Abstract
Background
Inadequate efficacy of current intranasal steroids in chronic rhinosinusitis (CRS) is attributable to ineffective and/or inconsistent drug delivery to target anatomic sites. A new exhalation delivery system with fluticasone (EDS‐FLU) may improve outcomes by significantly increasing superior/posterior corticosteroid delivery. A study was conducted to assess the long‐term efficacy and safety outcomes of EDS‐FLU in individuals with CRS.
Methods
This was a 12‐month, multicenter, single‐arm study evaluating the safety and efficacy of EDS‐FLU 372 μg twice daily in CRS patients (with [n = 34] or without [n = 189] nasal polyps [NP]). Efficacy assessments by serial nasal endoscopy and patient report included: 22‐item Sino‐Nasal Outcome Test (SNOT‐22), NP grade, standardized surgical indicator assessment, Lund‐Kennedy score, and Patient Global Impression of Change. Adverse event (AE) evaluations included nasal endoscopy. Additional safety and efficacy outcomes were assessed.
Results
Of 223 patients who received EDS‐FLU, 96% reported prior corticosteroid use and 29% prior sinus surgery. The EDS‐FLU AE profile was similar to conventional intranasal steroids studied in similar populations. Most patients (87%) reported symptom improvement. Through 12 months, mean SNOT‐22 scores improved by −21.5 and −21.1 for CRS with and without NP, respectively. Among patients with NP, 54.2% had polyp elimination in at least 1 nostril and 83.3% had ≥1‐point improvement in polyp grade.
Conclusion
Over 1 year of treatment in CRS with and without NP, EDS‐FLU 372 μg twice daily was well tolerated and produced improvements across a broad range of objective and subjective measures. EDS‐FLU may be a desirable new option for patients with this condition.
Keywords: chronic rhinosinusitis, corticosteroid, intranasal steroid, nasal congestion, nasal inflammation, nasal polyps, sinus surgery
Chronic rhinosinusitis (CRS) with and without nasal polyps (NPs) (the subgroups are sometimes referred to as nasal polyposis and chronic rhinosinusitis, respectively), is the second most prevalent chronic health condition in the United States, affecting up to 31 million people, or an estimated 10% to 15% of the adult population.1, 2, 3 In 2014, the annual direct and indirect costs of CRS were estimated at $22 billion in the United States.4 Patients with CRS experience substantial disease burden due to multiple symptoms, including those cardinal to defining the disease: persistent nasal congestion/obstruction, rhinorrhea and postnasal drip, facial pain/pressure, and hyposmia.5, 6, 7 Extrasinus complications and comorbidities are also very common, including headache, fatigue and body pain, sleep dysfunction, asthma, gastroesophageal reflux disease, adenotonsillitis, and depression.8 These symptoms and comorbidities contribute to a substantial disease burden9 and significant reduction in patient quality of life (QoL).8, 10, 11 The overall impairment of multiple domains of QoL (eg, bodily pain, general health perception) is similar in magnitude to other serious chronic diseases such as congestive heart failure (CHF), chronic obstructive pulmonary disease (COPD), and Parkinson's disease.7, 12
CRS is characterized by widespread nasal inflammation, involving deep anatomical sites difficult to reach with conventional nasal steroids.5, 13 Chronic mucosal inflammation is present throughout the nasal/sinus cavities, notably including the ostiomeatal complex (OMC) region, where the sinus ostia normally drain and ventilate.14, 15 NPs, when present, most commonly develop in the OMC and can exacerbate the inflammatory blockage of ventilation and clearance/drainage from the paranasal sinuses.16 Chronic sinusitis is a term also commonly used in the literature to describe this population. CRS treatment guidelines recommend intranasal corticosteroids as standard care for patients both with and without NPs.5, 7 The most commonly used method of delivery for these medications is traditional nasal sprays, which have long been recognized to be suboptimal for delivery of topically acting drugs to intranasal sites beyond the nasal valve, including the OMC.13, 17, 18, 19 Additionally, it has been shown that traditional nasal sprays deposit most of the medication on the nasal valve and head of the inferior turbinate, and that sniffing draws medication off‐target along the floor of the nasal cavity toward the pharynx where it is swallowed.20, 21 Other forms of delivery, such as nasal drops, high‐volume lavage, or nasal nebulizers are associated with significant challenges, particularly in light of the need for long‐term outpatient compliance, variously including difficulty with optimal head positions, discomfort associated with administration, lung or gastrointestinal exposure, poor dose control, and other factors. It is unsurprising that suboptimal symptom control, inadequate polyp regression, and side effects such as nasal steroid dripping out of the nose and down the back of the throat are common with these treatments.17, 22
Exhalation delivery systems (EDSs) utilize a mechanism for intranasal drug delivery that has been shown to deliver medication high and deep to superior/posterior sites in the nasal passages, including the OMC.19, 23 An EDS with fluticasone (EDS‐FLU) offers potential to improve treatment efficacy by enabling placement of a high‐potency steroid (fluticasone propionate) on sites of chronic inflammation in CRS that are not effectively or consistently accessed with standard nasal delivery approaches during chronic outpatient care23, 24, 25, 26 (Fig. 1). In addition to a different approach to delivery, the EDS‐FLU contains a different drug formulation than Flonase® and has been shown to be not bioequivalent (even at comparable doses).27 The EDS‐FLU formulation does not include alcohol or fragrance and has a significantly higher concentration of fluticasone. This 12‐month, prospective study is 1 of the largest and longest‐duration treatment trials in CRS of which we are aware, and assessed longitudinal outcomes (safety and efficacy) of treatment with EDS‐FLU (372 μg twice daily) in patients who met symptom criteria for CRS with or without NPs.
