Abstract
Stability towards protease degradation combined with modular synthesis has made peptoids of considerable interest in the fields of chemical biology, medicine, and biomaterials. Given their tertiary amide backbone, peptoids lack the capacity to hydrogen‐bond, and as such, controlling secondary structure can be challenging. The incorporation of bulky, charged, or chiral aromatic monomers can be used to control conformation but such building blocks limit applications in many areas. Through NMR and X‐ray analysis we demonstrate that non‐chiral neutral fluoroalkyl monomers can be used to influence the Kcis/trans equilibria of peptoid amide bonds in model systems. The cis‐isomer preference displayed is highly unprecedented given that neither chirality nor charge is used to control the peptoid amide conformation. The application of our fluoroalkyl monomers in the design of a series of linear peptoid oligomers that exhibit stable helical structures is also reported.
Keywords: fluorine, NMR spectroscopy, peptidomimetics, peptoids, secondary structure
Peptoids (Figure 1) are a class of foldamers that are being developed as potential therapeutics,1 biomaterials,2 chemical sensors,3 and organocatalysts.4 They represent an attractive platform for biological and pharmaceutical applications as they are highly resistant to protease degradation.5 However, given their tertiary amide backbone, peptoids lack the capacity to form hydrogen bonds so that their secondary structures are dominated by relatively weak interactions. Considerable efforts have been devoted to try and understand the relationships between a peptoid primary sequence and its folded structure.6, 7, 8, 9, 10 The cis/trans isomerization of the tertiary amide bond is the major cause of conformational heterogeneity in peptoid oligomers. Despite this, the groups of Zuckermann and Barron have demonstrated that α‐chiral aromatic monomers, such as NSpe (1), can stabilize the cis configuration of the peptoid amide bond largely through steric effects (Figure 1 b, c).6, 7 Peptoid oligomers of NSpe (1) fold into stable all cis‐amide helices, structurally similar to that of a peptide PPI helix.6, 7 Gorske and Blackwell found that the synergistic application of steric and non‐covalent n→π* interactions (NCIs) in aromatic systems could also be used to design stable cis‐amide peptoid monomers (e.g., Ns1npe, 2).8 However, it is not possible to use the aforementioned NCIs to stabilize the cis‐amide conformation of alkyl peptoid monomers, and thus the design of stable peptoid helices remains dominated by the use of chiral aromatic residues (e.g., 1 and 2).9 Recently, Faure, Taillefumier, and co‐workers exploited steric effects in the design of a non‐chiral tBu alkyl monomer that has a clear cis‐amide preference (NtBu, 3).10 Whereas 3 offers a route to control peptoid structure that avoids the use of aromatic building blocks, the design of non‐chiral but stable cis‐amide alkyl monomers is an area that is still highly underdeveloped.
Figure 1.
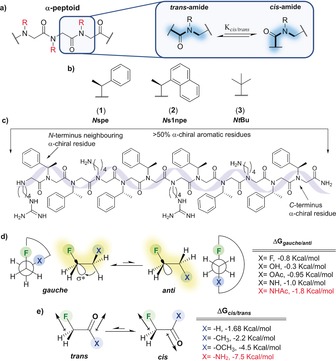
a) General structure of an α‐peptoid and the amide cis/trans isomerization process. b) The cis‐inducing α‐methyl chiral aromatic monomers 1 and 2 and the sterically demanding tBu monomer 3. c) Summary of the general sequence requirements for helical secondary structure induction in peptoids. d) The β‐fluorine gauche effect. e) Dipole interactions in α‐fluoroamides.
