Abstract
Tobacco smoking and alcohol consumption are well‐established risk factors for head and neck cancer. The prognostic role of smoking and alcohol intake at diagnosis have been less well studied. We analysed 1,393 people prospectively enrolled into the Head and Neck 5000 study (oral cavity cancer, n=403; oropharyngeal cancer, n=660; laryngeal cancer, n=330) and followed up for a median of 3.5 years. The primary outcome was all‐cause mortality. We used Cox proportional hazard models to derive minimally adjusted (age and gender) and fully adjusted (age, gender, ethnicity, stage, comorbidity, body mass index, HPV status, treatment, education, deprivation index, income, marital status, and either smoking or alcohol use) mortality hazard ratios (HR) for the effects of smoking status and alcohol intake at diagnosis. Models were stratified by cancer site, stage and HPV status. The fully‐adjusted HR for current versus never‐smokers was 1.7 overall (95% confidence interval [CI] 1.1, 2.6). In stratified analyses, associations of smoking with mortality were observed for oropharyngeal and laryngeal cancers (fully adjusted HRs for current smokers: 1.8 (95% CI=0.9, 3.40 and 2.3 (95% CI=0.8, 6.4)). We found no evidence that people who drank hazardous to harmful amounts of alcohol at diagnosis had a higher mortality risk compared to non‐drinkers (HR=1.2 (95% CI=0.9, 1.6)). There was no strong evidence that HPV status or tumour stage modified the association of smoking with survival. Smoking status at the time of a head and neck cancer diagnosis influenced all‐cause mortality in models adjusted for important prognostic factors.
Keywords: head and neck cancer, smoking, alcohol, human papillomavirus, survival
Short abstract
What's new?
Smoking and alcohol use are risk factors for developing head‐and‐neck cancer (HNC) and are known to influence mortality in general. However, the prognostic role of smoking status and alcohol intake at time of diagnosis on HNC survival is less clear. In this study, the authors provide a comprehensive, prospective analysis of mortality risk in different tumour sites, adjusting for important prognostic factors such as stage, comorbidity, and HPV infection. These results may provide insight to inform and improve prediction of clinical outcomes.
Abbreviations
- BMI
body mass index
- CI
confidence interval
- HNC
head and neck cancer
- HPV
human papillomavirus
- HR
hazard ratio
- HSCIC
Health and Social Care Information Centre
- ICD
International Classification of Diseases
- IMD
index of multiple deprivation
- MIF
Median fluorescence intensity
- TNM
tumor, node, metastases
Head and neck cancers (HNCs) are a heterogeneous group of tumours that arise from the mucosal epithelium of the upper aerodigestive tract. Collectively, they represent the sixth leading cause of cancer worldwide.1 Within the UK, HNC incidence has increased by almost a quarter in the last decade, with an estimated annual burden of ∼11,400 new cases.2 Since the early 1990s, oropharyngeal cancers (OPCs, tonsil and base of tongue) have seen the biggest rise of any HNC, with incidence rates more than doubling.3 In contrast, there has been a 20% decrease in the incidence of laryngeal cancers in the same period,4 though rates have levelled off more recently.5
Lifestyle factors play an important role in the aetiology of these cancers.6 Around 75% of HNCs have been attributed to the combined effects of tobacco and alcohol use.7 Human papillomavirus (HPV) infection, predominantly HPV‐16 infection, is also recognised as a primary risk factor for OPCs, especially in younger age groups.8
Despite an overall decline in HNC mortality rates,3 survival remains poor. The overall 5‐year survival rate is around 50%, but ranges from 33% for hypopharyngeal cancers to 60% for laryngeal cancers.9 People with HPV‐positive oropharyngeal tumours have consistently demonstrated improved survival compared to their HPV‐negative counterparts, despite the fact that they are frequently diagnosed at a later tumour stage.10 This is largely due to improved therapeutic response.8 People with HPV‐positive OPCs also tend to have distinct risk factor profiles, including higher socioeconomic status and a lower comorbidity,11 which may favour survival.
Although tobacco and alcohol drinking are responsible for the majority of new HNC cases, the prognostic role of smoking status and alcohol intake at the time of cancer presentation remains unclear, especially for people with HPV‐associated oropharyngeal tumours. In general, smoking and heavy alcohol use are both related to increased mortality risk, but estimates of the magnitude of the effects in this population are hugely inconsistent. Moreover, it has yet to be established whether smoking and alcohol use provide any additional prognostic information beyond the tumour, node, metastasis (TNM) staging system, which currently forms the basis for clinical decision making in people with HNC.
In general, the HNC literature describes a dose‐dependent increase in mortality risk with increasing exposure to tobacco pre‐diagnosis.12, 13, 14, 15, 16, 17, 18, 19 Studies have frequently been undertaken in single cancer sites, however, typically the larynx or oropharynx.17, 18, 19 Where studies have included multiple sites, analyses have rarely stratified on this. Both factors may help explain why estimates of the effect of smoking status on HNC survival have varied so considerably. In addition to this, studies have frequently been unable to adjust for important prognostic factors, such as comorbidity,13, 14, 15 body mass index13, 15, 17, 18 or HPV status,12, 13, 14, 15 often because they were conducted retrospectively.
Evidence of an association between pre‐treatment alcohol use and HNC mortality risk is conflicting. Some studies report an inverse association between alcohol intake and survival,13, 19, 20, 21 whilst others have found little or no evidence of an effect.17, 22 Consequently, it is unclear whether any association of alcohol consumption with HNC cancer mortality is genuine, or the result of residual confounding by smoking (or other factors). Recently, it was suggested that the effects of alcohol intake on HNC survival may differ by treatment method and primary site,21 but this study only included 427 individuals from a single cancer centre in Japan, emphasising the need for further research in this area.
An improved understanding of the prognostic significance of drinking and smoking status by site could help improve HNC outcome prediction. This could in turn help inform the lifestyle advice clinicians give to people upon a diagnosis of HNC. In the present study, we used data collected as part of a large, prospective study of over 5,500 people with HNC from across 76 UK sites (Head and Neck 5000),23 to examine the effect of smoking status and alcohol intake on survival in different cancer sites. To our knowledge, this is the largest study of its kind. Larger sample sizes generally lead to increased precision, and therefore our results are arguably the most accurate estimates of the effects of smoking status and alcohol intake at diagnosis on HNC mortality to date. Furthermore, given the wealth of clinical, biological and lifestyle data available, owing to the prospective study design, we were able to investigate possible interactions between smoking, alcohol and HPV status in determining mortality risk.
Methods
Study population
The study population included individuals enrolled in the Head and Neck 5000 clinical cohort study. Full details of the study methods and population are described in detail elsewhere.23 Briefly, 5,511 people with a new HNC diagnosis were recruited from 76 centres across the UK between April 2011 and December 2014. At the time of recruiting, there were approximately 180 HNC centres nationally; 78 were approached. Recruitment rates to the study varied by centre, from around 20% to around 90% of eligible HNC cases. Overall, we estimate that when all study centres were open, the study captured a third of all incident cases in the UK. Individuals were recruited before they started treatment, unless their treatment was their diagnostic procedure. At baseline, 5,474 (99%) data capture forms and 4,099 (74%) health and lifestyle questionnaires were completed.24 Baseline blood samples were obtained from 4,676 (85%) individuals. Full ethical approval was granted by The South West – Frenchay Regional Ethics Committee granted (ref: 10/H0107/57).
Baseline data collection
Participants were asked to complete three self‐administered questionnaires at baseline, which included questions on social and economic circumstances, lifestyle behaviours, general health and past sexual behaviours.23, 25 Research nurses collected a blood sample from all consenting participants. Samples were frozen and stored at −80°C in the Avon Longitudinal Study of Parents and Children (ALSPAC) bio‐sample repository (http://www.bristol.ac.uk/alspac/). At each site, information on stage at diagnosis, tumour stage, treatment and various other clinical and pathologic prognostic variables was abstracted from participants’ medical records. Diagnoses were coded using the International Classification of Diseases (ICD) version 10.26 Clinical staging of the tumour was based on the American Head and Neck Society TNM staging of HNC.27
Assessment of tobacco and alcohol exposure
Detailed information on tobacco and alcohol history was obtained at baseline via the self‐reported questionnaire. Participants were asked about their current smoking and drinking status and their use of tobacco and alcohol products pre‐diagnosis.25 Smoking status was defined as “current,” “former” or “never.” Former smokers were defined as having smoked at least one cigarette a day for a period of at least a year. Never smokers were defined as having never smoked at least one daily cigarette during a whole year. The questionnaire differentiated between use of cigarettes, hand‐rolled cigarettes, cigars and smokeless tobacco. Respondents were asked to report their average weekly alcohol consumption of a range of alcoholic beverage types before they became ill.
