Abstract
Objective
In dermatology, patient and physician adoption of light‐emitting diode (LED) medical technology continues to grow as research indicates that LEDs may be used to treat skin conditions. The goal of this systematic review is to critically analyze published randomized controlled trials (RCTs) and provide evidence‐based recommendations on the therapeutic uses of LEDs in dermatology based on published efficacy and safety data.
Methods
A systematic review of the published literature on the use of LED treatments for skin conditions was performed on September 13th 2017.
Results
Thirty‐one original RCTs were suitable for review.
Conclusions
LEDs represent an emerging modality to alter skin biology and change the paradigm of managing skin conditions. Acne vulgaris, herpes simplex and zoster, and acute wound healing received grade of recommendation B. Other skin conditions received grade of recommendation C or D. Limitations of some studies include small patient sample sizes (n < 20), absent blinding, no sham placebo, and varied treatment parameters. Due to few incidences of adverse events, affordability, and encouraging clinical results, we recommend that physicians use LEDs in clinical practice and researchers continue to explore the use of LEDs to treat skin conditions. Lasers Surg. Med. 50:613–628, 2018. © 2018 The Authors. Lasers in Surgery and Medicine Published by Wiley Periodicals, Inc.
Keywords: light‐emitting diode, phototherapy, photobiomodulation, skin therapy
Abbreviations
- CO2
Carbon dioxide
- ER:YAG
Erbium‐doped yttrium aluminum garnet
- FDA
Federal Drug Administration
- IPL
Intense pulsed light
- HSV
Herpes simplex virus
- HZV
Herpes zoster virus
- LED
Light‐emitting diode
- LED‐BL
Light‐emitting diode blue light
- LED‐nIR
Light‐emitting diode near infrared
- LED‐RL
Light‐emitting diode red light
- LED‐WL
Light‐emitting diode white light
- LED‐YL
Light‐emitting diode yellow light
- PDT
Photodynamic therapy
- RCT
Randomized Controlled Trial
- PRISMA
Preferred Reporting Items for Systematic Reviews and Meta‐Analyses
- UV
Ultraviolet
- WHO
World Health Organization
INTRODUCTION
In dermatology, patient and physician adoption of light‐emitting diode (LED) medical technology continues to grow as research indicates that LEDs may be used to treat skin conditions. This increased level of interest is evidenced by a doubling of the number of articles published and PubMed indexed on LEDs per year since 2010 (Fig. 1). LEDs are combinable with systemic and topical therapies and may be clinically advantageous due to efficacy, excellent safety of non‐ionizing wavelengths, low cost, ease of home use by patients, and portability.
Figure 1.
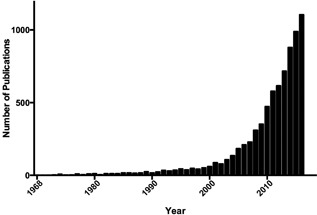
PubMed cited articles on light‐emitting diodes (1968–2016). The number of PubMed indexed articles on light‐emitting diodes by publication year (1968–2016). Since 2010, the total number of articles published on light‐emitting diodes per year has more than doubled.
LEDs utilize high‐efficiency semiconductors to produce non‐coherent, non‐collimated light in the ultraviolet (UV), visible, and near‐infrared ranges of the electromagnetic spectrum (approximately 255–1300 nm) 1. LEDs may treat skin conditions by altering intrinsic cellular activity according to the principles of photobiomodulation 1. Chromophores in the skin, such as mitochondrial cytochrome C, endogenous protoporphyrins, and melanin, absorb photons, and cause downstream alterations in skin biophysiology that can manifest as changes in cellular proliferation, differentiation, migration, inflammation, or collagen production 2, 3, 4. When comparing LED therapy, the following descriptive treatment parameters are commonly used: (i) the wavelength or color of light; (ii) the fluence or the amount of energy received per unit of skin surface area (unit: J/cm2); (iii) the power density or energy delivered per surface area of skin (W/cm2); (iv) treatment period (Seconds); and (v) duty cycle or fraction of treatment length in which light is delivered (expressed as a percentage of treatment period). Each wavelength has unique biophysiological properties due to differences in chromophore targets and how deeply each wavelength penetrates the skin 2. The relationship between power density, session length, and fluence can be described using this general equation:
The goal of this systematic review is to critically analyze published randomized controlled trials (RCTs) and provide evidence‐based recommendations on the therapeutic uses of LEDs in dermatology based on published efficacy and safety data.
METHODS
We performed a search strategy according to Preferred Reporting Items for Systematic Reviews and Meta‐analysis (PRISMA) protocol on September 13th, 2017. The bibliographies of included publications were checked for additional relevant articles that were not identified in the database search. Each article was independently reviewed by two of the authors. We included published RCTs that used LEDs therapeutically for skin conditions. We excluded articles pertaining to UV light as its therapeutic effects and mechanism of action have been well studied. We excluded studies that lacked an LED‐only treatment arm when other photoactive drugs, photosensitizers, lasers, and light‐based devices were used. Reviews, conference abstracts, presentations, basic science manuscripts, animal studies, and non‐English articles were excluded. A research librarian assisted with the systematic search and the accuracy and completeness of included and excluded articles (Fig. 2).
Figure 2.
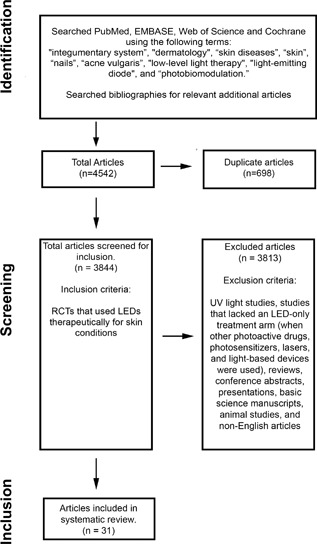
PRISMA search strategy. Search strategy according to preferred reporting items for systematic Reviews and meta‐analysis (PRISMA) protocol.
RESULTS
Our systematic search identified 4,542 articles. After screening titles, abstracts, and full text articles, 31 original RCTs using LED blue light (LED‐BL), LED red light (LED‐RL), LED near‐infrared light (LED‐nIR) and/or yellow light (LED‐YL) were suitable for review: acne vulgaris (8), herpes simplex and zoster [HSV, HZV] (3), skin rejuvenation (6), acute wound healing (5), psoriasis (3), atopic dermatitis (1), chronic wound healing (2), oral mucositis (1), radiation dermatitis (1), and thigh cellulite reduction (1) (Table 1). Grades of recommendation were assigned based on the Oxford Centre for Evidence‐based Medicine—Levels of Evidence 5. Table 1 provides a detailed summary of the identified studies and highlights the grades of recommendation, study designs, treatment parameters, results, and adverse events.
Table 1.
