Abstract
Aims
To compare the efficacy and safety of once‐weekly glucagon‐like peptide‐1 receptor agonist dulaglutide 1.5 and 0.75 mg with glimepiride in East‐Asian patients with type 2 diabetes (T2D).
Materials and methods
In this phase III, multinational, multicentre, double‐blind, randomized, parallel‐arm, 26‐week study, patients with inadequate glycaemic control were randomized 1:1:1 to once‐weekly dulaglutide 1.5 or 0.75 mg or daily glimepiride (1‐3 mg/d). The primary endpoint was assessment of the non‐inferiority of dulaglutide (1.5 mg), as measured by change in glycated haemoglobin (HbA1c), compared with glimepiride using a 0.4% non‐inferiority margin.
Results
A total of 737 patients were randomized (dulaglutide 1.5 mg, n = 244; dulaglutide 0.75 mg, n = 248; glimepiride, n = 245). At week 26, both doses of dulaglutide were non‐inferior and also superior to glimepiride for HbA1c reduction from baseline with a least squares mean difference of −6.34 mmol/mol (95% confidence interval [CI] −8.31, −4.26) or ‐0.58% (95% CI −0.76, −0.39) for dulaglutide 1.5 mg and −3.50 mmol/mol (95% CI −5.47, −1.42) or −0.32% (95% CI −0.50, −0.13) for dulaglutide 0.75 mg (P < .001). A greater proportion of patients in the dulaglutide 1.5 mg group achieved the HbA1c target of <53 mmol/mol (<7.0%) compared with the glimepiride group (74.1% vs 57.4%; P < .001). The mean body weight decreased (P < .005) and total hypoglycaemia rates were lower (P < .001) in the dulaglutide groups compared with the glimepiride group. The most common drug‐related adverse events in both dulaglutide groups (≥5% of patients) included diarrhoea, nausea, increased lipase, decreased appetite, abdominal distension and vomiting.
Conclusions
Dulaglutide (both doses) demonstrated superior glycaemic control vs glimepiride, with a favourable tolerability and safety profile in East‐Asian patients with T2D.
Keywords: dulaglutide, glimepiride, glucagon‐like peptide‐1 receptor agonist, HbA1c, type 2 diabetes
1. INTRODUCTION
The prevalence of type 2 diabetes (T2D) has been increasing at an alarming rate in East‐Asian countries over recent decades.1 A national epidemiological survey in 2010 on diabetes among Chinese adults revealed that the estimated overall prevalence of diabetes was ~11.6%.2 Faced with this huge population‐based challenge, emerging antidiabetic agents must not only provide robust blood glucose (BG) control, but also enhance adherence to treatment and effectively prevent the micro‐ and macrovascular complications of diabetes.3, 4
Glucagon‐like peptide‐1 receptor agonists (GLP‐1RAs), with their unique pharmacological effects, including enhancement of insulin secretion in a glucose‐dependent pattern, delay of gastric emptying and diminishment of appetite, have demonstrated efficacy with regard to glycated haemoglobin (HbA1c) reduction, with a lower risk of hypoglycaemia and weight loss.4, 5 Importantly, significant cardiovascular benefits have been demonstrated in dedicated cardiovascular outcome studies of several GLP‐1RAs with high homology.6, 7, 8 These accumulated results suggest a better risk‐to‐benefit ratio of GLP‐1RAs compared with traditional antidiabetic drugs, such as glimepiride, which is currently widely used across East Asia.9, 10
Dulaglutide, a once‐weekly GLP‐1RA approved for the treatment of T2D, consists of two identical disulfide‐linked chains, each containing an N‐terminal glucagon‐like peptide‐1 (GLP‐1) analogue sequence covalently linked to a modified human immunoglobulin G4 Fc fragment by a small peptide linker. The GLP‐1 analogue portion of dulaglutide is 90% homologous to native human GLP‐1 and contains amino acid substitutions designed to optimize its clinical profile, including protection from dipeptidyl peptidase‐4 (DPP‐4) inactivation, increased solubility and reduction of immunogenicity. In addition, the larger size of dulaglutide slows absorption and reduces renal clearance. These features result in a soluble formulation and a prolonged half‐life of ~5 days, making it suitable for once‐weekly subcutaneous (s.c.) administration.4, 11, 12
In phase II studies, dulaglutide demonstrated significant dose‐dependent improvements in glycaemic control and body weight, and a low rate of hypoglycaemia.13, 14 In a completed global phase III study, AWARD‐3 (Assessment of Weekly AdministRation of Dulaglutide), dulaglutide demonstrated significant HbA1c reductions as monotherapy, with both fasting and postprandial glucose improvements, and weight loss.15 The present study is the first to examine the efficacy and safety of dulaglutide (0.75 and 1.5 mg) as monotherapy compared with glimepiride in East‐Asian patients with T2D who had inadequate glycaemic control after treatment with lifestyle modifications and were either oral antihyperglycaemic medication (OAM)‐naïve or on OAM monotherapy.
2. MATERIALS AND METHODS
2.1. Study design
This was a randomized, double‐blind, double‐dummy, parallel‐arm, active comparator, non‐inferiority phase III study conducted over 26 weeks at 48 centres in three East‐Asian countries and regions (China, South Korea and Taiwan). The study consisted of four periods: screening (2 weeks), lead‐in (2 weeks; during which patients discontinued any previous OAMs), treatment (26 weeks), and a post‐treatment safety follow‐up (30 days; Figure S1). The study (http://clinicaltrials.gov: NCT01644500) was conducted in accordance with the Declaration of Helsinki,16 Good Clinical Practice guidelines, and applicable laws and regulations. The ethical review board approved the protocol before patient enrolment, and written informed consent was obtained from all patients.
2.2. Study participants
Eligible patients included men and non‐pregnant, non‐breastfeeding women aged ≥18 years (≥20 years in Taiwan) with a body mass index of 19 to 35 kg/m2 and a diagnosis of T2D, and patients who were OAM‐naïve with an HbA1c concentration at screening of 53 to 91 mmol/mol (7.0−10.5%), or patients who were taking OAM monotherapy for at least 3 months before screening with an HbA1c concentration at screening of 48 to 86 mmol/mol (6.5−10.0%).
Exclusion criteria included: type 1 diabetes; a prescription for incretin‐based medications (such as GLP‐1RA and DPP‐4 inhibitors); thiazolidinediones and insulin <3 months before screening; unstable body weight between screening and randomization; a history of clinically significant gastric emptying abnormality, hepatitis, clinical signs or symptoms of pancreatitis and liver disease; or impaired renal function (serum creatinine concentration ≥ 133 µmol/L for men or ≥ 124 µmol/L mg/dL for women, or creatinine clearance <60 mL/min) and elevated serum calcitonin concentration (> 20 ng/L) at screening.
