Abstract
Verinurad (RDEA3170) is a selective uric acid reabsorption inhibitor in clinical development for treatment of hyperuricemia and gout. This phase 1b, multiple‐dose, drug‐drug interaction study evaluated the pharmacokinetics, pharmacodynamics, and tolerability of verinurad in combination with allopurinol. Adult males with gout were randomized to receive once‐daily oral doses of allopurinol 300 mg or verinurad 10 mg alone for 7 days, allopurinol 300 mg + verinurad 10 mg on days 8 to 14, and the alternative single agent on days 15 to 21. Colchicine 0.6 mg was taken prophylactically for gout flares. Plasma/serum and urine samples were assayed for verinurad, allopurinol, oxypurinol (allopurinol active metabolite), colchicine (plasma only), and uric acid. Safety was assessed by adverse events (AEs) and laboratory tests. Verinurad plasma exposure was unaffected by allopurinol. Verinurad increased the maximum observed plasma concentration (Cmax) for allopurinol by 33%; the area under the plasma concentration‐time curve (AUC) was unaffected. Oxypurinol Cmax and AUC were reduced 32% and 38%, respectively, by verinurad. Colchicine plasma exposure was unaltered by verinurad. The maximum decrease in serum urate was greater with verinurad + allopurinol (65%) than with verinurad (51%) or allopurinol (43%) alone. Compared with the baseline rate, the maximum rate of uric acid excreted in urine was +56% with verinurad, −46% with allopurinol, and unchanged with verinurad + allopurinol. No serious AEs, discontinuations due to AEs, or clinically significant laboratory abnormalities were noted. Despite decreased systemic exposure of allopurinol and oxypurinol in the presence of verinurad, the combination resulted in greater serum urate reduction compared with either drug alone and was well tolerated at the studied doses.
Keywords: Pharmacokinetics, pharmacodynamics, tolerability, combination therapy, serum uric acid, gout
Gout is a chronic inflammatory arthritis characterized by deposition of monosodium urate crystals in the joints, tendons, and other connective tissues secondary to chronic hyperuricemia in which the concentration of serum uric acid is ≥6.8 mg/dL.1, 2 Reducing serum uric acid below its saturation point leads, over time, to the dissolution of crystals and alleviation of gout symptoms.3, 4 To achieve this, most patients require long‐term urate‐lowering therapy in addition to lifestyle and dietary changes to reduce serum uric acid to <6.0 mg/dL or <5.0 mg/dL in patients with severe gout symptoms.
The recommended first line of urate‐lowering therapy includes the xanthine oxidase inhibitors allopurinol and febuxostat, which lower serum uric acid by reducing urate production.4, 5 Allopurinol is the most widely prescribed xanthine oxidase inhibitors for gout. Although allopurinol is approved at dosages up to 800 mg (in the United States) or 900 mg (in the European Union) daily, the vast majority of patients receive no more than 300 mg.6 Whatever the xanthine oxidase inhibitors or dosage, the majority of patients fail to achieve target serum uric acid using an xanthine oxidase inhibitors alone.6, 7 For these patients, guidelines recommend switching to another treatment or combining treatments with complementary mechanisms of action, such as an xanthine oxidase inhibitors with a uricosuric.4, 5
The uricosurics—probenecid, benzbromarone, sulfinpyrazone, and lesinurad—increase renal excretion of uric acid by inhibiting its reabsorption.2 Lesinurad, a selective uric acid reabsorption inhibitor, was recently approved in the United States and Europe in combination with an xanthine oxidase inhibitors for the treatment of hyperuricemia associated with gout in patients who fail to achieve target serum uric acid levels on an xanthine oxidase inhibitors alone.8 Lesinurad inhibits the uric acid transporter URAT1, which is responsible for most reabsorption of urate in the renal tubule.9, 10 In combination with febuxostat, treatment with lesinurad 200 mg and 400 mg resulted in more patients with tophaceous gout achieving target serum uric acid levels and experiencing reduction in overall tophus area compared with febuxostat alone.11 However, there were only trends toward reductions in gout flares at the 400‐mg dose, a dose with more renal side effects compared with lesinurad 200 mg + febuxostat and febuxostat alone. The lesinurad 400‐mg dose was neither submitted to nor approved by US and European regulatory agencies.
The clinical trial experience with lesinurad and other urate‐lowering agents has demonstrated that achieving serum uric acid levels below recommended targets may lead to improved outcomes (eg, lower gout flare incidence, more rapid tophus area reduction).12, 13, 14, 15, 16
Verinurad, a selective next‐generation reabsorption inhibitor, demonstrated high potency in inhibiting URAT117 and has shown significant serum uric acid lowering in humans at doses as low as 2.5 mg.18 The potency and pharmacokinetic properties of verinurad may enable it to be administered at a low dose that could potentially lessen drug‐drug interactions (DDIs) in patients with gout, many of whom also receive treatment for 1 or more comorbidities.
Elevated urinary uric acid concentrations and low rates of serum creatinine (sCr) elevation have been observed with verinurad monotherapy.18 In contrast, an xanthine oxidase inhibitors reduces urinary uric acid excretion by inhibiting urate production.19, 20 Therefore, the combination of verinurad with an xanthine oxidase inhibitors has the potential to reduce the incidence of renal uric acid crystallization and sCr elevation, while it reduces serum uric acid levels to a greater extent than by an xanthine oxidase inhibitors alone.
The current study investigated the pharmacokinetics (PK), pharmacodynamics (PD), and safety of combining verinurad with allopurinol in adult male subjects with gout.
Methods
The protocol, informed consents, and amendments were approved by the institutional review board (Midlands Independent Review Board, Overland Park, Kansas) with jurisdiction over the study site. All participants signed informed consent at study entry. The study (RDEA3170‐107; NCT02279641) was conducted in accordance with Good Clinical Practice guidelines of the International Conference on Harmonisation and the Declaration of Helsinki.
