Abstract
A series of novel matrinic thiadiazole derivatives were designed, synthesized and evaluated for their inhibitory effect on COL1A1 promotor. The SAR indicated that: (i) the introduction of a thiadiazole on the 11-side chain was beneficial for activity; (ii) a 12-N-benzyl moiety was favorable for activity. Among them, compound 6n displayed a high activity with an inhibitory rate of 39.7% at a concentration of 40 μM. It also effectively inhibited the expression of two representative collagen proteins (COL1A1 and α-SMA) on both the mRNA and protein levels and showed a high safety profile in vivo, indicating its great promise as an anti-liver fibrosis agent. Further study indicated that it might repress hepatic fibrogenesis via the TGFβ/Smad pathway. This study provided powerful information for further strategic optimization and the top compound 6n was selected for further study as an ideal liver fibrosis lead for next investigation.
Keywords: liver fibrosis, matrinic, thiadiazole, structure-activity relationship, COL1A1, TGFβ/Smad pathway
1. Introduction
Liver fibrosis is a general stage in the process of cirrhosis, which is characterized by the excessive accumulation of extracellular matrix (ECM) and abnormal hyperplasia of intrahepatic connective tissue [1]. Fibrillation is the scarring response to chronic liver injury [2]. It can be controlled in the early phase after effective treatment, but most often, fibrous septa, pseudolobules or nodules would form and eventually develop into liver cirrhosis. Unfortunately, to date, treatments for liver fibrosis are quite limited and therefore, there is still an urgent need to develop new effective anti-liver fibrosis candidates.
The activation and proliferation of hepatic stellate cells (HSCs) leads to the excess production and abnormal deposition of ECM components [3,4], as indicated in the cytological basis for liver fibrosis [5].Transforming growth factor β1 (TGFβ1) is closely related to liver fibrosis, and it is one of the most important fibrotic cytokines known so far [6]. It promotes the production of liver fibrosis mainly by activating HSCs and up-regulating the secretion of collagen, especially collagen type I (COL1) and α-smooth muscle actin (α-SMA). Since the transcription of key gene collagen type I α1 chain (COL1A1) for liver fibrosis could be activated by TGFβ1 through TGFβ/Smad pathway [7], a high-throughput drug screening cell model based on COL1A1 promotor was established earlier in our group, in which the activity of COL1A1 promotor and luciferase reporter gene could be elevated by TGFβ1, and inhibited by candidate agents [8].
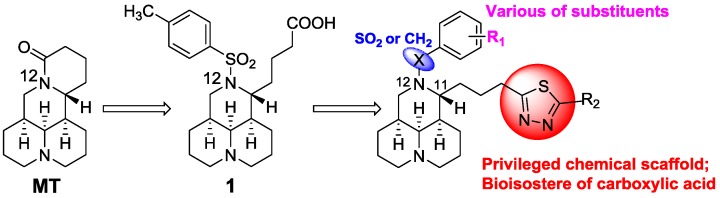
Our group has been working on the discovery of innovative candidates agents with novel structure skeletons from Chinese natural products such as matrine (MT) [9,10,11]. Since MT is an anti-liver fibrosis monomer [12], the matrinic acid compound library constructed in our lab [9,10,13] was screened by the high-throughput screening model based on COL1A1 promotor as mentioned above, taking epigallocatechin gallate (EGCG) as the positive control [14]. To our great delight, the hit compound 12-N-p-methyl benzenesulfonyl matrinic acid (1, Figure 1) [15] had been identified to display a reasonable inhibitory effect against COL1A1 at a rate of 18.5% at the concentration of 40 μM. It was also worth mentioning that compound 1 possesses a special tricyclic flexible scaffold, and appealing druglike characteristics. These studies prompted our interest in continuing our structure-activity relationship (SAR) study of this compound class taking compound 1 as the lead, in an effort to develop and discover novel anti-liver fibrosis candidates.
Figure 1.
Chemical structures of matrine and the lead compound 1, and structure modification strategy.
The 1,3,4-thiadiazole group is recognized as a bioisostere of the carboxyl group [16], and also a privileged chemical scaffold [17,18,19], therefore, in the present study, the carboxyl was replaced with 1,3,4-thiadiazole group on the 11-side chain, whereby a series of novel tricyclic matrinic thiadiazole derivatives was generated and evaluated for their anti-COL1A1 activity. Herein, we describe the synthesis of 19 novel thiadiazole derivatives, SAR analysis, as well as in vivo safety profile and primary mechanism of action of key compounds.
2. Results and Discussion
2.1. Chemistry
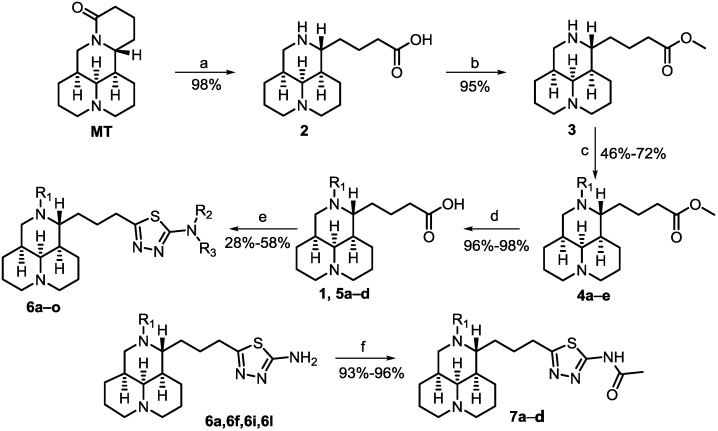
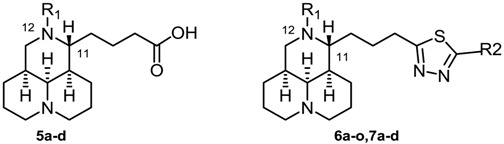
The semi-synthetic routes of all target compounds were outlined in Scheme 1, taking MT as the starting material. 12-N-substituted matrinic acids 1, 5a–d were obtained in high yields of 42–66% by a four-step procedure including hydrolysis, esterification, 12-N substitution and hydrolysis instead of the original diphenyldiazomethane protection method [9,15]. Then a series of matrinic thiadiazole derivatives 6a–o were obtained by the cyclization of 1 or 5a–d with thiosemicarbazide or various of N-substituted thiosemicarbazides in the presence of phosphorus pentoxide/methanesulfonic acid (1:5) in yields of 28–58% [20,21]. The acylation of 6a, 6f, 6i and 6l in triethylamine generated compounds 7a–d with yields of 93–96%. The desired products were purified with flash column chromatography on silica gel using dichloromethane/methanol or petroleum/ethyl acetate as eluents.
Scheme 1.
Reagents and conditions: (a) 5 N NaOH, reflux, 9 h; 10 N HCl, pH = 3–5; (b) 2 N MeOH/HCl, reflux, 2 h; (c) Substituted benzensulfonyl chloride or benzyl bromide, TEA or K2CO3, CH2Cl2 or MeCN, r.t., 6–8 h; (d) 5 N NaOH, reflux, 6 h; 10 N HCl, pH = 7–7.5; (e) P2O5/MsOH (1:5), thiosemicarbazide, 70 °C, 8 h; (f) Acetyl chloride, TEA, THF, r.t., 0.5 h.
2.2. Inhibition of COL1A1 Promotor in Human Hepatic Stellate LX-2 Cells by Target Compounds
A single luciferase reporter gene detection model was applied to screen the inhibitory effects towards COL1A1 promotor of all target compounds in human hepatic stellate LX-2 cells at the concentration of 40 μM, taking EGCG and compound 1 as the positive controls. The LX2-COL monoclone cell line was treated simultaneously with TGFβ1 and a target compound [13]. The cytotoxicity was determined using MTT assay in HepG2 cells. The structures and inhibitory effects (%) of all target compounds are shown in Table 1.
Table 1.
Inhibition of COL1A1 promotor of target compounds and cytotoxicity of key compounds.
| Code | R1 | R2 | Inhibition Percentage a | CC50 (μM) b |
|---|---|---|---|---|
| 1 | p-CH3C6H4SO2 | - | 18.5% ± 1.1% | >320 |
| 5a | p-CF3C6H4SO2 | - | −3.7% ± 3.0% | NT c |
| 5b | C6H5CH2 | - | −33.6% ± 10.6% | NT c |
| 5c | p-CH3C6H4CH2 | - | −116.2% ± 45.7% | NT c |
| 5d | p-BrC6H4CH2 | - | 6.4%± 0.9% | NT c |
| 6a | p-CH3C6H4SO2 | NH2 | 21.2% ± 10.4% | >320 |
| 6b | p-CH3C6H4SO2 | NHCH3 | 12.8% ± 7.3% | NT c |
| 6c | p-CH3C6H4SO2 | NHCH(CH3)2 | −84.2% ± 63.1% | NT c |
| 6d | p-CH3C6H4SO2 | N(CH3)2 | −36.7% ± 17.3% | NT c |
| 6e | p-CH3C6H4SO2 | NHPh | 69.0% ± 13.5% | 82.55 |
| 6f | p-CF3C6H4SO2 | NH2 | −19.0% ± 7.8% | NT c |
| 6g | p-CF3C6H4SO2 | NHCH3 | 6.1% ± 0.8% | NT c |
| 6h | p-CF3C6H4SO2 | N(CH3)2 | −35.2% ± 2.1% | NT c |
| 6i | C6H5CH2 | NH2 | 47.8% ± 29.0% | >320 |
| 6j | C6H5CH2 | NHCH3 | 29.5% ± 6.1% | NT c |
| 6k | C6H5CH2 | N(CH3)2 | 15.2% ± 7.1% | NT c |
| 6l | p-CH3C6H4CH2 | NH2 | 38.7% ± 4.4% | >320 |
| 6m | p-CH3C6H4CH2 | NHCH3 | −29.6% ± 13.6% | NT c |
| 6n | p-CH3C6H4CH2 | N(CH3)2 | 39.7%± 12.3% | >320 |
| 6o | p-BrC6H4CH2 | NH2 | 7.8% ± 3.0% | NT c |
| 7a | p-CH3C6H4SO2 | NHCOCH3 | 33.9% ± 9.9% | NT c |
| 7b | p-CF3C6H4SO2 | NHCOCH3 | −6.0% ± 5.0% | NT c |
| 7c | C6H5CH2 | NHCOCH3 | 12.9% ± 10.6% | NT c |
| 7d | p-CH3C6H4CH2 | NHCOCH3 | 27.0% ± 9.5% | NT c |
| EGCG | - | - | 27.5% ± 7.9% | - |
| DMSO | - | - | 2.9% ± 0 | - |
a The inhibition rate of COL1A1 promotor’s activity in LX2 cells; b Cytotoxic concentration required to inhibit HepG2 cells cell growth by 50%; c Not tested.
