Abstract
Proteinase 3 (P3), a serine protease expressed by myeloid cells, localized within azurophil granules, and also expressed on the cellular membrane of polymorphonuclear neutrophils (PMN), is the target of autoimmunity in granulomatosis with polyangiitis. PR1, an HLA-A2 restricted nonameric peptide derived from P3, has been targeted effectively in myeloid leukemia. We previously showed (Molldrem et al. 2003. J. Clin. Invest. 111: 639–647) that overexpression of P3 in chronic myeloid leukemia induces apoptosis of high-affinity PR1-specific T cells, leading to deletional tolerance and leukemia outgrowth. In this study, we investigated the effect of membrane P3 (mP3)–expressing PMN and acute myeloid leukemia (AML) blasts on the proliferation of CD4 and CD8 T cells in vitro. We demonstrate that mP3-expressing PMN significantly inhibits autologous healthy donor T cell proliferation but does not affect cytokine production in activated T cells and that this effect requires cell proximity and was abrogated by P3 blockade. This inhibition required P3 enzyme activity. However, suppression was not reversed by either the addition of catalase or the inhibition of arginase I. In addition to P3 blockade, anti–low density lipoprotein receptor-related protein 1 (LRP1) Ab also restored T cells’ capacity to proliferate. Last, we show dose-dependent inhibition of T cell proliferation by mP3-expressing AML blasts. Together, our findings demonstrate a novel mechanism whereby PMN- and AML-associated mP3 inhibits T cell proliferation via direct LRP1 and mP3 interaction, and we identify P3 as a novel target to modulate immunity in myeloid leukemia and autoimmune disease.
Introduction
Polymorphonuclear neutrophils (PMN) are the most abundant leukocytes, accounting for 50–70% of leukocytes in circulating blood. PMN express numerous serine proteases, including proteinase 3 (P3), neutrophil elastase, and cathepsin G (1). These enzymes mediate intracellular killing of phagocytosed pathogens at sites of inflammation (1, 2). There is also evidence that PMN-derived serine proteases and their products impact the tumor microenvironment and play an important role in regulating tumor cell proliferation, angiogenesis, and metastasis (3, 4). A number of studies have found increased numbers of PMN in the peripheral blood and tumors of patients with various cancers (5, 6). Intratumoral PMN are associated with poor prognosis in multiple tumor types, including renal cell carcinoma and lung cancer (3).
P3 has been shown to regulate cell proliferation. P3 can stimulate the proliferation of basophils and mast cells (7) and can activate apoptosis in HUVECs (8). P3 also plays a role in the autoimmune disease granulomatosis with polyangiitis (GPA), in which it serves as an autoantigen (9). Moreover, P3 is overexpressed in acute myeloid leukemia (AML) and chronic myeloid leukemia (CML) (10) and has been shown to play a role in leukemogenesis (11).
Although P3 has a defined role in innate immunity, GPA, and leukemogenesis, little is known about the effect of P3 on T cells or how this protease might modulate host immune responses to cancer. In the current study, we investigate the effect of membrane P3 (mP3)–expressing PMN and AML on T lymphocytes in vitro and elucidate the mechanism by which mP3 exerts inhibitory effects on T cells. We show that mP3-expressing PMN inhibit proliferation of T cells via an enzyme-dependent mechanism. Inhibition is dose-dependent and correlates with mP3 expression. AML blasts expressing high levels of mP3 inhibit T cell proliferation, and such inhibition is abrogated by P3 blockade. Together, these data support a new role for membrane-bound P3 expressed on PMN and AML in regulating adaptive T cell immunity.
Materials and Methods
Cells and patients
Blood of healthy adult donors was provided as leukocyte concentrates by Gulf Coast Regional Blood Bank and the University of Texas MD Anderson Cancer Center (MDACC) Blood Bank. Patient AML samples were collected after informed written consent was obtained in accordance with the Declaration of Helsinki under protocols approved by the MDACC Institutional Review Board. Before use, the AML cells were thawed, washed, and recovered by overnight incubation at 37°C in RPMI 1640 medium (Life Technologies, Grand Island, NY) supplemented with 10% FCS.
Reagents
Anti-CD3 mAb, anti-CD28 mAb, anti-CD4-allophycocyanin, anti-CD8-allophycocyanin-H7, anti-CD16-PE/Cy7, and anti-CD45-PB mAbs were purchased from BD Pharmingen (San Jose, CA). Anti-CD4-PB and anti-CD8 allophycocyanin-Cy7 mAbs were from Tonbo Biosciences (San Diego, CA). Anti-CD177 allophycocyanin was purchased from AbD Serotec (Munich, Germany). Anti-P3 Abs included MCPR3-2 (Thermo Fisher Scientific, Fremont, CA), WGM2 (Abcam, Cambridge, MA), and human polyclonal anti-neutrophil cytoplasmic Abs (cANCA) (LSBio, Seattle, WA). Mouse isotype control was purchased from R&D Systems (Minneapolis, MN). Catalase was purchased from Athens Research and Technology (Athens, GA). N-hydroxy-nor-l-arginine (nor-NOHA) was purchased from Cayman Chemical (Ann Arbor, MI). CFSE was obtained from Molecular Probes (Eugene, OR). Anti- LRP1 mAb was purchased from Thermo Fisher Scientific (Waltham, MA).
Isolation and sort-purification of PBMC and PMN
PBMC and PMN were isolated from EDTA–anti-coagulated buffy coats of healthy donors by centrifugation on double-layer Histopaque-1077/1119 (Sigma-Aldrich). The erythrocytes in the granulocyte layer were lysed with ammonium chloride buffer. All subsequent steps were performed at 4°C. The purity of the isolated PMN typically was >90% as determined by CD16 staining. Cell sorting of mP3+ and mP3− PMN was done on a BD Influx high-speed sorter with use of BD software (BD Biosciences, Franklin Lakes, NJ). Briefly, PMN were stained with anti-CD16-PE/Cy7 (Clone 3G8) for 15 min prior to addition of mouse anti-CD177-allophycocyanin (Clone MEM-166) for 30 min, followed by subsequent washing steps. Cells were sorted for CD177+CD16+ and CD177−CD16+ populations to obtain mP3-expressing and mP3-lacking PMN, respectively (12); the purity of the sorted PMN typically was >90%. PBMC were stained with anti-CD4-PE (Clone RPA-T4; BioLegend, San Diego, CA) and anti-CD8-allophycocyanin (Clone RPA-T8; BioLegend) mAbs, then CD4 and CD8 T cells were sort purified using a BD Influx high-speed sorter; the purity of CD4 and CD8 cells was >95%.
Flow cytometric analysis of mP3
All steps were performed at 4°C. A total of 106 PMN were fixed with 0.5% paraformaldehyde (PFA) for 10 min, washed with PBS containing 1% BSA, and stained with anti-CD16-PE/Cy7 for 15 min prior to addition of mouse anti-P3 mAb (Clone MCPR3-2) conjugated with Alexa Fluor 488 or isotype control and anti-CD177-allophycocyanin for 30 min, followed by subsequent washing steps. To exclude dead cells from the analysis, a LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Invitrogen, Carlsbad, CA) was used. PMN were gated on live CD16+ cells. For mP3 expression on AML cells, anti-CD45 (Clone HI30) mAb was used instead of anti-CD16. Blast cells were identified by low to intermediate expression of CD45 (13). Cells were acquired on a BD LSRFortessa flow cytometer (BD Biosciences). Data were analyzed using FlowJo analysis software (FlowJo, Ashland, OR).
