Abstract
Background
In response to noxious stimulation, pupillary dilation reflex (PDR) occurs even in anaesthetized patients. The aim of the study was to evaluate the ability of pupillometry with an automated increasing stimulus intensity to monitor intraoperative opioid administration.
Methods
Thirty‐four patients undergoing elective surgery were enrolled. Induction by propofol anaesthesia was increased progressively until the sedation depth criteria (SeD) were attained. Subsequently, a first dynamic pupil measurement was performed by applying standardized nociceptive stimulation (SNS). A second PDR evaluation was performed when remifentanil reached a target effect‐site concentration. Automated infrared pupillometry was used to determine PDR during nociceptive stimulations generating a unique pupillary pain index (PPI). Vital signs were measured.
Results
After opioid administration, anaesthetized patients required a higher stimulation intensity (57.43 mA vs 32.29 mA, P < .0005). Pupil variation in response to the nociceptive stimulations was significantly reduced after opioid administration (8 mm vs 28 mm, P < .0005). The PPI score decreased after analgesic treatment (8 vs 2, P < .0005), corresponding to a 30% decrease. The elicitation of PDR by nociceptive stimulation was performed without changes in vital signs before (HR 76 vs 74/min, P = .09; SBP 123 vs 113 mm Hg, P = .001) and after opioid administration (HR 63 vs 62/min, P = .4; SBP 98.66 vs 93.77 mm Hg, P = .032).
Conclusions
During propofol anaesthesia, pupillometry with the possibility of low‐intensity standardized noxious stimulation via PPI protocol can be used for PDR assessment in response to remifentanil administration.
Keywords: analgesia, assessment, monitoring, reflex
Editorial Comment.
Acute pain and analgesics can influence autonomic pupillary responses. This study assesses an application for measuring the degree of analgesia with pupillometry by using standardized noxious stimuli during general anaesthesia. Further research is needed in order to assess the possible usefulness of this method in clinical situations.
1. INTRODUCTION
Despite the availability of numerous innovative technologies,1, 2 analgesia assessment in anaesthetized patients is not integrated in routine perioperative patient care.3 Patients are frequently unable to communicate as a result of sedative administration. For evaluation of a nociceptive/anti‐nociceptive balance and subsequent optimal analgesic (mostly opioids) treatment, anaesthesiologists still use non‐specific changes in heart rate (HR) or blood pressure (BP) in combination with the locomotor response as a surrogate for nociception.4 It has been recently demonstrated that PDR can be elicited under general anaesthesia with an automated generated electrical stimulation protocol with increasing intensity.5, 6 These study results were consistent with findings from previous studies with a single (high) tetanic stimulation for PDR elicitation in non‐communicative patients.7, 8, 9
Given the development of pupilometers with an integrated automated pupil tracking system,10 PDR can be used during surgical procedures in the operation room for nociceptive state evaluation.11, 12, 13
Recent research has revealed a PDR effect measured by single tetanic noxious stimulation, of anti‐emetics14 and respiratory distress with hypoxia and/or hypercarbia.15 To date, little is known about PDR evaluation after multiple increasing standardized noxious stimulations starting at 10 mA generated by an inbuilt PPI protocol (PPI, pupillary pain index) as an alternative for high tetanic stimulation. Although significant research is devoted to nociceptive monitoring, less attention has been paid to different techniques for PDR elicitation.
The aim of this study was to evaluate the PPI stimulation protocol for PDR measurement before and after opioid administration in adult patients undergoing general anaesthesia.
2. METHODS
2.1. Study design
This single‐centre interventional cohort study was performed in accordance with the ethical standards of ICH‐GCP and the Declaration of Helsinki after study approval by the institutional review board and ethics committee of Antwerp University Hospital, Belgium (study identifier: 16/40/410‐2). Registration at Clinicaltrials.gov (NCT03140241) occurred before study inclusion.
After written consent, patients who planned for elective abdominal or gynaecological surgery with American Society of Anaesthesiologists physical status classification system (ASA) I and II were recruited for study inclusion from May 2017 until June 2017. Open surgery (laparotomy), body mass index >30 kg m−2, a history of ophthalmologic surgery, known pupil reflex disorders, Horner's or Adie's syndrome, previous eye trauma, cranial nerve lesions, expected difficult airway management, chronic opioid use (>3 months) and preoperative use of topical eye drops (atropine, phenylephrine), β antagonists or anti‐emetics were defined as exclusion criteria. The patients did not receive premedication.