Figure 1.
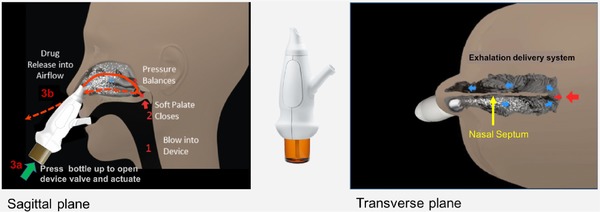
EDS mechanism. The EDS has a flexible mouthpiece and a nosepiece. The sealing nosepiece is shaped to transfer pressure from the mouth, to avoid compression of soft tissue in a way that could obstruct air flow, and to “stent” the nasal valve, particularly superiorly. Exhalation through the EDS (1) creates an airtight seal of the soft palate, isolating the nose from the mouth and lungs, (2) transfers proportional air pressure into the nose, and (3) helps “float” medication around obstructions to high/deep sites in the nasal labyrinth, such as the OMC. The transferred intranasal pressure is proportional, across various exhalation forces, to oral pressure, counterbalancing pressure on the soft palate. This assures a patent communication behind the nasal septum and allows air to escape through the opposite nostril. “Positive‐pressure” expands passages narrowed by inflammation (vs negative pressure delivery, “sniffing”). Use is simple and quick. A patient inserts the nosepiece into 1 nostril and starts blowing through the mouthpiece. This elevates and seals the soft palate, as with inflating a balloon, separating the oral and nasal cavities. The patient completes use by pressing the bottle to actuate. This causes a coordination‐reducing valve to release the exhaled breath concurrently with aerosol spray in a “burst” of naturally humidified air.
Patients and methods
Study patients
This prospective, 12‐month, single‐arm, multicenter study enrolled patients meeting CRS symptom criteria, either with or without NPs (CRSwNP or CRSsNP, respectively), as defined by history and nasal endoscopy. Patients were recruited from 21 geographically diverse U.S. centers. The first patient was enrolled on September 6, 2013, and the last patient completed the study on August 10, 2015.
Eligible patients were ≥18 years of age and had CRS diagnostic symptoms for ≥12 weeks. The presence of nasal polyps was determined by nasal endoscopy at screening. Patients with a history of CRSsNP were required to be currently experiencing ≥2 defining CRS symptoms (nasal congestion/obstruction, rhinorrhea, facial pain/pressure, hyposmia), 1 of which had to be nasal congestion/obstruction or rhinorrhea. Patients with comorbid asthma or COPD were required to be stable, with no exacerbations in the 3 months prior to screening. Inhaled corticosteroid use (eg, for asthma) had to be limited to stable doses of beclomethasone (or equivalent) ≤1000 μg/day for at least 3 months prior to screening and with no plans to change dosage throughout the study. Apart from these permitted inhaled steroids, exposure to any glucocorticoid with potential for systemic effects (eg, oral or parenteral, intraarticular, or epidural steroids, high‐dose/potency topical steroids) within 1 month prior to visit 1 (screening) through completion of the study was prohibited. Patients were required to stop treatment with intranasal inhaled steroids at trial entry. Antihistamines, decongestants, ipratropium, and oxymetazoline were permitted; however, intranasally administered medications were not allowed within 15 minutes before or after administration of study drug.
Exclusion criteria included nasal septum perforation; ≥1 episode of epistaxis with frank bleeding in the month prior to screening; evidence of significant mucosal injury, ulceration, or erosion on nasal endoscopy at screening; history of sinus or nasal surgery within 6 months prior to screening, or planned sinonasal surgery during the study period; current or ongoing rhinitis medicamentosa; or complete or near‐complete obstruction of nasal cavities.
Once selected for the study, all patients received EDS‐FLU 372 μg twice daily. At each study visit, compliance was assessed by examining the study drug liquid level in returned delivery systems.
Safety and efficacy endpoints
Safety assessments included standard capture and Medical Dictionary for Regulatory Activities (MedDRA) dictionary coding of adverse events (AEs) at all study visits, from serial nasal endoscopy, from serial ocular examinations by an ophthalmologist, and from spontaneous reports. Efficacy assessments included symptoms, functioning, and QoL as measured with the 22‐item Sino‐Nasal Outcome Test (SNOT‐22); summed bilateral polyp score (in those patients with nasal polyps at baseline), measured with a Nasal Polyp Grading Scale28; surgical indicator criteria, measured with a standardized assessment; and endoscopic assessment of the nasal cavity, measured using the Lund‐Kennedy nasal endoscopy assessment.29 The Patient Global Impression of Change (PGIC), a 7‐point balanced Likert scale, was also utilized.30, 31 Surgical indicator criteria did not necessarily reflect a physician determination to offer surgery to individual patients, but were intended to identify a standard group of patients reasonable to be considered for surgical intervention. To be eligible for surgery for the purposes of this study, a patient must have met all of the following criteria: at least moderate symptoms of congestion for ≥3 months, use of topical steroids at conventional doses for ≥6 weeks, current or previous use of saline lavage for ≥6 weeks, and (for patients with NPs) endoscopically visualized bilateral nasal polyposis of at least moderate severity (NP grading score ≥2 in at least 1 nostril). Last, a questionnaire assessing EDS‐FLU device attributes in comparison with previous nasal sprays was also completed by patients.
