Abstract
Amaryllidaceae plants are the commercial source of galanthamine, an alkaloid approved for the clinical treatment of Alzheimer’s disease. The chemistry and bioactivity of Chilean representatives of Rhodophiala genus from the family of Amaryllidaceae have not been widely studied so far. Ten collections of five different Chilean Rhodophiala were analyzed in vitro for activity against enzymes such as acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) as well as for their alkaloid composition by GC-MS. To obtain an insight into the potential AChE and BuChE inhibitory activity of the alkaloids identified in the most active samples, docking experiments were carried out. Although galanthamine was found neither in aerial parts nor in bulbs of R. splendens, these plant materials were the most active inhibitors of AChE (IC50: 5.78 and 3.62 μg/mL, respectively) and BuChE (IC50: 16.26 and 14.37 μg/mL, respectively). Some 37 known alkaloids and 40 still unidentified compounds were detected in the samples, suggesting high potential in the Chilean Amaryllidaceae plants as sources of both novel bioactive agents and new alkaloids.
Keywords: Rhodophiala, alkaloids, molecular docking, AChE, BuChE, GC-MS
1. Introduction
The vast structural and chemical diversity of natural products gives them a significant role in drug discovery [1]. Alkaloids are of particular interest in biomedicine and drug discovery research [2] due to their structural diversity and specific biological potential [3]. The Amaryllidaceae is a plant family that contains an exclusive, large and still expanding alkaloid group known as the Amaryllidaceae alkaloids, which are characterized by unique skeleton arrangements and a broad spectrum of biological activities [4,5]. Amaryllidaceae plants have been used in folk medicine for their therapeutic and toxic properties [6]. Hippocrates of Kos (460–370 BP), considered the father of modern medicine, recommended the oil of Narcissus (Amaryllidaceae) species for the treatment of symptoms that today would be recognized as cancer [7].
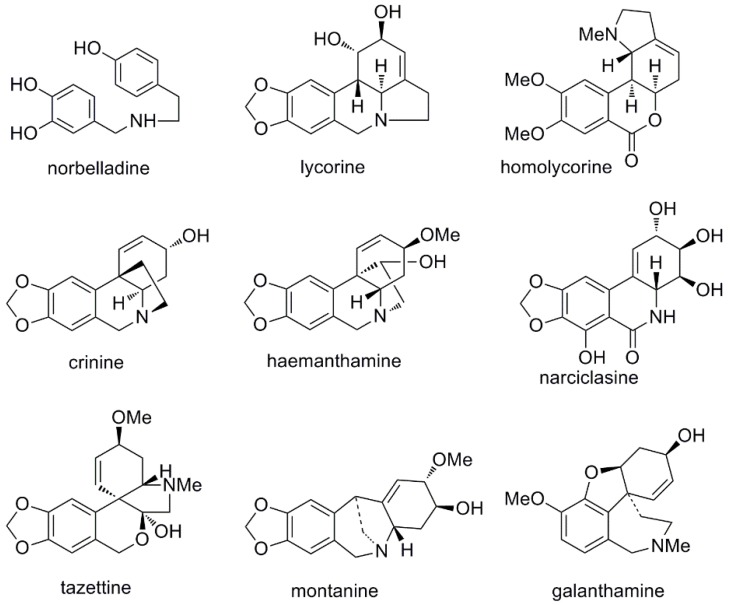
The Amaryllidaceae alkaloids are classified mainly into nine skeleton types: norbelladine, lycorine, homolycorine, crinine, haemanthamine, narciclasine, tazettine, montanine and galanthamine (Figure 1) [4]. The most important Amaryllidaceae alkaloid is galanthamine, which was isolated for the first time from Galanthus woronowii in the 1950s and was approved for the clinical treatment of mild to moderate Alzheimer’s disease (AD) by the Food and Drug Administration (FDA) at the beginning of this century [8,9]. Alzheimer’s disease (AD), characterized by severe and progressive memory loss, is the most common form of dementia and is becoming increasingly prevalent in people older than 65 years [10,11]. It is estimated that 47 million people live with dementia in the world today, with an economic impact estimated at 818 billion dollars [12]. Known factors involved in the development of AD include a reduced cholinergic neurotransmission level, oxidative stress, amyloid-β-peptide (Aβ) and tau protein aggregation [13].
Figure 1.
Amaryllidaceae alkaloid types.
Acetylcholinesterase (AChE) and butyrylcholinesterase (BuChE) are involved in the hydrolysis of the neurotransmitter acetylcholine (ACh) [14]. Acetylcholinesterase is highly selective for ACh hydrolysis, and BuChE can metabolize different substrates [15]. In the brain of AD patients, AChE activity tends to decrease while that of BuChE increases [16]. Consequently, cholinesterase inhibitors that suppress both AChE and BuChE may provide a better therapeutic response rather than AChE-selective agents [17].
Little is known on the chemistry and bioactivity of the South American endemic Amaryllidaceae genus Rhodophiala. The Rhodophiala species have high ornamental potential due to its attractive red, yellow, white or orange flowers [18,19,20,21,22]. The approximately 40 species described at present occur in Argentina, Bolivia, southern Brazil, Chile and Uruguay [22]. Rhodophiala plants have a tunicate bulb of 4–6 cm diameter, which is set 20–30 cm underground, a single umbel holding up to six flowers, each flower being 4–6 cm wide and a flower stem 35 to 50 cm long [18].
Rhodophiala species are well known ornamental plants. Propagation of the Chilean species was reported [19] as well as phylogenetic [20] and morphological studies [21,22]. The renewed interest on galanthamine sources including the search for additional alkaloids with inhibitory activity towards the enzymes AChE and BuChE has prompted research work on the South American species of this family. Brazilian [23] and Argentinean wild Amaryllidaceae [24] showed high structural diversity in the alkaloid patterns and encouraged work on the Chilean species of this family. The knowledge of the chemical composition and anti-cholinesterase activity of the Chilean species belonging to genus Rhodophiala is limited. The comparative studies of their chemical composition are needed to identify the best potential sources of bioactive alkaloids in the South American species.
The aim of this work was to disclose the potential of Chilean Rhodophiala species as inhibitors of the enzymes AChE and BuChE as well as to analyze the alkaloid content and composition of native species by gas chromatography coupled to mass spectrometry (GC-MS) looking for galanthamine sources and searching for other compounds with effect of AChE and BuChE. Molecular docking studies were also carried out to investigate the affinity of the alkaloids identified in the most promising sample at the active sites of AChE and BuChE.
2. Results and Discussion
Twenty alkaloid extracts from five different Chilean Rhodophiala species (Figure 2 and Figure 3) were assessed for inhibitory activity towards the enzymes AChE and BuChE. The alkaloid composition of the extracts was analyzed by GC-MS and the single alkaloid content was quantified and reported as mg GAL/g alkaloid extract (AE). The extracts have been obtained as described in Section 2.2. Briefly, the (fresh) plant material was lyophilized to obtain the dry tissues content (values included in the Table) of the different plant parts investigated. The extraction yields are calculated and reported from the lyophilized plant material.
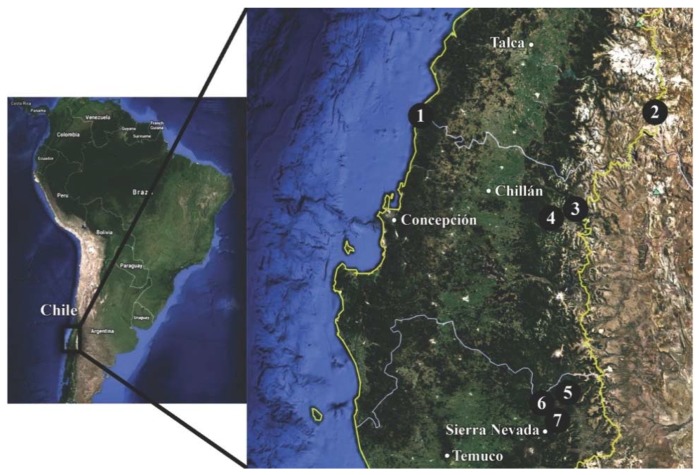
Figure 2.
Map of Chile showing the collection sites of the Rhodophiala species. 1: Arcos de Calán; 2: Laguna del Maule; 3: Nevado de Chillán; 4: Las Trancas; 5: Volcán Lonquimay; 6: Malalcahuello; 7: Sierra Nevada. Map source: Google Earth.
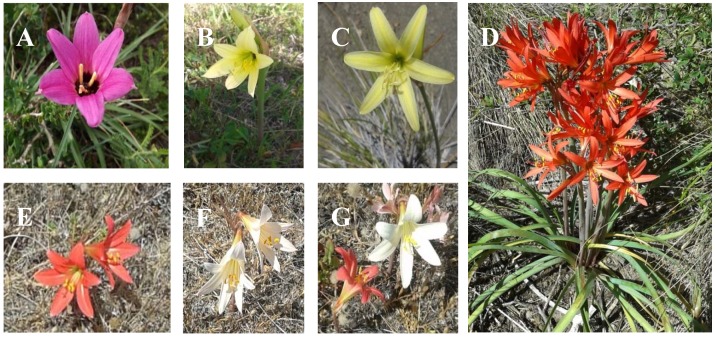
Figure 3.
Flowering Rhodophiala species from central-southern Chile. (A) R. andicola (Poepp.) Traub; (B) R. araucana (Phil.) Traub; (C) R. montana (Phil.) Traub; (D) R. splendens (Renjifo) Traub; (E) R. pratensis (Poepp.) Traub.; (F) R. pratensis white flowers and (G) R. pratensis plants with red and white flowers.
2.1. AChE and BuChE Inhibitory Activities
The crude alkaloid samples from the different Chilean Rhodophiala were tested in vitro for AChE and BuChE-inhibitory activity. The percent dry weight of the samples, w/w extraction yields, and cholinesterase inhibition are summarized in Table 1. Galanthamine was used as a control and presented AChE and BuChE inhibition with IC50 values of 0.48 ± 0.07 and 3.70 ± 0.24 μg/mL, respectively. All the alkaloid extracts tested were active against AChE. The highest AChE inhibitory potential was found in bulbs of R. pratensis (sample Q) followed by R. splendens (sample S) with IC50 values of 3.32 ± 0.26 and 3.62 ± 0.02 μg/mL, respectively. Lowest activity was measured for the aerial part of R. pratensis (sample N) (IC50 value: 102.27 ± 6.61 μg/mL). Nearly 50% of the samples showed some activity against BuChE, with better effect for the bulbs of R. splendens (sample S) (IC50 14.37 ± 1.94 μg/mL).
Table 1.