RCTs Using Light‐Emitting Diodes
| Author | Total # of Patients/Drop‐Out | Study Design and Biases | Follow‐Up | Primary Outcome | Treatment Parameters | Treatment Regimen | Results | Adverse Events |
|---|---|---|---|---|---|---|---|---|
| FDA‐cleared LED treatments of skin conditions | ||||||||
| Acne vulgaris (8)—Grade of recommendation: B | ||||||||
| Ash et al. 6 | 41/5 | Rater‐blinded, no placebo | 12‐week | Lesion count | LED‐BL (414‐nm, 17.6 J/cm2)* | Every other day for 8 weeks | 50.08% decrease | None reported |
| No treatment | 2.45% increase | |||||||
| Gold et al. 7 | 30/0 | Placebo‐controlled, split‐face | 10‐day or until resolution | Lesion size | LED‐BL (414‐nm)* | Four treatments over 2 days | Lesion size—76% decrease Clearance—37% | None reported |
| Sham placebo | Lesion size—41% decrease Clearance—10% | |||||||
| Kwon et al. 8 | 35/3 | Double‐blind, placebo‐controlled | 12‐week | Lesion count | LED‐BL (420‐nm, 6.1 mW/cm2, 0.91 J/cm2) and LED‐RL (660‐nm, 8.1 mW/cm2 1.22 J/cm2) for 2.5 minutes (100% duty cycle) | Twice daily for 4 weeks | Inflammatory lesions—77% decrease Non‐inflammatory lesions—54% decrease | Mild dryness, erythema, and desquamation |
| Sham placebo | No significant change from baseline | |||||||
| Liu et al. 9 | 20/0 | Rater‐blinded, no placebo | 8‐week | Inflammatory lesion count | LED‐BL (405‐nm, 6.0 mW/cm2, 7.2 J/cm2) for 20 minutes. Five regions of face received 20% each of total irradiation | Twice weekly for 4 weeks | 71.4% decrease | Skin dryness |
| LED‐RL (630‐nm, 9.6 mW/cm2, 11.52 J/cm2) for 20 minites. Five regions of face received 20% each of total irradiation | 19.5% decrease | |||||||
| Liu et al. 10 | 150/0 | Split‐face, no placebo, no blinding | 4‐month | Sessions till 90% clearance of inflammatory lesions | 5% ALA PDT (633‐nm, 105 mW/cm2, 126 J/cm2) for 20 minutes | Weekly until 90% clearance | 3 ± 1.52 sessions | PDT: Pain, erythema, and edema LED and IPL: Minimal erythema and stinging |
| IPL (420‐nm, 11‐15 J/cm2, 30‐40 ms pulses) | 6 ± 2.15 sessions | |||||||
| LED‐RL (633‐nm, 105 mW/cm2, 126 J/cm2, 50% duty cycle) and LED‐BL (415‐nm, 40 mW/cm2, 48 J/cm2, 50% duty cycle) for 40 minutes | Twice weekly until 90% clearance | 9 ± 3.34 sessions | ||||||
| Na et al. 11 | 30/2 | Split‐face, rater‐blinded, no placebo | 16‐week | Lesion count | LED‐RL (635‐670‐nm; 6 mW/cm2, 5.4 J/cm2, 100% duty cycle) for 15 minutes | Twice a day for 8 weeks | Inflammatory lesions—66% decrease Non‐inflammatory lesions—59% decrease | Burning sensation |
| No treatment | Inflammatory lesions—74% increase Non‐inflammatory lesions—3% increase | |||||||
| Nestor et al. 12 | 105/13 | Double‐blinded, no placebo, missing control groups | 12‐week | Lesion count | LED‐BL (445‐nm) and LED‐RL (630‐nm)* | N/A | Inflammatory lesions—24.4% decrease Non‐inflammatory lesions—19.5% decrease | No adverse events |
| LED‐BL and LED‐RL and 1% salicylic acid/retinol* | Inflammatory lesions—22.7% decrease Non‐inflammatory lesions—4.8% decrease | |||||||
| Topical benzoyl peroxide | Inflammatory lesions—17.2% decrease Non‐inflammatory lesions—6.3% decrease | |||||||
| Sami et al. 13 | 45/0 | Split‐face, rater‐blinded, no placebo | 1‐month following last treatment | Sessions till 90% clearance of inflammatory lesions | PDL (595‐nm, 6‐8 J/cm2, 40 ms pulse, 75% duty cycle) | Weekly until 90% clearance | 4.1 ± 1.39 sessions | PDL: Mild purpura and PIH LED: No adverse events IPL: Slight stinging and erythema |
| IPL (550‐1200‐nm, 22 J/cm2, 30 ms pulses) | 6.0 ± 2.05 sessions | |||||||
| LED‐RL (623‐nm, 40 mW/cm2, 48 J/cm2, 50% duty cycle) and LED‐BL (470‐nm, 10 mW/cm2,12 J/cm2, 50% duty cycle) for 20 minutes | Twice weekly until 90% clearance | 10 ± 3.34 sessions | ||||||
| Herpes simplex and zoster (3)—Grade of recommendation: B | ||||||||
| Dougal and Lee 14 | 87/7 | Double‐blind, placebo‐controlled | 16‐day | Healing time | LED‐nIR (1072‐nm) for 3 minutes* | Six times over 2 days | 5.9 ± 2.6 days | None reported |
| Sham Placebo | 7.5 ± 3.0 days | |||||||
| Hargate 15 | 32/5 | Double‐blind, placebo‐controlled, self‐reported | 12‐day | Healing time | LED‐nIR (1072‐nm)* | Six times over 2 days | 6.3 ± 2.99 days | No adverse events |
| Sham Placebo | 9.4 ± 4.58 days | |||||||
| Park et al. 16 | 28/0 | Rater‐blinded, no placebo | 20‐day | Healing time | LED‐nIR (830‐nm, 55 mW/cm2, 33 J/cm2, 100% duty cycle) for 10 minutes and oral famciclovir | LED‐nIR on days 0, 4, 7, and 10 | 13.14 ± 2.34 days | No adverse events |
| oral famciclovir | 15.92 ± 2.55 days | |||||||
| Skin rejuvenation (6)—Grade of recommendation: C | ||||||||
| Bhat et al. 17 | 23/1 | Split‐face, rater‐blinded, no placebo | 12‐week | Elasticity and hydration | LED‐RL (630‐nm, 80 mW/cm2, 96 J/cm2, 100% duty cycle) for 20 minutes | Three times a week for 3 weeks | No difference between LED‐RL and control side | None reported |
| No treatment | ||||||||
| Lee et al. 18 | 112/36 | Split‐face, double‐blinded, placebo‐controlled | 16‐week | Wrinkles and elasticity | LED‐RL (633‐nm, 126 J/cm2, 55 mW/cm2, 100% duty cycle) for 20 minutes | Twice weekly for 4 weeks | Wrinkles: 26% improvement, elasticity: 14% improvement | No adverse events |
| LED‐nIR (830‐nm, 55 mW/cm2, 66 J/cm2, 100% duty cycle) for 20 minutes | Wrinkles: 33% improvement, elasticity: 19% improvement | |||||||
| LED‐RL and LED‐nIR | Wrinkles: 36% improvement, elasticity: 16% improvement | |||||||
| Sham Placebo | Wrinkles: No difference, elasticity: no difference | |||||||
| Miglardi et al. 19 | 30/0 | Patient rated outcomes, no blinding, no placebo | 2‐month after last treatment | Patient satisfaction | LED‐RL (633‐nm, 50% duty cycle) and LED‐nIR (880‐nm, 50% duty cycle) for 1.17 minutes* | Every 5 days for 40 days | 100% satisfaction | No adverse events |
| RF | Every 10 days for 50 days | 100% satisfaction | ||||||
| LED‐RL, LED‐nIR, and RF* | 1 RF and 2 LED treatments every 5 days for 45 days | 100% satisfaction | ||||||