2.2. Definition of outcome parameters
The primary outcome was the difference in stimulation intensity necessary for pupil dilation of >13% before and after opioid (ie, remifentanil) administration, as defined by the inbuilt PPI stimulation protocol. Secondary outcome measurements were changes in vital signs before and after standardized nociceptive stimulation. HR and BP were recorded before and immediately after stimulation.
2.3. Study protocol
The enrolled subjects underwent 2 consecutive, by convention left, pupil measurements under general anaesthesia. Pupil assessments were executed before surgery. Anaesthesia was induced with propofol (propolipid 1%) by target controlled infusion (TCI; Marsh‐model; injectomat TIVA Agilia, Fresenius Kabi, Germany),16, 17 and the target effect‐site concentration (Ce) was progressively increased until loss of consciousness (LOC). The sedation depth (SeD) ranged from 40 to 50 on the sedation depth brain monitor NeuroSense® (NeuroWave Systems Inc., Cleveland, OH). Thereafter, the first PDR measurement was performed. Consequently, the subjects received remifentanil by continuous infusion up to Ce 5 ng mL−1 using the pharmacokinetics of Minto.18 Manually assisted ventilation using a facemask with 100% oxygen was initiated as soon as the subjects became apnoeic. Then, 0.6 mg kg−1 rocuronium was administered to facilitate orotracheal intubation when considered necessary by the attending anaesthesiologist. No deep neuromuscular block was used during surgery. Airway management was performed by laryngeal mask (LMA Unique™; LMA Deutschland GmbH, Bonn, Germany) placement or endotracheal intubation (Tracheal Tube Mallinckrodt™, Covidien™, Tullamore, Ireland). A second pupil assessment was conducted after reaching a remifentanil plateau level of Ce 5 ng mL−1. Propofol adjustments were executed to maintain the defined SeD criteria during both pupil measurements. In the awake state and during the entire study period (Figure 1), SeD variables and HR were registered continuously, and the BP was recorded routinely every 2 minutes and after maximal stimulation intensity.
Figure 1.
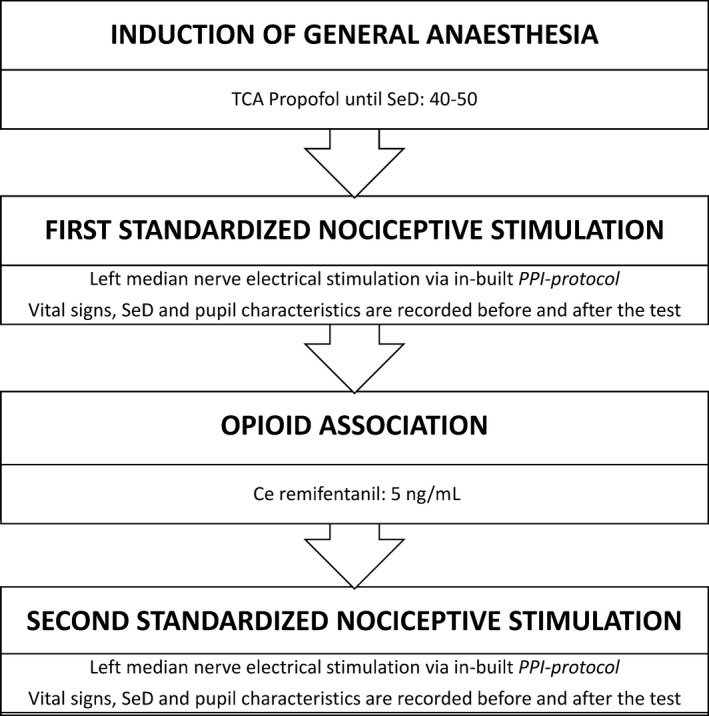
Summary of study timeline. TCA, target controlled analgesia; SeD, sedation depth; PPI, pupillary pain index; Ce, effect‐site concentration
2.4. Standardized nociceptive stimulation and measurements of pupil characteristics
For PDR measurement, we used the CE‐approved NeuroLight AlgiScan® (IDMed, Marseille, France) pupillometre using infrared video recording to allow quantitative pupil size assessment during steady‐state anaesthesia. For nociceptive stimulation, 2 Ag‐AgCl electrodes with low impedance were optimally placed at the skin area innervated by the median nerve. Constant current stimulations were generated during pupil measurement, and the voltage was automatically increased according to the resistance. The voltage is limited to a maximum of 300 V. Therefore, for a current fixed at 60 mA, the maximum acceptable resistance is 5 kΩ. The time to reach the medication plateau level and therefore pupil analyses were recorded.