Taking 1 as the lead, the carboxyl group was retained, and the SAR investigation was initiated with the variation of 12-N substitution, by which 4 matrinic acid derivatives 5a–d were obtained. As depicted in Table 1, the replacement of the methyl group on the benzene ring with a trifluoromethyl group (compound 5a) or the replacement of a benznesulfonyl with a benzyl motif (compounds 5b–d) caused a significant decrease in activity.
The carboxyl group at the end of the 11-side chain was then replaced by its 1,3,4-thiadiazole bioisostere, and a series of target matrinic thiadiazole compounds was prepared and evaluated. To start, the 12-N-p-methylbenzenesulfonyl was retained, and five matrinic aminothiadiazoles 6a–e were obtained. Compound 6a showed higher inhibitory effects than 6b–d and the lead 1, which indicated that 5-amino-1,3,4-thiadiazole motif was a beneficial group, and the introduction of an extra alkyl group on the amino group was not helpful for activity in this series. In the meantime, the introduction of a phenyl group into the amino (compound 6e) resulted in an obvious increase in activity. It was indicated that the aminothiadiazole motif was more beneficial, which might result from the smaller spatial hindrance. Then, the 12-N-substituent was replaced with a p-trifluoromethyl benzenesulfonyl moiety to prepare target compounds 6f–h, which displayed decreased activities in varied degrees, as anticipated.
Next, the aminothiadiazole motif was retained and the 12-N-benzenesulfonyl was replaced with a 12-N-benzyl group to generate the corresponding compounds 6i–o. To our great delight, most of them showed a significant improvement in activity, and compounds 6i, 6l and 6n gave inspiring inhibitory rates of 47.8%, 38.7% and 39.7%, respectively, significantly higher than that of 1 (18.5%) and EGCG (27.5%). These results suggested that benzyl was a favorable 12-N substitution.
Finally, an acyl group was condensed to the amino group of 6a, 6f, 6i and 6l to generate 7a–d, respectively, and 12-N-benzenesulfonyl compounds 7a and 7b displayed higher activity than their amino counterparts 6a and 6f, while the 12-N-benzyl compounds 7c and 7d displayed lower activity than their counterparts 6i and 6l.
Five target compounds 6a, 6e, 6i, 6l and 6n with the highest activity were selected as the representative compounds to further evaluate their cytotoxicities. As indicated in Table 1, compounds 6a, 6i, 6l and 6n displayed high cellular safety with the concentration of half cellular toxicity (CC50) value of over 320 μM, comparable to that of 1, while compound 6e displayed a high cellular toxicity with a CC50 value of over 82.6 μM, indicating that the introduction of a benzylamino to the thiadiazole motif might contribute to a high cytotoxicity, which might result from its high lipophilicity.
2.3. Inhibition Effects of COL1A1 on RNA and Protein Levels by Key Compounds
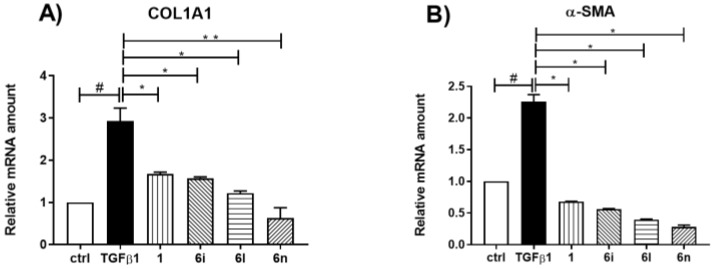
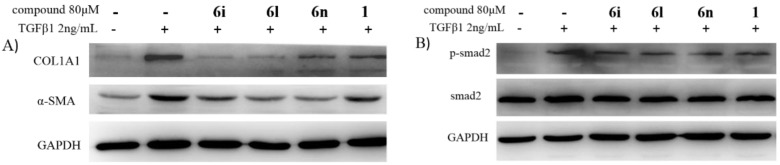
Next, compounds 6i, 6l and 6n were chosen as the key compounds to verify their inhibitory effects against COL1A1. LX-2 cells were stimulated with TGFβ1 (2 ng/mL), and then treated with compounds 6i, 6l and 6n (80 μM) respectively, taking 1 as the control. Their anti-COL1A1 effects were evaluated on RNA level by real-time PCR amplification. As indicated in Figure 2A, all three compounds 6i, 6l and 6n could significantly reduce COL1A1 mRNA level with the inhibitory rates of 46.1%,58.2% and 78.4%, respectively, prevailing over that of 1 (41.9%) to varying degrees. Then their anti-COL1A1 effects were investigated on the protein level by western blot assays. As indicated in Figure 3A, 6i, 6l and 6n could significantly reduce the COL1A1 protein level with inhibition rates of 65.0%, 99.3% and 47.6%, respectively, significantly higher than that of 1 (16.2%). These results suggested that these matrinic thiadiazole compounds could effectively reduce COL1A1 on both the mRNA and protein levels.
Figure 2.
Effects of compounds 6i, 6l and 6n inhibiting (A) COL1A1, (B) α-SMA mRNA levels in LX-2 cells. Data were analyzed by Real-time PCR and presented as the mean ± SEM, (#) p < 0.05 as compared to that of control group; (*) p < 0.05, (**) p < 0.01 as compared to that of TGF-β1 group. The mRNA expression levels were normalized against GAPDH.
Figure 3.
(A) Effects of compounds 6i, 6l and 6n on inhibiting fibrogenetic COL1A1 and α-SMA protein levels in LX-2 cells; (B) Effects of compounds 6i, 6l and 6n on p-smad2 and smad2 levels in LX-2 cells. Data were analyzed by western blot assay. The protein expression levels were normalized against GAPDH.
2.4. Inhibition Effects of α-SMA on RNA and Protein Levels by Key Compounds
α-SMA is a more commonly recognized indicator for liver fibrosis, and it could be induced by a stimulation of TGFβ1 [22], therefore, the amounts of α-SMA were also evaluated. In the same experiments as illustrated above, 6i, 6l and 6n significantly reduced α-SMA mRNA levels with inhibitory rates of 75.1%, 82.5% and 87.6% respectively, superior to that of 1 (69.7%), as indicated in Figure 2B. Compounds 6i, 6l and 6n significantly reduced the α-SMA protein level with inhibition rates of 64.9%, 93.8% and 104.1%, respectively, significantly superior to that of 1 (28.9%), as indicated in Figure 3A. These results disclosed that these kind of compounds also inhibited fibrogenetic α-SMA on both mRNA and protein levels.
2.5. Action on TGFβ/Smad Pathway of Key Compounds
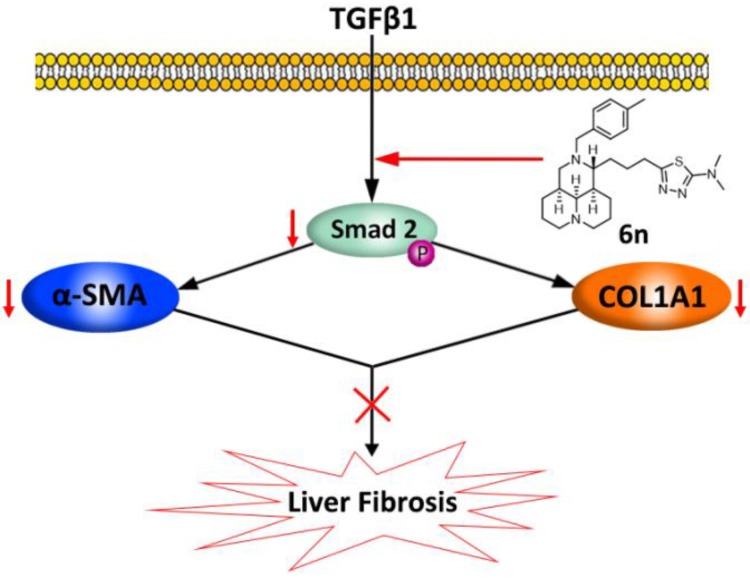
Considering the key role of TGFβ/Smad pathway in activating COL1A1, the effects of the three compounds on Smad and phosphorylated Smad were then evaluated. As indicated in Figure 2B, 6i, 6l and 6n did not affect the Smad level, while they repressed the phosphorylation level of Smad2 significantly, therefore, it was speculated that they might exert anti-liver fibrotic activity via repressing the TGFβ/Smad pathway, as depicted in Figure 4. Among them, compound 6n showed the highest inhibition rate of 49.8% as anticipated, and it was selected for the next investigation.
Figure 4.
Compound 6n exerted the anti-liver fibrotic activity via repressing the Smad/TGFβ pathway.
2.6. Safety Profile of Key Compounds
To evaluate the safety profile of 6n, an acute toxicity test was performed in Kunming mice. Compound 6n was given orally in a single-dosing experiment at 125, 250 or 500 mg·kg−1, respectively. The mice were closely monitored for 7 days. To our delight, it gave the median lethal dose (LD50) value over 500 mg·kg−1, indicating a good safety profile in vivo.
3. Experimental Section
3.1. Apparatus, Materials, and Analysis Reagents
All chemical reagents and anhydrous solvents were obtained from commercial sources and used without further purification. Melting points (mp) were obtained with a MP90 melting point apparatus and was uncorrected (Mettler-Toledo, Greifensee, Switzerland). Specific rotations were obtained with Rudolph Autopol IV automatic polarimeter (Jasco Products Company, Oklahoma City, OK, USA). 1H- and 13C-NMR spectra were recorded on Avance (600 MHz and 500 MHz for 1H-NMR; 151 MHz and 126 MHz for 13C-NMR) spectrometers (Bruker, Zürich, Switzerland). The 1H chemical shifts were referenced to the solvent peak: DMSO-d6 (2.49 ppm) and CDCl3 (7.26 ppm) and the 13C chemical shifts were referenced to the solvent peak: DMSO-d6 (40.5 ppm) and CDCl3 (77.4 ppm). ESI high-resolution mass spectra (HRMS) were recorded on an AutospecUltima-TOF spectrometer (Micromass UK Ltd., Manchester, UK). The method of the HPLC analysis was as follows: column, Inertsustain C18, 250 mm × 4.6 mm, id; flow rate, 1.0 mL/min; mobile phase, acetonitrile/water (0.01 mol·L−1 KH2PO4 in water, pH = 4.0) = 5:95; Flash column chromatography was performed on Combiflash Rf 200 (Teledyne, Lincoln, NE, USA), particle size 0.038 mm. The target compounds 1 and 5a–d were reported previously [9,15,23], but 1 and 5b–d were obtained in this manuscript by a new method with improved yields.
