Abstract
Objectives
To estimate associations between long‐term use of proton pump inhibitors (PPIs) and pneumonia incidence in older adults in primary care.
Design
Longitudinal analyses of electronic medical records.
Setting
England
Participants
Individuals aged 60 and older in primary care receiving PPIs for 1 year or longer (N=75,050) and age‐ and sex‐matched controls (N=75,050).
Measurements
Net hazard ratios for pneumonia incidence in Year 2 of treatment were estimated using the prior event rate ratio (PERR), which adjusts for pneumonia incidence differences before initiation of treatment. Inverse probability weighted models adjusted for 78 demographic, disease, medication, and healthcare usage measures.
Results
During the second year after initiating treatment, PPIs were associated with greater hazard of incident pneumonia (PERR‐adjusted hazard ratio=1.82, 95% confidence interval=1.27–2.54), accounting for pretreatment pneumonia rates. Estimates were similar across age and comorbidity subgroups. Similar results were also obtained from propensity score– and inverse probability–weighted models.
Conclusion
In a large cohort of older adults in primary care, PPI prescription was associated with greater risk of pneumonia in the second year of treatment. Results were robust across alternative analysis approaches. Controversies about the validity of reported short‐term harms of PPIs should not divert attention from potential long‐term effects of PPI prescriptions on older adults.
Keywords: proton pump inhibitors, pneumonia, primary care
Proton pump inhibitors (PPIs) are widely prescribed to reduce gastric acid production and for gastroprotection,1, 2 but there is evidence of frequent prescribing of PPIs without adequate indication.3 PPI use has been linked to higher rates of several conditions, including osteoporotic fractures,4, 5, 6 cardiovascular disease,7, 8 and Clostridium difficile infection.7, 8, 9
PPIs act directly on H+/K+‐ ATPase enzymes, reducing acid secretion, but gastric acid plays a vital role in the innate response to bacterial infection. Without this protection, bacterial colonization occurs,10 increasing the risk of bacterial micro‐aspiration and pulmonary colonization. Several studies, including meta‐analyses, have reported high risk of pneumonia soon after PPI therapy initiation,11, 12, 13, 14, 15 although negative associations have also been reported.16, 17 A recent observational study of the first year after PPI prescription16 used the prior event rate ratio (PERR),18, 19, 20, 21 which adjusts differences in postprescription pneumonia incidence between cases and controls with preexisting differences to correct for unmeasured confounding. That study used data from individuals in primary care in England and found that pneumonia rates increased during the first 30 days of PPI prescription (incidence rate ratio (IRR)=1.19, 95% confidence interval (CI)=1.14–1.25), but pneumonia rates were even higher during the 30 days immediately before the first PPI prescription (IRR=1.92, 95% CI=1.84–2.00), perhaps because the greater medical scrutiny of individuals with pneumonia led to more PPI prescribing for comorbidities, including medications commonly co‐prescribed with PPIs. The PERR analysis, accounting for these prior differences, indicated no additional effect of PPIs on pneumonia risk in the 30 days or first year of treatment periods, but no data on longer‐term effects have been reported.
Approximately 40% of elderly adults receive PPIs, and appropriate clinical indications may be lacking for up to 85% of PPIs prescribed.22, 23 Pneumonia is a major cause of death,24, 25 costing in excess of $17 billion per year in the United States alone.26 It is therefore important to clarify associations between PPIs and pneumonia for short‐ and long‐term exposure, especially in older adults, who may be most at risk from lack of barriers to respiratory infection. We therefore aimed to estimate long‐term associations between PPI prescription and risk of pneumonia in individuals in primary care aged 60 and older, similarly applying the PERR approach to account for prior differences between PPI‐treated individuals and controls.
Methods
Data source
Data in this analysis were from Clinical Practice Research Datalink (CPRD) for England, a large de‐identified database of primary care electronic medical records from participating practices.27 Clinicians entered data as the main record of each consultation; the extract available for research includes basic demographic characteristics, diagnoses, prescriptions, vaccinations, and specialist referrals. The primary care data are linked for research purposes to each individual's hospital discharge diagnoses (from the National Health Service Hospital Episode Statistics) and death certificates from the Office of National Statistics database for individuals in England. Virtually all older adults in England are registered with a primary care practice and receive all routine prescriptions (all dispensed free of charge) through that practice.