It is in this context that we sought to explore the potential application of fluorine incorporation as a tool to modulate the conformational preferences of alkyl peptoid monomers. Fluorine is a relatively small atom, close in size to hydrogen, but a H to F swap can give rise to significant changes in the electronic and structural properties of a molecule.11, 12 For example, fluorine may engage in stereoelectronic hyperconjugative interactions with neighbouring C−H bonds (σ(CH)→σ*(CF)). This ability of fluorine to enforce the preorganization of its local environment is most keenly observed when fluorine atoms are located β to electron‐withdrawing groups. In such an arrangement, the fluorine gauche effect is seen (Figure 1 d).12, 13 Notably, the fluorine gauche effect is more pronounced in β‐fluoroamides than in other related systems.12, 13 However, in α‐fluoroamides, CF/C=O dipolar interactions dominate, and the fluorine atom adopts a trans‐periplanar arrangement (Figure 1 e).14 The peptoid amide bond cis/trans equilibrium in our model systems (Figure 2; 10–14) was analysed by a range of established NMR methods (see the Supporting Information for the synthesis of 10–14).8b–8d The non‐fluorinated dipeptoid 10 exhibits a cis/trans equilibrium that highly favours the trans isomer (CD3CN; ΔG cis/trans=0.28, K cis/trans=0.66; Figure 3 a). Relative to this, all of the fluorinated dipeptoids (11–13) showed an enhanced preference for the cis‐amide conformation (Figure 3). Initial NMR analysis (in CD3CN) revealed that even the introduction of a single fluorine atom β to the amide bond enhanced the cis‐amide preference by 0.37 kcal mol−1 when compared to 10. Incorporation of a second fluorine atom further increased the cis‐amide preference. Indeed, unlike 10 and 11, the difluorinated dipeptoid 12 shows a highly predominant cis‐amide conformation in solution, with ΔG cis/trans=−0.42 kcal mol−1 and K cis/trans=2.05 (Figure 3 a). We were surprised to note that the K cis/trans value exhibited by 12 is comparable to those seen when cis‐inducing chiral aromatic monomers are used (e.g., for 14, K cis/trans=2.08 in CD3CN). Initial NMR analysis revealed a linear correlation between the ΔG cis/trans values observed and the electron‐withdrawing character of the Cα carbon substituent when one or two fluorine atoms were incorporated (e.g., 10 to 12; Figure 3 b, c). This correlation indicated a clear relationship between the inductive properties of the fluorinated groups and the cis/trans ratios produced (σI; Figure 3 c, e).15 An even greater cis‐isomer preference was observed when the N3fEt‐containing dipeptoid 13 was analysed (CD3CN; K cis/trans=2.24).
Figure 2.
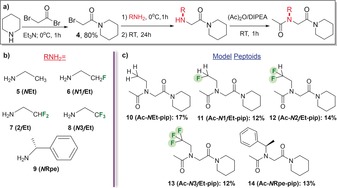
a) Synthesis of model piperinidyl acetamides 10–14. b) Peptoid monomers used in this study. c) Reference model dipeptoids (10, 14) and novel β‐fluoroethyl (11), β‐difluoroethyl (12), and β‐trifluoroethyl (13) systems.
Figure 3.
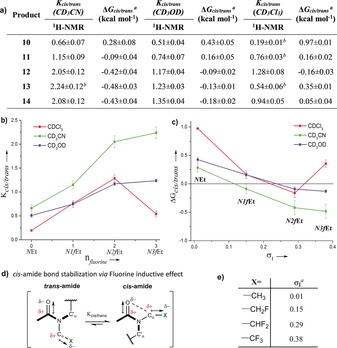
a) Average K cis/trans values in model systems 10–14. [a] From each replica, ΔG=−RT ln(K cis/trans) at 25 °C. Averages and standard deviation values are given for n=6 or n=4 (b). b) Average K cis/trans values vs. the number of fluorine atoms present (n f). c) Correlation between ΔG cis/trans and Cα‐substituent field/inductive constants (σ I). d) Schematic representation of the proposed peptoid cis‐amide isomer stabilization by inductive factors. e) Inductive constants of Cα groups.15
To determine the nature of the interactions present within 11–13, we next examined how the solvent polarity influenced the cis/trans ratios. When using protic MeOD, the K cis/trans values observed were collectively lower than those found in CD3CN (Figure 3 a, b). However, the general increases in the cis‐isomer preference produced upon fluorine incorporation were still clearly maintained. This outcome indicates that hydrogen bonding is not involved in the cis‐isomer stabilization observed in 11–13 (Figure 3 a–c). The use of CDCl3 also reduced the K cis/trans values recorded, and this general trend is in good agreement with previous observations reported for other model peptoid systems.8b–8d Despite this general decrease, the cis‐amide preferences of 11 and 12 in CDCl3 were still significantly greater than that of the control 10. Upon moving from no fluorine atoms (10) to either one (11) or two (12), relative changes in the free energy of −0.81 kcal mol−1 and −1.13 kcal mol−1, respectively, were seen.
