Abstract
Background
Icodextrin is a high molecular weight, starch-derived glucose polymer, which is capable of inducing sustained ultrafiltration over prolonged (12–16 hour) peritoneal dialysis (PD) dwells. The aim of this study was to evaluate the ability of icodextrin to alleviate refractory, symptomatic fluid overload and prolong technique survival in PD patients.
Methods
A prospective, open-label, pre-test/post-test study was conducted in 17 PD patients (8 females/9 males, mean age 56.8 ± 2.9 years) who were on the verge of being transferred to haemodialysis because of symptomatic fluid retention that was refractory to fluid restriction, loop diuretic therapy, hypertonic glucose exchanges and dwell time optimisation. One icodextrin exchange (2.5 L 7.5%, 12-hour dwell) was substituted for a long-dwell glucose exchange each day.
Results
Icodextrin significantly increased peritoneal ultrafiltration (885 ± 210 ml to 1454 ± 215 ml, p < 0.05) and reduced mean arterial pressure (106 ± 4 to 96 ± 4 mmHg, p < 0.05), but did not affect weight, plasma albumin concentration, haemoglobin levels or dialysate:plasma creatinine ratio. Diabetic patients (n = 12) also experienced improved glycaemic control (haemoglobin Alc decreased from 8.9 ± 0.7% to 7.9 ± 0.7%, p < 0.05). Overall PD technique survival was prolonged by a mean of 11.6 months (95% CI 6.0–17.3 months). On multivariate Cox proportional hazards analysis, extension of technique survival by icodextrin was only significantly predicted by baseline net daily peritoneal ultrafiltration (adjusted HR 2.52, 95% CI 1.13–5.62, p < 0.05).
Conclusions
Icodextrin significantly improved peritoneal ultrafiltration and extended technique survival in PD patients with symptomatic fluid overload, especially those who had substantially impaired peritoneal ultrafiltration.
Background
Icodextrin is an isosmolar, starch-derived, high molecular weight glucose polymer, which is minimally absorbed across the peritoneal membrane. Two previous randomised, controlled trials have shown that this agent promotes sustained ultrafiltration and small solute clearances equivalent to that achieved with hypertonic (3.86%/4.25%) glucose exchanges [1,2]. Moreover, in subjects with higher peritoneal membrane transport characteristics, icodextrin appears to achieve superior fluid removal compared with glucose-based dialysates [3,4]. Ultrafiltration is produced by colloidal, rather than crystalline, osmotic pressure and is sustained over prolonged (12 to 16 hour) dwells [5]. There is also emerging evidence that this isosmolar solution may be less damaging to the peritoneal membrane than glucose-based dialysates [6].
Two small, retrospective studies in CAPD patients [7][8]have suggested that substitution of icodextrin for a long-dwell glucose exchange may result in respective extensions of technique survival by median values of approximately 8 months [7] and 22 months [8]. However, these studies were potentially limited by recall bias and by the lack of a clear definition of "ultrafiltration failure," thereby introducing uncertainty as to the true extent of PD prolongation. Furthermore, neither of these studies attempted to examine which patient characteristics predicted a satisfactory ultrafiltration response to icodextrin and subsequent enhancement of technique survival. The aim of the present study was to prospectively evaluate the ability of icodextrin to alleviate fluid overload and extend technique survival in PD patients on the verge of being transferred to haemodialysis because of refractory, symptomatic fluid retention. Subsequent analyses were also performed to determine which patients were most likely to respond to icodextrin therapy.
Methods
Patients
All patients over the age of 18 years who were receiving PD at the Princess Alexandra Hospital between 30 January 1999 and 31 May 2001 were included in the study if they had pulmonary or peripheral oedema that was refractory to a combination of (i) fluid restriction (≤ 800 ml/day), (ii) frusemide therapy (≥ 250 mg daily), (iii) dwell time optimisation according to transport status, and, (iv) hypertonic glucose exchanges (consisting of at least one daily 4.25% exchange). All patients were considered by their treating physicians to be at the point of transfer to haemodialysis because of refractory fluid overload. CAPD patients had received a minimum of 4 exchanges and 8 litres of dialysis fluid per day. Those individuals on automated PD (APD) were treated with a continuous cycling PD (CCPD) regimen consisting of at least 12.5 L of dialysis fluid per day. Informed consent was obtained from all patients prior to their participation in the trial and the study protocol was reviewed and approved by the Princess Alexandra Hospital Research Ethics Committee.