Study design
Patients were assessed in person at baseline and at months 1, 3, 6, 9, and 12, and via phone during all other months. An end‐of‐study (EOS) termination visit was conducted at month 12 or at the last visit for participants who discontinued early.
Physicians experienced in nasal endoscopy performed a nasal examination and endoscopy at each study visit and completed forms explicitly querying for evidence of potential local adverse effects, such as new or old bleeding, septal ulceration, candidiasis, atypical swelling, and erythema. Ocular examinations performed by an ophthalmologist to assess for potential steroid‐related risks included intraocular pressure (IOP) measurement for emergence of glaucoma and slit‐lamp evaluation for subcapsular cataracts and were conducted at screening, month 6, and month 12.
The efficacy of EDS‐FLU in alleviating CRS was assessed using the Lund‐Kennedy assessment. This validated, objective measure of intranasal disease based on direct endoscopic visualization is used to evaluate changes in common pathology associated with CRS.29 The Lund‐Kennedy assessment rates evidence of pathology, including edema, discharge, crusting, scarring/adhesions, and nasal polyps, on a 0 to 2 scale for each sign.
The PGIC is a patient‐reported rating of improvement on a 7‐point balanced Likert scale.30, 31 Patients report their change in symptoms as “very much improved,” “much improved,” “minimally improved,” “no change,” “minimally worse,” “much worse,” or “very much worse.” Last, a medication evaluation questionnaire was used to assess ease of use and compare EDS‐FLU to previously used nasal sprays.
Statistical methods
Safety and efficacy data were summarized using descriptive statistics. All reported AEs were coded using MedDRA version 13.1 or higher.
Descriptive statistics for continuous variables included the number of observations, mean, standard deviation (SD), median, minimum, and maximum. Descriptive statistics for categorical variables included patient counts and percentages. Percentages were based on the number of patients with nonmissing data, unless otherwise specified. Mean changes in SNOT‐22, bilateral Lund‐Kennedy, and bilateral NP grade were summarized by time point using reported values and at EOS. Surgical eligibility assessment was conducted at various time points and analyzed as a categorical variable.
Results
A total of 224 subjects were enrolled. Of the 224 subjects, 194 entered the study directly after screening, the remaining 30 enrolled after completing a shorter‐term study,32 which was identical with respect to inclusion/exclusion criteria, measures, and treatment (dose and regimen). One enrolled patient was not treated; therefore, a total of 223 patients received study drug. Patient demographics and baseline characteristics are outlined in Table 1. The majority of patients, 64.6% (n = 144), completed the year‐long study. Reasons for discontinuation were sought and the most specific reason based on available information was assigned. The most common reasons for discontinuation included withdrawal by subject (25 subjects [11.2%]), AEs (21 subjects [9.4%]), lost to follow‐up (18 subjects [8.1%]), lack of efficacy and protocol deviation (6 subjects [2.7%] each), and other (3 subjects [1.3%]).
Table 1.
Patient demographics and baseline characteristics
| Characteristics | CRS with NP (n = 34) | CRS without NP (n = 189) | Total enrolled (n = 223) |
|---|---|---|---|
| Age (years), mean ± SD | 46 ± 13.7 | 45.3 ± 12.5 | 45.4 ± 12.6 |
| Male sex, n (%) | 20 (58.8) | 76 (40.2) | 96 (43.0) |
| White race, n (%) | 30 (88.2) | 145 (76.7) | 175 (78.5) |
| Corticosteroids used for CRS in last 10 years, n (%) | 34 (100) | 180 (95.2) | 214 (96.0) |
| Fluticasone propionate | 28 (82.4) | 123 (65.1) | 151 (67.7) |
| Mometasone furoate | 24 (70.6) | 80 (42.3) | 104 (46.6) |
| Intranasal steroid in last 30 days, n (%) | 19 (55.9) | 48 (25.4) | 67 (30.0) |
| Fluticasone propionate | 5 (14.7) | 23 (12.2) | 28 (12.6) |
| Mometasone furoate | 7 (20.6) | 9 (4.8) | 16 (7.2) |
| Bilateral endoscopic NP score, mean ± SD | 2.8 ± 1.2 | – | – |
| Lund‐Kennedy total score, mean ± SD | 1.7 ± 1.5 | 1.4 ± 1.2 | 1.4 ± 1.3 |
| SNOT‐22 total score, mean ± SD | 41.9 ± 23.3 | 39.7 ± 21.7 | 40 ± 21.9 |
| ≥1 sinus surgery for polyp removal or sinus surgery, n (%) | 15 (44.1) | 49 (25.9) | 64 (28.7) |
CRS = chronic rhinosinusitis; NP = nasal polyps; SD = standard deviation; SNOT‐22 = 22‐item Sino‐Nasal Outcome Test.
Mean SNOT‐22 scores decreased monotonically over 12 months, with the largest improvement in the first month and with greater improvement with longer duration of treatment: the magnitude of improvement was similar in CRS patients with and without NPs (−21.5 and −21.1 at month 12) (Fig. 2). In patients with and without NPs, the median SNOT‐22 at month 12 reached 8.5 and 9.0, respectively, a substantial improvement from the baseline median of 39.0 and 40.0, respectively. Among patients with NPs at entry, the baseline bilateral polyp score was 2.8. After 12 months of treatment, the average was reduced to 1.3 (Fig. 3).
Figure 2.