Subjects
Eligible men aged ≥18 to 75 years, with a diagnosis of gout as per the American Rheumatism Association Criteria for the Classification of Acute Arthritis of Primary Gout,21 were eligible. Subjects had body weight ≥50 kg, a body mass index ≥18 and ≤45 kg/m2, and a screening serum uric acid level ≥8 mg/dL and ≤10 mg/dL. Eligible subjects were free of any clinically significant disease or medical condition based on the investigator's judgment and had no clinically relevant abnormalities in blood pressure, heart rate, body temperature, or respiratory rate. Subjects were excluded if they were unable to take colchicine for gout‐flare prophylaxis, had a history or suspicion of kidney stones, were taking any moderate or strong enzyme‐inducing drug or product, CYP3A inhibitors, or p‐glycoprotein inhibitors, digoxin, or >81 mg of aspirin daily, and had an estimated creatinine clearance <60 mL/min based on the Cockcroft‐Gault equation using ideal body weight. Chronic and stable doses of losartan, fenofibrate, guaifenesin, and sodium‐glucose linked transporter‐2 inhibitors were permitted if the dose had been stable for at least 14 days before the start of the study.
Study Design
This was a phase 1b, randomized, DDI study to evaluate the potential PK and PD interactions between allopurinol and verinurad in adult male subjects with gout. Subjects were admitted to the study site on day −2 and were randomized on day 1. Subjects were randomized to 1 of 2 treatment sequences (sequence A or B) in a 1:1 ratio (Figure 1) and received once‐daily oral doses of allopurinol 300 mg or verinurad 10 mg alone for 7 days, allopurinol 300 mg + verinurad 10 mg on days 8 to 14, and the alternative single agent (verinurad 10 mg or allopurinol 300 mg) on days 15 to 21. Gout‐flare prophylaxis with colchicine 0.6 mg was taken once daily from day −14 through follow‐up.
Figure 1.
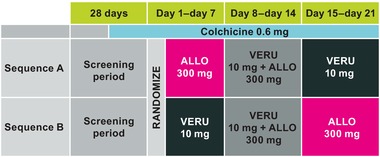
Study design. ALLO indicates allopurinol; VERU, verinurad.
Study medication was orally administered with approximately 240 mL of water, 30 to 35 minutes after initiating a standardized moderate‐fat (approximately 30% to 40% of calories) and moderate‐calorie (approximately 642 to 800 calories) breakfast. On PK/PD sampling days, subjects fasted overnight before consuming their breakfast, and no food was allowed for 4 hours after the administration of study medication. Water was allowed as desired, except for 1 hour before and 1 hour after the administration of any study medication. On PK/PD urine collection days, subjects drank 240 mL of water immediately after waking (within 2 hours predose) and 22 hours postdose, and 160 mL of water at frequent intervals postdose.
Serial plasma PK samples were collected on days −1, 7, 14, and 21 at predose and up to 24 hours postdose. Serial serum PD samples were collected on days 7, 14, and 21 at predose and up to 24 hours postdose with additional predose time points on day 1. Urine (total catch) samples for PK/PD were collected on days 1, 7, 8 (PD only), 14 and 21 at 0 to 1, 1 to 2, 2 to 3, 3 to 4, 4 to 5, 5 to 6, 6 to 8, 8 to 10, 10 to 12, 12 to 22, and 22 to 24 hours postdose. Subjects were discharged from the study site on day 22 and returned to the study site for follow‐up assessments on day 28 ± 1 day.
Analytical Methods
The analysis of verinurad, allopurinol, and oxypurinol (the active metabolite of allopurinol) in plasma samples and verinurad in urine samples was performed by Ardea Biosciences, Inc (San Diego, California). Plasma samples were acidified with 1% phosphoric acid (v/v) to prevent conversion of 1 metabolite to another. Verinurad and internal standard [13C6]verinurad were extracted from the acidified plasma using a protein precipitation procedure with acetonitrile containing 0.2% formic acid (v/v). Extracted samples were injected onto a Kinetex C18, 4.6 × 50 mm, 2.6 μm column (Phenomenex, Torrance, California), running 0.1% formic acid in water, v/v (mobile phase A) with 0.1% formic acid in acetonitrile, v/v (mobile phase B) at a flow rate of 0.9 mL/min into an API 5000 triple quadrupole mass spectrometer (AB Sciex, Framingham, Massachusetts) operated in positive TurboIonSpray mode. Under unit/low resolution (Q1/Q3), selected reaction monitoring (SRM) was used to monitor the precursor → product ion transitions of m/z 349→ 261 (verinurad), 355→ 261 ([13C6]verinurad) with a 100‐millisecond dwell time for verinurad and 30‐millisecond dwell times for its internal standard. The lower limit of quantification (LLOQ) was 0.1 ng/mL and QCs showed percentage theoretical (%CV) during study conduct of 99.3% to 101.7% (7.0%‐16.0%) for verinurad.
Analytical methodology for the quantitation of verinurad in urine samples has been described previously.18 Urine samples were prepared by dilution and quantified using high performance liquid chromatography‐mass spectrometry. During the current study analysis, verinurad QCs showed % Theoretical (%CV) during study conduct of 96.7% to 103.0% (1.6%‐2.7%).