2.3. Study treatment
Eligible patients were randomly assigned (1:1:1) to one of three double‐blind groups according to a computer‐generated random sequence using an interactive voice response system: once‐weekly dulaglutide 1.5 mg (s.c.), once‐weekly dulaglutide 0.75 mg (s.c.), or daily glimepiride capsules 1 to 3 mg/d (orally). Randomization was stratified by country or region, baseline HbA1c (<69 mmol/mol or ≥ 69 mmol/mol [<8.5% or ≥8.5%]), and pre‐study OAM. Patients, investigators and clinical trial staff members were masked to treatment assignment throughout the study. The double‐dummy design was accomplished by use of placebo formulations. Patients in the dulaglutide group received oral placebo capsules each day. Patients in the glimepiride group received a single placebo injection (s.c.) each week.
Patients in the glimepiride group received 1 glimepiride capsule/d (1 mg) at randomization. If no tolerability or safety issues occurred, the dose of glimepiride was increased to 2 capsules/d at visit 5 (week 4) and to 3 capsules/day at visit 6 (week 8), and subsequently maintained at that level if tolerated. Before randomization, participants were taught injection techniques and glucose monitoring. In addition, a trained caregiver could assist patients with their study drug injections.
2.4. Study endpoints
The primary efficacy endpoint was change in HbA1c from baseline at week 26 between once‐weekly 1.5 mg dulaglutide and once‐daily glimepiride, with a 0.4% non‐inferiority margin. Key secondary efficacy endpoints assessed at week 26 included demonstration of non‐inferiority of dulaglutide 0.75 mg to glimepiride and superiority of dulaglutide (both doses) to glimepiride in achieving glycaemic control, as measured by change in HbA1c from baseline. Other efficacy measures at week 26 included the proportion of patients attaining HbA1c <53 mmol/mol or ≤ 48 mmol/mol (<7% or ≤ 6.5%), fasting BG (FBG), 7‐point self‐monitored BG (SMBG) profiles, BG excursions, β‐cell function and insulin sensitivity assessed using the updated version of homeostasis model assessment (HOMA2; calculated using BG, insulin and C‐peptide concentrations).
Safety and tolerability were evaluated throughout by the assessment of weight change, hypoglycaemic episodes, serious adverse events (SAEs), treatment‐emergent adverse events (TEAEs), discontinuations attributable to adverse events (AEs), laboratory tests, vital signs, 12‐lead electrocardiograms, dulaglutide antidrug antibodies (ADAs), and allergic/hypersensitivity reactions. All cases of definite or possible acute pancreatitis were adjudicated by an independent committee of expert physicians. Deaths (from any cause) and non‐fatal cardiovascular AEs were adjudicated by a committee of external physicians with cardiology expertise.
2.5. Statistical analyses
The study was designed with 90% power to confirm non‐inferiority of dulaglutide 1.5 mg vs glimepiride (1‐3 mg/d) for change in HbA1c from baseline to 26 weeks with a non‐inferiority margin of 0.4%, standard deviation (SD) of 1.3, and one‐sided significance level of .025, assuming no true difference between treatments. This corresponds to 223 patients/group with an assumed dropout rate of 15%. If non‐inferiority was achieved, tree‐gatekeeping17 was used to control the type 1 error rate at .025 while assessing the superiority of dulaglutide 1.5 mg vs glimepiride, and non‐inferiority and superiority of dulaglutide 0.75 mg vs glimepiride for change in HbA1c from baseline to 26 weeks. Two‐sided 95% confidence intervals (CIs) were included in the presentation of the results.
Efficacy analyses were conducted on the modified intention‐to‐treat (mITT) analysis set, which included all randomized patients who had a baseline HbA1c measurement and ≥ 1 postbaseline HbA1c measurement and received ≥1 dose of study drug. Safety analyses were conducted on an as‐treated analysis set (safety analysis set) which included patients who received ≥1 dose of study drug and were analysed according to the treatment they received, regardless of their planned treatment.
A mixed‐model repeated measure (MMRM) analysis using the mITT analysis set was used as the primary analysis, with change in HbA1c as the dependent variable, treatment, country or region, pre‐study therapy, visit and treatment by visit interaction as fixed effects, baseline HbA1c as a covariate, and patient as a random effect. The MMRM included HbA1c measurements from all post‐baseline visits. Imputation of missing HbA1c data was not performed. Other efficacy and safety markers (continuous variables) were assessed using an MMRM model similar to that used for the primary analysis. Categorical variables were assessed using Fisher's exact test from an analysis of variance model. TEAEs were coded using the Medical Dictionary for Regulatory Activities (version 19.1). Type 1 error was controlled for primary and gated secondary objectives. As per convention, P values <.05 were taken to indicate statistical significance, but that should be mostly interpreted as descriptive.
3. RESULTS
3.1. Patient disposition and baseline characteristics
Of 1150 patients screened, 737 eligible patients were randomized (1:1:1) to one of three treatment groups. Overall, 61 patients discontinued the study, with 676 (91.7%) patients completing the study treatment (Figure 1).
Figure 1.
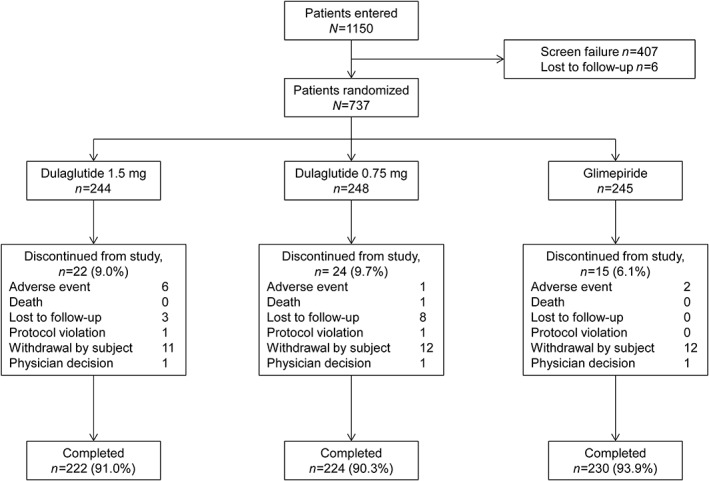
Patient disposition. N, total population; n, number of patients in each category
Patient demographics and baseline characteristics are described in Table 1. A total of 54.3% of participants were men. The mean (SD) HbA1c was 63 (11) mmol/mol or 8.0 (1.00)% and the mean (SD) duration of T2D was 3.7 (4.20) years. A total of 56.9% of patients had been previously treated with ≥1 OAM. The percentage of patients with previous OAMs was similar among treatment groups (Table S1). Baseline characteristics were similar in the three treatment groups.
Table 1.