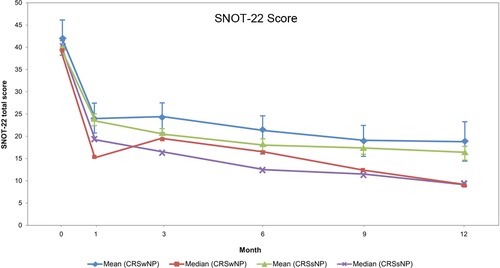
Changes in mean and median in the total score from the SNOT‐22. Month 12 includes recorded assessments at Month 12/EOS for patients who completed the study. The SNOT‐22 is a validated 22‐item questionnaire addressing symptoms, functioning, and QoL, with each item scored 0 (no problem) to 5 (as bad as it can be). The SNOT‐22 total score ranges from 0 to 110. The MCID for SNOT‐22 total score has been reported to be 8.90, and healthy volunteers report a mean score of 9.3.41, 42 CRSsNP = chronic rhinosinusitis without nasal polyps; CRSwNP = chronic rhinosinusitis with nasal polyps; EOS = end of study; MCID = minimally clinically important difference; QoL = quality of life; SNOT‐22 = 22‐item Sino‐Nasal Outcome Test.
Figure 3.
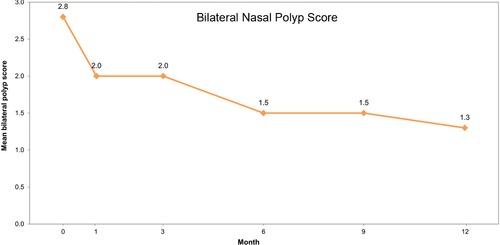
Mean bilateral polyp score at study time points. Month 12 includes recorded assessments at Month 12/EOS for patients who completed the study. Polyp grade was based on the anatomic landmark reached by the largest visualized polyp in a single (vertical) dimension: a score of 0 = no polyps; a score of 1 = polyps not below the inferior border of the middle turbinate; a score of 2 = polyps below the inferior border of the middle concha, but not the inferior border of the inferior turbinate; and a score of 3 = polyps below the lower inferior border of the inferior turbinate.
The percentage of patients with polyp elimination on at least 1 side of the nasal cavity increased steadily over the 12‐month study period. At 12 months, slightly over one‐half of patients with polyps at baseline (54.2%) had no observable polyps in at least 1 nostril (Fig. 4). After 12 months of treatment, 83.3% of patients were observed to have a ≥1‐point improvement in polyp grade. At screening, 16 of 34 (47.1%) patients with CRSwNP and 11 of 189 (5.8%) patients with CRSsNP met surgical indicator criteria. The percentage of patients meeting surgical indicator criteria steadily declined over the course of the study. By month 12, only 1 of 23 (4.3%) patients with CRSwNP and 3 of 120 (2.5%) patients with CRSsNP met surgical indicator criteria. The end‐of‐study assessment also indicated a significant reduction: CRSwNP = 5 of 34 (14.7%) and CRSsNP = 6 of 184 (3.3%).
Figure 4.
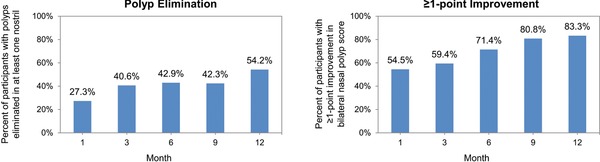
(A) Percentages of participants with polyps eliminated in at least 1 nostril at study time points. (B) Percentage of participants with ≥1‐point improvement in bilateral nasal polyp score at study time points. Month 12 includes recorded assessments at Month 12/EOS for patients who completed the study. EOS assessments include the last recorded post‐baseline information regardless if the patient completed the study. At end of study, polyps were eliminated in at least 1 nostril in 47.1% of patients and 70.6% achieved ≥1‐point improvement in polyp grade. EOS = end of study.
At month 12, Lund‐Kennedy scores indicated an improvement from baseline in multiple objective signs of nasal inflammation, including edema (−0.7 in CRSwNP and −0.8 in CRSsNP), discharge (−0.4 in CRSwNP and −0.4 in CRSsNP), and crusting (−0.2 in CRSwNP and −0.2 in CRSsNP). A large majority of patients reported global improvement in their symptoms with treatment, as assessed by the PGIC (Fig. 5), with few reporting worsening; results were similar for patients with and without NPs. On the Medication Evaluation Questionnaire, large majorities of enrolled patients (85.8% at month 1 and EOS) also reported that EDS‐FLU was “easy” or “somewhat easy” to use and that it was “somewhat” or “very” comfortable (74.5% at month 1 and 73.9% at EOS). Most patients (59% at month 1 and 57.3% at EOS) reported “less” or “much less” drip out of their nose, and 73.1% and 71.1% at month 1 and EOS, respectively, reported “less” or “much less” drip down the back of their throat with EDS‐FLU compared with their prestudy conventional nasal steroid spray.
Figure 5.
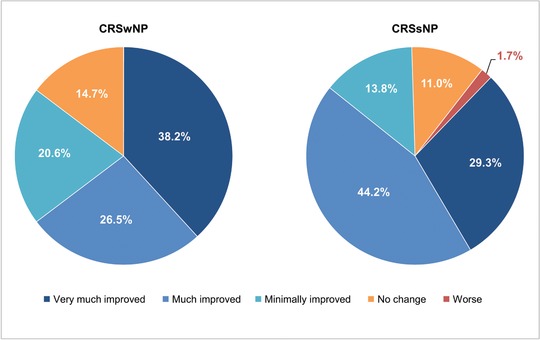
Patient Global Impression of Change: symptom reduction at end of study. CRSsNP = chronic rhinosinusitis without nasal polyps; CRSwNP = chronic rhinosinusitis with nasal polyps.
