Abstract
Fatty acids play a major role in determining membrane biophysical properties. Staphylococcus aureus produces branched-chain fatty acids (BCFAs) and straight-chain saturated fatty acids (SCSFAs), and can directly incorporate exogenous SCSFAs and straight-chain unsaturated fatty acids (SCUFAs). Many S. aureus strains produce the triterpenoid pigment staphyloxanthin, and the balance of BCFAs, SCSFAs and staphyloxanthin determines membrane fluidity. Here, we investigated the relationship of fatty acid and carotenoid production in S. aureus using a pigmented strain (Pig1), its carotenoid-deficient mutant (Pig1ΔcrtM) and the naturally non-pigmented Staphylococcus argenteus that lacks carotenoid biosynthesis genes and is closely related to S. aureus. Fatty acid compositions in all strains were similar under a given culture condition indicating that staphyloxanthin does not influence fatty acid composition. Strain Pig1 had decreased membrane fluidity as measured by fluorescence anisotropy compared to the other strains under all conditions indicating that staphyloxanthin helps maintain membrane rigidity. We could find no evidence for correlation of expression of crtM and fatty acid biosynthesis genes. Supplementation of medium with glucose increased SCSFA production and decreased BCFA and staphyloxanthin production, whereas acetate-supplementation also decreased BCFAs but increased staphyloxanthin production. We believe that staphyloxanthin levels are influenced more through metabolic regulation than responding to fatty acids incorporated into the membrane.
Keywords: membrane fatty acids composition, Staphylococcus aureus, staphyloxanthin, membrane fluidity, metabolic regulation
1. Introduction
It is generally accepted that the fatty acid composition of membrane phospholipids have a major impact on determining membrane biophysical and, thereby, physiological properties [1]. Staphylococcus aureus is a Gram-positive bacterial pathogen that shows a significant growth environment-dependent plasticity in its membrane fatty acid composition [2]. The fatty acids of S. aureus are generally considered to be a mixture of saturated branched-chain fatty acids (BCFAs) and straight-chain fatty acids (SCSFAs) that are synthesized by fatty acid biosynthesis system II (FAS II). Some bacteriological media such as Mueller-Hinton broth (MHB) promote a high proportion of BCFAs, whereas others such as Tryptic Soy Broth (TSB) result in decreased BCFAs and increased SCSFAs. BCFAs fluidize the membrane and SCSFAs have the opposite effect [1,3,4,5]. The majority of BCFAs are anteiso- having a methyl group at the antepenultimate position while iso-fatty acids have a methyl group at the penultimate position. S. aureus lacks a fatty acid desaturase enzyme that converts saturated fatty acids into unsaturated products [2,6], and, hence, BCFAs play a critical role in maintaining membrane fluidity. However, when cells are grown ex vivo in serum straight-chain unsaturated fatty acids (SCUFAs) become a significant fraction of the total fatty acid composition [2], due to direct incorporation of exogenous preformed SCUFAs from serum into the phospholipid biosynthesis pathway [6]. SCUFAs are known to significantly increase membrane fluidity [1].
The membrane composition and properties of S. aureus are further complicated by the unique production of the triterpene staphyloxanthin carotenoid responsible for the typical golden color of S. aureus colonies. Staphyloxanthin is thought to be important in the life of S. aureus decreasing membrane fluidity [2,7,8,9,10,11,12,13], influencing membrane permeability [8], susceptibility to oxidative stress and neutrophil killing [14,15,16]. Thus staphyloxanthin biosynthesis has been explored as a potential drug target [16]. Fatty acid biosynthesis is one of the highly energy demanding biosynthetic pathways and acetyl-CoA is the common precursor for both fatty acid and staphyloxanthin synthesis [17]. Recently, we found a correlation between a high content of the membrane fluidizing fatty acids (BCFAs and SCUFAs) and an increase in staphyloxanthin content, possibly to counterbalance potential membrane hyperfluidity conferred by BCFAs or SCUFAs [2]. Similarly, a S. aureus mutant in brnQ1 that was defective in transport of BCFA precursor amino acids had higher anteiso-fatty acids and increased pigmentation [18]. Here, we were interested in probing further the relationship between carotenoid and fatty acid production and membrane biophysical properties in pigmented (Pig1) and non-pigmented (Pig1ΔcrtM and MSHR1132) staphylococcal strains. S. aureus Pig1ΔcrtM is deleted in the crtM gene that encodes a key enzyme in staphyloxanthin production [14]. Strain MSHR1132 is a close relative of S. aureus that naturally lacks the carotenoid biosynthesis operon (crtOPQMN) that is known as Staphylococcus argenteus (the silver Staphylococcus, [19,20].
