Summary
Postoperative pulmonary complications are common after cardiothoracic surgery and are associated with adverse outcomes. The ability to detect postoperative pulmonary complications using chest X‐rays is limited, and this technique requires radiation exposure. Little is known about the diagnostic accuracy of lung ultrasound for the detection of postoperative pulmonary complications after cardiothoracic surgery, and we therefore aimed to compare lung ultrasound with chest X‐ray to detect postoperative pulmonary complications in this group of patients. We performed this prospective, observational, single‐centre study in a tertiary intensive care unit treating adult patients who had undergone cardiothoracic surgery. We recorded chest X‐ray findings upon admission and on postoperative days 2 and 3, as well as rates of postoperative pulmonary complications and clinically‐relevant postoperative pulmonary complications that required therapy according to the treating physician as part of their standard clinical practice. Lung ultrasound was performed by an independent researcher at the time of chest X‐ray. We compared lung ultrasound with chest X‐ray for the detection of postoperative pulmonary complications and clinically‐relevant postoperative pulmonary complications. We also assessed inter‐observer agreement for lung ultrasound, and the time to perform both imaging techniques. Subgroup analyses were performed to compare the time to detection of clinically‐relevant postoperative pulmonary complications by both modalities. We recruited a total of 177 patients in whom both lung ultrasound and chest X‐ray imaging were performed. Lung ultrasound identified 159 (90%) postoperative pulmonary complications on the day of admission compared with 107 (61%) identified with chest X‐ray (p < 0.001). Lung ultrasound identified 11 out of 17 patients (65%) and chest X‐ray 7 out of 17 patients (41%) with clinically‐relevant postoperative pulmonary complications (p < 0.001). The clinically‐relevant postoperative pulmonary complications were detected earlier using lung ultrasound compared with chest X‐ray (p = 0.024). Overall inter‐observer agreement for lung ultrasound was excellent (κ = 0.907, p < 0.001). Following cardiothoracic surgery, lung ultrasound detected more postoperative pulmonary complications and clinically‐relevant postoperative pulmonary complications than chest X‐ray, and at an earlier time‐point. Our results suggest lung ultrasound may be used as the primary imaging technique to search for postoperative pulmonary complications after cardiothoracic surgery, and will enhance bedside decision making.
Keywords: cardiothoracic surgery and cardiac surgery, chest X‐ray, lung ultrasound, postoperative pulmonary complications
Introduction
Postoperative pulmonary complications (PPCs), including atelectasis, pneumonia and pulmonary oedema, are common after cardiothoracic surgery, and are associated with adverse outcomes 1, 2, 3. Early recognition of PPCs might be important for intervention and/or monitoring, as these patients often have compromised physiological reserves. The time from surgery until PPC detection is approximately three days using the most commonly used diagnostics such as chest auscultation, chest X‐ray (CXR), arterial oxygen tension, PaO₂/FIO₂ (P/F) ratio, leucocyte count and temperature 4. Chest X‐ray remains the current standard diagnostic imaging technique to detect PPCs in the intensive care unit (ICU) setting 5, 6. However, the diagnostic accuracy of CXR is limited, and CXR requires radiation exposure and considerable costs 6.
Bedside lung ultrasound, an alternative imaging modality with high sensitivity and specificity, is increasingly used for the diagnosis of pulmonary pathology in the ICU. Lung ultrasound is without the downside of radiation exposure, and creates distinctive artefacts through the interplay between fluid, air and pleurae 7. Combinations of these artefacts can help differentiate between various pathological processes, and are combined and deciphered in the bedside lung ultrasound in emergency (BLUE) protocol. In the BLUE protocol, profiles have been designed for the main pulmonary complications (pneumonia; congestive heart failure; chronic obstructive pulmonary disease (COPD); asthma; pulmonary embolism; and pneumothorax) have been validated with an accuracy > 90%, and are therefore extensively used in daily critical care 8. For example, the lung sliding artefact, which is a ‘twinkling’ visible at the pleural line during inspiration, excludes a pneumothorax and is part of the definition of a normal lung surface (see also Supporting Information, Video S1). A‐lines are the main horizon artefact and represent a reverberation of the pleural line, indicating air at the pleural line. B‐lines are the result of many to‐and‐fro movements of ultrasound beams between air and fluid, generating this long, vertical hyperechoic artefact (see also Supporting Information, Fig. S1). More than two anterior B‐lines are pathological; this is the definition of a B‐profile in the BLUE protocol, indicating interstitial syndrome or pulmonary oedema. Furthermore, detection of pleural effusions and consolidation are part of the postero‐lateral alveolar and/or pleural syndrome (PLAPS) (see also Supporting Information, Fig. S2). At the PLAPS‐point, a disorder can be described when an anechoic pleural effusion is visible or a disorder within the lung tissue is seen; for example, a shredded deep border immediately indicates lung consolidation 8.