The majority of spontaneously reported AEs were local in nature, mild in severity, and resolved spontaneously with continued use of EDS‐FLU. The most common AEs (≥5%) included those coded as epistaxis (11.2%); nasal erythema (17.5%); nasal septum disorder (nasal septal erythema, 14.3%); acute sinusitis (13.9%); nasal septal erosion or ulceration (11.2%); headache (9.4%); and upper respiratory infection (7.6%).
Ocular examinations, including measurement of IOP and slit‐lamp evaluation, indicated no evidence of emergence of glaucoma or subcapsular cataracts.
Discussion
EDS‐FLU is a new treatment with potential to improve medical care of CRS in patients who do not respond satisfactorily to conventional nasal steroids by enabling reliable and consistent outpatient delivery of steroid to high and deep target sites of inflammation.33, 34 In addition, studies have suggested the possibility that direct EDS device benefits could be produced by the effects of carbon dioxide in exhaled breath (influencing inflammatory mediator and neuropeptide activity), by removal of nitric oxide, by positive pressure, or by change in pH.35 This 1‐year study assessed the long‐term efficacy and safety of EDS‐FLU in patients with CRS with and without NPs, more than 90% of whom had previously used corticosteroids, and a significant minority of whom had undergone prior surgery. This study is 1 of the longest prospective treatment trials in CRS with and without NPs to date, most of which are 2 to 24 weeks in duration,5 and it included intensive safety monitoring. Broad entry criteria, a diversity of treatment sites, and realistic treatment conditions (eg, no blinding) increase generalizability and ability to inform “real‐world” outcomes.
The majority of AEs identified over 1 year were local in nature, mild in severity, and did not increase in frequency or severity with continued EDS‐FLU use. In fact, AE rates tended to decrease with continued use of EDS‐FLU. Most local AEs that occurred were endoscopically observed to resolve with continued use of EDS‐FLU. Fluticasone is a high‐potency steroid selected for this product in part due to its extremely low systemic absorption.36, 37 This is consistent with the almost exclusively local nature of reported AEs and the fact that systemic adverse effects associated with steroids, including glaucoma and subcapsular cataracts, were not identified. The pattern and frequency of AEs were generally similar to those reported previously with conventional nasal steroid sprays studied in similar populations,38, 39, 40 suggesting that the EDS mechanism of action for delivery and the drug deposition profile produced did not introduce previously unrecognized risks. The systemic exposure to fluticasone resulting from use of EDS‐FLU was evaluated separately in a formal 2‐phase randomized pharmacokinetic study comparing EDS‐FLU to Flonase and Flovent®. The results of the study demonstrated clearly that EDS‐FLU is not bioequivalent to Flonase (even at comparable doses). Specifically, the study showed that EDS‐FLU 372 μg produces higher systemic exposure than Flonase nasal spray 400 μg and substantially lower systemic exposure than 440 μg of inhaled fluticasone (Flovent), suggesting that any systemic effects would be comparable to or less than those expected with Flovent 440 μg.27
Two large, randomized controlled trials were performed to establish the efficacy of EDS‐FLU in patients with CRSwNP.33, 34 However, practical data that more closely resemble “real‐world evidence” for the benefits of long‐term treatment are also valuable. This trial found that EDS‐FLU improved signs and symptoms of CRS similarly in patients who had symptoms of CRS either with or without NPs, as measured by a variety of both patient‐reported outcomes and objective investigator assessments. The magnitude of improvement appears meaningful. The study population generally started with moderate to severe symptoms, as measured by baseline mean SNOT‐22 total score (40.0), which is similar to that reported in some studies for patients prior to undergoing sinus surgery. EDS‐FLU treatment was associated with an improvement of −21.5 and −21.1 for patients with and without polyps, respectively, over 1 year. This magnitude of improvement is similar to that reported by patients after endoscopic sinus surgery—an intervention known to be effective—and greatly exceeds the minimum clinically important difference.41 At 12 months, the median SNOT‐22 score was 9.0, which is comparable with the average score for healthy individuals (9.3).42 The PGIC is directly reported by patients and is another approach to determining if the treatment benefit is meaningful to patients. A very high proportion of these moderate to severe patients reported improvement (87.0%), and very few reported worsening (1.4%).
Patients with CRS who fail medical therapy are often offered surgery.43 The observed reduction in the proportion of patients meeting surgical indicator criteria in a population in which most had previously used steroid nasal sprays suggests that EDS‐FLU could play an important role in maximizing appropriate medical care.
Intranasal steroids are first‐line treatment of CRS with or without NPs and are usually administered by conventional nasal spray. Several alternatives to nasal spray administration (eg, nasal drops, locally compounded high‐volume medicated lavage) have been studied but are subject to serious practical challenges, particularly in a context in which long‐term outpatient compliance is needed. These may include difficulty with training and optimal head positions, control of local dose exposure, lung or gastrointestinal exposure, time and effort associated with use, discomfort, and other challenges. Steroid nasal sprays offer some symptom benefit and have been shown to reduce polyp size, particularly for larger (easier‐to‐access) polyps; however, evidence suggests that there are limitations to the degree of polyp reduction that can be obtained with this approach. Clinical trials with nasal steroid sprays38, 39, 44 suggest that improvement in mean bilateral polyp grade (0‐6 scale) appears to plateau at an average score of ∼3 for treated groups and does not improve further with longer treatment. A longer‐term study with conventional fluticasone nasal spray suggests that patients with a polyp grade lower than 3 will actually experience polyp growth during treatment.44 This is not surprising because conventional sprays are able to reach larger polyps that protrude farther inferiorly or anteriorly, but are not able to continue to access polyps as they regress into the OMC, where polyps continue to obstruct sinus drainage and ventilation. In this study, CRSwNP patients entered with a mean bilateral polyp grade of only 2.8, and EDS‐FLU treatment produced a continual decrease in mean bilateral polyp grade, reaching 1.3 after 12 months, with 47.1% of patients experiencing complete polyp elimination in at least 1 nostril by the end of study. This progressive decrease in polyp size‐in some cases, elimination‐is consistent with reliable long‐term deposition of steroid on the inflamed tissue, including at the site of origin of most polyps in the OMC region.