CPRD received multiple research ethics committee approval (05/MRE04/87) for observational studies. National Information Governance Board's Ethics and Confidentiality Committee approval ECC 5–05 (a) 2012 also covers CPRD. Our study had gained prior approval from the CPRD Independent Scientific Advisory Committee (Protocol 15/210R).
Study population and follow‐up
We selected individuals aged 60 and older who had PPI prescriptions recorded in each calendar quarter for 1 year or longer and had clinical records for 1 year or longer before first PPI prescription. Seventy‐five thousand fifty individuals (Supplementary Figure 1S) in the database met these criteria (n=31,202 men, n=43,848 women). PPI‐treated individuals were 1‐to‐1 matched with 75,050 controls who had not received a PPI prescription. Matching was according to sex and year of birth only. The date of first PPI prescription was designated as the index date for analyses. After the matching, each treated individual's date of first PPI prescription was copied to his or her matched control.
Exposure and study end point
PPIs (esomeprazole, lansoprazole, omeprazole, pantoprazole, rabeprazole sodium) were identified in records based on the British National Formulary28 Chapter 1.3.5, regardless of dosage.
Community‐acquired pneumonia (pneumonia) has been defined previously elsewhere29, 30, 31 based on ascertainment in linked primary care and hospital admission records. Pneumonia diagnosis 14 days or less after discharge was regarded as hospital‐acquired pneumonia and excluded.29, 30 Diagnoses of pneumonia 28 days or less from each other were considered the same event. A pneumonia diagnosis 30 days or less from an individual's registration with a practices was also excluded as possibly reflecting historical records.30, 31
Covariates
Disease burden status was defined according to the Charlson Comorbidity Index32 as low (0) or high (>0) comorbidity. In sensitivity analyses, we adjusted survival estimates for propensity scores33, 34, 35 based on 78 variables summarizing demographic characteristics, diseases, drugs, and healthcare usage. (See Supplementary Table S1 for full list.) Missing data were coded as separate categories.
Details of statistical methods
An intention‐to‐treat approach including all individuals who received PPIs for at least 1 year, irrespective of details of treatment in the second year, was used for analyses to avoid biases that excluding those who stopped taking PPIs because of related adverse events might cause. As noted, 75,050 individuals with a PPI prescription for at least 1 year were included. In the second year, 64,364 of the 75,050 (85.8%) received prescriptions in at least 1 calendar quarter of the second year, with 55,961 (64.1%) receiving treatment during 3 of 4 calendar quarters of the second year.
PERR analyses were based on unadjusted Cox proportional hazard models, in accordance with validation studies.19, 20, 36 Post‐PPI hazard ratios (HRs) were adjusted by pre‐PPI HRs using the PERR method (Supplementary Figure 2S) to account for unmeasured confounding and presented as PERR HRs and 95% confidence intervals (95% CIs) obtained using bootstrapping.18, 19, 37 PERR analyses require independence of events in the pre‐PPI period from risk of subsequent exposure. PERR also assumes that unmeasured confounding is time invariant.20 PERR analyses analyzed post‐PPI pneumonia risk adjusted by unmeasured confounding from the 24‐ to 13‐month period before a PPI (Supplementary Figure 2S). The number needed to harm was computed using an established formula38 and bootstrapped to obtain 95% confidence limits. Subgroup analyses according to age and comorbidity were specified a priori in our original analysis application to CPRD.
Several alternative approaches were used in sensitivity analyses to test the robustness of our findings. Cox proportional hazard models adjusted by propensity scores33, 34 (details in Supplementary Table 1) were performed in sensitivity analyses for the post‐PPI period. Inverse‐weighted probability models included 78 putative, measured confounders covering demographic characteristics, diagnoses, medication, and healthcare usage measures (details in Supplementary Table S1). The PERR‐ALT method,36 a variant of PERR analysis using a cross‐over‐like design with clustered (or paired) Cox models, with posttreatment events in treated individuals compared with pretreatment events in controls and vice versa, was also used. The PERR and propensity scoring approaches for addressing confounding in nonrandomized longitudinal studies have been reviewed elsewhere.39 All statistical analyses were performed in Stata version 14 (Stata Corp., College Station, TX).