Allopurinol and oxypurinol, with internal standards [13C3 15N3]allopurinol and [13C2 15N]oxypurinol, were extracted from plasma using a protein precipitation procedure with methanol and from urine using a dilution procedure with methanol. Extracted samples were injected onto a Luna 3 μm PFP(2), 4.6 × 50 mm column (Phenomenex), running 0.01% formic acid in water, v/v (mobile phase A) with methanol (mobile phase B) at a flow rate of 0.9 mL/min into an API 4000 triple quadrupole mass spectrometer (AB Sciex) operated in negative TurboIonSpray mode. Under unit/unit resolution (Q1/Q3), SRM was used to monitor the precursor → product ion transitions of m/z 135 → 92 (allopurinol), 141 → 97 ([13C3 15N3]allopurinol), 151 → 42 (oxypurinol), and 154 → 42 ([13C2 15N]oxypurinol), with 150‐millisecond dwell times for each analyte transition and 50‐millisecond dwell times for each internal standard. The LLOQ from plasma was 25 and 100 ng/mL, respectively, and QCs showed % Theoretical (%CV) during study conduct of 96.1% to 100.0% (4.6%‐5.6%) for allopurinol and 95.7% to 99.7% (4.1%‐4.8%) for oxypurinol. The LLOQ from urine was 40 and 1000 ng/mL, respectively, and QCs showed % Theoretical (%CV) during study conduct of 94.8% to 103.3% (2.8%‐9.7%) for allopurinol and 99.3% to 107.0% (3.2%‐6.2%) for oxypurinol. It is additionally noted that both plasma and urine methods were demonstrated to chromatographically separate allopurinol and oxypurinol from their endogenous isomers, hypoxanthine and xanthine, respectively.
The analyses of colchicine in plasma were performed by inVentiv Health Clinique, Inc. (Québec, Canada). Colchicine and [2H6]colchicine were extracted from a 0.2 mL plasma using solid phase extraction (Strata X 30 mg, Phenomenex) with methanol. The eluant was evaporated and reconstituted with Mobile Phase A (0.1% formic acid in methanol/water, 50:50, v/v). Extracted samples were injected onto an ACE C18‐AR 3 μm, 3 × 30 mm column (Advanced Chromatography Technologies, Ltd, Aberdeen, Scotland), running a gradient of mobile phase A and 0.2% acetic acid in water/methanol, 2:98, v/v (Mobile Phase B) at a flow rate of 0.8 mL/min into an API 4000 triple quadrupole mass spectrometer (AB Sciex) operated in positive TurboIonSpray mode. Under unit/unit resolution (Q1/Q3), SRM was used to monitor the precursor → product ion transitions of m/z 400→358 (colchicine) and 406→362 ([2H6]colchicine), with a 450 millisecond dwell time for colchicine and a 200‐millisecond dwell time for its internal standard. The LLOQ was 0.04 ng/mL, and QCs showed % Theoretical (%CV) during study conduct of 95.5% to 96.8% (3.0%‐5.4%). All methods were validated according to the US Food and Drug Administration Bioanalytical Method Validation guidelines (2001).22
Serum samples were analyzed for serum uric acid and creatinine and urine samples for uric acid and creatinine by Covance Central Laboratory Services (Indianapolis, Indiana).
Pharmacokinetic Assessments
Parameters from individual plasma concentration−time profiles of verinurad and allopurinol were determined using noncompartmental methods. Plasma PK parameters estimated for verinurad and allopurinol included the time to maximum concentration (Tmax), the maximum observed plasma concentration (Cmax), and the area under the plasma concentration−time curve from time 0 to 24 hours postdose (AUC0‐24). Urine PK parameters included the amount of drug excreted in urine unchanged (Ae), fraction of drug excreted in urine unchanged (fe), and the renal clearance of drug (CLR). Ae was calculated as concentration measured × volume, and CLR was calculated as Ae divided by plasma AUC over the same time interval. Plasma PK parameters were derived using the validated program Phoenix WinNonlinProfessional, version 6.3 (Pharsight, Mountain View, California).
Pharmacodynamic Assessments
The PD parameters for urate were calculated by the Covance Clinical Research Unit (Madison, Wisconsin) using SAS version 8.2 or later (SAS Institute, Cary, North Carolina). To evaluate serum uric acid, percentage change from baseline (time‐matched, day −1) was calculated for serum uric acid concentrations and parameters. Percentage change from baseline (time‐matched, day −1) was also calculated for urine uric acid parameters. PD parameters included the maximum percent change from baseline in serum uric acid, amount (AeUR) and rate of uric acid excretion recovered in urine, renal clearance of uric acid (CLUR), and 24 hr fractional excretion of urate as well as the percentage change from baseline in AeUR, CLUR, and 24 hr fractional excretion of urate. CLUR was calculated as AeUR divided by plasma urate AUC over the same time interval, whereas 24 hr fractional excretion of urate was calculated as (CLUR/CrCl) × 100. Rate of uric acid excretion was calculated as AeUR divided by the number of hours in the urine collection interval.
Safety Assessments
Subjects were monitored throughout the study, and any adverse events (AEs) or remedial actions were recorded in the subject's clinical report form. The severity of an AE was assessed by the investigator according to Rheumatology Common Toxicity Criteria version 2.0. Blood and urine samples were collected for clinical laboratory evaluations at specific times during the study. Supine blood pressure, pulse rate, respiratory rate, and oral body temperature were also measured at specific times during the study. A physical examination and standard 12‐lead electrocardiograms (ECGs) were recorded at screening and follow‐up. The ECGs were viewed by the investigator.
Assessment of Xanthine Oxidase Inhibition
The method followed the published procedure by Obach et al.23 The incubation mixtures contained 0.1 mg/ml human liver cytosol (male pool of 10) and 12 μM phthalazine in 100 mM phosphate buffer (pH 7.4) with ethylenediaminetetraacetic acid (EDTA). The incubation volume in each tube was 200 μL. DMSO remained at a concentration of 0.1%. All tubes were incubated for a total of 5 minutes, and the reactions were terminated with acetonitrile containing 7‐hydroxycoumarin as an internal standard. The incubation mixtures were vortexed for 10 seconds and centrifuged at 3300g at 4°C for a total of 15 minutes. The supernatants were analyzed using LC‐MS/MS. A 10 μL sample was injected onto a Synergi 4u Polar‐RP column (80A 50 × 4.60 mm) (Princeton, New Jersey) and analyzed by Sciex triple quadrupole MS/MS (API 4000).