Percent dry weight, w/w extraction yields from dry starting material, percent alkaloid extract (from the crude extract), acetyl-(AChE) and butyryl- (BuChE) cholinesterase inhibitory activity of alkaloid-enriched extracts from Chilean Rhodophiala.
| Scientific Names, Abbreviated Collection Place and Reference Letter | % Dry Weight *a | w/w Extraction Yield *b | % Alkaloid Extract *c | AChE IC50 (μg/mL) *d |
BuChE IC50 (μg/mL) *d |
|---|---|---|---|---|---|
| Aerial parts | |||||
| R. andicola, SN (B) | 15.46 | 30.63 | 3.16 | 18.16 ± 2.94 | 138.27 ± 6.83 |
| R. andicola, NC (D) | 18.70 | 22.10 | 2.86 | 12.30 ± 0.74 | 43.41 ± 2.64 |
| R. andicola, VL (F) | 20.00 | 17.50 | 2.07 | 74.44 ± 5.53 | >200 |
| R. araucana, M, (H) | 17.70 | 25.30 | 3.78 | *e | *e |
| R. montana, LM (J) | 21.40 | 9.40 | 1.14 | 33.57 ± 2.16 | 16.38 ± 0.78 |
| R. pratensis, AC, RF, nl, sand dunes (L) | 14.84 | 27.08 | 1.93 | 72.59 ± 4.26 | >200 |
| R. pratensis, AC, RF, L (N) | 14.53 | 10.10 | 3.72 | 102.27 ± 6.61 | >200 |
| R. pratensis, AC, RF, nl (P) | 11.68 | 32.39 | 2.87 | 31.97 ± 3.24 | >200 |
| R. pratensis, AC, WF (R) | 10.67 | 16.47 | 1.15 | 8.39 ± 0.27 | >200 |
| R. splendens, LT, (T) | 14.81 | 44.26 | 1.53 | 5.78 ± 0.93 | 16.26 ± 3.34 |
| Bulbs | |||||
| R. andicola, SN (A) | 25.41 | 7.53 | 2.15 | 13.29 ± 1.01 | 45.76 ± 9.72 |
| R. andicola, NC (C) | 28.90 | 6.00 | 2.23 | 7.26 ± 0.16 | 47.38 ± 4.08 |
| R. andicola, VL (E) | 23.50 | 7.40 | 2.37 | 22.77 ± 1.57 | 113.24 ± 2.77 |
| R. araucana, M, (G) | 19.80 | 7.90 | 2.73 | 6.23 ± 0.24 | 45.71 ± 3.51 |
| R. montana, LM, (I) | 28.00 | 3.26 | 1.50 | 18.13 ± 0.51 | 40.05 ± 9.03 |
| R. pratensis, AC, RF, nl, sand dunes (K) | 18.87 | 19.73 | 1.50 | 11.81 ± 0.17 | >200 |
| R. pratensis, AC, RF, L (M) | 20.98 | 11.73 | 2.84 | 44.23 ± 4.08 | >200 |
| R. pratensis, AC, RF, nl (O) | 18.76 | 7.36 | 2.33 | 47.66 ± 1.78 | >200 |
| R. pratensis, AC, WF (Q) | 19.64 | 4.84 | 2.29 | 3.32 ± 0.26 | 52.16 ± 0.57 |
| R. splendens, LT, (S) | 21.35 | 35.05 | 1.60 | 3.62 ± 0.02 | 14.37 ± 1.94 |
*a after lyophilization; *b from lyophilized material; *c from the crude extract; *d all IC50 were calculated using R2 ≥ 0.99; *e insufficient sample; WF: white flowers; RF: red flowers; L: with leaves; nl: no leaves; Collection place: AC: Arcos de Calán, Región del Maule; LM: Laguna del Maule; LT: Las Trancas; M: Malalcahuello, Región de la Araucanía; NC: Nevado de Chillán; SN: Sierra Nevada; VL: volcan Lonquimay.
The bulb (I) and leaf (J) extracts of R. montana presented moderate activity against both enzymes with better effect of (I) against AChE and (J) towards BuChE. The differences in the chemical profiles of I and J could explain these results. However, the high number of unknown alkaloids in the extracts precludes further discussion. The bulb (Q) and leaf (R) extracts of white flowering R. pratensis were active towards AChE, with IC50 of 3.32 and 8.39 μg/mL, respectively but with mild to low effect against BuChE (Table 1), reducing the pharmacological interest of this species. The high AChE and BuChE inhibitory activity of R. splendens bulb (S) and aerial parts/leaves (T) renders this plant as the most promising species in the search for active molecules for AD therapy (Table 1).
2.2. Alkaloid Identification by GC-MS
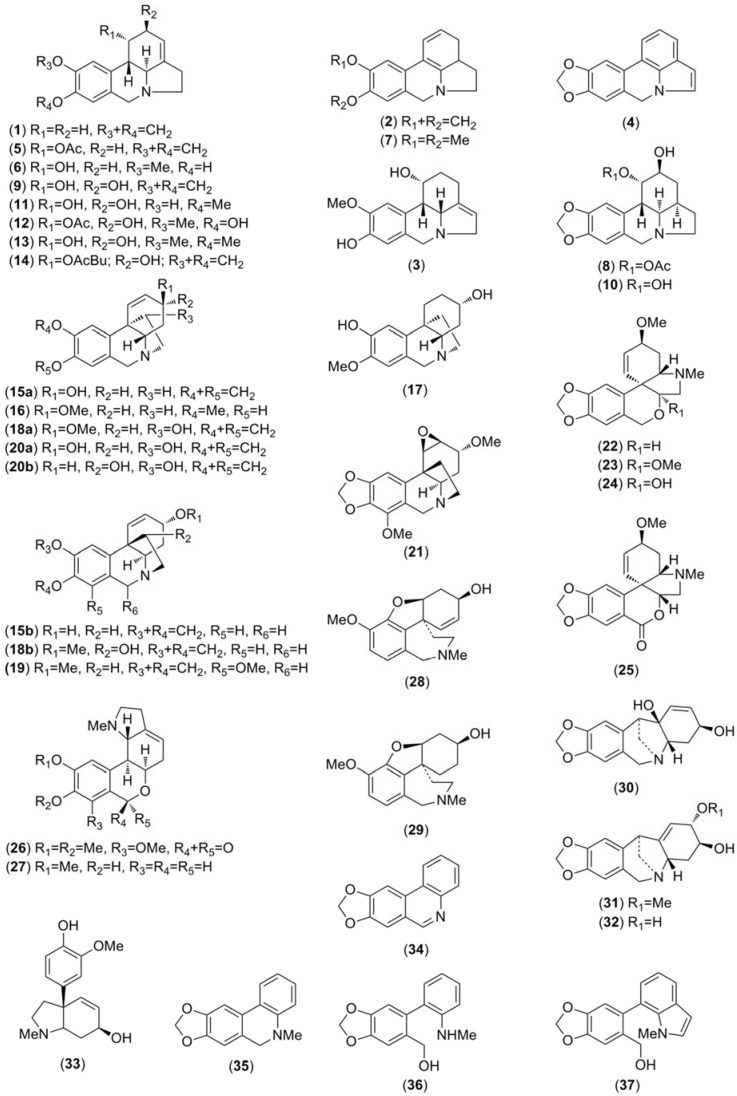
The activity of the Chilean Rhodophiala towards acetylcholinesterase is a consequence of the chemical composition of the extracts. Therefore, the alkaloid composition is a key point to understand the chemical diversity of these plants as a source of potential therapies for AD. The alkaloids occurring in the different extracts were identified by comparing their GC-MS spectra and Kovats Retention Index (RI) values with those of authentic samples. Thirty-seven known alkaloids were identified in these samples (Figure 4). About 50% of them belong to three different alkaloid types, namely: lycorine, haemanthamine and crinine. The others belong to six different alkaloid types: tazettine, homolycorine, galanthamine, montanine, mesembrenone and narciclasine. Two unusual alkaloids known as ismine and galanthindole were also found. The occurrence and quantification of the alkaloids in the samples is summarized in Table 2: (A) (aerial parts) and (B) (bulbs). The number of alkaloids detected varied among extracts, ranging from 8 in the aerial parts of R. andicola collected in Sierra Nevada (B) to 23 in the bulb of R. pratensis (K). Forty structures found in these samples could not be identified, suggesting high potential of Chilean Rhodophiala species in the search for new alkaloids. The number of unidentified compounds ranged from 3 in aerial parts of R. andicola (A, B and D), R. pratensis (P) and R. splendens (T) to 12 in aerial parts of R. montana (J). Representative chromatograms of the samples are shown in Figure 5, Figure 6, Figure 7, Figure 8 and Figure 9.
Figure 4.
Alkaloids identified in Chilean Rhodophiala species by GC-MS.
Table 2.
(A) Identification of alkaloids occurring in the aerial parts of Chilean Rhodophiala species by GC-MS. Values are expressed as mg GAL/g AE; (B) Identification of alkaloids occurring in the bulbs of Chilean Rhodophiala species by GC-MS. Values are expressed as mg GAL/g AE.