The upper eyelid of the measured eye was opened during pupil assessment. A rubber cup was placed on the orbit ensured optimal device position, pupil‐camera distance and environmental darkness. Direct contact with the cornea never occurred. The contralateral eye was closed, reducing the effect of the consensual light response.
2.5. Pupillary pain index protocol
Via the touch screen display, the PPI‐modus was selected for dynamic pupil measurement. This inbuilt measurement protocol generates an automatic electrical stimulation pattern. The operating principle is the application of a standardized noxious stimulation (from 10 to 60 mA by incremental steps of 10 mA, with a duration of 1 second, and pulse width of 200 μs) starting at low stimulation intensity in increasing steps until a pupillary dilation of >13% is achieved ([maximal diameter − minimal diameter]/maximal diameter × 100). When the defined criteria are achieved, stimulation is automatically stopped, reducing unnecessary high stimulation. Then, the PPI score is determined (Table 1). The generated PPI score is calculated depending on the necessary stimulation intensity to provoke a pupil dilation of >13% (ie, inbuilt cut‐off criteria) and pupil reflex amplitude. One point is added to the 9‐level PPI score if the dilation of the pupil is >20% despite a halt of stimulation at 13%. The measurable pupil size (diameter) ranges between 0.1 and 10 mm. Furthermore, the baseline (minimum) and maximum amplitude are recorded. Depending on the necessary stimulation intensity, the pupil measurement duration is between 2 and 16 seconds.
Table 1.
PPI scoring algorithm
| Maximum stimulation intensity (mA) | Pupil reactivity | Generated PPI score |
|---|---|---|
| 10 | Pupil dilation is >13% during 10‐mA stimulation | 9 |
| 20 | Pupil dilation is >13% during 20‐mA stimulation | 8 |
| 30 | Pupil dilation is >13% during 30‐mA stimulation | 7 |
| 40 | Pupil dilation is >13% during 40‐mA stimulation | 6 |
| 50 | Pupil dilation is >13% during 50‐mA stimulation | 5 |
| 60 | Pupil dilation is >13% during 60‐mA stimulation | 4 |
| 60 | Pupil dilation is >13% during the second 60‐mA stimulation | 3 |
| 60 (5% < dilation < 13%) | Pupil dilation is >13% during the third 60‐mA stimulation | 2 |
| 60 (dilation ≤ 5%) | Pupil dilation is >13% during the last 60‐mA stimulation | 1 |
If the pupil dilation is over 20% during stimulation, the PPI score is increased with one point.
2.6. Statistical analysis
From an earlier pilot study, data were available to make assumptions for the sample size calculation, which included 34 subjects (α = .05, 1−β = .9, difference to detect = 10 mA).6 Data analyses were screened for quality by a statistical department member.
Pupil characteristics were based on median and quartiles. Heart rate and blood pressure variables were reported as means ± standard deviation (SD). Pupil size variation was tested using non‐parametric analysis methods, as a normal distribution is unlikely in the study population. Mean stimulation intensity and sedation depth before and after opioid administration were compared using the unpaired Wilcoxon tests. These tests were also employed for comparisons of pupil diameter, HR, and SBP before and after nociceptive stimulation. Statistical analyses were performed with SPSS Statistics software, version 20.0 for Mac (IBM Corp., Armonk, NY, USA). Statistical significance was considered as P < .05.