Statistical Analysis
Sample size (N = 12) is typical for a phase 1 study and was not based on formal power calculations because this study was designed only to provide an assessment of the potential PK and PD interactions between verinurad and allopurinol. To assess the potential DDIs, a mixed‐effects model with fixed effects for treatment (combination versus single‐drug treatment alone) and subject as random effects was used to analyze the natural log‐transformed PK parameters Cmax, AUC0‐24, Ae0‐24 (verinurad and oxpurinol only), and CLR0‐24 (verinurad and oxypurinol only) for verinurad, allopurinol, oxypurinol, or colchicine for subjects receiving single‐drug treatment (day 7 or day 21) versus in combination with the other drug (day 14). On back‐transformation, the comparisons between 2 treatments (combination versus alone) were presented in the form of geometric mean ratios (GMRs) of the above parameters with the corresponding 90% CI. No DDI was deemed to have occurred if the GMR was contained within the boundaries of equivalence (80%‐125%).
Results
Study Subjects
A total of 81 subjects were screened, 12 were randomized and completed the study, and 67 failed at screening. An additional 2 subjects were screened but not enrolled. Subjects were males with a mean age of 51 years with a body mass index of 32.6 kg/m2 (Table 1). The majority of subjects were white (7 [58.3%]) and not Hispanic or Latino (11 [91.7%]). Two subjects were receiving allopurinol therapy at screening for the treatment of gout, which they stopped taking 16 days before the start of dosing on day 1.
Table 1.
Demographic Characteristics of Subjects
| Total (N = 12) | |
|---|---|
| Age, y, mean (SD) | 51 (10.0) |
| Body weight, kg, mean (SD) | 101.5 (13.88) |
| Body mass index, kg/m2, mean (SD) | 32.6 (4.33) |
| Race, n (%) | |
| Black | 4 (33.3) |
| Native Hawaiian or other Pacific Islander | 1 (8.3) |
| White | 7 (58.3) |
| Ethnicity, n (%) | |
| Hispanic or Latino | 1 (8.3) |
| Not Hispanic or Latino | 11 (91.7) |
| sUA at screening, mg/dL, mean (95% CI) | 8.4 (7.7‐9.0) |
sUA, serum urate.
Pharmacokinetics
Mean plasma concentration‐time profiles of verinurad, allopurinol, oxypurinol, and colchicine following once daily oral administration of a single agent or combination of verinurad and allopurinol or colchicine are depicted in Figure 2A‐D, and the PK parameters are summarized in Table 2. The PK parameters for verinurad were unaffected by the coadministration of allopurinol. The geometric mean ratios for all parameters were within the range of 0.8‐1.25.
Figure 2.
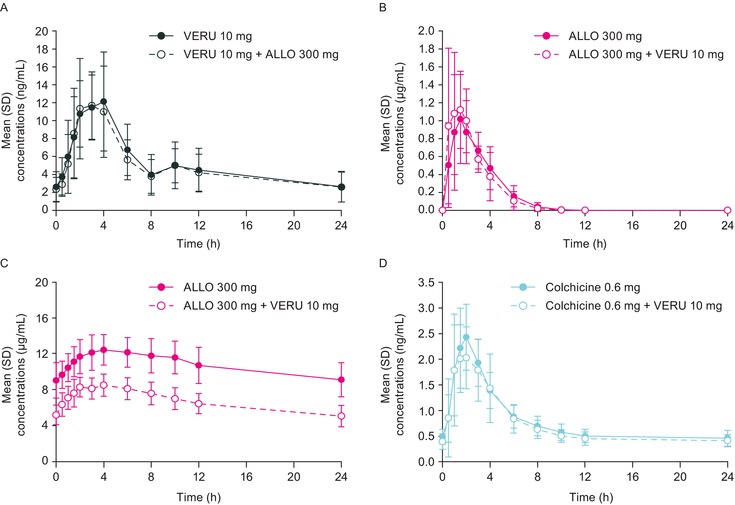
Mean (SD) plasma concentration‐time profiles of verinurad (A), allopurinol (B), oxypurinol (C), and colchicine (D) following once‐daily administration of verinurad, allopurinol, and colchicine alone or in combination. ALLO indicates allopurinol; VERU, verinurad.
Table 2.
