Summary
Objective
Inhibition of microRNA‐134 by an oligonucleotide antagomir (ant‐134) has been shown to produce powerful antiseizure effects in multiple models of epilepsy. However, to successfully translate the treatment to the clinic, it is important to assess what potential adverse effects it may have on naive brain tissue.
Methods
To investigate this, adult male Sprague‐Dawley rats were treated with either ant‐134 or a scrambled control sequence. Animals were later assessed for spatial navigation, before ex vivo slices were taken to assess the effects of microRNA‐134 knockdown on well‐defined measures of intrinsic and synaptic properties.
Results
Hippocampal field potential recordings determined that silencing of microRNA‐134 by ant‐134 injection was associated with a reduction in epileptiform activity following application of 9 mmol/L K+. Nevertheless, rats performed normally in the novel object location test. Action potential waveforms and miniature excitatory synaptic currents recorded in CA1 pyramidal neurons were unaffected by ant‐134.
Significance
These results demonstrate that ant‐134 confers a seizure‐protective effect without obvious interference with hippocampal neuronal properties or network function. These findings support further development of this novel approach to epilepsy treatment.
Keywords: antagomir, epilepsy, hippocampus, microRNA‐134
Key Points.
Ant‐134 is a promising disease‐modifying therapeutic for epilepsy, but it is not clear whether it has other impacts on brain function
In vivo injection of ant‐134 lowered microRNA‐134 levels, leading to reduced epileptiform activity in ex vivo naive brain slices
There was no impact on the hippocampal‐dependent novel object location test, indicating retained hippocampal function
Action potentials and miniature excitatory postsynaptic currents in hippocampal pyramidal neurons were also unchanged
Ant‐134 is antiepileptic but seems to have limited adverse effects, enhancing its therapeutic potential
1. INTRODUCTION
Epilepsy is a common neurologic disorder, manifesting as the susceptibility to recurrent spontaneous seizures, and affecting approximately 50 million people throughout the world. Epilepsy comprises a number of disorders, and for this reason the outcome of clinical treatment is variable, despite the number of antiepileptic drugs (AEDs) available. Around 25% of patients with epilepsy do not experience seizure freedom with existing treatments.1 In addition, adverse effects are common because most drugs work by either reducing neuronal excitation or increasing inhibition, with relatively limited specificity.2 A second limitation of treatment with AEDs is that they are not disease‐modifying; they reduce seizures without changing the underlying pathophysiology. For these reasons, there is an urgent need for novel therapeutics that can be effective against a broad range of epilepsy syndromes, reversing epileptic pathophysiology with minimal off‐target effects.
One promising new therapeutic approach is manipulation of microRNAs (or miRNAs).3 These are short (~22nt) noncoding RNAs that typically suppress protein translation at the posttranscriptional stage4 through specific binding to sequences in the 3′ untranslated region (UTR) of targeted mRNAs.5 Among several microRNAs that have been functionally manipulated to date, a locked nucleic acid, cholesterol‐tagged antagomir targeting microRNA‐134 (ant‐134) has shown consistent efficacy in experimental models of epilepsy. This includes seizures evoked by intraamygdala kainic acid,6 pilocarpine,7 and pentylenetetrazol8 in mice. Ant‐134 also suppresses the development of spontaneous recurrent seizures when given after status epilepticus in mice and rats6, 8 and reduces epileptiform activity generated by high potassium in naive ex vivo rat brain slices.8 The latter is particularly relevant because it demonstrates that ant‐134 may also affect seizure threshold in nonepileptic tissue. Although the mechanism of action is not fully understood, ant‐134 has been reported to reduce density and increase volume of dendritic spines in hippocampal pyramidal cells,6, 7 likely mediated by an increase in LimK1, a target of microRNA‐134.9, 10 This suggests that ant‐134 could reduce seizures through a disease‐modifying effect. Prior to clinical translation, it is critical to determine whether ant‐134 has potential adverse effects on normal neuronal functions that could preclude its use in clinic.
We asked whether ant‐134 treatment in nonepileptic rats could have potentially negative functional impacts on naive tissue. We explored rats’ ability to complete a hippocampus‐dependent novel object location (NOL) test (based on Hattiangady et al11) and subsequently tested hippocampal seizure susceptibility and pyramidal cell biophysics in ex vivo slices. We confirmed that ant‐134 is protective against epileptiform activity in this preparation, and, crucially, we show that this antiepileptic treatment is not associated with impaired spatial navigation or with pyramidal cell dysfunction. Together, our findings suggest that ant‐134 may have selective effects on seizure threshold without inducing gross adverse effects in healthy hippocampal neurons.
2. METHODS
2.1. Ethics
All experimental procedures were carried out in accordance with the UK Animals (Scientific Procedures) Act 1986 and following the principles outlined in the ARRIVE (Animal Research Reporting In Vivo Experiments) guidelines.