Results
Records for 75,050 PPI‐treated individuals and 75,050 matched controls were eligible for the primary analyses. Mean age in treated individuals and controls at index date was 71±7.3, and 58% of PPI‐treated and control participants were female (Table 1). Treated participants had greater comorbidity (54.2% with Charlson Index ≥1) than controls (36.2%). Details of diagnosis and prescription differences between the treated and control groups can be found in Supplementary Table S1.
Table 1.
Descriptive Characteristics at Index Date
| Characteristic | Controls, n = 75,050 | Treated, n = 75,050 | Difference Between Controls and Treated |
|---|---|---|---|
| % | |||
| Age | |||
| 60–74 | 70.7 | 70.7 | 0 |
| ≥75 | 29.3 | 29.3 | 0 |
| Female | 58.4 | 58.4 | 0 |
| Race | |||
| White | 60.3 | 79.2 | 31.3 |
| Non‐white | 1.4 | 2.6 | 85.7 |
| Unreported or undisclosed | 38.2 | 18.2 | –52.4 |
| Low socioeconomic status (Index of Multiple Deprivation 3rd to 5th quintile) | 50.2 | 52.4 | 4.4 |
| Charlson Comorbidity Index ≥1 | 36.2 | 54.2 | 49.7 |
| Smoking status | |||
| Never | 46.0 | 42.8 | –7.0 |
| Previous | 15.7 | 20 | 27.4 |
| Current | 25.9 | 30.1 | 16.2 |
| Undetermined | 12.5 | 7.1 | –43.2 |
| Alcohol consumption | |||
| Never, currently not | 9.6 | 11.9 | 24.0 |
| Current, known amount | 43.7 | 47.7 | 9.2 |
| Heavy | 9.1 | 11.6 | 27.5 |
| Current, unknown amount | 0.8 | 0.9 | 12.5 |
| Former | 1.8 | 2.4 | 33.3 |
| Undetermined | 35.0 | 25.4 | –27.4 |
| Body mass index, kg/m2 | |||
| <18.5 (underweight) | 0.9 | 0.9 | 0 |
| 18.5–24.9 (normal) | 16.7 | 17.5 | 4.8 |
| 25.0–29.9 (overweight) | 19.7 | 25.0 | 26.9 |
| ≥30.0 (obese) | 12.0 | 17.1 | 42.5 |
| Unreported | 50.8 | 39.5 | –22.2 |
| Coronary heart disease | 9.1 | 17.5 | 92.3 |
| Atrial fibrillation | 4.1 | 5.5 | 34.1 |
| Heart failure | 2.1 | 4.0 | 90.5 |
| Antiplatelet medication | 17.2 | 31.4 | 82.6 |
| Diuretic thiazide or related diuretic | 16.7 | 18.8 | 12.6 |
| Benzodiazepine | 5.4 | 12.2 | 125.9 |
| Corticosteroid | 25.8 | 44.1 | 70.9 |
| Nonsteroidal antiinflammatory drug | 16.2 | 35.3 | 117.9 |
Incidence rates
In the PPI‐treated group, unadjusted pneumonia incidence rates were less than 6 per 1,000 person‐years from 24 to 6 months before treatment but then rose considerably in the last 6 months before treatment (peak: 22 PPI‐treated individuals per 1,000 person‐years) (Figure 1). In the post‐PPI treatment period, pneumonia incidence declined to a stable incidence of approximately 8 per 1,000 person‐years in the first year but then showed a generally upward trend in the second year after PPI treatment. Pneumonia incidence in controls was lower (<4/1,000 person‐years) and more stable throughout. Numbers of pneumonia events according to participant subgroup are shown in Supplementary Table S2.
Figure 1.