Patient demographics and baseline characteristics
| Characteristics | Dulaglutide 1.5 mg N = 239 | Dulaglutide 0.75 mg N = 239 | Glimepiride N = 242 | Total N = 720 | P‐value* |
|---|---|---|---|---|---|
| Gender, n | 239 | 239 | 242 | 720 | .794 |
| Female, n (%) | 105 (43.9) | 112 (46.9) | 112 (46.3) | 329 (45.7) | |
| Male, n (%) | 134 (56.1) | 127 (53.1) | 130 (53.7) | 391 (54.3) | |
| Age, years, n | 239 | 239 | 242 | 720 | .150 |
| Mean (SD) | 52.7 (10.75) | 53.8 (10.09) | 52.0 (10.05) | 52.8 (10.32) | |
| Age group, n | 239 | 239 | 242 | 720 | .132 |
| <65 years, n (%) | 213 (89.1) | 208 (87.0) | 224 (92.6) | 645 (89.6) | |
| ≥65 years, n (%) | 26 (10.9) | 31 (13.0) | 18 (7.4) | 75 (10.4) | |
| Country/region, n | 239 | 239 | 242 | 720 | >.999 |
| China, n (%) | 184 (77.0) | 186 (77.8) | 186 (76.9) | 556 (77.2) | |
| Korea, n (%) | 26 (10.9) | 25 (10.5) | 27 (11.2) | 78 (10.8) | |
| Taiwan, n (%) | 29 (12.1) | 28 (11.7) | 29 (12.0) | 86 (11.9) | |
| BMI, kg/m2, n | 239 | 238 | 242 | 719 | .213 |
| Mean (SD) | 25.8 (3.43) | 26.2 (3.49) | 25.7 (3.14) | 25.9 (3.36) | |
| HbA1c, n | 239 | 239 | 242 | 720 | .317 |
| Mean (SD), mmol/mol | 63.6 (10.42) | 64.04 (11.27) | 62.51 (11.07) | 63.38 (10.93) | |
| Mean (SD), % | 8.0 (0.95) | 8.0 (1.03) | 7.9 (1.01) | 8.0 (1.00) | |
| HbA1c group, n | 239 | 239 | 242 | 720 | .506 |
| <69 mmol/mol (<8.5%), n (%) | 171 (71.5) | 165 (69.0) | 179 (74.0) | 515 (71.5) | |
| ≥69 mmol/mol (≥8.5%), n (%) | 68 (28.5) | 74 (31.0) | 63 (26.0) | 205 (28.5) | |
| Duration of T2D, years, n | 239 | 239 | 241 | 719 | .383 |
| Mean (SD) | 4.0 (4.44) | 3.5 (4.06) | 3.8 (4.09) | 3.7 (4.20) | |
| History of ≥1 previous OAM, n | 239 | 239 | 242 | 720 | .977 |
| Yes, n (%) | 135 (56.5) | 136 (56.9) | 139 (57.4) | 410 (56.9) | |
| No, n (%) | 104 (43.5) | 103 (43.1) | 103 (42.6) | 310 (43.1) | |
| Current alcohol use, n | 239 | 239 | 241 | 719 | .139 |
| Yes, n (%) | 56 (23.4) | 43 (18.0) | 40 (16.6) | 139 (19.3) | |
| No, n (%) | 183 (76.6) | 196 (82.0) | 201 (83.4) | 580 (80.7) | |
| Current tobacco use, n | 237 | 239 | 241 | 717 | .032 |
| Yes, n (%) | 70 (29.5) | 46 (19.2) | 60 (24.9) | 176 (24.5) | |
| No, n (%) | 167 (70.5) | 193 (80.8) | 181 (75.1) | 541 (75.5) | |
| Vital signs (sitting position), n | 239 | 239 | 242 | 720 | |
| Systolic BP, mm Hg, Mean (SD) | 128 (13.3) | 128 (13.7) | 126 (14.7) | 127 (13.9) | 0.260 |
| Diastolic BP, mm Hg, Mean (SD) | 79 (8.7) | 79 (9.3) | 78 (8.6) | 78 (8.8) | 0.312 |
| Pulse rate, bpm, Mean (SD) | 76 (9.6) | 75 (9.3) | 77 (9.7) | 76 (9.5) | 0.131 |
Abbreviations: BMI, body mass index; BP, blood pressure; bpm, beats per minute; HbA1c, glycated haemoglobin; N, total number of patients in specified treatment group; n, number of patients in specified category; OAM, oral antidiabetic medication; T2D, type 2 diabetes.
P‐value is from Fisher's exact test for categorical variables, and is from an analysis of variance (ANOVA) model for continuous variables.
3.2. Efficacy variables
Of the 737 patients randomized, 720 patients comprised the mITT population. At week 26, the least‐squares mean (LSM; [SE]) change from baseline in HbA1c was greater with dulaglutide 1.5 mg (−16.18 [0.75] mmol/mol or −1.48 [0.07]%) and dulaglutide (−13.34 [0.75] mmol/mol or −1.22 [0.07]%) than with glimepiride (−9.84 [0.75] mmol/mol or −0.90 [0.07]%). The LSM for the difference of dulaglutide vs glimepiride was −6.34 mmol/mol (95% CI −8.31, −4.26) or −0.58% (95% CI –0.76, −0.39) for dulaglutide 1.5 mg and −3.50 mmol/mol (95% CI −5.47, −1.42) or − 0.32% (95% CI –0.50, −0.13) for dulaglutide 0.75 mg at week 26 (Figure 2A). Overall, the HbA1c reduction with both doses of dulaglutide was non‐inferior (P < .001) and superior (P < .001) to that achieved with glimepiride. Figure 2B shows LSM (SE) change in HbA1c by visit from baseline to week 26 in all treatment groups. The HbA1c reduction was significantly greater with both doses of dulaglutide compared with glimepiride in both OAM‐naïve patients and those on OAM monotherapy (P < .05; Figure S2).
Figure 2.