2.2. Surgery
Stereotaxic injection for microRNA‐134 knockdown was performed on adult male Sprague‐Dawley rats (weight range 270‐380 g). Rats were anesthetized with isoflurane (5% induction, ~2.5% maintenance) and given meloxicam (0.2 mL subcutaneous) prior to beginning surgery, followed by 0.15 mL buprenorphine and 2.5 mL saline (both subcutaneous) during recovery. We injected 0.12 nmol in 1 mL Tris‐EDTA buffer of either mmu‐miR‐134‐5p miRCURY LNA power inhibitor (ant‐134; Exiqon, Denmark, product no. 4104449‐101) or miRCURY LNA Power microRNA negative control A (scramble; Exiqon, Vedbaek, Denmark, product no. 199006‐101) at the following coordinates (mm relative to bregma: anteroposterior [AP] −0.92, mediolateral [ML] +1.3, DV −3.3) to target the lateral ventricle. Experimenters were blinded to treatments throughout all experimental procedures and analysis. Injection rate was controlled at 200 nL/min, and the needle was left in place for 5 minutes postinjection to minimize backflow through the injection tract. Rats were allowed to recover from surgery with food and water freely available.
2.3. Novel object location test
Rats were habituated to the behavioral arena (1 m × 1 m; Tracksys, Nottingham, UK) for 5 minutes each day over 5 days. On day 6, rats underwent stereotaxic surgery as described earlier to inject either ant‐134 or scramble control. Experimenters were blinded to treatments prior to injection and throughout all experiments and analysis. On day 7, rats completed the novel object location (NOL) test. Rats were allowed to explore 2 identical objects for 5 minutes. After 1 hour, rats were returned to the arena with one object moved to a novel location within the arena (see Figure 4). This design was based on that used by Hattiangady et al.11 Exploration was measured manually and defined as when the nose was within roughly 2 cm of the object, excluding time spent climbing on top of the object. Task performance was assessed using 2 measures12: D1 (T novel‐T familiar) and discrimination index ([T novel‐T familiar]/[T novel+T familiar]).
Figure 4.
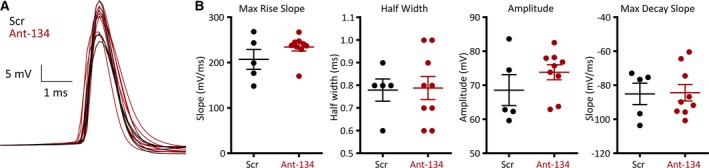
Ant‐134 does not alter action potential waveforms in CA1 pyramidal neurons. A, Overlay of the threshold action potentials obtained from scramble‐ (black) and ant‐134‐ (red) treated neurons. B, Scatter plots show no significant changes in any action potential properties measured (amplitude‐independent samples t test P = .19; Mann‐Whitney U tests: Rise slope P = .181, half‐width P = .428, decay slope P = .635)
2.4. Slice preparation
Ex vivo brain slices were prepared between 2 and 4 days after surgery to coincide with the maximal microRNA‐134 silencing effect of ant‐134.6 Rats were anesthetized briefly with isoflurane and heavily with an intraperitoneal injection of sodium pentobarbital, prior to cardiac perfusion with ice‐cold oxygenated sucrose artificial cerebrospinal fluid (ACSF) slicing solution (in mmol/L: 205 sucrose, 10 glucose, 26 NaHCO3, 1.2 NaH2PO4.H2O, 2.5 KCl, 5 MgCl2, 0.1 CaCl2). The brain was quickly extracted and sliced in 350 μm horizontal sections using a Campden 7000 smz slicer (Campden Instruments, Loughborough, UK). Slices for electrophysiology were held in a submerged style holding chamber filled with oxygenated recording ACSF (in mmol/L: 125 NaCl, 10 glucose, 26 NaHCO3, 1.25 NaH2PO4.H2O, 3 KCl, 2 CaCl2, 1 MgCl2) and allowed to recover at room temperature for 1 hour before recording. Slices for TaqMan (Thermo Fisher, Waltham, MA, USA) assay were transferred immediately to a −80°C freezer.