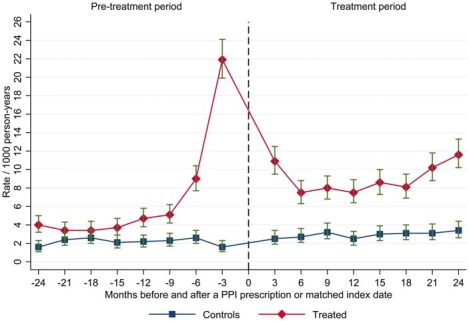
Incidence rates of community acquired pneumonia according to months before and after index date in proton pump inhibitor–treated participants and controls (N=151,952).
In Cox survival estimates for months 24 to 13 before a PPI prescription, pneumonia was more common in PPI‐treated individuals than controls (HR=1.67, 95% CI=1.37–2.02), but in the second year of the post‐PPI period, this excess pneumonia risk was substantially larger (HR=3.03, 95% CI=2.6–3.53). The estimate of net long‐term association according to the PERR calculation was significant (HR=1.82, 95% CI=1.27–2.54), suggesting substantial excess long‐term pneumonia risk (Table 2). The number needed to harm estimate for this result was 420 (95% CI=113–1,996).
Table 2.
Cox Model Hazard Ratios and Corresponding Prior Event Rate Ratio Net Hazards Estimates for Community Acquired Pneumonia
| Sample | Before Treatment | After Treatment | PERR |
|---|---|---|---|
| Hazard Ratio (95% Confidence Interval) | |||
| Full sample | 1.7 (1.4–2.0) | 3.0 (2.6–3.5) | 1.8 (1.3–2.5) |
| Age | |||
| 60–74 | 1.6 (1.2–2.0) | 3.3 (2.7–4.1) | 2.1 (1.4–3.6) |
| ≥75 | 1.8 (1.4–2.4) | 2.8 (2.2–3.4) | 1.5 (0.9–2.5) |
| Charlson Comorbidity index | |||
| 0 | 1.1 (0.8–1.6) | 3.0 (2.4–3.9) | 2.8 (1.7–5.7) |
| ≥1 | 1.5 (1.2–1.9) | 2.4 (2.0–2.9) | 1.6 (1.1–2.4) |
The results in our prespecified age and comorbidity subgroups (Table 2) show broadly similar PERR estimates for the association between PPIs and pneumonia. Inspection of PERR CIs suggested no interactions and no main effect by these subgroups, although some subgroup‐specific estimates had wide CIs, reflecting smaller numbers (Table 2).
Sensitivity analysis
We used several alternative analysis approaches to check the robustness of our findings. Inverse probability weighting models and propensity scoring models were based on 78 measured potential confounders covering demographic characteristics, diagnoses, medication, and healthcare usage measures (details in Supplementary Table S1). The inverse probability–weighted models yielded results similar to those of PERR (Table 3), as did propensity score modelling and adjustment for baseline variables. Primary analyses were reproduced using the PERR‐ALT method (Supplementary Table S5), which also produced similar results. Models of alternative time windows (months 7 to 24 before and after PPI treatment) also yielded similar results (Supplementary Tables S3 and S4).
Table 3.
Propensity Score Adjusted and Inverse Probability Weighting Adjusted Proportional Hazard Cox Model Hazard Ratios for Community Acquired Pneumonia
| Sample | Before Treatment | After Treatment |
|---|---|---|
| Hazard Ratio (95% Confidence Interval) | ||
| Propensity scoring | ||
| Full sample | 1.1 (0.9–1.4) | 1.8 (1.5–2.2) |
| Age | ||
| 60–74 | 0.9 (0.7–1.2) | 1.8 (1.4–2.3) |
| ≥75 | 1.4 (1.0–1.9) | 1.8 (1.4–2.4) |
| Charlson Comorbidity Index | ||
| 0 | 0.7 (0.5–1.1) | 2.1 (1.5–2.9) |
| ≥1 | 1.3 (1.0–1.7) | 1.7 (1.3–2.1) |
| Inverse probability weighting | ||
| Full sample | 1.1 (0.9–1.5) | 1.9 (1.5–2.5) |
| Age | ||
| 60–74 | 0.9 (0.6–1.3) | 2.1 (1.5–3.0) |
| ≥75 | 1.6 (1.1–2.2) | 2.0 (1.3–3.0) |
| Charlson Comorbidity Index | ||
| 0 | 0.8 (0.5–1.2) | 2.7 (2.0–3.6) |
| ≥1 | 1.3 (0.9–1.8) | 1.7 (1.2–2.4) |
| Covariate adjustment | ||
| Full sample | 1.1 (0.9–1.4) | 1.8 (1.5–2.1) |
| Age | ||
| 60–74 | 0.9 (0.7–1.3) | 1.9 (1.5–2.4) |
| ≥75 | 1.4 (1.0–1.9) | 1.8 (1.4–2.3) |
| Charlson Comorbidity Index | ||
| 0 | 0.7 (0.5–1.1) | 2.0 (1.5–2.7) |
| ≥1 | 1.3 (1.0–1.7) | 1.6 (1.3–2.0) |
Discussion
Main findings
