Summary
Background
The catheter lock solutions 2% taurolidine and 0.9% saline are both used to prevent catheter‐related bloodstream infections (CRBSIs) in home parenteral nutrition patients.
Aims
To compare the effectiveness and safety of taurolidine and saline.
Methods
This multicentre double‐blinded trial randomly assigned home parenteral nutrition patients to use either 2% taurolidine or 0.9% saline for 1 year. Patients were stratified in a new catheter group and a pre‐existing catheter group. Primary outcome was the rate of CRBSIs/1000 catheter days in the new catheter group and pre‐existing catheter group, separately.
Results
We randomised 105 patients, of which 102 were analysed as modified intention‐to‐treat population. In the new catheter group, rates of CRBSIs/1000 catheter days were 0.29 and 1.49 in the taurolidine and saline arm respectively (relative risk, 0.20; 95% CI, 0.04‐0.71; P = 0.009). In the pre‐existing catheter group, rates of CRBSIs/1000 catheter days were 0.39 and 1.32 in the taurolidine and saline arm respectively (relative risk, 0.30; 95% CI, 0.03‐1.82; P = 0.25). Excluding one outlier patient in the taurolidine arm, mean costs per patient were $1865 for taurolidine and $4454 for saline (P = 0.03). Drug‐related adverse events were rare and generally mild.
Conclusions
In the new catheter group, taurolidine showed a clear decrease in CRBSI rate. In the pre‐existing catheter group, no superiority of taurolidine could be demonstrated, most likely due to underpowering. Overall, taurolidine reduced the risk for CRBSIs by more than four times. Given its favourable safety and cost profile, taurolidine locking should be considered as an additional strategy to prevent CRBSIs.
Trial registration: Clinicaltrials.gov, identifier: NCT01826526.
1. INTRODUCTION
Home parenteral nutrition entered the clinical arena in the late 1960s and has since remained the mainstay for the support of patients with chronic intestinal failure.1, 2, 3 This type of nutritional support requires the presence of a central venous access device (CVAD), most commonly a catheter, for the supplementation of nutrients and fluids at home. Despite ongoing technical improvements, catheter‐related bloodstream infections (CRBSIs) remain the Achilles’ heel of home parenteral nutrition treatment.4 The reported CRBSI incidence ranges from 0.38 to 2.99 CRBSI per 1000 catheter days and accounts for approximately 70% of all home parenteral nutrition‐related hospital admissions.5, 6 As such, CRBSIs compromise venous access and patient survival, and may lead to home parenteral nutrition failure because of a permanent loss of vascular access. Strict adherence to aseptic protocols when handling CVADs remains the key strategy to prevent CRBSIs. The use of anti‐microbial catheter lock solutions has also been advocated, but the search for the optimal locking agent remains ongoing.4
Several catheter lock solutions have been studied for their effectiveness in preventing CRBSIs, including antiseptic agents, antibiotics and anticoagulants. Most catheter lock solutions have been abandoned because of side effects, concern about the development of microbial resistance or mere lack of effect.4 Historically, heparin was the most commonly used catheter lock solution, also because of the supposed need for an anticoagulant. However, its use potentially increases the risk for CRBSIs by promoting intraluminal biofilm formation and is no longer recommended.4, 7, 8 In 1998, use of the broad‐spectrum antiseptic agent taurolidine as catheter lock solution was first described.9 The background for the effect of taurolidine is that this agent prevents endoluminal biofilm formation by inhibiting microbial adhesion to the inner surface of the catheter and that it destroys microbial cell membranes and toxins.10, 11, 12, 13 Subsequent studies and a meta‐analysis confirmed that taurolidine, when compared to heparin, decreases CRBSI incidence in patients with catheters.14, 15, 16 None of these previous studies, however, discriminated between beneficial effects of taurolidine and/or the detrimental effects of heparin. Currently, 0.9% saline solution is commonly used as an alternative to taurolidine because of its safety profile and low cost, despite a lack of evidence from well‐powered studies.4, 17 These issues, and the fact that it remains unclear whether the use of taurolidine should be restricted to patients with a high risk for CRBSIs, urged us to test the hypothesis that 2% taurolidine is a superior catheter lock solution to 0.9% saline in preventing CRBSIs. This hypothesis was tested in home parenteral nutrition patients who were stratified in two patient categories. Patients in the first group had a new catheter without any biofilm, and in this group, the potency of taurolidine to prevent the development of biofilms (and hence CRBSIs) could be shown. Patients in the second group had previously experienced CRBSIs and had a pre‐existing CVAD that possibly had acquired an infection‐promoting biofilm on the inner catheter surface. These patients were at a higher risk to develop a CRBSI.
2. METHODS
2.1. Study oversight
This multicentre, double‐blinded superiority trial was conducted in six referral centres (Denmark, Israel, Italy, the Netherlands and the United Kingdom), in which home parenteral nutrition patients were randomly assigned to use either a catheter lock solution containing 2% taurolidine or 0.9% saline for 1 year. The study design was conceived by the final author and followed close consultation with members of the Home Artificial Nutrition and Chronic Intestinal Failure special interest group of the European Society for Clinical Nutrition and Metabolism. The institutional review board or ethics committee at each participating centre approved the study protocol. Geistlich Pharma AG (Wolhusen, Switzerland) provided 2% taurolidine (TauroSept) and 0.9% saline solution, but had no role in the study design, data analysis or preparation of the manuscript. Acromion GmbH (Frechen, Germany), an independent contract research organisation, provided data management. Acromion GmbH and the first author performed the data analyses. The CONSORT guidelines were followed to report this study.18
2.2. Participants
Eligible patients were between 18 and 80 years of age and had a benign underlying disease leading to intestinal failure. Patients received ≥2 bags per week of nutrition and/or fluids (saline and/or glucose) over a single‐lumen CVAD. Other eligibility criteria included an estimated life expectancy ≥1 year and complete understanding of the nature of the proposed trial.
Patients were stratified into two groups. Patients in the first group, called “new catheter group”, received a new CVAD (ie without biofilm) and were either new patients (home parenteral nutrition naive) or patients who already used home parenteral nutrition. The latter patients may or may not have had a history of CRBSIs. The second group was called “pre‐existing catheter group”, and consisted of supposed “high‐risk” patients, defined as patients having a CVAD that had already been in place for at least 6 months and thus possibly contained an intraluminal biofilm. In addition, patients had been on home parenteral nutrition ≥1 year and had previously experienced a CRBSI rate of ≥0.82/1000 catheter days during their treatment period. The cut‐off rate of 0.82/1000 catheter days was chosen based on a systematic review from Dreesen et al who found that the median CRBSI rate of studies including 50% or more intestinal failure patients with a benign underlying disease was 0.82 episodes per 1000 catheter days.19 The pre‐existing catheter group was designed based on expert opinion, since no exact criteria for high‐risk patients have been defined in the home parenteral nutrition literature.
Exclusion criteria were antibiotic therapy <2 months prior to trial inclusion, implantation of antibiotic coated, silver impregnated or antimicrobial cuff catheters, a current CRBSI, compromised skin integrity of catheter exit site, use of taurolidine locks in the current CVAD, known hypersensitivity to taurolidine and significant cardiovascular disease (unstable angina pectoris, acute myocardial infarction or recent cerebral vascular accident (within 6 weeks), or a cardiac rhythm, which in the investigators judgement may result in significant hemodynamic effects). Other exclusion criteria included clinically significant abnormalities in coagulation requiring intervention, thrombolytic therapy 6 weeks prior to CVAD insertion (80‐325 mg acetylsalicylic acid daily was acceptable), and concurrent pregnancy or lactation.