3. RESULTS
Thirty‐four patients were enrolled in the study. Five subjects were found to require maximal stimulation intensity for the primary measurement (ie, 60 mA). Nevertheless, the PPI varied in this subgroup from 4 to 2. In the enrolled patients, the male/female ratio was 9/26, with a mean age of 45 ± 14 years and a mean BMI of 24.47 ± 3.53 kg m−2. The mean Ce of propofol was 7.34 ± 1.27 μg mL−1 to establish a mean overall sedation depth of 45.70 ± 2.76. Propofol adjustments were made if necessary to fulfil sedation depth criteria. All pupil measurements were performed in the absence of hypoxia or hypercarbia. No anti‐emetic treatment or premedication (benzodiazepines) was administered prior to pupil analyses. The pupil characteristics are presented in Table 2. Differences in the baseline pupil measurements, stimulation intensity and PPI scores are presented in Figure 2. The BP and HR decreased from the awake state to the LOC (Table 3).
Table 2.
Changes in pupil characteristics before and after opioid administration
| Parameter | LOC | Remifentanil Ce 5 ng mL−1 | P‐valuea |
|---|---|---|---|
| Baseline pupil diameter (mm) | 4.00 [IQR 3.30‐4.50] | 1.90 [IQR 1.70‐2.00] | <.0005 |
| Stimulation intensity (mA) | 30.00 [IQR 20.00‐40.00] | 60.00 [IQR 60.00‐60.00] | <.0005 |
| PDRA (mm) | 1.11 [IQR 0.91‐1.47] | 0.16 [IQR 0.11‐0.23] | <.0005 |
| Pupil variation (%) | 28 [IQR 21‐39] | 8 [IQR 6‐12] | <.0005 |
| PPI score | 8 [IQR 7‐9] | 2 [IQR 1‐2] | <.0005 |
PDRA, pupillary dilation reflex amplitude; PPI, pupillary pain index.
Data are expressed as the overall median and interquartile range [IQR]. Loss of consciousness (LOC) and remifentanil Ce 5 ng mL−1 are reported for the first and second PDR assessment, respectively.
Statistically significant for P < .05.
Figure 2.
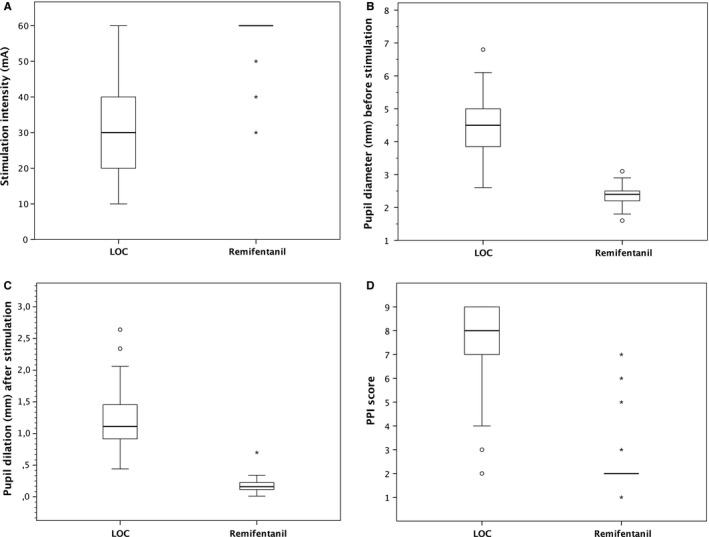
(A), Boxplots of necessary stimulation intensity to elicit PDR via a PPI stimulation protocol. The thick horizontal line indicates the median, the limits of the box indicate the 25th (Q1) and 75th (Q3) percentiles, and the whiskers denote the extreme values (Q1 − 1.5*[IQR]; Q3 + 1.5*[IQR]). (B), Boxplots of baseline pupil diameter in millimetres (mm) before stimulation. (C), Boxplots of pupil dilation in millimetres (mm) evoked by the standardized noxious stimulation. (D), Boxplots of the pupillary pain index (PPI) score based on stimulation intensity and pupil variation
Table 3.