| (A) | ||||||||||||||
| Alkaloid | M+ | BP | RI | B | D | F | H | J | L | N | P | R | T | |
| Lycorine-type | 8.6 | 7.5 | 25.1 | 46.0 | 46.9 | 35.5 | 37.3 | 35.4 | 60.8 | 13.1 | ||||
| lycorene (1) | 255 | 254 | 2346.8 | - | - | - | - | 9.4 | 5.1 | 7.2 | T | - | - | |
| anhydrolycorine (2) | 251 | 250 | 2543.1 | - | T | 5.9 | 11.2 | 9.6 | 9.9 | 8.3 | 11.8 | 13.2 | - | |
| kirkine (3) | 253 | 252 | 2588.2 | - | - | - | - | 7.2 | 5.3 | - | 6.0 | 7.2 | - | |
| 11,12-dehydroanhydrolycorine (4) | 249 | 248 | 2646.4 | 8.6 | 7.5 | 13.5 | 8.4 | 15.6 | 8.1 | 21.8 | 7.5 | 9.4 | 7.8 | |
| 1-O-acetylcaranine (5) | 313 | 226 | 2653.1 | - | - | - | - | - | - | - | - | - | - | |
| norpluviine (6) | 273 | 228 | 2683.6 | - | - | - | - | - | - | - | - | 6.2 | - | |
| assoanine (7) | 267 | 266 | 2708.9 | - | T | - | - | - | - | - | - | - | - | |
| 3,4-dihydro 1-acetyllycorine (8) | 331 | 330 | 2723.3 | - | - | - | - | - | - | - | - | - | - | |
| lycorine (9) | 287 | 226 | 2791.7 | - | T | 5.7 | 14.7 | 5.1 | 7.1 | - | 10.1 | 24.8 | 5.3 | |
| dihydrolycorine (10) | 289 | 288 | 2833.9 | - | - | - | - | - | - | - | T | T | - | |
| pseudolycorine (11) | 289 | 228 | 2856.4 | - | - | - | - | - | - | - | - | - | - | |
| sternbergine (12) | 331 | 228 | 2838.8 | - | - | - | 6.4 | - | - | - | T | - | - | |
| methylpseudolycorine (13) | 303 | 242 | 2911.2 | - | - | - | - | - | - | - | - | - | ||
| 1-O-(3′acetoxybutanoyl)lycorine (14) | 415 | 226 | 3248.8 | - | - | - | 5.3 | - | - | - | - | - | - | |
| Haemanthamine/crinine type | - | 11.6 | 53.1 | 34.4 | 14.8 | 17.2 | 21.4 | 38.7 | 35.7 | 48.0 | ||||
| vittatine (15a)/crinine (15b) | 271 | 271 | 2512.4 | - | T | 7.7 | - | - | - | - | 6.2 | 5.6 | - | |
| 8-O-demethylmaritidine (16) | 273 | 273 | 2540.0 | - | - | - | - | - | - | - | T | - | 6.4 | |
| deacetylcantabricine (17) | 275 | 275 | 2573.1 | - | - | - | - | - | - | - | - | - | T | |
| haemanthamine (18a)/crinamine (18b) | 301 | 272 | 2673.4 | - | 11.6 | 45.4 | 34.4 | 7.7 | 17.2 | 21.4 | 32.5 | 30.1 | 25.0 | |
| buphanidrine (19) | 315 | 315 | 2748.3 | - | - | - | - | - | - | - | - | - | - | |
| 11-hydroxyvittatine (20a)/hamayne (20b) | 287 | 258 | 2750.5 | - | - | - | - | - | - | - | - | - | 16.6 | |
| undulatine (21) | 331 | 331 | 2892.5 | - | - | - | - | 7.1 | - | - | - | - | - | |
| Tazettine-type | 60.7 | 59.1 | 95.8 | 22.3 | - | 11.2 | 16.3 | 16.0 | 29.9 | 55.6 | ||||
| deoxytazettine (22) | 315 | 231 | 2575.6 | 6.1 | 5.5 | 6.7 | 5.0 | - | T | 5.2 | - | 5.2 | - | |
| O-methyltazettine (23) | 345 | 261 | 2641.1 | 54.6 | 36.0 | 66.2 | 10.3 | - | 11.2 | 11.1 | 10.1 | 14.9 | 30.7 | |
| tazettine (24) | 331 | 247 | 2685.1 | - | 12.5 | 17.0 | 7.0 | - | - | - | 5.9 | 9.8 | 24.9 | |
| epimacronine (25) | 329 | 245 | 2848.0 | - | 5.1 | 5.9 | T | - | T | - | - | T | T | |
| Homolycorine-type | - | - | 9.1 | - | - | 8.6 | 22.4 | 6.5 | 19.5 | - | ||||
| nerinine (26) | 347 | 109 | 2513.5 | - | - | - | - | - | 8.6 | 22.4 | 6.5 | 19.5 | - | |
| 8-O-demethylhomolycorine (27) | 301 | 109 | 2856.4 | - | - | 9.1 | - | - | - | - | - | - | - | |
| Galanthamine-type | - | 5.1 | 14.1 | 7.1 | 5.8 | - | - | - | - | - | ||||
| galanthamine (28) | 287 | 286 | 2519.9 | - | - | 8.6 | 7.1 | 5.8 | - | - | - | - | - | |
| lycoramine (29) | 289 | 288 | 2544.6 | - | 5.1 | 5.5 | - | - | - | - | - | - | - | |
| Montanine-type | - | - | - | - | 8.1 | 28.3 | 19.7 | 15.2 | 26.7 | - | ||||
| pancratinine C (30) | 287 | 176 | 2623.5 | - | - | - | - | - | - | - | - | - | ||
| montanine (31) | 301 | 301 | 2663.1 | - | - | - | - | 8.1 | 28.3 | 19.7 | 15.2 | 26.7 | - | |
| pancracine (32) | 287 | 287 | 2737.4 | - | - | - | - | - | - | - | - | - | - | |
| Mesembrenone-type | - | - | - | - | - | - | - | - | - | - | ||||
| demethylmesembrenol (33) | 275 | 205 | 2343.2 | - | - | - | - | - | T | - | T | T | - | |
| Narciclasine-type | 12.7 | 11.2 | 13.5 | 5.8 | 6.2 | 5.5 | 44.5 | - | 10.4 | 32.2 | ||||
| trisphaeridine (34) | 223 | 223 | 2322.9 | 12.7 | 5.1 | 6.0 | 5.8 | 6.2 | 5.5 | 39.4 | T | 5.2 | 22.7 | |
| dihydrobicolorine (35) | 239 | 238 | 2366.1 | T | 6.1 | 7.5 | T | - | T | 5.1 | T | 5.2 | 9.5 | |
| Other-type | 5.2 | 32.9 | 62.1 | 8.5 | - | 5.9 | - | 5.7 | 17.7 | 34.5 | ||||
| ismine (36) | 257 | 238 | 2304.6 | - | 7.6 | 17.0 | T | - | T | - | T | 6.0 | 14.3 | |
| galanthindole (37) | 281 | 281 | 2534.8 | 5.2 | 25.3 | 45.1 | 8.5 | - | 5.9 | - | 5.7 | 11.7 | 20.2 | |
| Not identified | 46.0 | 26.8 | 46,1 | 42.0 | 126.9 | 35.9 | 78.5 | 20.6 | 73.4 | 21.8 | ||||
| unknown (ismine-derivate) * | 227 | 226 | 2232.2 | - | - | - | - | - | - | 8.8 | - | - | - | |
| unknown (ismine-derivate) * | 227 | 225 | 2232.2 | - | 5.6 | 6.8 | 5.6 | - | - | 8.9 | - | - | 6.2 | |
| unknown | 269 | 238 | 2258.9 | - | 5.1 | 6.9 | - | - | - | - | - | - | 6.4 | |
| unknown (mesembrenone-type) * | 245 | 175 | 2280.8 | - | - | - | - | 6.0 | - | - | 10.5 | - | ||
| unknown | 269 | 268 | 2285.5 | - | - | - | - | - | - | 9.7 | - | - | - | |
| unknown | 253 | 252 | 2313.1 | 5.9 | T | - | - | 5.9 | - | 13.7 | T | - | - | |
| unknown | 251 | 251 | 2335.2 | - | - | - | - | 6.2 | - | 9.9 | - | - | - | |
| unknown (lycorine-type) * | 257 | 256 | 2379.0 | - | - | - | - | - | - | - | - | - | - | |
| unknown | 253 | 252 | 2405.0 | - | - | - | - | 8.9 | 6.2 | 10.3 | - | 5.5 | - | |
| unknown (lycorine-type) * | 269 | 211 | 2480.5 | - | - | - | T | 28.1 | - | 6.1 | T | 7.1 | - | |
| unknown | 271 | 238 | 2492.7 | - | - | - | - | - | - | - | - | - | - | |
| unknown (homolycorine-type) * | 331 | 109 | 2557.5 | - | - | - | - | - | 5.3 | 5.7 | 7.0 | 33.0 | - | |
| unknown (crinine/haemanthamine-type) * | 329 | 329 | 2564.1 | - | - | - | - | - | 5.3 | - | T | 7.3 | - | |
| unknown | 257 | 225 | 2579.2 | - | - | - | T | - | - | 5.4 | - | - | - | |
| unknown | 299 | 238 | 2584.0 | - | - | - | - | - | - | - | - | - | - | |
| unknown (crinine/haemanthamine-type) * | 315 | 254 | 2616.4 | - | - | - | - | 17.5 | 6.0 | - | 5.7 | - | - | |
| unknown (tazettine-type) * | 315 | 231 | 2616.9 | - | - | - | - | - | - | - | - | - | - | |
| unknown (lycorine-type) * | 329 | 268 | 2646.1 | - | - | - | - | 5.1 | - | - | - | - | - | |
| unknown | 297 | 297 | 2655.7 | - | - | - | - | 5.4 | - | - | - | - | - | |
| unknown (tazettine-type) * | 345 | 261 | 2662.6 | 17.9 | 16.1 | 24.6 | 5.6 | - | - | - | - | - | 9.2 | |
| unknown (crinine/haemanthamine-type) * | 345 | 272 | 2671.8 | 22.2 | - | - | - | - | - | - | - | - | - | |
| unknown (crinine/haemanthamine-type) * | 283 | 283 | 2692.5 | - | - | - | - | 6.9 | - | - | - | - | - | |
| unknown | 303 | 302 | 2703.2 | - | - | - | - | - | 7.1 | - | - | - | - | |
| unknown (crinine/haemanthamine-type) * | 315 | 315 | 2724.2 | - | - | - | 16.8 | - | - | - | - | - | ||
| unknown (lycorine-type) * | 253 | 252 | 2735.3 | - | - | - | - | - | - | - | - | - | - | |
| unknown (homolycorine-type) * | 345 | 109 | 2735.6 | - | - | - | - | 5.9 | - | - | - | - | - | |
| unknown (crinine/haemanthamine-type) * | 347 | 331 | 2795.5 | - | - | - | - | 6.5 | - | - | - | - | - | |
| unknown (crinine/haemanthamine-type) * | 345 | 331 | 2795.7 | - | - | - | 5.6 | - | - | - | - | - | - | |
| unknown (crinine/haemanthamine-type) * | 347 | 331 | 2795.8 | - | - | - | - | - | - | - | - | - | - | |
| unknown (lycorine-type) * | 251 | 250 | 2846.6 | - | - | 7.8 | - | - | - | - | - | - | - | |
| unknown | 335 | 335 | 2860.6 | - | - | - | - | - | - | - | - | - | - | |
| unknown (lycorine-type) * | 345 | 242 | 2868.8 | - | - | - | 5.3 | - | - | - | - | - | - | |
| unknown | 373 | 372 | 2881.9 | - | - | - | - | - | - | - | - | - | - | |
| unknown (lycorine-type) * | 357 | 356 | 2942.1 | - | - | - | - | - | - | - | 7.9 | 10.0 | - | |
| unknown (lycorine-type) * | 279 | 278 | 3016.0 | - | - | - | - | 13.7 | - | - | - | - | - | |
| unknown (galanthamine-type) * | 375 | 330 | 3027.5 | - | - | - | - | - | - | - | - | - | - | |
| unknown (lycorine-type) * | 267 | 266 | 3032.9 | - | - | - | - | - | - | - | - | - | - | |
| unknown (lycorine-type) * | 375 | 374 | 3049.2 | - | - | - | - | - | - | - | - | - | - | |
| unknown (lycorine-type) * | 355 | 226 | 3066.6 | - | - | - | 6.1 | - | - | - | - | - | - | |
| unknown (lycorine-type) * | 373 | 226 | 3161.0 | - | - | - | 13.8 | - | - | - | - | - | - | |
| Total | 133.2 | 154.2 | 311.1 | 166.1 | 208.7 | 148.1 | 240.1 | 138.1 | 274.1 | 205.3 | ||||
| (B) | ||||||||||||||
| Alkaloid | M+ | BP | RI | A | C | E | G | I | K | M | O | Q | S | |
| Lycorine-type | 44.1 | 58.2 | 43.2 | 79.0 | 78.6 | 42.6 | 26.1 | 33.8 | 41.2 | 38.5 | ||||
| lycorene (1) | 255 | 254 | 2346.8 | - | - | - | - | 7.6 | - | T | - | - | - | |