2.3. Randomisation and blinding
Patients were screened for eligibility during regular outpatient clinical check‐ups or during hospitalisation. After the local principal investigator determined eligibility and obtained written informed consent, the patients were stratified into the new catheter group or pre‐existing catheter group, and randomised according to a random computer‐permuted block scheme (1:1, with block sizes of four patients) to either 2% taurolidine or 0.9% saline by the sponsor in a validated environment. All vials were identical in appearance, smell and method of administration. All patients, investigators and site personnel as well as persons involved in field monitoring, data handling or the conduct of the trial were blinded to the randomisation data and the trial medication.
2.4. Study treatment
Patients or caregivers were instructed according to local training procedures on how to instil the lock solution at home. The catheter lock solutions were stored at room temperature. Each time an infusion of parenteral nutrition or fluids was completed, the CVAD was flushed with 10 mL sterile physiological saline solution. Subsequently, an identical vial containing 5 mL 2% taurolidine or 0.9% saline solution was instilled into the CVAD. The frequency of administration depended on the patient's parenteral nutrition schedule and ranged from twice per week to once daily. Patients were seen every 3 months by an investigator during regular outpatient clinic visits or during unscheduled visits and hospitalisations. At these check‐ups, CVAD‐related complications, concomitant medication, catheter function, catheter exit sites and adverse events were assessed. In cases where a CRBSI was proven during the study, the patient was withdrawn from the trial after a final visit procedure.
2.5. Outcomes
A CRBSI was suspected when a patient presented with clinical evidence of systemic infection (ie body temperature >38.5°C or <36°C, chills, hypotension (systolic blood pressure <90 mm Hg or a decrease in systolic blood pressure >40 mm Hg), tachycardia (>90 beats per minute), elevated white blood cell count (>12 × 109/L) and/or C‐reactive protein rise), in the absence of any other apparent source of infection than the CVAD.4 In this event, paired quantitative or qualitative blood cultures from the CVAD and from a peripheral vein were taken. A CRBSI was proven when at least one blood culture was positive from the CVAD or a peripheral vein. In the absence of positive blood cultures, defervescence after removal of an implicated catheter from a patient with clinical infectious symptoms was considered indirect evidence of a CRBSI.4 Blood cultures were performed, analysed and treated according to investigational site protocols.
The primary outcome was the rate of CRBSIs/1000 catheter days between taurolidine and saline for the new catheter group and pre‐existing catheter group, separately. Predefined secondary outcomes included time to CRBSIs or CVAD removals due to CRBSIs, number of CVAD removals due to CRBSIs, exit site infections, CVAD occlusions, patient satisfaction with the assigned catheter lock solution (rated on a scale: not at all satisfied, somewhat unsatisfied, satisfied, very satisfied), adverse events and cost. The removal of a CVAD due to a CRBSI was indicated in case of a port abscess, tunnel infection, in patients with septic shock, or in case of complicated infections, such as metastatic infections, septic thrombosis or when blood cultures were positive for fungi or virulent bacteria.4 An exit site infection was defined as a local CVAD infection (local erythema, induration, tenderness around the CVAD exit site and/or purulent discharge). A CVAD occlusion was defined as a complete obstruction of the CVAD lumen (failure to flush or aspirate or the inability to infuse sufficiently into the CVAD). Patient satisfaction and costs were assessed at the end of the trial. All other outcomes were assessed during all scheduled and unscheduled visits.
2.6. Statistical analysis
The sample size calculation was based on assumptions for the primary endpoint in the new catheter group. At an 80% power and a 5% significance level, a minimum of 21 patients per arm would be required to detect a mean numerical difference of 1.5 between the treatment arms. A standard deviation of 0.3 in the taurolidine arm and 2 in the saline arm was incorporated, based on the study by Bisseling et al, and we assumed a dropout rate of 20%.14 To allow testing for differences in the pre‐existing catheter group, an additional 42 patients were required, resulting in a total of 84 patients. We aimed to include up to 20 patients from seven participating referral centres, resulting in a maximum of 140 patients included in the trial.
A statistical analysis plan was finished before unblinding. Originally, the primary analysis (CRBSI rate between taurolidine and sa‐ line) was tested using a two‐sided Mann‐Whitney U test. However, after consultation of both an epidemiologist and a statistician, new insights urged us to submit an amendment (after unblinding) of the statistical analysis plan and to use a two‐sided Fisher exact test via the website OpenEpi instead. This website compares incidence rates by incorporating both events and person‐time.20, 21 Of note: use of the Fisher exact test did not change any of the results and conclusions obtained with the Mann‐Whitney U test (Table S2).
The primary analysis was tested in a modified intention‐to‐treat population, which included all randomised patients exposed to taurolidine or saline and who had at least one effectiveness assessment available. Furthermore, a per‐protocol analysis was performed in which, in addition to the modified intention‐to‐treat criteria, patients had to meet the eligibility criteria and complete the trial for planned visits with complete test results in accordance with all relevant aspects of the protocol. Safety analyses were based on all randomised patients. Baseline characteristics, effectiveness and safety measurements were summarised using descriptive statistical methods. Time‐to‐event endpoints were described by Kaplan‐Meier curves and were compared using a log‐rank test. Costs were based on Dutch prices and consisted of catheter lock solution costs and CRBSI resource use costs (hospitalisations, unscheduled outpatient clinic consultations, CVAD changes and drug treatment).22, 23 The mean costs per patient were calculated by means of an independent samples t test after bootstrapping (1000 simulations) with 95% confidence intervals. All other outcomes were analysed using a two‐sided Fisher exact test. In a post hoc analysis, the results of both groups were summarised in a combined group analysis. All analyses, except for the primary analysis, were performed using Statistical Analysis System software, version 9.4.
3. RESULTS
3.1. Study population
Between October 2013 and March 2015, a total of 124 patients were screened. Following the eligibility criteria, 105 patients were enrolled in the trial (Figure 1). A total of 102 (97%) patients qualified for the modified intention‐to‐treat analysis. In the new catheter group, 36 and 35 patients received taurolidine and saline respectively. The median follow‐up period was 362 days (interquartile range, 201‐369) for taurolidine and 348 days (interquartile range 79‐368) for saline. In the pre‐existing catheter group, 16 and 15 patients received taurolidine and saline respectively. The median follow‐up period was 363 days (interquartile range, 331‐370) for taurolidine and 343 days (interquartile range 119‐370) for saline. Baseline characteristics between taurolidine and saline were similar in each patient group (Table 1). A total of 85 patients were included in the per‐protocol analysis.
Figure 1.