3.2. Chemistry
3.2.1. General Procedure for the Synthesis of Compounds 1, 5b–d
Matrine (5.0 g, 20 mmol) was added to 5 N NaOH solution (30 mL), and the reaction mixture was refluxed for 9 h, cooled to room temperature and then acidified with HCl (2 N) to pH 2–3. The solvent was removed in vacuo and the residue was dissolved with 2% HCl in methanol and then refluxed for 2 h. The solvent was removed under reduced pressure to give a crude product, which was purified by recrystallization from ethanol to achieve intermediate 3 (6.6 g, 93.1%). To a suspension of compound 3 (5.0 g, 14 mmol) in dichloromethane (50 mL), triethylamine (4.34 g, 43 mmol) and substituted benzenesulfonyl chloride (17 mmol) or substituted benzyl bromide (17 mmol) were added, the reaction mixture was stirred at room temperature for 6 h until TLC analysis showed completion of the reaction. The reaction mixture was then washed by water (50 mL), saturated aqueous ammonium chloride (50 mL) and saturated aqueous sodium chloride (50 mL) subsequently, dried over anhydrous sodium sulfate and concentrated under reduced pressure. The residue was purified by recrystallization from methanol to give 4. Compound 4 (10 mmol) were refluxed in 5 N NaOH solution (30 mL) for 4 h, cooled and acidified with HCl (2 N) to pH 4–5. The solvent was removed by condensation, and the residue was suspended in methanol, and the precipate was filtered off. The filtrate was evapotared to give a crude. The desired compounds (1 or 5b–d) were gained by flash column chromatography purification on silica gel with dichloromethane/methanol as the eluent.
12-N-p-Methylbenzenesulfonyl matrinic acid (1). Total yield: 64%, m.p.: 239–240 °C (Total yiled: 6%, m.p.: 239–241 °C [15]).
12-N-Benzyl matrinic acid (5b). Total yield: 41%, m.p.: 95–96 °C (Total yield: 15%, m.p.: 95–96 °C [15]).
12-N-p-Methylbenzyl matrinic acid (5c). Total yield: 46%, m.p.: 106–108 °C (Total yield: 34%, m.p.: 106–108 °C [9]).
12-N-p-Bromobenzyl matrinic acid (5d). Total yield: 59%, m.p.: 134–135 °C (Total yield: 27%, m.p.: 133–135 °C [9]).
3.2.2. General Procedure for the Synthesis of Compounds 6a–o
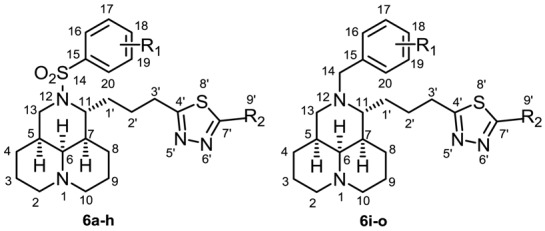
A mixture of 1 or 5a–d (2.5 mmol), thiosemicarbazide or N-substituted thiosemicarbazide (2.5 mmol), methanesulfonic acid (12.5 mmol) and phosphorus pentoxide (2.5 mmol) were heated at 70 °C for 8 h. The reaction mixture was poured into ice water (40 mL) and neutralized with aqueous ammonia to pH 8. The solution was extracted with dichloromethane (50 mL), and the organic layer was washed by saturated aqueous sodium chloride (50 mL), dried over anhydrous sodium sulfate, and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel with dichloromethane/methanol or petroleum ether/ethyl acetate as the eluent.
12-N-p-Methlybenzenesulfonyl-3′-(5-amino-1,3,4-thiadiazol-2-yl)-matrinic propane (6a). Compound 1 (1.04 g, 2.5 mmol) was treated with thiosemicarbazide (0.23 g, 2.5 mmol) according to the general procedure, then purified by flash column chromatography with dichloromethane/methanol as the eluent to give the desired product 6a as a white solid, yield: 40.9%; m.p.: 237 °C (dec.); = −6.16; 1H-NMR (500 MHz, DMSO-d6) δ 7.68 (d, J = 8.2 Hz, 2H, 16-CH, 20-CH), 7.38 (d, J = 8.2 Hz, 2H, 17-CH, 19-CH), 7.00 (s, 2H, 9′-NH2), 3.42–3.36 (m, 2H, 3′-CH2), 3.16–3.08 (m, 1H, 6-CH), 2.73–2.69 (m, 1H, 5-CH), 2.65–2.61 (m, 1H, 7-CH), 2.59–2.56 (m, 1H, 11-CH), 2.38 (s, 3H, Ph-CH3), 1.95–1.90 (m, 1H, 1′a-CH), 1.87–1.80 (m, 2H, 2′-CH2), 1.76–1.61 (m, 6H, 2-CH2, 10-CH2, 13-CH2), 1.51–1.45 (m, 2H, 4-CH2), 1.39–1.23 (m, 7H, 3-CH2, 8-CH2, 9-CH2, 1′b-CH); 13C-NMR (126 MHz, DMSO-d6) δ 168.2 (7′-C), 158.2 (4′-C), 142.9 (18-C), 136.7 (15-C), 129.5 (17-CH, 19-CH), 127.2 (16-CH, 20-CH), 62.3 (6-CH), 56.6 (11-CH), 56.2 (2-CH2), 56.1 (10-CH2), 47.1 (13-CH2), 38.6 (7-CH), 34.0 (5-CH), 29.7 (1′-CH2), 29.6 (3′-CH2), 27.6 (4-CH2), 27.5 (8-CH2), 24.8 (2′-CH2), 21.1 (Ph-CH3), 20.3 (3-CH2), 20.2 (9-CH2); ESI-HRMS: m/z Calcd for C23H34O2N5S2 [M + H]+, 476.2148; Found, 476.2149; HPLC: tR = 10.29 min, normalization method purity 97.11%.
12-N-p-Mmethlybenzenesulfonyl-3′-(N-methyl-5-amino-1,3,4-thiadiazol-2-yl)-matrinic propane (6b). Compound 1 (1.04 g, 2.5 mmol) was treated with 4-methyl-3-thiosemicarbazide (0.26 g, 2.5 mmol) according to the general procedure, then purified by flash column chromatography with dichloromethane/methanol as the eluents to give to give the desired product 6b as a white solid, yield: 33.4%; m.p.: 169–170 °C; = −7.98; 1H-NMR (500 MHz, DMSO-d6) δ 7.68 (d, J = 7.9 Hz, 2H, 16-CH, 20-CH), 7.49 (q, J = 4.8 Hz, 1H, 9′-NH), 7.38 (d, J = 7.9 Hz, 2H, 17-CH, 19-CH), 3.43–3.35 (m, 2H, 3′-CH2), 3.14–3.09 (m, 1H, 6-CH), 2.83 (d, J = 4.8 Hz, 3H, 9′-NCH3), 2.76–2.70 (m, 1H, 5-CH), 2.67–2.61 (m, 1H, 7-CH), 2.57 (m 1H, 11-CH), 2.38 (s, 3H, Ph-CH3), 1.95–1.90 (m, 1H, 1′a-CH), 1.86–1.80 (m, 2H, 2′-CH2), 1.75–1.62 (m, 6H, 2-CH2, 10-CH2, 13-CH2), 1.49–1.23 (m, 9H, 3-CH2, 4-CH2, 8-CH2, 9-CH2, 1′b-CH ); 13C-NMR (126 MHz, DMSO-d6) δ 169.1 (7′-C), 157.6 (4′-C), 142.9 (18-C), 136.7 (15-C), 129.5 (17-CH, 19-CH), 127.2 (16-CH, 20-CH), 62.3 (6-CH), 56.6 (11-CH), 56.1 (2-CH2), 56.0 (10-CH2), 47.0 (13-CH2), 38.6 (7-CH), 34.0 (5-CH), 31.2 (9′-NCH3), 29.7 (1′-CH2), 29.6 (3′-CH2), 27.5 (4-CH2), 27.4 (8-CH2), 24.9 (2′-CH2), 21.0 (Ph-CH3), 20.3 (3-CH2), 20.2 (9-CH2); ESI-HRMS: m/z Calcd for C24H36O2N5S2 [M + H]+, 490.2304; Found, 490.2304; HPLC: tR = 10.78 min, normalization method purity 98.74%.
12-N-p-Methylbenzenesulfonyl-3′-(N-iso-propyl-5-amino-1,3,4-thiadiazol-2-yl)-matrinic propane (6c). Compound 1 (1.04 g, 2.5 mmol) was treated with 4-iso-propyl-3-thiosemicarbazide (0.33 g, 2.5 mmol) according to the general procedure, then purified by flash column chromatography with dichloromethane/methanol as the eluents to give the desired product 6d as a white solid, yield: 34.1%; m.p.: 142–143 °C; = −15.79; 1H-NMR (600 MHz, DMSO-d6) δ 7.68 (d, J = 8.1 Hz, 2H, 16-CH, 20-CH), 7.44 (d, J = 7.2 Hz, 1H, 9′-NH), 7.38 (d, J = 8.1 Hz, 2H, 17-CH, 19-CH), 3.77–3.69 (m, 1H, 9′-NCH), 3.42–3.38 (m, 2H, 3′-CH2), 3.13 (t, J = 11.6 Hz, 1H, 6-CH), 2.74–2.70 (m, 1H, 5-CH), 2.66–2.61 (m, 1H, 7-CH), 2.58–2.53 (m, 1H, 11-CH), 2.38 (s, 3H, Ph-CH3), 1.94 (m, 1H, 1′a-CH), 1.86–1.80 (m, 2H, 2′-CH2), 1.75–1.61 (m, 6H, 2-CH2, 10-CH2, 13-CH2), 1.49–1.46 (m, 2H, 4-CH2), 1.39–1.23 (m, 7H, 3-CH2, 8-CH2, 9-CH2, 1′b-CH), 1.17 (d, J = 6.4 Hz, 6H, NC(CH3)2); 13C-NMR (151 MHz, DMSO-d6) δ 167.4 (7′-C), 157.2 (4′-C), 142.9 (18-C), 136.7 (15-C), 129.5 (17-CH, 19-CH), 127.2 (16-CH, 20-CH), 62.3 (6-CH), 56.6 (11-CH), 56.1 (2-CH2), 56.0 (10-CH2), 47.0 (13-CH2), 46.5 (NCH), 38.6 (7-CH), 34.0 (5-CH), 29.8 (1′-CH2), 29.5 (3′-CH2), 27.5 (4-CH2), 27.4 (8-CH2), 24.9 (2′-CH2), 22.2 (NC(CH3)2), 21.0 (Ph-CH3), 20.3 (3-CH2), 20.2 (9-CH2); ESI-HRMS: m/z Calcd for C26H40O2N5S2 [M + H]+, 518.2617; Found, 518.2607; HPLC: tR = 12.11 min, normalization method purity 97.99%.