Assessment of HPV status
HPV serologic testing was conducted at the German Cancer Research Center (DKFZ, Heidelberg, Germany) using glutathione S‐transferase multiplex assays.28 Plasma was analysed for antibodies against the HPV16 E6 oncoprotein (a marker of HPV‐transformed tumour cells29, 30), using a median fluorescence intensity (MFI) cut‐off of ≥1,000 MFI.31 The detection of antibodies against HPV early proteins in serum has been shown to be highly sensitive and specific for HPV16‐driven OPSCC, and consequently provides a good surrogate marker in the absence of appropriate histologic specimens.32
Study follow‐up
Nurses at each site extracted up‐to‐date treatment and cancer recurrence information from participants’ medical records.23 All participants were flagged with NHS Digital (formerly the Health and Social Care Information Centre (HSCIC)) for ongoing notification of deaths and provision of information recorded on the death certificate.
Statistical analysis
All analyses were performed using the data release H&N024_H&N dataset_v2.3.
The included population
The study involved participants with cancers of the oral cavity, oropharynx and larynx (C01‐C06; C09‐C10; C32).
Defining exposure
From the questionnaire data, we derived an average intake of alcohol consumption in units per week. Baseline drinking categories were then defined as none, moderate (men and women drinking <14 units/week), hazardous (men consuming 14–50 units/week; women consuming 14–35 units/week) and harmful (men consuming >50 units/week; women consuming >35 units/week), where one unit of alcohol = 8 g/10 mL ethanol.33 Smoking categories were consistent with those of the questionnaire (never, former and current).
Defining outcome
The outcome of interest was death from any cause. Follow‐up for survival analysis was defined as the time in years from study enrolment to date of death from any cause or the date of censorship (i.e., the last date of follow‐up). We only included all‐cause deaths in the current analysis as assignment of death as being due to HNC on death certificates is subject to misattribution bias.34
Descriptive analysis
Baseline descriptive data were stratified by tumour site and HPV status (oropharyngeal only). The χ 2‐test was used to compare the distribution of variables between groups. Kaplan–Meier survival curves were plotted to visualize survival probabilities, and differences in survival between tumour sites in relation to smoking and alcohol status were compared using the log‐rank test.
Missing data
Data were missing for smoking status, alcohol intake and the following covariates: body mass index (BMI), tumour stage, treatment group1, comorbidity2, ethnicity, annual household income, Index of Multiple Deprivation (IMD3),35 highest education level obtained and marital status. Missing values were imputed using the ICE package for multiple chained equations in STATA.36 Twenty imputed datasets were generated for each tumour site and were combined using Rubin's rule to obtain valid statistical inferences.37 Imputation models included the event indicator, the Nelson–Aalen estimator of the cumulative hazard,38 the tobacco and alcohol exposure variables, in addition to the confounders listed above.
Survival analysis
The primary analyses included complete cases only i.e. participants with complete data for confounders used in the adjusted models and information on smoking and alcohol consumption. Cox proportional hazards models, stratified by tumour site, stage and HPV status, were used to examine the associations of baseline smoking status and alcohol intake with survival. Only oropharyngeal cases were considered in the HPV stratified models because the role of HPV in tumours outside the oropharynx is uncertain, as is the ability of serology to detect HPV driven tumours in other anatomical sites. Hazard ratios (HRs) and 95% confidence intervals (CIs) for mortality were calculated for each category of smoking and drinking, using never‐smokers and non‐drinkers as the reference groups. The proportional hazard assumption was tested by plotting scaled Schoenfeld's residuals against survival time. Unadjusted Kaplan–Meier graphs were plotted to compare overall survival between cancer sites and between HPV seronegative and HPV seropositive cancers.
Minimally adjusted models included age and gender. Fully adjusted models included the following variables: clinical (tumour stage, BMI, comorbidity, treatment intent and HPV status), sociodemographic (education, annual household income, IMD and marital status, and ethnicity) and behavioural. Models evaluating the effects of smoking included adjustment for alcohol, whereas models evaluating the effects of alcohol included adjustment for smoking. To account for unobserved heterogeneity in recruitment centres, we fitted a Cox model with a shared frailty term (with gamma distribution). The significance of the frailty component was tested using a likelihood‐ratio test. To allow assessment of the effect of controlling for potential confounders, we qualitatively compared minimally adjusted with fully adjusted models.
As a sensitivity analysis, we performed the same cox regression models in the imputed dataset. Results, stratified by tumour site and HPV status can be found in Supporting Information Tables 6 and 7.
We further investigated potential interactions between: (i) tumour stage and smoking, (ii) tumour stage and alcohol consumption, (iii) HPV status and smoking, (iv) HPV status and alcohol consumption and (v) smoking and alcohol intake, by fitting an interaction term in the models and using a likelihood ratio test. As above, HPV analyses were restricted to the subset of participants with oropharyngeal tumours.
All reported p‐values are two‐sided, with α = 0.05. All analyses were conducted using Stata v15 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP).
Results
Baseline characteristics of study population
The raw dataset included 5,369 individuals with HNC (Fig. 1). Of these, 4,276 had cancers of the oral cavity (n = 1,296), oropharynx (n = 1,910) and larynx (n = 1,070). After selecting participants with complete data, the analytic sample consisted of 1,393 individuals (oral cavity n = 403; oropharynx n = 660; larynx n = 330).
Figure 1.
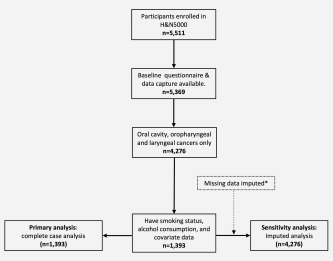
Flow of head and neck 5000 participants through the study.
Descriptive characteristics of the sample, stratified by tumour site and HPV status, are presented in Tables 1 and 2 (Supporting Information Tables 1 and 2 for participants included in the imputed analysis). There were differences across tumour groups with respect to gender, age, stage, HPV status, comorbidity, smoking status, education level, annual household income, IMD quintile and marital status across tumour groups (Table 1). There was no strong evidence of a difference in the amount of alcohol consumed per week (p for trend = 0.739; Table 1). At the time of diagnosis, the proportion of former or current smokers was 72.7%, 70.0% and 90.6% in the oral cavity, oropharyngeal and laryngeal cancer groups, respectively. Smoking and drinking histories were comparable for participants included in the imputed analysis (Supporting Information Table 1).
Table 1.