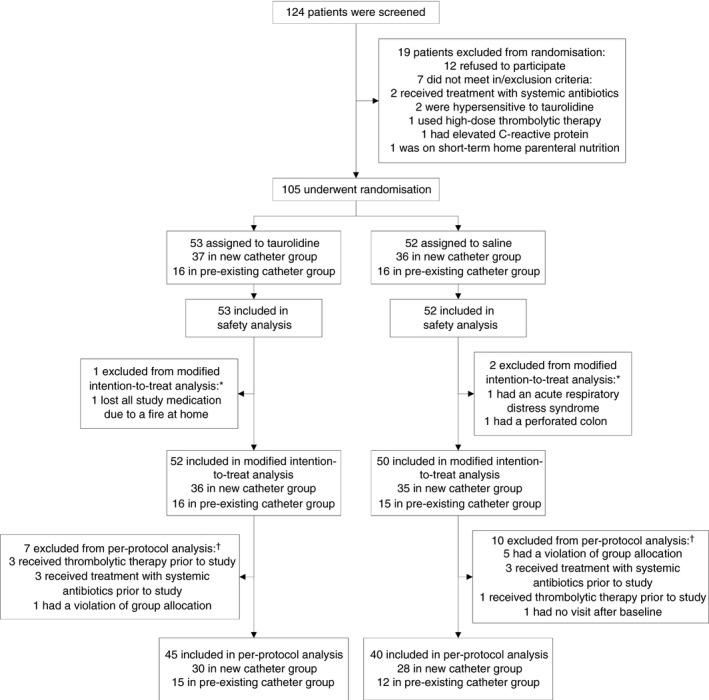
Enrollment and follow‐up. *Patients were excluded from the modified intention‐to‐treat analysis because no effectiveness assessments were available after the baseline visit. †Patients were excluded from the per‐protocol analysis because they either did not meet eligibility criteria or did not complete the trial before the planned visits with complete test results in accordance with the protocol. Some patients were excluded from the per‐protocol analysis for more than one reason but were included in only one exclusion category
Table 1.
Baseline characteristics of the new catheter group and the pre‐existing catheter group (modified intention‐to‐treat population)
| Characteristic | New catheter group | Pre‐existing catheter group | ||
|---|---|---|---|---|
| 2% taurolidine (n = 36) | 0.9% saline (n = 35) | 2% taurolidine (n = 16) | 0.9% saline (n = 15) | |
| Female—no. of patients (%) | 21 (58) | 21 (60) | 8 (50) | 12 (80) |
| Age—median years (IQR) | 59 (51–68) | 55 (38‐61) | 47 (32–62) | 47 (35‐63) |
| Current medical condition—n (%) | ||||
| Short‐bowel syndrome | 18 (50) | 21 (60) | 12 (75) | 9 (60) |
| Gastrointestinal motility disorder | 14 (39) | 9 (26) | 4 (25) | 4 (27) |
| Mechanical obstruction | 3 (8) | 1 (3) | 0 (0) | 0 (0) |
| Extensive small bowel mucosal disease | 1 (3) | 2 (6) | 0 (0) | 2 (13) |
| Intestinal fistula | 0 (0) | 2 (6) | 0 (0) | 0 (0) |
| Underlying disease—no. of patients (%) | ||||
| Crohn's disease | 6 (17) | 11 (31) | 3 (19) | 1 (7) |
| Chronic idiopathic pseudo obstruction | 9 (25) | 6 (17) | 1 (6) | 3 (20) |
| Surgical complications | 8 (22) | 4 (11) | 0 (0) | 2 (13) |
| Mesenteric Ischaemia | 5 (14) | 7 (20) | 1 (6) | 0 (0) |
| Radiation enteritis | 2 (6) | 2 (6) | 1 (6) | 1 (7) |
| Adhesions | 0 (0) | 1 (3) | 2 (13) | 2 (13) |
| Other | 6 (17) | 4 (11) | 8 (50) | 6 (40) |
| Medication—no. of patients (%) | ||||
| Anticoagulantsa | 11 (31) | 12 (34) | 2 (13) | 3 (20) |
| Immunosuppressantsb | 4 (11) | 2 (6) | 2 (13) | 1 (7) |
| Opiatesc | 19 (53) | 16 (46) | 5 (31) | 6 (40) |
| Medical history—no. of patients (%) | ||||
| Diabetes | 2 (6) | 2 (6) | 0 (0) | 1 (7) |
| CRBSIs | 18 (50) | 9 (26) | 13 (81)d | 12 (80)d |
| Home parenteral nutrition naïve—no. of patients (%) | 13 (36) | 13 (37) | 0 (0) | 0 (0) |
| Type of venous access device—no. of patients (%) | ||||
| Hickman catheter | 19 (53) | 16 (46) | 9 (56) | 13 (87) |
| Broviac catheter | 11 (31) | 11 (31) | 2 (13) | 0 (0) |
| Peripheral central venous catheter | 2 (6) | 2 (6) | 4 (25) | 1 (7) |
| Subcutaneous port system | 4 (11) | 6 (17) | 1 (6) | 1 (7) |
| New venous access device—no. of patients (%) | 36 (100) | 32 (91)e | 0 (0) | 0 (0) |
| Type of infusion components—no. of patients (%) | ||||
| Nutrition | 31 (86) | 25 (71) | 15 (94) | 12 (80) |
| Fluids | 5 (14) | 10 (29) | 1 (6) | 3 (20) |
| Number of infusions per week—Mean ± SD | 5.8 (1.6) | 6.5 (1.2) | 6.3 (1.2) | 5.9 (1.2) |
CRBSI, catheter‐related bloodstream infection; IQR, interquartile range; SD, standard deviation.
Anticoagulants comprise all anti‐thrombotic drugs, including acetylsalicylic acid, dipyridamole, phenprocoumon and warfarin.
Immunosuppressants comprise drugs that suppress or reduce the strength of the body's immune system, such as prednisolone, methotrexate or adalimumab.
Opiates comprise all drugs containing opium or its derivatives, such as codeine, tramadol, oxycodone or buprenorphine.
Six patients in the pre‐existing catheter group did not experience a CRBSI episode before study enrolment and were improperly included in the pre‐existing catheter group.
Three patients in the new catheter group received a CVAD already before study enrolment and were improperly included in the new catheter group.
3.2. Outcomes
In the new catheter group, three CRBSIs occurred during 10 248 catheter days in the taurolidine arm, while in the saline arm, 13 CRBSIs occurred in 8708 catheter days, resulting in 0.29 and 1.49 CRBSIs/1000 catheter days for taurolidine and saline respectively (relative risk, 0.20; 95% CI, 0.04‐0.71; P = 0.009). The cumulative proportion of CRBSI‐free patients after 1 year was 89% for taurolidine and 43% for saline (P = 0.002) (Figure 2). The remaining secondary outcomes were non‐significant between taurolidine and saline. The results of the per‐protocol analysis were generally similar to the modified intention‐to‐treat analysis (Table S1 and Figure S1).
Figure 2.