| anhydrolycorine (2) | 251 | 250 | 2543.1 | 18.1 | 15.4 | 25.0 | 27.3 | 24.6 | 9.4 | 10.1 | 13.3 | 14.9 | 7.7 | |
| kirkine (3) | 253 | 252 | 2588.2 | 5.0 | 6.0 | - | 5.1 | T | 6.1 | - | - | - | T | |
| 11,12-dehydroanhydrolycorine (4) | 249 | 248 | 2646.4 | 11.0 | 8.6 | 8.1 | 8.5 | 19.0 | 6.6 | 6.4 | 7.3 | 6.6 | 8.5 | |
| 1-O-acetylcaranine (5) | 313 | 226 | 2653.1 | - | - | - | - | 17.4 | - | - | - | - | - | |
| norpluviine (6) | 273 | 228 | 2683.6 | - | - | - | - | - | - | - | - | - | - | |
| assoanine (7) | 267 | 266 | 2708.9 | - | - | - | T | - | - | - | - | - | - | |
| 3,4-dihydro 1-acetyllycorine (8) | 331 | 330 | 2723.3 | - | - | - | - | - | - | T | - | - | T | |
| lycorine (9) | 287 | 226 | 2791.7 | 10.0 | 21.8 | 10.1 | 27.5 | 10.0 | 15.4 | T | 7.4 | 19.7 | 16.3 | |
| dihydrolycorine (10) | 289 | 288 | 2833.9 | - | - | - | - | - | T | 9.6 | T | T | T | |
| pseudolycorine (11) | 289 | 228 | 2856.4 | - | 6.4 | - | 10.6 | - | 5.1 | - | - | - | 6.0 | |
| sternbergine (12) | 331 | 228 | 2838.8 | - | - | - | - | - | - | - | 5.8 | - | - | |
| methylpseudolycorine (13) | 303 | 242 | 2911.2 | - | - | - | T | - | - | - | - | - | - | |
| 1-O-(3′acetoxybutanoyl)lycorine (14) | 415 | 226 | 3248.8 | - | - | - | - | - | - | - | - | - | - | |
| Haemanthamine/crinine type | 60.0 | 49.5 | 31.7 | 43.7 | 17.4 | 28.6 | 33.8 | 45.5 | 44.9 | 36.9 | ||||
| vittatine (15a)/crinine (15b) | 271 | 271 | 2512.4 | - | 10.8 | - | 7.1 | T | 5.6 | 6.2 | 7.3 | 7.3 | 6.3 | |
| 8-O-demethylmaritidine (16) | 273 | 273 | 2540.0 | - | T | - | - | - | - | - | - | - | 6.0 | |
| deacetylcantabricine (17) | 275 | 275 | 2573.1 | - | - | - | - | - | - | - | - | - | - | |
| haemanthamine (18a)/crinamine (18b) | 301 | 272 | 2673.4 | 60.0 | 31.7 | 31.7 | 36.6 | 5.8 | 23.0 | 27.6 | 38.2 | 31.0 | 16.4 | |
| buphanidrine (19) | 315 | 315 | 2748.3 | - | - | - | - | 5.9 | - | - | - | - | - | |
| 11-hydroxyvittatine (20a)/ hamayne (20b) | 287 | 258 | 2750.5 | - | 7.00 | - | T | - | - | - | - | 6.6 | 8.2 | |
| undulatine (21) | 331 | 331 | 2892.5 | - | - | - | - | 5.7 | - | - | - | - | - | |
| Tazettine-type | 74.8 | 56.1 | 85.9 | 33.1 | - | 21.8 | 18.0 | 8.9 | 17.9 | 21.9 | ||||
| deoxytazettine (22) | 315 | 231 | 2575.6 | 8.3 | 10.7 | 13.1 | 9.6 | - | - | - | - | - | T | |
| O-methyltazettine (23) | 345 | 261 | 2641.1 | 58.7 | 23.0 | 61.7 | 14.8 | - | 10.9 | 12.7 | 8.9 | 9.9 | 14.3 | |
| tazettine (24) | 331 | 247 | 2685.1 | 7.8 | 22.4 | 11.1 | 8.7 | - | 10.9 | 5.3 | T | 8.0 | 7.6 | |
| epimacronine (25) | 329 | 245 | 2848.0 | - | T | T | T | - | - | - | - | - | - | |
| Homolycorine-type | 9.4 | - | 10.6 | - | - | 13.9 | 14.1 | 10.1 | 28.5 | 9.8 | ||||
| nerinine (26) | 347 | 109 | 2513.5 | - | - | - | - | - | 13.9 | 14.1 | 10.1 | 21.1 | - | |
| 8-O-demethylhomolycorine (27) | 301 | 109 | 2856.4 | 9.4 | - | 10.6 | - | - | - | - | - | 7.4 | 9.8 | |
| Galanthamine-type | - | 19.0 | 11.9 | 12.3 | - | - | - | - | - | - | ||||
| galanthamine (28) | 287 | 286 | 2519.9 | - | 5.1 | 6.8 | 5.4 | T | - | - | - | - | - | |
| lycoramine (29) | 289 | 288 | 2544.6 | - | 13.9 | 5.1 | 6.9 | - | - | - | - | - | - | |
| Montanine-type | - | - | - | 5.2 | 7.8 | 41.7 | 23.1 | 12.5 | 24.7 | 6.4 | ||||
| pancratinine C (30) | 287 | 176 | 2623.5 | - | - | - | - | T | 5.3 | T | - | - | - | |
| montanine (31) | 301 | 301 | 2663.1 | - | - | - | 5.2 | 7.8 | 29.8 | 23.1 | 12.5 | 24.7 | - | |
| pancracine (32) | 287 | 287 | 2737.4 | - | - | - | - | - | 6.6 | - | - | - | 6.4 | |
| Mesembrenone-type | - | - | - | - | - | 7.2 | 5.2 | - | 5.1 | - | ||||
| demethylmesembrenol (33) | 275 | 205 | 2343.2 | - | - | - | - | - | 7.2 | 5.2 | T | 5.1 | - | |
| Narciclasine-type | 12.9 | 12.7 | 13.3 | 5.8 | 5.7 | - | - | - | - | 5.1 | ||||
| trisphaeridine (34) | 223 | 223 | 2322.9 | 5.5 | 6.3 | 5.3 | T | 5.7 | T | T | T | T | 5.1 | |
| dihydrobicolorine (35) | 239 | 238 | 2366.1 | 7.4 | 6.4 | 8.0 | 5.8 | T | T | T | T | T | T | |
| Other-type | 19.2 | 35.6 | 28.2 | 21.8 | - | 7.9 | 7.8 | 5.3 | 8.0 | 7.3 | ||||
| ismine (36) | 257 | 238 | 2304.6 | 6.6 | 9.9 | 9.5 | 6.6 | - | T | T | T | T | T | |
| galanthindole (37) | 281 | 281 | 2534.8 | 12.6 | 25.7 | 18.7 | 15.2 | - | 7.9 | 7.8 | 5.3 | 8.0 | 7.3 | |
| Not identified | 29.9 | 41.4 | 41.2 | 79.8 | 76.8 | 92.6 | 50.5 | 32.0 | 76.0 | 36.0 | ||||
| unknown (ismine-derivate) * | 227 | 226 | 2232.2 | - | - | - | - | - | - | - | - | - | - | |
| unknown (ismine-derivate) * | 227 | 225 | 2232.2 | - | T | 6.2 | 5.1 | - | - | - | T | - | - | |
| unknown | 269 | 238 | 2258.9 | - | T | - | - | - | - | - | - | - | - | |
| unknown (mesembrenone-type) * | 245 | 175 | 2280.8 | - | - | - | - | - | 15.3 | 11.8 | 6.1 | 11.4 | - | |
| unknown | 269 | 268 | 2285.5 | - | - | - | - | - | - | - | - | - | - | |
| unknown | 253 | 252 | 2313.1 | 5.4 | - | - | - | 6.7 | - | - | 5.1 | - | - | |
| unknown | 251 | 251 | 2335.2 | - | - | - | - | 6.2 | - | - | - | - | - | |
| unknown (lycorine-type) * | 257 | 256 | 2379.0 | - | - | - | 8.0 | - | - | - | - | - | - | |
| unknown | 253 | 252 | 2405.0 | - | 6.4 | - | 5.1 | 6.9 | 8.3 | 8.0 | 6.0 | 5.4 | 5.5 | |
| unknown (lycorine-type) * | 269 | 211 | 2480.5 | - | - | - | T | 34.5 | 5.3 | 5.1 | - | - | - | |
| unknown | 271 | 238 | 2492.7 | 5.7 | - | - | - | - | - | - | - | - | ||
| unknown (homolycorine-type) * | 331 | 109 | 2557.5 | - | - | - | - | - | 19.7 | 18.5 | 8.7 | 46.7 | - | |
| unknown (crinine/haemanthamine-type) * | 329 | 329 | 2564.1 | - | - | - | - | 6.2 | 7.1 | 6.1 | 7.2 | - | ||
| unknown | 257 | 225 | 2579.2 | - | - | - | - | - | - | - | - | - | - | |
| unknown | 299 | 238 | 2584.0 | - | - | T | - | - | - | - | - | - | - | |
| unknown (crinine/haemanthamine-type) * | 315 | 254 | 2616.4 | - | - | - | - | 5.4 | 8.6 | - | - | - | - | |
| unknown (tazettine-type) * | 315 | 231 | 2616.9 | - | - | 5.2 | - | - | - | - | - | - | - | |
| unknown (lycorine-type) * | 329 | 268 | 2646.1 | - | - | - | - | - | 7.9 | - | - | 5.3 | - | |
| unknown | 297 | 297 | 2655.7 | - | - | - | - | - | - | - | - | - | - | |
| unknown (tazettine-type) * | 345 | 261 | 2662.6 | 18.8 | 11.2 | 23.0 | - | - | - | - | - | - | 7.3 | |
| unknown (crinine/haemanthamine-type) * | 345 | 272 | 2671.8 | - | - | - | - | - | - | - | - | - | - | |
| unknown (crinine/haemanthamine-type) * | 283 | 283 | 2692.5 | - | - | - | - | - | - | - | - | - | - | |
| unknown | 303 | 302 | 2703.2 | - | - | - | - | - | 21.3 | - | - | - | - | |
| unknown (crinine/haemanthamine-type) * | 315 | 315 | 2724.2 | - | - | - | - | - | - | - | - | - | - | |
| unknown (lycorine-type) * | 253 | 252 | 2735.3 | - | - | - | 17.1 | - | - | - | - | - | - | |
| unknown (homolycorine-type) * | 345 | 109 | 2735.6 | - | - | - | - | - | - | - | - | - | - | |
| unknown (crinine/haemanthamine-type) * | 347 | 331 | 2795.5 | - | - | - | - | - | - | - | - | - | - | |
| unknown (crinine/haemanthamine-type) * | 345 | 331 | 2795.7 | - | - | - | - | - | - | - | - | - | - | |
| unknown (crinine/haemanthamine-type) * | 347 | 331 | 2795.8 | - | - | - | - | 6.9 | - | - | - | - | - | |
| unknown (lycorine-type) * | 251 | 250 | 2846.6 | - | - | 6.8 | 9.9 | - | - | - | - | - | - | |
| unknown | 335 | 335 | 2860.6 | - | 7.6 | - | - | - | - | - | - | - | - | |
| unknown (lycorine-type) * | 345 | 242 | 2868.8 | - | - | - | - | - | - | - | - | - | - | |
| unknown | 373 | 372 | 2881.9 | - | - | - | - | - | - | - | - | - | 7.8 | |
| unknown (lycorine-type) * | 357 | 356 | 2942.1 | - | - | - | - | - | - | - | - | - | 7.0 | |
| unknown (lycorine-type) * | 279 | 278 | 3016.0 | - | - | - | - | 10.2 | - | - | - | - | - | |
| unknown (galanthamine-type) * | 375 | 330 | 3027.5 | - | 7.0 | - | - | - | - | - | - | - | - | |
| unknown (lycorine-type) * | 267 | 266 | 3032.9 | - | 9.2 | - | 7.3 | - | - | - | - | - | - | |
| unknown (lycorine-type) * | 375 | 374 | 3049.2 | - | - | - | - | - | - | - | - | - | 8.4 | |
| unknown (lycorine-type) * | 355 | 226 | 3066.6 | - | - | - | 5.8 | - | - | - | - | - | - | |
| unknown (lycorine-type) * | 373 | 226 | 3161.0 | - | - | - | 21.5 | - | - | - | - | - | - | |
| Total | 250.3 | 272.5 | 265.9 | 280.4 | 186.0 | 256.3 | 178.6 | 148.1 | 246.3 | 161.9 | ||||
BP: Base Peak; T: Traces; B: R. andicola, SN; D: R. andicola, NC; F: R. andicola, VL; H: R. araucana; J: R. montana; L: R. pratensis, RF, nl, dunes; N: R. pratensis, AP, RF, L; P: R. pratensis, RF, nl; R: R. pratensis, WF; T: R. splendens; A: R. andicola, SN; C: R. andicola, NC; E: R. andicola, VL; G: R. araucana; I: R. montana; K: R. pratensis, RF, nl, dunes; M: R. pratensis, RF, L; O: R. pratensis, RF, nl; Q: R. pratensis, WF; S: R. splendens; - : not detected; * proposed structure-type according to the fragmentation pattern.