Summary PK for Verinurad, Allopurinol, Oxypurinol, and Colchicine
| Tmax, hra | Cmax, ng/mL | AUC0‐24, ng·h/mL | Ae0‐24, μg | CLR0‐24, mL/min | |
|---|---|---|---|---|---|
| Verinurad | |||||
| VERU 10 mg | 3.00 (1.50‐4.00) | 14.6 (12.2‐17.5) | 120 (98.1‐147) | 66.8 (51.8‐86.1) | 9.28 (7.43‐11.6) |
| VERU 10 mg + ALLO 300 mg | 3.00 (2.00‐10.00) | 14.5 (11.7‐17.9) | 115 (96.5‐137) | 67.2 (51.7‐87.4) | 9.75 (7.73‐12.3) |
| GMRb, % (90% CI) | − | 98.7 (87.7‐111) | 95.9 (88.4‐104) | 101 (86.7‐117) | 105 (94.4‐117) |
| Allopurinol | |||||
| Tmax, hra | Cmax, μg/mL | AUC0‐24, μg·h/mL | Ae0‐24, mg | CLR0‐24, mL/min | |
| ALLO 300 mg | 1.50 (1.00‐4.00) | 1.13 (0.89‐1.43) | 3.69 (3.24‐4.21) | 25.4 (22.1‐29.2) | 120 (106‐135) |
| VERU 10 mg + ALLO 300 mg | 1.25 (0.50‐4.00) | 1.51 (1.24‐1.82) | 3.68 (3.28‐4.13) | 22.7 (19.2‐26.9) | 103 (87.1‐122) |
| GMRb, % (90% CI) | − | 133 (111‐159) | 102 (93.3‐111) | 89.5 (81.8‐98.0) | 87.5 (79.3‐96.6) |
| Oxypurinol | |||||
| Tmax, hra | Cmax, μg/mL | AUC0‐24, μg·h/mL | Ae0‐24, mg | CLR0‐24, mL/min | |
| ALLO 300 mg | 4.00 (1.50‐6.00) | 12.8 (11.7‐14.0) | 255 (229‐284) | 231 (216‐248) | 15.1 (13.0‐17.6) |
| VERU 10 mg + ALLO 300 mg | 3.50 (1.50‐8.00) | 8.68 (7.99‐9.43) | 157 (140‐177) | 275 (262‐290) | 29.2 (25.1‐33.9) |
| GMRb, % (90% CI) | − | 67.9 (65.2‐70.7) | 61.8 (58.1‐65.7) | 119 (114‐125) | 193 (177‐210) |
| Colchicine | |||||
| Tmax, hra | Cmax, ng/mL | AUC0‐24, ng·h/mL | Ae0‐24, μg | CLR0‐24, mL/min | |
| VERU 10 mg | 1.75 (1.00‐4.00) | 2.32 (1.93‐2.78) | 17.0 (14.3‐20.1) | − | − |
| VERU 10 mg + ALLO 300 mg | 2.00 (1.00‐3.00) | 2.55 (2.17‐3.01) | 18.4 (15.6‐21.8) | − | − |
| GMRb, % (90% CI) | − | 110 (101‐120) | 109 (99.8‐119) | − | − |
Ae0–24 indicates urinary excretion from time zero to 24 hours postdose; ALLO, allopurinol; AUC0–24, area under the concentration‐time curve from time 0 to 24 hours postdose; Cmax, maximum observed concentration; PK, pharmacokinetics; Tmax, time of maximum observed concentration; VERU, verinurad.
Data are geometric least squares mean (95% CI).
aTmax, median (range).
bGeometric least squares mean ratio (combo/alone).
The Tmax and AUC0‐24 of allopurinol were unaltered following coadministration of verinurad, whereas Cmax of allopurinol was greater when compared to allopurinol alone (Table 2). The geometric mean ratios for Cmax and AUC0‐24 were 133% and 102%, respectively. After coadministration of allopurinol and verinurad, the Cmax of oxypurinol, the active metabolite of allopurinol, decreased by approximately 32% and the AUC0‐24 by approximately 38% compared with allopurinol alone.
In the presence of verinurad, both Ae0‐24 and CLR0‐24 for allopurinol were similar to allopurinol alone, with GMRs of 89.5% and 87.5%, whereas they were increased for oxypurinol (119% and 193%, respectively [Table 2]). In the presence of verinurad the Tmax, Cmax, and AUC0‐24 for colchicine were similar to those with colchicine alone. The GMRs for Cmax and AUC0‐24 were 110% and 109%, respectively.
Pharmacodynamics
Serum Urate Levels
Mean baseline serum uric acid concentrations ranged from 8.4 to 9.0 mg/dL over all time points in the 24‐hour period before the start of dosing. Verinurad, allopurinol, and the combination reduced serum uric acid over 24 hours postdose (Figure 3). The maximum mean (95% CI) change from baseline in serum uric acid was −65.2% (−70.3%, −60.2%) with verinurad + allopurinol compared with −50.5% (−55.9%, −45.2%) with verinurad alone or −43.0% (−46.5%, −39.6%) with allopurinol alone. The corresponding mean change from baseline at 24 hours was −51.7% (−56.7%, −46.6%) with verinurad + allopurinol, −37.7% (−42.9%, −32.5%) with verinurad alone and −36.0% (−39.0%, −33.0%) with allopurinol alone.
Figure 3.
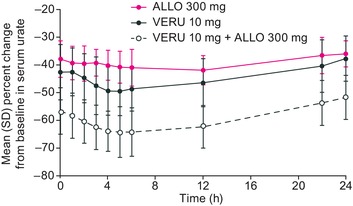
Mean (SD) percentage change from baseline in serum uric acid at steady state following VERU 10 mg or ALLO 300 mg or in combination. ALLO indicates allopurinol; serum uric acid, serum urate concentration; VERU, verinurad.
Urine Uric Acid
The rate of urinary uric acid excretion during each urine collection interval is shown in Figure 4. Rate of uric acid excretion was increased by verinurad alone, with the highest mean rate during the 2‐3 hour urine collection interval postdose being 56% greater than the maximum observed baseline rate. Allopurinol decreased rate of uric acid excretion 46% during the same interval. For the combination of verinurad and allopurinol, the highest mean uric acid excretion rates occurred during the 2‐3 and 3‐4 hour intervals postdose and were similar to the maximum observed baseline rate.
Figure 4.
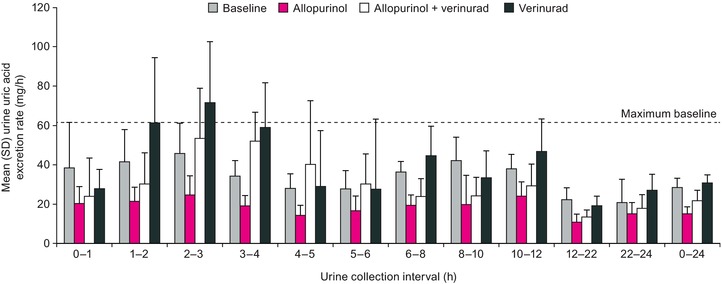
Mean (SD) rate of uric acid recovered in urine during each urine collection interval following administration of multiple oral doses of verinurad 10 mg and allopurinol 300 mg, alone and in combination, to male subjects with gout.