Baseline descriptive characteristics of participants, stratified by tumour site (n = 1,393)
| Oral Cavity (n = 403) | Oropharynx (n = 660) | Larynx (n = 330) | ||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic | N | % | N | % | N | % | Total | p valuesa |
| Survival status | N/A | |||||||
| Alive | 288 | 71.50% | 542 | 82.10% | 248 | 75.20% | 1,078 | |
| Died | 115 | 28.50% | 118 | 17.90% | 82 | 24.80% | 315 | |
| Gender | <0.001 | |||||||
| Male | 247 | 61.30% | 539 | 81.70% | 278 | 84.20% | 1,064 | |
| Female | 156 | 38.70% | 121 | 18.30% | 52 | 15.80% | 329 | |
| Age group | <0.001 | |||||||
| <50 | 66 | 16.40% | 93 | 14.10% | 17 | 5.20% | 176 | |
| 50–64 | 170 | 42.20% | 400 | 60.60% | 125 | 37.90% | 695 | |
| 65–79 | 138 | 34.20% | 153 | 23.20% | 159 | 48.20% | 450 | |
| 80+ | 29 | 7.20% | 14 | 2.10% | 29 | 8.80% | 72 | |
| Ethnicity group | 0.005 | |||||||
| White | 392 | 97.30% | 656 | 99.40% | 328 | 99.40% | 1,376 | |
| Non‐white | 11 | 2.70% | 4 | 0.60% | 2 | 0.60% | 17 | |
| TNM staging | <0.001 | |||||||
| Low | 252 | 62.50% | 93 | 14.10% | 227 | 68.80% | 572 | |
| High | 151 | 37.50% | 567 | 85.90% | 103 | 31.20% | 821 | |
| Serum HPV status | <0.001 | |||||||
| Negative | 392 | 97.30% | 178 | 27.00% | 323 | 97.90% | 893 | |
| Positive | 11 | 2.70% | 482 | 73.00% | 7 | 2.10% | 500 | |
| Comorbidity | <0.001 | |||||||
| None | 177 | 43.90% | 360 | 54.50% | 138 | 41.80% | 675 | |
| Mild | 138 | 34.20% | 207 | 31.40% | 111 | 33.60% | 456 | |
| Moderate/severe | 88 | 21.80% | 93 | 14.10% | 81 | 24.50% | 262 | |
| BMI | 0.002 | |||||||
| <18.5 | 14 | 3.50% | 16 | 2.40% | 13 | 4.00% | 43 | |
| 18.5–24.9 | 179 | 45.10% | 220 | 33.50% | 115 | 35.40% | 514 | |
| 25–29.9 | 133 | 33.50% | 275 | 41.90% | 128 | 39.40% | 536 | |
| 30+ | 71 | 17.90% | 146 | 22.20% | 69 | 21.20% | 286 | |
| Treatment group | <0.001 | |||||||
| Surgery only | 314 | 77.90% | 59 | 8.90% | 75 | 22.70% | 448 | |
| Surgery + adjunct | 61 | 15.10% | 130 | 19.70% | 22 | 6.70% | 213 | |
| Chemorad only | 6 | 1.50% | 395 | 59.80% | 49 | 14.80% | 450 | |
| Radio only | 17 | 4.20% | 70 | 10.60% | 182 | 55.20% | 269 | |
| Palliative/supportive | 5 | 1.20% | 6 | 0.90% | 2 | 0.60% | 13 | |
| Smoking | <0.001 | |||||||
| Never | 110 | 27.30% | 198 | 30.00% | 31 | 9.40% | 339 | |
| Former | 197 | 48.90% | 366 | 55.50% | 230 | 69.70% | 793 | |
| Current | 96 | 23.80% | 96 | 14.50% | 69 | 20.90% | 261 | |
| Alcohol consumption | 0.739 | |||||||
| Non‐drinker | 119 | 29.50% | 175 | 26.50% | 92 | 27.90% | 386 | |
| Moderate | 84 | 20.80% | 156 | 23.60% | 70 | 21.20% | 310 | |
| Hazardous to harmful | 200 | 49.60% | 329 | 49.80% | 168 | 50.90% | 697 | |
| Highest education level | 0.005 | |||||||
| School education | 181 | 44.90% | 284 | 43.00% | 179 | 54.20% | 644 | |
| College | 142 | 35.20% | 243 | 36.80% | 110 | 33.30% | 495 | |
| Degree | 80 | 19.90% | 133 | 20.20% | 41 | 12.40% | 254 | |
| Annual household income | <0.001 | |||||||
| <£18.000 | 200 | 49.60% | 223 | 33.80% | 184 | 55.80% | 607 | |
| £18.000 to £34.999 | 114 | 28.30% | 209 | 31.70% | 94 | 28.50% | 417 | |
| £35.000+ | 89 | 22.10% | 228 | 34.50% | 52 | 15.80% | 369 | |
| IMD group | 0.005 | |||||||
| Low deprivation | 184 | 45.70% | 288 | 43.60% | 115 | 34.80% | 587 | |
| Moderate deprivation | 83 | 20.60% | 155 | 23.50% | 70 | 21.20% | 308 | |
| High deprivation | 136 | 33.70% | 217 | 32.90% | 145 | 43.90% | 498 | |
| Marital status | 0.021 | |||||||
| Single | 51 | 12.70% | 65 | 9.80% | 39 | 11.80% | 155 | |
| In a relationship | 260 | 64.50% | 485 | 73.50% | 218 | 66.10% | 963 | |
| Separated/divorced/widow | 92 | 22.80% | 110 | 16.70% | 73 | 22.10% | 275 | |
N = number of participants.
p values for trend.
Table 2.
Baseline descriptive characteristics of participants with oropharyngeal tumours, stratified by HPV status
| HPV negative | HPV positive | ||||
|---|---|---|---|---|---|
| Characteristic | N | % | N | % | p valuea |
| Survival status | <0.001 | ||||
| Alive | 120 | 67.40% | 422 | 87.60% | |
| Died | 58 | 32.60% | 60 | 12.40% | |
| Gender | 0.149 | ||||
| Male | 139 | 78.10% | 400 | 83.00% | |
| Female | 39 | 21.90% | 82 | 17.00% | |
| Age group | 0.339 | ||||
| <50 | 29 | 16.30% | 64 | 13.30% | |
| 50–64 | 98 | 55.10% | 302 | 62.70% | |
| 65–79 | 46 | 25.80% | 107 | 22.20% | |
| 80+ | 5 | 2.80% | 9 | 1.90% | |
| Ethnicity group | 0.298 | ||||
| White | 176 | 98.90% | 480 | 99.60% | |
| Non‐White | 2 | 1.10% | 2 | 0.40% | |
| TNM staging | <0.001 | ||||
| Low | 43 | 24.20% | 50 | 10.40% | |
| High | 135 | 75.80% | 432 | 89.60% | |
| Comorbidity | <0.001 | ||||
| None | 79 | 44.40% | 281 | 58.30% | |
| Mild | 59 | 33.10% | 148 | 30.70% | |
| Moderate/severe | 40 | 22.50% | 53 | 11.00% | |
| BMI | <0.001 | ||||
| <18.5 | 10 | 5.60% | 6 | 1.30% | |
| 18.5–24.9 | 81 | 45.50% | 139 | 29.00% | |
| 25–29.9 | 62 | 34.80% | 213 | 44.50% | |
| 30+ | 25 | 14.00% | 121 | 25.30% | |
| Treatment group | 0.001 | ||||
| Surgery only | 20 | 11.20% | 39 | 8.10% | |
| Surgery + adjunct | 30 | 16.90% | 100 | 20.70% | |
| Chemorad only | 95 | 53.40% | 300 | 62.20% | |
| Radio only | 28 | 15.70% | 42 | 8.70% | |
| Palliative/supportive | 5 | 2.80% | 1 | 0.20% | |
| Smoking | <0.001 | ||||
| Never | 25 | 14.00% | 173 | 35.90% | |
| Former | 88 | 49.40% | 278 | 57.70% | |
| Current | 65 | 36.50% | 31 | 6.40% | |
| Alcohol consumption | 0.052 | ||||
| Non‐drinker | 43 | 24.20% | 132 | 27.40% | |
| Moderate | 33 | 18.50% | 123 | 25.50% | |
| Hazardous to harmful | 102 | 57.30% | 227 | 47.10% | |
| Highest education level | 0.693 | ||||
| School education | 78 | 43.80% | 206 | 42.70% | |
| College | 68 | 38.20% | 175 | 36.30% | |
| Degree | 32 | 18.00% | 101 | 21.00% | |
| Annual household income | <0.001 | ||||
| <£18.000 | 87 | 48.90% | 136 | 28.20% | |
| £18.000 to £34.999 | 51 | 28.70% | 158 | 32.80% | |
| £35 000 + | 40 | 22.50% | 188 | 39.00% | |
| IMD group | 0.555 | ||||
| Low deprivation | 74 | 41.60% | 214 | 44.40% | |
| Moderate deprivation | 47 | 26.40% | 108 | 22.40% | |
| High deprivation | 57 | 32.00% | 160 | 33.20% | |
| Marital status | <0.001 | ||||
| Single | 23 | 12.90% | 42 | 8.70% | |
| In a relationship | 109 | 61.20% | 376 | 78.00% | |
| Seperated/divorced /widow | 46 | 25.80% | 64 | 13.30% | |
N = number of participants.
p values for trend.
The proportion of OPCs that were HPV‐positive was 73%. People with HPV‐positive tumours were less likely to be current smokers (6.4% compared to 36.5% in the HPV‐negative group; p trend <0.001). There was evidence of a difference in alcohol consumption between groups (p trend = 0.052), with a higher proportion of hazardous to harmful drinkers in the HPV‐negative group.