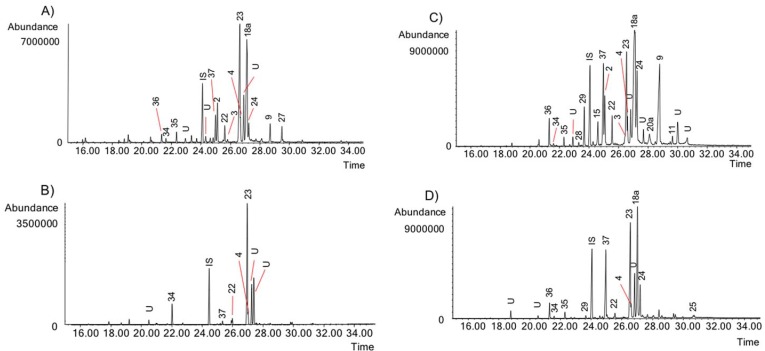
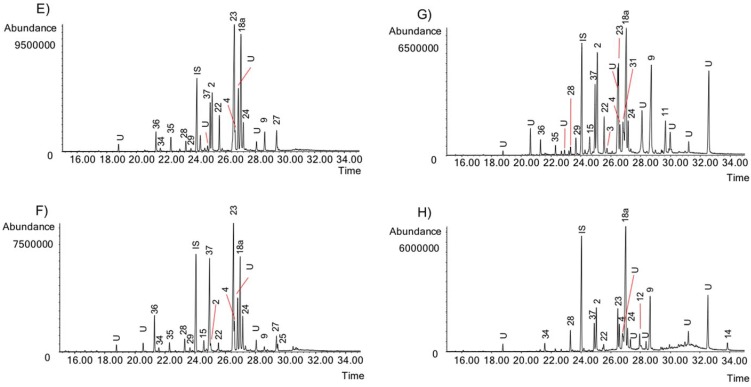
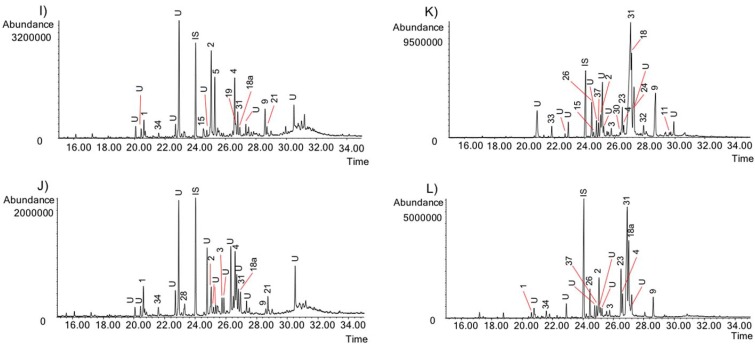
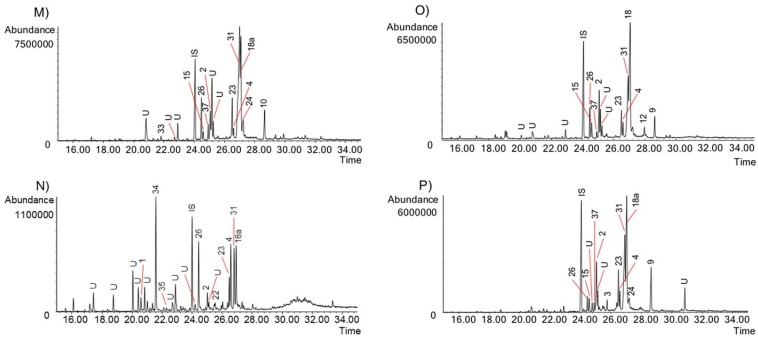
Figure 5.
GC chromatograms of the alkaloids from Chilean Rhodophiala species. Numbers refer to Table 2. (A) Bulbs of R. andicola (Sierra Nevada); (B) Aerial parts of R. andicola (Sierra Nevada); (C) Bulbs of R. andicola (Nevado de Chillán); (D) Aerial parts of R. andicola (Nevado de Chillán). IS: internal standard; U: unknown.
Figure 6.
GC chromatograms of the alkaloids from Chilean Rhodophiala species. Numbers refer to Table 2. (E) Bulbs of R. andicola (Volcán Lonquimay); (F) Aerial parts of R. andicola (Volcán Lonquimay); (G) Bulbs of R. araucana; (H) Aerial parts of R. araucana. IS: internal standard; U: unknown.
Figure 7.
GC chromatograms of the alkaloids from Chilean Rhodophiala species. Numbers refer to Table 2. (I) Bulbs of R. montana; (J) Aerial parts of R. montana; (K) Bulbs of R. pratensis (red flowers, without leaves, growing on sand dunes); (L) Aerial parts of R. pratensis (red flowers, without leaves, growing on sand dunes). IS: internal standard; U: unknown.
Figure 8.
GC chromatograms of the alkaloids from Chilean Rhodophiala species. Numbers refer to Table 2. (M) Bulbs of R. pratensis (red flowers, with leaves); (N) Aerial parts of R. pratensis (red flowers, with leaves); (O) Bulbs of R. pratensis (red flowers, without leaves); (P) Aerial parts of R. pratensis (red flowers, without leaves). IS: internal standard; U: unknown.
Figure 9.
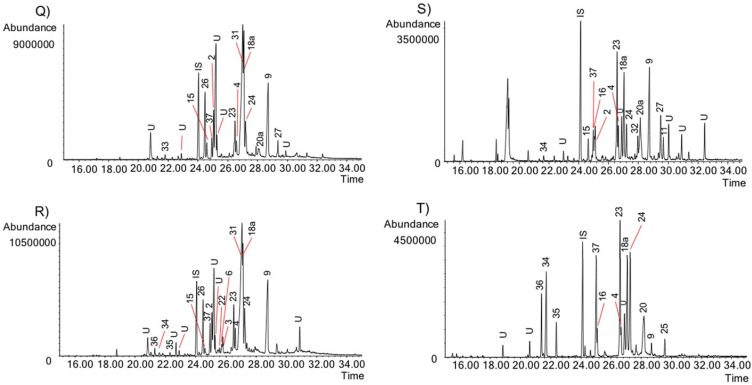
GC chromatograms of the alkaloids from Chilean Rhodophiala species. Numbers refer to Table 2. (Q) Bulbs of R. pratensis (white flowers); (R) Aerial parts of R. pratensis (white flowers); (S) Bulbs of R. splendens; (T) Aerial parts of R. splendens. IS: internal standard; U: unknown.
The highest alkaloid concentration was detected in the aerial parts of R. andicola (F) and in the aerial parts of R. pratensis (R) (311.1 and 274.1 mg GAL/g AE, respectively). Lowest content was found in the aerial parts of R. andicola (B) and in the aerial parts of R. pratensis (P) (133.2 and 138.1 mg GAL/g AE, respectively). In 70% of the samples, lycorine-, haemanthamine/crinine- and tazettine-type alkaloids were predominant. Lycorine-type alkaloids were present in all species with higher content in R. araucana (G) and R. montana bulbs (I) (79.0 and 78.6 mg GAL/g AE, respectively) and lowest values in the aerial parts of R. andicola (B) and (D) (8.6 and 7.5 mg GAL/g AE, respectively).
Haemanthamine/crinine-alkaloids occur in all samples except the aerial parts of R. andicola (B). However, the higher content was found in the bulbs of R. andicola from the same collection place (A) and in the aerial parts of the plant collected at Volcan Lonquimay (F). Compounds from the tazettine-type were not detected in the aerial parts and bulbs of R. montana (I and J). From the different Amaryllidaceae alkaloids groups, tazettine-type alkaloids were the main compounds in several samples, occurring in highest concentration in R. andicola (E, F) with values of 85.9 and 95.8 mg GAL/g AE of tazettine-type alkaloids in bulbs and aerial parts, respectively.
Galanthamine-type alkaloids were detected in low quantities in three species, namely R. andicola, R. araucana and R. montana (samples C, D, E, F, G, H and J) ranging between 5.1 to 19.0 mg GAL/g AE. Montanine-type alkaloids were present in all species, except R. andicola. The highest level of montanine-type compounds was detected in R. pratensis (K) (41.7 mg GAL/g AE), which presented three different alkaloids: pancratinine C, montanine and pancracine (5.3, 29.8 and 6.6 mg GAL/g AE, respectively). Mesembrenone-type was the least representative alkaloid-type. It was represented by demethylmesembrenol, detected in low quantities in three different samples of R. pratensis (K, M and Q) (7.2, 5.2 and 5.1 mg GAL/g AE, respectively). Narciclasine-type occurs in most samples in a range between 5.1 mg GAL/g AE in bulbs of R. splendens (S) to 44.5 and 32.2 mg GAL/g AE in aerial parts of R. pratensis with red flowers and leaves (N) and aerial parts of R. splendens (T), respectively. All species investigated presented ismine and/or galanthindole alkaloids, except R. montana. Forty structures occurring in the extracts could not be identified using the available databases. Three of the unidentified compounds were highly representative among the samples.
The compound with m/z 252 [M+ = 253] (RI 2405.0) occurs in 60% of the samples. The m/z 109 with [M+ = 331] (RI 2557.5), which probably belongs to the homolycorine-type alkaloids, was detected in 40% of the samples and in high amounts in bulbs of R. pratensis with white flowers (46.7 mg GAL/g AE). Finally, m/z 261 with [M+ = 345] (RI 2662.6) was detected in 45% of the samples and in high quantity in aerial parts of R. andicola collected at Volcan Lonquimay (24.6 mg GAL/g AE).
The highest content of non-identified alkaloids was detected in the aerial parts of R. montana (J) and in the bulbs of R. pratensis (red flowers and without leaves) collected in the sand dunes at the sea shore (K) (126.9 and 92.6 mg GAL/g AE, respectively). The lowest content was detected in aerial parts of R. pratensis with red flowers and without leaves (P) and in aerial parts of R. splendens (T) (20.6 and 21.8 mg GAL/g AE, respectively).