T cell proliferation
Proliferation of T lymphocytes was measured using CFSE (catalog no. V12883; Thermo Fisher Scientific) dye dilution assay. Human PBMC (107 cells/ml) were labeled with CFSE at a final concentration of 2 μM for 5 min at 25°C. CFSE labeling was quenched by incubating cells in RPMI 1640 medium supplemented with 10% FCS for 5 min at 37°C. Cells were then washed three times in FCS-containing RPMI 1640 medium. To prevent leakage of intracellular proteinases (14), PMN used in the CFSE assay were fixed for 10 min at 4°C with 0.5% PFA unless indicated otherwise. A total of 4 × 105 CFSE-labeled PBMC or sort-purified CD8 or CD4 T cells were cocultured with PMN, sorted mP3-expressing, or mP3-lacking PMN at indicated PMN/PBMC ratios, for up to 5 d in the presence of anti-CD3/CD28 (Clone HIT3a and Clone 28.2, respectively) mAbs (5 μg/ml) in a 96-well U-bottom plate. In all proliferation assays, cells were gated on live CD4 and CD8 T cells, and CFSElow cells as a percentage of CD4 or CD8 T cells were reported as a percentage of proliferating cells. CFSElow gate placement was set just below the CFSE-labeling level in unstimulated, undivided T cells. T cell apoptosis induced by PMN was determined using annexin V (eBioscience, San Diego, CA) staining by flow cytometry on days 4 and 5 of PBMC/PMN cocultures at various ratios.
In some experiments, PMN were fixed before being incubated with anti-P3 mAb (Clone WGM2) (10 μg/107 PMN) for 30 min on ice and then added to the cell culture. In other experiments, the proliferation of T cells was assessed by flow cytometry using a BrdU Flow kit (BD Pharmingen). Seventy-two hours after stimulation, BrdU was added to the coculture for an additional 16 h. CFSE-labeled PBMC activated by anti-CD3/CD28 mAbs were cocultured with prefixed allogeneic AML cells at the indicated ratios with or without anti-P3 Ab human polyclonal cANCA (LS-C38480; LSBio) for 5 d. Catalase (1000 U/ml) or nor-NOHA (100 μM) was added to the cell cultures along with unfixed PMN. Transwell experiments were performed in 96-well plates with 0.4-μm pore size membranes (Corning, Corning, NY). PMSF (329-98-6) and α1-antitrypsin (AAT) (A-6150) were purchased from Sigma-Aldrich. Elafin (ALX-201-240-C100) was purchased from Enzo Biochem (Farmingdale, NY).
Stimulated T cells were collected and stained with fluorochrome-conjugated Abs against CD4 and CD8 in PBS containing 1% FBS for 30 min, then fixed in PBS with 1% PFA. A LIVE/DEAD Fixable Aqua Dead Cell Stain Kit (Thermo Fisher Scientific) was used to exclude dead cells from the analysis. Cells were acquired on a BD LSRFortessa or FACSCanto II flow cytometer. PBMC stimulated with anti-CD3/CD28 mAbs were collected at various time points and stained for LRP1 (Clone 8G1; Calbiochem) surface expression.
Intracellular flow cytometry assessment of cytokine production
To assess cytokine production by T cells, stimulated PBMC were incubated with PMN at the indicated ratio. Cells were cocultured for a total of 16 h with the addition of brefeldin A at a final concentration of 10 μg/ml after the initial hour. Cells were washed and surface stained with fluorochrome-conjugated Abs against CD4 (Clone SK4), CD8 (Clone RPA-T8) (BD Biosciences), and LIVE/DEAD Fixable Violet Dead Cell Stain (Invitrogen). Subsequently, the cells were washed, treated with Cytofix/Cytoperm and Perm/Wash (BD Biosciences) according to manufacturer instructions, and then stained intracellularly with IFN-γ–FITC (Clone B27; BD Biosciences), IL-2–PE (Clone MQ1-17H12), and TNF-α–PerCP-Cy5.5 (Clone Mab11) (both eBioscience). Data were acquired on the LSRFortessa flow cytometer (BD Biosciences) and analyzed with FlowJo software.
Statistical analysis
An unpaired t test was used to compare two discrete groups. The p values <0.05 were considered statistically significant. Data analysis was performed using GraphPad Prism 5.0 (GraphPad Software, La Jolla, CA). Results were expressed as mean ± SEM.
Results
PMN inhibit T cell proliferation in a dose-dependent manner
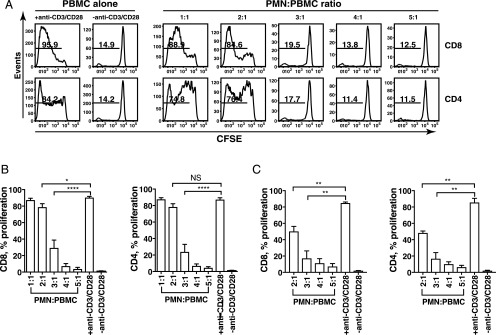
Tumor-associated neutrophils (TAN) can mediate broad immunosuppressive effects (3, 15), and the presence of TAN has been associated with poor clinical outcome (3). However, the precise mechanism of TAN-mediated immunosuppression is unknown (16–18). To investigate whether PMN from healthy donors affect T cell proliferation, we cocultured PMN with autologous anti-CD3/CD28–stimulated PBMC (containing >90% lymphocytes). At a ratio of 3:1 (PMN/PBMC), a significant decrease in proliferating cells was observed (19.5% CD8 and 17.7% CD4, respectively) compared with that seen at the 2:1 ratio (84.6% CD8 and 76.4% CD4, respectively) (Fig. 1A). This inhibition of proliferation was even more pronounced in the 4:1 and 5:1 ratios, establishing a dose-dependent relationship between PMN and PBMC in coculture. Similar results were obtained from assays performed with nine different donors following coculture of PBMC with PMN (Fig. 1B). Compared with activated PBMC alone (proliferating CD8 cells, 90.2 ± 1.5% and CD4 cells, 87.1 ± 2.4%, mean ± SEM, respectively), coincubation of PMN with PBMC at a 3:1 ratio resulted in significant inhibition of proliferation in both CD8 (29.2 ± 9.6% proliferating, mean ± SEM) and CD4 (23.7 ± 9.2% proliferating, mean ± SEM) T cells. Accordingly, coincubation of PBMC with PMN decreased the percentage of T cells in S phase while increasing the fraction of T cells in G2/M phase in a BrdU incorporation assay (Supplemental Fig. 1). We used fixed PMN in most assays to prevent leakage of intracellular proteinases; experiments were also carried out using unfixed PMN to reflect the physiological and pathological conditions (Fig. 1C). Unfixed PMN mediated dose-dependent inhibition of T cell proliferation similar to the results obtained with fixed PMN (Fig. 1B).
FIGURE 1.
PMN inhibit T cell proliferation in a dose-dependent manner. CFSE-labeled PBMC stimulated with or without anti-CD3/CD28 mAbs were cocultured with increasing numbers of fixed PMN (A and B) or unfixed PMN (C) for 5 d. Flow cytometry was used to determine CFSE dilution in live CD8 and CD4 T cells. (A) The result is representative of nine different donors. Numbers on histograms represent the percentage of proliferating T cells. (B) Results from nine different donors (n = 9) are expressed as mean ± SEM. ****p < 0.0001, *p < 0.05, p = NS. (C) CFSE-labeled PBMC were cocultured with unfixed PMN in the presence of anti-CD3/CD28 mAbs at indicated ratios. Results are shown as mean ± SEM (n = 3) from three different donors. **p < 0.01.