Missing data
The distribution of missing data for all participants with cancers of the oral cavity, oropharynx and larynx (n = 4,276), stratified by site and HPV status, are shown in Supporting Information Tables 3 and 4. Gender and age data were available for all participants. BMI data were missing for 41.9% overall (as height and weight measurements were not routinely collected at the start of the study). Smoking and alcohol data were missing for 29.0% and 30.0% of participants, respectively. Gender, age, stage, comorbidity and treatment group were comparable in people with and without missing smoking and alcohol data (Supporting Information Table 5). Those individuals with missing exposure data were more likely to live in deprived areas (high deprivation = 50.4% vs. 37.2% in people with smoking and alcohol data.
Survival analysis
We found no violations of the proportionality assumption for any of the covariates included in the multivariable models. There were 315 deaths (oral cavity n = 115 (28.5%); oropharynx n = 118 (17.9%); larynx n = 82 (24.8%)) during a median follow‐up time of 3.5 years (25% IQR = 2.9 years; 75% IQR = 4.2 years). The proportion of deaths across tumour groups was comparable in the imputed analysis (32.9%, 24.0% and 27.7% for oral cavity, oropharyngeal and laryngeal cancers respectively). Kaplan–Meier estimates of survival by smoking and alcohol intake at diagnosis, stratified by tumour site and HPV status, are presented in Supporting Information Figures 1 and 2.
Inclusion of a frailty term did not improve the proportional hazards model (p values for the one‐sided likelihood ratio test = 0.13), suggesting that there is no heterogeneity in effect‐estimates by recruitment centre.
Smoking status and survival
For all cancer sites combined (n = 1,393), there was strong evidence of an association between smoking status at diagnosis and survival. Compared to never smokers, the HR for current smokers was 3.0 (95% CI = 2.1, 4.3, 3.6; p‐trend <0.001) in the minimally adjusted model and attenuated to 1.71 (95% CI = 1.1, 2.6; p‐trend = 0.001) in the fully adjusted model (Table 3). For former smokers, the HR was 1.6 (95% CI = 1.2, 2.3; p‐trend <0.001) in the minimally adjusted model and attenuated to 1.4 (95% CI = 1.0, 2.0; p‐trend 0.001) on full adjustment.
Table 3.
Association of smoking status and alcohol intake with mortality risk, stratified by tumour site (n = 1,393)
| All sites (n = 1.393) | Oral cavity (n = 403) | Oropharyngeal (n = 660) | Larynx (n = 330) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| HR | Lower CI | Upper CI | p valuea | HR | Lower CI | Upper CI | p valuea | HR | Lower CI | Upper CI | p valuea | HR | Lower CI | Upper CI | p valuea | |
| Model 1 | ||||||||||||||||
| Smoking | <0.001 | 0.036 | <0.001 | <0.001 | ||||||||||||
| Former | 1.66 | 1.20 | 2.30 | 2.32 | 1.39 | 3.89 | 1.51 | 0.92 | 2.46 | 1.24 | 0.49 | 3.12 | ||||
| Current | 3.03 | 2.12 | 4.32 | 1.91 | 1.06 | 3.44 | 3.93 | 2.30 | 6.73 | 3.23 | 1.24 | 8.41 | ||||
| Alcohol amount | 0.072 | 0.186 | 0.486 | 0.616 | ||||||||||||
| Moderate | 0.77 | 0.55 | 1.09 | 0.72 | 0.40 | 1.29 | 0.85 | 0.49 | 1.46 | 0.70 | 0.36 | 1.39 | ||||
| Hazardous/harmful | 1.22 | 0.93 | 1.60 | 1.29 | 0.83 | 2.01 | 1.13 | 0.72 | 1.75 | 1.09 | 0.65 | 1.83 | ||||
| Model 2 | ||||||||||||||||
| Smoking | <0.001 | 0.142 | 0.015 | 0.007 | ||||||||||||
| Former | 1.48 | 1.06 | 1.06 | 2.28 | 1.35 | 3.85 | 1.25 | 0.76 | 2.06 | 1.10 | 0.43 | 2.78 | ||||
| Current | 1.84 | 1.26 | 2.69 | 1.55 | 0.83 | 2.87 | 2.18 | 1.19 | 3.98 | 2.28 | 0.86 | 6.05 | ||||
| Alcohol amount | 0.162 | 0.183 | 0.491 | 0.708 | ||||||||||||
| Moderate | 0.77 | 0.54 | 1.08 | 0.76 | 0.42 | 1.38 | 0.86 | 0.49 | 1.49 | 0.62 | 0.31 | 1.24 | ||||
| Hazardous/harmful | 1.15 | 0.88 | 1.50 | 1.40 | 0.89 | 2.21 | 1.06 | 0.68 | 1.66 | 1.00 | 0.59 | 1.71 | ||||
| Model 3 | ||||||||||||||||
| Smoking | 0.001 | 0.081 | 0.045 | 0.010 | ||||||||||||
| Former | 1.48 | 1.05 | 1.07 | 2.60 | 1.51 | 4.49 | 1.26 | 0.76 | 2.08 | 1.14 | 0.43 | 3.01 | ||||
| Current | 1.82 | 1.22 | 1.70 | 1.70 | 0.27 | 3.34 | 1.82 | 0.96 | 3.47 | 2.40 | 0.85 | 6.78 | ||||
| Alcohol amount | 0.160 | 0.092 | 0.315 | 0.665 | ||||||||||||
| Moderate | 0.79 | 0.56 | 1.12 | 0.79 | 0.43 | 1.45 | 0.95 | 0.54 | 1.67 | 0.63 | 0.31 | 1.26 | ||||
| Hazardous harmful | 1.17 | 0.89 | 1.53 | 1.44 | 0.91 | 2.30 | 1.19 | 0.75 | 1.88 | 1.04 | 0.61 | 1.80 | ||||
| Model 4 | ||||||||||||||||
| Smoking | 0.001 | 0.145 | 0.049 | 0.011 | ||||||||||||
| Former | 1.42 | 1.01 | 2.00 | 2.45 | 1.41 | 4.25 | 1.23 | 0.74 | 2.04 | 1.10 | 0.42 | 2.91 | ||||
| Current | 1.71 | 1.14 | 2.55 | 1.55 | 0.78 | 3 07 | 1.79 | 0.93 | 3.43 | 2.25 | 0.80 | 6.39 | ||||
| Alcohol amount | 0.314 | 0.164 | 0.355 | 0.960 | ||||||||||||
| Moderate | 0 81 | 0.57 | 1.14 | 0.75 | 0.41 | 1.39 | 1.02 | 0.58 | 1.80 | 0.64 | 0.32 | 1.29 | ||||
| Hazardous/harmful | 1.12 | 0.85 | 1.47 | 1.31 | 0.82 | 2. 12 | 1.20 | 0.75 | 1.90 | 0.97 | 0.56 | 1.67 | ||||
Model 1 (minimally adjusted): adjusted for age and gender.
Model 2: additionally adjusted for clinical features (stage, treatment, comorbidity, BMI, HPV).
Model 3: additionally adjusted for social features (education, annual household income, IMD, marital status, ethnicity).
Model 4 (fully adjusted): additionally includes smoking or drinking.
HR = hazard ratio; CI = confidence interval.
p values for trend.
The associations of smoking status with survival were present for oropharyngeal and laryngeal cancer groups in the subgroup analysis. In the OPC group, the HR for current smokers was 3.9 (95% CI = 2.3, 6.7; p‐trend <0.001) in the minimally adjusted model and 1.8 (95% CI = 0.9, 3.4; p‐trend 0.008) in the fully adjusted model (Table 3); for people with laryngeal cancers, the respective HRs were 3.2 (95% CI = 1.2, 8.4; p‐trend <0.001) and 2.3 (95% CI = 0.8, 6.4; p‐trend = 0.011).
Results of the imputed analysis (n = 4,276) were comparable to those of the complete case analysis. In minimally adjusted models, the hazard ratio was 2.9 (95% CI = 2.3, 3.7) and 1.6 (95% CI = 1.3, 1.9; p for trend = <0.001) for current and former smokers, respectively. In fully adjusted models, the corresponding hazard ratios were 1.6 (95% CI = 1.2, 2.1) and 1.3 (95% CI = 1.1, 1.6; p for trend = <0.001) (Supporting Information Table 6). A similar pattern of association was seen in the stratified analysis.