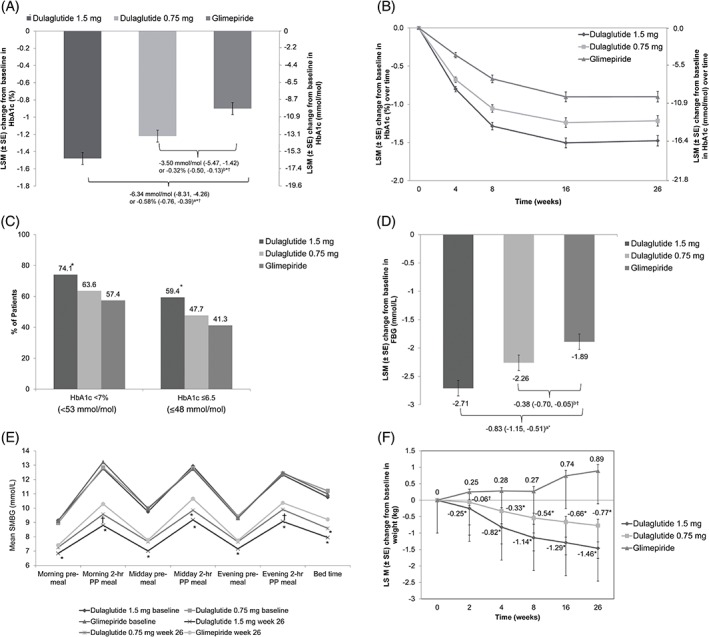
Efficacy and safety outcome measures through the treatment period. A, Change in glycated haemoglobin (HbA1c) from baseline to 26 weeks. aLeast Square Mean (LSM) difference (95% confidence interval [CI]) of dulaglutide 1.5 mg with glimepiride. bLSM difference (95% CI) of dulaglutide 0.75 mg with glimepiride. *Dulaglutide non‐inferior to glimepiride (1‐sided P value <.001). †Dulaglutide superior to glimepiride (2‐sided P value <.001). B, Change in HbA1c from baseline over time. C, Percentage of patients achieving HbA1c targets at week 26. *P < .001 dulaglutide vs glimepiride. D, Change in fasting blood glucose (FBG) from baseline to week 26. aLSM difference (95% CI) of dulaglutide 1.5 mg with glimepiride. bLSM difference (95% CI) of dulaglutide 0.75 mg with glimepiride. * P < .001 dulaglutide vs glimepiride. † P < .05 dulaglutide vs glimepiride. E, Seven‐point self‐monitored blood glucose (SMBG) profiles by time of day. PP, postprandial. *P < .001 dulaglutide vs glimepiride. † P < .05 dulaglutide vs glimepiride. F, Change in body weight from baseline to 26 weeks. *P < .001 dulaglutide vs glimepiride. † P < .05 dulaglutide vs glimepiride. Abbreviation: SE, standard error
At week 26, a significantly greater proportion of patients in the dulaglutide 1.5 mg group compared with the glimepiride group achieved a decrease in HbA1c level to <53 mmol/mol or <7.0% (74.1% vs 57.4%; P < .001) and to ≤ 48 mmol/mol or ≤6.5% (59.4% vs 41.3%; P < .001). The proportion of patients achieving HbA1c of <53 mmol/mol or ≤48 mmol/mol (<7% or ≤6.5%) at week 26 did not differ significantly between the dulaglutide 0.75 mg and glimepiride groups, although the proportion was numerically greater in the dulaglutide 0.75 mg group (Figure 2C).
The LSM (SE) change in FBG from baseline to week 26 was significantly greater (P < .05) with dulaglutide than with glimepiride: −2.71 (0.14) and − 2.26 (0.14) mmol/L with dulaglutide 1.5 mg and 0.75 mg, respectively, vs −1.89 (0.14) mmol/L with glimepiride (Figure 2D).
The mean BG values on the 7‐point SMBG profile at week 26 decreased at each time point compared with baseline in all treatment groups (Figure 2E). At week 26, the change from baseline in 7‐point SMBG values was statistically greater for dulaglutide 1.5 mg compared with glimepiride at all time points (P < .001; Table S2). Reductions in BG levels from baseline in 7‐point SMBG were also significantly greater with dulaglutide 0.75 mg compared with glimepiride at morning 2‐hour postprandial, midday 2‐hour postprandial, evening 2‐hour postprandial, and bed time assessments (all P < .05; Table S2).
Patients receiving dulaglutide 1.5 mg had greater reductions in the mean change of all 2‐hour postprandial glucose (PPG) excursions compared with glimepiride (P < .001); while changes in evening 2‐hour PPG excursions were similar between the dulaglutide 0.75 mg and glimepiride groups (P = .052). The decreases in morning (P = .017) and midday (P < .001) 2‐hour PPG excursions were significantly greater with dulaglutide 0.75 mg than with glimepiride (Table S2).
At week 26, significantly greater increases in insulin‐ and C‐peptide‐based HOMA2 for β‐cell function were observed with both dulaglutide groups compared with glimepiride (P < .05), and a significant decrease in C‐peptide‐based HOMA2 for insulin sensitivity was noticed in the dulaglutide 0.75 mg group (P = .007; Table S3).
3.3. Safety and tolerability
Two of the 737 randomized patients did not receive the study drug (glimepiride) and were excluded from the safety analysis population.
Overall, 60.8% of patients (447/735) experienced at least 1 TEAE by the end of 26 weeks (Table 2). Among these patients, 66.9% (299/447) experienced mild TEAEs and 17.9% (80/447) experienced moderate TEAEs. The most frequently reported TEAEs (≥5% of patients in any group) were hyperlipidaemia, diarrhoea, nasopharyngitis, nausea, increased lipase levels, decreased appetite, abdominal distension and vomiting (Table 2).
Table 2.
Safety assessments
| Dulaglutide 1.5 mg | Dulaglutide 0.75 mg | Glimepiride | Total | Overall P valuea | |
|---|---|---|---|---|---|
| N = 244 | N = 248 | N = 243 | N = 735 | ||
| n (%) | n (%) | n (%) | n (%) | ||
| Patients with ≥1 TEAE | 166 (68.0) | 142 (57.3) | 139 (57.2) | 447 (60.8) | .018 |
| Patients with ≥1 SAE | 6 (2.5) | 4 (1.6) | 3 (1.2) | 13 (1.8) | .612 |
| Patients who discontinued treatment because of a TEAE | 9 (3.7) | 5 (2.0) | 3 (1.2) | 17 (2.3) | .203 |
| Patients who died on therapy | 0 (0.0) | 1 (0.4) | 0 (0.0) | 1 (0.1) | >.999 |
| Patients with ≥1 drug‐related TEAE | 90 (36.9) | 53 (21.4) | 21 (8.6) | 164 (22.3) | <.001 |
| TEAEs (in ≥5% patients) | |||||
| Hyperlipidaemiab | 27 (11.1) | 30 (12.1) | 41 (16.9) | 98 (13.3) | .146 |
| Diarrhoea | 42 (17.2) | 18 (7.3) | 7 (2.9) | 67 (9.1) | <.001 |
| Nasopharyngitis | 16 (6.6) | 13 (5.2) | 13 (5.3) | 42 (5.7) | .814 |
| Nausea | 25 (10.2) | 13 (5.2) | 1 (0.4) | 39 (5.3) | <.001 |
| Lipase increased | 15 (6.1) | 14 (5.6) | 6 (2.5) | 35 (4.8) | .108 |
| Decreased appetite | 15 (6.1) | 13 (5.2) | 1 (0.4) | 29 (3.9) | <.001 |
| Abdominal distension | 16 (6.6) | 6 (2.4) | 5 (2.1) | 27 (3.7) | .019 |
| Vomiting | 19 (7.8) | 4 (1.6) | 1 (0.4) | 24 (3.3) | <.001 |
| All hypoglycaemic episodes | |||||
| Number of episodes | 16 | 11 | 84 | 111 | |
| Incidencea, n (%) | 14 (5.7)* | 9 (3.6)* | 38 (15.6) | 61 (8.3) | <.001 |
| Ratec, events/patient/year, Mean (SD) | 0.14 (0.64)* | 0.09 (0.50)* | 1.13 (7.07) | <.001 | |
| Severe hypoglycaemic episodesd | |||||
| Number of episodes | 0 | 0 | 0 | 0 | |
| Incidencea, n (%) | 0 (0.0) | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA |
| Nocturnal hypoglycaemic episodese | |||||
| Number of episodes | 1 | 1 | 9 | 11 | |
| Incidencea, n (%) | 1 (0.4) | 1 (0.4) | 5 (2.1) | 7 (1.0) | .190 |
| Documented symptomatic hypoglycaemic episodesf | |||||
| Number of episodes | 2 | 2 | 34 | 38 | |
| Incidencea, n (%) | 2 (0.8)* | 2 (0.8)* | 15 (6.2) | 19 (2.6) | <.001 |
| Asymptomatic hypoglycaemic episodesg | |||||
| Number of episodes | 7 | 4 | 33 | 44 | |
| Incidencea, n (%) | 6 (2.5)* | 3 (1.2)* | 26 (10.7) | 35 (4.8) | <.001 |
| Probable hypoglycaemic episodesh | |||||
| Number of episodes | 7 | 5 | 17 | 29 | |
| Incidencea, n (%) | 7 (2.9) | 4 (1.6)* | 12 (4.9) | 23 (3.1) | .109 |
| Patients with treatment‐emergent pancreatic enzymes >1 × ULN | |||||
| Pancreatic amylase | 12 (5.0) | 11 (4.6) | 2 (0.8) | ||
| Total amylase | 14 (5.9) | 4 (1.7) | 5 (2.1) | ||
| Lipase | 43 (18.0) | 38 (15.9) | 18 (7.4) | ||
| Patients with treatment‐emergent pancreatic enzymes >3 × ULN | |||||
| Pancreatic amylase | 0 | 0 | 0 | ||
| Total amylase | 0 | 0 | 0 | ||
| Lipase | 3 (1.3) | 3 (1.3) | 3 (1.2) | ||
Abbreviations: AE, adverse event; N, number of patients in the analyses population in specified treatment arm; OAM, oral anti‐hyperglycaemic medication; PG, plasma glucose; SAE, serious adverse event; TEAE, treatment‐emergent adverse event; ULN, upper limit of normal.