Reported associations between PPI treatment and community‐acquired pneumonia incidence during the first 30 days or 1 year of treatment may be due to biases,16, 17 but little was known about longer‐term risks. We have demonstrated for the first time a clear increase in long‐term pneumonia incidence associated with ongoing PPI use after accounting for preexisting differences in pneumonia rates between treated and control groups. Reassuringly, the inverse probability–weighted and propensity score approaches accounting for 78 demographic, disease, medication, and healthcare usage measures in treated and control participants (in post‐PPI Cox models) gave similar results, suggesting that the findings are robust. Although the number needed to harm was relatively large (420 participants for 12 months), given the high prevalence and frequent continuation of PPIs without good indication,3 this number needed to harm represents a significant risk to older adults. Analyses according to age and comorbidity subgroups produced similar estimates, although larger samples are needed to confirm this.
Strengths and limitations
Our study has several strengths. First, the CPRD dataset used is representative of the general U.K. population,27 so our results should have good external validity. As previously demonstrated,19, 36 the PERR and PERR‐ALT can approximate results of randomized controlled trials when suitably applied to observational clinical records. Our analyses were also designed to reduce confounding by focusing on long‐term outcomes.
Inevitably there are limitations. Our matching of treatment groups (according to date of birth and sex only) may seem crude, but many potentially important differences between treatment and control groups go unrecorded in clinical records. The PERR method seeks to account for measured and unmeasured differences between groups by adjusting for differences in the outcome (pneumonia rates) before treatment started, so complex matching on baseline measures is redundant and was not used in validation of the PERR method. Our sensitivity analyses using propensity scoring and inverse probability–weighting approaches accounted for many measured potential confounders and provided similar results, but our list of potential measures did not include, for example, neurological diseases other than stroke that might increase risk of outpatient aspiration, and better measures of this and other risks should be used in future studies. Addition of a falsification analysis, using a condition not related to PPI exposure, could strengthen future work, although identifying a repeated‐event condition suitable for PERR analysis and potentially being exposed to similar biases to pneumonia would be challenging.
This study used prescription records from primary care and therefore omitted individuals who obtained over‐the‐counter PPIs, but PPIs are sold in small packs over the counter in England, and long‐term use would be expensive. Because older adults receive free prescriptions and free consultations in the U.K. National Health Services, it is likely that our treatment group selection identified the great majority of long‐term PPI use. We were also not able to control for adherence to treatment, but over‐the‐counter use and nonadherence would, in general, tend to reduce estimated effect sizes and are implausible explanations for the excess pneumonia risks identified. Data on indications and daily doses are complex and incomplete in the CPRD data, but accounting for these factors may strengthen future work. There may be drug‐drug interactions modifying the effects of PPIs, which could be examined in future work. Furthermore, the PERR method cannot address time‐dependent confounding. By excluding the year before PPI therapy in the main analysis, we reduced the risk of indication biases, but not longer‐term time‐dependent confounding, although the consistency of our results across the differing analysis approaches used suggests that such effects are likely to be small.