Coincubation of PBMC with PMN does not affect their cytokine production
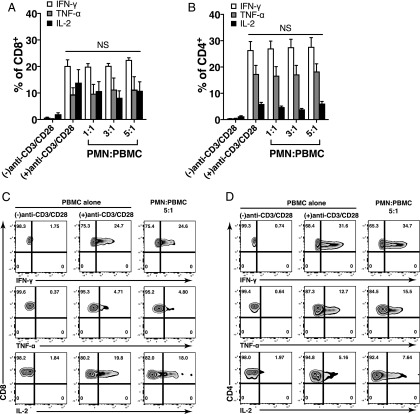
Next, we used intracellular cytokine flow cytometry to investigate cytokine production of IFN-γ, TNF-α, and IL-2 by T cells in response to coculture of PMN. Cytokine profiles illustrated in Fig. 2A and 2B revealed upon stimulation with anti-CD3/CD28 mAbs that the percentage of IFN-γ–, TNF-α–, or IL-2–producing cells was 20.1 ± 2.4%, 9.4 ± 2.6%, and 13.7 ± 5% (mean ± SEM) for CD8 and 26.3 ± 3.4%, 17.3 ± 3.3%, and 5.9 ± 0.7% (mean ± SEM) for CD4, respectively. As compared with T cells stimulated with anti-CD3/CD28 mAbs alone, coculture of PBMC with PMN did not significantly change the percentage of IFN-γ–, TNF-α–, or IL-2–producing CD8 and CD4 cells. A representative dot plot of IFN-γ–, TNF-α–, or IL-2–producing CD8 (Fig. 2C) and CD4 cells (Fig. 2D) stimulated with or without anti-CD3/CD28 mAbs or cocultured with PMN at 5:1 (PMN/PBMC) ratio are shown. These data suggest that PMN coculture inhibits T cell proliferation but does not affect cytokine production in activated T cells.
FIGURE 2.
PMN coculture does not affect cytokine production in stimulated T cells. (A and B) PBMC stimulated with or without anti-CD3/CD28 mAbs were cocultured with increasing numbers of PMN for 16 h, and the percentage of T cells producing cytokines was determined using intracellular staining of flow cytometry. Live CD8 and CD4 T cells were gated. Results are shown as mean ± SEM (n = 3) from three different donors. p = NS. (C and D) Dot plots show representative data of three different donors as shown in the cumulative data graph (top panels). Fixed PMN were used.
PMN-mediated inhibition of T cell proliferation is not associated with production of reactive oxygen species or depletion of l-arginine
PMN are reported to inhibit T cell proliferation through the production of reactive oxygen species (ROS) and the depletion of l-arginine by the PMN-associated enzyme arginase I (4, 19, 20). To determine the role, if any, played by ROS and arginase I in PMN-mediated suppression of T cell proliferation, we treated unfixed PMN with inhibitors in our coculture system. Briefly, CFSE-labeled anti-CD3/CD28–activated PBMC were cocultured for 5 d with PMN alone or with PMN treated with either catalase, a ROS inhibitor, or with nor-NOHA, an inhibitor of arginase I. Neither the addition of catalase nor nor-NOHA prevented the PMN-mediated suppression of T cell proliferation (Supplemental Fig. 2). The addition of catalase or nor-NOHA to PBMC alone had no effect on T cell proliferation induced by anti-CD3/CD28. Our data suggest that the inhibition of T cell proliferation after coculture with freshly isolated PMN is not mediated by ROS or depletion of l-arginine.
PMN inhibit T cell proliferation in a cell contact–dependent manner
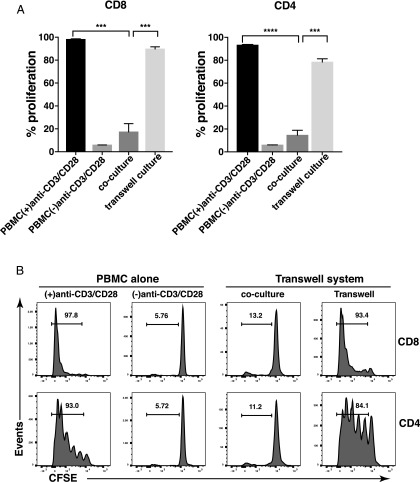
Next, we asked whether cell–cell contact was required for PMN-mediated T cell suppression. We used a Transwell system with anti-CD3/CD28–stimulated PBMC in the lower compartment and PMN in the upper compartment, physically separated from the lower compartment by a 0.4-μm membrane. As shown in Fig. 3A, coculture of PMN with PBMC at a 4:1 ratio significantly decreased proliferation of CD8 and CD4 T cells by 82.3 ± 7.2% and 85 ± 4.3%, mean ± SEM, respectively, whereas PMN-mediated inhibition of T cell proliferation was merely 8.5 ± 1.9% for CD8 and 15.8 ± 2.9% for CD4, mean ± SEM, in the Transwell culture. There was no significant soluble P3 detected in the culture media (data not shown). These data demonstrate that cell contact is required for maximal T cell inhibition by PMN. Representative histogram plots of the CFSE profile are shown in Fig. 3B. Similar results were obtained when unfixed PMN were used (data not shown).
FIGURE 3.
PMN-mediated inhibition of T cell proliferation requires cell-cell contact. Flow cytometric analysis of T cell proliferation in the presence of PMN using a Transwell system. (A) PMN were mixed with CFSE-labeled PBMC at a 4:1 ratio in coculture with anti-CD3/CD28 (coculture). To separate PBMC and PMN, PBMC were cultured in the bottom chamber and PMN were placed in the top chamber of a 96-well Transwell culture plate. Representative results from one of three different donors performed in triplicate are expressed as mean ± SEM. ****p < 0.0001, ***p < 0.01. (B) Representative results of three different donors are shown. Numbers on histograms represent the percentage of proliferating T cells. Fixed PMN were used.