12-N-p-Methylbenzenesulfonyl-3′-(N,N-dimethyl-5-amino-1,3,4-thiadiazol-2-yl)-matrinic propane (6d). Compound 1 (1.04 g, 2.5 mmol) was treated with 4,4-dimethyl-3-thiosemicarbazide (0.31 g, 2.5 mmol) according to the general procedure, then purified by flash column chromatography with dichloromethane/methanol as the eluents to give the desired product 6e as a white solid, yield: 24.2%; m.p.: 171–172 °C; = −9.66; 1H-NMR (500 MHz, DMSO-d6) δ 7.68 (d, J = 8.2 Hz, 2H, 16-CH, 20-CH), 7.39 (d, J = 8.2 Hz, 2H, 17-CH, 19-CH), 3.45–3.36 (m, 2H, 3′-CH2), 3.18–3.07 (m, 1H, 6-CH), 3.03 (s, 6H, N(CH3)2), 2.81–2.72 (m, 1H, 5-CH), 2.72–2.63 (m, 1H, 7-CH), 2.57 (d, J = 11.4 Hz, 1H, 11-CH), 2.38 (s, 3H, Ph-CH3), 1.94 (m, 1H, 1′a-CH), 1.90–1.79 (m, 2H, 2′-CH2), 1.78–1.60 (m, 6H, 2-CH2, 10-CH2, 13-CH2), 1.54–1.18 (m, 9H, 3-CH2, 4-CH2, 8-CH2, 9-CH2, 1′b-CH); 13C-NMR (151 MHz, DMSO-d6) δ 171.2 (7′-C), 158.5 (4′-C), 142.8 (18-C), 136.8 (15-C), 129.5 (17-CH, 19-CH), 127.1 (16-CH, 20-CH), 62.3 (6-CH), 56.6 (11-CH), 56.1 (2-CH2), 56.0 (10-CH2), 47.0 (13-CH2), 41.1 (N(CH3)2), 38.7 (7-CH), 34.0 (5-CH), 29.7 (1′-CH2), 29.6 (3′-CH2), 27.5 (4-CH2), 27.4 (8-CH2), 25.1 (2′-CH2), 21.0 (Ph-CH3), 20.3 (3-CH2), 20.2 (9-CH2); ESI-HRMS: m/z Calcd for C25H38O2N5S2 [M + H]+, 504.2441; Found, 504.2447; HPLC: tR = 11.59 min, normalization method purity 98.45%.
12-N-p-Methylbenzenesulfonyl-3′-(N-phenyl-5-amino-1,3,4-thiadiazol-2-yl)-matrinic propane (6e). Compound 1 (1.04 g, 2.5 mmol) was treated with 4-phenyl-3-thiosemicarbazide (0.42 g, 2.5 mmol) according to the general procedure, then purified by flash column chromatography with petroleum ether/ethyl acetate as the eluents to give the desired product 6c as a white solid, yield: 48.2%; m.p.: 193–194 °C; = −13.89; 1H-NMR (500 MHz, DMSO-d6) δ 10.26 (s, 1H, 9′-NH), 7.68 (d, J = 8.0 Hz, 2H, 16-CH, 20-CH), 7.60 (d, J = 8.0 Hz, 2H, 17-CH, 19-CH), 7.38 (d, J = 7.5 Hz, 2H, 2 × 9′-NCHarom), 7.33 (t, J = 7.5 Hz, 2H, 2 × 9′-NCHarom), 6.97 (t, J = 7.5 Hz, 1H, 9′-NCHarom ), 3.43–3.38 (m, 2H, 3′-CH2), 3.13 (t, J = 11.6 Hz, 1H, 6-CH), 2.87–2.81 (m, 1H, 5-CH), 2.79–2.72 (m, 1H, 7-CH), 2.57 (d, J = 11.1 Hz, 1H, 11-CH), 2.35 (s, 3H, Ph-CH3), 1.93 (m, 1H, 1′a-CH), 1.86–1.83 (m, 2H, 2′-CH2), 1.75–1.68 (m, 6H, 2-CH2, 10-CH2, 13-CH2), 1.56–1.23 (m, 9H, 3-CH2, 4-CH2, 8-CH2, 9-CH2, 1′b-CH); 13C-NMR (151 MHz, DMSO-d6) δ 163.9 (7′-C), 159.5 (4′-C), 142.8 (18-C), 140.8 (9′N-Ph), 136.8 (15-C), 129.5 (17-CH, 19-CH), 129.0 (9′N-Ph), 127.1 (16-CH, 20-CH), 121.6 (9′N-Ph), 117.1 (9′N-Ph), 62.3 (6-CH), 56.6 (11-CH), 56.1 (2-CH2), 56.0 (10-CH2), 46.9 (13-CH2), 38.8 (7-CH), 34.0 (5-CH), 29.8 (1′-CH2), 29.4 (3′-CH2), 27.6 (4-CH2), 27.5 (8-CH2), 24.9 (2′-CH2), 21.0 (Ph-CH3), 20.3 (3-CH2), 20.2 (9-CH2); ESI-HRMS: m/z Calcd for C29H38O2N5S2 [M + H]+, 552.2461; Found, 552.2462; HPLC: tR = 13.39 min, normalization method purity 96.69%.
12-N-p-Ttrifluoromethlybenzenesulfonyl-3′-(5-amino-1,3,4-thiadiazol-2-yl)-matrinic propane (6f). Compound 5a (1.19 g, 2.5 mmol) was treated with thiosemicarbazide (0.23 g, 2.5 mmol) according to the general procedure, then purified by flash column chromatography with dichloromethane/methanol as the eluents to give the desired product 6f as a white solid, yield: 48.0%; m.p.: 206 °C (dec.); = −0.65; 1H-NMR (500 MHz, CDCl3) δ 7.96 (d, J = 8.2 Hz, 2H, 16-CH, 20-CH), 7.73 (d, J = 8.2 Hz, 2H, 17-CH, 19-CH), 5.56 (s, 2H, 9′-NH2), 3.67–3.57 (m, 1H, 3′a-CH), 3.53–3.45 (m, 1H, 3′b-CH), 3.22 (t, J = 11.5 Hz, 1H, 6-CH), 2.93–2.75 (m, 2H, 5-CH, 7-CH), 2.54 (d, J = 11.5 Hz, 1H, 13a-CH), 2.45 (d, J = 11.5 Hz, 1H, 13b-CH), 1.96 (m, 2H, 2′-CH2), 1.89–1.62 (m, 8H, 2-CH2, 4-CH2, 10-CH2, 11-CH, 1′a-CH), 1.49–1.26 (m, 7H, 3-CH2, 8-CH2, 9-CH2, 1′b-CH); 13C-NMR (126 MHz, CDCl3) δ 168.2 (7′-C), 161.2 (4′-C), 143.9 (15-C), 133.9 (18-C), 128.1 (17-CH, 19-CH), 125.9 (16-CH, 20-CH), 123.4 (Ph-CF3), 62.7 (6-CH), 57.5 (11-CH), 56.6 (2-CH2), 56.5 (10-CH2), 46.9 (13-CH2), 39.7 (7-CH), 34.4 (5-CH), 31.6 (1′-CH2), 30.3 (3′-CH2), 28.3 (4-CH2), 27.9 (8-CH2), 25.8 (2′-CH2), 20.9 (3-CH2), 20.6 (9-CH2); ESI-HRMS: m/z Calcd for C23H31O2N5F3S2 [M + H]+, 530.1865; found, 530.1864; HPLC: tR = 11.41 min, normalization method purity 98.85%.
12-N-p-Trifluoromethlybenzenesulfonyl-3′-(N-methyl-5-amino-1,3,4-thiadiazol-2-yl)-matrinic propane (6g). Compound 5a (1.19 g, 2.5 mmol) was treated with 4-methyl-3-thiosemicarbazide (0.26 g, 2.5 mmol) according to the general procedure, then purified by flash column chromatography with dichloromethane/methanol to give the desired product 6g as a white solid, yield: 45.0%; m.p.: 181 °C (dec.); = −1.89; 1H-NMR (500 MHz, CDCl3) δ 7.97 (d, J = 8.2 Hz, 2H, 16-CH, 20-CH), 7.73 (d, J = 8.2 Hz, 2H, 17-CH, 19-CH), 5.79 (s, 1H, 9′-NH), 3.68–3.57 (m, 1H, 3′a-CH ), 3.53–3.46 (m, 1H, 3′b-CH), 3.24 (t, J = 11.4 Hz, 1H, 6-CH), 3.01 (s, 3H, 9′-NCH3), 2.91–2.77 (m, 2H, 5-CH, 7-CH), 2.54 (d, J = 11.4 Hz, 1H, 13a-CH), 2.46 (d, J = 11.4 Hz, 1H, 13b-CH), 2.02–1.91 (m, 2H, 2′-CH2), 1.86–1.63 (m, 8H, 2-CH2, 4-CH2, 10-CH2, 11-CH, 1′a-CH), 1.51–1.31 (m, 7H, 3-CH2, 8-CH2, 9-CH2, 1′b-CH); 13C-NMR (126 MHz, CDCl3) δ 171.4 (7′-C), 159.2 (4′-C), 144.1 (15-C), 133.9 (18-C), 128.1 (17-CH, 19-CH), 125.8 (16-CH, 20-CH), 123.4 (Ph-CF3), 62.7 (6-CH), 57.6 (11-CH), 56.6 (2-CH2), 56.5 (10-CH2), 47.0 (13-CH2), 39.8 (7-CH), 34.5 (5-CH), 33.3 (9′-NCH3), 31.6 (1′-CH2), 30.4 (3′-CH2), 28.3 (4-CH2), 28.0 (8-CH2), 25.9 (2′-CH2), 20.9 (3-CH2), 20.7 (9-CH2); ESI-HRMS: m/z Calcd for C24H33O2N5F3S2 [M + H]+, 544.2022; found, 544.2019; HPLC: tR = 11.89 min, normalization method purity 98.90%.