Limitations
The single‐arm design permitted generalizability of the population and intervention, which is desirable; however, by the same token, the lack of a control group and blinding limits certain types of interpretation or comparisons, poses risk for certain types of bias, and limits hypothesis‐testing conclusions. It should be noted that although CRS symptoms were required at baseline, there was no requirement to confirm disease by imaging. Dropout rates are always a concern in prospective clinical trials: the rate in this trial was consistent with expectations for a study of this duration in a population with a chronic symptomatic disease. At entry, the proportion of participants with polyps who met the standardized surgical indicator criteria was substantially higher than for those without polyps (47% vs 6%). The low rate in nonpolyp patients in this trial is due to the fact that, in order to meet criteria, patients without polyps were required to have obstructive edema/mucus in the middle meatus or ethmoid region on endoscopic examination. Although this is an acceptable method for objectively identifying sinus disease, it is less sensitive than imaging, which is more commonly employed in clinical practice (but was not performed for this study). As a result, this study provides limited information to inform reduction in surgical eligibility in nonpolyp patients.
Conclusion
EDS‐FLU combines a proven, high‐potency, topically acting steroid with a new approach to achieving the superior and posterior intranasal deposition needed to reach target sites for treatment of CRS. This multicenter, prospective treatment study evaluated the safety and efficacy of EDS‐FLU under conditions that more closely approximate long‐term, “real‐world” treatment than typical controlled trials. In a population of patients who had almost all previously used steroids, and some of whom had undergone previous surgery, this study found that over 1 year, treatment with EDS‐FLU was well tolerated and substantially improved both symptoms and objective disease measures. The profile of treatment‐related AEs was consistent with expectations based on data for conventional nasal sprays or steroid drops reported from studies in similar patient populations. EDS‐FLU was associated with clinically significant symptom improvements in a large majority of treated patients. Benefits increased with increasing duration of exposure, whereas the proportion of patients with AEs did not appear to increase. Interestingly for CRSwNP patients, this is the first study of an intranasal steroid that shows elimination of polyps in more than one‐half of patients and a large reduction in the fraction meeting standardized surgical indicator criteria. These data, in conjunction with findings from other studies, suggest that EDS‐FLU may offer meaningful benefits as part of long‐term care and should be considered when optimizing appropriate medical therapy, including in patients before or after surgery.
Acknowledgments
We thank the patients who participated in this study, the principal study investigators, and the following individuals from OptiNose US, Inc.: Jen Carothers and Jean Van Dyke. Editorial assistance with drafting the report following the authors’ guidance, incorporating comments according to authors’ feedback, and providing support with submission was provided by Benjamin J. Epstein of ECIR Medical Communications, funded by OptiNose US, Inc.
How to Cite this Article: Palmer JN, Jacobson KW, Messina JC, Kosik‐Gonzalez C, Djupesland PG, Mahmoud RA. EXHANCE‐12: 1‐year study of the exhalation delivery system with fluticasone (EDS‐FLU) in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2018;8:869–876.
Funding sources for the study: OptiNose US, Inc. The sponsor contributed to the study design, data interpretation, and writing of the report, as well as coordinating the data collection and analysis. All authors signed confidentiality agreements with the sponsor, had full access to the data, and vouch for the accuracy of the findings. The corresponding author had final responsibility for the decision to submit for publication.
Potential conflict of interest: J.C.M., R.A.M., and P.G.D. are employees of and have stock interest in OptiNose US, Inc. C.K‐G. was an employee at OptiNose US, Inc. when the trial was conducted and has stock interest. J.N.P. and K.W.J. received writing assistance in the preparation of this manuscript and are consultants for OptiNose US, Inc.
Presented orally at the Annual ARS Meeting on September 26, 2016 in San Diego, CA.
Public clinical trial registration: http://clinicaltrials.gov/show/NCT01623310. 12‐Month OL Evaluating the Safety of Intranasal Administration Fluticasone BID Using OptiNose Device in Subjects With CS With or Without Nasal Polyps.
[Correction added on 6/20, after first online publication: Updates to references, Table 1, Figures 2 and 3 have been made.]
[Correction added on 7/19, after first online publication: An update to text in the reference section has been made.]