PMN-mediated T cell inhibition is prevented by anti-P3 blocking Ab and requires P3 enzyme activity
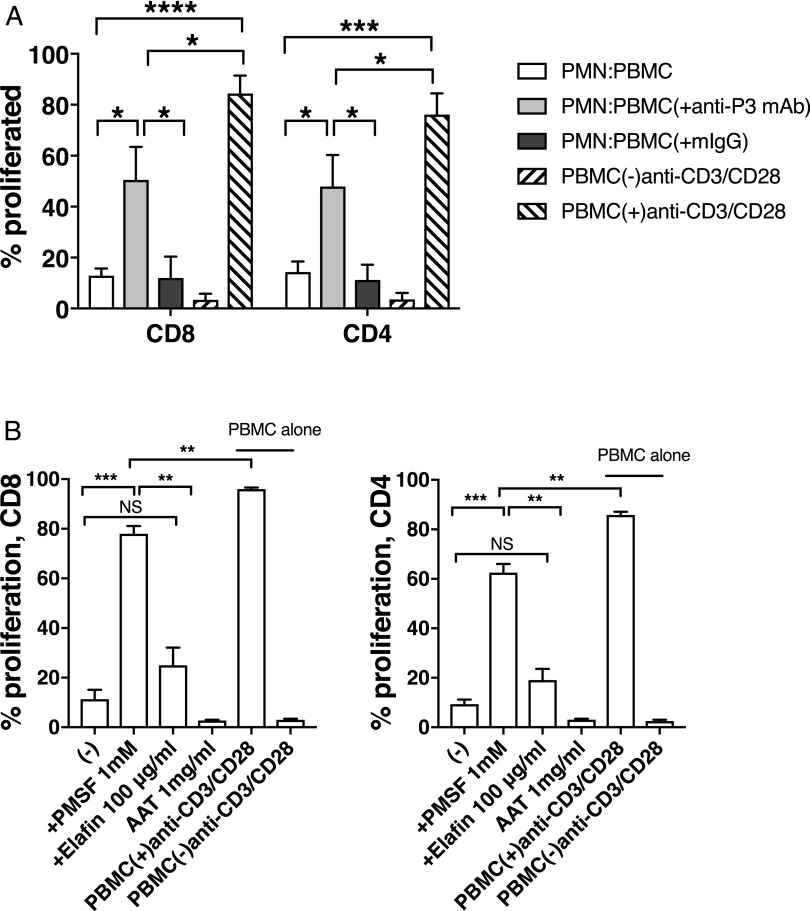
We previously showed that high expression of P3 by CML selectively deletes high-avidity PR1–CTLs (21), and AML and CML patients had higher levels of soluble P3 in their serum compared with healthy donors (22). We examined whether mP3 negatively regulates T cell proliferation and, if so, whether this inhibition could be blocked with an anti-P3 Ab. PBMC stimulated with anti-CD3/CD28 mAbs were cocultured with PMN in the presence of anti-P3 mAb or isotype control. Compared with significant inhibition of T cell proliferation observed (85 ± 2.7% for CD8 and 81 ± 4.1% for CD4, mean ± SEM, respectively) at 3:1 ratio of PMN/PBMC, addition of anti-P3 Ab resulted in significant reduction in inhibition for CD8 (53 ± 12.9%, mean ± SEM) and CD4 (54 ± 12.3%, mean ± SEM) T cells, respectively (Fig. 4A). The anti-P3 Ab significantly reduced PMN-mediated suppression of T cell proliferation, whereas isotype control showed no effect. These data indicate that membrane-bound P3 contributes to the inhibition of T cell proliferation by PMN. Representative histogram plots are shown in Supplemental Fig. 3.
FIGURE 4.
PMN-mediated T cell inhibition is prevented by anti-P3 blocking Ab and requires P3 enzyme activity. CFSE-labeled PBMC stimulated with anti-CD3/CD28 mAbs were cocultured with PMN at the indicated PMN/PBMC ratio. (A) PMN were treated with anti-P3 mAb (clone WGM2) or mIgG (clone 11711). The PMN/PBMC ratio is 3:1. Results from five different donors (n = 5) are shown as mean ± SEM. (B) PMN were preincubated with or without PMSF, elafin, or AAT before adding to PBMC cultures. The PMN/PBMC ratio is 4:1. Results shown as mean ± SEM are representative of three different donors performed in triplicates. Fixed PMN were used. ****p < 0.0001, ***p < 0.0005, **p < 0.005, *p < 0.05, p = NS.
It has been shown that the fixation process does not affect catalytic activity of membrane-bound serine proteinases (14, 23). To determine whether the protease activity of mP3 was required for the inhibition of T cell proliferation, the serine proteinase inhibitors PMSF, elafin, and AAT were used. In the presence of PMSF, at a ratio of 4:1 (PMN/PBMC), PMN-mediated inhibition of T cell proliferation was largely abrogated (69.3 ± 3.1 and 62 ± 3.5% reduction in inhibition for CD8 and CD4, mean ± SEM, respectively) (Fig. 4B). Added at large molar excess (14), elafin, a reversible inhibitor for P3, resulted in a 14 ± 7.1% and 11.3 ± 4.5% reduction in inhibition in CD8 and CD4 T cells, respectively, whereas AAT had no effect on the inhibitory activity of PMN (Fig. 4B). The result with elafin and AAT is consistent with a previous report demonstrating that mP3 is not readily inhibited by circulating physiological inhibitors such as AAT (14). The addition of the inhibitors alone to PBMC did not have any effect on CD4 or CD8 T cell proliferation (data not shown). Taken together, these data demonstrate the P3 enzyme dependence of the PMN-mediated antiproliferative effect.
mP3-expressing PMN mediate inhibition of T cell proliferation
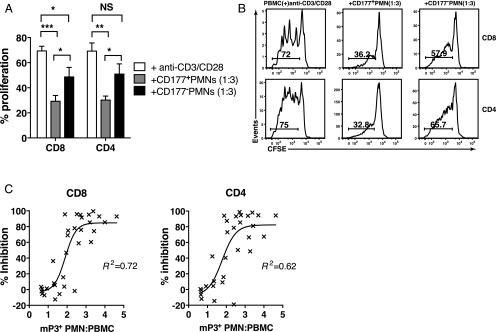
Because the GPI-anchored CD177 (NB1) membrane protein and mP3 were coexpressed on the same subset of PMN (12) (Supplemental Fig. 4), we used an anti-CD177 mAb to sort PMN (98% purity) into mP3-expressing and mP3-lacking populations to confirm that T cell inhibition by PMN was mediated by mP3. PMN were fixed before sorting to prevent activation-induced changes in mP3 expression during cell sorting. Compared with activated PBMC alone, proliferation of T cells was significantly reduced by 57.6 ± 4.2% and 56.1 ± 2.8% (mean ± SEM) for CD8 and CD4, respectively, in the presence of CD177-expressing PMN at a 3:1 PMN/PBMC ratio (Fig. 5A). A significant reduction in the inhibition of T cell proliferation was observed when the same ratio of CD177-negative PMN was added, with only a 29.7 ± 7.2% and 26.2 ± 7.8% (mean ± SEM) reduction in CD8 and CD4 T cell proliferation, respectively. The lower suppression might be due to low-level mP3 expression on CD177-negative PMN (24), (Supplemental Fig. 4). Maximum inhibition of T cell proliferation observed in the CD177-expressing PMN cocultures further supports a role for mP3 in mediating T cell inhibition. Representative CFSE profiles of CD8 and CD4 T cells are shown (Fig. 5B). To determine whether mP3-expressing PMN-mediated inhibition of T cell proliferation is sustained after removal of PMN, sorted CD177-expressing PMN were cocultured with anti-CD3/CD28–activated, CFSE-labeled PBMC from healthy donors at a ratio of 3:1 for 24 h. PMN were then depleted using anti-CD16 beads, and PBMC cultures were continued for a total of 5 d in the presence of anti-CD3/CD28 mAbs. The proliferation of CD8 and CD4 T cells was restored following PMN depletion, demonstrating that PMN-mediated suppression of T cell proliferation was not permanent (data not shown).
FIGURE 5.
mP3+ PMN mediate the inhibition of T cell proliferation. CFSE-labeled PBMC were activated with anti-CD3/CD28 mAbs in all cell cultures. Sort-purified PMN were fixed with 0.5% PFA. (A) Sorted CD177-expressing and CD177-negative PMN were cocultured with PBMC at a ratio of 3:1. T cells alone were used as control. Data from three different donors (n = 3) are shown as mean ± SEM. ***p < 0.001, **p < 0.003, *p < 0.05, p = NS. (B) Representative histogram plots of three different donors are shown. Numbers on histograms represent the percentage of proliferating T cells. (C) Nonlinear regression analysis with results from seven different donors (n = 7) shows the correlation between percentage inhibition and the relative mP3-expressing PMN/PBMC ratio. R2 = 0.72 for CD8 and R2 = 0.62 for CD4, respectively.