| Nam et al. 20 | 52/2 | Double‐blind, no placebo | 12‐week | Skin roughness and physician assessment | LED‐RL (660‐nm, 5.17 J/cm2, 7.5 mW/cm2, 15% duty cycle) for 11.5 minutes | Daily for 12 weeks | Improvements in 3/5 roughness parameters compared to baseline. No difference in physician assessment | Ocular symptoms |
| LED‐WL (411‐777‐nm, 7.5 mW/cm2, 15% duty cycle) for 11.5 minutes | Improvements in 4/5 roughness parameters compared to baseline. No difference in physician assessment | |||||||
| Nikolis et al. 21 | 32/2 | Placebo‐controlled, single‐blind, split‐faced | 12‐week | Total wrinkle score | LED‐BL (446‐nm, 45 J/cm2, 150 mW/cm2, 100% duty cycle) for 5 minutes and chromophore gel | Weekly for 4 weeks | Significant improvement | Edema and erythema |
| LED‐BL and placebo gel | Significant improvement | |||||||
| LED‐WL and chromophore gel* | Significant worsening | |||||||
| 0.1% retinol‐based cream | No difference | |||||||
| Stirling and Haslam 22 | 79/1 | Double‐blind, placebo‐controlled, patient rated outcomes. | 6‐10 week | Patient assessment | LED‐nIR (1072‐nm) for 3 minutes* | Daily for 8–10 weeks | 52% reported improvement | No adverse events |
| Sham placebo | 20% reported improvement | |||||||
| Non‐FDA cleared LED treatments of skin conditions | ||||||||
| Acute wound healing (4)—Grade of recommendation: B | ||||||||
| Alster and Wanitphakdeedecha 23 | 20/0 | Split‐face, rater‐blinded, no placebo | 96‐hour | Erythema | LED‐YL (590‐nm, 0.1 J/cm2, 2.86 mW/cm2) for 35 seconds following erbium‐doped fiber laser | Once following laser treatment | At 24 hours less erythema in 20/20 patients in LED‐YL treatment group. At 48 hours, less erythema in 6/20 patients. No difference at 96‐hour follow‐up | None reported |
| No treatment following erbium‐doped fiber laser | ||||||||
| Bay et al. 27 | 20/0 | Split‐body, double‐blind | 11‐day | Physician assessment, erythema and hyperpigmentation | LED‐nIR (830‐nm, 65 J/cm2, 109 mW/cm2) and LED‐YL (595‐nm, 0.13 J/cm2, 0.19 mW/cm2) for 11 minutes following CO2 laser assisted red light PDT. Unclear duty cycle for LED‐YL and LED‐nIR | Daily for 5 days starting one day before CO2 assisted PDT | No difference in physician assessment, erythema, or hyperpigmentation | None reported |
| LED‐YL (595‐nm, 0.13 J/cm2, 0.19 mW/cm2) for 11 minutes following CO2 laser assisted red light PDT | ||||||||
| Chaves et al. 26 | 16/6 | Double‐blind, placebo‐controlled, small population (< 20) | 4‐week | Sessions to heal and pain | LED‐nIR (860‐nm, 4 J/cm2, 50 mW/cm2, 50% duty cycle) for 79 seconds | Twice weekly for 4 weeks | 2‐4 sessions, clinically significant reduction in pain following 6 out of 8 treatment sessions. 5‐8 sessions, no change in pain after sessions | No adverse events |
| Sham placebo | ||||||||
| Khoury and Goldman 24 | 15/0 | Split‐face, rater‐blinded, small patient population (< 20), no placebo | 1‐week | Erythema score | LED‐YL (590‐nm, 71.4% duty cycle) for 35 seconds following IPL (16‐22 J/cm2)* | Once following laser treatment and once at 24 hours post treatment | 43.3 ± 21.9 erythema score immediately after treatment. 16.0 ± 15.9 after 24 hours. No difference after 1 week | None reported |
| No treatment following IPL | 52.7 ± 24.6 erythema score immediately after treatment. 20.0 ± 18.5 after 24 hours. No difference after 1 week | |||||||
| Trelles et al. 25 | 28/0 | Split‐face, rater‐blinded, no placebo | 6‐month | Physician assessment | LED‐RL (633‐nm, 96 J/cm2, 80 mW/cm2, 100% duty cycle) for 20 minutes and LED‐nIR (830‐nm, 60 J/cm2, 55 mW/cm2, 100% duty cycle) following ER:YAG/CO2 laser | LED‐nIR immediately and 72 hours following ER:YAG/CO2 laser. Then three LED‐RL treatments in following 2 weeks | 93% efficacy at 3‐month follow‐up.100% efficacy at 6‐month follow‐up. 50% increase in healing time | None reported |
| No treatment following ER:YAG/CO2 laser | 86% efficacy at 3‐month follow‐up. 97% efficacy at 6‐month follow‐up | |||||||
| Psoriasis (3)—Grade of recommendation: C | ||||||||
| Kleinpenning et al. 28 | 27/0 | Split‐face, double‐blind, no placebo | 4‐week | SUM score | LED‐RL (630‐nm, 60 J/cm2, 50 mW/cm2, 100% duty cycle) for 20 minutes and salicylic acid | LED − 3 times a week for 4 weeks; salicylic acid‐ daily for 4 weeks | 26.7% improvement | Burning sensation and hyperpigmentation |
| LED‐BL (420‐nm, 120 J/cm2, 50 mW/cm2, 100% duty cycle) for 20 minutes and salicylic acid | 33.9% improvement | |||||||
| 10% Salicylic acid | 39.4% improvement | |||||||
| Pfaff et al. 29 | 47/2 | Split‐face, double‐blind, no placebo | 16‐week | LPSI | LED‐BL (453‐nm, 90 J/cm2, 200 mW/cm2) for 30 minutes. Duty cycle differed between treatments but is not directly stated* | Daily (5–7 days) for 4 weeks followed by thrice weekly for 8 weeks | −0.92 ± 1.1 LPSI change | Changes in pigmentation |
| LED‐BL (453‐nm, 90 J/cm2, 100 mW/cm2) for 30 minutes. Duty cycle differed between treatments but is not directly stated* | −0.74 ± 1.18 LPSI change | |||||||
| Weinstbl et al. 30 | 40/3 | Split face, double‐blind, no placebo | 6‐week | LPSI | LED‐BL (420‐nm, 90 J/cm2, 100 mW/cm2) for 15 minutes | Daily for 4 weeks | Significant improvement compared to untreated plaque at week‐4, but not week‐6 | Hyperpigmentation |
| LED‐BL (453‐nm, 90 J/cm2,100 mW/cm2) for 15 minutes | Significant improvement compared to untreated plaque at week‐4, but not week‐6 | |||||||
| Atopic dermatitis (1)—Grade of recommendation: D | ||||||||
| Keemss et al. 31 | 21/1 | Split‐face, no placebo | 6‐week | Eczema severity index | LED‐BL (453‐nm, 90 J/cm2)* | Thrice weekly for 4 weeks | 30.4% improvement following LED‐BL | Mild hyperpigmentation |
| No treatment | ||||||||
| Chronic wound healing (2)—Grade of recommendation: D | ||||||||
| Frangez et al. 33 | 80/1 | Double‐blind, placebo‐controlled | 8‐week | Circulation and Falanga wound bed score | Diabetic chronic wound: LED‐RL (625‐nm, 24% of power density and 660‐nm, 71% of power density) and LED‐nIR (850‐nm, 5% of power density). Total 2.4 J/cm2, 50% duty cycle for 5 minutes. Power density not specified* | Three times weekly for 8 weeks | 29% increase in blood flow. Significant improvement in Falanga wound bed score compared to placebo. | None reported |