2.3. Molecular Docking
In this study, R. splendens was the most active inhibitor of AChE and BuChE. Alkaloid analysis by GC-MS allowed the identification of 17 compounds in the leaf extract of R. splendens (T) including two unidentified constituents (Table 2). The 15 alkaloids identified in the extract were evaluated for their theoretical AChE and BuChE inhibitory potential by molecular docking (Table 3 and Table 4). As expected, no alkaloid identified in sample T presented better theoretical AChE inhibitory activity than galanthamine. Molecular simulation of six alkaloids identified in sample T on the 4BDS structure theoretically showed higher enzymatic inhibition against BuChE than galanthamine by 0.80 kcal/mol.
Table 3.
AChE and BuChE inhibitory activities of some alkaloids identified in the aerial parts of R. splendens (T) and the reference compound galanthamine. Values are expressed as IC50 (μg/mL).
| Alkaloid | AChE | BuChE |
|---|---|---|
| 11-hydroxyvittatine (20a) | 122.17 ± 22.03 | >200 |
| lycorine (9) | 101.70 ± 23.79 | >200 |
| 8-O-demethylmaritidine (16) | 113.21 ± 8.21 | 127.87 ± 2.45 |
| hamayne (20b) | 135.09 ± 15.33 | 48.40 ± 1.13 |
| deacetylcantabricine (17) | >200 | >200 |
| haemanthamine (18a) | 184.68 ± 11.58 | >200 |
| galanthamine (28) | 0.48 ± 0.07 | 3.70 ± 0.24 |
Table 4.
Estimated free energy binding of molecular docking between alkaloids identified in aerial parts of R. splendens and cholinesterases (AChE and BuChE). Values are expressed in kcal/mol.
| Alkaloid | AChE *a | BuChE *b |
|---|---|---|
| 11-hydroxyvittatine (20a) | −8.43 | −9.03 |
| lycorine (9) | −8.82 *c | −8.94 |
| 8-O-demethylmaritidine (16) | −8.74 *d | −8.93 |
| hamayne (20b) | −8.28 | −8.54 |
| deacetylcantabricine (17) | −7.90 | −8.43 |
| haemanthamine (18a) | −8.80 | −8.34 |
| galanthamine (28) | −9.55 *c | −8.23 *c |
| epimacronine (25) | −9.36 *c | −7.63 |
| tazettine (24) | −8.66 *c | −7.87 |
| O-methyltazettine (23) | −8.54 | −7.87 |
| 11,12-dehydroanhydrolycorine (4) | −8.41 | −7.44 |
| galanthindole (37) | −7.81 | −7.41 |
| trisphaeridine (34) | −7.38 | −7.27 |
| dihydrobicolorine (35) | −7.33 | −7.38 |
| ismine (36) | −6.78 | −7.08 |
*a PBD code: 1DX6; *b PBD code: 4BDS; *c Cortes et al., 2015; *d Cortes et al., 2017.
To gain further insight into the molecular docking results, an experiment was carried out to check the AChE and BuChE inhibitory activities of 11-hydroxyvittatine (20), lycorine (9), 8-O-demethylmaritidine (16), hamayne (20b), deacetylcantabricine (17) and haemanthamine (18a) (Table 3). The best AChE and BuChE inhibitory activities were obtained for lycorine (9) (IC50 101.70 ± 23.79 μg/mL) and hamayne (20b) (IC50 48.40 ± 1.13 μg/mL), respectively. However, their theoretical BuChE inhibition was not supported by the experimental assays. The difference in origin of the BuChE structure used in the molecular docking (human) and experimental assays (equine serum), as well as the inability of these compounds to arrive at the BuChE active site of the enzyme could help to explain the difference between theoretical and practical results.
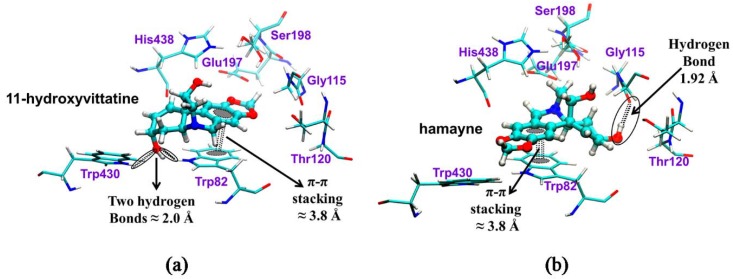
Two important regions in the active sites of the hBuChE enzymes have been located: the first corresponding to the catalytic triad composed by the residues His438, Ser198, and Glu325 [25], while the second corresponds to a choline binding site (α-anionic site), composed principally by the residues Trp82 and Phe329 [25]. A graphical representation of molecular binding of 11-hydroxyvittatine (20a) and hamayne (20b) alkaloids with the hBuChE protein is presented in Figure 10. The alkaloid 11-hydroxyvittatine (20a) shows two strong interactions, hydrogen bonds, with the residues Trp82 and Trp430; however, this molecule does not present any interactions close to the catalytic triad His438, Ser198, and Glu325. On the other hand, hamayne (20b) shows one hydrogen bond interaction with the residue Gly115, an amino acid located close to the catalytic triad His438, Ser198, and Glu325. In the case of the interactions at the choline binding site (α-anionic site), both alkaloids show the same π–π stacking interaction with the residue Trp82. These molecular interactions suggest that the β-orientation of the hydroxyl group at C-3 in 11-hydroxyvittatine (20a) could theoretically increase the BuChE inhibition on the 4BDS structure by 0.49 kcal/mol, compared to the α-orientation of the hydroxyl group at the C-3 position in hamayne (20b). However, in the experimental assays, hamayne (20b) showed BuChE inhibitory activity (48.40 ± 1.13 μg/mL). It can be hypothesized that the β-orientation of the hydroxyl group at C-3 in 11-hydroxyvittatine (20a) probably makes it difficult for the compound to arrive at the catalytic triad in the active site of the BuChE.
Figure 10.
Graphical representations of the binding of (a) 11-hydroxivittatine (20a) and (b) hamayne (20b) in the gorge of the active site of hBuChE.
Studies on alkaloid composition associated with cholinesterase inhibition and binding-mode prediction have been reported [26,27]. A work on Argentinean Amaryllidaceae [24] reported the composition and acetylcholinesterase inhibition of four wild growing species, including Rhodophiala mendocina. Two R. mendocina samples collected in different locations presented similar activity towards AChE with IC50 values of 2.0 μg/mL but with relevant differences in the qualitative and quantitative alkaloid composition. The sample from the Provincia de San Juan showed high content of haemanthamine/crinamine (31.2%), tazettine (32.9%) and lycorine (13.3%) while the plant collected in the Provincia de Neuquen presented 6.8% haemanthamine/crinamine and 20.4% of lycorine, respectively. Galanthamine was found in both samples with 0.6 and 0.8% for the San Juan and Neuquen plants, respectively. In a report from acetylcholinesterase inhibitory alkaloids from Brazilian Amaryllidaceae [23] the bulbs of Rhodophiala bifida (Herb.) Traub were investigated. The activity on AChE was moderate with an IC50 value of 8.45 μg/mL, being lower than that from R. mendocina [24]. The alkaloid extract of R. bifida bulbs contained high amounts of montanine (91.94%). The alkaloid composition of the Chilean Rhodophiala ananuca (formerly: Hippeastrum ananuca) was described [28,29]. The bulbs contained phenantridine alkaloids, including hippeastidine and epi-homolycorine.
The alkaloid montanine isolated from R. bifida showed activity towards a panel of eight human cancer cell lines. According to [30], montanine at 2.5 μg/mL was more active than doxorubicine on the multi-drug resistant breast cell line NCLADR. Montanine also showed antimicrobial effect with MIC of 5 μg/mL against S. aureus ATCC 6538 and E. coli ATCC 24922 and 20 μg/mL against P. aeruginosa ATCC 27853, respectively [31]. In a screening towards Trichomonas vaginalis, dichloromethane and n-butanol extracts from Brazilian Hippeastrum species and Rhodophiala bifida showed activity against the protozoa [32]. The most active fractions contained the alkaloids lycorine and lycosinine.
In a study on the alkaloids of Zephyranthes robusta (Amaryllidaceae), the compounds isolated were evaluated as inhibitors of human cholinesterases [33]. The authors used human erythrocye AChE and serum BuChE. The compounds were tested in a range of 0.5–500 μg/mL and the inhibition was reported as IC50 values in μMolar concentration. While the activity of the reference compound galanthamine was similar in both studies, 11-hydroxyvitattine, lycorine and haemanthamine were not active on the human AChE and BuChE. 8-O-demethylmaritidine and hamayne were inactive on human BuChE but presented activity on erythrocyte AChE. The differences in the results can be explained by the biological source of the enzymes (human cholinesterases for [33] and electric eel AChE and horse (equine serum) BuChE in this work. For a better comparison of results, the use of enzymes from the same biological source should be recommended.
In summary, the AChE and BuChE inhibitory activity of the Chilean Rhodophiala species investigated led to an interesting source of inhibitors that do not contain the alkaloid galanthamine. Our results suggest that Chilean Rhodophiala could be a promising source of new alkaloids with effect towards cholinesterases. The difficulty in finding a species with high activity against AChE and BuChE, the similarity of the AChE and BuChE inhibitory values, the low complexity of the alkaloid profile of aerial parts of R. splendens, together with the absence of galanthamine-type alkaloids in this sample, prompted us to further explore the results.
3. Materials and Methods
3.1. Plant Material
The samples were collected in central-southern Chile and were identified following the reference [19,34]. Rhodophiala andicola (Poepp.) Traub, was collected at Sierra Nevada (Región de la Araucanía, 27 January 2016), the slopes of Volcán Lonquimay (Región de la Araucanía, Provincia del Malleco, 19 December 2016) and the slopes of Nevado de Chillán (Región del Bio-Bio, 30 December 2016). Rhodophiala araucana (Phil.) Traub was collected at Malalcahuello, Región de la Araucanía (19 December 2016), and Rhodophiala montana (Phil.) Traub at the roadside to Laguna del Maule, Región del Maule (2 January 2017). Samples from Rhodophiala pratensis (Poepp.) Traub were collected at Arcos de Calán, Región del Maule (12 December 2016) including plants growing on sand dunes and grasslands close to the sea. Plants with red and white flowers were collected separately. According to [34], the plant with red flowers fits the description of R. pratensis. Rhodophiala splendens (Renjifo) Traub was collected at Las Trancas, Región del Bio-Bio (2 January 2016). The plants were identified by Dr. Patricio Peñailillo, Herbario de la Universidad de Talca. Voucher herbarium specimens have been deposited at the Universidad de Talca as follows: R. andicola (N° 4081); R. araucana (N° 4083); R. montana (N° 4080); R. pratensis (N° 4084); R. pratensis (white flower) (N° 8085); R. splendens (N° 4082). A map with the collection places is shown in Figure 2. Pictures of the species investigated are illustrated in Figure 3.
3.2. Extraction
The freshly collected plant material was cleaned and separated into bulbs and aerial parts, frozen and lyophilized before extraction. The dry weight percentage was determined. The lyophilized plant material was extracted with MeOH under sonication for 10 min (3×) changing the solvent each time. The plant to solvent ratio ranged from 1:10 to 1:60 and was selected according to the volume of plant material for extraction. The combined MeOH solubles were taken to dryness under reduced pressure to afford the crude extracts. The crude extracts were then acidified to pH 3 with diluted H2SO4 (2%, v/v) and the neutral material was removed with Et2O. The aqueous solutions were basified up to pH 9–10 with NH4OH (25%, v/v) and extracted with EtOAc to provide the alkaloid extracts which were used for all experiments (enzyme inhibition assays and chemical analysis by GC-MS).