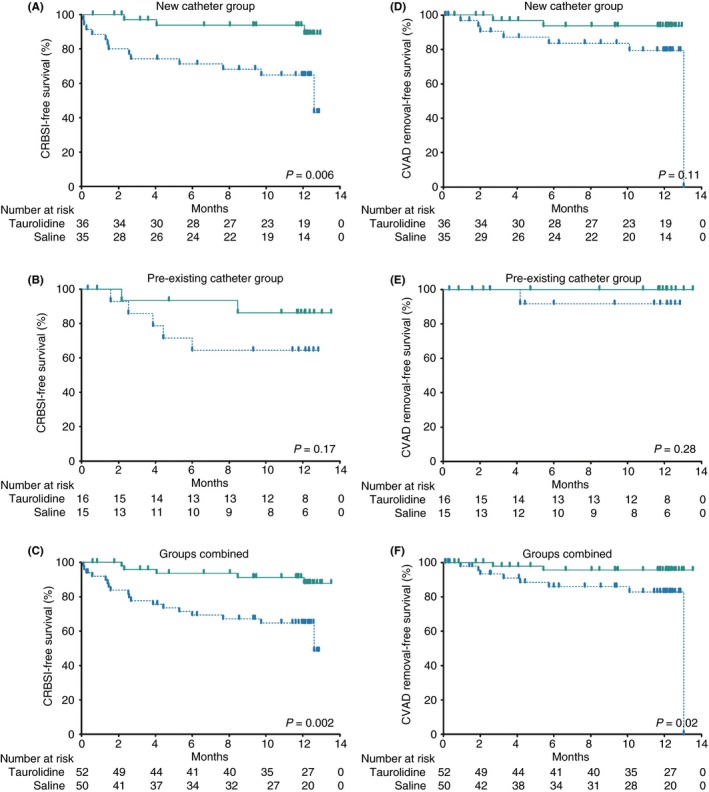
Kaplan‐Meier survival curves of modified intention‐to‐treat population. CRBSI, catheter‐related bloodstream infection; CVAD, catheter‐related access device. Kaplan‐Meier survival curves presenting the time to CRBSIs (A‐C) and time to CVAD removals due to CRBSIs (D‐F) with CVADs locked with 2% taurolidine (green continuous line) versus 0.9% saline (blue interrupted line) in the new catheter group, pre‐existing catheter group, and the groups combined. Survival curves were compared using log‐rank testing
In the pre‐existing catheter group, two CRBSIs occurred during 5070 catheter days in the taurolidine arm, while in the saline arm, five CRBSI occurred in 3785 catheter days, resulting in 0.39 and 1.32 CRBSIs/1000 catheter days for taurolidine and saline respectively (relative risk, 0.30; 95% CI, 0.03‐1.82; P = 0.25). The remaining secondary outcomes were non‐significant between taurolidine and saline. The results of the per‐protocol analysis were generally similar to the modified intention‐to‐treat analysis (Table S1 and Figure S1).
In the combined group analyses, five CRBSIs occurred during 15 318 catheter days in the taurolidine arm, while in the saline arm, 18 CRBSIs occurred in 12 493 catheter days, resulting in 0.33 and 1.44 CRBSI/1000 catheter days for taurolidine and saline respectively (relative risk, 0.23; 95% CI, 0.07‐0.63; P = 0.002) (Table 2). The cumulative proportion of CRBSI‐free patients after 1 year was 88% in the taurolidine arm and 49% in the saline arm (P = 0.002) (Figure 2). The number of patients with a CRBSI‐related catheter removal was two (4%) in the taurolidine arm and eight (16%) in the saline arm (P = 0.049). The time to CVAD removal due to CRBSI was significantly prolonged in the taurolidine arm (P = 0.02) (Figure 2). The remaining secondary outcomes were non‐significant between taurolidine and saline. The per‐protocol analyses were generally similar to the modified intention‐to‐treat analyses (Table S1 and Figure S1).
Table 2.
Outcomes of the modified intention‐to‐treat populationa
| New catheter group | Pre‐existing catheter group | Groups combined | |||||||
|---|---|---|---|---|---|---|---|---|---|
| 2% taurolidine (n = 36) | 0.9% saline (n = 35) | P value | 2% taurolidine (n = 16) | 0.9% saline (n = 15) | P value | 2% taurolidine (n = 52) | 0.9% saline (n = 50) | P value | |
| CRBSIs—no. | 3 | 13 | 2 | 5 | 5 | 18 | |||
| Total treatment time—daysPer patient—median days (IQR) | 10 248362 (201‐369) | 8708348 (79‐368) | 5070363 (331‐370) | 3785343 (119‐370) | 15 318363 (249‐369) | 12 493346 (109‐368) | |||
| CRBSIs/1000 catheter days—(95% CI) | 0.29 (0.06‐0.86) | 1.49 (0.79‐2.55) | 0.009 | 0.39 (0.04‐1.42) | 1.32 (0.43‐3.08) | 0.25 | 0.33 (0.11‐0.76) | 1.44 (0.85‐2.23) | 0.002 |
| CVAD removal due to CRBSI—no. (%) | 2 (6) | 7 (20) | 0.08 | 0 (0) | 1 (7) | 0.48 | 2 (4) | 8 (16) | 0.049 |
| Exit site infection—no. (%)b | 6 (17) | 4 (11) | 0.74 | 1 (6) | 1 (7) | >0.99 | 7 (14) | 5 (10) | 0.76 |
| CVAD occlusion—no. (%)b | 2 (6) | 1 (3) | >0.99 | 1 (6) | 2 (13) | 0.60 | 3 (6) | 3 (6) | >0.99 |
| Patient satisfaction—no. (%)b | |||||||||
| Not at all satisfied | 0 (0) | 0 (0) | 0.41 | 0 (0) | 0 (0) | >0.99 | 0 (0) | 0 (0) | 0.80 |
| Somewhat unsatisfied | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 (0) | |||
| Satisfied | 9 (25) | 6 (17) | 6 (38) | 6 (40) | 15 (29) | 12 (24) | |||
| Very satisfied | 15 (42) | 12 (34) | 7 (44) | 3 (20) | 22 (42) | 15 (30) | |||
CI, confidence interval; CRBSI, catheter‐related bloodstream infection; CVAD, central venous access device; IQR, interquartile range.
All P values were calculated with the use of the Fisher's exact test. CRBSI rates were calculated with the use of the Fisher's exact test from the website OpenEpi.20
For exit site infections, data were missing for one patient in the saline arm of the pre‐existing catheter group. For CVAD occlusions, data were missing for one patient in the saline arm of the pre‐existing catheter group. For patient satisfaction in the new catheter group, data were missing for 12 and 17 patients in the taurolidine and saline groups, respectively. For patient satisfaction in the pre‐existing catheter group, data were missing for three and six patients in the taurolidine and saline groups respectively.
3.3. CRBSI‐causing microorganisms
During the study, all CRBSIs were proven by at least one positive blood culture. In the taurolidine arm, three patients experienced a monobacterial Gram‐positive CRBSI, one patient a polybacterial CRBSI and one patient had an episode with a microorganism that was not specified (Table 3). In the saline arm, six monobacterial Gram‐positive and eight Gram‐negative CRBSIs occurred. One patient experienced a polybacterial CRBSI, one patient had an isolated fungaemia and two patients experienced an episode with a microorganism that was not specified. The CVAD salvage rate in patients with a CRBSI was 60% for taurolidine and 56% for saline.
Table 3.
Microorganisms causing catheter‐related bloodstream infectionsa
| 2% taurolidine (n = 52) | 0.9% saline (n = 50) | P value | |
|---|---|---|---|
| Infection type – no. of CRBSIs | |||
| Monobacterial bloodstream infection | 3 | 14 | 0.21 |
| Gram‐positive – % | 3 (100) | 6 (43) | |
| Gram‐negative – % | 0 (0) | 8 (57) | |
| Polybacterial bloodstream infectionb | 1 | 1 | |
| Isolated fungemia | 0 | 1 | |
| Unknownc | 1 | 2 | |
| Total | 5 | 18 | |
| Gram–positive – no. of microorganisms d | |||
| Bacillus cereus | 0 | 1 | |
| Staphylococcus aureus | 1 | 2 | |
| Staphylococcus epidermidis | 1 | 4 | |
| Streptococcus salivarius | 1 | 0 | |
| Species not specified | 1 | 0 | |
| Total | 4 | 7 | |
| Gram–negative – no. of microorganisms e | |||
| Acinetobacter ursingii | 0 | 1 | |
| Citrobacter koseri | 0 | 1 | |
| Klebsiella oxytoca | 0 | 1 | |
| Klebsiella pneumonia | 0 | 2 | |
| Morganella morganii | 0 | 1 | |
| Neiserna elongate | 1 | 0 | |
| Stenotrophomonas maltophilia | 0 | 1 | |
| Serratia marcescens | 0 | 1 | |
| Species not specified | 0 | 2 | |
| Total | 1 | 10 | |
| Fungemia—no. of microorganisms | |||
| Candida albicans | 0 | 1 | |
CRBSI, catheter‐related bloodstream infection.