2.5. Electrophysiology
All slice electrophysiology was performed using a membrane chamber13, 14 perfused with oxygenated recording ACSF and heated to 34°C, at a rate of 16 mL/min. Patch clamp recordings used ~5 MΩ glass electrodes filled with intracellular solution (in mmol/L: 135 K‐gluconate, 4 KCl, 10 HEPES, 4 Mg‐ATP, 0.3 Na‐GTP, 10 Na2‐phosphocreatine; pH 7.3; 290 mOsm). Patch‐clamp data were acquired with WinEDR (John Dempster, University of Strathclyde) and exported to MatLab (MathWorks, Natick, MA, USA) for custom automated analysis. All recordings were made with access resistance <20 MΩ. Current clamp recordings were rejected if action potentials did not overshoot 0 mV; voltage‐clamp recordings were rejected if leak current exceeded 100 pA. Miniature excitatory postsynaptic potentials (mEPSCs) were recorded in the presence of 30 μmol/L picrotoxin (PTX), 300 nmol/L tetrodotoxin (TTX), and 50 μmol/L d‐2‐amino‐5‐phosphonovalerate (APV) to block γ‐aminobutyric acid (GABA)A receptors, voltage‐gated sodium channels, and N‐methyl‐d‐aspartate (NMDA) receptors, respectively. Local field potential (LFP) data from a previous study8 were reblinded and reanalyzed with a custom MatLab script. Briefly, slices were prepared using the protocol above and allowed to equilibrate in the membrane chamber for 1 hour. Local field potential recordings were made from CA1 stratum pyramidale, and epileptiform activity was elicited by raising extracellular K+ concentration from 3 to 9 mmol/L. Data were bandpass‐filtered at 1‐500 Hz to remove DC baseline shifts and to limit the bandwidth to relevant frequencies. Coastline was calculated for the 10 minutes following K+ application. Three recordings (one scramble [Scr] and 2 Ant‐134) were excluded prior to unblinding due to the presence of large extracellular spikes and other artifacts that were not removed by the filter and contaminated the calculation of the coastline.
2.6. RNA extraction, reverse transcription, and quantitative polymerase chain reaction (qPCR) analysis of microRNA‐134 levels
RNA was isolated from individual ex vivo brain slices using a standard phenol‐chloroform extraction protocol to isolate total RNA. Briefly, Trizol was added to each slice before pipetting up and down to homogenize the tissue, which was allowed to rest at room temperature for 5 minutes. Chloroform was then added, and the samples were vortexed and again allowed to sit at room temperature for 3 minutes. Samples were centrifuged to separate the phases, and the upper aqueous phase was retained. RNA was precipitated with isopropanol overnight at −20°C before ethanol purification. RNA was then quantified using a nanodrop and 260/280 and 260/230 measurements were made. Samples were used only if both ratios were between 1.8 and 2.2. Reverse transcription and qPCR were performed using TaqMan small‐scale microRNA assays for microRNA‐134 and microRNA‐124. 250 ng of total RNA was used as input. Real‐time qPCR was performed on a QuantStudio 12k flex (Thermo Fisher) in triplicate with a negative control for each primer. MicroRNA levels were then quantified using microRNA‐124 as a housekeeping gene and using the ΔΔC t method.
2.7. Statistics
All data are expressed as mean ± standard deviation (SD). Data were tested for normality using a Kolmogorov‐Smirnov test. Comparisons between ant‐134 and control parameters were made using Student's t test or Mann‐Whitney U test, as appropriate. Statistical tests were performed using IBM SPSS statistics (version 24, IBM, Armonk, NY, USA) or GraphPad Prism (version 5; GraphPad, La Jolla, CA, USA). Ant‐134 and scramble control treatments were blinded throughout all experiments and analysis.