Cardiac ultrasound has been shown to be valuable in the peri‐operative assessment and therapeutic management of patients undergoing surgery 9. In non‐cardiothoracic surgery patients, lung ultrasound performed better than CXR in diagnosing pneumonia, pulmonary oedema, pneumothorax and pleural effusion 10, 11, 12, 13. Until now, however, there have been limited data available for the diagnostic value of lung ultrasound in cardiothoracic surgical patients. In addition, it has to date been unknown whether repeated lung ultrasound is able to detect PPCs at an earlier stage compared with routine CXR. The primary outcome measure of this study was to compare the performance of lung ultrasound with CXR in detecting PPCs and clinically‐relevant PPCs, defined as PPCs that required treatment, after cardiothoracic surgery. We hypothesised that lung ultrasound would detect more PPCs and clinically‐relevant PPCs than CXR. Secondary outcome measures were to evaluate the time to detection of clinically‐relevant PPCs by lung ultrasound and CXR, and to assess the relationship between lung ultrasound findings and outcome measures.
Methods
We conducted this prospective, observational cohort study at the VU University Medical Center Amsterdam, a tertiary referral hospital. The local Human Subjects Committee of the VUmc approved the study and waived the requirement for informed consent. We included consecutive adult patients (≥ 18 years old) who had undergone cardiothoracic surgery between April 2015 and January 2016 upon their admission to the ICU. Patients were not included if there was no investigator available to perform lung ultrasound examination upon ICU admission. Cardiothoracic procedures included coronary artery bypass grafting and/or valve surgery, Bentall procedures or pulmonary endarterectomy. All patients admitted to the ICU had their tracheas intubated and were sedated following surgery. Their tracheas were extubated according to local protocol, followed by the administration of supplemental oxygen via nasal cannulae. Monitoring, including pulse oximetry, heart rate, electrocardiographic, invasive blood pressure and CXR, was performed according to standard clinical practice.
Anteroposterior bedside CXRs were obtained using a DRX‐Revolution mobile X‐ray unit (Carestream Health, Inc. © Toronto, ON, Canada) upon admission to the ICU, and at postoperative days two and three, according to local protocol. We retrieved CXR findings, assessed by a radiologist blinded to the lung ultrasound findings, from the electronic patient data management system. The Nomenclature Committee of the Fleischner Society recommended terminology was used to describe pathological entities according to the diagnostic criteria for bedside CXR 14, 15.
A trained member of the research team (n = 5), which consisted of two investigators, one ICU physician and two intensivists, performed lung ultrasound upon admission to the ICU on day 0, and at postoperative days two and three. All ultrasonographers were trained according to the Netherlands Society of Intensive Care Programme for Intensive Care Ultrasound (NVIC ICARUS) training course (http://www.nvic.nl). The level of experience of lung ultrasound varied from three months to over five years. The ultrasonographer was blinded to clinical details and CXR findings, and did not contribute to the diagnostic and treatment strategy of the patient. Lung ultrasound was performed immediately before or after CXR. We used a CX50 ultrasound machine (Koninklijke Philips NV®, Eindhoven, the Netherlands) with both the cardiac phased array (1–5 MHz) and the linear vascular probe (> 10 MHz). The ultrasonographer could choose a particular probe for a particular view in a particular patient, according to individual preference. Lung sliding was determined using the vascular probe. Lung ultrasound views were obtained according to the BLUE protocol 16, 17, 18.
In short, we determined the BLUE profile for each BLUE point and BLUE profile per hemithorax (BLUE 1 and 2) as follows: A; B; A’; B’ or C‐profile. A‐profile means predominantly A‐lines (see also Supporting Information, Fig. S1 and Video S1). B‐profile means predominantly multiple (> 2) anterior diffuse B‐lines. A’ or B’ means the corresponding BLUE profile with absence of, or abolished lung sliding. C‐profile means anterior alveolar consolidation. Furthermore, we determined the postero‐lateral alveolar and/or pleural syndrome (PLAPS) on each side at the lateral subposterior ultrasound examination and scored this as positive or negative (see also Supporting Information, Fig. S2). When positive, consolidation and/or pleural effusion were scored separately and the diagnosis of atelectasis was added to the flowchart. The final conclusion was made according to the flowchart of the modified BLUE protocol after cardiothoracic surgery (see also Supporting Information, Fig. S3). For differentiation between pneumonia and atelectasis, the ultrasonographer was allowed to look for fever and C‐reactive protein (CRP) in the medical chart of the patient.
We retrieved patient characteristics from the electronic patient data management system. These included: sex; age; body mass index (BMI); surgery type; and relevant cardiopulmonary comorbidities. Peri‐operative data consisted of extracorporeal circulation and aortic clamp time, total peri‐operative blood loss and blood product transfusions. Respiratory data at time of lung ultrasound included: arterial oxygen saturation; mode of ventilation; positive end‐expiratory pressure (PEEP); O2 supplementation; P/F‐ ratio; and hours of mechanical ventilation during ICU stay. Parameters retrieved from the documentation by the treating ICU physician included: chest auscultation findings; conclusions/diagnoses concerning pulmonary status; and treatments. Routine data collection during ICU and medium care stay included: temperature; fluid balance; drains; drain production; haemoglobin levels; white blood cell count; lactate; CRP levels; and ICU/medium care stay in hours, corrected for prolonged stay due to delayed transfer to the ward due to capacity problems.