P value was calculated with the use of Fisher's exact test.
In the taurolidine and saline group, respectively two (Streptococcus salivarius and Neiserna elongata) and three (Acinetobacter ursingii, Stenotrophomonas maltophilia and Staphylococcus epidermidis) microorganisms were grown from one blood culture.
A positive blood culture was reported; however, the microorganism involved was not documented.
Gram–positive species cultured from both monobacterial and polybacterial blood cultures.
Gram–negative species cultured from both monobacterial and polybacterial blood cultures.
3.4. Adverse events
A total of 71 (68%) patients reported adverse events. Except for occurrence of CRBSIs, there was no difference in adverse events between taurolidine and saline (Table 4). CVAD‐related complications were the most frequently reported adverse event. Drug‐related adverse events were rare and either mild to moderate. Two patients deceased in the taurolidine arm, but neither demise was considered to be drug‐related.
Table 4.
Clinical adverse events (safety analysis)a
| 2% taurolidine (n = 53) | 0.9% saline (n = 52) | P value | |
|---|---|---|---|
| No. of patients – (%) | |||
| Adverse events | |||
| Patients with ≥1 adverse events (≥5% of patients) | 32 (60) | 39 (75) | 0.14 |
| Abdominal pain | 4 (8) | 4 (8) | >0.99 |
| Catheter dislocation | 2 (4) | 3 (6) | 0.68 |
| Catheter exit site infection | 5 (9) | 4 (8) | >0.99 |
| CVAD occlusion | 2 (4) | 5 (10) | 0.27 |
| CRBSI | 5 (9) | 18 (35) | 0.002 |
| Pneumonia | 4 (8) | 1 (2) | 0.36 |
| Pyrexia | 1 (2) | 4 (8) | 0.21 |
| Urinary tract infection | 5 (9) | 5 (10) | >0.99 |
| Patients with drug‐related adverse events (Intensity) | 2 (4) | 2 (4) | >0.99 |
| Dysgeusia (moderate) | 1 | 0 | |
| Dizziness (moderate) | 1 | 0 | |
| Erythema to catheter exit site (moderate) | 1 | 0 | |
| Flushing (mild) | 0 | 1 | |
| Reduced catheter patency (mild) | 0 | 1 | |
| Serious adverse events | |||
| Patients with ≥1 serious adverse event (≥2% of patients) | 23 (43) | 34 (65) | 0.03 |
| Abdominal pain | 1 (2) | 2 (4) | 0.62 |
| Catheter dislocation | 2 (4) | 3 (6) | 0.68 |
| Catheter exit site infection | 4 (8) | 3 (6) | >0.99 |
| CVAD occlusion | 0 (0) | 3 (6) | 0.12 |
| CRBSI | 4 (8) | 18 (35) | 0.001 |
| Pneumonia | 4 (8) | 0 (0) | 0.12 |
| Patients with serious drug‐related adverse event | 0 (0) | 0 (0) | >0.99 |
| Discontinuation of treatment due to adverse event | 11 (21) | 23 (44) | 0.02 |
| Death | 2 (4) | 0 (0) | 0.50 |
| Euthanasia after bowel obstruction | 1 | 0 | |
| Ruptured abdominal aneurysm | 1 | 0 | |
CRBSI, catheter‐related bloodstream infection; CVAD, central venous access device.
P values were calculated with the use of Fisher's exact test.
3.5. Resource use and costs
The mean costs per patient were comparable for taurolidine ($4422) and saline ($4454) (Table 5). Importantly, however, one patient was the main cost driver in the taurolidine arm, causing 67% ($153 435) of all taurolidine costs. This patient was hospitalised for 4 months because of a disseminated Staphylococcus aureus infection including endocarditis, during which time a second CRBSI (Candida albicans) developed, resulting in the patient being admitted to the intensive care unit. A cost sensitivity analysis, in which was adjusted for this single patient, showed a significant cost reduction for taurolidine ($1865, 95% CI, $1016‐2931) compared to saline ($4454, 95% CI, $2631‐6579) (P = 0.03).
Table 5.
Cost and sensitivity analysis of modified intention‐to‐treat population
| 2% taurolidine (n = 52) | 0.9% saline (n = 50) | P value | |
|---|---|---|---|
| Catheter lock solution costs | |||
|
Study treatment—days CLS price per daya Total cost of catheter lock solution—USD Mean cost of catheter lock solution per patient—(95% CI)b |
15 318 3.48 45 949 884 (772‐998) |
12 493 0.70 7879 158 (131‐183) |
<0.001 |
| Resource use | |||
|
Hospital admissions due to CRBSIs—no. Total hospital admission days Ward Intensive care unit Total cost of hospital admissions—USDc Mean cost of hospital admissions per patient—(95% CI)b |
5 161 145 16 122 701 2360 (42‐6810) |
18 229 229 0 160 970 3219 (1865‐4798) |
0.70 |
|
Outpatient clinic consultations—no. Total cost of consultations—USDc Mean cost of consultations per patient—(95% CI)b |
0 0 0 (0‐0) |
1 178 4 (0‐12) |
0.12 |
|
Antimicrobial treatment—days Cost of drug treatment—USDd Mean cost of drug treatment per patient—(95% CI)b |
325 53 339 1026 (12‐3329) |
423 32 423 648 (176‐1376) |
0.71 |
|
CVAD changes Cost of CVAD changes—USDe Mean cost of CVAD changes per patient—(95% CI)b |
3 7974 153 (0‐412) |
8 21 264 425 (170‐702) |
0.12 |
|
Total costs—USD Costs per treatment day—USD Costs per treatment year—USD Total costs per patient in USD—mean (95% CI)b |
229 963 15.0 5491 4422 (978‐11 350) |
222 715 17.8 6507 4454 (2631‐6579) |
>0.99 |
| Sensitivity analysis f | |||
|
Total costs—USD Costs per treatment day—USD Costs per treatment year—USD Total costs per patient in USD—mean (95% CI)b |
96 994 6.3 2311 1865 (1016‐2931) |
222 715 17.8 6507 4454 (2631‐6579) |
0.03 |
CI, confidence interval; CRBSI, catheter‐related bloodstream infection; CVAD, central venous access device; USD, United States dollar.
Price of 2% taurolidine (TauroSept) per day in the Netherlands: $3.48. Price of 0.9% saline solution per day: $0.70.
Costs (USD) were compared using an independent samples t test after bootstrapping (1000 simulations).
Hospital admission and outpatient clinic consultation prices are based on the Dutch guidelines for conducting economic evaluations in healthcare (2016) from Zorginstituut Nederland (The Netherlands Healthcare Institute).22 Price of 1‐day ward admission: $703. Price of 1‐day intensive care unit: $1299. Price of outpatient clinic consultation: $178.
CRBSI drug treatment prices are based on medicijnkosten.nl (2016) from Zorginstituut Nederland (The Netherlands Healthcare Institute).23
Price of CVAD change in the Netherlands (including costs CVAD, operation room, surgeon, anaesthetics): $2658.