Alcohol intake and survival
In the minimally adjusted model, the hazard ratio for hazardous to harmful drinkers compared to non‐drinkers was 1.2 (95% CI = 0.9, 1.6; p‐trend = 0.072) (Table 3). On full adjustment, there was no evidence for an increase in mortality risk (HR = 1.1 (95% CI = 0.9, 1.5; p‐trend = 0.314). There was weak evidence to suggest that moderate drinkers experienced improved survival compared to non‐drinkers in the minimally adjusted model (HR = 0.8, 0.6, 1.1; p for trend = 0.072), but this association was not robust to adjustment.
When models were stratified by tumour site, there was no evidence of an association between alcohol consumption and survival.
In the imputed analyses, a comparable pattern of association between alcohol drinking and survival was observed (minimally adjusted model: HR = 1.2 (95% CI= 1.0, 1.4) and 0.9 (95% CI = 0.7, 1.1; p for trend = <0.036) for hazardous/harmful and moderate drinkers, respectively, fully adjusted: HR = 1.1 (95% CI = 0.9, 1.3) and 0.9 (95% CI = 0.7, 1.1) (Supporting Information Table 6).
Influence of tumour stage on the associations of smoking and alcohol intake with survival
There were 572 people with low stage tumours and 821 with high stage tumours in the analysis. On full adjustment, the hazard of death for current versus never‐smokers was 2.3 in the low stage group (95% CI = 1.1, 4.9; p for trend = 0.075), and 1.7 in the high stage group (95% CI = 1.0, 2.8; p for trend = <0.004) (Table 4). There was no evidence of an association between the amount of alcohol consumed at diagnosis and survival in either the low or high stage subgroups.
Table 4.
Association of smoking status and alcohol intake with mortality risk, stratified by tumour stage (n = 1,393)
| Low stage (n = 572) | High stage (n = 821) | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | Lower CI | Upper CI | p valuea | HR | Lower CI | Upper CI | p valuea | |
| Model 1 | ||||||||
| Smoking | <0.001 | |||||||
| Former | 2.02 | 1.05 | 3.86 | 1.65 | 1.13 | 2.41 | ||
| Current | 2.90 | 1.44 | 5.82 | 0.002 | 3.50 | 2.31 | 5.29 | |
| Alcohol amount | 0.445 | |||||||
| Moderate | 0.67 | 0.35 | 1.30 | 0.75 | 0.50 | 1.12 | ||
| Hazardous/harmful | 1.45 | 0.90 | 2.34 | 0.074 | 1.08 | 0.78 | 1.50 | |
| Model 2 | ||||||||
| Smoking | 0.005 | |||||||
| Former | 2.05 | 1.06 | 3.97 | 1.35 | 0.92 | 1.99 | ||
| Current | 2.38 | 1.16 | 4.90 | 0.052 | 1.75 | 1.11 | 2.75 | |
| Alcohol amount | 0.619 | |||||||
| Moderate | 0.72 | 0.37 | 1.40 | 0.80 | 0.53 | 1.19 | ||
| Hazardous/harmful | 1.50 | 0.92 | 2.45 | 0.121 | 1.05 | 0.76 | 1.45 | |
| Model 3 | ||||||||
| Smoking | 0.004 | |||||||
| Former | 2.15 | 1.09 | 4 23 | 1.40 | 0.94 | 2.09 | ||
| Current | 2.54 | 1.19 | 5.45 | 0.075 | 1.77 | 1.09 | 2.86 | |
| Alcohol amount | 0.655 | |||||||
| Moderate | 0.71 | 0.36 | 1.40 | 0.81 | 0.54 | 1.23 | ||
| Hazardous/harmful | 1.58 | 0.96 | 2.60 | 0.086 | 1.04 | 0.75 | 1.46 | |
| Model 4 | ||||||||
| Smoking | 0.004 | |||||||
| Former | 2.00 | 1.01 | 3.97 | 1.37 | 0.92 | 2.05 | ||
| Current | 2.29 | 1.06 | 4.94 | 0.075 | 1.70 | 1.04 | 2.77 | |
| Alcohol amount | 0.904 | |||||||
| Moderate | 0.68 | 0.34 | 1.33 | 0.85 | 0.56 | 1.29 | ||
| Hazardous/harmful | 1.44 | 0.88 | 2.36 | 0.135 | 1.02 | 0.73 | 1.42 | |
Model 1 (minimally adjusted): adjusted for age and gender.
Model 2: additionally adjusted for clinical features (stage, treatment, comorbidity, BMI).
Model 3: additionally adjusted for social features (education, annual household income, IMD, marital status, ethnicity).
Model 4 (fully adjusted): additionally includes smoking or drinking.
HR = hazard ratio; CI = confidence interval.
p values for trend.
In the imputed analysis, there was a 2.2‐fold higher mortality risk (95% CI = 1.4, 3.6; p‐trend = <0.001) for current versus never smokers in the low stage tumour group (n = 1,701) compared to a 1.4‐fold higher mortality risk (95% CI = 1.1, 2.0; p < 0.007) in the high stage tumour group (n = 2,562). The HRs for hazardous to harmful drinkers were similar to those in the primary analyses (Supporting Information Table 7).
Influence of HPV status on the associations of smoking and alcohol intake with survival
Results of the HPV‐stratified analyses are presented in Table 5. In fully adjusted models (n = 660), the HR for current versus never smokers was 1.3 (95% CI = 0.3, 5.1; p‐trend = 0.481) in the HPV‐negative group (n = 178) and 2.1 (95% CI = 0.8, 5.3; p‐trend = 0.263) in the HPV‐positive group (n = 482). The mortality hazards for hazardous to harmful drinkers versus non‐drinkers were 2.6 (95% CI = 1.2, 5.6; p‐trend = 0.012) and 0.6 (95% CI = 0.3, 1.1; p‐trend = 0.160) in HPV‐negative and HPV‐positive groups, respectively. Results of the imputed analyses (n = 1,595) are presented in Supporting Information Table 8. Here, there was evidence that current smokers were at increased risk of death compared to non‐smokers, regardless of HPV status (fully adjusted HR = 1.9 (95% CI = 0.9, 4.1; p for trend = 0.070) and 2.0 (95% CI = 1.0, 4.0; p for trend = 0.049) for HPV‐negative and HPV‐positive groups, respectively).
Table 5.
Association of smoking status and alcohol intake with mortality risk, stratified by HPV status (n = 660)
| HPV negative (n = 178) | HPV positive (n = 482) | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | Lower CI | Upper CI | p valuea | HR | Lower CI | Upper CI | p valuea | |
| Model 1 | ||||||||
| Smoking | 0.002 | 0.313 | ||||||
| Former | 3.02 | 0.91 | 10.05 | 1.08 | 0.61 | 1.89 | ||
| Current | 5.12 | 1.53 | 17.18 | 1.82 | 0.75 | 4.42 | ||
| Alcohol amount | 0.038 | 0.096 | ||||||
| Moderate | 0.70 | 0.26 | 1.90 | 0.89 | 0.46 | 1.71 | ||
| Hazardous/harmful | 1.84 | 0.92 | 3.65 | 0.60 | 0.33 | 1.12 | ||
| Model 2 | ||||||||
| Smoking | 0.188 | 0.271 | ||||||
| Former | 2.16 | 0.64 | 7.23 | 1.05 | 0.59 | 1.87 | ||
| Current | 2.58 | 0.75 | 8.91 | 1.88 | 0.77 | 4.61 | ||
| Alcohol amount | 0.016 | 0.093 | ||||||
| Moderate | 0.76 | 0.26 | 2.24 | 0.93 | 0.48 | 1.79 | ||
| Hazardous/harmful | 2.01 | 1.00 | 4.03 | 0.59 | 0.32 | 1.09 | ||
| Model 3 | ||||||||
| Smoking | 0.493 | 0.265 | ||||||
| Former | 2.30 | 0.67 | 7.96 | 1.03 | 0.57 | 1.85 | ||
| Current | 1.71 | 0.45 | 6.50 | 2.02 | 0.80 | 5.10 | ||
| Alcohol amount | 0.008 | 0.119 | ||||||
| Moderate | 1.19 | 0.38 | 3.76 | 0.90 | 0.46 | 1.77 | ||
| Hazardous/harmful | 2.82 | 1.34 | 5.91 | 0.57 | 0.30 | 1.11 | ||
| Model 4 | ||||||||
| Smoking | 0.481 | 0.263 | ||||||
| Former | 1.53 | 0.43 | 5.48 | 1.07 | 0.59 | 1.94 | ||
| Current | 1.30 | 0.33 | 5.14 | 2.06 | 0.81 | 5.25 | ||
| Alcohol amount | 0.012 | 0.160 | ||||||
| Moderate | 1.11 | 0.34 | 3.59 | 0.93 | 0.47 | 1.83 | ||
| Hazardous/harmful | 2.59 | 1.20 | 5.61 | 0.58 | 0.30 | 1.12 | ||
Model 1 (minimally adjusted): adjusted for age and gender.