P ≤ .05 dulaglutide vs glimepiride.
P value is calculated based on Fisher's exact test.
Lipid profile was measured at randomization (visit 3) for the first time with the TEAE collected from lead‐in phase, leading to the high incidence of hyperlipidaemia.
Overall P value is based on a negative binomial regression model: patient's hypoglycaemia count = OAM strata + treatment + country/region, with log of patient's total number of days of exposure/30 as an offset variable.
Severe hypoglycaemia: an episode requiring the assistance of another person to actively administer carbohydrate, glucagon, or other resuscitative actions. These episodes may be associated with sufficient neuroglycopenia to induce seizure or coma. Plasma glucose measurements may not be available during such an event, but neurological recovery attributable to the restoration of PG to normal is considered sufficient evidence that the event was induced by a low PG concentration.
Nocturnal hypoglycaemia: any hypoglycaemic event that occurs between bedtime and waking.
Documented symptomatic hypoglycaemia: any time a patient feels that he/she is experiencing symptoms and/or signs associated with hypoglycaemia and has a PG concentration of ≤3.9 mmol/L (≤70 mg/dL).
Asymptomatic hypoglycaemia: an event not accompanied by typical symptoms of hypoglycaemia but with a measured PG concentration of ≤3.9 mmol/L (≤70 mg/dL).
Probable hypoglycaemia: an event during which symptoms of hypoglycaemia are not accompanied by a PG determination (but that was presumably caused by a PG concentration of ≤3.9 mmol/L [≤70 mg/dL]).
During the 26‐week treatment period, 17 patients (2.3%) discontinued study treatment because of AEs (Table 2). The incidence of TEAEs leading to discontinuation was similar among the three treatment groups (Table 2).
During the 26‐week treatment period, 13 patients (1.8%) reported SAEs; six patients (2.5%) in the dulaglutide 1.5 mg group, four patients (1.6%) in the dulaglutide 0.75 group, and three patients (1.2%) in the glimepiride group (Table S4). One death occurred in the dulaglutide 0.75 mg group but was a result of intentional injury and was not considered to be related to the study drug (Table 2).
The most frequently reported drug‐related TEAEs corresponded to the gastrointestinal system organ class: diarrhoea (6.7%), nausea (4.2%), abdominal distension (3.3%) and vomiting (2.7%), with a higher incidence reported in the dulaglutide groups. The incidence of gastrointestinal TEAEs was high during the first 2 weeks of dulaglutide treatment but decreased gradually from the fourth week of dulaglutide treatment (data not shown).
A total of 61 (8.3%) patients (dulaglutide 1.5 mg, n = 14 [5.7%]; dulaglutide 0.75 mg, n = 9 [3.6%]; glimepiride, n = 38 [15.6%]) experienced any hypoglycaemia during the 26 weeks, with notably fewer patients in both dulaglutide groups compared with the glimepiride group. The mean rates of total hypoglycaemia were 0.14, 0.09 and 1.13 events/patient/year in the dulaglutide 1.5 mg, dulaglutide 0.75 mg and glimepiride groups, respectively. No episodes of severe hypoglycaemia were reported during this study (Table 2).
Over the 26‐week treatment period, patients in both dulaglutide groups experienced weight loss, while those in the glimepiride group gained weight (Figure 2F). At week 26, the LSM (SE) change from baseline in body weight for dulaglutide 1.5 mg, dulaglutide 0.75 mg and glimepiride was −1.46 (0.192) kg, −0.77 (0.192) kg and 0.89 (0.190) kg, respectively.
At week 26, a numerically greater reduction from baseline in systolic blood pressure (~2‐5 mm Hg) was observed in both dulaglutide groups. The mean pulse rate increased in both dulaglutide groups (0.75‐3.76 beats per minute [bpm]) and decreased in the glimepiride group (0.07‐0.22 bpm). At week 26, a notably greater increase in heart rate from baseline was reported in both dulaglutide groups (1.90‐3.99 bpm) compared with the glimepiride group (0.44 bpm). Also, a notable difference was reported in PR interval among the three groups, with an increase in both dulaglutide groups (3.29‐3.73 milliseconds) and a decrease in the glimepiride group (0.23 milliseconds).
Two patients in the dulaglutide groups had adjudicated cardiovascular events of lacunar infarction and transient ischaemic attack. Of these, only the event of transient ischaemic attack was confirmed upon adjudication.
There were no cases of adjudicated acute or chronic pancreatitis during the study. At week 26, notably greater mean changes in p‐amylase, total amylase and lipase were observed with dulaglutide compared with glimepiride (Table S5). No patient in any group had pancreatic amylase and total amylase levels of >3 × the upper limit of normal at week 26, whereas three patients in each group had lipase levels of >3 × the upper limit of normal during the study (Table 2).
The mean change in serum calcitonin levels was negligible in all three treatment groups during the 26‐week treatment period. No cases of thyroid neoplasms, C‐cell hyperplasia, or medullary thyroid carcinoma were reported during this study.