12-N-p-Trifluoromethlybenzenesulfonyl-3′-(N,N-dimethyl-5-amino-1,3,4-thiadiazol-2-yl)-matrinic propane (6h). Compound 5a (1.19 g, 2.5 mmol) was treated with 4,4-dimethyl-3- thiosemicarbazide (0.31 g, 2.5 mmol) according to the general procedure, then purified by flash column chromatography with petroleum ether/ethyl acetate to give the desired product 6h as a white solid, yield: 33.3%; m.p.: 147–148 °C; = −3.30; 1H-NMR (500 MHz, CDCl3) δ 7.97 (d, J = 8.2 Hz, 2H, 16-CH, 20-CH), 7.72 (d, J = 8.2 Hz, 2H, 17-CH, 19-CH), 3.66–3.62 (m, 1H, 3′a-CH), 3.51 (dd, J = 12.6, 6.2 Hz, 1H, 3′b-CH), 3.27–3.22 (m, 1H, 6-CH), 3.09 (s, 6H, 9′-N(CH3)2), 2.90–2.71 (m, 2H, 5-CH, 7-CH), 2.56–2.53 (m, 1H, 13a-CH), 2.49–2.45 (m, 1H, 13b-CH), 1.99–1.94 (m, 2H, 2′-CH2), 1.86–1.61 (m, 8H, 2-CH2, 4-CH2, 10-CH2, 11-CH, 1′a-CH), 1.52–1.29 (m, 7H, 3-CH2, 8-CH2, 9-CH2, 1′b-CH); 13C-NMR (126 MHz, CDCl3) δ 172.0 (7′-C), 159.3 (4′-C), 144.2 (15-C), 133.8 (18-C), 128.1 (17-CH, 19-CH), 125.8 (16-CH, 20-CH), 123.4 (Ph-CF3), 62.8 (6-CH), 57.6 (11-CH), 56.6 (2-CH2), 56.5 (10-CH2), 47.1 (13-CH2), 41.5 (9′N(CH3)2), 39.8 (7-CH), 34.5 (5-CH), 31.5 (1′-CH2), 30.4 (3′-CH2), 28.3 (4-CH2), 28.0 (8-CH2), 25.9 (2′-CH2), 20.9 (3-CH2), 20.7 (9-CH2); ESI-HRMS: m/z Calcd for C25H35O2N5F3S2 [M + H]+, 558.2178; found, 558.2169; HPLC: tR = 12.71 min, normalization method purity 98.60%.
12-N-Benzyl-3′-(5-amino-1,3,4-thiadiazol-2-yl)-matrinic propane (6i) Compound 5b (1.19 g, 2.5 mmol) was treated with thiosemicarbazide (0.23 g, 2.5 mmol) according to the general procedure, then purified by flash column chromatography with dichloromethane/methanol to give the desired product 6i as a white solid, yield: 34.7%; m.p.: 170–171 °C; = −29.79; 1H-NMR (600 MHz, CDCl3) δ 7.30 (dt, J = 15.0, 7.2 Hz, 4H, 16-CH, 17-CH, 19-CH, 20-CH), 7.20 (t, J = 7.2 Hz, 1H, 18-CH), 5.67 (s, 2H, 9′-NH2), 4.00 (d, J = 13.4 Hz, 1H, 14a-CH), 3.12 (d, J = 13.4 Hz, 1H, 14b-CH), 2.94–2.72 (m, 5H, 3′-CH2, 5-CH, 7-CH, 11-CH ), 2.64 (t, J = 11.9 Hz, 1H, 6-CH), 2.33 (m, 1H, 1′a-CH), 1.95–1.57 (m, 12H, 2-CH2, 4-CH2, 8-CH2, 10-CH2, 13-CH2, 2′-CH2), 1.45–1.28 (m, 5H, 3-CH2, 9-CH2, 1′b-CH); 13C-NMR (151 MHz, CDCl3) δ 168.3 (7′-C), 161.6 (4′-C), 140.6 (15-C), 128.7 (17-CH, 19-CH), 128.3 (16-CH, 20-CH), 126.7 (18-CH), 64.6 (6-CH), 57.6 (11-CH), 57.4 (14-CH2), 57.3 (13-CH2), 56.7 (2-CH2), 52.4 (10-CH2), 37.9 (7-CH), 34.0 (5-CH), 30.8 (1′-CH2), 28.3 (3′-CH2), 28.2 (4-CH2), 27.4 (8-CH2), 23.9 (2′-CH2), 21.7 (3-CH2), 21.4 (9-CH2); ESI-HRMS: m/z Calcd for C23H34N5S [M + H]+, 412.2529; found, 412.2530; HPLC: tR = 8.26 min, normalization method purity 98.65%.
12-N-Benzyl-3′-(N-methyl-5-amino-1,3,4-thiadiazol-2-yl)-matrinic propane (6j) Compound 5b (1.19 g, 2.5 mmol) was treated with 4-methyl-3-thiosemicarbazide (0.26 g, 2.5 mmol) according to the general procedure, then purified by flash column chromatography with dichloromethane/methanol to give the desired product 6j as a white solid, yield: 32.3%; m.p.: 137–138 °C; = −28.80; 1H-NMR (600 MHz, CDCl3) δ 7.32–7.27 (m, 4H, 16-CH, 17-CH, 19-CH, 20-CH), 7.20 (t, J = 7.2 Hz, 1H, 18-CH), 5.86 (m, 1H, 9′-NH), 4.01 (d, J = 13.0 Hz, 1H, 14a-CH), 3.12 (d, J = 13.0 Hz, 1H, 14b-CH), 2.97–2.60 (m, 9H, 3′-CH2, 5-CH, 6-CH, 7-CH, 9′-NCH3, 11-CH), 2.34 (d, J = 11.6 Hz, 1H, 1′a-CH), 1.99–1.52 (m, 12H, 2-CH2, 4-CH2, 8-CH2, 10-CH2, 13-CH2, 2′-CH2), 1.47–1.29 (m, 5H, 3-CH2, 9-CH2, 1′b-CH); 13C-NMR (151 MHz, CDCl3) δ 171.4 (7′-C), 159.7 (4′-C), 140.6 (15-C), 128.7 (17-CH, 19-CH), 128.3 (16-CH, 20-CH), 126.7 (18-CH), 64.6 (6-CH), 57.7 (11-CH), 57.4 (14-CH2), 57.3 (13-CH2), 56.7 (2-CH2), 52.3 (10-CH2), 37.9 (7-CH), 34.0 (5-CH), 33.3 (9′-NCH3), 30.8 (1′-CH2), 28.3 (3′-CH2), 28.2 (4-CH2), 27.4 (8-CH2), 24.0 (2′-CH2), 21.7 (3-CH2), 21.4 (9-CH2); ESI-HRMS: m/z Calcd for C24H36N5S [M + H]+, 426.2686; found, 426.2685; HPLC: tR = 9.14 min, normalization method purity 98.91%.
12-N-Benzyl-3′-(N,N-dimethyl-5-amino-1,3,4-thiadiazol-2-yl)-matrinic propane hydrochloride (6k). Compound 5b (1.19 g, 2.5 mmol) was treated with 4,4-dimethyl-3-thiosemicarbazide (0.31 g, 2.5 mmol) according to the general procedure, then purified by flash column chromatography with petroleum ether/ethyl acetate to give the crude product and treated with 2 N hydrochloride/ether (3 mL) to give the desired product 6k as a white solid, yield: 16.3%; m.p.: 189–190 °C; = −14.64; 1H-NMR (500 MHz, DMSO-d6) δ 11.81 (s, 1H, 1-NH+), 7.66–7.62 (m, 2H, 16-CH, 20-CH), 7.47–7.43 (m, 3H, 17-CH, 18-CH, 19-CH), 5.00 (d, J = 12.7 Hz, 2H, 14-CH2), 4.26 (t, J = 10.3 Hz, 1H, 6-CH), 3.99–3.93 (m, 2H, 13-CH2), 3.61 (d, J = 10.3 Hz, 1H, 7-CH), 3.32–3.20 (m, 2H, 3′-CH2), 3.05–2.87 (m, 6H, 9′-N(CH3)2), 2.66–2.60 (m, 2H, 5-CH, 11-CH), 2.57–2.52 (m, 1H, 1′a-CH), 2.18–1.92 (m, 6H, 2-CH2, 10-CH2, 2′-CH2), 1.89–1.55 (m, 8H, 3-CH2, 4-CH2, 8-CH2, 9-CH2), 1.43 (d, J = 13.6 Hz, 1H, 1′b-CH); 13C-NMR (126 MHz, DMSO-d6) δ 171.0 (7′-C), 158.7 (4′-C), 132.0 (18-C), 130.7 (15-C), 123.0 (17-CH, 19-CH), 129.4 (16-CH, 20-CH), 61.0 (6-CH), 60.8 (14-CH2), 58.1 (11-CH), 54.8 (2-CH2), 54.7 (10-CH2), 49.3 (13-CH2), 43.0 (9′-N(CH3)2), 36.5 (7-CH), 30.5 (5-CH), 29.9 (1′-CH2), 28.2 (3′-CH2), 26.4 (4-CH2), 24.7 (8-CH2), 24.2 (2′-CH2), 18.5 (3-CH2), 18.4 (9-CH2); ESI-HRMS: m/z Calcd for C25H38N5S [M-HCl+H]+, 440.2842; found, 440.2839; HPLC: tR = 9.40 min, normalization method purity 98.49%.