One patient was a major outlier in the taurolidine arm with 67% ($153 435) of the total taurolidine costs. In a sensitivity analysis, the patients costs were adjusted by winsorising (the original value was replaced by the nearest value of an observation not seriously suspect) the hospital admission ($14 059 instead of $96 693) and drug treatment costs ($785 instead of $51 120) in the taurolidine arm.
3.6. Post hoc analysis new catheter group
Of 71 patients included in the new catheter group, 27 (38%) had a history of at least one CRBSI. Of the remaining 44 patients without a history of CRBSIs, 13 (72%) in the taurolidine and 13 (50%) in the saline arm, respectively, were home parenteral nutrition‐naïve at inclusion. In the group of patients with a history of CRBSIs, three (17%) in the taurolidine arm and four (44%) in the saline arm experienced a new CRBSI during the study (P = 0.22) (Table 6). Notably, of 44 patients with no record of CRBSIs in the past, none of the patients in the taurolidine arm experienced a CRBSI, while nine patients (35%) in the saline arm experienced their first CRBSI during the study (P = 0.01). Of these nine patients, five patients were home parenteral nutrition‐naïve.
Table 6.
Post hoc analysis of the new catheter groupa
| History of CRBSIs | No history of CRBSIs | |||||
|---|---|---|---|---|---|---|
| 2% Taurolidine (n = 18) | 0.9% Saline (n = 9) | P value | 2% Taurolidine (n = 18) | 0.9% Saline (n = 26) | P value | |
| CRBSIs—no. | 3 | 4 | 0 | 9 | ||
| Total treatment time—days | 4992 | 1996 | 5256 | 6712 | ||
| CRBSIs/1000 catheter days—(95% CI) | 0.60 (0.12‐1.76) | 2.00 (0.54‐5.13) | 0.22 | 0 (0‐0) | 1.34 (0.61‐2.55) | 0.01 |
CI, confidence interval; CRBSI, catheter‐related bloodstream infection.
P values were calculated with the use of Fisher's exact test from the website OpenEpi.20
4. DISCUSSION
This study is by far the largest multicentre double‐blind randomised clinical trial ever performed in the home parenteral nutrition setting, and the first to demonstrate that 2% taurolidine prevents the development of CRBSIs in home parenteral nutrition support, when compared with 0.9% saline. Specifically in the new catheter group, the CRBSI rate was significantly decreased by taurolidine, and the time to CRBSIs was prolonged. In the pre‐existing catheter group, however, a statistically significant difference in CRBSI rate could not be demonstrated for these high‐risk patients. This result is most likely because of the limited number of patients that could be enrolled into this group (underpowering). In addition, we cannot rule out that taurolidine might have a lower effectiveness to prevent CRBSIs in the pre‐existing catheter group because of an already present biofilm when initiating the taurolidine catheter lock, since such adhesive intraluminal layers have been shown to be important precursors for the development of CRBSIs in long‐term CVADs.10, 24 If this were the case, these findings would underscore the need for strategies—such as taurolidine locking—that aim to prevent intraluminal biofilm formation in long‐term CVADs.10, 11, 12 In the combined group analysis, the CRBSI rate was lowered by taurolidine as well, and additional beneficial effects on (time to) CVAD removals due to CRBSIs were observed.
It is important to emphasise that adequate catheter care with strict adherence to aseptic protocols when handling CVADs remains the key strategy to prevent CRBSIs, whereas catheter locking only comes second in line in this respect. Our results suggest that, in the most likely clinical setting, that is, home parenteral nutrition patients starting locking of a new catheter, taurolidine seems most effective and as such this agent promises to be an important addition to our armamentarium to prevent CRBSIs.
When implementing any new preventive strategy, cost‐effectiveness is an issue on the population level, especially since the background risk for the development of CRBSIs is a quality indicator for the care provided by an intestinal failure centre. This implies that to be cost‐effective in a centre with a very low background risk for CRBSIs, a substantial number of patients will receive this locking solution who will never develop an episode of CRBSI, while in those centres with a high background risk for CRBSIs, cost‐effectiveness will be easily achieved. At the individual patient level, however, the prevention of any episode of CRBSI may be crucial in light of the associated morbidity, risk for loss of options to obtain venous access and mortality. In this vein, it is also important to mention that so far, evidence for serious side effects of long‐term catheter locking with taurolidine in the clinical setting is lacking.25, 26 This finding is corroborated by the absence of such deleterious effects when taurolidine is infused in large volumes (>1 L/d) in humans in the setting of treatment of oncologic diseases.27, 28 In light of the trial results, it is our opinion that there is no reason to withhold taurolidine in any patient with a new CVAD that is without biofilm for CRBSI prevention. However, the counterbalance of the previously mentioned issues and the consideration to implement an effective preventive strategy in this specific patient category may eventually vary per treating specialist team. As previously stated, our study results preclude any conclusions on the use of taurolidine in high‐risk patients who already had a CVAD in place, be it due to statistical issues (underpowering) and/or because of decreased effectiveness in CVADs with a pre‐existing intraluminal biofilm.
It is often difficult, if not impossible, to discriminate between CVAD occlusions (either thrombotic or due to parenteral nutrition components (especially lipids)) on one side, and venous thrombotic occlusions on the other. In this study, taurolidine showed that no additional effect on CVAD occlusion rates compared to saline. In addition, a meta‐analysis comparing 1.35% taurolidine and 4% citrate with heparin did not find any beneficial effects on CVAD occlusion rates either.15 However, some in vitro and retrospective studies have reported beneficial effects of taurolidine over heparin, without using any concomitant anticoagulant protection.11, 25, 29, 30 This decrease in CVAD occlusions may be explained by a parallel decrease in CRBSIs, and thus of infection‐induced activation of the coagulation system, rather than anti‐coagulant properties of taurolidine.
Despite prolonged use of taurolidine, some patients still developed CRBSIs. One reason might be breaches or non‐compliance of patients or caregivers to antiseptic CVAD care protocols. Another reason could be selective growth of microorganisms with a phenotypic adaptation to taurolidine. This matter was previously addressed when we evaluated microbicidal concentrations of taurolidine in bacterial strains that caused CRBSIs in a heparin‐controlled trial but found no evidence for altered bacterial susceptibility to taurolidine.14, 31 This was not unexpected, given taurolidines’ non‐specific chemical aseptic mode of action. To date, no microbial resistance to taurolidine has been reported.11, 15, 32
The CVAD salvage rate of 60% in patients with a CRBSI was similar in both arms and is comparable to rates observed in previous studies (55%‐80%).6, 33, 34 With regard to the microbial pathogens leading to CRBSIs, several cohort studies reported a preponderance of infections caused by Gram‐positive (60%‐80%) over Gram‐negative bacteria (20%‐40%).4, 19, 35, 36 One meta‐analysis showed that the use of taurolidine significantly decreased CRBSIs caused by Gram‐negative bacteria, whereas CRBSIs from Gram‐positive organisms did not change.15 In this trial, there was no difference in the type of pathogens leading to CRBSIs between taurolidine and saline.
Although taurolidine showed a clear beneficial effect in terms of CRBSI prevention in the new catheter group and the groups combined, patient satisfaction did not differ between taurolidine and saline, and was high in both arms. The low number of drug‐related adverse events in both groups may be a likely explanation for this.
The use of taurolidine increased catheter lock solution costs compared with saline, but the mean total costs per patient were similar between the two treatment arms. The increase in taurolidine costs was offset by the obviously much higher CRBSI resource use (more hospital admissions, CRBSI drug treatment and CVAD changes) in the saline arm. However, if the extraordinary high costs in one patient in the taurolidine arm (who was responsible for two‐thirds of all costs in this arm) were adjusted in a sensitivity cost analysis, taurolidine showed a decrease in costs per patient.