We then performed a nonlinear regression analysis to determine the correlation between the number of mP3-expressing PMN as measured by flow cytometry and the percentage inhibition of T cell proliferation in CFSE assays in seven healthy donors (Fig. 5C). A sigmoidal function fit of the data resulted in a lower sum of residual errors (R2 = 0.72 or R2 = 0.62 for CD8 and CD4 T cells, respectively) compared with a linear fit (R2 = 0.65 or R2 = 0.55, p < 0.0001). This suggests that there is a threshold effect of mP3-expressing PMN-mediated inhibition of T cell proliferation at a ratio of ∼2.2. This interpretation also suggests that the inhibitory effects of mP3-expressing PMN would be operative at sites of acute inflammation, where the ratio of PMN to PBMC is above this threshold, but not in peripheral blood during normal homeostatic conditions, where the ratio is typically ≤2.2 (25).
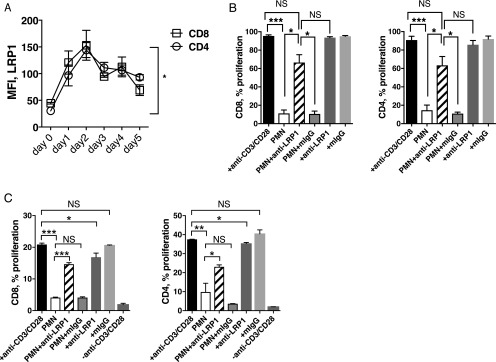
LRP1 blockade prevents PMN-mediated inhibition of T cell proliferation
mP3 coexpresses with calreticulin (CRT) on apoptotic PMN. Their association on PMN disrupts binding of CRT to LRP1 on macrophages and impairs CRT–LRP1-mediated phagocytosis (26). Moreover, LRP1 was shown to involve regulating T cell motility (27). Therefore, we sought to determine whether mP3-expressing PMN-mediated T cell inhibition involved LRP1 on T cells. Flow cytometry analysis revealed expression of LRP1 increased upon stimulation with anti-CD3/CD28 mAbs. Upregulation of LRP1 surface expression was most significant on day 2 on the surface of activated CD8 (mean = 3.4-fold) and CD4 (mean = 4.8-fold) T cells compared with unstimulated CD8 and CD4 T cells (Fig. 6A). Next, we examined the role of LRP1 in PMN-mediated inhibition of T cell proliferation. PMN were added in a 3:1 ratio to CFSE-labeled PBMC stimulated with anti-CD3/CD28 mAbs in the presence of anti-LRP1 mAb or an isotype control mIgG for 5 d. Blockade of LRP1 significantly reduced PMN-mediated inhibition of CD8 and CD4 T cells when compared with PBMC cultured with PMN (Fig. 6B) (33.1 ± 9.7% versus 88.4 ± 3.9% and 29.5 ± 13.1% versus 84.5 ± 5.4% for CD8 and CD4, mean ± SEM, respectively). Isotype control did not affect the inhibitory effect of PMN. To exclude the possible indirect or direct role of non-T cells, especially monocytes comprised in PBMC, we performed coculture experiments with sort-purified CD8 and CD4 T cells from PBMC (Fig. 6C). Compared with significant inhibition of T cell proliferation observed (80.2 ± 0.1% for CD8 and 73.7 ± 3.2% for CD4, mean ± SEM, respectively) at 3:1 ratio of PMN/T cells, addition of anti-LRP1 mAb resulted in significant reduction in inhibition for CD8 (30 ± 0.4%, mean ± SEM) and CD4 (38.5 ± 0.7%, mean ± SEM) T cells, respectively. These results are comparable to the results obtained using PBMC as the source of T cells. This demonstrates a role for LRP1 in PMN-mediated inhibition of T cell proliferation, possibly through the interaction between LRP1 on activated T cells and mP3 on PMN.
FIGURE 6.
LRP1 blockade prevents PMN-mediated inhibition of T cell proliferation. (A) PBMC from healthy donors were stimulated with anti-CD3/CD28 mAbs for up to 5 d, and LRP1 surface expression was determined by flow cytometry. Graph shows the median fluorescence intensity (MFI) of LRP1 on CD8 and CD4 T cells, respectively. Representative results from one of two different donors performed in duplicates are shown. Error bars represent mean ± SEM. *p < 0.05. (B) Fixed PMN were cocultured with activated PBMC at a ratio of 3:1 for 5 d in the presence or absence of anti-LRP1 mAb (clone 8G1) or mIgG isotype control. Data from three different donors (n = 3) are expressed as mean ± SEM. (C) Fixed PMN were cocultured with sort-purified CD8 or CD4 T cells activated with anti-CD3/CD28 mAbs at 3:1 ratio for 5 d in the presence or absence of anti-LRP1 mAb (clone 8G1) or mIgG isotype control. Results shown as mean ± SEM are representative of three different donors performed in duplicates. ***p < 0.001, **p < 0.01, *p < 0.05, p = NS.
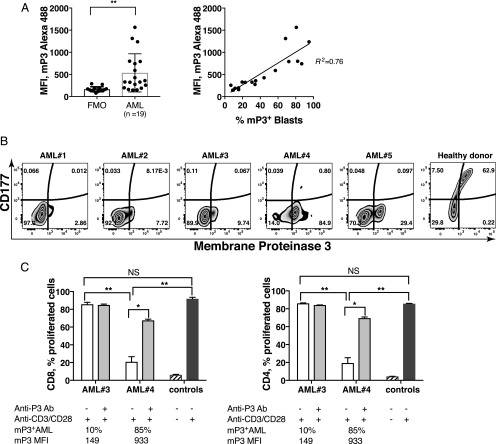
mP3+ AML mediates inhibition of T cell proliferation, and proliferation is restored via P3 blockade
We sought to examine whether primary AML blasts suppressed T cell proliferation in a manner analogous to mP3-expressing PMN. Because P3 is coexpressed with CD177 on the membrane of resting PMN, we first examined whether coexpression also occurs on primary AML blasts. The level of mP3 expression varied greatly between AML blasts from the 19 patients we investigated, and the fraction of mP3-expressing blasts correlates with the median fluorescence intensity of mP3 on AML blasts (Fig. 7A). In contrast to the coexpression on PMN from healthy donors, mP3 expression was not associated with CD177 on AML. Fig. 7B shows representative flow cytometry plots of blasts from five AML patients and PMN from one healthy donor. We further investigated the impact of AML blasts on T cell proliferation by comparing mP3high (AML#4) with mP3low (AML#3); both contained >90% blast cells as documented in patients’ medical records. Before being cocultured with CFSE-labeled PBMC in the presence of anti-CD3/CD28 mAbs, allogeneic AML blasts were fixed with 0.5% PFA for 10 min at 4°C to prevent T cell activation (<5% T cells present within the samples) and to keep in line with the treatment of PMN. Proliferation of CD8 and CD4 T cells cocultured with mP3high AML was significantly inhibited, whereas the percentage of proliferating CD8 and CD4 T cells cocultured with mP3low AML was comparable with PBMC stimulated with anti-CD3/CD28 mAbs alone (Fig. 7C). As expected, anti-P3 Ab significantly restored T cell capacity to proliferate. In conclusion, mP3high AML demonstrated suppression of T cell proliferation that could be abrogated by P3 blockade.