Compared with baseline, verinurad increased 24‐hour AeUR, 24 hr fractional excretion of urate, and CLUR by 9.88%, 77.4%, and 122%, respectively (Table 3). In contrast, allopurinol decreased all 3 parameters by 47.4%, 12.0%, and 8.5%, respectively. The decrease in AeUR with the combination of verinurad + allopurinol was less (21.6%) than with allopurinol alone, whereas the increase in 24 hr fractional excretion of urate (78.4%) and in CLUR (125%) was similar to that with verinurad alone. The increase in AeUR, 24 hr fractional excretion of urate, and CLUR with verinurad was greater in the first 12‐hour interval postdose than during the 12‐24 hour interval, whereas the decrease in all 3 parameters with allopurinol was similar in both intervals. The decrease in AeUR with verinurad + allopurinol was greater in the second 12 hours, whereas the increase in 24 hr fractional excretion of urate and CLUR was greater in the first 12 hours and similar to that observed with verinurad alone.
Table 3.
Mean (SD) and Percentage Change From Baseline for AeUR, FEUA, and CLUR During Urine Collection Intervals After Multiple Daily Doses of Verinurad, Allopurinol, and the Combination
| 0‐ to 12‐Hour Interval | 12‐ to 24‐Hour Interval | 0‐ to 24‐Hour Interval | ||||
|---|---|---|---|---|---|---|
| Treatment | Mean (SD) | Change From Baseline (%) | Mean (SD) | Change From Baseline (%) | Mean (SD) | %Change From Baseline |
| AeUR, mg | ||||||
| Baseline | 418 (43) | 264 (68) | 683 (98) | |||
| VERU 10 mg | 490 (71) | 21.4 (23.3) | 250 (52) | 4.2 (48.0) | 739 (84) | 9.9 (21.5) |
| ALLO 300 mg | 231 (45) | −46.8 (7.3) | 180 (46) | −45.4 (14.5) | 363 (73) | −47.4 (7.6) |
| VERU 10 mg + ALLO 300 mg | 360 (73) | −11.4 (20.1) | 170 (48) | −27.8 (41.4) | 522 (105) | −21.6 (15.8) |
| FEUA, % | ||||||
| Baseline | 4.84 (0.56) | 3.58 (0.76) | 4.45 (0.54) | |||
| VERU 10 mg | 11.6 (3.77) | 160 (96.6) | 5.88 (1.78) | 49.9 (21.9) | 9.19 (3.28) | 77.4 (36.0) |
| ALLO 300 mg | 4.06 (0.47) | −9.7 (9.2) | 3.19 (0.62) | −7.6 (14.6) | 3.69 (0.59) | −12.0 (12.2) |
| VERU 10 mg + ALLO 300 mg | 11.0 (2.16) | 128 (67.6) | 5.17 (2.12) | 31.8 (26.0) | 7.93 (2.0) | 78.4 (48.9) |
| CLUR, mL/min | ||||||
| Baseline | 7.03 (0.44) | 4.18 (1.23) | 5.67 (0.92) | |||
| VERU 10 mg | 16.3 (4.57) | 153 (77.0) | 6.74 (0.96) | 95.5 (148) | 11.5 (2.74) | 122 (105) |
| ALLO 300 mg | 6.50 (1.18) | −6.0 (12.0) | 3.62 (1.30) | −9.4 (23.2) | 5.00 (1.13) | −8.50 (14.8) |
| VERU 10 mg + ALLO 300 mg | 17.9 (6.69) | 163 (99.0) | 6.41 (2.19) | 85.0 (164) | 11.8 (4.5) | 125 (103) |
AeUR, amount of uric acid recovered in urine; ALLO, allopurinol; CLUR, renal clearance of uric acid; FEUA, 24‐hour fractional excretion of uric acid; VERU, verinurad.
Safety
Verinurad was well tolerated by male subjects with gout when administered as multiple doses alone, and in combination with allopurinol, in the fed state. The overall incidence of treatment‐emergent AEs (TEAEs) during the study was low, and no deaths, other serious AEs, or withdrawals due to AEs were reported. Seven of the 10 TEAEs were mild in severity, although 3 were moderate. Only 1 TEAE (petechiae) was considered by the investigator to be possibly related to both verinurad and allopurinol because it occurred following the first dose of verinurad + allopurinol. The petechiae resolved without treatment after approximately 19 days. Three subjects received concomitant therapy for treatment of AEs (toothache, headache, and right elbow pain), all of which were considered to be unlikely related to study medication. Two colchicine‐emergent AEs (ie, those starting on or after the date of the first dose of colchicine but before initiation of randomized study treatment) were reported; the AEs were constipation and nasal congestion.
No clinically meaningful changes in any laboratory values over time were observed, except for changes in serum uric acid. Two subjects experienced an increase in sCr, but neither experienced a sCr value ≥1.5 × baseline sCr value. There were no clinically significant changes from baseline in vital signs, in ECG, or on physical examination.
Discussion
This study evaluated the combination of verinurad, a selective uric acid reabsorption inhibitor, and allopurinol, an xanthine oxidase inhibitors, in adult male subjects with gout for impact on PK and PD parameters of the 2 drugs. Following multiple doses of verinurad administered with or without allopurinol, verinurad plasma exposure (Cmax and AUC) was unchanged compared with verinurad alone. Allopurinol also did not alter the urinary excretion of verinurad. In contrast, the plasma Cmax of allopurinol was 33% higher following coadministration of verinurad and allopurinol compared with allopurinol alone. Verinurad decreased the plasma Cmax and AUC of oxypurinol, the active metabolite of allopurinol, by approximately 32% and 38%, respectively, and oxypurinol urinary excretion and renal clearance were increased by 19% and 93%, respectively. The PK changes in oxypurinol are consistent with the mechanism of action of verinurad since oxypurinol, like urate, is a substrate for URAT1.24, 25 A similar decrease in oxypurinol exposure was observed in subjects with gout on coadministration of allopurinol and the URAT1 inhibitor, benzbromarone,26 or lesinurad.27 Verinurad did not alter the plasma exposure of colchicine, an important concomitant medication in this patient population.