References
- 1. Hamilos DL. Chronic rhinosinusitis: epidemiology and medical management. J Allergy Clin Immunol. 2011;128:693–707; quiz 708–709. [DOI] [PubMed] [Google Scholar]
- 2. Anand VK. Epidemiology and economic impact of rhinosinusitis. Ann Otol Rhinol Laryngol Suppl. 2004;193:3–5. [DOI] [PubMed] [Google Scholar]
- 3. Mahmoud R, Palmer J, Biletch R, Grosel K, Messina JC. Healthcare for chronic rhinosinusitis (CRS) symptoms ‐ a cross‐sectional population‐based survey of U.S. adults meeting symptom criteria for CRS. J Allergy Clin Immunol. 2017;139;AB68. [Google Scholar]
- 4. Smith KA, Orlandi RR, Rudmik L. Cost of adult chronic rhinosinusitis: a systematic review. Laryngoscope. 2015;125:1547–1556. [DOI] [PubMed] [Google Scholar]
- 5. Fokkens WJ, Lund VJ, Mullol J, et al. European Position Paper on Rhinosinusitis and Nasal Polyps 2012. Rhinol Suppl. 2012;(23):3 p preceding table of contents, 1–298. [PubMed] [Google Scholar]
- 6. Meltzer EO, Hamilos DL, Hadley JA, et al. Rhinosinusitis: establishing definitions for clinical research and patient care. J Allergy Clin Immunol. 2004;114(6 Suppl):155–212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Orlandi RR, Kingdom TT, Hwang PH, et al. International consensus statement on allergy and rhinology: rhinosinusitis. Int Forum Allergy Rhinol. 2016;6(Suppl 1):S22–S209. [DOI] [PubMed] [Google Scholar]
- 8. Tan BK, Chandra RK, Pollak J, et al. Incidence and associated premorbid diagnoses of patients with chronic rhinosinusitis. J Allergy Clin Immunol. 2013;131:1350–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Bhattacharyya N. The economic burden and symptom manifestations of chronic rhinosinusitis. Am J Rhinol. 2003;17:27–32. [PubMed] [Google Scholar]
- 10. Alt JA, Smith TL, Mace JC, Soler ZM. Sleep quality and disease severity in patients with chronic rhinosinusitis. Laryngoscope. 2013;123:2364–2370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Lange B, Holst R, Thilsing T, Baelum J, Kjeldsen A. Quality of life and associated factors in persons with chronic rhinosinusitis in the general population: a prospective questionnaire and clinical cross‐sectional study. Clin Otolaryngol. 2013;38:474–480. [DOI] [PubMed] [Google Scholar]
- 12. Soler ZM, Wittenberg E, Schlosser RJ, Mace JC, Smith TL. Health state utility values in patients undergoing endoscopic sinus surgery. Laryngoscope. 2011;121:2672–2678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Merkus P, Ebbens FA, Muller B, Fokkens WJ. The ‘best method’ of topical nasal drug delivery: comparison of seven techniques. Rhinology. 2006;44:102–107. [PubMed] [Google Scholar]
- 14. Akdis CA, Bachert C, Cingi C, et al. Endotypes and phenotypes of chronic rhinosinusitis: a PRACTALL document of the European Academy of Allergy and Clinical Immunology and the American Academy of Allergy, Asthma and Immunology. J Allergy Clin Immunol. 2013;131:1479–1490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. DeMarcantonio MA, Han JK. Nasal polyps: pathogenesis and treatment implications. Otolaryngol Clin North Am. 2011;44:685–695, ix. [DOI] [PubMed] [Google Scholar]
- 16. Larsen PL, Tos M. Origin of nasal polyps: an endoscopic autopsy study. Laryngoscope. 2004;114:710–719. [DOI] [PubMed] [Google Scholar]
- 17. Aggarwal R, Cardozo A, Homer JJ. The assessment of topical nasal drug distribution. Clin Otolaryngol Allied Sci. 2004;29:201–205. [DOI] [PubMed] [Google Scholar]
- 18. Laube BL. Devices for aerosol delivery to treat sinusitis. J Aerosol Med. 2007;20(Suppl 1):S5‐S17; discussion S17–S18. [DOI] [PubMed] [Google Scholar]
- 19. Djupesland PG. Nasal drug delivery devices: characteristics and performance in a clinical perspective‐a review. Drug Deliv Transl Res. 2013;3:42–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leach CL, Kuehl PJ, Chand R, McDonald JD. Nasal deposition of HFA–beclomethasone, aqueous fluticasone propionate and aqueous mometasone furoate in allergic rhinitis patients. J Aerosol Med. 2015;28:334–340 [DOI] [PubMed] [Google Scholar]
- 21. Harvey RJ, Goddard JC, Wise SK, Schlosser RJ. Effects of endoscopic sinus surgery and delivery device on cadaver sinus irrigation. Otolaryngol Head Neck Surg. 2008;139:137–142. [DOI] [PubMed] [Google Scholar]
- 22. Dijkstra MD, Ebbens FA, Poublon RM, Fokkens WJ. Fluticasone propionate aqueous nasal spray does not influence the recurrence rate of chronic rhinosinusitis and nasal polyps 1 year after functional endoscopic sinus surgery. Clin Exp Allergy. 2004;34:1395–1400. [DOI] [PubMed] [Google Scholar]
- 23. Djupesland PG, Messina JC, Mahmoud R. New exhalation delivery systems (EDS) enhance topical steroid delivery in chronic rhinosinusitis with nasal polyps. J Allergy Clin Immunol. 2017;139:AB65. [Google Scholar]
- 24. Djupesland PG, Vlckova I, Hewson G. Impact of baseline nasal polyp size and previous surgery on efficacy of fluticasone delivered with a novel device: a subgroup analysis. Am J Rhinol Allergy. 2010;24:291–295. [DOI] [PubMed] [Google Scholar]