Comparison with previous literature
The safety of PPIs has been assessed using records from CPRD (e.g.),16 retrospective data from randomized controlled trials,40 and several meta‐analytical and population‐based studies,12, 13, 41, 42 including a meta‐analytical multiple‐database study of individuals treated with nonsteroidal antiinflammatory drugs1 and a study of individuals with nontraumatic intracranial hemorrhage.2 Conclusions on PPI safety have been derived mainly from observational research and have led to conflicting suggestions on the role of confounding by indication in the association between PPIs and pneumonia.15, 16, 17 Observational studies to date, including meta‐analyses, have reported on approximately 30% greater PPI‐related risk of pneumonia.11, 12, 13 As noted, a study16 of the first year of PPI treatment found higher rates of pneumonia before PPI initiation, which biased posttreatment HR estimates. A meta‐analytical study17 suggested similar confounding as an explanation for the perceived link between PPIs and pneumonia in the field. That study focused on effects of PPIs in users of nonsteroidal antiinflammatory drugs. Using data from several databases and a high‐dimensional propensity‐scoring approach, no statistically significantly greater risk of hospitalization for pneumonia was found except in one population in primary analysis. Our own results suggest that although short‐term PPI pneumonia associations may be confounded, longer‐term associations in older adults in primary care were present and consistent across a range of analysis approaches.
Clinical and research implications
Our findings support the need for caution in long‐term prescribing of PPIs to older adults. Further work is needed to clarify indication biases in PPI prescribing, especially in older adults hospitalized for pneumonia. Although application of the PERR method strengthened our analysis, observational designs provide less certain estimates than well‐conducted clinical trials and may suffer from residual bias or confounding, although a randomized controlled trial would have to be large, given that incident pneumonia is relatively uncommon. Also, recruiting typical older adults to clinical trials is challenging. Thus, observational estimates in large representative populations based on robust statistical methods are likely to provide valuable estimates of effect (and are often the only available estimates) for informing safe prescribing practice, especially for older adults.
Conclusions
Pneumonia risk is greater with long‐term PPI therapy in older adults in primary care, independent of excess pneumonia rates immediately before first PPI receipt. In our cohort, the excess risk was statistically similar across age and comorbidity groups, but more work is needed to identify individuals at highest risk.
Supporting information
Tables S1–S6
Figure S1. Flow diagram: pre‐selection of PPI‐treated individuals and controls.
Figure S2. The retrospective cohort study design with the prior event rate ratio (PERR) adjustment.
Acknowledgments
The views expressed are those of the authors and not necessarily those of the National Health Service, the NIHR, or the U.K. Department of Health.
Financial Disclosure: This research was funded by National Institute for Health Research (NIHR) Grant PB‐PG‐0214‐3309. JM is supported by NIHR Doctoral Research Fellowship 2014–07–177.
Conflict of Interest: A.B. is a former employee of Pfizer (until November 2012) and is currently employed by Alfasigma.
Author Contributions: Dr. Zirk‐Sadowski and Dr. Ble had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Ble, Melzer, Zirk‐Sadowski. Acquisition and interpretation of data, drafting of manuscript, critical revision of manuscript for important intellectual content, approval of final draft submitted: all authors. Statistical analysis: Zirk‐Sadowski, Henley, Melzer, Ble.
Sponsor's Role: None.
References
- 1. Katz PO, Gerson LB, Vela MF. Guidelines for the diagnosis and management of gastroesophageal reflux disease. Am J Gastroenterol 2013;108:308–328. [DOI] [PubMed] [Google Scholar]
- 2. National Institute for Health and Care Excellence (NICE) . Gastro‐Oesophageal Reflux Disease and Dyspepsia in Adults: Investigation and Management, 2014, https://www.nice.org.uk/guidance/cg184/chapter/1-Recommendations [PubMed]