12-N-p-Methylbenzyl-3′-(5-amino-1,3,4-thiadiazol-2-yl)-matrinic propane (6l) Compound 5c (0.93 g, 2.5 mmol) was treated with thiosemicarbazide (0.23 g, 2.5 mmol) according to the general procedure, then purified by flash column chromatography with dichloromethane/methanol to give the desired product 6l as a white solid, yield: 41.4%; m.p.:167–168 °C; = −30.62; 1H-NMR (600 MHz, CDCl3) δ 7.19 (d, J = 7.7 Hz, 2H, 16-CH, 20-CH), 7.09 (d, J = 7.7 Hz, 2H, 17-CH, 19-CH), 5.68 (s, 2H, 9′-NH2), 3.96 (d, J = 13.4 Hz, 1H, 14a-CH), 3.08 (d, J = 13.4 Hz, 1H, 14b-CH), 2.89–2.73 (m, 4H, 3′-CH2, 5-CH, 7-CH), 2.62 (t, J = 11.9 Hz, 1H, 6-CH), 2.35–2.32 (m, 1H, 11-CH), 2.31 (s, 3H, Ph-CH3), 2.04–2.00 (m, 1H, 1′a-CH), 1.92–1.60 (m, 12H, 2-CH2, 4-CH2, 8-CH2, 10-CH2, 13-CH2, 2′-CH2 ), 1.43–1.29 (m, 5H, 3-CH2, 9-CH2, 1′b-CH ); 13C-NMR (151 MHz, CDCl3) δ 168.3 (7′-C), 161.6 (4′-C), 137.3 (18-C), 136.2 (15-C), 129.0 (17-CH, 19-CH), 128.7 (16-CH, 20-CH), 64.6 (6-CH), 57.6 (14-CH2), 57.4 (11-CH), 56.4 (2-CH2), 52.2 (10-CH2), 37.9 (13-CH2), 33.9 (7-CH), 30.8 (5-CH), 28.3 (1′-CH2), 28.2 (3′-CH2), 27.4 (4-CH2), 23.9 (8-CH2), 21.7 (2′-CH2), 21.4 (3-CH2), 21.2 (9-CH2), 21.1 (Ph-CH3); ESI-HRMS: m/z Calcd for C24H36N5S [M + H]+, 426.2686; found; 426.2685; HPLC: tR =8.78 min, normalization method purity 97.53%.
12-N-p-Methylbenzyl-3′-(N-methyl-5-amino-1,3,4-thiadiazol-2-yl)-matrinic propane (6m). Compound 5c (0.93 g, 2.5 mmol) was treated with 4-methyl-3-thiosemicarbazide (0.26 g, 2.5 mmol) according to the general procedure, then purified by flash column chromatography with dichloromethane/methanol to give the desired product 6m as a white solid, yield: 34.1%; m.p.: 163–164 °C; = −35.69; 1H-NMR (600 MHz, CDCl3) δ 7.19 (d, J = 7.6 Hz, 2H, 16-CH, 20-CH), 7.08 (d, J = 7.6 Hz, 2H, 17-CH, 19-CH), 6.28 (s, 1H, 9′-NH), 3.96 (d, J = 13.3 Hz, 1H, 14a-CH), 3.06 (d, J = 13.3 Hz, 1H, 14b-CH), 2.94 (s, 3H, 9′-NCH3), 2.89–2.77 (m, 4H, 3′-CH2, 5-CH, 7-CH), 2.61 (t, J = 12.0 Hz, 1H, 6-CH), 2.35–2.32 (m, 1H, 11-CH), 2.30 (s, 3H, Ph-CH3), 2.01 (m, 1H, 1′a-CH), 1.95–1.55 (m, 12H, 2-CH2, 4-CH2, 8-CH2, 10-CH2, 13-CH2, 2′-CH2), 1.44–1.29 (m, 5H, 3-CH2, 9-CH2, 1′b-CH); 13C-NMR (151 MHz, CDCl3) δ 171.6 (7′-C), 159.5 (4′-C), 137.4 (18-C), 136.1 (15-C), 128.9 (17-CH, 19-CH), 128.6 (16-CH, 20-CH), 64.6 (6-CH), 57.7 (14-CH2), 57.4 (11-CH), 56.4 (2-CH2), 52.2 (10-CH2), 38.0 (13-CH2), 34.0 (7-CH), 33.3 (9′-NCH3), 30.8 (5-CH), 28.3 (1′-CH2), 28.2 (3′-CH2), 27.4 (4-CH2), 24.0 (8-CH2), 21.7 (2′-CH2), 21.4 (3-CH2), 21.2 (9-CH2), 21.1 (Ph-CH3); ESI-HRMS: m/z Calcd for C25H38N5S [M + H]+, 440.2842; found, 440.2845; HPLC: tR =10.09 min, normalization method purity 95.47%.
12-N-p-Methylbenzyl-3′-(N,N-dimethyl-5-amino-1,3,4-thiadiazol-2-yl)-matrinic propane hydrochloride (6n). Compound 5c (0.93 g, 2.5 mmol) was treated with 4,4-dimethyl-3-thiosemicarbazide (0.31 g, 2.5 mmol) according to the general procedure, then purified by flash column chromatography with petroleum ether/ethyl acetate to give the crude product and treated with 2 N hydrochloride/ether (3 mL) to give the desired product 6n as a white solid, yield: 21.1%; m.p.: 199–200 °C; = −17.19; 1H-NMR (500 MHz, DMSO-d6) δ 7.51 (d, J = 7.6 Hz, 2H, 16-CH, 20-CH), 7.25 (d, J = 7.6 Hz, 2H, 17-CH, 19-CH), 5.76 (s, 1H, 12-NH), 4.94 (m, 1H, 5-CH), 4.24 (m, 1H, 6-CH), 3.98–3.84 (m, 2H, 14-CH2), 3.62 (m, 1H, 11-CH), 3.24 (m, 2H, 13-CH2), 3.17 (s, 6H, 9′-N(CH3)2), 3.05–2.86 (m, 4H, 2-CH2,10-CH2 ), 2.62 (d, J = 10.3 Hz, 2H, 3′-CH2), 2.32 (s, 3H, Ph-CH3), 2.17–2.07 (m, 1H, 7-CH), 1.98 (m, 3H, 1′a-CH, 2′-CH2), 1.87–1.55 (m, 8H, 3-CH2, 4-CH2, 8-CH2, 9-CH2), 1.43 (d, J = 13.8 Hz, 1H, 1′b-CH).; 13C-NMR (126 MHz, DMSO-d6) δ 170.5 (7′-C), 158.0 (4′-C), 138.8 (18-C), 131.4 (15-C), 129.4 (17-CH, 19-CH), 126.9 (16-CH, 20-CH), 60.3 (6-CH), 60.2 (14-CH2), 57.2 (11-CH), 55.0 (2-CH2), 54.2 (10-CH2), 48.5 (13-CH2), 42.3 (N(CH3)2), 35.9 (7-CH), 29.9 (5-CH), 29.2 (1′-CH2), 27.6 (3′-CH2), 25.8 (4-CH2), 24.1 (8-CH2), 23.5 (2′-CH2), 20.9 (3-CH2), 17.9 (9-CH2), 17.8 (Ph-CH3); ESI-HRMS: m/z Calcd for C26H40N5S [M − HCl + H]+, 454.2999 found, 454.2996; HPLC: tR = 9.84 min, normalization method purity 96.00%.
12-N-p-Bromobenzyl-3′-(5-amino-1,3,4-thiadiazol-2-yl)-matrinic propane (6o) Compound 5d (1.09 g, 2.5 mmol) was treated with thiosemicarbazide (0.23 g, 2.5 mmol) according to the general procedure, then purified by flash column chromatography with dichloromethane/methanol to give the desired product 6o as a white solid, yield: 44.1%; m.p.: 193–194 °C; = −19.58; 1H-NMR (500 MHz, CDCl3) δ 7.39 (d, J = 8.0 Hz, 2H, 16-CH, 20-CH), 7.19 (d, J = 8.0 Hz, 2H, 17-CH, 19-CH), 5.61 (s, 2H, 9′-NH2), 3.92 (d, J = 13.8 Hz, 1H, 14a-CH), 3.06 (d, J = 13.8 Hz, 1H,14b-CH), 2.91–2.75 (m, 5H, 3′-CH2, 5-CH, 7-CH, 11-CH), 2.64 (t, J = 11.8 Hz, 1H, 6-CH), 2.27 (m, 1H, 1′a-CH), 1.94–1.55 (m, 12H, 2-CH2, 4-CH2, 8-CH2, 10-CH2, 13-CH2, 2′-CH2), 1.45–1.30 (m, 5H, 3-CH2, 9-CH2, 1′b-CH); 13C-NMR (126 MHz, CDCl3) δ 168.2 (7′-C), 161.5 (4′-C), 139.6 (15-C), 131.4 (17-CH, 19-CH), 130.3 (16-CH, 20-CH), 120.3 (18-C), 64.5 (6-CH), 57.6 (11-CH), 57.4 (14-CH2), 57.2 (13-CH2), 55.9 (2-CH2), 52.3(10-CH2), 37.9 (7-CH), 33.9 (5-CH), 30.7(1′-CH2), 28.2 (3′-CH2), 28.1 (4-CH2), 27.4 (8-CH2), 23.8 (2′-CH2), 21.6 (3-CH2), 21.4 (9-CH2); ESI-HRMS: m/z Calcd for C23H33N5BrS [M + H]+, 490.1635; found, 490.1635; HPLC: tR = 9.88 min; normalization method purity 97.24%.
3.2.3. General Procedure for the Synthesis of Compounds 7a–d
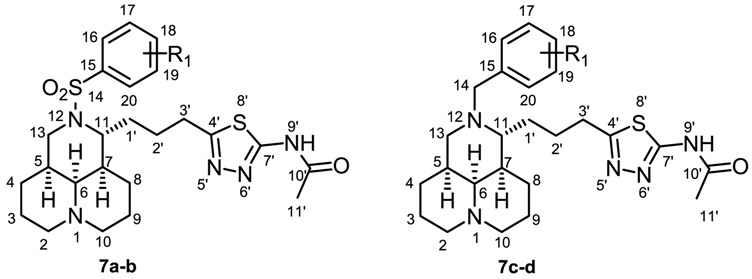
To a solution of 6a, 6f, 6i or 6l (0.40 mmol) in dichloromethane (10 mL), triethylamine (0.25 g, 2.5 mmol) and acetyl chloride (0.16 g, 2 mmol) were added at 0 °C and stirred at room temperature, until the TLC analysis showed completion of the reaction. The reaction mixture was washed by saturated aqueous ammonium chloride (10 mL × 2) and saturated aqueous sodium chloride (10 mL), dried over anhydrous sodium sulfate and concentrated under reduced pressure. The residue was purified by flash column chromatography on silica gel with dichloromethane/methanol as the eluents to give 7a–d.