Model 2: additionally adjusted for clinical features (stage, treatment, comorbidity, BMI).
Model 3: additionally adjusted for social features (education, annual household income, IMD, marital status, ethnicity).
Model 4 (fully adjusted): additionally includes smoking or drinking.
HR = hazard ratio; CI = confidence interval.
p values for trend.
There was no strong evidence that HPV status modified the association of smoking with survival in the sensitivity analyses (p‐interaction = 0.563). The effect of alcohol may differ by HPV status (p‐interaction = 0.024), but this may be due to chance as the number of deaths was small (58 in the HPV negative group and 60 in the HPV positive group).
Interaction of tobacco and alcohol
We found no evidence of an interaction between smoking and alcohol consumption on survival (p‐interaction = 0.233 in the fully adjusted model).
Discussion
Principle findings
The major finding of this large, prospective study is that, even after adjusting for a wide‐range of prognostic factors (confounders), smoking status at the time of a HNC diagnosis is associated with worse survival. In fully adjusted models, current smokers had around a 70% higher all‐cause mortality risk compared to people who had never smoked, whilst former smokers were over 40% more likely to die during follow‐up. Drinking behaviour around the time of diagnosis was not associated with overall mortality risk in this analysis.
Our findings are in line with those of earlier studies, which suggest that smoking at the time of a HNC diagnosis may result in poorer clinical outcomes and reduced survival.12, 13, 15, 39 Estimates of the size of the effect have varied considerably however, ranging from a 2.4‐fold higher all‐cause mortality risk in current versus never‐smokers12 to an almost fivefold higher mortality risk in people with >60 pack‐years of smoking versus never‐smokers.13 There are a number of possible explanations for this. First, much of the evidence is based on retrospective analyses of population‐level cancer registries, which are often incomplete or incorrect.40 Consequently, studies have frequently been missing information on important clinical and lifestyle factors such as comorbidity, BMI and socioeconomic position, which could potentially confound the association of smoking with survival. Those studies which have been conducted prospectively are small—typically five hundred persons or fewer,12, 13, 14 and as a result they have limited statistical power to detect an accurate measure of the effect. Second, estimates have been derived from different subpopulations of people, i.e. different HNC sites or tumour stages, which are often not considered separately. This could bias estimates of the effect of smoking on survival if mortality risk is greater or lesser in certain tumour groups.
With respect to alcohol consumption, the existing literature is limited and conflicting. Our results support those of Duffy et al. who found no difference in mortality risk between active drinkers and non‐drinkers after adjusting for a wider range of confounders.12 In contrast to this however, Mayne et al. reported a fivefold increased mortality risk for persons who drank >35 drinks per week compared to those who abstained.13 Both studies were relatively small (504 and 204 people, respectively), and enrolled participants from either a single centre12 or a single clinical trial,13 limiting their generalisability.
It is biologically plausible that, as well as being risk factors for HNC, smoking could reduce survival following a diagnosis. One way in which smoking could influence survival is through its effects on treatment response. A growing body of evidence suggests that smokers have an increased risk of treatment‐related adverse events and poorer clinical outcomes following radiotherapy, compared to non‐smokers.41, 42 The biological mechanisms underpinning this association are not fully understood, but increased tumour hypoxia, resulting from increased carboxyhaemoglobin (the binding of carbon monoxide to haemoglobin) in smokers, is a likely explanation.43 In addition to this, it has been suggested that tobacco reduces the efficacy of radiotherapy through triggering a p53 mutation that could promote resistance to apoptosis.44 Smoking is also known to effect inflammatory responses45 and immune competence,46 which could increase the likelihood of adverse clinical outcomes.
Strengths and limitations of the study
This study has several strengths. These include the prospective population‐based cohort design, the relatively large sample size and our ability to adjust for multiple biological, clinical and lifestyle covariates, including HPV. In addition to this, we explored the risk of bias due to missing data by employing a multiple imputation approach.47 Results of the imputed analysis was broadly consistent with those of the complete case analysis.
The study has several limitations. First, as in most previous studies, assessments of smoking and alcohol intake were based on participants’ self‐reports. Prior work has shown that self‐reports can often provide an inaccurate assessment of tobacco and alcohol use, particularly in people who have recently been diagnosed with cancer.48 This would most likely result in an underestimation of the effects of smoking and drinking on HNC survival.
Second, although we adjusted for several confounders in our models, residual confounding by unmeasured or poorly measured factors, such as a delay in receiving treatment or other lifestyle factors related to smoking and drinking behaviours (e.g., physical activity and dietary intake), is possible. Furthermore, it is possible that the individuals included in the complete case analysis were different to those in the imputed analysis, since those with missing smoking and alcohol data lived in more deprived areas overall. However, given that results were comparable using both approaches, it seems unlikely that the complete case analysis was biased by socioeconomic position.
Third, whilst the sample size was sufficient for us to detect the main effects of baseline smoking status and alcohol intake on survival, it was insufficient to examine interactions between these two exposures and HPV in determining mortality; it also limited our ability to investigate whether the effects of smoking and drinking on survival were modified by cancer site. This was because there were only a small number of events (deaths) in each subgroup. We had no prior hypotheses that smoking or alcohol intake would have a greater or lesser effect on survival in any one cancer group however, and therefore the analyses were exploratory by design.
Fourth, we used HPV‐specific antibody levels to identify individuals with HPV‐positive oropharyngeal tumours, but the presence of HPV16 DNA is considered the gold standard measure. Previous studies have confirmed, however, that detection of antibodies against E6 and E7 oncoproteins shows good correlation with HPV DNA in the tumour tissue.32 Kreimer et al. showed that high HPV viral load increased the odds of HPV16 E6 seropositivity 57‐fold and HPV16 E7 seropositivity 26‐fold. Moreover, HPV16 E6 antibodies have also been shown to be independent favourable prognostic factors in OPCs.49, 50 Some HPV‐related OPCs are mediated by other HPV genotypes, including HPV18 (1–8% of OPCs), and less commonly HPV33, −35, −56 and −67.51 We only considered HPV16 in the current analysis because 69% of the 1,910 participants with OPCs were HPV16 positive, compared to only 2% who were HPV18 positive.
Finally, we were unable to examine whether baseline smoking status and alcohol intake influenced cancer‐specific mortality as cause‐specific mortality data were not available for all participants. Previous studies suggest that death from non‐cancerous causes (competing mortality) and second primary malignancies are important events in HNC52 and could provide greater insight into the biological mechanisms that underlie the associations of smoking and drinking with survival. The cause of death information on a death certificate is often inaccurate however.53 Accuracy of all‐cause mortality is solely dependent on the number of deaths identified, and is arguably a more reliable outcome measure.
Policy implications
Our results emphasise the importance of clinicians recording information on peoples’ smoking status in their medical notes at the time of diagnosis, to help identify those at risk of poor survival. It is possible that smoking cessation and reduced alcohol consumption could reduce mortality in this population, which highlights the need to take advantage of the “teachable moment” that a cancer diagnosis presents.54 Clinicians routinely advise people with HNC to stop smoking, but our data provides an impetus for health providers and policy makers to ensure that this remains a focus of care.
Future research
There remain several unanswered questions concerning the role of smoking status and alcohol use in HNC survival. First, it is unclear whether the associations of smoking and alcohol use at the time of diagnosis with survival vary in different tumour sites. In the current study, the mortality hazard for current smokers was much lower in the oral cavity cancer group than it was in the oropharyngeal and laryngeal cancer groups, suggesting that tumour site may modify the relationship between smoking status and survival. This finding needs to be validated in other HNC cohorts.