Although the systemic exposure of oxypurinol was reduced in the presence of verinurad, the serum uric acid lowering over 24 hours was greater after coadministration of verinurad and allopurinol compared with either single agent alone. Similar serum uric acid results were observed with the coadministration of lesinurad and allopurinol.27
Consistent with the mechanism of action of verinurad, 24‐hour AeUR, 24 hr fractional excretion of urate, and CLUR were increased versus baseline or allopurinol. In contrast, allopurinol decreased AeUR compared with baseline, verinurad alone, or verinurad + allopurinol with smaller reductions in 24 hr fractional excretion of urate and CLUR. The decrease in AeUR can be attributed to a lowering of serum uric acid levels via xanthine oxidase inhibition, thereby reducing the amount of uric acid available for excretion by the kidney. The lower 24 hr fractional excretion of urate and CLUR with allopurinol have been observed previously.28 With verinurad + allopurinol, 24 hr fractional excretion of urate and CLUR were increased over baseline or allopurinol alone, whereas AeUR was less than baseline but greater than allopurinol alone
We hypothesize that the maximum AeUR postdosing may reflect the potential for renal damage. To better characterize the risk, urine collection periods as short as 1 hour were employed in this study, which allowed an assessment of the rate of uric acid excretion. Verinurad increased rate of uric acid excretion above baseline, particularly in the early hours after dosing. As expected, allopurinol decreased rate of uric acid excretion. The combination of allopurinol and verinurad did not result in rate of uric acid excretion that was above baseline, which suggests that verinurad + allopurinol treatment may lessen the potential for renal AEs.
Verinurad 10 mg was well tolerated by male subjects with gout when administered as multiple daily doses alone and with concurrent daily doses of allopurinol 300 mg in the fed state. No deaths, other serious AEs, AEs leading to study medication discontinuation, AEs leading to withdrawal from the study, or other significant AEs of interest were reported during the study. There were no safety concerns for any of the serum chemistry, hematology, urinalysis, or coagulation parameters assessed. No clinically significant findings for vital signs, 12‐lead ECGs, or physical examinations performed were reported during the study.
Limitations of the study include the small number of subjects and the short duration. The small number can limit the ability to generalize the results to larger populations. The relatively short duration (7 days) can limit the impact on safety assessments. However, both the small number of subjects and the short duration are standard for this type of study.
In summary, despite an observed DDI between verinurad and oxypurinol, the active metabolite of allopurinol, reduction in serum uric acid was greater with the combination than with either drug alone. The combination was safe and well tolerated. These data support continued development of verinurad and an xanthine oxidase inhibitors as a dual‐mechanism approach for treatment of patients with gout or asymptomatic hyperuricemia. Phase 2 studies employing verinurad in combination with either allopurinol or febuxostat have further evaluated the PK, PD, and safety of combination therapy in patients with this metabolic disorder. An ongoing clinical research study (NCT03118739) with verinurad and febuxostat will be looking for improvements in the kidney or cardiovascular status of patients with hyperuricemia, albuminuria, and type 2 diabetes.
Acknowledgments
Editorial support was provided by Tom Claus, PhD, of PAREXEL and funded by AstraZeneca.
Funding
Funding for this study was provided by Ardea Biosciences/AstraZeneca.
Contributions
Jeffrey N. Miner, Zancong Shen, Sha Liu, Michael Gillen, Vicki Clauson, Susan Walker, and Mai Nguyen were responsible for the conception or design of the work; acquisition, analysis, or interpretation of data for the work; review and revision of manuscript; and final approval of the version to be published. Caroline Lee, Jesse Hall, and Xiaojuan Yang contributed to the analysis and interpretation of data, the review and revision of the manuscript, and the final approval of the version to be published.
Disclosures
M.K. has nothing to disclose. J.H., X.Y., Z.S., C.L., S.L., J.N.M., S.W., V.C., D.W., and M.N. are former employees of Ardea Biosciences, Inc, a member of the AstraZeneca group. M.G. is an employee of AstraZeneca.
This article was presented at the 2016 ACR/ARHP Annual Meeting, November 11‐16, 2016, Washington, DC, as a poster presentation with interim findings. The poster's abstract was published in “Poster Abstracts” in Arthritis Rheum. 2016; 68 (suppl 10): http://acrabstracts.org/abstract/pharmacokinetics-pharmacodynamics-and-tolerability-of-concomitant-multiple-dose-administration-of-verinurad-rdea3170-and-allopurinol-in-adult-male-subjects-with-gout/
References
- 1. Perez‐Ruiz F, Dalbeth N, Bardin T. A review of uric acid, crystal deposition disease, and gout. Adv Ther. 2015;32:31–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Rees F, Hui M, Doherty M. Optimizing current treatment of gout. Nat Rev Rheumatol. 2014;10:271–283. [DOI] [PubMed] [Google Scholar]
- 3. Doherty M, Jansen TL, Nuki G, et al. Gout: why is this curable disease so seldom cured? Ann Rheum Dis. 2012;71:1765–1770. [DOI] [PubMed] [Google Scholar]
- 4. Richette P, Doherty M, Pascual E, et al. 2016 updated EULAR evidence‐based recommendations for the management of gout. Ann Rheum Dis. 2017;76:29–42. [DOI] [PubMed] [Google Scholar]
- 5. Khanna D, Fitzgerald JD, Khanna PP, et al. 2012 American College of Rheumatology guidelines for management of gout. Part 1: systematic nonpharmacologic and pharmacologic therapeutic approaches to hyperuricemia. Arthritis Care Res (Hoboken). 2012;64:1431–1446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Singh JA, Akhras KS, Shiozawa A. Comparative effectiveness of urate lowering with febuxostat versus allopurinol in gout: analyses from large U.S. managed care cohort. Arthritis Res Ther. 2015;17:120–132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Becker MA, Fitz‐Patrick D, Choi HK, et al. An open‐label, 6‐month study of allopurinol safety in gout: The LASSO study. Semin Arthritis Rheum. 2015;45:174–183. [DOI] [PubMed] [Google Scholar]
- 8. AstraZeneca. Zurampic (lesinurad). 2016. http://www.azpicentral.com/zurampic/zurampic.pdf#page=1. Accessed January 6, 2017.