| Diabetic chronic wound: placebo (580‐900‐nm, 0.72 J/cm2) for 5 minutes* | 11% increase in blood flow | |||||||
| Non‐diabetic chronic wound: LED‐RL and LED‐nIR | 48% increase in blood flow. Significant improvement in Falanga wound bed score compared to placebo | |||||||
| Non‐diabetic wound: placebo | 12% decrease in blood flow | |||||||
| Siqueira et al. 32 | 17/2 | Double‐blind, placebo‐controlled, small patient population (<20) | 30‐week | Ulcer surface area and healing rate | LED‐RL (625‐nm, 4 J/cm2, 25 mW/cm2) for 2.67 minutes and Unna boot. In large ulcers (>1 cm2), five areas of wound received of 4 J/cm2 for total of 20 J/cm2 for 800 seconds | Weekly for 30 weeks | Ulcer surface area change 9.8% of baseline (9.8% to 31.2% quartiles). Healing time hazard ratio of 0.89 (95%CI 0.4‐1.98) | None reported |
| Sham placebo and Unna boot | Ulcer surface area change 112% of baseline (18.7% to 417% quartiles) | |||||||
| Oral mucositis (1)—Grade of recommendation: D | ||||||||
| Hodgson et al. 35 | 80/0 | Double‐blind, placebo‐controlled | 2‐week | WHO pain assessment scale | LED‐RL (670‐nm, 4 J/cm2, 50 mW/cm2) for 80 s | Daily for 2 weeks | 44% less pain in LED‐RL high‐risk group compared to sham placebo high‐risk group. No difference for low‐risk group | None reported |
| Sham Placebo | ||||||||
| Radiation dermatitis (1)—Grade of recommendation: D | ||||||||
| Fife et al. 38 | 33/4 | Double‐blind, placebo‐controlled | 6‐week | NCI grading | LED‐YL (590‐nm, 71.4% duty cycle) for 35 seconds* | Before and after each radiation session and seven additional treatments for 2 weeks | No difference in NCI grades between groups | No adverse events |
| Sham Placebo (machine not turned on) | ||||||||
| Thigh cellulite reduction (1)—Grade of recommendation: D | ||||||||
| Sasaki et al. 39 | 9/0 | Split‐face, double‐blind, small patient population (<20) | 18‐month | Thigh cellulite grade | LED‐RL (660‐nm) and LED‐nIR (950‐nm) and placebo gel* | Twice weekly for 12 weeks | 0/9 thighs improved | None reported |
| LED‐RL and LED‐nIR and phosphatidylcholine gel* | 8/9 thighs improved at 3‐month follow‐up. Recurrence in 3/8 thighs | |||||||
LED, Light‐emitting diode; RL, Red light; BL, Blue light; YL, Yellow light; WL, White light; nIR, Near infrared; PDT, Photodynamic therapy; IPL, Intense pulsed light; RF, Radiofrequency; PDL, Pulsed dye light; ER:YAG, Erbium‐doped yttrium aluminum garnet; CO2, Carbon dioxide; WHO, World Health Organization; NCI, National Cancer Institute; LPSI, Local Psoriasis Severity Index; Min, Minimum; Max, Maximum; CI, Confidence interval, PIH, Post‐inflammatory Hyperpigmentation; OMI, Oral Mucositis Index.
If LED treatment parameters (ie, fluence, power density, or treatment length) were not included in the original article, an asterisk (*) marks the treatment parameters.
CHARACTERISTICS OF LED DEVICES
Among the reviewed studies, there were greater than 20 different LED devices used. A majority of reviewed studies used FDA‐cleared or commercially available LED devices (Table 2). LED treatment parameters (wavelength, power density, fluence, and session length) are included in the description of each study and Table 1. If LED treatment parameters were not included in the original article, an asterisk (*) marks the treatment parameters in text. Duty cycle is 100% unless otherwise indicated.
Table 2.
FDA‐Cleared LED Treatments of Skin Conditions
| Device Wavelength | Device Names (Manufacturer) | Skin Indication |
|---|---|---|
| LED‐BL | Tanda Zap (Syneron), Illumask (La Lumiere/Neutrogena/Johnson & Johnson), Omnilux Blue (Photo Therapeutics) | Mild to moderate acne |
| LED‐RL | Young Again (Espansione), Omnilux Revive (Photo Therapeutics) | Acne vulgaris, vascular/pigmented lesions, and rhytides |
| LED‐YL | Gentlewaves (Light Bioscience) | Rhytides |
| LED‐nIR | Young Again (Espansione), Virtulite cold sore machine (Virtulite) | Rhytides and facial herpes simplex |
FDA‐CLEARED LED TREATMENTS OF SKIN CONDITIONS
Acne Vulgaris—Grade of Recommendation: B
Eight RCTs used LEDs for acne vulgaris (2 LED‐BL; 1 LED‐RL; 5 LED‐BL and LED‐RL) 6, 7, 8, 9, 10, 11, 12, 13. One RCT of 41 patients used LED‐BL* (414‐nm, 17.6 J/cm2) every other day for 8 weeks and demonstrated a 52% reduction in lesion count compared to no treatment control 6. In a placebo‐controlled RCT of 30 patients, LED‐BL* (414‐nm) decreased lesion size by 35% after twice‐daily treatment for 2 days 7.
In one split‐face RCT of twice daily LED‐RL (635–670‐nm, 6 mW/cm2, 5.4 J/cm2,15 minutes) for 8 weeks, there was a 66% and 59% reduction in inflammatory and non‐inflammatory lesion count, respectively. However, by 16‐week follow‐up, 21 out of 22 patients complained of acne recurrence 11. One RCT of 20 patients compared twice weekly LED‐RL (630‐nm, 9.6 mW/cm2, 11.52 J/cm2, 20 minutes) to LED‐BL (405‐nm, 6.0 mW/cm2, 7.2 J/cm2, 20 minutes) for 4 weeks in which five regions of the face received 20% of total irradiation each; LED‐BL reduced lesion count by 71.4% compared to 19.5% in LED‐RL 9.
Two RCTs of 105 and 35 patients used combination LED‐BL* (445‐nm or 420‐nm, 6.1 mW/cm2, 0.91 J/cm2, 2.5 minutes) and LED‐RL* (630‐nm or 660‐nm, 8.1 mW/cm2 1.22 J/cm2, 2.5 minutes). LED‐BL and LED‐RL reduced inflammatory lesion count (24–77%) compared to placebo control (0%) or topical benzoyl peroxide treatment (17.2%) groups at 12 week follow‐up 8, 12. Two RCTs of 150 and 45 patients compared time to achieve 90% clearance with combination twice weekly LED‐RL (623‐nm, 40 mW/cm2, 48 J/cm2, 50% duty cycle, 20 minutes or 633‐nm, 105 mW/cm2, 126 J/cm2, 50% duty cycle, 40 minutes) and LED‐BL (470‐nm, 10 mW/cm2,12 J/cm2, 50% duty cycle, 20 minutes or 415‐nm, 40 mW/cm2, 48 J/cm2, 50% duty cycle, 40 minutes) compared to weekly photodynamic therapy (PDT), intense pulse light (IPL) or pulsed dye laser therapy (PDL) 10, 13. All treatments improved acne compared to baseline, but LED‐BL and LED‐RL required 2–3 times as many sessions to achieve 90% clearance compared to PDL, IPL, and PDT.