Postoperative pulmonary complications were defined as previously described by Canet et al.: respiratory infection; respiratory failure; pleural effusion; atelectasis; pneumothorax; and bronchospasm, and scored accordingly 1. A distinction was made between PPCs and clinically‐relevant PPCs, defined as a PPC that required treatment, as judged by the treating physician. We considered the following to be clinically‐relevant treatments for PPCs: high flow oxygen therapy; a higher PEEP level or recruitment manoeuvres; continuous positive airway pressure (CPAP); re‐intubation; bronchoscopy; extra bronchodilator therapy; thoracic drain placement; and the use of diuretics and/or antibiotics, in addition to local antibiotic protocols for cardiothoracic surgery. Scoring of clinically‐relevant PPCs was based on the documentation by the treating ICU physician. The physician who diagnosed clinically‐relevant PPCs was taking into account physical examination, conventional monitoring, laboratory results and CXR. This reflected daily clinical practice. The treating ICU physician was blinded to lung ultrasound findings. Clinically‐relevant PPCs were considered the composite reference standard. The diagnostic accuracy of CXR alone is debated and is therefore an imperfect reference standard for lung ultrasound. The diagnostic accuracy of the reference standard was improved by using a composite reference standard, including multiple tests, one of which was CXR.
To assess the relationship between lung ultrasound findings and patient outcome parameters, we compared the number of B‐lines, depth (cm) of pleural effusion and total atelectasis (cm2) between patients with clinically‐relevant PPCs and patients without clinically‐relevant PPCs. Furthermore, we compared P/F ratio and length of ICU stay between both groups.
We performed a predefined subgroup analysis of patients in whom all lung ultrasound and CXR examinations were successfully completed on both the day of surgery and admission to ICU (day 0), and days two and three. This allowed for comparison of the time to detection of PPC and clinically‐relevant PPC using both techniques. Not all patients could be included in this subgroup, as sometimes no researcher was available to perform lung ultrasound, mostly at weekends. In addition, CXRs were not always performed for logistical reasons or at the discretion of the treating physician, bypassing the departmental protocol.
We assessed the inter‐observer agreement in a random subset of the study population. We calculated that a sample size of 70 measurements would be required, according to a difference in overall agreement and change probability of 0.6, and an accepted relative error of 20%. Seventy lung ultrasound points (BLUE points and PLAPS) were assessed to determine inter‐observer agreement. Lung ultrasound was performed by two research team members (HT and KP). Both investigators were blinded to mutual lung ultrasound findings. Agreement between the findings was assessed at 10 different points per patient, per hemithorax (BLUE profile per BLUE point), PLAPS (positive or negative), atelectasis (positive or negative) and pleural effusion (positive or negative).
The time to perform CXR and lung ultrasound, after CXR was ordered, was compared. We hypothesised that lung ultrasound would detect an incidence of PPCs of 80% and that CXR would detect an incidence of 60% (our primary endpoint). We calculated that a sample size of 162 subjects would be required with an alpha of 0.05 and a power of 80%. With an anticipated 10% loss to follow‐up, we set our sample size at 180 patients 1. We carried out analyses using SPSS statistical software package version 19.0 (IBM, New York, NY, USA). We used the Chi‐square test to compare unpaired categorical data, and the McNemar test for paired data. The accuracy of lung ultrasound and CXR for the detection of clinically‐relevant PPCs was expressed as sensitivity, specificity, positive and negative predictive values and diagnostic accuracy.
We used the Wilcoxon signed‐rank test for paired ordinal data (repeated measures) in order to analyse earlier detection of clinically‐relevant PPCs with lung ultrasound vs. CXR. Detection of day 0 PPCs was ranked as 1, detection of day 2 PPCs as 2, detection of day 3 PPCs as 3 and undetected PPCs were ranked as 4. To assess the inter‐observer agreement for lung ultrasound, Cohen's kappa (κ) statistics were used with κ < 0.40 indicating low agreement, 0.40 to 0.75 low‐to‐good agreement, and > 0.75 good‐to‐perfect agreement.
Results
During the study period, 360 patients underwent cardiothoracic surgery, and we recruited 177 of these patients to the study. The other patients were not included because no member of the research team was available to perform lung ultrasound on admission. Table 1 shows the baseline characteristics and clinical characteristics of the included patients.
Table 1.
Peri‐operative characteristics of 177 patients after cardiothoracic surgery. Values are number (proportion), mean (SD), or median (IQR [range])
| Men | 125 (71%) |
| Age; years | 68 (10) |
| Body mass index; kg.m−2 | 27.5 (4.9) |
| Type of surgery | |
| CABG | 69 (40%) |
| Valve surgery | 54 (31%) |
| CABG and valve surgery | 32 (18%) |
| Pulmonary endarterectomy | 10 (6%) |
| Bentall surgery | 11 (6%) |
| Type of comorbidity | |
| Obstructive pulmonary airway disease | 11 (6%) |
| Heart failure | 49 (28%) |
| ECC duration; min; n = 166 | 129 (68) |
| Aortic clamp time; min; n = 166 | 94 (47) |
| Mechanical ventilation time on ICU; h | 6.5 (4.5–9.0 [0.5–263.0]) |
| ICU stay; h | 24 (22–46 [8–264]) |
CABG, coronary artery bypass graft; ECC, extracorporeal circulation time; ICU, intensive care unit.