3. RESULTS
3.1. Locked nucleic acid antagomir reduces microRNA‐134 level in naive tissue
We first confirmed that intracerebroventricular injection of ant‐134 reduced microRNA‐134 levels in the rat hippocampus. Levels of microRNA‐134 were profiled in individual rat brain slices from those treated with scrambled antagomirs and those treated with ant‐134. We saw a robust reduction in microRNA‐134 levels in slices treated with ant‐134 (Figure 1; Mann‐Whitney U test, P = .041). This confirmed knockdown of microRNA‐134 in naive tissue by ant‐134 treatment and provided a unique tool for further cellular analysis of the role of microRNA‐134 in excitability.
Figure 1.
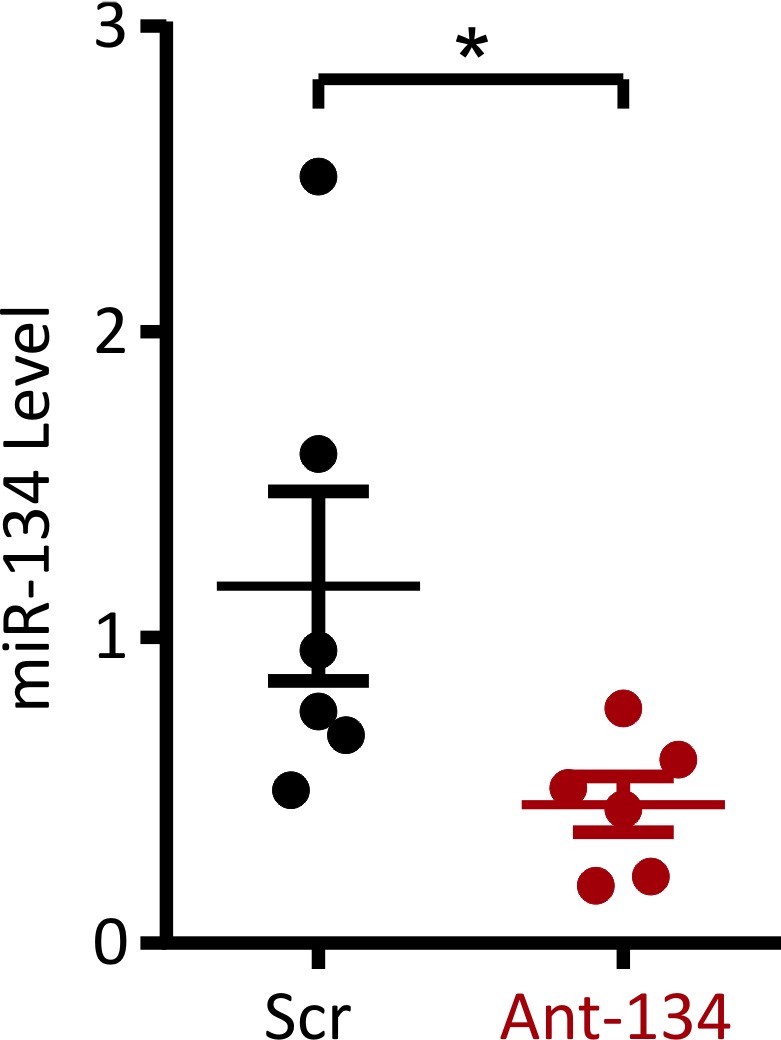
MicroRNA‐134 level is reduced by locked nucleic acid antagomir targeting microRNA‐134. MicroRNA‐134 level was measured using a TaqMan assay targeting microRNA‐134 and normalized to microRNA‐124 levels. This confirmed that microRNA‐134 levels were reduced in slices pretreated with ant‐134. *Mann‐Whitney U test, P = .041
3.2. Ant‐134 reduces epileptiform activity induced by 9 mmol/L potassium
We previously showed that brain slices from rats pretreated with ant‐134 displayed a delay to the onset of epileptiform activity caused by 9 mmol/L K+.8 However, the signal in that initial analysis was obscured by signal artifacts above 500 Hz (the highest physiologically relevant frequency15). Here, we have removed these artifacts and carried out coastline analysis, which reveals that the overall level of epileptiform activity in the 10 minutes following 9 mmol/L K+ application was reduced in slices treated with ant‐134 (Figure 2; Scr coastline: 1948 ± 114 mV, Ant‐134: 1805 ± 223 mV, independent samples t test P = .047). This confirms and extends our previous observation that ant‐134 reduces acutely induced epileptiform activity in naive hippocampal slices.
Figure 2.
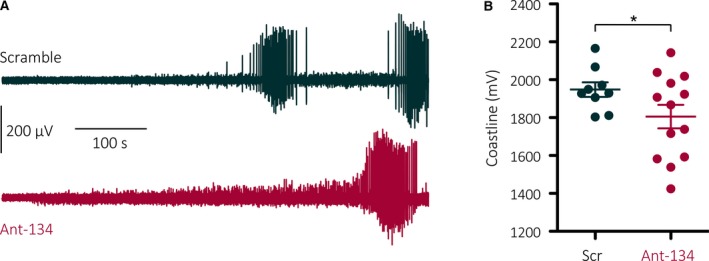
Ant‐134 reduces epileptiform activity following high potassium application. A, Representative traces showing local field potentials (1‐500 Hz bandpass‐filtered) recorded from CA1 stratum pyramidale for the 10 minutes after 9 mmol/L K+ was applied. B, The coastline of these traces was significantly reduced by pretreatment with ant‐134. *Independent samples t test, P = .047
3.3. Ant‐134 does not alter the frequency or amplitude of miniature EPSCs in hippocampal pyramidal neurons
The mechanism of the antiseizure effect of ant‐134 is unknown but may be linked to a reduction in spine density in hippocampal pyramidal neurons.6 We used patch‐clamp electrophysiology to ask whether ant‐134 disrupted miniature EPSCs, which may be one consequence of a reduction in spine density. Miniature EPSCs were recorded from CA1 hippocampal pyramidal neurons in voltage‐clamp mode. Action potential firing was blocked with 300 nmol/L TTX. AMPA receptor–mediated mEPSCs were recorded with the neuron clamped at −80 mV and isolated pharmacologically by blocking GABAA receptors with 30 μmol/L picrotoxin and NMDA receptors with 50 μmol/L APV. Both mEPSC frequency (Figure 3A and C; Scr: 1.6 ± 1.4 Hz; Ant‐134: 2.4 ± 1.6 Hz; t test, P = .174) and amplitude (Figure 3B and C; Scr: 37.8 ± 12.8 pA; Ant‐134: 38.6 ± 12.4 pA; t test, P = .875) were unchanged (Scr = 10 cells; Ant‐134 = 23 cells) by pretreatment with ant‐134. These findings suggest that ant‐134 does not interfere with postsynaptic function under nonpathophysiologic conditions.
Figure 3.