Clinical recommendation
We recommend LED‐BL or LED‐RL with power densities of 6–40 mW/cm2 or 8–100 mW/cm2, respectively, for 20 minutes to safely reduce inflammation and lesion count. Treatments may be offered twice weekly for 4–8 weeks for best efficacy. The reviewed studies used heterogeneous treatment parameters, and it is difficult to state the exact optimal power density or fluence. We identified more than 10 case series demonstrating similar trends, which support our recommendation. PDL, PDT, and IPL required fewer treatment sessions to achieve clearance, but LEDs may be safe for home use. LEDs may be especially beneficial for pregnant women with acne vulgaris as retinoid treatments are pregnancy class C (ie, animal studies have shown harm, but there are not enough high quality studies in humans to judge safety).
Herpes Simplex and Zoster—Grade of Recommendation: B
Three RCTs used LED‐nIR for the treatment of recurrent facial HSV or HZV 14, 15, 16. In two placebo‐controlled, double‐blind RCTs of 87 and 32 patients, six treatments of LED‐nIR* (1072‐nm) over 2 days resulted in a 2–3 days reduction in re‐epithelialization time in patients with labial HSV infections by 12–16 days follow‐up 14, 15. In a RCT of 28 patients with HZV, LED‐nIR (830‐nm, 55 mW/cm2, 33 J/cm2,10 minutes) for four treatments over 10 days with oral famciclovir resulted in reduced healing time, less atrophic scarring, and fewer incidences of post‐inflammatory hyperpigmentation compared to famciclovir alone treatment 16.
Clinical recommendation
LED‐nIR treatment significantly and consistently reduced healing time by at least 2 days in patients with HSV and HZV. Two of these studies did not describe treatment parameters used and it is therefore difficult to translate the findings to clinical practice. Thrice daily LED‐nIR for 3 days may be a useful at‐home adjunct with standard‐of‐care oral anti‐viral medications to enhance recovery. Based on the results of one of the RCTs the following treatment parameters may be safe and effective: 830‐nm, 55 mW/cm2, 33 J/cm2 for 10 minutes.
Skin Rejuvenation—Grade of Recommendation: C
Six RCTs used LEDs for skin rejuvenation (2 LED‐RL; 1 LED‐nIR; 1 LED‐BL; 2 LED‐RL and LED‐nIR) 17, 18, 19, 20, 21, 22. In a RCT of 23 patients, LED‐RL (630‐nm, 80 mW/cm2, 96 J/cm2, 20 minutes) did not significantly improve skin elasticity or hydration (assessed using cutometers and corneometers) compared to untreated controls after thrice daily treatments for 3 weeks 17. In a different RCT of 52 patients, LED‐RL (660‐nm, 5.17 J/cm2, 7.5 mW/cm2, 15% duty cycle, 11.5 minutes) or LED white light (LED‐WL; 411–777‐nm, 7.5 mW/cm2, 15% duty cycle, 11.5 minutes) improved wrinkles in three out of five parameters using digital analysis but there were no changes in physician assessment 20. In a double‐blind, placebo‐controlled RCT of 79 patients, there was a 32% improvement in skin texture following daily LED‐nIR* (1072‐nm, 3 minutes) treatment for 8–10 weeks by patient self‐assessment. In a RCT of 32 patients, LED‐BL (446‐nm, 45 J/cm2, 150 mW/cm2, 5 minutes) and a placebo gel improved wrinkles compared to a 0.1% retinol‐based cream after four weekly treatments 21.
One placebo‐controlled RCT of 112 patients found that LED‐RL (633‐nm, 126 J/cm2, 55 mW/cm2, 20 minutes), LED‐nIR (830‐nm, 55 mW/cm2, 66 J/cm2, 20 minutes), or combination LED‐RL (50% duty cycle) and LED‐nIR (50% duty cycle) twice weekly for 4 weeks improved wrinkles by 26%, 33%, and 36%, respectively.18 In another RCT, 30 patients were satisfied when receiving LED‐RL* (633‐nm, 50% duty cycle, 1.17 minutes) and LED‐nIR * (880‐nm, 50% duty cycle, 1.17 minutes), radiofrequency, or combination (LED with radiofrequency) treatments after 5–27 treatments over 40–50 days 19.
Clinical recommendation
Clinical evidence indicates that daily LED‐nIR with LED‐RL for 8–10 weeks has the best efficacy in improving rhytides. There is a high level of variability in treatment parameters and future studies may seek to optimize power densities, fluences, and session lengths. Several researchers have used LED‐YL with success in case series, but our search did not reveal any RCTs studying LED‐YL for skin rejuvenation 4. Therapies for skin rejuvenation often have gradual results, and 6‐month or longer follow‐up may be required to assess the efficacy of LEDs for long‐term skin rejuvenation.
NON‐FDA CLEARED LED TREATMENTS OF SKIN CONDITIONS
Acute Wound Healing—Grade of Recommendation: B
Five RCTs used LEDs (1 LED‐nIR; 2 LED‐YL; 1 LED‐RL and LED‐nIR; 1 LED‐nIR and LED‐YL) for enhanced wound healing and recovery following acute trauma or laser skin procedures 23, 24, 25, 26. One double‐blind, placebo‐controlled RCT used twice weekly LED‐nIR (860‐nm, 4 J/cm2, 50 mW/cm2, 50% duty cycle; 1.31 minutes) for 4 weeks to treat nipple trauma in sixteen breastfeeding female patients. There was a reduction in lesion area and pain after LED‐nIR therapy 26. Two split‐face RCTs used LED‐YL* (590‐nm, 0.1 J/cm2, 2.86 mW/cm2; 35 seconds or 590‐nm, 71.4% duty cycle) to improve wound healing and erythema immediately following erbium‐doped laser or IPL therapy for photodamaged skin 23, 24. LED‐YL improved erythema in 20 out of 20 patients and there was a physician‐evaluated reduction in erythema at 24 hours follow‐up 23, 24. In a split‐face RCT of 28 female patients treated with ER:YAG or CO2 laser for photodamaged skin, healing time was 50% faster on the combination LED‐RL (633‐nm, 96 J/cm2, 80 mW/cm2, 50% duty cycle, 20 minutes) and LED‐nIR (830‐nm, 60 J/cm2, 55 mW/cm2, 50% duty cycle, 20 minutes) treated side compared to no treatment after 15 treatments over 3 weeks 25. One double‐blind, split‐body RCT compared combined LED‐nIR (830‐nm, 65 J/cm2, 109 mW/cm2, unclear duty cycle, 11 minutes) and LED‐YL (595‐nm, 0.13 J/cm2, 0.19 mW/cm2, 11 minutes) to LED‐YL alone for reduced erythema and pigmentation following CO2 assisted red light PDT 27. There was no significant difference between LED‐nIR and LED‐YL compared the LED‐YL in physician assessment, erythema, or hyperpigmentation. The authors considered “ultra‐low fluence” LED‐YL as a “placebo,” but low fluence and power density LED‐YL may improve wound healing. As a result, this study is lacking a true placebo.