Lung ultrasound identified more patients with a PPC compared with CXR (p < 0.001) upon ICU admission. The PPCs identified were pulmonary oedema, consolidation, pneumothorax, atelectasis and pleural effusion. The incidences of PPCs on day 0, day 2 and day 3, as detected by CXR and lung ultrasound, are shown in Table 2. The prevalence of pleural effusion increased during the postoperative course for both groups, but there was a higher prevalence detected using lung ultrasound compared with CXR, with rates of 44% and 71% on day 2 (p ≤ 0.001) and 71% and 90% on day 3 (p = 0.013) for CXR and lung ultrasound, respectively. Lung ultrasound on admission mainly detected more cases of atelectasis (87% vs. 41%, p < 0.001) and pulmonary oedema (20% vs. 15%, p = 0.143) when compared with CXR.
Table 2.
Presence of postoperative pulmonary complications (PPC) based on lung ultrasound and chest X‐ray and on days 0, 2 and 3 after cardiothoracic surgery. Values are number (proportion)
| Day 0 | Day 2 | Day 3 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| n = 177 | n = 99 | n = 58 | |||||||
| Lung ultrasound | Chest X‐ray | p value | Lung ultrasound | Chest X‐ray | p value | Lung ultrasound | Chest X‐ray | p value | |
| Total PPCs | 159 (89.8%) | 107 (60.5%) | < 0.001 | 90 (90.9%) | 80 (80.8%) | 0.031 | 58 (100%) | 52 (89.7%) | – |
| Pulmonary oedema | 36 (20.3%) | 26 (14.7%) | 0.143 | 14 (14.1%) | 9 (9.1%) | 0.383 | 20 (34.5%) | 11 (19.0%) | 0.049* |
| Consolidation | 14 (7.9%) | 0 | – | 16 (16.2%) | 2 (2.0%) | 0.001 | 16 (27.6%) | 0 | – |
| Pneumothorax | 7 (4.0%) | 2 (1.1%) | 0.180 | 0 | 2 (2.0%) | – | 3 (5.2%) | 3 (5.2%) | 1.000 |
| Atelectasis | 154 (87.0%) | 72 (40.6%) | < 0.001 | 84 (84.8%) | 65 (65.7%) | 0.002 | 57 (98.3%) | 39 (67.8%) | < 0.001* |
| Pleural effusion | 60 (33.9%) | 51 (28.8%) | 0.328 | 70 (70.7%) | 44 (44.4%) | < 0.001 | 52 (89.7%) | 41 (70.7%) | 0.013* |
The incidence of clinically‐relevant PPCs on day 0, 2 and 3 are shown in Table 3. Seventeen patients out of 177 patients (10%) developed a clinically‐relevant PPC at day 0. The incidence of clinically‐relevant PPCs increased over the days; 15 patients out of 99 patients (15%) on day 2 and 13 patients out of 58 patients (22%) on day 3.
Table 3.
Presence of clinically‐relevant postoperative pulmonary complications (PPC) on days 0, 2 and 3 after cardiothoracic surgery. Values are number (proportion)
| Day 0 | Day 2 | Day 3 | |
|---|---|---|---|
| n = 177 | n = 99 | n = 58 | |
| Total clinically‐relevant PPCs | 17 (9.6%) | 15 (15.2%) | 13 (22.4%) |
| Pulmonary oedema | 2 (1.1%) | 8 (8.1%) | 10 (17.2%) |
| Bronchospasm | 4 (2.3%) | 2 (2.0%) | 1 (1.7%) |
| Pneumonia | 0 | 1 (1.0%) | 1 (1.7%) |
| Pneumothorax | 0 | 0 | 1 (1.7%) |
| Atelectasis | 11 (6.2%) | 4 (4.0%) | 0 |
| Pleural effusion | 0 | 0 | 0 |
Table 4 shows the sensitivity, specificity, PPV and NPV, and diagnostic accuracy for lung ultrasound and CXR in detecting clinically‐relevant PPCs. Pulmonary oedema and atelectasis were the two clinically‐relevant PPCs that occurred sufficiently commonly to allow us to calculate the sensitivity and specificity of diagnosis by lung ultrasound and CXR. The sensitivity was higher and specificity was lower for lung ultrasound for detecting clinically‐relevant atelectasis compared with CXR. The diagnostic accuracy for detecting pulmonary oedema was comparable during the study period.
Table 4.