12-N-p-Methylblenzenesulfonyl-3′-(5-acetylamino-1,3,4-thiadiazol-2-yl)-matrinic propane (7a). White solid, yield: 93.7%; m.p.: 273 °C (dec,); = −4.83; 1H-NMR (500 MHz, DMSO-d6) δ 12.39 (s, 1H, 9′-NH), 7.68 (d, J = 8.0 Hz, 2H, 16-CH, 20-CH), 7.37 (d, J = 8.0 Hz, 2H, 17-CH, 19-CH), 3.46–3.38 (m, 2H, 3′-CH2), 3.14 (t, J = 11.7 Hz, 1H, 6-CH), 2.92–2.84 (m, 1H, 5-CH), 2.83–2.74 (m, 1H, 7-CH), 2.62–2.51 (m, 1H, 11-CH), 2.37 (s, 3H, Ph-CH3), 2.17 (s, 3H, 11′CH3), 1.96–1.92 (m, 1H, 1a’-CH), 1.89–1.81 (m, 2H, 2′-CH2), 1.77–1.66 (m, 8H, 2-CH2, 4-CH2, 10-CH2, 13-CH2), 1.58–1.22 (m, 7H, 3-CH2, 8-CH2, 9-CH2, 1′b-CH); 13C-NMR (126 MHz, DMSO-d6) δ 168.4 (7′-C), 163.7 (10′-CO), 158.1 (4′-C), 142.9 (18-C), 136.8 (15-C), 129.5 (17-CH, 19-CH), 127.2 (16-CH, 20-CH), 62.4 (6-CH), 56.7 (11-CH), 56.2 (2-CH2), 56.1 (10-CH2), 47.2 (13-CH2), 38.7 (7-CH), 34.2 (5-CH), 29.6 (1′-CH2), 29.0 (3′-CH2), 27.5 (4-CH2), 27.4 (8-CH2), 25.1(2′-CH2), 22.4 (11′-CH3), 21.0 (Ph-CH3), 20.4 (3-CH2), 20.3 (9-CH2); ESI-HRMS: m/z Calcd for C25H36O3N5S2 [M + H]+, 518.2254; found, 518.2252; HPLC: tR = 11.66 min, normalization method purity 95.35%.
12-N-p-Trifluoromethlybenzenesulfonyl-3′-(5-acetylamino-1,3,4-thiadiazol-2-yl)-matrinic propane (7b). White solid, yield: 99.1%; m.p.: 197 °C (dec,); = −1.30; 1H-NMR (600 MHz, CDCl3) δ 13.27 (s, 1H, 9′-NH), 7.98 (d, J = 8.2 Hz, 2H, 16-CH, 20-CH), 7.73 (d, J = 8.2 Hz, 2H, 17-CH, 19-CH), 3.66–3.57 (m, 1H, 3′a-CH), 3.53–3.42 (m, 1H, 3′b-CH), 3.25 (t, J = 11.6 Hz, 1H, 6-CH), 3.03–2.91 (m, 2H, 5-CH, 7-CH), 2.54 (d, J = 11.4 Hz, 1H, 13a-CH), 2.49–2.42 (m, 4H, 13b-CH, 11′-CH3), 1.99–1.96 (m, 2H, 2′-CH2), 1.88–1.74 (m, 8H, 2-CH2, 4-CH2, 10-CH2, 11-CH, 1′a-CH), 1.52–1.29 (m, 7H, 3-CH2, 8-CH2, 9-CH2, 1′b-CH); 13C-NMR (151 MHz, CDCl3) δ 168.9 (7′-C), 164.6 (10′-CO), 160.5 (4′-C), 144.1 (15-C), 134.0 (18-C), 128.1 (17-CH, 19-CH), 125.8 (16-CH, 20-CH), 123.4 (Ph-CF3), 62.7 (6-CH), 57.5 (11-CH), 56.7 (2-CH2), 56.6 (10-CH2), 46.9 (13-CH2), 39.8 (7-CH), 34.4 (5-CH), 31.8 (1′-CH2), 29.8 (3′-CH2), 28.4 (4-CH2), 28.0 (8-CH2), 25.8 (2′-CH2), 23.3 (11′-CH3), 20.9 (3-CH2), 20.7 (9-CH2); ESI-HRMS: m/z Calcd for C25H33O3N5F3S2 [M + H]+, 572.1971; found, 572.1970; HPLC: tR =11.91 min, normalization method purity 99.53%.
12-N-Benzyl-3′-(5-acetylamino-1,3,4-thiadiazol-2-yl)-matrinic propane (7c). White solid, yield: 93.5%; m.p.: 188–189 °C; = −27.65; 1H-NMR (500 MHz, CDCl3) δ 13.31 (s, 1H, 9′-NH), δ 7.32–7.27 (m, 4H, 16-CH, 17-CH, 19-CH, 20-CH), 7.20 (t, J = 7.2 Hz, 1H, 18-CH), 3.98 (d, J = 13.4 Hz, 1H, 14a-CH), 3.10 (d, J = 13.4 Hz, 1H, 14b-CH), 2.97–2.73 (m, 5H, 3′-CH2, 5-CH, 7-CH, 11-CH), 2.63 (t, J = 12.0 Hz, 1H, 6-CH), 2.42 (s, 3H, 11′-CH3), 2.37–2.31 (m, 4H, 2-CH2,10-CH2), 2.06–1.78 (m, 8H, 4-CH2, 8-CH2, 13-CH2, 2′-CH2), 1.47–1.22 (m, 6H, 3-CH2, 9-CH2, 1′-CH2); 13C-NMR (126 MHz, CDCl3) δ 169.0 (7′-C), 165.0 (10′-CO), 160.5 (4′-C), 140.4 (15-C), 128.6 (17-CH, 19-CH), 128.4 (16-CH, 20-CH), 126.8 (18-CH), 64.6 (6-CH), 57.6 (11-CH), 57.4 (14-CH2), 56.9 (13-CH2), 56.7 (2-CH2), 52.4 (10-CH2), 37.9 (7-CH), 34.0 (5-CH), 30.2 (1′-CH2), 28.4 (3′-CH2), 28.2 (4-CH2), 27.4 (8-CH2), 23.9 (11′-CH3), 23.3 (2′-CH2), 21.6 (3-CH2), 21.4 (9-CH2); ESI-HRMS: m/z Calcd for C25H36ON5S [M + H]+, 454.2662; found, 454.2662; HPLC: tR = 8.96 min, normalization method purity 98.36%.
12-N-p-Methylbenzyl-3′-(5-acetylamino-1,3,4-thiadiazol-2-yl)-matrinic propane (7d). White solid, yield: 91.5%; m.p.: 226 °C (dec.); = −31.74; 1H-NMR (500 MHz, CDCl3) δ 13.34 (s, 1H, 9′-NH), 7.19 (d, J = 7.6 Hz, 2H, 16-CH, 20-CH), 7.09 (d, J = 7.6 Hz, 2H, 17-CH, 19-CH), 3.98 (d, J = 13.3 Hz, 1H, 14a-CH), 3.10 (d, J = 13.3 Hz, 1H, 14b-CH), 2.95–2.86 (m, 2H, 3′-CH2), 2.83 (m, 1H, 5-CH), 2.76 (m, 1H, 7-CH), 2.63 (t, J = 12.0 Hz, 1H, 6-CH), 2.42 (s, 3H, 11′-CH3), 2.36 (d, J = 11.0 Hz, 1H, 11-CH), 2.31 (s, 3H, Ph-CH3), 2.03 (s, 1H, 1′a-CH), 1.92–1.78 (m, 6H, 2-CH2, 4-CH2, 8-CH2,), 1.75–1.62 (m, 4H, 10-CH2, 2′-CH2 ), 1.48–1.30 (m, 6H,3-CH2, 9-CH2, 13-CH2), 1.25 (s, 1H, 1′b-CH); 13C-NMR (126 MHz, CDCl3) δ 169.0 (7′-C), 165.1 (10′-CO), 160.5 (4′-C), 137.4 (18-C), 136.2 (15-C), 129.0 (17-CH, 19-CH), 128.6 (16-CH, 20-CH), 64.6 (6-CH), 57.7 (14-CH2), 57.5 (11-CH), 56.7 (2-CH2), 52.4 (10-CH2), 38.0 (13-CH2), 34.1 (7-CH), 30.2 (5-CH), 29.8 11′-CH3), 28.5 (1′-CH2), 28.2 (3′-CH2), 27.4 (4-CH2), 24.0 (8-CH2), 23.3 (2′-CH2), 21.7 (3-CH2), 21.5 (9-CH2), 21.2 (Ph-CH3); ESI-HRMS: m/z Calcd for C26H38ON5S [M + H]+, 468.2792; found, 468.2789; HPLC: tR =9.34 min, normalization method purity 98.77%.
3.3. Biology Assay
3.3.1. Cell Culture and Screening of Compound
Cells were laid on 96-well plate, cultured in Dulbecco’s Modified Eagle’s medium (DMEM), containing 10% fetal bovine serum (FBS) in a 5% CO2 atmosphere at 37 °C. Moreover, serum-free culture was required until the cells at 90–95% confluence. After 24 h, cells were treated with a matrinic derivative (80 μM) for 24 h. The COL1A1 promotor activity was determined using the Bright-Glo luciferase assay system (Promega Corporation, Madison, WI, USA) [8].
3.3.2. Cell Survival Assay
The cell survival was evaluated by MTT assay. HepG2 cells were seeded at density of 6 × 103 cells/well in 96-well plate. Cells were treated with various concentrations of matrinic derivate until the cells at 50% confluence. After 24 h of incubation, 20 µL of the MTT (5 mg/mL) solution was added into each plate and incubated for 4 h at 37 °C, 5% CO2. Subsequently, the culture supernatant was replaced with 150 µL DMSO to dissolve the formazan crystal. The absorbance at 570 nm was measured using a microplate reader (ELx800, BioTek Instruments, Winooski, VT, USA).
3.3.3. RT-qPCR Assay
LX-2 cells were seeded in 6-well plate, cultured in DMEM, containing 10% fetal bovine serum (FBS) in a 5% CO2 atmosphere at 37 °C. And serum-free culture was required until the cells at 90–95% confluence. After 24 h, cells were treated with TGFβ1 (2 ng/mL) and matrinic derivatives (80 μM) for 24 h. Total RNA from the LX-2 cells was extracted using Trizol reagent, purified by NucleoSpin RNA Clean-up. Reverse transcription was performed with a Transcriptor first strand cDNA synthesis kit. The cDNA was then analysis by ABI 7500 Fast Real-Time PCR System (ThermoFisher Scientific, Singapore) using TaqMan probes of TGFβ1, COL1A1, α-SMA, and GAPDH (sequence reserved by ABI, Foster City, CA ,USA) and FastStart Universal Probe master mix (Roche, Indianapolis, IN, USA).