Clinical recommendation
Daily LED‐YL (590‐nm) or LED‐nIR (830‐nm) until wound resolution may reduce healing time and erythema in acute wound healing processes of different etiologies. For LED‐YL, data indicates that one to 2 minutes of 5 mW/cm2 LED‐YL help acute wound healing process. Higher fluences (5–40 J/cm2), power densities (∼50 mW/cm2), and session length (∼20 minutes) may be required for LED‐nIR treatments. The included RCTs have short follow‐up (7 days or less) and future studies using LED‐YL or LED‐nIR may assess patients at later time points to determine reduction of scarring following LED therapy.
Psoriasis—Grade of Recommendation: C
Three double‐blind, split‐body RCTs used LEDs (2 LED‐BL; 1 LED‐BL and LED‐RL) to manage psoriasis 28, 29, 30. Two split‐body RCTs compared daily LED‐BL of different wavelengths (420‐nm or 453‐nm), irradiances (200 or 100 mW/cm2), and duty cycles (100% or not specified)* for 4 weeks, and both studies showed a significant improvement in local psoriasis severity index compared to the contralateral untreated control plaques 29, 30. In both studies fluence was consistent at 90 J/cm2. Lesions recurred in one of these studies after treatment cessation. One split‐body RCT of 27 patients found that thrice weekly LED‐RL (630‐nm, 60 J/cm2, 50 mW/cm2, 20 minutes) and LED‐BL (420‐nm, 120 J/cm2, 50 mW/cm2, 20 minutes) for 4 weeks reduced patient psoriatic plaque erythema and induration by 26.7% and 33.9%, respectively, but not significantly compared to daily salicylic acid in petroleum after 4 weeks 28 Salicylic acid had the greatest effect on plaque desquamation, while LED‐RL and LED‐BL decreased erythema.
Clinical recommendation
LED‐BL (at least 90 J/cm2, 50 mW/cm, 20 minutes) may be effective for the treatment of psoriasis with best results achieved with daily treatments. The reviewed studies do not provide enough evidence to recommend whether 50, 100, or 200 mW/cm2 power densities are most effective. According to clinical evidence, the treatment parameters and regimens studied have greatest effect on the inflammatory component of psoriasis and not the hyperproliferative component of the psoriatic plaques. Lesions recurred following LED‐BL treatment cessation in one study, a common issue associated with discontinuation of psoriasis treatment.
Atopic Dermatitis—Grade of Recommendation: D
In a split‐face RCT of 21 patients, thrice weekly LED‐BL (453‐nm, 90 J/cm2)* for 4 weeks improved erythema, edema, lichenification, and crusts by 30.4%, according to the eczema severity index 31.
Clinical recommendation
LED‐BL may improve atopic dermatitis. There is limited evidence to make clinical recommendations and additional RCTs are required. We did not identify any non‐RCTs studying LEDs for atopic dermatitis.
Chronic Wound Healing—Grade of Recommendation: D
Two RCTs used LEDs (1 LED‐RL; 1 LED‐RL and LED‐nIR) for chronic wounds 32, 33. One RCT compared LED‐RL (625‐nm, 4–20 J/cm2, 25 mW/cm2 2.67–13.33 minutes) and Unna boot. plus Unna boot to Unna boot alone in patients with chronic venous ulcers 32. Overall healing time was not improved in the LED treatment group. One double‐blind RCT used combination LED‐RL* (625‐nm, 12% duty cycle and 660‐nm, 35.1% duty) and LED‐nIR (850‐nm, 2.5% of power density) for 5 minutes for a total fluence of 2.4 J/cm2 to treat 80 patients with diabetic or non‐diabetic chronic ulcer. Wound healing and blood flow improved by 18–60% compared to LED‐WL* (580–900‐nm, 0.72 J/cm2, 5 minutes) 33.
Clinical recommendation
There is insufficient evidence to recommend LEDs for chronic wounds. We have previously published a review of photobiomodulation therapy of diabetic ulcers, and evidence from case reports and case series show that light therapy may provide benefit 34. Differences in treatment regimen and study sample size powering may be responsible for the contradictory results. Researchers may consider reevaluating successful treatment parameters in larger studies 33.
Oral Mucositis—Grade of Recommendation: D
In one double‐blind RCT of 80 bone‐marrow transplant patients, daily LED‐RL (LED‐RL (670‐nm, 4 J/cm2, 50 mW/cm2,1.33 minutes) for 2 weeks did not alter the onset of oral mucositis compared to placebo 35. One subset of patients, those with regular risk for developing oral mucositis, reported 44% less pain using the World Health Organization (WHO) pain assessment scale following LED‐RL therapy 35.
Clinical recommendation
There is insufficient evidence to suggest that LEDs improve or prevent oral mucositis. RCTs, expert opinion, and anecdotal evidence supports the use of low‐level laser and light‐based therapy over LEDs for patients at high risk for oral mucositis 36.
Radiation Dermatitis—Grade of Recommendation: D
One double‐blind, placebo‐controlled RCT examined the use of LED‐YL* (590‐nm, 71.4% duty cycle, 35 seconds) treatment for 2 weeks to prevent radiation dermatitis in 33 breast cancer patients 37, 38. LED‐YL was applied before and after each radiation session and seven additional times in a 2 week regimen. LED‐YL did not alter the onset or severity of dermatitis as assessed by the National Cancer Institute grading system.
Clinical recommendation
There is insufficient evidence to recommend LEDs for radiation dermatitis. A previous cohort study with the same LED‐YL treatment regimen showed decreased onset of radiation dermatitis, but this RCT was unable to replicate those results 37. Larger sample sizes may be needed to demonstrate benefit.
Thigh Cellulite Reduction—Grade of Recommendation: D
In a double‐blind, split‐face RCT of nine patients, twice weekly LED‐RL* (660‐nm) and LED‐nIR* (950‐nm) for 12 weeks did not improve cellulite with a placebo gel 39. Combination phosphatidylcholine gel, LED‐RL, and LED‐nIR reduced cellulite in eight patients.
Clinical recommendation
We do not recommend LEDs to reduce thigh cellulite, as LED alone did not result in improvement in thigh cellulite reduction.
DISCUSSION
Based upon our systematic review of 31 RCTs, we provide evidence based suggested treatment parameters and regimens for LED therapy for skin conditions which dermatologists may tailor to meet patient needs. Scientific evidence exists that supports that LEDs may improve outcomes in acne vulgaris, HSV, HZV, and acute wound healing. LED treatments were safe and well tolerated by patients. Adverse events were mild and included pigment changes, dryness, erythema, desquamation, and stinging. No severe adverse events were reported. There is a theoretical risk of malignancy and photoaging from LED‐BL as the wavelengths emitted by LED‐BL devices are near UVA, but based on the reviewed studies with a maximum follow‐up of 18 months, there were no reports of carcinogenesis or accelerated photoaging. Outside the scope of this review, LEDs may be used in PDT with topical or systemic medications.