The second unanswered question is, what influence does HPV have on the associations of tobacco and alcohol use with survival? It has been shown that smoking increases the risk of OPC, irrespective of HPV16 status,55 but evidence of an effect of joint exposure on survival after diagnosis is conflicting. Some studies report reduced survival in HPV‐positive smokers compared to their non‐smoking counterparts,18 whilst others report no difference in prognosis between HPV‐positive smokers and HPV‐positive non‐smokers.56 To the best of our knowledge, no studies have compared the prognostic value of alcohol use in people with HPV‐positive versus HPV‐negative OPCs. To adequately address these questions, further well‐designed studies in people with oropharyngeal tumours are needed, which are large enough to ensure that there is adequate power to detect an interaction. Furthermore, HPV testing needs to be included, where possible, in all future studies involving OPC cases.
The final question is whether survival can be improved with smoking cessation and reduced alcohol consumption after diagnosis. Given that at least one‐third of people with HNC continue to smoke and 16% continue to drink hazardously57 after receiving the primary HNC diagnosis, this is an important question to address. Few studies in the literature have examined patterns of smoking and alcohol drinking after diagnosis, but there is some evidence to suggest benefits of smoking cessation in terms of response to radiation therapy and reduced risk of second primary tumours.58 If smoking cessation and reduced alcohol consumption can improve survival rates in this population, then pilot behavioural intervention trials should be conducted to identify the most effective way of supporting individuals to make and sustain changes.
An issue which is pertinent to all future research in this area is the accurate measurement of smoking and alcohol use in observational studies. As highlighted above, self‐reported intake is frequently unreliable, but recent advances in genome‐wide methylation profiling have permitted the identification of robust biomarkers of tobacco and alcohol exposure,59, 60 circumventing issues of reporting bias. Another approach that could be used to obtain unbiased estimates of the causal effects of tobacco and alcohol consumption on HNC survival is Mendelian Randomization, whereby genetic variants which modulate an observed risk factor are used as a proxy measure for that exposure, eliminating problems of confounding and reverse causation.61
Conclusions
In this large prospective clinical cohort of people with HNC we showed that smoking status at the time of diagnosis is associated with poorer survival. Further research should explore whether interventions that encourage smoking cessation or reduced alcohol consumption result in improved outcomes.
Supporting information
Supporting Information 1
Supporting Information 2
Supporting Information 3
Acknowledgements
The authors are extremely grateful to all Head and neck 5000 participants, the Head and Neck 5000 study co‐ordination team and laboratory technicians at the Bristol Bioresource Laboratories. This publication included data from the Head and Neck 5000 study. The views expressed in this article are those of the authors and not necessarily any funding body. R.B. is funded by a Wellcome Trust 4‐year studentship (110021/Z/15/Z).
Conflict of interest: The authors have no conflicts to report.
Footnotes
Refers to the intent of the cancer care plan for the patient's head and neck cancer at the point of diagnosis e.g. radiotherapy or chemotherapy.
Comorbidity was measured using the Adult Co‐morbidity Evaluation (ACE‐27) assessment tool. Categories were defined as “mild‐“, “moderate”‐, and “severe decompensation”,
IMD is the official measure of relative deprivation ranks every small area (or neighbourhood) in England from 1 (most deprived area) to 32,844 (least deprived area).
References
- 1. Ferlay J, Shin HR, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer 2010;127:2893–917. [DOI] [PubMed] [Google Scholar]
- 2. Cancer Research UK (CRUK) . Oral cancer incidence statistics. Available from: http://www.cancerresearchuk.org/health-professional/cancer-statistics/statistics-by-cancer-type/oral-cancer/incidence (cited May 18, 2017).
- 3. Price G, Roche M, Crowther R. Profile of head and neck cancers in England: incidence, mortality and survival [Internet]. Natl Cancer Intelligence Netw 2011; Available from: http://www.ncin.org.uk/view?rid=69 (cited April 10, 2017). [Google Scholar]
- 4. Bosetti C, Garavello W, Levi F, et al. Trends in laryngeal cancer mortality in Europe. Int J Cancer 2006;119:673–81. [DOI] [PubMed] [Google Scholar]
- 5.(OCIU) OCIU. Profiles of head and neck cancers in England: incidence, mortality and survival; 2010. Available from: http://www.ociu.nhs.uk/sph-documents/Head_and_Neck_Profiles.pdf. Accessed 18 May 2017.
- 6. IARC . IARC Monographs on the evaluation of carcinogenic risks to Humans. [Internet]. 2004. Available from: https://monographs.iarc.fr/ENG/Monographs/vol83/mono83.pdf (cited April 10, 2017).
- 7. Hashibe M, Brennan P, Chuang SC, et al. Interaction between tobacco and alcohol use and the risk of head and neck cancer: pooled analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol Biomarkers Prev 2009;18:541–50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Elrefaey S, Massaro MA, Chiocca S, et al. HPV in oropharyngeal cancer: the basics to know in clinical practice. Acta Otorhinolaryngol Ital 2014;34:299–309. [PMC free article] [PubMed] [Google Scholar]
- 9. National Head and Neck Cancer Audit 2014 . DAHNO Tenth Annual Report [Internet]. 2015. Available from: http://digital.nhs.uk/catalogue/PUB18081 (cited April 10, 2017).
- 10. Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med 2010;363:24–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dahlstrom KR, Bell D, Hanby D, et al. Socioeconomic characteristics of patients with oropharyngeal carcinoma according to tumor HPV status, patient smoking status, and sexual behavior. Oral Oncol 2015;51:832–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Duffy SA, Ronis DL, McLean S, et al. Pretreatment health behaviors predict survival among patients with head and neck squamous cell carcinoma. JCO 2009;27:1969–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Mayne ST, Cartmel B, Kirsh V, et al. Alcohol and tobacco use prediagnosis and postdiagnosis, and survival in a cohort of patients with early stage cancers of the oral cavity, pharynx, and larynx. Cancer Epidemiol Biomarkers Prev 2009;18:3368–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Warren GW, Kasza KA, Reid ME, et al. Smoking at diagnosis and survival in cancer patients. Int J Cancer 2013;132:401–10. [DOI] [PubMed] [Google Scholar]
- 15. Sharp L, McDevitt J, Carsin AE, et al. Smoking at diagnosis is an independent prognostic factor for cancer‐specific survival in head and neck cancer: findings from a large, population‐based study. Cancer Epidemiol Biomarkers Prev 2014;23:2579–90. [DOI] [PubMed] [Google Scholar]
- 16. Peterson LA, Bellile EL, Wolf GT, et al. Cigarette use, comorbidities, and prognosis in a prospective head and neck squamous cell carcinoma population. Head Neck 2016;38:1810–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Boffetta P, Merletti F, Faggiano F, et al. Prognostic factors and survival of laryngeal cancer patients from Turin, Italy. A population‐based study. Am J Epidemiol 1997;145:1100–5. [DOI] [PubMed] [Google Scholar]
- 18. Hafkamp HC, Manni JJ, Haesevoets A, et al. Marked differences in survival rate between smokers and nonsmokers with HPV 16‐associated tonsillar carcinomas. Int J Cancer 2008;122:2656–64. [DOI] [PubMed] [Google Scholar]
- 19. Fortin A, Wang CS, Vigneault E. Influence of smoking and alcohol drinking behaviors on treatment outcomes of patients with squamous cell carcinomas of the head and neck. Int J Radiat Oncol Biol Phys 2009;74:1062–9. [DOI] [PubMed] [Google Scholar]
- 20. Leoncini E, Vukovic V, Cadoni G, et al. Clinical features and prognostic factors in patients with head and neck cancer: results from a multicentric study. Cancer Epidemiol 2015;39:367–74. [DOI] [PubMed] [Google Scholar]
- 21. Sawabe M, Ito H, Oze I, et al. Heterogeneous impact of alcohol consumption according to treatment method on survival in head and neck cancer: a prospective study. Cancer Sci 2017;108:91–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dikshit RP, Boffetta P, Bouchardy C, et al. Lifestyle habits as prognostic factors in survival of laryngeal and hypopharyngeal cancer: a multicentric European study. Int J Cancer 2005;117:992–5. [DOI] [PubMed] [Google Scholar]
- 23. Ness AR, Waylen A, Hurley K, et al. Establishing a large prospective clinical cohort in people with head and neck cancer as a biomedical resource: head and neck 5000. BMC Cancer 2014;14:973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Ness AR, Waylen A, Hurley K, et al. Recruitment, response rates and characteristics of 5511 people enrolled in a prospective clinical cohort study: head and neck 5000. Clin Otolaryngol 2016;41:804–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Head and Neck 5000. Information for Researchers. 2015. Available from: http://www.headandneck5000.org.uk/information-for-researchers/study-materials/. Last accessed 18 May 2018.