Diagnostic accuracy of lung ultrasound and chest X‐ray in detecting clinically‐relevant postoperative pulmonary complications on days 0, 2 and 3 after cardiothoracic surgery
| Sensitivity | Specificity | PPV | NPV | Diagnostic accuracy | |
|---|---|---|---|---|---|
| Lung ultrasound | |||||
| Pulmonary oedema (day 0) | 0.42 | 0.85 | 0.95 | 0.17 | 0.86 |
| Pulmonary oedema (day 2) | 0.25 | 0.87 | 0.93 | 0.14 | 0.82 |
| Pulmonary oedema (day 3) | 0.50 | 0.83 | 0.89 | 0.38 | 0.78 |
| Atelectasis (day 0) | 0.82 | 0.13 | 0.91 | 0.06 | 0.17 |
| Chest X‐ray | |||||
| Pulmonary oedema (day 0) | 0.27 | 0.84 | 0.95 | 0.10 | 0.81 |
| Pulmonary oedema (day 2) | 0.13 | 0.91 | 0.92 | 0.11 | 0.85 |
| Pulmonary oedema (day 3) | 0.30 | 0.83 | 0.85 | 0.27 | 0.74 |
| Atelectasis (day 0) | 0.45 | 0.59 | 0.94 | 0.07 | 0.58 |
PPV, positive predictive value; NPV, negative predictive value.
In total, lung ultrasound identified 26 (58%) clinically‐relevant PPCs and CXR identified 17 (38%) clinically‐relevant PPCs out of a total of 45 clinically‐relevant PPCs on day 0, 2 and 3 (p = 0.021). On day 0, lung ultrasound identified 11 out of 17 (65%) patients with a clinically‐relevant PPC, whereas CXR detected 7 out of 17 (41%) patients (p = 0.125). On day 2, lung ultrasound identified 8 out of 15 (53%) patients with a clinically‐relevant PPC and CXR identified 5 out of 15 (33%) patients (p = 0.250). On day 3, lung ultrasound identified seven (54%) patients and CXR identified five (39%) patients out of 13 patients with clinically‐relevant PPCs (p = 0.625).
In a subgroup of 42 patients, clinically‐relevant PPCs were detected earlier with lung ultrasound than with CXR (p = 0.024) (Fig. 1). Overall inter‐observer agreement for lung ultrasound showed excellent agreement (κ = 0.907, p < 0.001). Lung ultrasound was performed within a mean (SD) of 15 (5) min compared with 42 (16) min for CXR (p < 0.001).
Figure 1.
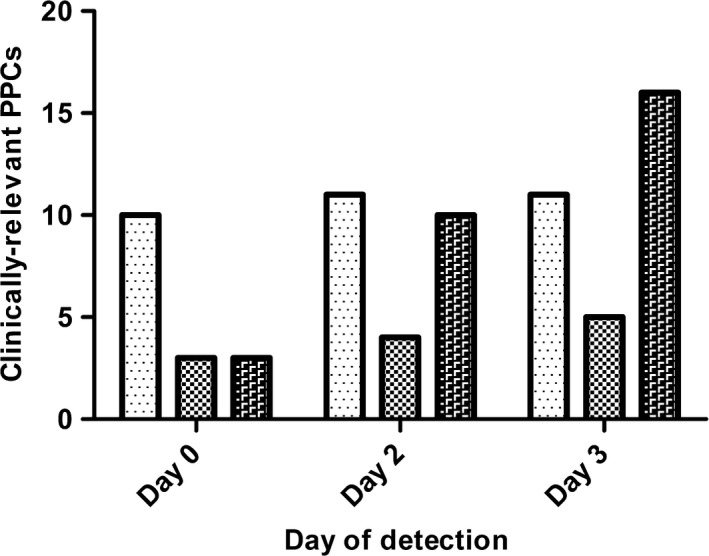
Cumulative number and time to detection of clinically‐relevant postoperative pulmonary complications (PPC) after cardiothoracic surgery in a subgroup of patients with both lung ultrasound and chest X‐ray findings available on days 0, 2 and 3 (n = 42). Bars represent number of cases of clinically‐relevant PPCs, or future clinically‐relevant PPCs detected with lung ultrasound ( ), chest X‐ray (
), chest X‐ray ( ), and actual clinically‐relevant postoperative complications (
), and actual clinically‐relevant postoperative complications ( ).
).
Patients with a clinically‐relevant PPC had a lower mean (SD) P/F ratio compared with patients without clinically‐relevant PPCs on day 1 (188 (58) vs. 250 (72) mmHg, p = 0.001) and on day 2 (190 (84) vs. 273 (71) mmHg, p = 0.001), respectively. Furthermore, ICU stay differed significantly between both groups (p = 0.083) (see also Supporting Information, Table S1). The total number of B‐lines and pleural effusion (in cm) did not differ between patients with and without clinically‐relevant PPCs. The degree of atelectasis (in cm2) was smaller only on day 2 in patients with clinically‐relevant PPCs compared with the group that were not clinically‐relevant (12.8 (6.4) cm2 vs. 7.7 (7.1) cm2, p = 0.045). (see also Supporting Information, Table S1)
Discussion
This study demonstrates that lung ultrasound detected more PPCs and clinically‐relevant PPCs, and at an earlier time‐point, when compared with CXR, in patients following cardiothoracic surgery. In addition, time to perform lung ultrasound was significantly shorter than for CXR, and lung ultrasound had excellent inter‐observer agreement. Lung ultrasound outperformed CXR in diagnosing clinically‐relevant PPCs following cardiothoracic surgery and may potentially replace CXR as the primary imaging technique in these patients.