LIMITATIONS
Limitations of some studies include small patient sample sizes (n < 20), absent blinding, no sham placebo, and varied treatment parameters which makes it difficult to compare study outcomes. Future studies using LEDs may address the aforementioned limitations through the use of sham placebo and temperature‐matched controls to ensure that the results are solely due to photobiomodulatory effects. However, with light‐based studies, it is sometimes difficult to blind both provider and patient, and placebo treatments are also challenging. There are several key factors that determine clinical outcomes, and all are important: peak wavelength and distribution range, power density at treatment site, treatment time period, total fluence, and treatment regimen. Although most studies used commercially available LED devices, differences in light output and power densities among manufacturers’ devices may contribute to outcome variability. It is possible that some clinical studies that did not achieve desired outcomes are using LEDs at a sub‐optimal regimen, wavelength, power density, or fluence for the desired therapeutic effect. For example, studies may have used similar wavelength(s) and fluences, but the power densities may be drastically different. A high power density or low power density light source may be used for different treatment session lengths to achieve the same fluences. Even though fluences will be the same, these differences in power densities may alter the results of a study. Pulsing versus continuous treatments may also be significant to clinical outcomes, but there is not enough data to make a recommendation. In the published literature, actual duty cycles may not necessarily equal device on/off time. Due to the angle of divergence inherent in many of the LEDs, the distance to treatment surface is often critical and the delivered power density may be very different than what is published. Surface area in cm2 and therefore power density (W/cm2) may change due to small differences in the distance from the LED to the skin surface. As a result, it is difficult to determine if heterogeneity in treatment parameters changes treatment efficacy. Photobiomodulation tends to have biphasic dose response and LED treatment parameters are often not tailored to specific indications 40. Low‐fluence LED therapies are usually appropriate when cell growth or collagen production is desired, while high‐fluence LED therapies may have inhibitory effects 40. There may be clinical exceptions to this biphasic response. As a result, future RCTs will need to clearly detail treatment parameters and optimize wavelength, fluence, and power density for each skin condition in order to determine the efficacy of LEDs for each skin condition.
CONCLUSION
LEDs represent an emerging modality to alter skin biology and change the paradigm of managing skin conditions. Based on the published evidence, acne vulgaris, HSV, HZV, and acute wound healing received grade of recommendation B. Other skin conditions received grade of recommendation C or D. Due to few adverse events, affordability, and encouraging clinical results, we recommend that physicians use LEDs in clinical practice and researchers continue to explore the use of LEDs to treat skin conditions. As therapeutic LED technology is further translated from a research setting to clinical practice, we anticipate that standardized treatment protocols with consistent treatment wavelengths, fluences, and regimens for additional dermatologic indications will be established.
ACKNOWLEDGMENTS
Bruce Abbot, a research librarian, provided valuable assistance in designing the systematic search and confirming the accuracy and completeness of included and excluded articles. Dr. Jagdeo is a recipient of a National Institutes of Health (NIH) K23 Career Development Award focused on the investigation of novel light‐based anti‐scar therapy. Information reported in this publication is based on research that was supported by the National Institute of General Medical Sciences of the NIH under Award No. K23GM117309.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest and have disclosed the following: Dr. Jagdeo and Dr. Siegel are on the scientific advisory board of Global Med Technologies. Dr. Jagdeo has received honoraria from Global Med Technologies.
Disclaimer: The views expressed in this article are those of the authors and do not necessarily reflect the official policy or position of the NIH, U.S. Department of Veterans Affairs, or the United States government.
REFERENCES
- 1. Barolet D. Light‐emitting diodes (LEDs) in dermatology. Semin Cutan Med Surg 2008;27(4):227–238. [DOI] [PubMed] [Google Scholar]
- 2. de Freitas LF, Hamblin MR. Proposed mechanisms of photobiomodulation or low‐level light therapy. IEEE J Sel Top Quantum Electron 2016;22(3):17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Mamalis A, Koo E, Garcha M, Murphy WJ, Isseroff RR, Jagdeo J. High fluence light emitting diode‐generated red light modulates characteristics associated with skin fibrosis. J Biophotonics 2016;9(11–12):1167–1179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Opel DR, Hagstrom E, Pace AK, et al. Light‐emitting diodes: A brief review and clinical experience. J Clin aesthetic Dermatol 2015;8(6):36. [PMC free article] [PubMed] [Google Scholar]
- 5. Phillips B, Ball C, Sackett D, et al. Oxford Centre for Evidence‐Based Medicine Levels of Evidence Grades of Recommendation (2001). Oxford (UK): Oxford Centre for Evidence‐Based Medicine Google Scholar; 2013. [Google Scholar]
- 6. Ash C, Harrison A, Drew S, Whittall R. A randomized controlled study for the treatment of acne vulgaris using high‐intensity 414 nm solid state diode arrays. J Cosmet Laser Ther 2015;17(4):170–176. [DOI] [PubMed] [Google Scholar]
- 7. Gold MH, Sensing W, Biron JA. Clinical efficacy of home‐use blue‐light therapy for mild‐to moderate acne. J Cosmet Laser Ther 2011;13(6):308–314. [DOI] [PubMed] [Google Scholar]
- 8. Kwon HH, Lee JB, Yoon JY, et al. The clinical and histological effect of home‐use, combination blue‐red LED phototherapy for mild‐to‐moderate acne vulgaris in Korean patients: A double‐blind, randomized controlled trial. Br J Dermatol 2013;168(5):1088–1094. [DOI] [PubMed] [Google Scholar]
- 9. Liu G, Pan C, Li K, Tan Y, Wei X. Phototherapy for mild to moderate acne vulgaris with portable blue and red LED. J innovative Opt Health Sci 2011;04(01):45–52. [Google Scholar]
- 10. Liu LH, Fan X, An YX, Zhang J, Wang CM, Yang RY. Randomized trial of three phototherapy methods for the treatment of acne vulgaris in Chinese patients. Photodermatol Photoimmunol Photomed 2014;30(5):246–253. [DOI] [PubMed] [Google Scholar]
- 11. Na JI, Suh DH. Red light phototherapy alone is effective for acne vulgaris: Randomized, single‐blinded clinical trial. Dermatol Surg 2007;33(10):1228–1232. [DOI] [PubMed] [Google Scholar]