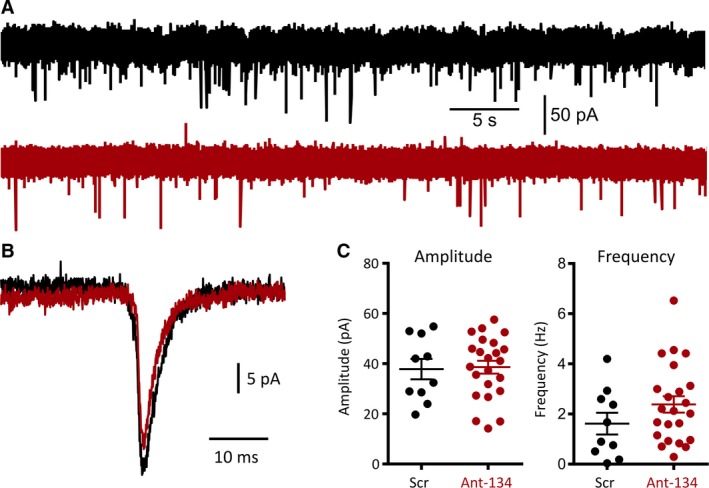
Ant‐134 does not alter miniature excitatory postsynaptic potentials (mEPSCs) in CA1 pyramidal neurons. A, Representative raw traces from scramble‐ (black) and ant‐134‐ (red) treated neurons clamped at −80 mV. B, Representative average mEPSCs for both conditions show that individual mEPSCs are unchanged by ant‐134 treatment. C, Scatter plots reveal no significant changes in either amplitude or frequency of mEPSCs between the 2 groups (independent samples t tests, frequency P = .174, amplitude P = .875)
3.4. Ant‐134 does not alter action potential properties in hippocampal pyramidal neurons
We next considered whether ant‐134 altered intrinsic properties of neurons in naive tissue.
We recorded in whole‐cell current clamp mode and elicited action potentials using a series of incremental depolarizing current steps. For each neuron, we defined the threshold action potential as the first to be triggered by the stimulus train and selected this action potential for further analysis. We did not observe significant changes in any of the action potential parameters measured (Figure 4; amplitude, half‐width, rising slope or decaying slope; n = 5 cells for control, 10 cells for ant‐134; comparisons summarized in Table 1). This suggests that microRNA‐134 knockdown with ant‐134 does not alter the firing of individual hippocampal neurons in healthy tissue.
Table 1.
Statistical summary of threshold action potential parameters measured in current clamp step recordings
| Parameter | Scr (5 cells) | Ant‐134 (9 cells) | Statistical test | P value |
|---|---|---|---|---|
| Amplitude | 68.6 ± 10.2 mV | 73.8 ± 6.6 mV | t test | .19 |
| Half‐width | 0.78 ± 0.1 msec | 0.79 ± 0.2 msec | Mann‐Whitney U | .43 |
| Rising slope | 207.0 ± 48.7 mV/msec | 234.6 ± 26.4 mV/msec | Mann‐Whitney U | .18 |
| Decaying slope | −85.1 ± 14.1 mV/msec | −84.4 ± 14.5 mV/msec | Mann‐Whitney U | .64 |
Values are means ± SD.
3.5. Ant‐134 does not affect performance in a novel object location test
If treatment with ant‐134 has gross effects on hippocampal activity, it would likely disrupt behavior reliant on hippocampal processing. For this reason, we assessed whether treatment of naive animals with ant‐134 was associated with any adverse effect on rats’ performance in the hippocampus‐dependent NOL test. Rats were allowed to explore 2 identical objects, with spatial cues to orient themselves within the arena. Following a delay, rats were reintroduced to the same environment with one of the objects moved to a new location (Figure 5A). Rats with intact spatial memory should favor exploration of the moved object, and so this allowed us to explore whether ant‐134 had an impact on spatial navigation, a key function of the hippocampus. We quantified preference for the novel location using 2 measures12: the absolute difference (D1) between time spent exploring each object [T Novel‐T Familiar] and the discrimination index (DI) [(T Novel‐T Familiar)/(T Novel+T Familiar)], which accounts for total exploration time during the task. We observed no difference in performance in the NOL task between ant‐134 and scramble‐treated animals using either measure (D1: Scramble = 4.6 ± 7.5, Ant‐134 = 7.6 ± 9.8, t test P = .54; DI: Scramble = 0.160 ± 0.30, Ant‐134 = 0.159 ± 0.20, t test P = .99; N = 6 scramble and 7 ant‐134). This is similar to observations in mice7 and suggests that knockdown of microRNA‐134 using ant‐134 does not cause a substantial deficit in hippocampal function in multiple species.
Figure 5.