Variation in vital signs during the study protocol
| SBP (mm Hg) | DBP (mm Hg) | HR (/min) | SeD | |
|---|---|---|---|---|
| Awake | 142 ± 29 | 76 ± 13 | 77 ± 12 | 92 ± 2 |
| LOC | ||||
| Before PDR | 123 ± 25 | 69 ± 14 | 76 ± 12 | 46 ± 3 |
| After PDR | 113 ± 19 | 64 ± 13 | 74 ± 11 | |
| Remifentanil | ||||
| Before PDR | 99 ± 19 | 52 ± 13 | 63 ± 12 | 45 ± 4 |
| After PDR | 94 ± 12 | 49 ± 8 | 62 ± 11 | |
SBP, Systolic blood pressure; DBD, diastolic blood pressure; HR, heart rate; SeD, sedation depth.
Data are expressed as the mean ± SD. Loss of consciousness (LOC) and remifentanil Ce 5 ng mL−1 are reported for the first and second PDR assessment, respectively.
In the absence of noxious stimulation, the pupil size (baseline diameter) decreased from the LOC to the point that remifentanil Ce 5 ng mL−1 was achieved. The sedation level (SeD) was comparable for both pupil assessments. The pupil dilation response to the built‐in noxious stimulation PPI protocol decreased from LOC to remifentanil Ce 5 ng mL−1, ie, stimulation intensity increased significantly after opioid administration. At the second PDR evaluation, the pupil variation (amplitude response after SNS) was remarkably reduced without frequent pupil “overshooting” (dilation of >13%). After opioid administration, maximal stimulation was necessary in 30 subjects to obtain a pupillary dilation of at least 13%. The PPI score, which was automatically coupled by the pupillometre to stimulation intensity, decreased by a mean of 5 points from the LOC to remifentanil Ce 5 ng mL−1.
4. DISCUSSION
This study suggests that pupillometry with a built‐in standardized PPI protocol for increasing stimulation intensity is a good alternative for single tetanic noxious stimulation PDR evaluation and therefore useful for analgesia level assessment in patients under general anaesthesia with propofol. The use of lower stimulation intensities in this pre‐scheduled PPI protocol may provide the anaesthesiologist with sufficient information about PDR without causing unnecessary changes in HR or BP.
In awake subjects, PDR occurs after sympathetic pathway stimulation with a dilatation response as a result of radial muscle contraction. Under general anaesthesia, the robust PDR is parasympathetically mediated. In the anaesthetized patient, sympathetic activity is depressed by the administration of sedative drugs, enhancing the parasympathetic influence towards the Edinger–Westphal (E.W.) nucleus. E.W. neurons are pacemaker cells with intrinsic firing characteristics to the sphincter pupillae muscle fibres. General anaesthesia, therefore, results in miosis. When applying a nociceptive stimulation sufficient to activate nociceptors via Aδ‐ or C fibres in an anaesthetized individual, pupil dilation occurs through E.W. neuron inhibition and consequently passive sphincter relaxation.19, 20
Propofol, lidocaine and neuromuscular blocking agents do not affect pupil reactivity in contrast to modern inhalation anaesthetics, such as sevoflurane and suprane, and nociceptive stimulation still induces mydriasis under general anaesthesia.21, 22 Opioids mediate pupil diameter under general anaesthesia by E.W. nucleus disinhibition, resulting in miosis, and depress PDR in a dose‐dependent manner.23 To date, the mechanisms of blocking this pupil reflex are not completely understood.
Larson et al11 demonstrated the superiority of pupillometry for assessing nociception above vital signs during isoflurane and propofol anaesthesia. More recent research from Barvais and colleagues confirms those findings in volunteers during propofol anaesthesia. PDR upon a single painful tetanic stimulation was a better indicator for remifentanil titration than a haemodynamic response or BIS measurements during propofol TCI in healthy individuals.23
In our study methodology, we used propofol TCI and remifentanil via continuous infusion, which is the most common technique for total intravenous anaesthesia (TIVA).