3.3. Acetylcholinesterase (AChE) and Butyrylcholinesterase (BuChE) Inhibitory Activity
Cholinesterase inhibitory activities were determined according to [35] with some modifications [36]. Stock solutions with 518U of AchE from Electrophorus electricus (Merck, Darmstadt, Germany) and BuChE from equine serum (Merck, Darmstadt, Germany), respectively, were prepared and kept at −20 °C. Acetylthiocholine iodide (ATCI), S-butyrylthiocholine iodide (BTCI) and 5,5′-dithiobis (2-nitrobenzoic acid) (DTNB) were obtained from Merck (Darmstadt, Germany). Fifty microliters of AChE or BuChE (both enzymes used at 6.24 U) in phosphate buffer (8 mM K2HPO4, 2.3 mM NaH2PO4, 0.15 NaCl, pH 7.5) and 50 μL of the sample dissolved in the same buffer were added to the wells. The plates were incubated for 30 min at room temperature. Then, 100 μL of the substrate solution (0.1 M Na2HPO4, 0.5 M DTNB, and 0.6 mM ATCI or 0.24 mM BTCI in Millipore water, pH 7.5) was added. After 10 min, the absorbance was read at 405 nm in a Labsystem microplate reader (Thermo Fischer, Waltham, MA, USA). Enzyme activity was calculated as percent compared to a control using buffer without any inhibitor. Galanthamine served as positive control. In a first step, samples were assessed at 10, 100 and 200 μg/mL towards both enzymes. Samples with an IC50 > 200 μg/mL were considered inactive. Samples with an IC50 < 200 μg/mL were further analyzed to determine the IC50 values. The cholinesterase inhibitory data were analyzed with the software Microsoft Office Excel 2010 (Microsoft, Redmond, WA, USA).
3.4. Alkaloids Identification and Quantification
3.4.1. Equipment
The equipment used for the identification and quantification of the alkaloids was a GC-MS 6890N apparatus (Agilent Technologies, Santa Clara, CA, USA) coupled with MSD5975 inert XL operating in the electron ionization (EI) mode at 70 eV. A Sapiens-X5 MS column (30 m × 0.25 mm i.d., film thickness 0.25 μm) was used. The temperature gradient was as follows: 12 min at 100 °C, 100–180 °C at 15 °C/min, 180–300 °C at 5 °C/min and 10 min hold at 300 °C. The injector and detector temperatures were 250 and 280 °C, respectively, and the flow-rate of carrier gas (He) was 1 mL/min. Two mg of each alkaloid extract was dissolved in 1 mL of MeOH:CHCl3 (1:1, v/v) and 1 μL was injected using the splitless mode. Codeine (0.05 mg/mL) was used as an internal standard in all the samples.
3.4.2. Alkaloids Identification
Amaryllidaceae alkaloids occurring in the samples were identified by comparison of the Rt, fragmentation patterns and data interpretation of the spectra. The database used was built using single alkaloids previously isolated and identified by spectroscopic and spectrometric methods (NMR, UV, CD, IR, MS) in the Natural Products Laboratory, Universidad de Barcelona, the NIST 05 Database and literature data [37,38,39,40,41,42].
3.4.3. Alkaloid Quantification
To quantify the single constituents, a calibration curve of galanthamine (10, 20, 40, 60, 80 and 100 μg/mL) was used. The same amount of codeine (0.05 mg/mL) was added to each solution sample as an internal standard. The peak areas were manually obtained considering selected ions for each compound (usually the base peak of their MS, i.e., m/z at 286 for galanthamine, at 299 for codeine). The ratio between the values obtained for galanthamine and codeine in each solution was plotted against the corresponding concentration of galanthamine to obtain the calibration curve and its equation (y = 0.0224x − 0.2037; R2 = 0.9977). All data were standardized to the area of the internal standard (codeine) and the equation obtained for the calibration curve of galanthamine was used to calculate the amount of each alkaloid. Results are expressed as mg GAL, which was finally related to the alkaloid extract weight. As the peak area does not only depend on the corresponding alkaloid concentration but also on the intensity of the mass spectra fragmentation, the quantification is not absolute. However, the methodology is considered suitable to compare the specific alkaloid amount between samples [39,42].
3.5. Molecular Docking
The molecular docking simulations for the alkaloids identified in Rhodophiala splendens, the most promising species found in this study, were performed to investigate the binding mode into the active site of two different enzymes, namely Torpedo californica AChE (TcAChE) and hBuChE: proteins with PDB codes 1DX6 [43] and 4BDS [44], respectively. The 3D-structures of the alkaloids were drawn using the Chemcraft program [45], and then submitted to a geometrical optimization procedure at PBE0 [46]/6-311+g* [47] level of theory using the Gaussian 09 program [48]. All optimized alkaloids were confirmed as a minimum on the potential energy surface. The docking simulations for the set of optimized ligands were performed using the AutoDock v.4.2 program [49]. AutoDock combines a rapid energy evaluation through pre-calculated grids of affinity potentials with a variety of search algorithms to find suitable binding positions for a ligand on a given macromolecule. To compare the results from the docking simulations, the water molecules, cofactors, and ions were excluded from each X-ray crystallographic structure. Likewise, the polar hydrogen atoms of the enzymes were added, and the non-polar hydrogen atoms were merged. Finally, the enzyme was treated as a rigid body. The grid maps of interaction energy for various atom types with each macromolecule were calculated by the auxiliary program AutoGrid, choosing a grid box with dimensions of 70 × 70 × 70 Å around the active site, which was sufficiently large to include the most important residues of each enzyme. The docking searches for the best orientations of the ligands binding to the active site of each protein were performed using the Lamarckian Genetic Algorithm (LGA) [50]. The LGA protocol applied a population size of 2000 individuals, while 2,500,000 energy evaluations were used for the 50 LGA runs. The best conformations were chosen from the lowest docked energy solutions in the cluster populated by the highest number of conformations. The best docking complex solutions (poses) were analyzed according to the potential intermolecular interactions (ligand/enzyme), such as hydrogen bonding and the cation–π, π–π stacking.
Acknowledgments
L.R.T. is thankful to CAPES (Coordenação de Pessoal de Nível Superior-Bolsista CAPES, Processo 13553135) for a doctoral fellowship.
Author Contributions
G.S.-H. and J.B. conceived and designed the experiments; L.R.T., N.C., E.H.O. and C.T. performed the experiments; L.R.T., J.B. and G.S.-H. analyzed the data; C.T. and G.S.-H. collected and processed the samples; G.S.-H., J.B., L.R.T. and E.H.O. wrote the paper.
Funding
This research was funded by PIEI-QUIM-BIO, Universidad de Talca, CCiTUB and Programa CYTED (416RT0511). L.R.T. and J.B. are members of the Research Group 2017-SGR-604 at the University of Barcelona.
Conflicts of Interest
The authors declare no conflict of interest. The founding sponsors had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, and in the decision to publish the results.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Newman D.J., Cragg G.M. Natural products as sources of new drugs from 1981 to 2014. J. Nat. Prod. 2016;79:629–661. doi: 10.1021/acs.jnatprod.5b01055. [DOI] [PubMed] [Google Scholar]
- 2.Lu J.-J., Bao J.-L., Chen X.-P., Huang M., Wang Y.-T. Alkaloids isolated from natural herbs as the anticancer agents. Evid.-Based Complement. Altern. Med. 2012;2012:485042. doi: 10.1155/2012/485042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Feher M., Schmidt J.M. Property Distributions: Differences between drugs, natural products, and molecules from combinatorial chemistry. J. Chem. Inf. Comput. Sci. 2003;43:218–227. doi: 10.1021/ci0200467. [DOI] [PubMed] [Google Scholar]
- 4.Bastida J., Lavilla R., Viladomat F. Chemical and biological aspects of Narcissus alkaloids. In: Cordell G.A., editor. The Alkaloids: Chemistry and Physiology. Volume 63. Elsevier; Amsterdam, The Netherlands: 2006. pp. 87–179. eBook ISBN 9780080466552; Hardcover ISBN 9780124695634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bastida J., Berkov S., Torras L., Pigni N.B., de Andrade J.P., Martínez V., Codina C., Viladomat F. Chemical and biological aspects of Amaryllidaceae alkaloids. In: Muñoz-Torrero D., editor. Recent Advances in Pharmaceutical Sciences. Transworld Research Network; Kerala, India: 2011. pp. 65–100. [Google Scholar]
- 6.Ingrassia L., Lefranc F., Mathieu V., Darro F., Kiss R. Amaryllidaceae isocarbostyril alkaloids and their derivatives as promising antitumor agents. Transl. Oncol. 2008;1:1–13. doi: 10.1593/tlo.08100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Van Goiestsenoven G., Mathieu V., Lefranc F., Kornienko A., Evidente A., Kiss R. Narciclasine as well as other Amaryllidaceae isocarnostyrils are promising GTP-ase targeting agents against brain cancers. Med. Res. Rev. 2013;33:439–455. doi: 10.1002/med.21253. [DOI] [PubMed] [Google Scholar]
- 8.Heinrich M., Teoh H.L. Galanthamine from showdrop—The development of a modern drug against Alzheimer’s disease from local Caucasian knowledge. J. Ethnopharmacol. 2004;9:147–162. doi: 10.1016/j.jep.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 9.Maelicke A., Samochocki M., Jostock R., Fehrenbacher A., Ludwig J., Albuquerque E.X., Zerlin M. Allosteric sensitization of nicotinic receptors by galanthamine, a new treatment strategy for Alzheimer’s disease. Biol. Psychiatry. 2001;49:279–288. doi: 10.1016/S0006-3223(00)01109-4. [DOI] [PubMed] [Google Scholar]
- 10.Craig L.A., Hong N.S., McDonald R.J. Revisiting the cholinergic hypothesis in the development of Alzheimer’s disease. Neurosci. Biobehav. Rev. 2011;35:1397–1409. doi: 10.1016/j.neubiorev.2011.03.001. [DOI] [PubMed] [Google Scholar]
- 11.Querfurth H.W., LaFerla F.M. Alzheimer’s disease. N. Engl. J. Med. 2010;362:329–344. doi: 10.1056/NEJMra0909142. [DOI] [PubMed] [Google Scholar]
- 12.Alzheimer’s Disease International. [(accessed on 13 October 2017)]; Available online: https://www.alz.co.uk/research/WorldAlzheimerReport2016.pdf.