Study protocol
The study followed a prospective, open label, pre-test/post-test design. One 12-hour 7.5% icodextrin exchange (2.5 L Extraneal®, Baxter Healthcare, Castlebar, Ireland) was substituted for either one overnight (CAPD patients) or daytime (APD patients) 4.25% glucose exchange. The remaining glucose exchanges (all 2.5% glucose exchanges except for 2 patients who each had an additional 4.25% glucose exchange) were not altered at any stage. Clinical and laboratory indices of fluid retention (weight, blood pressure, serum albumin concentration, plasma sodium concentration, haemoglobin concentration), peritoneal membrane transport status (peritoneal equilibration test [9]) and daily net peritoneal ultrafiltration were recorded prior to, and 1 month after, commencing icodextrin. Glycated hamoglobin (HbA1c) levels were additionally measured in diabetic patients prior to, and 3 months after, commencing icodextrin. The decision regarding whether subjects remained on PD or were transferred to haemodialysis was left to the discretion of their treating nephrologist. Patients were followed at monthly intervals until the end of the study (31 May 2001), at which point data were censored.
The primary outcome measure was extension of PD technique survival, which was regarded as the time interval between commencement of icodextrin and either PD completion or study termination. Secondary outcome measures included changes in daily ultrafiltration volume, indices of fluid retention, dialysate:plasma creatinine ratio at 4 hours (D:P Cr 4 h), and HbA1c during the initial month (or 3 months for HbA1c) of icodextrin therapy.
Statistical analysis
Normality of data was evaluated by the Kolmogorov-Smirnov test with Lilliefor's correction. Results are expressed as mean ± standard error (SEM) for continuous parametric data, median (interquartile range) for continuous non-parametric data, and frequencies and percentages for categorical data. PD technique survival curves, survival probabilities and estimated mean survival times were generated according to the Kaplan-Meier method. Data were not censored for death. Differences in the survival curves between the subgroups were evaluated using the log rank test. In order to ascertain the patient characteristics that independently predicted extension of PD technique survival, a multivariate Cox proportional hazards model regression analysis was subsequently performed, which included age, gender, racial status, diabetes, ultrafiltration and high transporter status as covariates. A backward elimination procedure was carried out with removal testing based on the probability of the Wald statistic until the most parsimonious model was identified. Adjusted survival curves were estimated using the Cox average covariate method, which calculates predicted survival probabilities at the mean levels of the covariates. Pre- and post-icodextrin changes in the secondary outcome measures of daily ultrafiltration volume, fluid retention indices, D:P Cr 4 h and HbA1c were assessed by paired t-test or Wilcoxon signed rank tests, depending on data distribution. Data were analysed using the using the statistical software package, SPSS release version 10.0.5 (SPSS Inc., Chicago, IL). P values less than 0.05 were considered significant.
Results
Patient characteristics
Seventeen patients out of a total PD population of 263 met the inclusion criteria for the study during the 28-month recruitment period. Their characteristics are shown in Table 1. Compared with the general PD population at the Princess Alexandra Hospital, patients with refractory, symptomatic fluid overload had significantly higher weights (80.8 ± 4.3 versus 72.5 ± 1.5 kg, p < 0.05), lower daily net ultrafiltration rates (855 ± 210 versus 1310 ± 100 ml, p < 0.05) and greater frequencies of diabetes mellitus (71% versus 26%, p < 0.001), ischaemic heart disease (71% versus 37%, p = 0.01) and either high or high-average peritoneal transport characteristics (89% versus 58%, p < 0.05). All 17 eligible subjects agreed to participate in the study and none were lost to follow-up. The total follow-up time on icodextrin was 98.7 patient-months.
Table 1.