- 9. Reginato AM, Mount DB, Yang I, Choi HK. The genetics of hyperuricaemia and gout. Nat Rev Rheumatol. 2012;8:610–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. So A, Thorens B. Uric acid transport and disease. J Clin Invest. 2010;120:1791–1799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dalbeth N, Jones G, Terkeltaub R, et al. Lesinurad, a selective uric acid reabsorption inhibitor, in combination with febuxostat in patients with tophaceous gout: a phase III clinical trial [published online ahead of print June 9, 2017]. Arthritis Rheumatol. 10.1002/art.40159 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Pascual E, Andres M, Vela P. Gout treatment: should we aim for rapid crystal dissolution? Ann Rheum Dis. 2013;72:635–637. [DOI] [PubMed] [Google Scholar]
- 13. Perez‐Ruiz F, Calabozo M, Pijoan JI, Herrero‐Beites AM, Ruibal A. Effect of urate‐lowering therapy on the velocity of size reduction of tophi in chronic gout. Arthritis Rheum. 2002;47:356–360. [DOI] [PubMed] [Google Scholar]
- 14. Perez‐Ruiz F. Treating to target: a strategy to cure gout. Rheumatology (Oxford). 2009;48(suppl 2):ii9–ii14. [DOI] [PubMed] [Google Scholar]
- 15. Shiozawa A, Buysman EK, Korrer S. Serum uric acid levels and the risk of flares among gout patients in a US managed care setting. Curr Med Res Opin. 2017;33:117–124. [DOI] [PubMed] [Google Scholar]
- 16. Terkeltaub R, Edwards NL. Gout: Diagnosis and Management of Gouty Arthritis and Hyperuricemia. 3rd ed. West Islip, NY: Professional Communications, Inc; 2013. [Google Scholar]
- 17. Tan PK, Liu S, Gunic E, Miner JN. Discovery and characterization of verinurad, a potent and specific inhibitor of URAT1 for the treatment of hyperuricemia and gout. Sci Rep. 2017;7:665–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Shen Z, Gillen M, Miner JN, Bucci G, Wilson D, Hall J. Pharmacokinetics, pharmacodynamics, and tolerability of verinurad, a selective uric acid reabsorption inhibitor, in healthy adult male subjects. Drug Design, Dev Ther. 2017;11:2077–2086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Goldfarb DS, MacDonald PA, Hunt B, Gunawardhana L. Febuxostat in gout: serum urate response in uric acid overproducers and underexcretors. J Rheumatol. 2011;38:1385–1389. [DOI] [PubMed] [Google Scholar]
- 20. Perez‐Ruiz F, Alonso‐Ruiz A, Calabozo M, Herrero‐Beites A, Garcia‐Erauskin G, Ruiz‐Lucea E. Efficacy of allopurinol and benzbromarone for the control of hyperuricaemia. A pathogenic approach to the treatment of primary chronic gout. Ann Rheum Dis. 1998;57:545–549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wallace SL, Robinson H, Masi AT, Decker JL, McCarty DJ, Yu TF. Preliminary criteria for the classification of the acute arthritis of primary gout. Arthritis Rheum. 1977;20:895–900. [DOI] [PubMed] [Google Scholar]
- 22. Guidance for Industry . Bioanalytical Method Validation. US Department of Heath and Human Services Food and Drug Administration. 2001. http://www.fda.gov/downloads/Drugs/Guidance/ucm070107.pdf. Accessed June 28, 2017.
- 23. Obach RS. Potent inhibition of human liver aldehyde oxidase by raloxifene. Drug Metab Dispos. 2004;32:89–97. [DOI] [PubMed] [Google Scholar]
- 24. Colin JN, Farinotti R, Fredj G, et al. Kinetics of allopurinol and oxipurinol after chronic oral administration. Interaction with benzbromarone. Eur J Clin Pharmacol. 1986;31:53–58. [DOI] [PubMed] [Google Scholar]
- 25. Iwanaga T, Kobayashi D, Hirayama M, Maeda T, Tamai I. Involvement of uric acid transporter in increased renal clearance of the xanthine oxidase inhibitor oxypurinol induced by a uricosuric agent, benzbromarone. Drug Metab Dispos. 2005;33:1791–1795. [DOI] [PubMed] [Google Scholar]
- 26. Muller FO, Schall R, Groenewoud G, Hundt HK, van der Merwe JC, van DM. The effect of benzbromarone on allopurinol/oxypurinol kinetics in patients with gout. Eur J Clin Pharmacol. 1993;44:69–72. [DOI] [PubMed] [Google Scholar]
- 27. Perez‐Ruiz F, Sundy JS, Miner JN, Cravets M, Storgard C. Lesinurad in combination with allopurinol: results of a phase 2, randomised, double‐blind study in patients with gout with an inadequate response to allopurinol. Ann Rheum Dis. 2016;75:1074–1080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Liu S, Perez‐Ruiz F, Miner JN. Patients with gout differ from healthy subjects in renal response to changes in serum uric acid. Joint Bone Spine. 2016;84:183–188. [DOI] [PubMed] [Google Scholar]