The PPC rate in our study is in line with previous research, which showed that 54–73% of patients had atelectasis, pleural effusion or both after cardiothoracic surgery when detected by CXR 2, 15, 19. Of interest, we found a higher incidence of atelectasis and pleural effusions detected by lung ultrasound in these patients. This finding could be explained by the higher sensitivity and specificity of lung ultrasound in detecting pleural effusion and atelectasis compared with CXR, when compared with the gold standard for thoracic imaging, computed tomography (CT) scans 5, 7, 13, 18. Furthermore, our results are comparable with the reported incidence of atelectasis of 90% found with thoracic CT scan in anaesthetised patients 20. The incidence of clinically‐relevant PPCs in our study was comparable with rates described before, making our results generalisable to the postoperative population 2, 4, 21. Of note, more clinically‐relevant PPCs were detected by lung ultrasound, although this clinically‐relevant composite reference standard was based on CXR findings, among others. This suggests that lung ultrasound would have performed even better when used as the primary imaging technique to screen for clinically‐relevant PPCs. Previous research comparing CXR with lung ultrasound in patients after cardiothoracic surgery found that lung ultrasound identified most of the PPCs detected by CXR. Unfortunately, they reported the diagnostic value of lung ultrasound compared with CXR as the reference standard 22. The use of a reference standard with potentially worse accuracy (CXR) than the index test (lung ultrasound) in the study, makes it difficult to interpret their results.
Early detection of PPCs may lead to improved outcomes. Previous research has shown that although PPC rates did not differ among hospitals, mortality rates did due to ‘failure to rescue’, a term coined by Silber et al. 23, 24. In a predefined subgroup analysis, we found that lung ultrasound identified clinically‐relevant PPCs at an earlier timepoint than CXR. In addition, lung ultrasound had superior sensitivity and almost comparable specificity in detecting PPCs, and thereby improved detection of PPCs. However, in the BLUE protocol we used, the extent of the complication was not determined, but only assessed as dichotomous; either a complication was present or not 17. An approach in which the extent of the PPC is quantified might be preferable. For assessing pleural effusions, considerable work has been done to quantify the volume of effusion according to lung ultrasound imaging, but not to determine the clinical significance 25. Our study found only that atelectasis in cm2 differed significantly on day 2 for patients with clinically‐relevant PPCs compared with patients without clinically‐relevant PPCs. Of note, quantification of PPCs detected by lung ultrasound should be evaluated in future studies, because our study was underpowered to detect such a difference.
Considering the great variability in imaging strategies after cardiothoracic surgery in current practice, an optimal imaging strategy is still warranted 26. Daily routine CXR performance has been largely abandoned in ICU patients whose lungs are ventilated, and has been replaced by an ‘on demand’ strategy 27. This has led to a significant decrease in the number of CXRs without any increase in adverse events 6, 26. Studies comparing a daily routine with an ‘on demand strategy’ using primarily CXR or lung ultrasound as the imaging technique are lacking in the cardiothoracic population.
Our data suggest that lung ultrasound might be a key modality to optimise the postoperative imaging strategy in these patients. Lung ultrasound potentially facilitates prompt diagnosis of PPCs, thereby enabling treatment to start early. In addition, lung ultrasound can be used to evaluate the efficacy of therapies initiated for PPCs, since lung ultrasound offers an accurate, non‐invasive, easily reproducible bedside evaluation. Alsaddique et al. found that repeated imaging with lung ultrasound after cardiothoracic surgery frequently altered diagnosis and management of PPCs 28. Considering the aforementioned, we suggest using lung ultrasound as an extension of the physical examination in patients after cardiothoracic surgery. The use of stethoscopes and CXRs for the detection of PPCs, with their debatable accuracy and downsides such as radiation exposure, may be superseded 5, although CXR remains the standard for confirmation of the position of central venous catheters, as well as tracheal tube placement.