These relative ΔG cis/trans changes are in fact larger in CDCl3 (non‐polar) than in CD3CN (polar), and this finding supports the hypothesis that an electronic cis‐stabilizing effect is occurring. Remarkably, in CDCl3, the N2fEt monomer (7) actually has a greater ability to stabilise a cis‐amide preference than the chiral aromatic NRpe monomer (9; K cis/trans=1.28 vs. 0.94; Figure 3 a). The N3fEt‐containing dipeptoid 13 was found to be more affected in CDCl3, and it produced a strong out‐of‐trend shift to the trans isomer (Figure 3 a, b). Given this observation, we hypothesised that the energetic penalty that 13 experiences in the cis conformation may arise from an increased solvation barrier as non‐polar solvents are well‐known to disfavour structures where large dipoles are present. As depicted in Figure 3 d, the overall dipolar moment within trans‐13 is likely to be lower than that within the corresponding cis isomer as the carbonyl and side‐chain dipoles are opposed. This solvation effect should be less pronounced in 11 and 12 as they have weaker dipoles (Figure 3 e).
Next, we explored the role that fluorine/amide gauche interactions could play in enhancing the cis‐isomer preferences. The vicinal (three‐bond coupling) 3 J HF coupling constants were thus analysed (Figure 4 a).17, 18 In 11, a 3 J HF,cal value of 20.0 Hz was calculated for an ideal fluorine/amide gauche conformation of the side chain (g). A significantly lower value of 8.0 Hz was obtained for the alternative fluorine/amide anti configuration (a). The experimental value found within the predominant cis‐11 isomer was 3 J HF,obs=25.7 Hz (CD3CN). This result strongly suggested an overall fluorine/amide gauche orientation within the side chain. Two staggered conformations for 12 were also examined, and the experimental value of 3 J HF,obs=14.9 Hz was in perfect agreement with an anti/gauche conformation (Figure 4 b). This finding indicates that only one F atom may be actually located gauche to the peptoid amide group, and this is contrary to the more intuitive (+g/−g) configuration that would be expected. No significant variations in the experimental 3 J HF,obs values were seen within each cis/trans pair in any of the solvents tested, indicating that the fluorine/amide relative arrangement is retained between conformers. The NMR results suggest that fluorine gauche effects are not solely responsible for the cis‐isomer preferences observed in 11 and 12. In 13, fast rotation of the CF3 group was inferred as the experimental 3 J HF,obs value greatly deviated from the calculated value, which assumes a static fluorine/amide arrangement (Figure 4 c).
Figure 4.
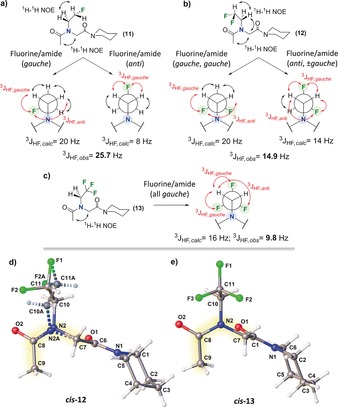
Theoretical versus experimental vicinal 3 J H‐F coupling constants within a) 11, b) 12, and c) 13 in their preferred cis conformations (CD3CN). Ball‐and‐stick representations of the crystal structures of cis‐amides d) 12 and e) 13.16
We were able to crystallize dipeptoids 12 and 13 from their EtOAc saturated solutions.19 The solid‐state structures for 12 and 13 and the conformations suggested by NMR analysis were in perfect agreement (Figure 4 d, e). It is worth noting that from the crystal structures of 12 and 13, it would appear that neither fluorine–oxygen repulsive interactions nor unfavourable steric clashes contribute substantially to the cis/trans conformation preferences observed in these systems. As shown in Figure 4 d, e, the fluorinated groups in 12 and 13 display a well‐defined orthogonal orientation relative to the amide bond planes. This orientation minimizes the potential steric clashes and/or electronic repulsion imposed by the CHF2/CF3 groups. Overall, our findings support the hypothesis that the enhanced cis‐amide preferences observed in 11–13 arise from the inductive effects imposed by the fluorine atom(s). As the polarization at Cα increases, the peptoid cis‐amide preference also increases. We propose that this is due to the fact that the δ+ on Cα can form a syn‐periplanar stabilising dipolar interaction with the amide C=O (Figure 3 d).