The strengths of this trial include its design with a low risk of bias, owing to the (patient and investigator) blinding and randomly allocated treatment assignments. The multicentre design in combination with only few major exclusion criteria ensured that the majority of screened patients were included and thus provided a good representation of a typical chronic intestinal failure population with benign underlying intestinal failure.4, 34 Another strength is the pragmatic design of this trial, where routine daily practice remained unchanged as much as possible; for example, patients or caregivers managed the venous catheter and infusion pump at home and instilled the catheter lock solutions according to local training procedures.
A clear limitation of this trial is the inclusion of the pre‐existing catheter group. The inclusion was partly hampered by participation of only six instead of seven centres due to local administrative issues, and because one of the six remaining centres was unable to include high‐risk patients due to previous use of taurolidine in the CVADs. This latter group, in whom any beneficial effect of a CRBSI‐preventing measure might be expected to be most noticeable, was based on expert opinion, since no strict criteria for high‐risk patients have been defined in the home parenteral nutrition literature. The assumptions on which this group was based may have been too strict (eg CVADs ≥6 months in place and no antibiotic therapy <2 months prior to trial inclusion), resulting in a lower than expected number of high‐risk patients in the study, and subsequently an underpowered group. In addition, some patients in the pre‐existing catheter group were improperly included as these patients did not have CRBSIs in their medical history and therefore did not meet the criteria for being a high‐risk patient. A second limitation of our study is that it was performed against the background of a certain infectious complication rate. As mentioned, this does not necessarily prove that the cost‐effectiveness of the strategy to use taurolidine also pertains to centres with an extremely low CRBSI rate.
Our study outcomes may be relevant for other fields (haemodialysis, oncology) where reliable venous access is paramount. Apart from the 2% taurolidine formulation that was used in this study, various other combinations of 1.35% taurolidine and anticoagulants (citrate, heparin or urokinase) are available. These agents are intended for use in areas with a risk for thrombotic occlusion, such as haemodialysis, where blood necessarily enters the catheter. In the absence of adequately powered studies comparing taurolidine formulations with and without concomitant anticoagulants, we can only speculate about their effectiveness in the setting of home parenteral nutrition.
Future research should focus on additional strategies to minimise CRBSI rates, apart from patient and caregiver training in catheter handling, including optimisation of (taurolidine‐containing) locking formulations and its selection in subgroups of patients with a higher risk for CBRSIs, and the use of devices such as antimicrobial catheter caps.37
In conclusion, using 2% taurolidine as catheter lock solution decreases the risk for CRBSIs in home parenteral nutrition patients with a new catheter compared with 0.9% saline. It remains unclear whether this also applies for patients with a supposed high CRBSI risk who already have had a catheter in place. Given its favourable safety and cost profile, our study supports the use of 2% taurolidine locking for CRBSI prevention in home parenteral nutrition care.
AUTHORSHIP
Guarantor of the article: GW.
Author contributions: GW conceived and designed the study, produced the protocol and managed the study. FR, GW, HR, LP, LV, MT, PJ, PS and ST acquired the data. Acromion GmbH and YW analysed the data. YW produced the first complete draft and updated subsequent drafts. GW, HR, LP, LV, MT, PJ, PS, ST and YW interpreted the data, critically revised the manuscript and approved all drafts and the authorship list.
ETHICS APPROVAL
This study was approved by the research ethics committee of the Radboudumc in Nijmegen, the Netherlands (reference number NL40910.091.12) and by the board of directors and/or the research ethics committee of each of the participating hospitals. The study was conducted in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki.
DATA SHARING
The full data set is available from the corresponding author at Yannick.Wouters@radboudumc.nl on reasonable request. Consent was not obtained for data sharing, but the presented data are anonymised and risk of identification is low.
TRANSPARENCY
The lead author (GW) affirms that the manuscript is an honest, accurate and transparent account of the study being reported; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained. All authors had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors operated independently from the funder. The lead author takes full responsibility for the integrity of the data, the accuracy of the data analysis and had final responsibility for the decision to submit for publication.
Supporting information
ACKNOWLEDGEMENTS
We thank the research nurses, investigators and administrative assistants of the participating centres for their help with participant recruitment and data collection; the members of the data safety monitoring committee, and the patients and their partners who participated in this study.
Declaration of personal interests: All authors have completed the ICMJE uniform disclosure at http://www.icmje.org/coi_disclosure.pdf and declare: financial support for the submitted work from Geistlich Pharma AG; GW reports grants from Fresenius Kabi, Baxter international and BBraun Medical outside the submitted work. GW is consultant for Shire. ST reports a grant from TauroPharm GmbH.
Wouters Y, Theilla M, Singer P, et al. Randomised clinical trial: 2% taurolidine versus 0.9% saline locking in patients on home parenteral nutrition. Aliment Pharmacol Ther. 2018;48:410–422. 10.1111/apt.14904
Funding information
Geistlich Pharma AG (Wolhusen, Switzerland) funded the study and provided 2% taurolidine and 0.9% saline solution. The funder had no involvement in the study design, collection, analysis and interpretation of data, writing of the report and the decision to submit the article for publication.
The Handling Editor for this article was Professor Peter Gibson, and it was accepted for publication after full peer‐review.