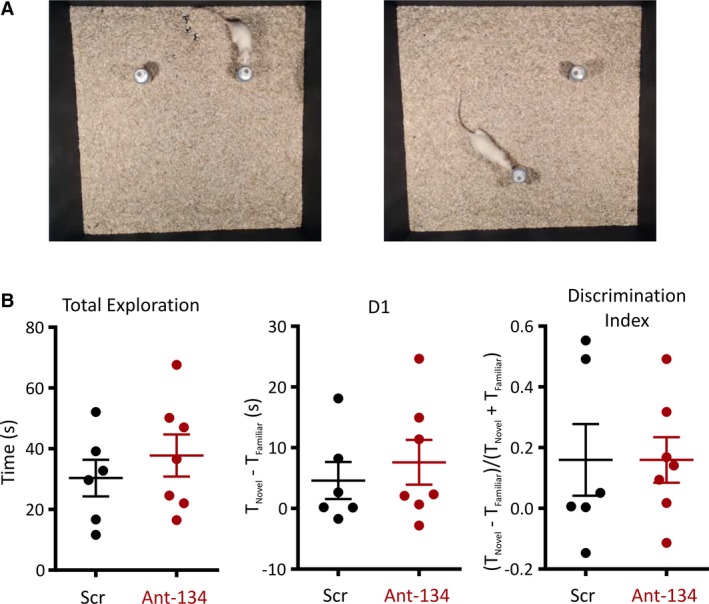
Ant‐134 does not affect performance in a novel object location test. A, 24 h after treatment with scramble or ant‐134, rats completed a novel object location test. Rats explored identical objects in their original positions (A—left panel) for 5 minutes and were reintroduced 1 hour later, with one of the objects moved to a novel location (A‐right panel). B, Scatter plots show that total time exploring in trial 2 (independent samples t test, P = .51), difference between time exploring novel and familiar objects (D1, independent samples t test P = .54) and discrimination index (independent samples t test P = .99) were not changed by ant‐134
4. DISCUSSION
Ant‐134 is a promising novel therapeutic for epilepsy,6, 8 and our data suggest that treatment with ant‐134 has relatively specific effects on seizure generation, with no obvious adverse effects on several measures of synaptic, intrinsic, and behavioral activity in naive rats. The lack of synaptic and biophysical changes is despite the observed reduction in acutely induced epileptiform activity in ex vivo slices taken from these animals and the confirmed reduction of microRNA‐134. Together, these findings should encourage continued preclinical development of this novel antiseizure therapy.
There remains an urgent and unmet need for anticonvulsant drugs with novel mechanisms of action for pharmacorefractory epilepsy patients, as well as treatments that disrupt the underlying pathophysiology. An oligonucleotide inhibitor targeting microRNA‐134 (ant‐134) is a promising approach to both therapeutic challenges, conferring antiseizure, neuroprotective, and possibly antiepileptogenic effects in multiple rodent models of epilepsy. Our results confirm a modest reduction in epileptiform activity induced acutely with high potassium. Although this effect is not as dramatic as those demonstrated in vivo,6, 8 it is similar to effects of oligonucleotide inhibitors of microRNA‐134 reported in rat hippocampal neurons in a low magnesium in vitro model.16 It is also consistent with the effects of several common AEDs on this model, where activity is modified but not abolished completely.17 However, microRNA‐134 serves a number of important physiologic roles in neuronal function, including negatively regulating dendritic spine morphology.9 Indeed, the precursor sequence for microRNA‐134 contains a trafficking sequence that directs accumulation to synapses.18 Understanding the consequences of inhibiting this microRNA is therefore a priority. Notably, mice lacking the microRNA cluster in which microRNA‐134 lies are viable.19 Nevertheless, given the previously reported alterations in dendritic spines,6, 7, 9 it is important to assess the function of individual hippocampal neurons after administration of ant‐134.
We used patch‐clamp recordings to explore the effect of ant‐134 on the active properties of hippocampal pyramidal neurons and observed no clear changes in action potential waveform (Figure 4) and mEPSC amplitude (Figure 3B and C) or frequency (Figure 3A and C). Although encouraging from the perspective of ant‐134 as a novel therapeutic, these results—especially the similarity of mEPSC frequency—are not consistent with previous reports of reduced spine density (which would be reflected as a change in mEPSC frequency) or increased spine volume (which would lead to greater mEPSC amplitude). We suggest several possible explanations for this apparent discrepancy.