Encouraged by the cis/trans ratios achieved in the model systems (11–13), we then moved to see if the non‐chiral fluoroalkyl monomers could be exploited to design stable peptoid helices. To this end, we designed a control 15‐mer peptoid, Pep.1, using non‐chiral alkyl ethylamine monomers (Figure 5). A single NSpe residue was introduced as a chiral reporter for circular dichroism (CD) spectroscopy. The Pep.1 sequence was then altered by substituting in the various fluorinated monomers (6–8) in place of some, but not all, of the NEt residues (group 1, Pep.2–4). In a second group of fluorinated peptoids, all of the NEt residues present were replaced (group 2, Pep.5–7). We were pleased to see that structural analysis of the peptoid oligomers Pep.2–Pep.7 by CD spectroscopy revealed the presence of stable peptoid helices (Figure 5 b, c). In all of the peptoids studied, substitution of the NEt residues by any of the fluorinated monomers clearly enhanced the CD minima at 218 nm (M θ,218), which is characteristic of an increase in helicity. When five substitutions (NEt for a fluoroalkyl monomer) were made in non‐consecutive positions (group 1, Pep.2–4, n f=5), the overall increases in molar ellipticity were found to correlate with the number of fluorine atoms within the side chain. For example, upon going from the non‐fluorinated peptoid (Pep.1) to the N1fEt‐based analogue (Pep.2), a change in molar ellipticity of ΔM θ,218=6660 deg cm2 dmol−1 was observed. Similarly, incorporation of N2fEt (7) and N3fEt (8) produced approximately two‐ and threefold higher increases in M θ,218 (Pep.3, ΔM θ,218=12 640; Pep.4, ΔM θ,218=17 000 deg cm2 dmol−1).
Figure 5.
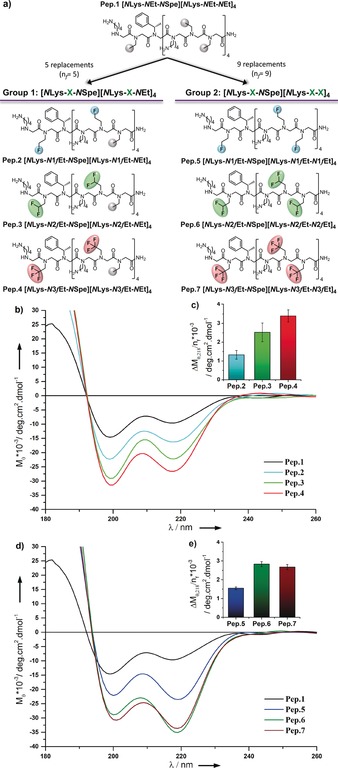
a) Peptoid oligomers Pep.1–Pep.7. b, d) Average CD spectra and c, e) average absolute increases in M θ,218 per fluorine residue incorporated (ΔM θ,218/n f) in peptoid sequences from group 1 (Pep.1–Pep.4, n f=5; parts b, c) and group 2 (Pep.5–Pep.7, n f=9; parts d, e).