- 25. Hansen FS, Djupesland PG, Fokkens WJ. Preliminary efficacy of fluticasone delivered by a novel device in recalcitrant chronic rhinosinusitis. Rhinology. 2010;48:292–299. [DOI] [PubMed] [Google Scholar]
- 26. Fokkens WJ. Recalcitrant rhinosinusitis, the diagnosis and treatment and evaluation of results. Rhinology. 2010;48:257–258. [DOI] [PubMed] [Google Scholar]
- 27. Messina JC, Carothers JL, Obaidi M, Offman E, Mahmoud RA. Intranasal fluticasone propionate delivered by exhalation delivery system (FLU‐EDS) versus Flonase® nasal spray and Flovent® HFA: a randomized comparison of bioavailability. J Allergy Clin Immunol. 2017;139:AB253. [DOI] [PubMed] [Google Scholar]
- 28. Lildholdt T, Rundcrantz H, Lindqvist N. Efficacy of topical corticosteroid powder for nasal polyps: a double‐blind, placebo‐controlled study of budesonide. Clin Otolaryngol Allied Sci. 1995;20:26–30. [DOI] [PubMed] [Google Scholar]
- 29. Lund VJ, Kennedy DW. Quantification for staging sinusitis. The Staging and Therapy Group. Ann Otol Rhinol Laryngol Suppl. 1995;167:17–21. [PubMed] [Google Scholar]
- 30. Ferguson L, Scheman J. Patient global impression of change scores within the context of a chronic pain rehabilitation program. J Pain. 2009;10:S73. [Google Scholar]
- 31. Kamper SJ, Maher CG, Mackay G. Global rating of change scales: a review of strengths and weaknesses and considerations for design. J Man Manip Ther. 2009;17:163–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Sher MR, Mair EA, Messina JC, Carothers J, Mahmoud R, Djupesland PG. EXHANCE‐3: a phase 3, three‐month study of safety and efficacy of fluticasone propionate exhalation delivery system (FLU‐EDS) in patients with chronic rhinosinusitis with (CRSwNP) and without nasal polyps (CRSsNP). J Allergy Clin Immunol. 2017;139(2 Suppl):AB66 10.1016/j.jaci.2016.12.261. [DOI] [Google Scholar]
- 33. Leopold DA, Elkayam D, Messina JC, Kosik‐Gonzalez C, Djupesland PG, Mahmoud RA, NAVIGATE II: randomized, double‐blind trial of the exhalation delivery system with fluticasone (EDS‐FLU) for nasal polyposis. Journal of Allergy and Clinical Immunology. (2018). 10.1016/j.jaci.2018.06.010. [DOI] [PubMed] [Google Scholar]
- 34. Soteres DF, Messina JC, Carothers J, Mahmoud R, Djupesland PG. NAVIGATE I: a randomized double‐blind trial of a Fluticasone Propionate Exhalation Delivery System (FLU‐EDS) for treatment of chronic rhinosinusitis with nasal polyps (CRSwNP). J Allergy Clin Immunol. 2017;139(2 Suppl):AB66. 10.1016/j.jaci.2016.12.262. [DOI] [Google Scholar]
- 35. Djupesland PG, Messina J, Mahmoud R. Novel nasal Exhalation Delivery Systems (EDS) may produce beneficial activity independent of delivered drug in inflammatory nasal diseases and migraine via exhaled CO2 and mucosal pH changes. Poster presented at: 62nd Annual Meeting of the American Rhinologic Society; September 16‐17, 2016; San Diego, CA.
- 36. FLONASE® (fluticasone propionate) Nasal Spray, 50 mcg [prescribing information]. Research Triangle Park, NC: GlaxoSmithKline; 2003.
- 37. Wiseman LR, Benfield P. Intranasal fluticasone propionate. A reappraisal of its pharmacology and clinical efficacy in the treatment of rhinitis. Drugs. 1997;53:885–907. [DOI] [PubMed] [Google Scholar]
- 38. Small CB, Hernandez J, Reyes A, et al. Efficacy and safety of mometasone furoate nasal spray in nasal polyposis. J Allergy Clin Immunol. 2005;116:1275–1281. [DOI] [PubMed] [Google Scholar]
- 39. Stjärne P, Mösges R, Jorissen M, et al. A randomized controlled trial of mometasone furoate nasal spray for the treatment of nasal polyposis. Arch Otolaryngol Head Neck Surg. 2006;132:179–185. [DOI] [PubMed] [Google Scholar]
- 40. Penttila M, Poulsen P, Hollingworth K, Holmstrom M. Dose‐related efficacy and tolerability of fluticasone propionate nasal drops 400 microg once daily and twice daily in the treatment of bilateral nasal polyposis: a placebo‐controlled randomized study in adult patients. Clin Exp Allergy. 2000;30:94–102. [DOI] [PubMed] [Google Scholar]
- 41. Hopkins C, Gillett S, Slack R, Lund VJ, Browne JP. Psychometric validity of the 22‐item Sinonasal Outcome Test. Clin Otolaryngol. 2009;34:447–454. [DOI] [PubMed] [Google Scholar]
- 42. Gillett S, Hopkins C, Slack R, Browne JP. A pilot study of the SNOT 22 score in adults with no sinonasal disease. Clin Otolaryngol. 2009;34:467–469. [DOI] [PubMed] [Google Scholar]
- 43. Bhattacharyya N. Ambulatory sinus and nasal surgery in the United States: demographics and perioperative outcomes. Laryngoscope. 2010;120:635–638. [DOI] [PubMed] [Google Scholar]
- 44. Vaidyanathan S, Barnes M, Williamson P, Hopkinson P, Donnan PT, Lipworth B. Treatment of chronic rhinosinusitis with nasal polyposis with oral steroids followed by topical steroids: a randomized trial. Ann Intern Med. 2011;154:293–302. [DOI] [PubMed] [Google Scholar]