FIGURE 7.
mP3+ AML mediates the inhibition of T cell proliferation, and proliferation is restored via P3 blockade. (A) Flow cytometric analysis of CD177 and mP3 expression on AML blasts and healthy donor PMN were performed. (B) Representative plots of AML blasts from AML patients (n = 19) and PMN of healthy donor (n = 20) are shown. (C) Following fixation with 0.5% PFA, allogeneic AML was preincubated with or without anti-P3 Ab for 30 min before they were cocultured with PBMC at a 5:1 ratio (experiment was performed in duplicates) for up to 5 d. PBMC stimulated with or without anti-CD3/CD28 mAbs were used as positive and negative controls, respectively. Error bars represent mean ± SEM. **p < 0.01, *p < 0.05, p = NS. FMO, fluorescence minus one.
Discussion
In this report, we demonstrate that cell-associated P3 has inhibitory properties on T cells. Specifically, mP3-expressing PMN inhibited T cell proliferation, which was abrogated through mP3 blockade. Coincubation of sort-purified mP3-expressing PMN with PBMC at a ratio of 3:1 significantly inhibited proliferation of both CD4 and CD8 T cells, whereas mP3dim/low PMN had a less potent effect. The inhibitory effect of PMN-associated mP3 was associated with altered T cell cycle kinetics. Gabillet et al. (26) showed that LRP blockade decreased the phagocytosis of apoptotic neutrophils from healthy donors with PMN coexpressing P3 and CRT on the membrane, but not from patients with GPA, who possess anti-P3 Abs that interfere with the CRT–LRP interaction. This suggests that a direct association between P3 and LRP (or LRP-containing complexes) might occur (26). In support of this hypothesis, we showed that LRP1 blockade effectively prevented suppression of T cells by mP3-expressing PMN. This indicates a potential mechanism for the inhibition of T cells that relies on the interaction between mP3 on PMN and LRP1 on activated T cells. We also showed that AML blasts inhibited T cell proliferation and that this effect was dependent on the expression of mP3 by AML. These findings provide one mechanism by which myeloid leukemia can evade the anti-leukemia T cell immune response. Taken together, our data suggest that P3 plays an important role in the regulation of adaptive immune responses by innate immunity.
Our data further indicate that PMN-mediated inhibition of T cell proliferation is dependent on the enzymatic activity of mP3. Although ROS and arginase I have been implicated in PMN-mediated T cell suppression (28), our data suggest that PMN-mediated inhibition of T cell proliferation is independent of these molecules. It is possible, however, that the discrepancy between our results and others are due to differences in the condition of PMN because activated PMN were used in other studies (20, 29, 30). Furthermore, our data indicate that the impact of mP3 on T cells is distinct from P3-mediated effects on other cell types, including endothelial cells (31, 32). For example, the catalytically inactive form of P3, as well as the active enzyme, was shown to induce P3-mediated apoptosis of endothelial cells. Of note, PMN-associated mP3 inhibited T cell proliferation but did not induce T cell apoptosis in our assays; therefore, the mP3-mediated inhibition of T cell proliferation described in this study is distinctive from the IDO pathway that inhibits proliferation of T cells and induces T cell apoptosis (33).
Clinical studies have demonstrated that the presence of P3 within the tumor microenvironment is associated with poor clinical outcomes, including reduced survival, further supporting the immunosuppressive role of P3 (34). Padrines et al. (35) showed that P3 enhances the biological activity of IL-8 by cleaving the N-terminal domain, suggesting that P3 may play a role in potentiating the protumor angiogenic properties of IL-8, an effect that has been demonstrated in human lung cancer (36). In addition, increased PMN were shown to be a poor prognostic factor for progression-free survival and overall survival in advanced-stage colorectal adenocarcinoma and melanoma (16–18). In this study, we showed that mP3 on PMN inhibited T cell proliferation at a low concentration, estimated at 7–8 nM (14), which suggests that mP3 may play an important role in regulating T cell immunity in inflammatory physiological conditions, including inflammation associated with malignant and autoimmune disease. Our data further suggest that in homeostatic conditions, when mP3 is constitutively expressed on a subset of PMN, there is a threshold effect that dictates the number of mP3-expressing PMN that are required to cause T cell inhibition (Fig. 5C).
Our data also suggest a mechanism by which autoimmunity may be regulated in GPA. Because cANCA are known to bind P3 and correlate with disease activity in GPA (37, 38), our data suggest that blockade of P3 by cANCA abrogates the physiologic inhibitory effect of P3 on T cells, thereby contributing to the autoimmune manifestations of GPA. This is in line with the observation that increased mP3 expression in GPA impairs phagocytosis of apoptotic neutrophils, thus leading to proinflammatory response (26). Taken together, these findings suggest a complex role for mP3 on PMN whereby P3 interacts with LRP1 on macrophages leading to proinflammatory responses or via LRP1 on T cells, resulting in inhibition, thus averting inflammatory responses. It is a balancing act of P3 depending on the types of cells involved.
We previously reported that serum P3 is increased in AML and CML patients compared with healthy donors (22). This finding is consistent with a prior report showing increased P3 expression by leukemia cells from patients with AML or CML (10). P3 was shown to play a role in leukemogenesis (39), and our findings suggest an additional leukemia-promoting effect of P3 via inhibition of the anti-leukemia T cell immune response. Importantly, we found that mP3high AML inhibited the proliferation of T cells, an effect that could be prevented via P3 blockade. To our knowledge, these results provide the first direct evidence that AML can inhibit T cell proliferation via membrane-bound P3, demonstrating one potential mechanism by which AML escapes T cell immunity. Such inhibition of T cell proliferation could shape patient outcomes after immune-based therapies such as allogeneic stem cell transplantation or vaccines.
Although our data indicate an inhibitory role for P3 on adaptive immune responses, previous reports suggested that P3 enhances adaptive immunity. For example, P3 was shown to induce dendritic cell maturation via protease-activated receptor-2 in patients with GPA, an effect hypothesized to promote autoimmunity (40). In addition, P3 can bind and activate IL-32, a recently discovered proinflammatory cytokine that has emerged as an important player in the innate and adaptive immune responses (41). Furthermore, we previously showed that P3 uptake by solid tumors enhanced their killing by PR1–CTL via cross-presentation (42). In the context of our current study, these reports emphasize the varying effects of P3 in different physiologic and pathologic states. The differences in the immune outcomes mediated by P3 could be determined by the cell type on which P3 exerts its effects and the other cytokines and proteases that are present within the tissue microenvironment. Moreover, P3 may be an alarmin (43) because it regulates the innate immune response (44–46), and our study expands on its role as an immune regulator by showing that it affects the adaptive immune response, specifically T cells.
In conclusion, our findings suggest that leukemia and neutrophil-associated P3 downregulate T cell activity by inhibiting T cell proliferation. These findings are highly important in expanding our understanding of the biology of immune regulation in leukemia and other cancers as well as in autoimmune disease. These results suggest that a novel therapeutic approach targeting P3 may be an effective immunotherapy for the treatment of leukemia and autoimmune disease.
Supplementary Material
Acknowledgments
We thank the MD Anderson South Campus Flow Cytometry Core for assisting in cell sorting and acquisition of the data, Tamara K. Locke (Department of Scientific Publications, MDACC) for editing the manuscript, and Annalea Elwell (Department of Stem Cell Transplantation and Cellular Therapy) for technical help.