- 12. Nestor MS, Swenson N, Macri A, Manway M, Paparone P. Efficacy and tolerability of a combined 445 nm and 630 nm over‐the‐counter light therapy mask with and without topical salicylic acid versus topical benzoyl peroxide for the treatment of mild‐to‐moderate acne vulgaris. J Clin Aesthetic Dermatol 2016;9(3):25–35. [PMC free article] [PubMed] [Google Scholar]
- 13. Sami NA, Attia AT, Badawi AM. Phototherapy in the treatment of acne vulgaris. J Drugs Dermatol: JDD 2008;7(7):627–632. [PubMed] [Google Scholar]
- 14. Dougal G, Lee SY. Evaluation of the efficacy of low‐level light therapy using 1072 nm infrared light for the treatment of herpes simplex labialis. Clin Exp Dermatol 2013;38(7):713–718. [DOI] [PubMed] [Google Scholar]
- 15. Hargate G. A randomised double‐blind study comparing the effect of 1072‐nm light against placebo for the treatment of herpes labialis. Clin Exp Dermatol 2006;31(5):638–641. [DOI] [PubMed] [Google Scholar]
- 16. Park KY, Han TY, Kim IS, Yeo IK, Kim BJ, Kim MN. The effects of 830 nm light‐Emitting diode therapy on acute herpes zoster ophthalmicus: A pilot study. Ann Dermatol 2013;25(2):163–167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Bhat J, Birch J, Whitehurst C, Lanigan SW. A single‐blinded randomised controlled study to determine the efficacy of Omnilux Revive facial treatment in skin rejuvenation. Lasers Med Sci 2005;20(1):6–10. [DOI] [PubMed] [Google Scholar]
- 18. Lee SY, Park KH, Choi JW, et al. A prospective, randomized, placebo‐controlled, double‐blinded, and split‐face clinical study on LED phototherapy for skin rejuvenation: Clinical, profilometric, histologic, ultrastructural, and biochemical evaluations and comparison of three different treatment settings. J Photochem Photobiol B: Biol 2007;88(1):51–67. [DOI] [PubMed] [Google Scholar]
- 19. Migliardi R, Tofani F, Donati L. Non‐invasive peri‐orbital rejuvenation: Radiofrequency dual radiowave energy source (rf) and light emission diode system (LED). Orbit 2009;28(4):214–218. [PubMed] [Google Scholar]
- 20. Nam CH, Park BC, Kim MH, Choi EH, Hong SP. The efficacy and safety of 660 nm and 411 to 777 nm light‐emitting devices for treating wrinkles. Dermatol Surg 2017;43(3):371–380. [DOI] [PubMed] [Google Scholar]
- 21. Nikolis A, Bernstein S, Kinney B, Scuderi N, Rastogi S, Sampalis JS. A randomized, placebo‐controlled, single‐blinded, split‐faced clinical trial evaluating the efficacy and safety of KLOX‐001 gel formulation with KLOX light‐emitting diode light on facial rejuvenation. Clin Cosmet Invest Dermatol 2016;9:115–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Stirling RJ, Haslam JD. A self‐reported clinical trial investigates the efficacy of 1072 nm light as an anti‐ageing agent. J Cosmet Laser Ther 2007;9(4):226–230. [DOI] [PubMed] [Google Scholar]
- 23. Alster TS, Wanitphakdeedecha R. Improvement of postfractional laser erythema with light‐emitting diode photomodulation. Dermatol Surg 2009;35(5):813–815. [DOI] [PubMed] [Google Scholar]
- 24. Khoury JG, Goldman MP. Use of light‐emitting diode photomodulation to reduce erythema and discomfort after intense pulsed light treatment of photodamage. J Cosmet Dermatol 2008;7(1):30–34. [DOI] [PubMed] [Google Scholar]
- 25. Trelles MA, Allones I, Mayo E. Combined visible light and infrared light‐emitting diode (LED) therapy enhances wound healing after laser ablative resurfacing of photodamaged facial skin. Med Laser Appl 2006;21(3):165–175. [Google Scholar]
- 26. Chaves ME, Araujo AR, Santos SF, Pinotti M, Oliveira LS. LED phototherapy improves healing of nipple trauma: A pilot study. Photomed Laser Surg 2012;30(3):172–178. [DOI] [PubMed] [Google Scholar]
- 27. Bay C, Vissing AC, Thaysen‐Petersen D, et al. Skin reactions after photodynamic therapy are unaffected by 839nm photobiomodulation therapy: A randomized, double‐blind, placebo‐controlled, clinical trial. Lasers Surg Medicine 2017. [DOI] [PubMed] [Google Scholar]
- 28. Kleinpenning M, Otero M, van Erp P, Gerritsen M, van de Kerkhof P. Efficacy of blue light vs. red light in the treatment of psoriasis: A double‐blind, randomized comparative study. J Eur Acad Dermatol Venereol 2012;26(2):219–225. [DOI] [PubMed] [Google Scholar]
- 29. Pfaff S, Liebmann J, Born M, Merk HF, Von Felbert V. Prospective randomized long‐term study on the efficacy and safety of UV‐free blue light for treating mild psoriasis vulgaris. Dermatology (Basel, Switzerland). 2015;231(1):24–34. [DOI] [PubMed] [Google Scholar]
- 30. Weinstabl A, Hoff‐Lesch S, Merk HF, von Felbert V. Prospective randomized study on the efficacy of blue light in the treatment of psoriasis vulgaris. Dermatology (Basel, Switzerland) 2011;223(3):251–259. [DOI] [PubMed] [Google Scholar]
- 31. Keemss K, Pfaff SC, Born M, Liebmann J, Merk HF, von Felbert V. Prospective, randomized study on the efficacy and safety of local UV‐free blue light treatment of eczema. Dermatology 2016;232(4):496–502. [DOI] [PubMed] [Google Scholar]
- 32. Siqueira CPCM, de Paula Ramos S, Gobbi CAA, et al. Effects of weekly LED therapy at 625 nm on the treatment of chronic lower ulcers. Lasers Med Sci 2014;30(1):367–373. [DOI] [PubMed] [Google Scholar]
- 33. Frangez I, Cankar K, Ban Frangez H, Smrke DM. The effect of LED on blood microcirculation during chronic wound healing in diabetic and non‐diabetic patients—A prospective, double‐blind randomized study. Lasers Med Sci 2017;32(4):887–894. [DOI] [PubMed] [Google Scholar]
- 34. Tchanque‐Fossuo CN, Ho D, Dahle SE, et al. A systematic review of low‐level light therapy for treatment of diabetic foot ulcer. Wound Repair Regen 2016;24(2):418–426. [DOI] [PubMed] [Google Scholar]
- 35. Hodgson B, Margolis D, Salzman D, et al. Amelioration of oral mucositis pain by NASA near‐infrared light‐emitting diodes in bone marrow transplant patients. Support Care Cancer 2012;20(7):1405–1415. [DOI] [PubMed] [Google Scholar]
- 36. Lalla RV, Bowen J, Barasch A, et al. MASCC/ISOO clinical practice guidelines for the management of mucositis secondary to cancer therapy. Cancer. 2014;120(10):1453–1461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. DeLand MM, Weiss RA, McDaniel DH, Geronemus RG. Treatment of radiation‐induced dermatitis with light‐emitting diode (LED) photomodulation. Lasers Surg Med 2007;39(2):164–168. [DOI] [PubMed] [Google Scholar]
- 38. Fife D, Rayhan DJ, Behnam S, et al. A randomized, controlled, double‐blind study of light emitting diode photomodulation for the prevention of radiation dermatitis in patients with breast cancer. Dermatol Surg 2010;36(12):1921–1927. [DOI] [PubMed] [Google Scholar]
- 39. Sasaki GH, Oberg K, Tucker B, Gaston M. The effectiveness and safety of topical PhotoActif phosphatidylcholine‐ based anti‐cellulite gel and LED (red and near‐infrared) light on Grade II‐III thigh cellulite: A randomized, double‐blinded study. J Cosmet Laser Ther 2007;9(2):87–96. [DOI] [PubMed] [Google Scholar]
- 40. Hamblin MR, Huang YY, Sharma SK, Carroll J. Biphasic dose response in low level light therapy—An update. Dose Response 2011;9(4):602–618. [DOI] [PMC free article] [PubMed] [Google Scholar]