Sedation depth monitoring equipment is being used more frequently for individual titration of anaesthesia depth. Previous research demonstrated that the bispectral index was a better indicator for sedation titration than haemodynamic parameters, and many anaesthesiologists are familiar with this technology.24 Despite what has been suggested in the past, sedation depth monitors are not as sensitive for nociceptive responses elicited by noxious stimulation and can therefore not help anaesthesiologists in assessing perioperative analgesia levels. The occurrence of perioperative movement suggests a lack of analgesia to many anaesthesiologists. However, an insufficient sedation level may contribute to this event. Furthermore, the presence of movement as a reaction to an SNS generated by the PPI protocol may indicate a patients’ need for increased opioid administration. Guglielminotti and colleagues concluded that a PDR evoked by SNS is accurate in the prediction of movement during a painful (surgical) stimulation.9
Our results indicate that PRD measurements during standardized nociceptive stimulation of the skin generated via a PPI protocol may demonstrate the effects of the endogenous opioid response in patients receiving propofol anaesthesia. To determine the effect of remifentanil, we used a gradual increase in stimulation intensity in anaesthetized patients per protocol. An advantage of this automated schedule is that there was no need for inappropriately high stimulation. When the device detects a pupil variation of >13%, the nociceptive stimulation is interpreted and stopped. The use of automated pupillometry for nociceptive PRD evaluation in non‐communicative adults may provide the caregiver the possibility to measure the reactivity of the autonomic system to nociceptive stimuli. PDR elicited by an SNS is at least as accurate as the estimated remifentanil Ce to predict movement, as evaluated upon cervix dilation by Guglielminotti.9 Recently, Jakuscheit et al25 used PDR as a nociceptive reflex and concluded that this assessment is a reflection of the analgesia‐nociception balance under general anaesthesia. Appropriate pain assessment and evidence‐based pain treatment may not only reduce over‐ or under‐dosing of opioids but may also even improve patient safety and outcome during hospital stays. Although current research addressing this complex issue provides some promising innovative techniques, no standardized objective pain monitoring protocols exist.2 Furthermore, there is a need for consensus to use and interpret different pupil assessment features, such as light‐induced PDR,15, 26 nociceptive stimulation‐induced PDR,8, 27 pupillary unrest,28 constriction velocity and reaction latency29 or PPI score.5
There are some limitations to this study. First, the unequal gender distribution caused by the inclusion of gynaecological patients may bias study results. Weak gender effects on pupillary light reflex have been suggested.30, 31 To date, no gender difference in PDR under general anaesthesia has been demonstrated. Second, no conclusions can be made regarding the assessment of the nociception level given that only 2 dosages of remifentanil are allowed. Moreover, the majority of the subjects had a PPI score of 2, suggesting the possibility of opioid dose reduction.
To date, whether a titratable analgesia level is assessable using PPI remains unknown. Additional research is needed to further clarify the sensitivity of the PPI protocol used and the discriminating value of a pupil dilation cut‐off of 13%. Third, the design of this proof of concept study does not include individual opioid management protocols. Ideally, adequately treating pain in patients under general anaesthesia is performed by multiple reproducible, objective analgesia assessments. In addition, it is essential to monitor patients during surgery to determine the adequacy of the therapeutic intervention (ie, opioid administration) based on individually derived PDR indices. Moreover, this study only validates the remifentanil Ce protocol and does not measure the adequacy of surgical analgesia. However, up to now there are no studies concerning analgesia level assessment via PDR‐PPI measurement during surgery evaluating opioid dosing and patient‐related outcome measures. Our findings must, therefore, be evaluated in larger comparative descriptive studies or randomized controlled trials.
In conclusion, if anaesthesiologists could improve opioid titration based on individual and more objective reflex parameters, adequate analgesic administration would be executed with less over‐ and under‐dosing. As a fast, straightforward, reliable and easy‐to‐use bedside device, PDR measurement in response to standardized nociceptive stimulation may help the anaesthesiologist to evaluate the autonomous component of nociception in anaesthetized adults undergoing painful procedures. Moreover, PDR could be of additional value in patients for whom anaesthesiologists’ cannot use classic pharmacokinetic algorithms based on the patient's age or body mass index.
Whether this technique, including PPI scoring, may be helpful in reducing perioperative opioids and whether it positively impacts the length of stay and the development of chronic pain after surgery requires additional clinical research.
ACKNOWLEDGEMENTS
The authors wish to thank the anaesthesiologists and the nursing staff of the operating theatre of the Antwerp University Hospital (UZA) for their support of this study.
CONFLICT OF INTEREST
The authors have no conflicts of interest.