- 13.Selkoe D.J. Alzheimer’s disease: Genes, proteins, and therapy. Physiol. Rev. 2001;81:741–766. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- 14.Basiri A., Murugaiyah V., Osman H., Kumar R.S., Kia Y., Awang K.B., Ali M.A. An expedient, ionic liquid mediated multi-component synthesis of novel piperidone grafted cholinesterase enzymes inhibitors and their molecular modeling study. Eur. J. Med. Chem. 2013;67:221–229. doi: 10.1016/j.ejmech.2013.06.054. [DOI] [PubMed] [Google Scholar]
- 15.Greig N.H., Lahiri D.K., Sambamurti K. Butyrylcholinesterase: An important new target in Alzheimer’s disease therapy. Int. Psychogeriatr. 2002;14:77–91. doi: 10.1017/S1041610203008676. [DOI] [PubMed] [Google Scholar]
- 16.Giacobini E. Cholinesterase inhibitors: New roles and therapeutic alternatives. Pharmacol. Res. 2004;50:433–440. doi: 10.1016/j.phrs.2003.11.017. [DOI] [PubMed] [Google Scholar]
- 17.Ballard C.G. Advances in the treatment of Alzheimer’s disease: Benefits of dual cholinesterase inhibition. Eur. Neurol. 2002;47:64–70. doi: 10.1159/000047952. [DOI] [PubMed] [Google Scholar]
- 18.Olate E., Bridgen M. Techniques for the in vitro propagation of Rhodophiala and Leucocoryne spp. Acta Hortic. 2005;673:335–342. doi: 10.17660/ActaHortic.2005.673.42. [DOI] [Google Scholar]
- 19.Schiappacasse F., Peñailillo P., Yáñez P. Propagación de Bulbosas Chilenas Ornamentals. Editorial Universidad de Talca; Talca, Chile: 2002. [Google Scholar]
- 20.Muñoz M., Riegel R., Seemann P., Penailillo P., Schiappacasse F., Nunez J. Phylogenetic relationships of Rhodolirium montanum Phil. and related species based on nucleotide sequences from ITS region and karyotype analysis. Gayana Bot. 2011;68:40–48. doi: 10.4067/S0717-66432011000100005. [DOI] [Google Scholar]
- 21.Schiappacasse F., Peñailillo P., Basoalto A., Seemann P., Riegel R., Muñoz M., Jara G., Durán C. Biotechnological applications on plant breeding of Chilean Rhodophiala species: Morphological and physiological studies. Agro Sur. 2007;35:65–67. doi: 10.4206/agrosur.2007.v35n2-31. [DOI] [Google Scholar]
- 22.Baeza C., Almendras F., Ruiz E., Peñailillo P. Comparative karyotype studies in species of Miltinea Ravenna, Phycella Lindl. and Rhodophiala C. Presl (Amaryllidaceae) from Chile. Rev. Fac. Cienc. Agrar. 2012;44:197–209. ISSN printed 0370-4661, ISSN online 1853-8665. [Google Scholar]
- 23.De Andrade J.P., Giordani R.B., Torras-Claveria L., Pigni N.B., Berkov S., Font-Bardia M., Calvet T., Konrath E., Bueno K., Sachett L.G., et al. The Brazilian Amaryllidaceae as a source of acetylcholinesterase inhibitory alkaloids. Phytochem. Rev. 2016;15:147–160. doi: 10.1007/s11101-015-9411-7. [DOI] [Google Scholar]
- 24.Ortiz J.E., Berkov S., Pigni N.B., Theoduloz C., Roitman G., Tapia A., Bastida J., Feresin G.E. Wild Argentinian Amaryllidaceae, a new renewable source of the acetylcholinesterase inhibitor galanthamine and other alkaloids. Molecules. 2012;17:13473–13482. doi: 10.3390/molecules171113473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nicolet Y., Lockridge O., Masson P., Fontecilla-Camps J.C., Nachon F. Crystal structure of human butyrylcholinesterase and of its complexes with substrate and products. J. Biol. Chem. 2003;278:41141–41147. doi: 10.1074/jbc.M210241200. [DOI] [PubMed] [Google Scholar]
- 26.Cortes N., Alvarez R., Osorio E.H., Alzate F., Berkov S., Osorio E. Alkaloid metabolite profiles by GC/MS and acetylcholinesterase inhibitory activities with binding-mode predictions of five Amaryllidaceae plants. J. Pharmaceut. Biomed. Anal. 2015;102:222–228. doi: 10.1016/j.jpba.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 27.Cortes N., Sierra K., Alzate F., Osorio E.H., Osorio E. Alkaloids of Amaryllidaceae as inhibitors of cholinesterases (AChEs and BuChEs): An integrated bioguided study. Phytochem. Anal. 2017;29:217–227. doi: 10.1002/pca.2736. [DOI] [PubMed] [Google Scholar]
- 28.Pacheco P., Silva M., Sammes P.G., Watson W.H. Estudio químico de las Amaryllidaceae chilenas. Nuevos alcaloides de Hippeastrum ananuca. Bol. Soc. Chil. Quim. 1982;27:289. [Google Scholar]
- 29.Pacheco P., Silva M., Steglich W., Watson W.H. Alkaloids of Chilean Amaryllidaceae I hippeastidine and epi-homolycorine two novel alkaloids. Rev. Latinoam. Quim. 1978;9:28–32. [Google Scholar]
- 30.Castilhos T.S., Giordani R., Dutilh J., Bastida J., de Carvalho J.E., Henriques A.T., Zuanazzi J.A.S. Chemical and biological investigation of the alkaloids from Rhodophiala bífida (Herb.) Traub (Amaryllidaceae); Proceedings of the 24° Reuniao Annual da Sociedade Brasileira de Química; Pocos de Caldas, Brazil. 28–31 May 2001. [Google Scholar]
- 31.Castilhos T.S., Giordani R., Henriques A.T., Menezes F.S., Zuanazzi J.A.S. Availacao in vitro das atividades antiinflamatoria, antioxidante e antimicrobiana do alcaloide montanina. Rev. Bras. Pharmacogn. 2007;17:209–214. doi: 10.1590/S0102-695X2007000200013. [DOI] [Google Scholar]
- 32.Vieira P., Giordani R., de Carli G., Tasca T., Zuanazzi J. Screening and bioguided fractionation of Amaryllidaceae species with activity against Trichomonas vaginalis. Planta Med. 2010;76:470. doi: 10.1055/s-0030-1264768. [DOI] [PubMed] [Google Scholar]
- 33.Kulhánková A., Cahlíková L., Novák Z., Macáková K., Kuneš J., Opletal L.P. Alkaloids from Zephyranthes robusta Baker and their acetylcholinesterase- and butyrylcholinesterase-inhibitory activity. Chem. Biodivers. 2013;10:1120–1127. doi: 10.1002/cbdv.201200144. [DOI] [PubMed] [Google Scholar]
- 34.Flora de Chile en su Habitat. [(accessed on 16 June 2018)]; Available online: www.floradechile.cl/monocoty/family/amaryllis.htm.
- 35.Ellman G.L., Courtney K.D., Andres V., Jr., Featherstone R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961;7:88–95. doi: 10.1016/0006-2952(61)90145-9. [DOI] [PubMed] [Google Scholar]
- 36.López S., Bastida J., Viladomat F., Codina C. Acetylcholinesterase inhibitory activity of some Amaryllidaceae alkaloids and Narcissus extracts. Life Sci. 2002;71:2521–2529. doi: 10.1016/S0024-3205(02)02034-9. [DOI] [PubMed] [Google Scholar]
- 37.De Andrade J.P., Pigni N.B., Torras-Claveria L., Berkov S., Codina C., Viladomat F., Bastida J. Bioactive alkaloid extracts from Narcissus broussonetii: Mass spectral studies. J. Pharmaceut. Biomed. 2012;70:13–25. doi: 10.1016/j.jpba.2012.05.009. [DOI] [PubMed] [Google Scholar]
- 38.De Andrade J.P., Guo Y., Font-Bardia M., Calvet T., Dutilh J., Viladomat F., Codina C., Nair J.J., Zuanazzi J.A.S., Bastida J. Crinine-type alkaloids from Hippeastrum aulicum and H. calyptratum. Phytochemistry. 2014;103:188–195. doi: 10.1016/j.phytochem.2014.03.007. [DOI] [PubMed] [Google Scholar]
- 39.Torras-Claveria L., Berkov S., Codina C., Viladomat F., Bastida J. Metabolomic analysis of bioactive Amaryllidaceae alkaloids of ornamental varieties of Narcissus by GC-MS combined with k-means cluster analysis. Ind. Crop. Prod. 2014;56:211–222. doi: 10.1016/j.indcrop.2014.03.008. [DOI] [Google Scholar]
- 40.Tallini L.R., de Andrade J.P., Kaiser M., Viladomat F., Nair J.J., Zuanazzi J.A.S., Bastida J. Alkaloid constituents of the Amaryllidaceae plant Amaryllis belladonna L. Molecules. 2017;22:1437. doi: 10.3390/molecules22091437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tallini L.R., Osorio E.H., dos Santos V.D., Borges W.d.S., Kaiser M., Viladomat F., Zuanazzi J.A.S., Bastida J. Hippeastrum reticulatum (Amaryllidaceae): Alkaloids profiling, biological activities and molecular docking. Molecules. 2017;22:2191. doi: 10.3390/molecules22122191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Guo Y., Pigni N.B., Zheng Y., de Andrade J.P., Torras-Claveria L., Borges W.S., Viladomat F., Codina C., Bastida J. Analysis of bioactive Amaryllidaceae alkaloid profiles in Lycoris species by GC-MS. Nat. Prod. Commun. 2014;9:1081–1086. [PubMed] [Google Scholar]
- 43.Greenblatt H.M., Kryger G., Lewis T., Silman I., Sussman J.L. Structure of acetylcholinesterase complexed with (−)-galanthamine at 2.3 Å resolution. FEBS Lett. 1999;463:321–326. doi: 10.1016/S0014-5793(99)01637-3. [DOI] [PubMed] [Google Scholar]
- 44.Nachon F., Carletti E., Ronco C., Trovaslet M., Nicolet Y., Jean L., Renard P.-Y. Crystal structures of human cholinesterases in complex with huprine W and tacrine: Elements of specificity for anti-Alzheimer’s drugs targeting acetyl- and butyryl-cholinesterase. Biochem. J. 2013;453:393–399. doi: 10.1042/BJ20130013. [DOI] [PubMed] [Google Scholar]
- 45.ChemCraft. [(accessed on 9 October 2017)]; Available online: http://www.chemcraftprog.com/citation.html.
- 46.Adamo C., Barone V. Toward reliable density functional methods without adjustable parameters: The PBE0 model. J. Chem. Phys. 1999;110:6158–6170. doi: 10.1063/1.478522. [DOI] [Google Scholar]
- 47.Petersson G.A., Bennett A., Tensfeldt T.G., Al-Laham M.A., Shirley W.A., Mantzaris J., Mantzaris J. A complete basis set model chemistry. I. The total energies of closed-shell atoms and hydrides of the first-row elements. J. Chem. Phys. 1988;89:2193–2218. doi: 10.1063/1.455064. [DOI] [Google Scholar]
- 48.Frisch M.J., Trucks G.W., Schlegel H.B., Scuseria G.E., Robb M.A., Cheeseman J.R., Scalmani G., Barone V., Mennucci B., Petersson G.A., et al. Gaussian 09, Revis. E.01. Gaussian, Inc.; Wallingford, CT, USA: 2013. [Google Scholar]
- 49.Moris G.M., Huey R., Lindstrom W., Sanner M.F., Belew R.K., Goodsell D.S., Olson A.J. Autodock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009;16:2785–2791. doi: 10.1002/jcc.21256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Thomsen R., Christensen M.H. MolDock: A new technique for high-accuracy molecular docking. J. Med. Chem. 2006;49:3315–3321. doi: 10.1021/jm051197e. [DOI] [PubMed] [Google Scholar]