Our study has some important limitations. Firstly, we could not report sensitivity and specificity of lung ultrasound and CXR compared with the gold standard for lung imaging, the thoracic CT scan, since this was not performed in our study. Therefore, we used clinically‐relevant PPCs as a composite reference standard, to minimise imperfect reference standard bias. However, a disadvantage is the introduction of the subjectivity of the treating physician 29. Secondly, lung ultrasound was not studied as the primary imaging technique following cardiothoracic surgery and compared with CXR in a blinded, randomised controlled trial. Possible variations in therapy initiated, and the incidence rate of detection of clinically‐relevant PPCs for lung ultrasound compared with CXR could not be studied. Theoretically, should therapy have been initiated according to more sensitive lung ultrasound findings, it could introduce an element of over‐treatment of clinically‐irrelevant PPCs. However, the positive effects of early treatment and potentially faster recovery were not studied either. Thirdly, the BLUE protocol was validated for patients in respiratory distress presenting to the emergency department 17. Strengths of the current study are the large sample size compared with previous studies in non‐cardiothoracic surgery, and the fact that this was the first study to show that lung ultrasound can detect PPCs at an earlier stage than CXR. Furthermore, lung ultrasound was performed by multiple investigators, with different levels of experience, thus mirroring daily clinical practice. In addition, lung ultrasound demonstrated high inter‐observer agreement, in concordance with previously reported findings, and was performed faster than CXR 22. Lastly, we have shown that lung ultrasound is feasible in patients after cardiothoracic surgery, who are considered less accessible to lung ultrasound, for example due to wound dressings and thoracic drain tubes, again in accordance with previous studies 22, 28. There is a need for larger (multicenter) trials investigating the cost effectiveness, the ease of implementation and the effect on patient outcomes and interventions performed when lung ultrasound is used a primary imaging technique, compared with CXR. Since different lung ultrasound protocols were used in previous studies, it would be of interest to determine if lung ultrasound protocols are comparable, or if one protocol is superior to another. In addition, we suggest quantification of lung ultrasound findings.
In conclusion, lung ultrasound detected more clinically‐relevant PPCs and at an earlier timepoint than CXR following cardiothoracic surgery. Our results suggest lung ultrasound can be used as the primary imaging technique to detect PPCs after cardiothoracic surgery, and will enhance bedside decision making. The next step is to test lung ultrasound as the primary imaging technique in these patients, and further quantify the extent of PPCs.
Supporting information
Figure S1 Lung ultrasound of the pleural line at anterior position demonstrating A‐line artefacts.
Figure S2 Lung ultrasound at the lateral subposterior position and detecting the postero‐lateral alveolar and/or pleural syndrome. C, consolidated lung; E, pleural effusion.
Figure S3 Bedside lung ultrasound in cardiothoracic surgery protocol.
Table S1 Lung ultrasound and clinical findings in patients without clinically‐relevant postoperative pulmonary complications compared with patients with clinically‐relevant postoperative pulmonary complications after cardiothoracic surgery.
Video S1 Lung ultrasound demonstrating normal lung sliding.
Acknowledgements
No external funding or competing interests declared.
Presented in part at the 29th Annual Congress of European Society of Intensive Care Medicine, October 2016, Milan, Italy.
You can respond to this article at http://www.anaesthesiacorrespondence.com
References
- 1. Canet J, Gallart L, Gomar C, et al. Prediction of postoperative pulmonary complications in a population‐based surgical cohort. Anesthesiology 2010; 113: 1338–50. [DOI] [PubMed] [Google Scholar]
- 2. Weissman C. Pulmonary complications after cardiac surgery. Seminars in Cardiothoracic and Vascular Anesthesia 2004; 8: 185–211. [DOI] [PubMed] [Google Scholar]
- 3. Marseu K, Slinger P. Peri‐operative pulmonary dysfunction and protection. Anaesthesia 2016; 71: 46–50. [DOI] [PubMed] [Google Scholar]
- 4. Mazo V, Sabaté S, Canet J, et al. Prospective external validation of a predictive score for postoperative pulmonary complications. Anesthesiology 2014; 121: 219–31. [DOI] [PubMed] [Google Scholar]
- 5. Lichtenstein D, Goldstein I, Mourgeon E, Cluzel P, Grenier P, Rouby J‐J. Comparative diagnostic performances of auscultation, chest radiography, and lung ultrasonography in acute respiratory distress syndrome. Anesthesiology 2004; 100: 9–15. [DOI] [PubMed] [Google Scholar]
- 6. Tolsma M, Rijpstra TA, Rosseel PM, et al. Defining indications for selective chest radiography in the first 24 hours after cardiac surgery. Journal of Thoracic and Cardiovascular Surgery 2015; 150: 225–9. [DOI] [PubMed] [Google Scholar]
- 7. Touw HR, Tuinman PR, Gelissen HP, Lust E, Elbers PW. Lung ultrasound: routine practice for the next generation of internists. Netherlands Journal of Medicine 2015; 73: 100–7. [PubMed] [Google Scholar]
- 8. Lichtenstein DA. BLUE‐Protocol and FALLS‐Protocol. Chest 2015; 147: 1659–70. [DOI] [PubMed] [Google Scholar]
- 9. Barber RL, Fletcher SN. A review of echocardiography in anaesthetic and peri‐operative practice. Part 1: impact and utility. Anaesthesia 2014; 69: 764–76. [DOI] [PubMed] [Google Scholar]
- 10. Ye X, Xiao H, Chen B, Zhang S. Accuracy of lung ultrasonography versus chest radiography for the diagnosis of adult community‐acquired pneumonia: review of the literature and meta‐analysis. PLoS ONE 2015; 10: e0130066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Martindale JL, Noble VE, Liteplo A. Diagnosing pulmonary edema: lung ultrasound versus chest radiography. European Journal of Emergency Medicine 2013; 20: 356–60. [DOI] [PubMed] [Google Scholar]
- 12. Alrajab S, Youssef AM, Akkus NI, Caldito G. Pleural ultrasonography versus chest radiography for the diagnosis of pneumothorax: review of the literature and meta‐analysis. Critical Care 2013; 17: R208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ashton‐Cleary DT. Is thoracic ultrasound a viable alternative to conventional imaging in the critical care setting? British Journal of Anaesthesia 2013; 111: 152–60. [DOI] [PubMed] [Google Scholar]
- 14. Tuddenham WJ. Glossary of terms for thoracic radiology: recommendations of the Nomenclature Committee of the Fleischner Society. American Journal of Roentgenology 1984; 143: 509–17. [DOI] [PubMed] [Google Scholar]
- 15. Rubinowitz AN, Siegel MD, Tocino I. Thoracic imaging in the ICU. Critical Care Clinics 2007; 23: 539–73. [DOI] [PubMed] [Google Scholar]
- 16. Lichtenstein DA, Mezière GA. Relevance of lung ultrasound in the diagnosis of acute respiratory failure: the BLUE protocol. Chest 2008; 134: 117–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Lichtenstein D. Lung ultrasound in acute respiratory failure an introduction to the BLUE‐protocol. Minerva Anestesiologica 2009; 75: 313–7. [PubMed] [Google Scholar]
- 18. Lichtenstein D. Whole body ultrasonography in the critically ill. Berlin, Germany: Springer‐Verlag, 2010. [Google Scholar]
- 19. Jain U, Rao TL, Kumar P, et al. Radiographic pulmonary abnormalities after different types of cardiac surgery. Journal of Thoracic and Cardiovascular Anesthesia 1991; 5: 592–5. [DOI] [PubMed] [Google Scholar]
- 20. Hedenstierna G, Edmark L. Mechanisms of atelectasis in the perioperative period. Best Practice and Research in Clinical Anaesthesiology 2010; 24: 157–69. [DOI] [PubMed] [Google Scholar]
- 21. Jindani A, Aps C, Neville E, Sonmez B, Tung K, Williams BT. Postoperative cardiac surgical care: an alternative approach. British Heart Journal 1993; 69: 59–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Vezzani A, Manca T, Brusasco C, et al. Diagnostic value of chest ultrasound after cardiac surgery: a comparison with chest X‐ray and auscultation. Journal of Thoracic and Cardiovascular Anesthesia 2014; 28: 1527–32. [DOI] [PubMed] [Google Scholar]
- 23. Ghaferi AA, Birkmeyer JD, Dimick JB. Complications, failure to rescue, and mortality with major inpatient surgery in medicare patients. Annals of Surgery 2009; 250: 1029–34. [DOI] [PubMed] [Google Scholar]
- 24. Silber JH, Williams SV, Krakauer H, Schwartz JS. Hospital and patient characteristics associated with death after surgery. A study of adverse occurrence and failure to rescue. Medical Care 1992; 30: 615–29. [DOI] [PubMed] [Google Scholar]
- 25. Balik M, Plasil P, Waldauf P, et al. Ultrasound estimation of volume of pleural fluid in mechanically ventilated patients. Intensive Care Medicine 2006; 32: 318–21. [DOI] [PubMed] [Google Scholar]
- 26. Lee J, Geyer B, Naraghi L, et al. Advanced imaging use in intensive care units has decreased, resulting in lower charges without negative effects on patient outcomes. Journal of Critical Care 2015; 30: 460–4. [DOI] [PubMed] [Google Scholar]
- 27. Hejblum G, Chalumeau‐Lemoine L, Ioos V, et al. Comparison of routine and on‐demand prescription of chest radiographs in mechanically ventilated adults: a multicentre, cluster‐randomised, two‐period crossover study. Lancet 2009; 374: 1687–93. [DOI] [PubMed] [Google Scholar]
- 28. Alsaddique A, Royse AG, Royse CF, et al. Repeated monitoring with transthoracic echocardiography and lung ultrasound after cardiac surgery: feasibility and impact on diagnosis. Journal of Thoracic and Cardiovascular Anesthesia 2016; 30: 406–12. [DOI] [PubMed] [Google Scholar]
- 29. Naaktgeboren CA, Bertens LC, de Groot JA, Moons KG, Reitsma JB. Value of composite reference standards in diagnostic research. British Medical Journal 2013; 25: 5605. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1 Lung ultrasound of the pleural line at anterior position demonstrating A‐line artefacts.
Figure S2 Lung ultrasound at the lateral subposterior position and detecting the postero‐lateral alveolar and/or pleural syndrome. C, consolidated lung; E, pleural effusion.
Figure S3 Bedside lung ultrasound in cardiothoracic surgery protocol.
Table S1 Lung ultrasound and clinical findings in patients without clinically‐relevant postoperative pulmonary complications compared with patients with clinically‐relevant postoperative pulmonary complications after cardiothoracic surgery.
Video S1 Lung ultrasound demonstrating normal lung sliding.