When the more heavily substituted peptoids from group 2 were analysed, higher values of M θ,218 were found, indicating that the secondary structure enhancement induced by the incorporation of fluorinated side chains has an overall accumulative behaviour (Pep.5–7; Figure 5 d, e). In fact, the average increases in M θ,218 produced by each N1fEt (6) and N2fEt (7) monomer introduced in these sequences were higher than those observed when only five replacements were made (ΔM θ,218/n f; Pep.2 vs. Pep.5 and Pep.3 vs. Pep.6; Figure 5 c–e). These results revealed a broadly cooperative effect between neighbouring fluorinated side chains. Interestingly, this synergy between consecutive monomers did not occur when N3fEt monomers were used (Pep.4 vs. Pep.7). Based on our crystal structure data, we could evaluate that the volumes of the CHF2 and CF3 groups are 29.63 and 40.47 Å3 respectively. Based on this, we hypothesise that the behaviour seen for Pep.7 may be related to unfavourable steric and/or repulsive interactions between the CF3 groups of adjacent N3fEt monomers. Overall, the results from the CD studies (Figure 5) are highly unprecedented owing to the fact that none of the fluorinated monomers investigated are either chiral, aromatic, or charged, and yet they can support the formation of stable peptoid helices.
In summary, we have shown that the selective and strategic incorporation of fluorine atom(s) offers a new route to control the amide bond isomerism in peptoids containing alkyl side chains. Through NMR and X‐ray analysis we demonstrated that simple non‐chiral fluoroalkyl monomers can be used to influence the key K cis/trans equilibria of a peptoid amide bond and induce a remarkable degree of cis‐amide preference. The cis‐isomer preference is highly unprecedented given that neither chirality nor charge was being used to control the peptoid amide conformation. The data gathered support the hypothesis that inductive effects imparted by the fluorine atom(s) and not fluorine gauche effects underpin the cis‐isomer stabilization observed. The novel fluoroalkyl monomers were also used to prepare a series of peptoid oligomers that exhibited stable helical structures despite only having one chiral aromatic residue. The application of fluorine in the design of alkyl monomers offers a new approach to control amide bond isomerism in peptoid sequences, overcoming the current need for high levels of chiral side chains. Given the lack of alternatives available, the N1fEt, N2fEt, and N3fEt alkyl monomers offer exciting new tools to design structurally stable peptoid systems with applications in a range of areas, including medicine and biomaterials.
Conflict of interest
The authors declare no conflict of interest.
Supporting information
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary
Acknowledgements
Financial support from the European Union's Seventh Framework Programme for research, technological development and demonstration under the Marie Curie ITN Scheme [Fluor21: grant number FP7‐PEOPLE‐2013‐ITN‐607787] is gratefully acknowledged. We thank D. S. Yufit and A. Batsanov for X‐ray data collection.
D. Gimenez, J. A. Aguilar, E. H. C. Bromley, S. L. Cobb, Angew. Chem. Int. Ed. 2018, 57, 10549.
References
- 1.
- 1a. Eggimann G. A., Bolt H. L., Denny P. W., Cobb S. L., ChemMedChem 2015, 10, 233–237; [DOI] [PubMed] [Google Scholar]
- 1b. Bolt H. L., Eggimann G. A., Denny P. W., Cobb S. L., MedChemComm 2016, 7, 799–805. [Google Scholar]
- 2.
- 2a. Statz A. R., Meagher R. J., Barron A. E., Messersmith P. B., J. Am. Chem. Soc. 2005, 127, 7972–7973; [DOI] [PubMed] [Google Scholar]
- 2b. Huang M. L., Ehre D., Jiang Q., Hu C., Kirshenbaum K., Ward M. D., Proc. Natl. Acad. Sci. USA 2012, 109, 19922–19927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.
- 3a. Reddy M. M., Kodadek T., Proc. Natl. Acad. Sci. USA 2005, 102, 12672–12677; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3b. Knight A. S., Zhou E. Y., Pelton J. G., Francis M. B., J. Am. Chem. Soc. 2013, 135, 17488–17493; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3c. Baskin M., Maayan G., Chem. Sci. 2016, 7, 2809–2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.