- 3. Lanas A. We are using too many PPIs, and we need to stop: A European perspective. Am J Gastroenterol 2016;111:1085–1086. [DOI] [PubMed] [Google Scholar]
- 4. Abrahamsen B, Vestergaard P. Proton pump inhibitor use and fracture risk—effect modification by histamine H1 receptor blockade. Observational case‐control study using National Prescription Data. Bone 2013;57:269–271. [DOI] [PubMed] [Google Scholar]
- 5. Elaine WY, Bauer SR, Bain PA, Bauer DC. Proton pump inhibitors and risk of fractures: A meta‐analysis of 11 international studies. Am J Med 2011;124:519–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Eom CS, Park SM, Myung SK, Yun JM, Ahn JS. Use of acid‐suppressive drugs and risk of fracture: A meta‐analysis of observational studies. Ann Fam Med 2011;9:257–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Charlot M, Ahlehoff O, Norgaard ML, et al. Proton‐pump inhibitors are associated with increased cardiovascular risk independent of clopidogrel use: A nationwide cohort study. Ann Intern Med 2010;153:378–386. [DOI] [PubMed] [Google Scholar]
- 8. Melloni C, Washam JB, Jones WS, et al. Conflicting results between randomized trials and observational studies on the impact of proton pump inhibitors on cardiovascular events when coadministered with dual antiplatelet therapy systematic review. Circ Cardiovasc Qual Outcomes 2015;8:47–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ghebremariam YT, LePendu P, Lee JC, et al. Unexpected effect of proton pump inhibitors: Elevation of the cardiovascular risk factor asymmetric dimethylarginine. Circulation 2013;128:845–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Fohl AL, Regal RE. Proton pump inhibitor‐associated pneumonia: Not a breath of fresh air after all. World J Gastrointest Pharmacol Ther 2011;2:17–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Eom CS, Jeon CY, Lim JW, Cho EG, Park SM, Lee KS. Use of acid‐suppressive drugs and risk of pneumonia: A systematic review and meta‐analysis. Can Med Assoc J 2011;183:310–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Johnstone J, Nerenberg K, Loeb M. Meta‐analysis: proton pump inhibitor use and the risk of community‐acquired pneumonia. Aliment Pharmacol Ther 2010;31:1165–1177. [DOI] [PubMed] [Google Scholar]
- 13. Giuliano C, Wilhelm SM, Kale‐Pradhan PB. Are proton pump inhibitors associated with the development of community‐acquired pneumonia? A meta‐analysis. Expert Rev Clin Pharmacol 2012;5:337–344. [DOI] [PubMed] [Google Scholar]
- 14. Lambert AA, Lam JO, Paik JJ, Ugarte‐Gil C, Drummond MB, Crowell TA. Risk of community‐acquired pneumonia with outpatient proton‐pump inhibitor therapy: A systematic review and meta‐analysis. PLoS One 2015;10:e0128004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Meijvis SCA, Cornips MCA, Voorn GP, et al. Microbial evaluation of proton‐pump inhibitors and the risk of pneumonia. Eur Respir J 2011;38:1165–1172. [DOI] [PubMed] [Google Scholar]
- 16. Othman F, Crooks CJ, Card TR. Community acquired pneumonia incidence before and after proton pump inhibitor prescription: Population based study. BMJ 2016;355:i5813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Filion KB, Chateau D, Targownik LE, et al. Proton pump inhibitors and the risk of hospitalisation for community‐acquired pneumonia: replicated cohort studies with meta‐analysis. Gut 2014;63:552–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Brophy S, Jones KH, Rahman MA, et al. Incidence of Campylobacter and Salmonella infections following first prescription for PPI: A cohort study using routine data. Am J Gastroenterol 2013;108:1094–1100. [DOI] [PubMed] [Google Scholar]
- 19. Tannen RL, Weiner MG, Xie D. Use of primary care electronic medical record database in drug efficacy research on cardiovascular outcomes: Comparison of database and randomised controlled trial findings. BMJ 2009;338:b81. d [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Uddin MJ, Groenwold RHH, van Staa TP, et al. Performance of prior event rate ratio adjustment method in pharmacoepidemiology: A simulation study. Pharmacoepidemiol Drug Saf 2015;24:468–477. [DOI] [PubMed] [Google Scholar]
- 21. Lin NX, Henley W. Prior event rate ratio adjustment for hidden confounding in observational studies of treatment effectiveness: A pairwise Cox likelihood approach. Stat Med 2016;35:5149–5169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Pasina L, Nobili A, Tettamanti M, et al. Prevalence and appropriateness of drug prescriptions for peptic ulcer and gastro‐esophageal reflux disease in a cohort of hospitalized elderly. Eur J Intern Med 2011;22:205–210. [DOI] [PubMed] [Google Scholar]