Twenty‐five patients (5.1%) developed treatment‐emergent dulaglutide ADAs at least once during the study. In 22 of these patients, no dulaglutide ADAs were observed at baseline but they developed ADAs post‐baseline, with the highest titre being 1:64.
Two patients (one in each dulaglutide group) experienced mild urticaria. Of the 13 patients who reported hypersensitivity reactions, five patients (dulaglutide 1.5 mg, n = 1; dulaglutide 0.75 mg, n = 3; glimepiride, n = 1) had study drug‐related hypersensitivity reactions. None of these patients developed treatment‐emergent dulaglutide ADAs.
4. DISCUSSION
The present double‐blind, double‐dummy study showed that monotherapy with either dulaglutide 1.5 or 0.75 mg was associated with a significantly greater decrease from baseline in HbA1c than with glimepiride (P < .001) after 26 weeks of treatment in East‐Asian patients with early stage T2D. Sensitivity analysis of the per‐protocol population confirmed the results of the primary objective of our study (data not included).
It is noteworthy that this greater HbA1c reduction occurred without an increased risk of hypoglycaemia (−16.18 mmol/mol [−1.48%] for dulaglutide 1.5 mg, −13.34 mmol/mol [−1.22%] for dulaglutide 0.75 mg) compared with the global AWARD‐3 study,15 which had a similar study design but included primarily white patients (−0.78% for dulaglutide 1.5 mg, −0.71% for dulaglutide 0.75 mg). Possible explanations for the observation of greater HbA1c reductions with dulaglutide monotherapy in the present study include: (i) a lower baseline mean body mass index (26.1 kg/m2) compared with AWARD‐3 (33.5 kg/m2), which may have led to a larger drug exposure in East‐Asian patients with acceptable safety profiles15, 18; (ii) a larger proportion of OAM‐naïve T2D patients19, 20, 21 and/or a higher mean HbA1c at baseline,22, 23, 24 which are well‐established baseline factors positively associated with HbA1c responses to antidiabetic drugs in clinical research; or (iii) as fewer resources for diabetic education are available in China compared with Western countries, the intensified diabetes self‐management might have played a role in the improved glycaemic control observed in the present study.25, 26
The 7‐point SMBG profiles showed significant reductions in BG at all time points from baseline to week 26 in the dulaglutide 1.5 mg group compared with the glimepiride group in the fasting and pre‐meal test points. In addition, decreases from baseline in PPG excursions for the mean morning, midday and evening assessments were all notably greater for dulaglutide 1.5 mg compared with glimepiride.
Similarly to earlier dulaglutide studies, the percentage of patients achieving HbA1c targets occurred with dulaglutide in a dose‐dependent manner.27, 28, 29, 30 The proportion of patients in the dulaglutide 1.5 mg group achieving an HbA1c target of <53 mmol/mol (<7%) and ≤48 mmol/mol (≤6.5%) at week 26 was 74.1% and 59.4% respectively, which was higher than monotherapy studies evaluating other GLP‐1RAs in both a global population31 and in Asian patients.32, 33 At week 26, reductions in mean body weight (0.8 to 1.5 kg) were observed in the dulaglutide groups and an increase (0.9 kg) was noted in the glimepiride group. These findings are in accordance with another phase III study evaluating other GLP‐1RAs vs glimepiride in Asian patients with T2D.32 At week 26, both dulaglutide groups had significantly greater reductions from baseline in FBG compared with glimepiride.
The safety profile of dulaglutide in the present study is consistent with previous data from AWARD studies15, 27, 28, 29, 30, 34, 35 and other compounds in the GLP‐1RA class.36, 37 The most common drug‐related AEs reported in the present study were gastrointestinal (eg, diarrhoea or nausea), which were transient and rarely led to treatment discontinuation. A similar pattern of pancreatic enzyme increase associated with dulaglutide15, 27, 28, 29, 30, 34, 35 and the GLP‐1RA class of drugs38 was also observed in the present study. Small elevations in the mean concentration of pancreatic enzymes within a normal range were observed over time. Several previous reports on GLP‐1RAs have suggested that the elevation in pancreatic enzymes is not predictive of pancreatitis.39 In the present study, no case of pancreatitis was reported by an investigator or adjudicated by an independent committee of expert physicians. The immunogenicity of dulaglutide was low, with 5.1% of patients developing treatment‐emergent dulaglutide ADAs, which is lower than with other GLP‐1RAs.33 No systemic hypersensitivity reaction was reported. No new safety concerns were identified for dulaglutide in the present study beyond those previously described.15, 35
The present study has some limitations. The short washout period of only 2 weeks, because of ethical considerations, is associated with unstable baseline HbA1c in patients taking OAMs before study entry, probably leading to an underestimation of HbA1c reduction from baseline in each single arm; however, our study primarily evaluated the treatment difference between dulaglutide and glimepiride, which is comparable with or without a washout period. The 26‐week treatment period is relatively short for the assessment of glycaemic control considering the chronic nature of T2D. Prospective long‐term studies are required to assess the durability of the observations noted in this study. Glimepiride 3 mg/d was set as the maximum dose in this study considering the early stage of T2D for eligible patients. Greater HbA1c reduction might be achieved with a higher glimepiride dose, but previous studies have indicated that this could put patients at greater risk of hypoglycaemia with limited additional efficacy.40, 41, 42
In conclusion, this 26‐week double‐blind study in East‐Asian patients with T2D showed that both doses of once‐weekly dulaglutide resulted in improved glycaemic control and a higher percentage of patients achieving the target, while also attaining a reduction in body weight and a substantially lower risk of hypoglycaemia compared with glimepiride. Both doses of dulaglutide were well tolerated and the safety profile of dulaglutide was similar to the GLP‐1RA class of drugs, suggesting a favourable benefit‐to‐risk profile for dulaglutide. The use of once‐weekly dulaglutide monotherapy can therefore be considered as a viable and clinically appropriate treatment option for East‐Asian patients with T2D.
Supporting information
Figure S1. Study design
Abbreviations: GLP‐1 RA, glucagon‐like peptide‐1 receptor agonist; LV30, poststudy therapy safety follow‐up visit; OAM, oral antihyperglycemic medication; TV1, telephone visit.
Figure S2. LSM (SE) change in HbA1c (%) from baseline to 26 weeks in OAM‐naïve patients and OAM monotherapy patients
Abbreviations: CI, confidence interval; HbA1c, glycated hemoglobin; LSM, least‐square mean; OAM, oral antihyperglycemic medication; SE, standard error.