- 4a. Pirrung M. C., Park K., Tumey L. N., J. Comb. Chem. 2002, 4, 329–344; [DOI] [PubMed] [Google Scholar]
- 4b. Maayan G., Ward M. D., Kirshenbaum K., Proc. Natl. Acad. Sci. USA 2009, 106, 13679–13684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Sun J., Zuckermann R. N., ACS Nano 2013, 7, 4715–4732. [DOI] [PubMed] [Google Scholar]
- 6. Armand P., Kirshenbaum K., Falicov A., Dunbrack R. L., Dill K. A., Zuckermann R. N., Cohen F. E., Folding Des. 1997, 2, 369–375. [DOI] [PubMed] [Google Scholar]
- 7. Wu C. W., Sanborn T. J., Huang K., Zuckermann R. N., Barron A. E., J. Am. Chem. Soc. 2001, 123, 6778–6784. [DOI] [PubMed] [Google Scholar]
- 8.
- 8a. Gorske B. C., Blackwell H. E., J. Am. Chem. Soc. 2006, 128, 14378–14387; [DOI] [PubMed] [Google Scholar]
- 8b. Gorske B. C., Bastian B. L., Geske G. D., Blackwell H. E., J. Am. Chem. Soc. 2007, 129, 8928–8929; [DOI] [PubMed] [Google Scholar]
- 8c. Gorske B. C., Stringer J. R., Bastian B. L., Fowler S. A., Blackwell H. E., J. Am. Chem. Soc. 2009, 131, 16555–16567; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8d. Stringer J. R., Crapster J. A., Guzei I. A., Blackwell H. E., J. Am. Chem. Soc. 2011, 133, 15559–15567; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8e. Gorske B. C., Nelson R. C., Bowden Z. S., Kufe T. A., Childs A. M., J. Org. Chem. 2013, 78, 11172–11183. [DOI] [PubMed] [Google Scholar]
- 9.An α-chiral alkyl monomer with cis-amide preference was recently reported; see:Roy O., Dumonteil G., Faure S., Jouffret L., Kriznik A., Taillefumier C., J. Am. Chem. Soc. 2017, 139, 13533–13540. [DOI] [PubMed] [Google Scholar]
- 10. Roy O., Caumes C., Esvan Y., Didierjean C., Faure S., Taillefumier C., Org. Lett. 2013, 15, 2246–2249. [DOI] [PubMed] [Google Scholar]
- 11.
- 11a. Welch J. T., Eswarakrishnan S., Fluorine in Bioorganic Chemistry, Wiley-Interscience, New York, 1991; [Google Scholar]
- 11b. O'Hagan D., Rzepa H. S., Chem. Commun. 1997, 645–652. [Google Scholar]
- 12. Hunter L., Beilstein J. Org. Chem. 2010, 6, 38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.
- 13a. Wiberg K. B., Murcko M. A., Laidig K. E., MacDougall P. J., J. Phys. Chem. 1990, 94, 6956–6959; [Google Scholar]
- 13b. O'Hagan D., Bilton C., Howard J. A. K., Knight L., Tozer D. J., J. Chem. Soc. Perkin Trans. 2 2000, 605–607. [Google Scholar]
- 14. Banks J. W., Batsanov A. S., Howard J. A. K., O'Hagan D., Rzepa H. S., Martin-Santamaria S., J. Chem. Soc. Perkin Trans. 2 1999, 2409–2411. [Google Scholar]
- 15. Hansch C., Leo A., Taft R. W., Chem. Rev. 1991, 91, 165–195. [Google Scholar]
- 16. Dolomanov O. V., Bourhis L. J., Gildea R. J., Howard J. a. K., Puschmann H., J. Appl. Crystallogr. 2009, 42, 339–341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ihrig A. M., Smith S. L., J. Am. Chem. Soc. 1972, 94, 34–41. [Google Scholar]
- 18. O'Hagan D., Rzepa H. S., Schüler M., Slawin A. M., Beilstein J. Org. Chem. 2006, 2, 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.CCDC https://www.ccdc.cam.ac.uk/services/structures?id=doi:10.1002/anie.201804488 contain the supplementary crystallographic data for this paper. These data can be obtained free of charge from http://www.ccdc.cam.ac.uk/.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
As a service to our authors and readers, this journal provides supporting information supplied by the authors. Such materials are peer reviewed and may be re‐organized for online delivery, but are not copy‐edited or typeset. Technical support issues arising from supporting information (other than missing files) should be addressed to the authors.
Supplementary