- 23. Jarchow‐Macdonald AA, Mangoni AA. Prescribing patterns of proton pump inhibitors in older hospitalized patients in a Scottish health board. Geriatr Gerontol Int 2013;13:1002–1009. [DOI] [PubMed] [Google Scholar]
- 24. Prina E, Ranzani OT, Torres A. Community‐acquired pneumonia. Lancet 2015;386:1097–1108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Musher DM, Thorner AR. Community‐acquired pneumonia. N Engl J Med 2014;371:1619–1628. [DOI] [PubMed] [Google Scholar]
- 26. File TM, Marrie TJ. Burden of community‐acquired pneumonia in North American adults. Postgrad Med 2010;122:130–141. [DOI] [PubMed] [Google Scholar]
- 27. Herrett E, Gallagher AM, Bhaskaran K, et al. Data resource profile: Clinical Practice Research Datalink (CPRD). Int J Epidemiol 2015;44:827–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. British National Formulary, 65th Ed London: BMJ Group and Pharmaceutical Press; 2010. [Google Scholar]
- 29. Lim WS, Van der Eerden MM, Laing R, et al. Defining community acquired pneumonia severity on presentation to hospital: An international derivation and validation study. Thorax 2003;58:377–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zingone F, Abdul Sultan A, Crooks CJ, Tata LJ, Ciacci C, West J. The risk of community‐acquired pneumonia among 9803 patients with coeliac disease compared to the general population: A cohort study. Aliment Pharmacol Ther 2016;44:57–67. [DOI] [PubMed] [Google Scholar]
- 31. Lewis JD, Bilker WB, Weinstein RB, Strom BL. The relationship between time since registration and measured incidence rates in the General Practice Research Database. Pharmacoepidemiol Drug Saf 2005;14:443–451. [DOI] [PubMed] [Google Scholar]
- 32. Khan NF, Perera R, Harper S, Rose PW. Adaptation and validation of the Charlson Index for Read/OXMIS coded databases. BMC Fam Pract 2010;11:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Austin PC. An introduction to propensity score methods for reducing the effects of confounding in observational studies. Multivariate Behav Res 2011;46:399–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Vansteelandt S, Daniel RM. On regression adjustment for the propensity score. Stat Med 2014;33:4053–4072. [DOI] [PubMed] [Google Scholar]
- 35. Brookhart MA, Schneeweiss S, Rothman KJ, Glynn RJ, Avorn J, Sturmer T. Variable selection for propensity score models. Am J Epidemiol 2006;163:1149–1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Yu M, Xie D, Wang X, Weiner MG, Tannen RL. Prior event rate ratio adjustment: Numerical studies of a statistical method to address unrecognized confounding in observational studies. Pharmacoepidemiol Drug Saf 2012;21 Suppl 2:60–68. [DOI] [PubMed] [Google Scholar]
- 37. Efron B, Tibshirani RJ. An introduction to the bootstrap. Boca Raton, Florida: Chapman & Hall; 1993. [Google Scholar]
- 38. Barratt AL Guyatt G, Simpson JM WP. NNT for studies with long‐term follow‐up. Can Med Assoc J 2005;172:613–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Streeter AJ, Lin NX, Crathorne L, et al. Adjusting for unmeasured confounding in nonrandomized longitudinal studies: A methodological review. J Clin Epidemiol 2017;87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Estborn L, Joelson S. Frequency and time to onset of community‐acquired respiratory tract infections in patients receiving esomeprazole: A retrospective analysis of patient‐level data in placebo‐controlled studies. Aliment Pharmacol Ther 2015;42:607–613. [DOI] [PubMed] [Google Scholar]
- 41. Eom C‐S, Park SM, Myung S‐K, Yun JM, Ahn J‐S. Use of acid‐suppressive drugs and risk of fracture: A meta‐analysis of observational studies. Ann Fam Med 9:257–267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Ho S‐W, Tsai M‐C, Teng Y‐H, et al. Population‐based cohort study on the risk of pneumonia in patients with non‐traumatic intracranial haemorrhage who use proton pump inhibitors. BMJ Open 2014;4:e006710. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Tables S1–S6
Figure S1. Flow diagram: pre‐selection of PPI‐treated individuals and controls.
Figure S2. The retrospective cohort study design with the prior event rate ratio (PERR) adjustment.