This work was supported by research funding from National Cancer Institute (NCI) CA100632 (to J.J.M.), NCI CA148600 (to J.J.M.), Leukemia and Lymphoma Society 6030-12 (to J.J.M.), NCI P30CA16672 (to K.C.-D.), and Leukemia and Lymphoma Society 7262-08 (to J.J.M.).
The online version of this article contains supplemental material.
- AAT
- α1-antitrypsin
- AML
- acute myeloid leukemia
- cANCA
- polyclonal anti-neutrophil cytoplasmic Ab
- CML
- chronic myeloid leukemia
- CRT
- calreticulin
- GPA
- granulomatosis with polyangiitis
- MDACC
- MD Anderson Cancer Center
- mP3
- membrane P3
- nor-NOHA
- N-hydroxy-nor-l-arginine
- P3
- proteinase 3
- PFA
- paraformaldehyde
- PMN
- polymorphonuclear neutrophil
- ROS
- reactive oxygen species
- TAN
- tumor-associated neutrophil.
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Pham C. T. 2008. Neutrophil serine proteases fine-tune the inflammatory response. Int. J. Biochem. Cell Biol. 40: 1317–1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.van der Geld Y. M., Limburg P. C., Kallenberg C. G. 2001. Proteinase 3, Wegener’s autoantigen: from gene to antigen. J. Leukoc. Biol. 69: 177–190. [PubMed] [Google Scholar]
- 3.Houghton A. M. 2010. The paradox of tumor-associated neutrophils: fueling tumor growth with cytotoxic substances. Cell Cycle 9: 1732–1737. [DOI] [PubMed] [Google Scholar]
- 4.Müller I., Munder M., Kropf P., Hänsch G. M. 2009. Polymorphonuclear neutrophils and T lymphocytes: strange bedfellows or brothers in arms? Trends Immunol. 30: 522–530. [DOI] [PubMed] [Google Scholar]
- 5.Dumitru C. A., Moses K., Trellakis S., Lang S., Brandau S. 2012. Neutrophils and granulocytic myeloid-derived suppressor cells: immunophenotyping, cell biology and clinical relevance in human oncology. Cancer Immunol. Immunother. 61: 1155–1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Powell D. R., Huttenlocher A. 2016. Neutrophils in the tumor microenvironment. Trends Immunol. 37: 41–52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Witko-Sarsat V., Canteloup S., Durant S., Desdouets C., Chabernaud R., Lemarchand P., Descamps-Latscha B. 2002. Cleavage of p21waf1 by proteinase-3, a myeloid-specific serine protease, potentiates cell proliferation. J. Biol. Chem. 277: 47338–47347. [DOI] [PubMed] [Google Scholar]
- 8.Pendergraft W. F., III, Rudolph E. H., Falk R. J., Jahn J. E., Grimmler M., Hengst L., Jennette J. C., Preston G. A. 2004. Proteinase 3 sidesteps caspases and cleaves p21(Waf1/Cip1/Sdi1) to induce endothelial cell apoptosis. Kidney Int. 65: 75–84. [DOI] [PubMed] [Google Scholar]
- 9.Kallenberg C. G. 2008. Pathogenesis of PR3-ANCA associated vasculitis. J. Autoimmun. 30: 29–36. [DOI] [PubMed] [Google Scholar]
- 10.Dengler R., Münstermann U., al-Batran S., Hausner I., Faderl S., Nerl C., Emmerich B. 1995. Immunocytochemical and flow cytometric detection of proteinase 3 (myeloblastin) in normal and leukaemic myeloid cells. Br. J. Haematol. 89: 250–257. [DOI] [PubMed] [Google Scholar]
- 11.Greiner J., Bullinger L., Guinn B. A., Döhner H., Schmitt M. 2008. Leukemia-associated antigens are critical for the proliferation of acute myeloid leukemia cells. Clin. Cancer Res. 14: 7161–7166. [DOI] [PubMed] [Google Scholar]
- 12.Bauer S., Abdgawad M., Gunnarsson L., Segelmark M., Tapper H., Hellmark T. 2007. Proteinase 3 and CD177 are expressed on the plasma membrane of the same subset of neutrophils. J. Leukoc. Biol. 81: 458–464. [DOI] [PubMed] [Google Scholar]
- 13.Sutherland D. R., Anderson L., Keeney M., Nayar R., Chin-Yee I., International Society of Hematotherapy and Graft Engineering 1996. The ISHAGE guidelines for CD34+ cell determination by flow cytometry. J. Hematother. 5: 213–226. [DOI] [PubMed] [Google Scholar]
- 14.Campbell E. J., Campbell M. A., Owen C. A. 2000. Bioactive proteinase 3 on the cell surface of human neutrophils: quantification, catalytic activity, and susceptibility to inhibition. J. Immunol. 165: 3366–3374. [DOI] [PubMed] [Google Scholar]
- 15.Fridlender Z. G., Sun J., Kim S., Kapoor V., Cheng G., Ling L., Worthen G. S., Albelda S. M. 2009. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 16: 183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schmidt H., Suciu S., Punt C. J., Gore M., Kruit W., Patel P., Lienard D., von der Maase H., Eggermont A. M., Keilholz U., American Joint Committee on Cancer Stage IV MelanomaEORTC 18951 2007. Pretreatment levels of peripheral neutrophils and leukocytes as independent predictors of overall survival in patients with American joint committee on cancer stage IV melanoma: results of the EORTC 18951 biochemotherapy trial. J. Clin. Oncol. 25: 1562–1569. [DOI] [PubMed] [Google Scholar]
- 17.Paesmans M., Sculier J. P., Lecomte J., Thiriaux J., Libert P., Sergysels R., Bureau G., Dabouis G., Van Cutsem O., Mommen P., et al. 2000. Prognostic factors for patients with small cell lung carcinoma: analysis of a series of 763 patients included in 4 consecutive prospective trials with a minimum follow-up of 5 years. Cancer 89: 523–533. [DOI] [PubMed] [Google Scholar]
- 18.Michael M., Goldstein D., Clarke S. J., Milner A. D., Beale P., Friedlander M., Mitchell P. 2006. Prognostic factors predictive of response and survival to a modified FOLFOX regimen: importance of an increased neutrophil count. Clin. Colorectal Cancer 6: 297–304. [DOI] [PubMed] [Google Scholar]
- 19.Kusmartsev S., Su Z., Heiser A., Dannull J., Eruslanov E., Kübler H., Yancey D., Dahm P., Vieweg J. 2008. Reversal of myeloid cell-mediated immunosuppression in patients with metastatic renal cell carcinoma. Clin. Cancer Res. 14: 8270–8278. [DOI] [PubMed] [Google Scholar]
- 20.Munder M., Schneider H., Luckner C., Giese T., Langhans C. D., Fuentes J. M., Kropf P., Mueller I., Kolb A., Modolell M., Ho A. D. 2006. Suppression of T-cell functions by human granulocyte arginase. Blood 108: 1627–1634. [DOI] [PubMed] [Google Scholar]