- 26.WHO. ICD‐10 Version: International Statistical Classification of Diseases and Related Health Problems 10th Revision. 2018. Available from: http://www.who.int/classifications/icd/icdonlineversions/en/ (cited April 10, 2018).
- 27. Deschler D, Day T. TNM staging of head and neck cancer/neck dissection classification. Alexandria (VA): American Academy of Head and Neck Surgery, 2008. [Google Scholar]
- 28. Waterboer T, Sehr P, Michael KM, et al. Multiplex human papillomavirus serology based on in situ‐purified glutathione S‐transferase fusion proteins. Clin Chem 2005;51:1845–53. [DOI] [PubMed] [Google Scholar]
- 29. Smith EM, Ritchie JM, Pawlita M, et al. Human papillomavirus seropositivity and risks of head and neck cancer. Int J Cancer 2007;120:825–32. [DOI] [PubMed] [Google Scholar]
- 30. Lang Kuhs KA, Kreimer AR, Trivedi S, et al. Human papillomavirus 16 E6 antibodies are sensitive for human papillomavirus‐driven oropharyngeal cancer and are associated with recurrence. Cancer 2017;123:4382–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Kreimer AR, Johansson M, Waterboer T, et al. Evaluation of human papillomavirus antibodies and risk of subsequent head and neck cancer. J Clin Oncol 2013;31:2708–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kreimer AR, Clifford GM, Snijders PJ, et al. HPV16 semiquantitative viral load and serologic biomarkers in oral and oropharyngeal squamous cell carcinomas. Int J Cancer 2005;115:329–32. [DOI] [PubMed] [Google Scholar]
- 33. National Institute for Health and Care Excellence (NICE) . Alcohol‐use disorders: preventing harmful drinking [Internet]. 2011. Available from: https://www.nice.org.uk/guidance/ph24/resources/alcoholuse-disorders-prevention-pdf-1996237007557 (cited May 25, 2017).
- 34. Hoel DG, Ron E, Carter R, et al. Influence of death certificate errors on cancer mortality trends. J Natl Cancer Inst 1993;85:1063–8. [DOI] [PubMed] [Google Scholar]
- 35.The English Index of Multiple Deprivation (IMD) 2015—guidance: Department for Communities and Local Government. Available from: https://www.gov.uk/government/uploads/system/uploads/attachment_data/file/464430/English_Index_of_Multiple_Deprivation_2015_-_Guidance.pdf (cited December 26, 2017).
- 36. Royston P. Multiple imputation of missing values: further update of ice, with an emphasis on categorical variables. Stata J 2009;9:466–77. [Google Scholar]
- 37. Rubin H. Synergistic mechanisms in carcinogenesis by polycyclic aromatic hydrocarbons and by tobacco smoke: a bio‐historical perspective with updates. Carcinogenesis 2001;22:1903–30. [DOI] [PubMed] [Google Scholar]
- 38. White IR, Royston P. Imputing missing covariate values for the Cox model. Stat Med 2009;28:1982–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Hatcher JL, Sterba KR, Tooze JA, et al. Tobacco use and surgical outcomes in head and neck cancer patients. Head Neck 2014;38:700–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Thygesen LC, Ersboll AK. When the entire population is the sample: strengths and limitations in register‐based epidemiology. Eur J Epidemiol 2014;29:551–8. [DOI] [PubMed] [Google Scholar]
- 41. Browman GP, Mohide EA, Willan A, et al. Association between smoking during radiotherapy and prognosis in head and neck cancer: a follow‐up study. Head Neck 2002;24:1031–7. [DOI] [PubMed] [Google Scholar]
- 42. Hoff CM, Grau C, Overgaard J. Effect of smoking on oxygen delivery and outcome in patients treated with radiotherapy for head and neck squamous cell carcinoma—a prospective study. Radiother Oncol 2012;103:38–44. [DOI] [PubMed] [Google Scholar]
- 43. Nordsmark M, Overgaard J. Tumor hypoxia is independent of hemoglobin and prognostic for loco‐regional tumor control after primary radiotherapy in advanced head and neck cancer. Acta Oncol 2004;43:396–403. [DOI] [PubMed] [Google Scholar]
- 44. Brennan JA, Boyle JO, Koch WM, et al. Association between cigarette smoking and mutation of the p53 gene in squamous‐cell carcinoma of the head and neck. N Engl J Med 1995;332:712–7. [DOI] [PubMed] [Google Scholar]
- 45. Yanbaeva DG, Dentener MA, Creutzberg EC, et al. Systemic effects of smoking. Chest 2007;131:1557–66. [DOI] [PubMed] [Google Scholar]
- 46. Szabo G. Consequences of alcohol consumption on host defence. Alcohol Alcohol 1999;34:830–41. [DOI] [PubMed] [Google Scholar]
- 47. Sterne JA, White IR, Carlin JB, et al. Multiple imputation for missing data in epidemiological and clinical research: potential and pitfalls. BMJ 2009;338:b2393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Morales NA, Romano MA, Michael Cummings K, et al. Accuracy of self‐reported tobacco use in newly diagnosed cancer patients. Cancer Causes Control 2013;24:1223–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Rubenstein LM, Smith EM, Pawlita M, et al. Human papillomavirus serologic follow‐up response and relationship to survival in head and neck cancer: a case‐comparison study. Infect Agents Cancer 2011;6:9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Koslabova E, Hamsikova E, Salakova M, et al. Markers of HPV infection and survival in patients with head and neck tumors. Int J Cancer 2013;133:1832–9. [DOI] [PubMed] [Google Scholar]
- 51. Burd EM. Human papillomavirus laboratory testing: the changing paradigm. Clin Microbiol Rev 2016;29:291–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Mell LK, Dignam JJ, Salama JK, et al. Predictors of competing mortality in advanced head and neck cancer. J Clin Oncol 2010;28:15–20. [DOI] [PubMed] [Google Scholar]
- 53. Smith Sehdev AE, Hutchins GM. Problems with proper completion and accuracy of the cause‐of‐death statement. Arch Intern Med 2001;161:277–84. [DOI] [PubMed] [Google Scholar]
- 54. McBride CM, Ostroff JS. Teachable moments for promoting smoking cessation: the context of cancer care and survivorship. Cancer Control 2003;10:325–33. [DOI] [PubMed] [Google Scholar]
- 55. Anantharaman D, Muller DC, Lagiou P, et al. Combined effects of smoking and HPV16 in oropharyngeal cancer. Int J Epidemiol 2016;45:752–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Applebaum KM, Furniss CS, Zeka A, et al. Lack of association of alcohol and tobacco with HPV16‐associated head and neck cancer. J Natl Cancer Inst 2007;99:1801–10. [DOI] [PubMed] [Google Scholar]
- 57. Duffy SA, Ronis DL, Valenstein M, et al. A tailored smoking, alcohol, and depression intervention for head and neck cancer patients. Cancer Epidemiol Biomarkers Prev 2006;15:2203–8. [DOI] [PubMed] [Google Scholar]
- 58. van Imhoff LC, Kranenburg GG, Macco S, et al. Prognostic value of continued smoking on survival and recurrence rates in patients with head and neck cancer: a systematic review. Head Neck 2016;38:E2214–E2220. [DOI] [PubMed] [Google Scholar]
- 59. Joehanes R, Just AC, Marioni RE, et al. Epigenetic signatures of cigarette smoking. Circ Cardiovasc Genet 2016;9:436–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Liu C, Marioni RE, Hedman AK, et al. A DNA methylation biomarker of alcohol consumption. Mol Psychiatry 2018;23:422–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Smith GD, Ebrahim S. Mendelian randomization: can genetic epidemiology contribute to understanding environmental determinants of disease? Int J Epidemiol 2003;32:1–22. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supporting Information 1
Supporting Information 2
Supporting Information 3