Table S1. Summary of Prior OAMs
Table S2. Summary of change from baseline to 26 weeks in the 7‐point self‐monitored blood glucose (SMBG)
Table S3. Summary of HOMA2‐%B and HOMA2‐%S using LOCF at baseline to week 26
Table S4. Summary of Serious Adverse Events by Preferred Term Safety Analysis Population
Table S5. Summary and analysis of pancreatic enzymes
ACKNOWLEDGMENTS
The authors thank the investigators and patients. The authors acknowledge Sahaja Banda for manuscript writing, Feyaz Mahammad and Rakesh Ojha for data integrity review, and Antonia Baldo for editorial support, all of Syneos Health (formerly INC Research/inVentiv Health Clinical), David Bradley Woodward, Li Shen, Luis‐Emilio Garcia, Mark C Lakshmanan and Ying Lou of Eli Lilly and Company for their critical review of the paper, and Fei Li and Wan Qi Zhao of Eli Lilly and Company for assistance in publication project management.
Conflict of interest
L.G., F.W. and J.Y. are employees of Eli Lilly and Company. P.L was an employee of Eli Lilly and Company at the time of manuscript preparation. Y.H.C., C‐N.H., Y.M.C. and W.Q.W. have nothing to disclose.
Author contributions
Author contributions to the paper were as follows: Y.H.C.: data interpretation; C‐N.H.: conception of work and data interpretation; Y.M.C.: acquisition and interpretation of data; W.Q.W.: conception, design, and acquisition of data; P.L.: analysis and data interpretation; L.G.: data interpretation; F.W.: data interpretation and drafting of manuscript; J.Y.: design of work and data interpretation. All authors were involved in critical revision of the paper.
Chen YH, Huang C‐N, Cho YM, et al. Efficacy and safety of dulaglutide monotherapy compared with glimepiride in East‐Asian patients with type 2 diabetes in a multicentre, double‐blind, randomized, parallel‐arm, active comparator, phase III trial. Diabetes Obes Metab. 2018;20:2121–2130. 10.1111/dom.13340
Funding information Eli Lilly and Company
Contributor Information
Jun Yang, Email: yang_jun_sh@lilly.com.
Wei Qing Wang, Email: wqingw@hotmail.com.
REFERENCES
- 1. Ma RC, Chan JC. Type 2 diabetes in East Asians: similarities and differences with populations in Europe and the United States. Ann N Y Acad Sci. 2013;1281:64‐91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Xu Y, Wang L, He J, et al. Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948‐959. [DOI] [PubMed] [Google Scholar]
- 3. Chatterjee S, Khunti K, Davies MJ. Type 2 diabetes. Lancet. 2017;389:2239‐2251. [DOI] [PubMed] [Google Scholar]
- 4. Thompson AM, Trujillo JM. Advances in the treatment of type 2 diabetes: impact of dulaglutide. Diabetes Metab Syndr Obes. 2016;9:125‐136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang L, Zhang M, Zhang Y, Tong N. Efficacy and safety of dulaglutide in patients with type 2 diabetes: a meta‐analysis and systematic review. Sci Rep. 2016;6:18904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375:311‐322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Marso SP, Bain SC, Consoli A, et al. Semaglutide and cardiovascular outcomes in patients with type 2 diabetes. N Engl J Med. 2016;375:1834‐1844. [DOI] [PubMed] [Google Scholar]
- 8. Mora PF, Johnson EL. Cardiovascular outcome trials of the incretin‐based therapies: what do we know so far? Endocr Pract. 2017;23:89‐99. [DOI] [PubMed] [Google Scholar]
- 9. Weng J, Ji L, Jia W, et al. Standards of care for type 2 diabetes in China. Diabetes Metab Res Rev. 2016;32:442‐458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Davies MJ. Insulin secretagogues. Curr Med Res Opin. 2002;18(suppl 1):s22‐s30. [DOI] [PubMed] [Google Scholar]
- 11. Glaesner W, Vick AM, Millican R, et al. Engineering and characterization of the long‐acting glucagon‐like peptide‐1 analogue LY2189265, an Fc fusion protein. Diabetes Metab Res Rev. 2010;26:287‐296. [DOI] [PubMed] [Google Scholar]
- 12. Trulicity® (dulaglutide) for injection [package insert]. Indianapolis, IN: Eli Lilly and Company; 2017.
- 13. Grunberger G, Chang A, Garcia Soria G, Botros FT, Bsharat R, Milicevic Z. Monotherapy with the once‐weekly GLP‐1 analogue dulaglutide for 12 weeks in patients with type 2 diabetes: dose‐dependent effects on glycaemic control in a randomized, double‐blind, placebo‐controlled study. Diabet Med. 2012;29:1260‐1267. [DOI] [PubMed] [Google Scholar]
- 14. Umpierrez GE, Blevins T, Rosenstock J, Cheng C, Anderson JH, Bastyr EJ III. The effects of LY2189265, a long‐acting glucagon‐like peptide‐1 analogue, in a randomized, placebo‐controlled, double‐blind study of overweight/obese patients with type 2 diabetes: the EGO study. Diabetes Obes Metab. 2011;13:418‐425. [DOI] [PubMed] [Google Scholar]
- 15. Umpierrez G, Tofé Povedano S, Pérez Manghi F, Shurzinske L, Pechtner V. Efficacy and safety of dulaglutide monotherapy versus metformin in type 2 diabetes in a randomized controlled trial (AWARD‐3). Diabetes Care. 2014;37:2168‐2176. [DOI] [PubMed] [Google Scholar]
- 16. World Medical Association Declaration of Helsinki . Recommendations guiding physicians in biomedical research involving human subjects. JAMA. 1997;277:925‐926. [PubMed] [Google Scholar]
- 17. Dmitrienko A, Tamhane AC. Mixtures of multiple testing procedures for gatekeeping applications in clinical trials. Stat Med. 2011;30:1473‐1488. [DOI] [PubMed] [Google Scholar]
- 18. Kim YG, Hahn S, Oh TJ, Park KS, Cho YM. Differences in the HbA1c‐lowering efficacy of glucagon‐like peptide‐1 analogues between Asians and non‐Asians: a systematic review and meta‐analysis. Diabetes Obes Metab. 2014;16:900‐909. [DOI] [PubMed] [Google Scholar]
- 19. Moretto TJ, Milton DR, Ridge TD, et al. Efficacy and tolerability of exenatide monotherapy over 24 weeks in antidiabetic drug‐naive patients with type 2 diabetes: a randomized, double‐blind, placebo‐controlled, parallel‐group study. Clin Ther. 2008;30:1448‐1460. [DOI] [PubMed] [Google Scholar]
- 20. Seino Y, Rasmussen MF, Nishida T, Kaku K. Efficacy and safety of the once‐daily human GLP‐1 analogue, liraglutide, vs glibenclamide monotherapy in Japanese patients with type 2 diabetes. Curr Med Res Opin. 2010;26:1013‐1022. [DOI] [PubMed] [Google Scholar]