3.3.4. Western Blot
LX-2 cells were cultured as described above. Briefly, cells were washed with phosphate-buffered saline (PBS) and were lysed in radioimmunoprecipitation assay (RIPA) lysis for 30 min in 4 °C; the supernatant was collected after centrifugation at 12,000× g, 4 °C for 15 min. Equal amounts of protein were quantified with Bradford assay, separated by SDS-PAGE and transferred to polyvinylidene difluoride membrane. The membranes were blocked for one hour at room temperature in PBST containing 5% milk and probed with specific first antibodies overnight at room temperature. Membrane was washed 3 times by PBST, followed by horseradish peroxidase-conjugated secondary antibodies and GAPDH. The proteins were visualized using chemiluminescence reagents.
3.4. Acute Toxicity
Female Kunming mice with weight of 20.0 (±1.0 g) were obtained from the Institute of Laboratory Animal Science (Beijing, China). Animals were cared according to the institutional guidelines of the Institute of Materia Medica, CAMS&PUMC (00000364). The mice were fed with regular rodent chow and housed in an air conditioned room. The mice were randomly divided into different groups with 6 mice each. Each compound was given orally in a single-dosing experiment at 0, 250, 500, 750 or 1000 mg·kg−1 (ddH2O as control), respectively. The mice were closely monitored for 7 days. Body weight as well as survival was monitored.
4. Conclusions
To summarize, a series of novel matrinic thiadiazole derivatives were designed, synthesized and evaluated for their inhibitory effect on the COL1A1 promotor. The SAR indicated that: (i) the introduction of a thiadiazole on the 11-side chain was beneficial for the activity; (ii) a 12-N-benzyl was favorable for activity. Among the new derivatives, compound 6n gave a high inhibitory effect on COL1A1 promotor at a rate of 39.7% on the cellular level at a concentration of 40 μM. It also effectively inhibited the expression of two representative fibrogenetic collagen proteins (COL1A1 and α-SMA) on both the mRNA and protein levels as well as a high safety profile with the LD50 value of over 500 mg·kg−1 in vivo. Further study indicated that it might exert anti-liver fibrotic activity via repressing the TGFβ/Smad pathway. Overall, this results offer powerful information for further structure optimization, in which compound 6n has been chosen as an ideal anti-liver fibrosis lead for further investigation by means of computer aided drug and other design strategies.
Acknowledgments
The authors thank center for analysis and testing of Institute of Materia Medica and Institute of Medicinal Biotechnology, Chinese Academy of Medical Sciences for their contributions to the determination of HR-MS, 1H-NMR and 13C-NMR, thank Animal Center of the Institute of Materia Medica, CAMS&PUMC for providing the animal experimental facilities, and thank Ms. Yanbin Wu from Institute of Medicinal Biotechnology for performing the animal experiment
Author Contributions
T.N. performed part of synthetic experiments and wrote the paper, W.N. and Y.B. performed the biological assay, T.L. was responsible for literature search, D.S. conceived and designed the chemistry experiments, Y.L. designed the target compounds and chemistry experiments, H.H. conceived and designed the biology experiments.
Funding
This work was supported by the National Natural Science Foundation of China (81321004 and 81473248), and CAMS initiative for innovative medicine (2017-12M-3-012).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds 1, 5a–d, 6a–o and 7a–d are available from the authors.
References
- 1.Sakata K., Eda S., Lee E.S., Hara M., Imoto M., Kojima S. Neovessel formation promotes liver fibrosis via providing latent transforming growth factor-β. Biochem. Biophys. Res. Commun. 2014;443:950–956. doi: 10.1016/j.bbrc.2013.12.074. [DOI] [PubMed] [Google Scholar]
- 2.Gressner A.M., Weiskirchen R. Modern pathogenetic concepts of liver fibrosis suggest stellate cells and TGF-β as major players and therapeutic targets. J. Cell. Mol. Med. 2006;10:76–99. doi: 10.1111/j.1582-4934.2006.tb00292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schuppan D., Ruehl M., Somasundaram R., Hahn E.G. Matrix as a modulator of hepatic fibrogenesis. Semin. Liver Dis. 2001;21:351–372. doi: 10.1055/s-2001-17556. [DOI] [PubMed] [Google Scholar]
- 4.Guo J., Friedman S.L. Hepatic fibrogenesis. Semin. Liver Dis. 2007;27:413–426. doi: 10.1055/s-2007-991517. [DOI] [PubMed] [Google Scholar]
- 5.Elpek G.O. Cellular and molecular mechanisms in the pathogenesis of liver fibrosis: An update. World J. Gastroenterol. 2014;20:7260–7276. doi: 10.3748/wjg.v20.i23.7260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikejima K., Okumura K., Kon K., Takei Y., Sato N. Role of adipocytokines in hepatic fibrogenesis. J. Gastroenterol. Hepatol. 2007;22(Suppl. 1):S87–S92. doi: 10.1111/j.1440-1746.2007.04961.x. [DOI] [PubMed] [Google Scholar]
- 7.Derynck R., Zhang Y.E. Smad-dependent and smad-independent pathways in TGF-β family signalling. Nature. 2003;425:577–584. doi: 10.1038/nature02006. [DOI] [PubMed] [Google Scholar]
- 8.Zhao S.S., Wang J.X., Wang Y.C., Shao R.G., He H.W. Establishment and application of a high-throughput drug screening model based on COL1A1 promoter for anti-liver fibrosis. Yao Xue Xue Bao. 2015;50:169–173. [PubMed] [Google Scholar]
- 9.Du N.N., Peng Z.G., Bi C.W., Tang S., Li Y.H., Li J.R., Zhu Y.P., Zhang J.P., Wang Y.X., Jiang J.D., et al. N-Substituted benzyl matrinic acid derivatives inhibit hepatitis c virus (HCV) replication through down-regulating host heat-stress cognate 70 (Hsc70) expression. PLoS ONE. 2013;8:e58675. doi: 10.1371/journal.pone.0058675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tang S., Peng Z.G., Li Y.H., Zhang X., Fan T.Y., Jiang J.D., Wang Y.X., Song D.Q. Synthesis and biological evaluation of tricyclic matrinic derivatives as a class of novel anti-HCV agents. Chem. Cent. J. 2017;11:94. doi: 10.1186/s13065-017-0327-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tang S., Peng Z.G., Zhang X., Cheng X.Y., Li W.J., Jiang J.D., Li Y.H., Song D.Q. Synthesis and biological evaluation of 12-benzyl matrinic amide derivatives as a novel family of anti-HCV agents. Chin. Chem. Lett. 2016;27:1052–1057. doi: 10.1016/j.cclet.2016.03.006. [DOI] [Google Scholar]
- 12.Liu A., Pang X., Gu Y., Wang K., Xun C. Clinical observation of matrine for antifibrosis. Chin. J. Integr. Tradit. West. Med. Liver Dis. 2001;2:003. [Google Scholar]
- 13.Wang S.G., Kong L.Y., Li Y.H., Cheng X.Y., Su F., Tang S., Bi C.W., Jiang J.D., Li Y.H., Song D.Q. Structure–activity relationship of N-benzenesulfonyl matrinic acid derivatives as a novel class of coxsackievirus b3 inhibitors. Bioorg. Med. Chem. Lett. 2015;25:3690–3693. doi: 10.1016/j.bmcl.2015.06.043. [DOI] [PubMed] [Google Scholar]
- 14.Tipoe G.L., Leung T.M., Liong E.C., Lau T.Y.H., Man L.F., Nanji A.A. Epigallocatechin-3-gallate (EGCG) reduces liver inflammation, oxidative stress and fibrosis in carbon tetrachloride (CCL4)-induced liver injury in mice. Toxicology. 2010;273:45–52. doi: 10.1016/j.tox.2010.04.014. [DOI] [PubMed] [Google Scholar]
- 15.Du N.N., Li X., Wang Y.P., Liu F., Liu Y.X., Li C.X., Peng Z.G., Gao L.M., Jiang J.D., Song D.Q. Synthesis, structure-activity relationship and biological evaluation of novel N-substituted matrinic acid derivatives as host heat-stress cognate 70 (Hsc70) down-regulators. Bioorg. Med. Chem. Lett. 2011;21:4732–4735. doi: 10.1016/j.bmcl.2011.06.071. [DOI] [PubMed] [Google Scholar]
- 16.Tosco P., Lolli M.L. Hydroxy-1,2,5-oxadiazolyl moiety as bioisoster of the carboxy function. A computational study on γ-aminobutyric acid (gaba) related compounds. J. Mol. Model. 2008;14:279–291. doi: 10.1007/s00894-008-0269-0. [DOI] [PubMed] [Google Scholar]
- 17.Kempegowda, Kumar G.P.S., Prakash D., Mani T.T. Thiadiazoles: Progress report on biological activities. Der Pharma Chemica. 2011;3:330–341. [Google Scholar]
- 18.Castro A., Castaño T., Encinas A., Porcal W., Gil C. Advances in the synthesis and recent therapeutic applications of 1,2,4-thiadiazole heterocycles. Bioorg. Med. Chem. 2006;14:1644–1652. doi: 10.1016/j.bmc.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 19.Gupta J.K., Dudhey R., Sharma P. Synthesis and pharmacological activity of substituted 1,3,4-thiadiazole derivatives. Medichemonline. 2010;1:1–9. [Google Scholar]
- 20.Tsuji T., Takenaka K. Convenient synthesis of 2,7-disubstituted 5H-1,3,4-thiadiazolo(3,2-α)-pyrimidin-5-ones and related compounds. Cheminform. 1982;13:637–638. doi: 10.1246/bcsj.55.637. [DOI] [Google Scholar]
- 21.Eaton P.E., Carlson G.R., Lee J.T. Phosphorus pentoxide-methanesulfonic acid. Convenient alternative to polyphosphoric acid. J. Org. Chem. 1973;38:4071–4073. doi: 10.1021/jo00987a028. [DOI] [Google Scholar]
- 22.Carson J.P., Ramm G.A., Robinson M.W., McManus D.P., Gobert G.N. Schistosome-induced fibrotic disease: The role of hepatic stellate cells. Trends Parasitol. 2018;34:524–540. doi: 10.1016/j.pt.2018.02.005. [DOI] [PubMed] [Google Scholar]
- 23.Tang S., Kong L., Li Y., Jiang J., Gao L., Cheng X., Ma L., Zhang X., Li Y., Song D. Novel N-benzenesulfonyl sophocarpinol derivatives as coxsackie B virus inhibitors. ACS Med. Chem. Lett. 2015;6:183–186. doi: 10.1021/ml500525s. [DOI] [PMC free article] [PubMed] [Google Scholar]