- 21.Molldrem J. J., Lee P. P., Kant S., Wieder E., Jiang W., Lu S., Wang C., Davis M. M. 2003. Chronic myelogenous leukemia shapes host immunity by selective deletion of high-avidity leukemia-specific T cells. J. Clin. Invest. 111: 639–647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Alatrash G., Ono Y., Sergeeva A., Sukhumalchandra P., Zhang M., St John L. S., Yang T. H., Ruisaard K., Armistead P. M., Mittendorf E. A., et al. 2012. The role of antigen cross-presentation from leukemia blasts on immunity to the leukemia-associated antigen PR1. J. Immunother. 35: 309–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Owen C. A., Campbell M. A., Sannes P. L., Boukedes S. S., Campbell E. J. 1995. Cell surface-bound elastase and cathepsin G on human neutrophils: a novel, non-oxidative mechanism by which neutrophils focus and preserve catalytic activity of serine proteinases. J. Cell Biol. 131: 775–789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hu N., Westra J., Kallenberg C. G. 2009. Membrane-bound proteinase 3 and its receptors: relevance for the pathogenesis of Wegener’s Granulomatosis. Autoimmun. Rev. 8: 510–514. [DOI] [PubMed] [Google Scholar]
- 25.Satomi A., Murakami S., Ishida K., Mastuki M., Hashimoto T., Sonoda M. 1995. Significance of increased neutrophils in patients with advanced colorectal cancer. Acta Oncol. 34: 69–73. [DOI] [PubMed] [Google Scholar]
- 26.Gabillet J., Millet A., Pederzoli-Ribeil M., Tacnet-Delorme P., Guillevin L., Mouthon L., Frachet P., Witko-Sarsat V. 2012. Proteinase 3, the autoantigen in granulomatosis with polyangiitis, associates with calreticulin on apoptotic neutrophils, impairs macrophage phagocytosis, and promotes inflammation. J. Immunol. 189: 2574–2583. [DOI] [PubMed] [Google Scholar]
- 27.Bergström S. E., Bergdahl E., Sundqvist K. G. 2013. A cytokine-controlled mechanism for integrated regulation of T-lymphocyte motility, adhesion and activation. Immunology 140: 441–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leliefeld P. H., Koenderman L., Pillay J. 2015. How neutrophils shape adaptive immune responses. Front. Immunol. 6: 471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hock B. D., Taylor K. G., Cross N. B., Kettle A. J., Hampton M. B., McKenzie J. L. 2012. Effect of activated human polymorphonuclear leucocytes on T lymphocyte proliferation and viability. Immunology 137: 249–258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rotondo R., Bertolotto M., Barisione G., Astigiano S., Mandruzzato S., Ottonello L., Dallegri F., Bronte V., Ferrini S., Barbieri O. 2011. Exocytosis of azurophil and arginase 1-containing granules by activated polymorphonuclear neutrophils is required to inhibit T lymphocyte proliferation. J. Leukoc. Biol. 89: 721–727. [DOI] [PubMed] [Google Scholar]
- 31.Pederzoli M., Kantari C., Gausson V., Moriceau S., Witko-Sarsat V. 2005. Proteinase-3 induces procaspase-3 activation in the absence of apoptosis: potential role of this compartmentalized activation of membrane-associated procaspase-3 in neutrophils. J. Immunol. 174: 6381–6390. [DOI] [PubMed] [Google Scholar]
- 32.Yang J. J., Preston G. A., Pendergraft W. F., Segelmark M., Heeringa P., Hogan S. L., Jennette J. C., Falk R. J. 2001. Internalization of proteinase 3 is concomitant with endothelial cell apoptosis and internalization of myeloperoxidase with generation of intracellular oxidants. Am. J. Pathol. 158: 581–592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Timosenko E., Hadjinicolaou A. V., Cerundolo V. 2017. Modulation of cancer-specific immune responses by amino acid degrading enzymes. Immunotherapy 9: 83–97. [DOI] [PubMed] [Google Scholar]
- 34.Desmedt C., Ouriaghli F. E., Durbecq V., Soree A., Colozza M. A., Azambuja E., Paesmans M., Larsimont D., Buyse M., Harris A., et al. 2006. Impact of cyclins E, neutrophil elastase and proteinase 3 expression levels on clinical outcome in primary breast cancer patients. Int. J. Canc. 119: 2539–2545. [DOI] [PubMed] [Google Scholar]
- 35.Padrines M., Wolf M., Walz A., Baggiolini M. 1994. Interleukin-8 processing by neutrophil elastase, cathepsin G and proteinase-3. FEBS Lett. 352: 231–235. [DOI] [PubMed] [Google Scholar]
- 36.Smith D. R., Polverini P. J., Kunkel S. L., Orringer M. B., Whyte R. I., Burdick M. D., Wilke C. A., Strieter R. M. 1994. Inhibition of interleukin 8 attenuates angiogenesis in bronchogenic carcinoma. J. Exp. Med. 179: 1409–1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.van der Woude F. J., Rasmussen N., Lobatto S., Wiik A., Permin H., van Es L. A., van der Giessen M., van der Hem G. K., The T. H. 1985. Autoantibodies against neutrophils and monocytes: tool for diagnosis and marker of disease activity in Wegener’s granulomatosis. Lancet 1: 425–429. [DOI] [PubMed] [Google Scholar]
- 38.Dolman K. M., Jager A., Sonnenberg A., von dem Borne A. E., Goldschmeding R. 1995. Proteolysis of classic anti-neutrophil cytoplasmic autoantibodies (C-ANCA) by neutrophil proteinase 3. Clin. Exp. Immunol. 101: 8–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sköld S., Rosberg B., Gullberg U., Olofsson T. 1999. A secreted proform of neutrophil proteinase 3 regulates the proliferation of granulopoietic progenitor cells. Blood 93: 849–856. [PubMed] [Google Scholar]
- 40.Csernok E., Ai M., Gross W. L., Wicklein D., Petersen A., Lindner B., Lamprecht P., Holle J. U., Hellmich B. 2006. Wegener autoantigen induces maturation of dendritic cells and licenses them for Th1 priming via the protease-activated receptor-2 pathway. Blood 107: 4440–4448. [DOI] [PubMed] [Google Scholar]
- 41.Csernok E., Holle J. U., Gross W. L. 2008. Proteinase 3, protease-activated receptor-2 and interleukin-32: linking innate and autoimmunity in Wegener’s granulomatosis. Clin. Exp. Rheumatol. 26(3, Suppl. 49)S112–S117. [PubMed] [Google Scholar]
- 42.Alatrash G., Mittendorf E. A., Sergeeva A., Sukhumalchandra P., Qiao N., Zhang M., St John L. S., Ruisaard K., Haugen C. E., Al-Atrache Z., et al. 2012. Broad cross-presentation of the hematopoietically derived PR1 antigen on solid tumors leads to susceptibility to PR1-targeted immunotherapy. J. Immunol. 189: 5476–5484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bianchi M. E. 2007. DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 81: 1–5. [DOI] [PubMed] [Google Scholar]
- 44.Bank U., Küpper B., Reinhold D., Hoffmann T., Ansorge S. 1999. Evidence for a crucial role of neutrophil-derived serine proteases in the inactivation of interleukin-6 at sites of inflammation. FEBS Lett. 461: 235–240. [DOI] [PubMed] [Google Scholar]
- 45.Novick D., Rubinstein M., Azam T., Rabinkov A., Dinarello C. A., Kim S. H. 2006. Proteinase 3 is an IL-32 binding protein. Proc. Natl. Acad. Sci. USA 103: 3316–3321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Meyer-Hoffert U. 2009. Neutrophil-derived serine proteases modulate innate immune responses. Front. Biosci. 14: 3409–3418. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.