Baseline patient characteristics. Results are presented as mean ± SEM, percentage or median (range), depending on data type.
| Characteristic | Value |
| Demographic | |
| Age (years) | 56.8 ± 2.9 |
| Male | 9 (53%) |
| Caucasian | 9 (53%) |
| Weight (kg) | 80.8 ± 4.3 |
| Diabetes Mellitus | 12 (71%) |
| Ischaemic Heart Disease | 12 (71%) |
| Dialysis | |
| Dialysis Duration (months) | 18.6 ± 3.5 |
| APD | 5 (29%) |
| Prescribed Dialysate Volume (L/day) | 12(8–21) |
| Ultrafiltration (mL/day) | 855 ± 210 |
| Residual Renal Creatinine Clearance (L/wk) | 17.1 ± 5.1 |
| Transport Status (H / HA / LA / L) | 18%/71%/12%/0% |
| ESRF Cause | |
| Diabetic Nephropathy | 11(65%) |
| Chronic Glomerulonephritis | 2 (12%) |
| Diffuse Cortical Necrosis | 1 (6%) |
| Renovascular Nephrosclerosis | 1 (6%) |
| Systemic Lupus Erythematosus | 1 (6%) |
| Bladder Cancer | 1 (6%) |
Extension of PD technique survival
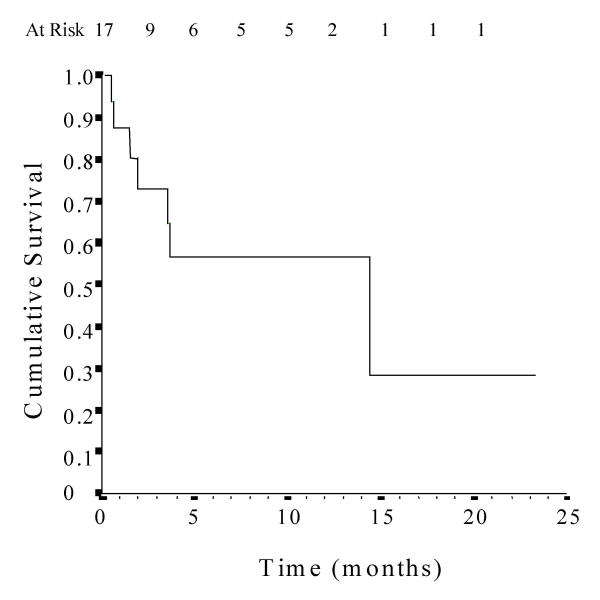
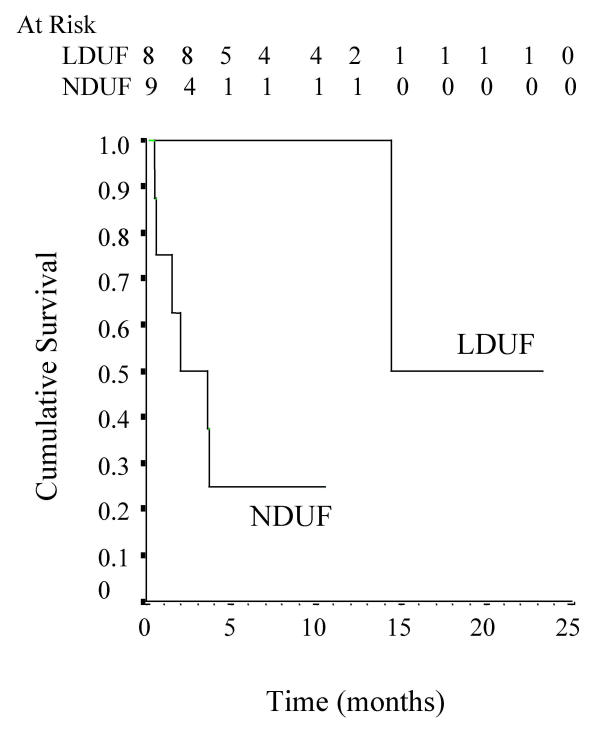
Substitution of an icodextrin exchange for a glucose exchange was associated with a significant prolongation of technique survival (mean 11.6 months, 95% CI 6.0–17.3 months) in patients who were otherwise about to be transferred to haemodialysis for refractory, symptomatic fluid overload (Fig. 1). The causes of technique failure included fluid overload (n = 4), peritonitis (n = 1) and death (peritonitis n = 1, acute myocardial infarction n = 1). No individuals underwent renal transplantation. Patients who had low daily ultrafiltration, defined as a net daily ultrafiltration of less than 1 L [10], remained on PD for a considerably longer time period following icodextrin administration than those who had normal daily ultrafiltration (Fig. 2). Using a multivariate Cox proportional hazards model analysis, baseline daily ultrafiltration (L/day) was the only independent predictor of technique survival on icodextrin (adjusted hazard ratio 2.52, 95% CI 1.13–5.62, p < 0.05). Extension of time on PD was not significantly associated with age, gender, ethnicity, diabetes mellitus or high transporter status.