First, our patch‐clamp experiments were carried out in CA1 pyramidal neurons, whereas the reduction in spine density was reported in CA3 pyramidal neurons.6 It is possible that ant‐134 can have a selective effect only in CA3, although this seems unlikely given that in situ hybridization shows that microRNA‐134 is also present in CA1, as well as the neocortex and amygdala.6 A species difference is also possible and we did not confirm whether our ant‐134 treatment, using the same dose as was effective in mice, affects dendritic spine number or volume in rats. An alternate explanation is that the spine reduction is either insufficient to produce measurable changes in mEPSCs or is compensated by the co‐occurrence of increased spine volume in the same neurons.7 It is also possible that the 2‐4 day time window between ant‐134 administration and functional measures could have been too short to see an effect of the antagomir. However, this time window was chosen to coincide with the maximal reduction of microRNA‐134 by ant‐134, as studied previously.6 However, our findings do not exclude longer‐lasting effects. Indeed, we have shown that posttreatment with the antimir can suppress seizures for many weeks.6 We might also consider that ant‐134 could have a larger functional impact on neuronal properties in diseased tissue. It is well documented that microRNA‐134 is consistently upregulated in multiple animal models of epilepsy as well as in tissue samples from patients with epilepsy.6, 7, 8,20 It could be that the lower level of microRNA‐134 in naive tissue means that its knockdown with ant‐134 has a smaller functional effect. In contrast, microRNA‐134 reaches a higher level and appears to play a more significant role in epileptogenesis, meaning that ant‐134 could have a greater impact in epileptic networks. The suggestion that ant‐134 selectively targets diseased tissue is an important characteristic of a potential treatment, but one which would require further experimental validation. Although we saw no obvious effect of ant‐134 in our preparation, we tested a relatively limited number of parameters. Further avenues for investigation could include the inhibitory system and plasticity within the hippocampus. Indeed, there is evidence that microRNA‐134 is enriched in subpopulations of inhibitory neurons21 and regulates learning and memory via control of cAMP response element‐binding protein (CREB).22
Although ant‐134 reduced epileptiform activity in our preparation, it was not clear whether the change in microRNA‐134 level could also be associated with nonspecific adverse effects on hippocampal processing. One of the key roles of the hippocampus is in spatial memory and navigation.23 We therefore tested the impact of ant‐134 on the ability of rats to perform a NOL test.11 In this test, rats use spatial cues to determine which of 2 identical objects has been moved to a novel location and typically spend more time exploring this object. We did not see any difference between rats treated with ant‐134 or scramble control in their ability to identify the moved object (Figure 5C). This is a key finding, indicating that ant‐134 treatment does not impair the ability of the naive hippocampus to process spatial memory. It is worth considering that our experimental design necessitated completing the behavior task 24 hours following surgery for stereotaxic injection. This should be sufficient time for animals to recover completely from the procedure and to metabolize the analgesics given, but we cannot rule out an impact of the surgery on the results of the NOL test. Regardless, both control and ant‐134‐treated animals showed the expected bias toward the novel object (Figure 5C), and our results are consistent with the lack of effect of ant‐134 in a similar test in mice.7
The present experimental approach could be used to screen for antiseizure efficacy in human tissue, using acute brain slices prepared from tissue obtained during surgical resections.24 This would be an important step toward clinical translation, as the antiseizure effect of ant‐134 has not yet been validated in a human model. Despite this, we suggest that ant‐134 treatment is likely to translate to the clinic. MicroRNA‐134 is upregulated is tissue resected from both the neocortex6 and hippocampus,8 although some profiling studies have not reported this finding.25, 26 Nevertheless, our study shows that ant‐134 can have antiseizure effects in tissue where microRNA‐134 is not upregulated and our previous work has shown a disease‐modifying action of ant‐134 in a model in which we did not detect upregulation of microRNA‐134.6 More broadly, this approach can be used as a low‐throughput screen of additional microRNA inhibitors with potential antiseizure actions—to date over a dozen microRNAs have been functionally interrogated,3 but the electrophysiologic consequences of their inhibition are largely unknown.
Taken together, our results show that ant‐134 does not appear to alter physiologic brain function, despite its potent disease‐modifying antiseizure effect in diseased tissue. This suggests that using ant‐134 in the treatment of epilepsy would have few adverse effects and raises the possibility that ant‐134 has a stronger effect in diseased tissue.
DISCLOSURE OF CONFLICT OF INTEREST
The Royal College of Surgeons in Ireland (D Henshall) holds a patent on the inhibition of microRNA‐134 for the treatment of seizure‐related disorders and other neurologic injuries (US9803200B2). The remaining authors have no potential conflicts of interest to disclose. We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
ACKNOWLEDGMENTS
This work was funded by the European Union Seventh Framework Programme “EpimiRNA” project under grant agreement number 602130, by Epilepsy Research UK, Science Foundation Ireland (13/IA/1891), and the Health Research Board (HRA_POR/2013/325). SS was funded by a fellowship from the Royal Society.
Morris G, Brennan GP, Reschke CR, Henshall DC, Schorge S. Spared CA1 pyramidal neuron function and hippocampal performance following antisense knockdown of microRNA‐134. Epilepsia. 2018;59:1518–1526. 10.1111/epi.14475
Contributor Information
Gareth Morris, Email: gareth.morris@ucl.ac.uk.
Stephanie Schorge, Email: s.schorge@ucl.ac.uk.