AUTHORS’ CONTRIBUTIONS
D. W.: Study design, patient recruitment, data collection, data analysis and writing. N. P.: Patient recruitment and data collection. M. V.: Data interpretation and writing. V. S.: Writing. G. H.: Study design, data analysis and writing
Wildemeersch D, Peeters N, Saldien V, Vercauteren M, Hans G. Pain assessment by pupil dilation reflex in response to noxious stimulation in anaesthetized adults. Acta Anaesthesiol Scand. 2018;62:1050–1056. 10.1111/aas.13129
Funding information
This work was supported by University of Antwerp–Belgium only.
Clinicaltrials.gov Identifier NCT03140241.
REFERENCES
- 1. De Jonckheere J, Bonhomme V, Jeanne M, et al. Physiological signal processing for individualized anti‐nociception management during general anesthesia: a review. Yearb Med Inform. 2015;10:95‐101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Cowen R, Stasiowska MK, Laycock H, Bantel C. Assessing pain objectively: the use of physiological markers. Anaesthesia. 2015;70:828‐847. [DOI] [PubMed] [Google Scholar]
- 3. Constant I, Sabourdin N. Monitoring depth of anesthesia: from consciousness to nociception. A window on subcortical brain activity. Paediatr Anaesth. 2015;25:73‐82. [DOI] [PubMed] [Google Scholar]
- 4. Guignard B. Monitoring analgesia. Best Pract Res Clin Anaesthesiol. 2006;20:161‐180. [DOI] [PubMed] [Google Scholar]
- 5. Ly‐Liu D, Reinoso‐Barbero F. Immediate postoperative pain can also be predicted by pupillary pain index in children. Br J Anaesth. 2015;114:345‐346. [DOI] [PubMed] [Google Scholar]
- 6. Wildemeersch D, Baeten M, Peeters N, Saldien V, Vercauteren M, Hans G. Pupillary dilation reflex and pupillary pain index evaluation during general anaesthesia: a pilot study. Rom J Anaesth Intensive Care. 2018;25:1‐5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Larson MD, Kurz A, Sessler DI, Dechert M, Bjorksten AR, Tayefeh F. Alfentanil blocks reflex pupillary dilation in response to noxious stimulation but does not diminish the light reflex. Anesthesiology. 1997;87:849‐855. [DOI] [PubMed] [Google Scholar]
- 8. Paulus J, Roquilly A, Beloeil H, Theraud J, Asehnoune K, Lejus C. Pupillary reflex measurement predicts insufficient analgesia before endotracheal suctioning in critically ill patients. Crit Care. 2013;17:R161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Guglielminotti J, Grillot N, Paule M, et al. Prediction of movement to surgical stimulation by the pupillary dilatation reflex amplitude evoked by a standardized noxious test. Anesthesiology. 2015;122:985‐993. [DOI] [PubMed] [Google Scholar]
- 10. Couret D, Boumaza D, Grisotto C, et al. Reliability of standard pupillometry practice in neurocritical care: an observational, double‐blinded study. Crit Care. 2016;20:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Larson MD, Sessler DI, Washington DE, Merrifield BR, Hynson JA, McGuire J. Pupillary response to noxious stimulation during isoflurane and propofol anesthesia. Anesth Analg. 1993;76:1072‐1078. [DOI] [PubMed] [Google Scholar]
- 12. Larson MD, Sessler DI. Pupillometry to guide postoperative analgesia. Anesthesiology. 2012;116:980‐982. [DOI] [PubMed] [Google Scholar]
- 13. Larson MD, Behrends M. Portable infrared pupillometry: a review. Anesth Analg. 2015;120:1242‐1253. [DOI] [PubMed] [Google Scholar]
- 14. Larson MD. The effect of antiemetics on pupillary reflex dilation during epidural/general anesthesia. Anesth Analg. 2003;97:1652‐1656. [DOI] [PubMed] [Google Scholar]