REFERENCES
- 1. Scribner BH, Cole JJ, Christopher TG, et al. Long‐term total parenteral nutrition. The concept of an artificial gut. JAMA 1970;212:457‐463. [PubMed] [Google Scholar]
- 2. Shils ME, Wright WL, Turnbull A, et al. Long‐term parenteral nutrition through an external arteriovenous shunt. N Engl J Med. 1970;283:341‐344. [DOI] [PubMed] [Google Scholar]
- 3. Pironi L, Arends J, Baxter J, et al. ESPEN endorsed recommendations. Definition and classification of intestinal failure in adults. Clin Nutr. 2015;34:171‐180. [DOI] [PubMed] [Google Scholar]
- 4. Pironi L, Arends J, Bozzetti F, et al. ESPEN guidelines on chronic intestinal failure in adults. Clin Nutr. 2016;35:247‐307. [DOI] [PubMed] [Google Scholar]
- 5. Wanten G, Calder PC, Forbes A. Managing adult patients who need home parenteral nutrition. BMJ. 2011;342:d1447. [DOI] [PubMed] [Google Scholar]
- 6. Dibb MJ, Abraham A, Chadwick PR, et al. Central venous catheter salvage in home parenteral nutrition catheter‐related bloodstream infections: long‐term safety and efficacy data. JPEN J Parenter Enteral Nutr. 2016;40:699‐704. [DOI] [PubMed] [Google Scholar]
- 7. Shanks RM, Donegan NP, Graber ML, et al. Heparin stimulates Staphylococcus aureus biofilm formation. Infect Immun. 2005;73:4596‐4606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Allon M. Prophylaxis against dialysis catheter‐related bacteremia: a glimmer of hope. Am J Kidney Dis. 2008;51:165‐168. [DOI] [PubMed] [Google Scholar]
- 9. Jurewitsch B, Lee T, Park J, et al. Taurolidine 2% as an antimicrobial lock solution for prevention of recurrent catheter‐related bloodstream infections. JPEN J Parenter Enteral Nutr 1998;22:242‐244. [DOI] [PubMed] [Google Scholar]
- 10. Handrup MM, Fuursted K, Funch P, et al. Biofilm formation in long‐term central venous catheters in children with cancer: a randomized controlled open‐labelled trial of taurolidine versus heparin. APMIS. 2012;120:794‐801. [DOI] [PubMed] [Google Scholar]
- 11. Shah CB, Mittelman MW, Costerton JW, et al. Antimicrobial activity of a novel catheter lock solution. Antimicrob Agents Chemother 2002;46:1674‐1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Luther MK, Mermel LA, LaPlante KL. Comparison of ML8‐X10 (a prototype oil‐in‐water micro‐emulsion based on a novel free fatty acid), taurolidine/citrate/heparin and vancomycin/heparin antimicrobial lock solutions in the eradication of biofilm‐producing staphylococci from central venous catheters. J Antimicrob Chemother. 2014;69:3263‐3267. [DOI] [PubMed] [Google Scholar]
- 13. Calabresi P, Goulette FA, Darnowski JW. Taurolidine: cytotoxic and mechanistic evaluation of a novel antineoplastic agent. Can Res 2001;61:6816‐6821. [PubMed] [Google Scholar]
- 14. Bisseling TM, Willems MC, Versleijen MW, et al. Taurolidine lock is highly effective in preventing catheter‐related bloodstream infections in patients on home parenteral nutrition: a heparin‐controlled prospective trial. Clin Nutr. 2010;29:464‐468. 10.1016/j.clnu.2009.12.005 [DOI] [PubMed] [Google Scholar]
- 15. Liu Y, Zhang AQ, Cao L, et al. Taurolidine lock solutions for the prevention of catheter‐related bloodstream infections: a systematic review and meta‐analysis of randomized controlled trials. PLoS ONE. 2013;8:e79417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Tribler S, Brandt CF, Petersen AH, et al. Taurolidine‐citrate‐heparin lock reduces catheter‐related bloodstream infections in intestinal failure patients dependent on home parenteral support: a randomized, placebo‐controlled trial. Am J Clin Nutr. 2017;106:839‐848. [DOI] [PubMed] [Google Scholar]
- 17. Klek S, Szczepanek K, Hermanowicz A, et al. Taurolidine lock in home parenteral nutrition in adults: results from an open‐label randomized controlled clinical trial. JPEN J Parenter Enteral Nutr. 2015;39:331‐335. [DOI] [PubMed] [Google Scholar]
- 18. Schulz KF, Altman DG, Moher D. CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMJ. 2010;340:c332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Dreesen M, Foulon V, Spriet I, et al. Epidemiology of catheter‐related infections in adult patients receiving home parenteral nutrition: a systematic review. Clin Nutr. 2013;32:16‐26. [DOI] [PubMed] [Google Scholar]
- 20. OpenEpi.com. http://www.openepi.com/PersonTime2/PersonTime2.htm.
- 21. Rijksinstituut voor Volksgezondheid en Milieu (RIVM) . http://www.rivm.nl/Onderwerpen/P/PREZIES/Infectiecijfers_betrouwbaar_gebruiken/Statistische_betrouwbaarheid_van_infectiecijfers.
- 22. Dutch guidelines for conducting economic evaluations in healthcare: Zorginstituut Nederland; 2016 [updated 29‐02‐2016]. https://www.zorginstituutnederland.nl/over-ons/publicaties/publicatie/2016/02/29/richtlijn-voor-het-uitvoeren-van-economische-evaluaties-in-de-gezondheidszorg.
- 23. Medicijnkosten.nl: Zorginstituut Nederland; 2016 [updated 01‐04‐2017]. http://www.medicijnkosten.nl.
- 24. Raad I, Costerton W, Sabharwal U, et al. Ultrastructural analysis of indwelling vascular catheters: a quantitative relationship between luminal colonization and duration of placement. J Infect Dis 1993;168:400‐407. [DOI] [PubMed] [Google Scholar]
- 25. Olthof E, Versleijen M, Huisman‐De Waal G, et al. Taurolidine lock is superior to heparin lock in the prevention of catheter related bloodstream infections and occlusions. Clin Nutr. 2014;33:S104‐S105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lambe C, Poisson C, Talbotec C, et al. Strategies to Reduce Catheter&hyphen ;Related Bloodstream Infections in Pediatric Patients Receiving Home Parenteral Nutrition: The Efficacy of Taurolidine&hyphen ;Citrate Prophylactic&hyphen ;Locking. JPEN Journal of parenteral and enteral nutrition. 2018; 10.1002/jpen.1043. [DOI] [PubMed] [Google Scholar]
- 27. Stendel R, Scheurer L, Schlatterer K, et al. Pharmacokinetics of taurolidine following repeated intravenous infusions measured by HPLC‐ESI‐MS/MS of the derivatives taurultame and taurinamide in glioblastoma patients. Clin Pharmacokinet 2007;46:513‐524. [DOI] [PubMed] [Google Scholar]
- 28. Braumann C, Winkler G, Rogalla P, et al. Prevention of disease progression in a patient with a gastric cancer‐re‐recurrence. Outcome after intravenous treatment with the novel antineoplastic agent taurolidine. Report of a case. World J Surg Oncol. 2006;4:34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Reinmuller J. [The influence of taurolidine on physiological and pathological blood coagulation and implications for its use]. Zentralbl Chir. 1999;124 (Suppl 4):13‐18. [PubMed] [Google Scholar]
- 30. Kaptanoglu L, Kucuk HF, Colak E, et al. The effect of taurolidine on experimental thrombus formation. Eur J Pharmacol. 2008;578:238‐241. [DOI] [PubMed] [Google Scholar]
- 31. Olthof ED, Rentenaar RJ, Rijs AJ, et al. Absence of microbial adaptation to taurolidine in patients on home parenteral nutrition who develop catheter related bloodstream infections and use taurolidine locks. Clin Nutr. 2013;32:538‐542. [DOI] [PubMed] [Google Scholar]
- 32. Chu HP, Brind J, Tomar R, et al. Significant reduction in central venous catheter‐related bloodstream infections in children on HPN after starting treatment with taurolidine line lock. J Pediatr Gastroenterol Nutr. 2012;55:403‐407. [DOI] [PubMed] [Google Scholar]
- 33. Clare A, Teubner A, Shaffer JL. What information should lead to a suspicion of catheter sepsis in HPN? Clin Nutr. 2008;27:552‐556. [DOI] [PubMed] [Google Scholar]
- 34. Brandt CF, Tribler S, Hvistendahl M, et al. Home parenteral nutrition in adult patients with chronic intestinal failure: catheter‐related complications over 4 decades at the main Danish Tertiary Referral Center. JPEN J Parenter Enteral Nutr. 2018;42:95‐103. [DOI] [PubMed] [Google Scholar]
- 35. Santarpia L, Alfonsi L, Tiseo D, et al. Central venous catheter infections and antibiotic therapy during long‐term home parenteral nutrition: an 11‐year follow‐up study. JPEN J Parenter Enteral Nutr. 2010;34:254‐262. [DOI] [PubMed] [Google Scholar]
- 36. Tribler S, Brandt CF, Hvistendahl M, et al. Catheter‐related bloodstream infections in adults receiving home parenteral nutrition. JPEN J Parenter Enteral Nutr. 2017;148607116686290. [DOI] [PubMed] [Google Scholar]
- 37. Voor In ‘t Holt AF, Helder OK, Vos MC, et al. Antiseptic barrier cap effective in reducing central line‐associated bloodstream infections: a systematic review and meta‐analysis. Int J Nurs Stud 2017;69:34‐40. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials


