adult participants. Interestingly, the pharmacokinetics of other new drugs for treatment of young people with Type 2 diabetes have also shown similar or mildly reduced drug exposures compared with values in adults with Type 2 diabetes 17, 18, 19. In addition to demonstrating the rapid and potent inhibition of renal SGLT2 activity in the young people, the drug was well tolerated.
The fraction of the dose eliminated in the urine as well as the renal clearance of empagliflozin was higher in the paediatric vs adult population. This might be explained by a higher baseline eGFR in the paediatric population with Type 2 diabetes compared with the adults; however, as mentioned above, the main pharmacokinetic and pharmycodynamic characteristics of the 10‐ and 25‐mg doses of empagliflozin were generally similar to those in young people with Type 2 diabetes. Hence, a higher eGFR in young people with Type 2 diabetes would not be expected to result in altered drug effects.
The present study has a number of limitations, including the single‐dose approach and the relatively small sample sizes of the three treatment groups, where one or two outliers can confound comparisons among the three dose groups. Larger, randomized controlled studies over 24–52 weeks of treatment will be required to establish the long‐term efficacy and safety of treatment with empagliflozin in children and adolescents with Type 2 diabetes.
In summary, after a single oral dose of empagliflozin, adults and young people with Type 2 diabetes had similar exposure–response relationships (after accounting for significant covariates), along with an acceptable short‐term safety profile. The dose‐dependent increases in urinary glucose excretion, along with decreases in fasting plasma glucose, suggest that SGLT2 inhibition with empagliflozin may become an important treatment tool to improve glycaemic control and possibly assist with weight management in the growing population of young people with Type 2 diabetes. This study supports the suggestion that 10‐mg and/or 25‐mg daily doses of empagliflozin should be considered for a pivotal phase III paediatric study.
Funding sources
The study was funded by Boehringer Ingelheim & Eli Lilly and Company Diabetes Alliance.
Competing interests
A.Y., G.S., J.W., V.N., D.H., S.K. and J.M. are employees of Boehringer Ingelheim. L.M.B.L. has served as Consultant for Eli Lilly, Sanofi, NovoNordisk, Bristol Myers Squibb, AstraZeneca, Boehringer Ingelheim, Menarini, J & J, LifeScan/Animas, Insulet, Roche Diagnostics and Dexcom. W.V.T. has served as Consultant for AstraZeneca, Boehringer Ingelheim, Janssen, Novo Nordisk, Sanofi and Takeda.
Supporting information
Figure S1. Trial design.
Figure S2. Arithmetic mean concentration‐time profiles of empagliflozin in plasma (a, linear scale, sensitivity analysis for 5 mg dose excluding 2 physiologically implausible values; b, linear scale, individual empagliflozin concentrations of affected patient).
Figure S3. Mean changes from baseline in micturition volume (including 95% confidence intervals) on Day 1 and Day 2 (adjusted for baseline micturition volume).
Table S1. Arithmetic mean values of pharmacokinetic parameters of empagliflozin 5mg in plasma: Comparison of the results of the main analysis and sensitivity analysis.
Table S2. Adverse events: overall summary.
Table S2. Adverse events: overall summary.
Acknowledgements
The authors thank the young people, investigators and staff who participated in this study.
Diabet. Med. 35, 1096–1104 (2018)
References
- 1. Mayer‐Davis EJ, Lawrence JM, Dabelea D, Divers J, Isom S, Dolan L et al Incidence Trends of Type 1 and Type 2 Diabetes among Youths, 2002‐2012. N Engl J Med 2017; 376: 1419–1429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. TODAY Study Group , Zeitler P, Hirst K, Pyle L, Linder B, Copeland K et al A clinical trial to maintain glycemic control in youth with type 2 diabetes. N Engl J Med 2012; 366: 2247–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Barnett AH, Mithal A, Manassie J, Jones R, Rattunde H, Woerle HJ et al Efficacy and safety of empagliflozin added to existing antidiabetes treatment in patients with type 2 diabetes and chronic kidney disease: a randomised, double‐blind, placebo‐controlled trial. Lancet Diabetes Endocrinol 2014; 2: 369–384. [DOI] [PubMed] [Google Scholar]
- 4. Häring HU, Merker L, Seewaldt‐Becker E, Weimer M, Meinicke T, Broedl UC et al Empagliflozin as add‐on to metformin in patients with type 2 diabetes: a 24‐week, randomised, double‐blind, placebo‐controlled trial. Diabetes Care 2014; 37: 1650–1659. [DOI] [PubMed] [Google Scholar]
- 5. Kovacs CS, Seshiah V, Swallow R, Jones R, Rattunde H, Woerle HJ et al Empagliflozin improves glycaemic and weight control as add‐on therapy to pioglitazone or pioglitazone plus metformin in patients with type 2 diabetes: a 24‐week, randomised, placebo‐controlled trial. Diabetes Obes Metab 2014; 16: 147–158. [DOI] [PubMed] [Google Scholar]
- 6. Roden M, Weng J, Eilbracht J, Delafont B, Kim G, Woerle HJ et al Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet Diabetes Endocrinol 2013; 1: 208–219. [DOI] [PubMed] [Google Scholar]
- 7. Rosenstock J, Jelaska A, Frappin G, Salsali A, Kim G, Woerle HJ et al Improved glucose control with weight loss, lower insulin doses, and no increased hypoglycemia with empagliflozin added to titrated multiple daily injections of insulin in obese inadequately controlled type 2 diabetes. Diabetes Care 2014; 37: 1815–1823. [DOI] [PubMed] [Google Scholar]
- 8. Rosenstock J, Jelaska A, Zeller C, Kim G, Broedl UC, Woerle HJ et al Impact of empagliflozin added on to basal insulin in type 2 diabetes inadequately controlled on basal insulin: a 78‐week randomised, double‐blind, placebo‐controlled trial. Diabetes Obes Metab 2015; 17: 936–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tikkanen I, Narko K, Zeller C, Green A, Salsali A, Broedl UC et al Empagliflozin reduces blood pressure in patients with type 2 diabetes and hypertension. Diabetes Care 2015; 3: 420–428. [DOI] [PubMed] [Google Scholar]
- 10. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S et al Empagliflozin, Cardiovascular Outcomes, and Mortality in Type 2 Diabetes. N Engl J Med 2015; 373: 2117–2128. [DOI] [PubMed] [Google Scholar]
- 11. Wanner C, Inzucchi SE, Lachin JM, Fitchett D, von Eynatten M, Mattheus M et al Empagliflozin and Progression of Kidney Disease in Type 2 Diabetes. N Engl J Med 2016; 375: 323–334. [DOI] [PubMed] [Google Scholar]
- 12. Heise T, Seman L, Macha S, Jones P, Marquart A, Pinnetti S et al Safety, tolerability, pharmacokinetics, and pharmacodynamics of multiple rising doses of empagliflozin in patients with type 2 diabetes mellitus. Diabetes Ther 2013; 4: 331–345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Heise T, Seewaldt‐Becker E, Macha S, Hantel S, Pinnetti S, Seman L et al Safety, tolerability, pharmacokinetics and pharmacodynamics following 4 weeks’ treatment with empagliflozin once daily in patients with type 2 diabetes. Diabetes Obes Metab 2013; 15: 613–621. [DOI] [PubMed] [Google Scholar]
- 14. Kanada S, Koiwai K, Taniguchi A, Sarashina A, Seman L, Woerle HJ. Pharmacokinetics, pharmacodynamics, safety and tolerability of 4 weeks’ treatment with empagliflozin in Japanese patients with type 2 diabetes mellitus. J Diabetes Investig 2013; 4: 613–617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. U.S. Food and Drug Administration . Empagliflozin product information.. 2016. Available at https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/204629s008lbl.pdf Last accessed 15 November 2017.
- 16. Copeland KC, Zeitler P, Geffner M, Guandalini C, Higgins J, Hirst K et al Characteristics of adolescents and youth with recent‐onset type 2 diabetes: the TODAY cohort at baseline. J Clin Endocrinol Metab 2011; 96: 159–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jones KL, Arslanian S, Peterokova VA, Park JS, Tomlinson MJ. Effect of metformin in pediatric patients with type 2 diabetes: a randomised controlled trial. Diabetes Care 2002; 25: 89–94. [DOI] [PubMed] [Google Scholar]
- 18. Gottschalk M, Danne T, Vlajnic A, Cara JF. Glimepiride versus metformin as monotherapy in pediatric patients with type 2 diabetes: a randomised, single‐blind comparative study. Diabetes Care 2007; 30: 790–794. [DOI] [PubMed] [Google Scholar]
- 19. Christensen ML, Meibohm B, Capparelli EV, Velasquez‐Mieyer P, Burghen GA, Tamborlane WV. Single‐ and multiple‐dose pharmacokinetics of pioglitazone in adolescents with type 2 diabetes. J Clin Pharmacol 2005; 45: 1137–1144. [DOI] [PubMed] [Google Scholar]
- 20. Tamborlane WV, Klingensmith G. Crisis in care: limited treatment options for type 2 diabetes in adolescents and youth. Diabetes Care 2013; 36: 1777–1778. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Trial design.
Figure S2. Arithmetic mean concentration‐time profiles of empagliflozin in plasma (a, linear scale, sensitivity analysis for 5 mg dose excluding 2 physiologically implausible values; b, linear scale, individual empagliflozin concentrations of affected patient).
Figure S3. Mean changes from baseline in micturition volume (including 95% confidence intervals) on Day 1 and Day 2 (adjusted for baseline micturition volume).
Table S1. Arithmetic mean values of pharmacokinetic parameters of empagliflozin 5mg in plasma: Comparison of the results of the main analysis and sensitivity analysis.
Table S2. Adverse events: overall summary.
Table S2. Adverse events: overall summary.