- 15. Rollins MD, Feiner JR, Lee JM, Shah S, Larson M. Pupillary effects of high‐dose opioid quantified with infrared pupillometry. Anesthesiology. 2014;121:1037‐1044. [DOI] [PubMed] [Google Scholar]
- 16. Marsh B, White M, Morton N, Kenny GN. Pharmacokinetic model driven infusion of propofol in children. Br J Anaesth. 1991;67:41‐48. [DOI] [PubMed] [Google Scholar]
- 17. Shafer SL, Gregg KM. Algorithms to rapidly achieve and maintain stable drug concentrations at the site of drug effect with a computer‐controlled infusion pump. J Pharmacokinet Biopharm. 1992;20:147‐169. [DOI] [PubMed] [Google Scholar]
- 18. Minto CF, Schnider TW, Egan TD, et al. Influence of age and gender on the pharmacokinetics and pharmacodynamics of remifentanil. I. Model development. Anesthesiology. 1997;86:10‐23. [DOI] [PubMed] [Google Scholar]
- 19. Larson MD, Tayefeh F, Sessler DI, Daniel M, Noorani M. Sympathetic nervous system does not mediate reflex pupillary dilation during desflurane anesthesia. Anesthesiology. 1996;85:748‐754. [DOI] [PubMed] [Google Scholar]
- 20. Larson MD, Major MA. The effect of hexobarbital on the duration of the recurrent IPSP in cat motoneurons. Brain Res. 1970;21:309‐311. [DOI] [PubMed] [Google Scholar]
- 21. Gray AT, Krejci ST, Larson MD. Neuromuscular blocking drugs do not alter the pupillary light reflex of anesthetized humans. Arch Neurol. 1997;54:579‐584. [DOI] [PubMed] [Google Scholar]
- 22. Ebert TJ, Muzi M, Berens R, Goff D, Kampine JP. Sympathetic responses to induction of anesthesia in humans with propofol or etomidate. Anesthesiology. 1992;76:725‐733. [DOI] [PubMed] [Google Scholar]
- 23. Barvais L, Engelman E, Eba JM, Coussaert E, Cantraine F, Kenny GN. Effect site concentrations of remifentanil and pupil response to noxious stimulation. Br J Anaesth. 2003;91:347‐352. [DOI] [PubMed] [Google Scholar]
- 24. Guignard B, Menigaux C, Dupont X, Fletcher D, Chauvin M. The effect of remifentanil on the bispectral index change and hemodynamic responses after orotracheal intubation. Anesth Analg. 2000;90:161‐167. [DOI] [PubMed] [Google Scholar]
- 25. Jakuscheit A, Weth J, Lichtner G, Jurth C, Rehberg B, von Dincklage F. Intraoperative monitoring of analgesia using nociceptive reflexes correlates with delayed extubation and immediate postoperative pain: a prospective observational study. Eur J Anaesthesiol. 2017;34:297‐305. [DOI] [PubMed] [Google Scholar]
- 26. Larson MD. Mechanism of opioid‐induced pupillary effects. Clin Neurophysiol. 2008;119:1358‐1364. [DOI] [PubMed] [Google Scholar]
- 27. Sabourdin N, Peretout JB, Khalil E, Guye ML, Louvet N, Constant I. Influence of Depth of Hypnosis on Pupillary Reactivity to a Standardized Tetanic Stimulus in Patients Under Propofol‐Remifentanil Target‐Controlled Infusion: a Crossover Randomized Pilot Study. Anesth Analg. 2018;126:70‐77. [DOI] [PubMed] [Google Scholar]
- 28. Neice AE, Behrends M, Bokoch MP, Seligman KM, Conrad NM, Larson MD. Prediction of Opioid Analgesic Efficacy by Measurement of Pupillary Unrest. Anesth Analg. 2017;124:915‐921. [DOI] [PubMed] [Google Scholar]
- 29. Larson MD, Berry PD, May J, Bjorksten A, Sessler DI. Latency of pupillary reflex dilation during general anesthesia. J Appl Physiol. 1985;2004:725‐730. [DOI] [PubMed] [Google Scholar]
- 30. Fan X, Hearne L, Lei B, Miles JH, Takahashi N, Yao G. Weak gender effects on transient pupillary light reflex. Auton Neurosci. 2009;147:9‐13. [DOI] [PubMed] [Google Scholar]
- 31. Fan X, Yao G. Modeling transient pupillary light reflex induced by a short light flash. IEEE Trans Biomed Eng. 2011;58:36‐42. [DOI] [PMC free article] [PubMed] [Google Scholar]