- 21. Russell‐Jones D, Cuddihy RM, Hanefeld M, et al. Efficacy and safety of exenatide once weekly versus metformin, pioglitazone, and sitagliptin used as monotherapy in drug‐naive patients with type 2 diabetes (DURATION‐4): a 26‐week double‐blind study. Diabetes Care. 2012;35:252‐258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Jeon HJ, Oh TK. Comparison of vildagliptin‐metformin and glimepiride‐metformin treatments in type 2 diabetic patients. Diabetes Metab J. 2011;35:529‐535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Chapell R, Gould AL, Alexander CM. Baseline differences in A1C explain apparent differences in efficacy of sitagliptin, rosiglitazone and pioglitazone. Diabetes Obes Metab. 2009;11:1009‐1016. [DOI] [PubMed] [Google Scholar]
- 24. Bloomgarden ZT, Dodis R, Viscoli CM, Holmboe ES, Inzucchi SE. Lower baseline glycemia reduces apparent oral agent glucose‐lowering efficacy: a meta‐regression analysis. Diabetes Care. 2006;29:2137‐2139. [DOI] [PubMed] [Google Scholar]
- 25. Guo XH, Yuan L, Lou QQ, et al. A nationwide survey of diabetes education, self‐management and glycemic control in patients with type 2 diabetes in China. Chin Med J (Engl). 2012;125:4175‐4180. [PubMed] [Google Scholar]
- 26. Wilson A, Gyi AA. The status and perspective of diabetes health education in China: inspiration from Australia. Int J Nurs Pract. 2010;16:92‐98. [DOI] [PubMed] [Google Scholar]
- 27. Wysham C, Blevins T, Arakaki R, et al. Efficacy and safety of dulaglutide added onto pioglitazone and metformin versus exenatide in type 2 diabetes in a randomized controlled trial (AWARD‐1). Diabetes Care. 2014;37:2159‐2167. [DOI] [PubMed] [Google Scholar]
- 28. Giorgino F, Benroubi M, Sun JH, Zimmermann AG, Pechtner V. Efficacy and safety of once‐weekly dulaglutide versus insulin glargine in patients with type 2 diabetes on metformin and glimepiride (AWARD‐2). Diabetes Care. 2015;38:2241‐2249. [DOI] [PubMed] [Google Scholar]
- 29. Blonde L, Jendle J, Gross J, et al. Once‐weekly dulaglutide versus bedtime insulin glargine, both in combination with prandial insulin lispro, in patients with type 2 diabetes (AWARD‐4): a randomised, open‐label, phase 3, non‐inferiority study. Lancet. 2015;385:2057‐2066. [DOI] [PubMed] [Google Scholar]
- 30. Nauck M, Weinstock RS, Umpierrez GE, Guerci B, Skrivanek Z, Milicevic Z. Efficacy and safety of dulaglutide versus sitagliptin after 52 weeks in type 2 diabetes in a randomized controlled trial (AWARD‐5). Diabetes Care. 2014;37:2149‐2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Garber A, Henry R, Ratner R, et al. Liraglutide versus glimepiride monotherapy for type 2 diabetes (LEAD‐3 Mono): a randomised, 52‐week, phase III, double‐blind, parallel‐treatment trial. Lancet. 2009;373:473‐481. [DOI] [PubMed] [Google Scholar]
- 32. Yang W, Chen L, Ji Q, et al. Liraglutide provides similar glycaemic control as glimepiride (both in combination with metformin) and reduces body weight and systolic blood pressure in Asian population with type 2 diabetes from China, South Korea and India: a 16‐week, randomized, double‐blind, active control trial(*). Diabetes Obes Metab. 2011;13:81‐88. [DOI] [PubMed] [Google Scholar]
- 33. Ji L, Onishi Y, Ahn CW, et al. Efficacy and safety of exenatide once‐weekly vs exenatide twice‐daily in Asian patients with type 2 diabetes mellitus. J Diabetes Investig. 2013;4:53‐61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Dungan KM, Povedano ST, Forst T, et al. Once‐weekly dulaglutide versus once‐daily liraglutide in metformin‐treated patients with type 2 diabetes (AWARD‐6): a randomised, open‐label, phase 3, non‐inferiority trial. Lancet. 2014;384:1349‐1357. [DOI] [PubMed] [Google Scholar]
- 35. Dungan KM, Weitgasser R, Perez Manghi F, et al. A 24‐week study to evaluate the efficacy and safety of once‐weekly dulaglutide added on to glimepiride in type 2 diabetes (AWARD‐8). Diabetes Obes Metab. 2016;18:475‐482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Garber AJ. Long‐acting glucagon‐like peptide 1 receptor agonists: a review of their efficacy and tolerability. Diabetes Care. 2011;34(suppl 2):S279‐S284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Wysham CH, Lin J, Kuritzky L. Safety and efficacy of a glucagon‐like peptide‐1 receptor agonist added to basal insulin therapy versus basal insulin with or without a rapid‐acting insulin in patients with type 2 diabetes: results of a meta‐analysis. Postgrad Med. 2017;129:436‐445. [DOI] [PubMed] [Google Scholar]
- 38. Wewer Albrechtsen NJ, Albrechtsen R, Bremholm L, et al. Glucagon‐like peptide 1 receptor signaling in acinar cells causes growth‐dependent release of pancreatic enzymes. Cell Rep. 2016;17:2845‐2856. [DOI] [PubMed] [Google Scholar]
- 39. Shihab HM, Akande T, Armstrong K, Singh S, Loke YK. Risk of pancreatic adverse events associated with the use of glucagon‐like peptide‐1 receptor agonist and dipeptidyl peptidase‐4 inhibitor drugs: a systematic review and metaanalysis of randomized trials. World J Meta‐Anal. 2015;3:254‐283. [Google Scholar]
- 40. Goldberg RB, Holvey SM, Schneider J. A dose‐response study of glimepiride in patients with NIDDM who have previously received sulfonylurea agents. The glimepiride protocol #201 study group. Diabetes Care. 1996;19:849‐856. [DOI] [PubMed] [Google Scholar]
- 41. http://Drugs.com [internet]. Glimepiride: Indications and Usage. 2017https://www.drugs.com/pro/glimepiride.html. Accessed October 3, 2017.
- 42. Guo XH, Lv XF, Han P, et al. Efficacy and safety of glimepiride as initial treatment in Chinese patients with type 2 diabetes mellitus. Curr Med Res Opin. 2013;29:169‐174. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Figure S1. Study design
Abbreviations: GLP‐1 RA, glucagon‐like peptide‐1 receptor agonist; LV30, poststudy therapy safety follow‐up visit; OAM, oral antihyperglycemic medication; TV1, telephone visit.
Figure S2. LSM (SE) change in HbA1c (%) from baseline to 26 weeks in OAM‐naïve patients and OAM monotherapy patients
Abbreviations: CI, confidence interval; HbA1c, glycated hemoglobin; LSM, least‐square mean; OAM, oral antihyperglycemic medication; SE, standard error.
Table S1. Summary of Prior OAMs
Table S2. Summary of change from baseline to 26 weeks in the 7‐point self‐monitored blood glucose (SMBG)
Table S3. Summary of HOMA2‐%B and HOMA2‐%S using LOCF at baseline to week 26
Table S4. Summary of Serious Adverse Events by Preferred Term Safety Analysis Population
Table S5. Summary and analysis of pancreatic enzymes