Figure 1.
Kaplan-Meier technique survival curve in 17 fluid-overloaded PD patients following commencement of icodextrin.
Figure 2.
Relataive effects of low daily ultrafiltration (LDUF, peritoneal ultrafiltration < 1000 mL/day) and normal daily ultrafiltration (NDUF) on icodextrin technique survival (log rank 7.2, p < 0.01).
Effect of icodextrin on ultrafiltration, fluid status indices and glycaemic control
Prescription of icodextrin for 1 month resulted in a significant 599 ml increase in mean daily ultrafiltration rate and a 10 mmHg reduction in mean arterial pressure (Table 2). Patient weight decreased from 80.8 ± 4.3 to 79.6 ± 4.2 kg, but the difference did not achieve statistical significance (p = 0.14). No significant changes were observed in haemoglobin levels or serum concentrations of sodium, chloride, osmolality, urea, creatinine and glucose.
Table 2.
Effect of prescription of icodextrin for 1 month on ultrafiltration, fluid retention indices and selected biochemical parameters in 17 PD patients with refractory fluid overload. * p < 0.05 versus baseline
| Parameter | Baseline | 1 month Post-Icodextrin |
| Net daily ultrafiltration (ml) | 855 ± 210 | 1454 ± 215* |
| Mean arterial pressure (mmHg) | 106 ± 4 | 96 ± 4* |
| Weight (kg) | 80.8 ± 4.3 | 79.6 ± 4.2 |
| Haemoglobin (g/L) | 107 ± 3 | 104 ± 4 |
| Serum albumin (g/L) | 33.6 ± 1.0 | 32.1 ± 0.9 |
| Serum sodium (mmol/L) | 137 ± 1 | 136 ± 1 |
| Serum chloride (mmol/L) | 94 ± 1 | 93 ± 1 |
| Serum osmolalitv (mOsm/kg) | 290 ± 3 | 288 ± 3 |
| Serum urea (mmol/L) | 16.1 ± 1.6 | 16.7 ± 2.2 |
| Serum creatinine (mmol/L) | 0.80 ± 0.07 | 0.71 ± 0.06 |
| Serum glucose (mmol/L) | 9.8 ± 1.6 | 7.8 ± 1.2 |
In the 12 patients with diabetes mellitus, substitution of icodextrin for glucose in one exchange daily led to a significant fall in HbA1c from 8.9 ± 0.7 to 7.9 ± 0.7% (p < 0.05). Seven out of 12 patients required a reduction in insulin prescription, whilst dosages in the remaining patients were not changed.
Adverse effects
Icodextrin therapy was not associated with a significant alteration in D:P Cr 4 h (pre-icodextrin 0.72 ± 0.05 versus post-icodextrin 0.74 ± 0.05, p = 0.55). Peritonitis rates following the institution of icodextrin were also not significantly different from antecedent rates (pre-icodextrin 0.20 versus post-icodextrin 0.21 episodes per patient-year, p = 1.00). No skin rashes or other adverse effects were observed.
Discussion
The results of the present study suggest that icodextrin may play a useful role in alleviating symptomatic fluid overload and extending technique survival in patients who have failed PD because of intractable hypervolaemia. Within the first month of substituting one icodextrin exchange for a glucose exchange, net daily peritoneal ultrafiltration was increased by nearly 600 ml and mean arterial pressure fell back towards normotensive levels by an average of 10 mmHg. The degree of symptomatic improvement obtained was sufficient to prolong time on PD by an average of 1 year.