REFERENCES
- 1. Brodie MJ, Barry SJE, Bamagous GA, et al. Patterns of treatment response in newly diagnosed epilepsy. Neurology. 2012;78:1548–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sirven JI, Noe K, Hoerth M, et al. Antiepileptic drugs 2012: recent advances and trends. Mayo Clin Proc. 2012;87:879–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Henshall DC, Hamer HM, Pasterkamp RJ, et al. MicroRNAs in epilepsy: pathophysiology and clinical utility. Lancet Neurol. 2016;15:1368–76. [DOI] [PubMed] [Google Scholar]
- 4. Kosik KS. The neuronal microRNA system. Nat Rev Neurosci. 2006;7:911–20. [DOI] [PubMed] [Google Scholar]
- 5. Bartel DP. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Jimenez‐Mateos EM, Engel T, Merino‐Serrais P, et al. Silencing microRNA‐134 produces neuroprotective and prolonged seizure‐suppressive effects. Nat Med. 2012;18:1087–94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Jimenez‐Mateos EM, Engel T, Merino‐Serrais P, et al. Antagomirs targeting microRNA‐134 increase hippocampal pyramidal neuron spine volume in vivo and protect against pilocarpine‐induced status epilepticus. Brain Struct Funct. 2015;220:2387–99. [DOI] [PubMed] [Google Scholar]
- 8. Reschke CR, Silva LFA, Norwood BA, et al. Potent anti‐seizure effects of locked nucleic acid antagomirs targeting miR‐134 in multiple mouse and rat models of epilepsy. Mol Ther Nucleic Acids. 2017;6:45–56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Schratt GM, Tuebing F, Nigh EA, et al. A brain‐specific microRNA regulates dendritic spine development. Nature. 2006;439:283–9. [DOI] [PubMed] [Google Scholar]
- 10. Sun J, Gao X, Meng D, et al. Antagomirs targeting MicroRNA‐134 increase Limk1 levels after experimental seizures in vitro and in vivo. Cell Physiol Biochem. 2017;43:636–43. [DOI] [PubMed] [Google Scholar]
- 11. Hattiangady B, Mishra V, Kodali M, et al. Object location and object recognition memory impairments, motivation deficits and depression in a model of Gulf War illness. Front Behav Neurosci. 2014;8:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Antunes M, Biala G. The novel object recognition memory: neurobiology, test procedure, and its modifications. Cogn Process. 2012;13:93–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Morris G, Jiruska P, Jefferys JGR, et al. A new approach of modified submerged patch clamp recording reveals interneuronal dynamics during epileptiform oscillations. Front Neurosci. 2016;10:519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Hill MRH, Greenfield SA. The membrane chamber: a new type of in vitro recording chamber. J Neurosci Methods. 2011;195:15–23. [DOI] [PubMed] [Google Scholar]
- 15. Jiruska P, Finnerty GT, Powell AD, et al. Epileptic high‐frequency network activity in a model of non‐lesional temporal lobe epilepsy. Brain. 2010;133:1380–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Wang XM, Jia RH, Wei D, et al. MiR‐134 blockade prevents status epilepticus like‐activity and is neuroprotective in cultured hippocampal neurons. Neurosci Lett. 2014;572:20–5. [DOI] [PubMed] [Google Scholar]
- 17. Georg Margineanu D, Klitgaard H. Inhibition of neuronal hypersynchrony in vitro differentiates levetiracetam from classical antiepileptic drugs. Pharmacol Res. 2000;42:281–5. [DOI] [PubMed] [Google Scholar]
- 18. Bicker S, Khudayberdiev S, Weiß K, et al. The DEAH‐box helicase DHX36 mediates dendritic localization of the neuronal precursor‐microRNA‐134. Genes Dev. 2013;27:991–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Valluy J, Bicker S, Aksoy‐Aksel A, et al. A coding‐independent function of an alternative Ube3a transcript during neuronal development. Nat Neurosci. 2015;18:666–73. [DOI] [PubMed] [Google Scholar]
- 20. Peng J, Omran A, Ashhab MU, et al. Expression patterns of miR‐124, miR‐134, miR‐132, and miR‐21 in an immature rat model and children with mesial temporal lobe epilepsy. J Mol Neurosci. 2013;50:291–7. [DOI] [PubMed] [Google Scholar]
- 21. Chai S, Cambronne XA, Eichhorn SW, et al. MicroRNA‐134 activity in somatostatin interneurons regulates H‐Ras localization by repressing the palmitoylation enzyme, DHHC9. Proc Natl Acad Sci. 2013;110:17898–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gao J, Wang WY, Mao YW, et al. A novel pathway regulates memory and plasticity via SIRT1 and miR‐134. Nature. 2010;466:1105–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Burgess N, Maguire EA, O'Keefe J. The human hippocampus and spatial and episodic memory. Neuron. 2002;35:625–41. [DOI] [PubMed] [Google Scholar]
- 24. Wickham J, Brödjegård NG, Vighagen R, et al. Prolonged life of human acute hippocampal slices from temporal lobe epilepsy surgery. Sci Rep. 2018;8:1–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kan AA, van Erp S, Derijck AAHA, et al. Genome‐wide microRNA profiling of human temporal lobe epilepsy identifies modulators of the immune response. Cell Mol Life Sci. 2012;69:3127–45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Miller‐Delaney SFC, Bryan K, Das S, et al. Differential DNA methylation profiles of coding and non‐coding genes define hippocampal sclerosis in human temporal lobe epilepsy. Brain. 2014;138:616–31. [DOI] [PMC free article] [PubMed] [Google Scholar]