These results support the previous findings of an analysis by Peers et al [7] of 56 ultrafiltration failure patients entered into a compassionate-use programme, in which icodextrin therapy extended median technique survival by approximately 8 months. Based on the cost differential between icodextrin-based PD and in-centre haemodialysis, this translated into a saving of approximately £1500 per year of extended PD life. A subsequent report by Wilkie and colleagues [8] similarly demonstrated that icodextrin extended median PD technique survival by 22 months in 33 patients with ultrafiltration failure. However, both of these retrospective studies were significantly limited by the fact that there had been no a priori well-defined indications or protocols for icodextrin administration. Thus, the true degree of prolongation of PD technique survival was uncertain. Moreover, the univariate survival analysis performed by Wilkie and associates [8] was censored for mortality, even though it is conceivable that fluid overload potentially contributed to some or all of the 8 deaths that occurred in the study. Both of these limitations were addressed in the present study by not censoring PD technique survival for patient death and by prospectively and precisely defining refractory, symptomatic fluid overload as a study inclusion criterion.
The extension of PD life in our study appears to have been related to a substantial increase in peritoneal fluid removal and was most marked in those patients who had poor initial daily ultrafiltration (<1 L/day). For each additional litre/day of peritoneal ultrafiltration prior to icodextrin commencement, the adjusted risk of subsequent early technique failure was increased by approximately 150%. These results are supported by Imholz et al [11], who demonstrated that icodextrin promoted a greater net ultrafiltration in 5 CAPD patients with low ultrafiltration than those with normal ultrafiltration (918 ± 85 vs 657 ± 104 ml, respectively, p = 0.06).
In keeping with previous studies of patients with refractory fluid overload [12-14], most (89%) of the patients in this investigation were high or high-average transporters. Their generally favourable response to icodextrin is consistent with the reported observation in stable peritoneal dialysis patients that the mass transfer area coefficient of creatinine was positively correlated with transcapillary ultrafiltration induced by icodextrin, but not glucose dialysate [3]. Similarly, Woodrow et al [4] noted in euvolaemic CCPD patients that the difference in daytime ultrafiltration between icodextrin and 3.86% glucose was positively correlated with the dialysate:plasma creatinine ratio, suggesting that icodextrin achieves superior fluid removal compared with glucose-based dialysates in subjects with higher peritoneal membrane transport characteristics. Such a correlation was unable to be demonstrated in our patients with symptomatic fluid overload, possibly because of the great preponderance of high and high-average transporters and the consequent narrowing of the range of dialysate:plasma creatinine ratios.
Inadequate ultrafiltration contributes directly to 8% of PD technique failure in Australia and New Zealand [15] and probably indirectly to an even larger number of patients. The risk of ultrafiltration failure increases progressively with time on dialysis and has been reported to be as high as 31% by 6 years [12]. It is possible to extend time on PD to a limited extent by increasing the number of hypertonic (3.86%/4.25%) glucose exchanges, but this is believed to cause further peritoneal damage [16]. Icodextrin on the other hand, has been shown to promote equivalent or superior ultrafiltration to glucose PD solutions, whilst avoiding the local and systemic sequelae of peritoneal glucose exposure [1,2,6,17-19]. The improvement in peritoneal ultrafiltration per gram of carbohydrate absorbed seen with icodextrin may be particularly advantageous to diabetic PD patients, who have an increased risk of type I ultrafiltration failure [20-24] and who frequently experience destabilisation of their glycaemic control following the use of hypertonic glucose dialysis solutions. Our study indicated that exchanging icodextrin for glucose dialysate resulted in a reduction in both insulin requirements and HbA1c levels in hypervolaemic diabetic PD patients. This issue has not been previously formally evaluated in icodextrin trials.
One of the weaknesses of our study is the lack of a parallel control group, which makes it difficult to be precise about the degree of extra PD time that was afforded by commencing icodextrin therapy. However, there clearly was a pronounced extension of PD technique survival in this well-defined group of patients with refractory fluid overload who had failed glucose-based dialysis. The important question that remains unanswered by this study is whether patient outcomes are optimised by giving such individuals a trial of icodextrin therapy or immediately transferring them to haemodialysis.
Conclusions
Icodextrin can significantly augment peritoneal ultrafiltration, alleviate fluid overload, improve diabetic glycaemic control and extend technique survival in PD patients with refractory, symptomatic fluid overload. Those individuals with low net daily ultrafiltration volumes (< 1 L/day) appear to be more likely to derive benefit from incorporation of icodextrin into their PD regimens than patients with higher ultrafiltration volumes.
List of abbreviations
APD Automated peritoneal dialysis
CAPD Continuous ambulatory peritoneal dialysis
CCPD Continuous cycling peritoneal dialysis
D:P Cr 4 h Dialysate:plasma creatinine ratio at 4 hours
HbA1c Glycated haemoglobin
LDUF Low daily ultrafiltration
NDUF Normal Daily ultrafiltration
PD Peritoneal dialysis
Declaration of competing interests
Dr Johnson has previously received consultancy fees from Baxter Healthcare Pty Ltd.
Pre-publication history
The pre-publication history for this paper can be accessed here:
Acknowledgments
Acknowledgements
The authors are grateful to the Princess Alexandra Hospital renal physicians for their assistance with the execution of this study.
Contributor Information
David Wayne Johnson, Email: david_johnson@health.qld.gov.au.
Mary Arndt, Email: Cot_Ambulatory_Dialysis_CAPD@health.qld.gov.au.
Amanda O'Shea, Email: Cot_Ambulatory_Dialysis_CAPD@health.qld.gov.au.
Rhonda Watt, Email: Cot_Ambulatory_Dialysis_CAPD@health.qld.gov.au.
Jan Hamilton, Email: Cot_Ambulatory_Dialysis_CAPD@health.qld.gov.au.
Kaia Vincent, Email: Kaia_Vincent@health.qld.gov.au.
References
- Mistry CD, Gokal R, Peers E. A randomized multicenter clinical trial comparing isosmolar icodextrin with hyperosmolar glucose solutions in CAPD. MIDAS Study Group. Multicenter Investigation of Icodextrin in Ambulatory Peritoneal Dialysis. Kidney Int. 1994;46:496–503. doi: 10.1038/ki.1994.300. [DOI] [PubMed] [Google Scholar]
- Posthuma N, ter Wee PM, Verbrugh HA, Oe PL, Peers E, Sayers J, et al. Icodextrin instead of glucose during the daytime dwell in CCPD increases ultrafiltration and 24-h dialysate creatinine clearance. Nephrol Dial Transplant. 1997;12:550–553. doi: 10.1093/ndt/12.3.550. [DOI] [PubMed] [Google Scholar]
- Ho-dac-Pannekeet MM, Schouten N, Langendijk MJ, Hiralall JK, de Waart DR, Struijk DG, et al. Peritoneal transport characteristics with glucose polymer based dialysate. Kidney Int. 1996;50:979–986. doi: 10.1038/ki.1996.399. [DOI] [PubMed] [Google Scholar]
- Woodrow G, Stables G, Oldroyd B, Gibson J, Turney JH, Brownjohn AM. Comparison of icodextrin and glucose solutions for the daytime dwell in automated peritoneal dialysis. Nephrol Dial Transplant. 1999;14:1530–1535. doi: 10.1093/ndt/14.6.1530. [DOI] [PubMed] [Google Scholar]
- Krediet RT, Ho-dac-Pannekeet MM, Imholz AL, Struijk DG. Icodextrin's effects on peritoneal transport. Pent Dial Int. 1997;17:35–41. [PubMed] [Google Scholar]
- Coles GA. Biocompatibility and new fluids. Pent Dial Int. 1999;19 Suppl 2:S267–70. [PubMed] [Google Scholar]
- Peers EM, Scrimgeour AC, Haycox AR. Cost-containment in CAPD patients with ultrafiltration failure. Clin Drug Invest. 1995;10:53–58. [Google Scholar]
- Wilkie ME, Plant MJ, Edwards L, Brown CB. Icodextrin 7.5% dialysate solution (glucose polymer) in patients with ultrafiltration failure: extension of CAPD technique survival. Perit Dial Int. 1997;17:84–87. [PubMed] [Google Scholar]
- Twardowski ZJ. PET – a simpler approach for determining prescriptions for adequate dialysis therapy. Adv Perit Dial. 1990;6:186–191. [PubMed] [Google Scholar]
- Davies SJ. Monitoring of long-term peritoneal membrane function. Perit Dial Int. 2001;21:225–230. [PubMed] [Google Scholar]
- Imholz AL, Brown CB, Koomen GC, Arisz L, Krediet RT. The effect of glucose polymers on water removal and protein clearances during CAPD. Adv Perit Dial. 1993;9:25–30. [PubMed] [Google Scholar]
- Heimburger O, Waniewski J, Werynski A, Tranaeus A, Lindholm B. Peritoneal transport in CAPD patients with permanent loss of ultrafiltration capacity. Kidney Int. 1990;38:495–506. doi: 10.1038/ki.1990.231. [DOI] [PubMed] [Google Scholar]
- Krediet RT, Imholz AL, Struijk DG, Koomen GC, Arisz L. Ultrafiltration failure in continuous ambulatory peritoneal dialysis. Perit Dial Int. 1993;13 Suppl 2:S59–66. [PubMed] [Google Scholar]
- Mujais S, Nolph K, Gokal R, Blake P, Burkart J, Coles G, et al. Evaluation and management of ultrafiltration problems in peritoneal dialysis. International Society for Peritoneal Dialysis Ad Hoc Committee on Ultrafiltration Management in Peritoneal Dialysis. Pent Dial Int. 2000;20 Suppl 4:S5–21. [PubMed] [Google Scholar]
- Disney APS, Russ GR, Walker R, Collins J, Herberrt K, Kerr P. Twenty-second Annual Report of the Australian and New Zealand Dialysis and Transplant Registry Adelaide: Australian and New Zealand Dialysis and Transplant Registry; 1999.
- Davies SJ, Phillips L, Naish PF, Russell GI. Peritoneal glucose exposure and changes in membrane solute transport with time on peritoneal dialysis. J Am Soc Nephrol. 2001;12:1046–1051. doi: 10.1681/ASN.V1251046. [DOI] [PubMed] [Google Scholar]
- Posthuma N, ter Weel PM, Donker AJ, Peers EM, Oe PL, Verbrugh HA. Icodextrin use in CCPD patients during peritonitis: ultrafiltration and serum disaccharide concentrations. Nephrol Dial Transplant. 1998;13:2341–2344. doi: 10.1093/ndt/13.9.2341. [DOI] [PubMed] [Google Scholar]
- Posthuma N, ter Wee PM, Donker AJ, Oe PL, Peers EM, Verbrugh HA. Assessment of the effectiveness, safety, and biocompatibility of icodextrin in automated peritoneal dialysis. The Dextrin in APD in Amsterdam (DIANA) Group. Perit Dial Int. 2000;20 Suppl 2:S106–S113. [PubMed] [Google Scholar]
- Breborowicz a, Oreopoulos DG. Biocompatibility of peritoneal dialysis solutions. Am J Kidney Dis. 1996;27:738–743. doi: 10.1016/s0272-6386(96)90114-6. [DOI] [PubMed] [Google Scholar]
- Tzamaloukas AH, Murata GH, Malhotra D, Rao P, Piraino B, Bernardini J, et al. Small-solute clearances in diabetic subjects on continuous ambulatory peritoneal dialysis: comparison to nondiabetic subjects. Adv Perit Dial. 1999;15:179–182. [PubMed] [Google Scholar]
- Cueto Manzano AM, Correa Rotter R. Is high peritoneal transport rate an independent risk factor for CAPD mortality? Kidney Int. 2000;57:314–320. doi: 10.1046/j.1523-1755.2000.00817.x. [DOI] [PubMed] [Google Scholar]
- Kjellstrand C, Hylander B, Collins A. Mortality on dialysis: on the influence of early start, patient characteristics, and transplantation and acceptance rates. Perit Dial Int. 1999;19:483–490. doi: 10.1016/s0272-6386(12)70365-7. [DOI] [PubMed] [Google Scholar]
- Hung KY, Lin TJ, Tsai TJ, Chen WY. Impact of peritoneal membrane transport on technique failure and patient survival in a population on automated peritoneal dialysis. ASAIO J. 1999;45:568–573. doi: 10.1097/00002480-199911000-00011. [DOI] [PubMed] [Google Scholar]
- Churchill DN, Thorpe KE, Nolph KD, Keshaviah PR, Oreopoulos DG, Page D. Increased peritoneal membrane transport is associated with decreased patient and technique survival for continuous peritoneal dialysis patients. The Canada-USA (CANUSA) Peritoneal Dialysis Study Group. J Am Soc Nephrol. 1998;9:1285–1292. doi: 10.1681/ASN.V971285. [DOI] [PubMed] [Google Scholar]