The level of staphyloxanthin production varies between strains and under different environmental conditions and the role of the pigment in the life of S. aureus remains somewhat enigmatic. The alternative sigma factor SigB is a positive regulator of expression of the crtMNOPQ operon [21], and direct or indirect effects on the expression and activity of SigB regulate pigment production in the bacterium. A cold-shock protein (CspA) [22] and an aeration sensing response regulator (AirR) [23] have been shown to positively affect SigB activity and increase pigmentation. Mutations and/or altered activities of some regulators (such as SarA, Agr, argR) and ClpP protease may also affect SigB expression altering pigmentation [17,24]. Also, a transfer-messenger antisense RNA has been reported that negatively regulates the crtMN transcript [25]. Additionally, a carbohydrate catabolite regulator (CcpE) has been shown to regulate pigment production without affecting crtM transcription [26,27]. On the other hand, addition of fatty acids or mevalonate in the growth medium [28], blocking polyprenyl synthetase [24] and inactivation of the tricarboxylic acid (TCA) cycle [17] have been reported to increase pigmentation. A S. aureus pyruvate dehydrogenase mutant defective in conversion of pyruvate to acetyl CoA had decreased staphyloxanthin production, whereas a branched-chain α-keto acid dehydrogenase mutant had decrease production of C4 and C5 iso and anteiso CoA precursors of BCFAs showed increased pigmentation [29].
The membrane lipid composition of S. aureus is complex due to the variable production and incorporation of BCFAs, SCSFAs and SCUFAs, as well as being overlain with the variable production of staphyloxanthin. In earlier work, we observed an association between increased content of membrane fluidizing BCFAs and SCUFAs and increased content of membrane rigidifying staphyloxanthin. In this work we probed this potential relationship further through studies of fatty acid composition, staphyloxanthin content, and membrane fluidity in a carotenoid-deficient S. aureus mutant and a naturally occurring staphylococcal species closely related to S. aureus that had lost the crt carotenoid biosynthesis operon. We found no evidence that carotenoid biosynthesis responded to membrane fatty acid composition. Carotenoid deficiency led to membranes with increased fluidity. However, medium carbon source influenced membrane fatty acid composition and staphyloxanthin content, with evidence that excess acetyl CoA led to increased staphyloxanthin production.
2. Results
2.1. The Fatty Acid Composition of S. aureus Pig1 Varies with the Growth Medium
To understand variations in fatty acid composition in a pigmented S. aureus strain grown in various media, we analyzed the fatty acid composition of TSB-, MHB- and serum-grown S. aureus Pig1 by fatty acid methyl-ester analysis. We observed nearly half each of SCSFAs (51%) and BCFAs (49%) in log phase cells grown in TSB (Table 1). However, MHB-grown cells had 81% BCFAs and the serum-grown cells had a significant proportion of SCUFAs (41%) (Table 1). Stationary phase cells grown in TSB and MHB had increased proportions of BCFAs (76% and 94% respectively) with a decrease in SCSFAs. However, cells grown in serum at stationary phase had similar proportion of the fatty acid types found in the log phase cells. Cells grown in serum at log phase had 30% oleic acid (18:1Δ9) and 8% gondoic acid (20:1Δ11) as major SCUFAs. Notably, stationary phase cells grown in serum had 12% linoleic acid (18:2Δ9, 12) besides oleic acid (17%), and gondoic acid (5%) as major SCUFAs (Supplementary Materials Table S2). In summary, fatty acid composition varied considerably with growth medium and growth phase.
Table 1.
Fatty acid composition (%) 1 of strain Pig1 grown in various media.
| Fatty Acids | Log 2 | Stationary 3 | ||||
|---|---|---|---|---|---|---|
| TSB | MHB | Serum | TSB | MHB | Serum | |
| SCSFAs | 50.7 ± 0.28 | 19.0 ± 0.12 | 43.9 ± 0.25 | 24.1 ± 0.11 | 5.7 ± 0.03 | 44.0 ± 0.39 |
| BCFAs | 49.2 ± 0.20 | 80.9 ± 0.22 | 14.8 ± 0.18 | 75.9 ± 0.11 | 94.4 ± 0.04 | 19.9 ± 0.02 |
| SCUFAs | nd | nd | 41.4 ± 0.42 | nd | nd | 38.1 ± 0.40 |
1 Standard error of the mean (SEM) in the % of each fatty acid was determined from two independent fatty acid analysis experiments; 2 OD600 ~0.8; 3 12 h. nd, not detected.
2.2. The Carotenoid Content of Pig1 Varies with the Growth Medium
To understand the variations in cellular pigmentation in different growth media, staphyloxanthin content in Pig1 cells grown in TSB, MHB and serum was determined by the spectrophotometric method. Staphyloxanthin content was expressed as OD465 per mg dry wt. of cell pellets. Compared to staphyloxanthin content (0.017 ± 0.001) in the TSB-grown cells at log phase, the MHB and serum grown cells had higher pigmentation (0.051 ± 0.003 and 0.036 ± 0.003 respectively) (Table 2). However, cells grown to stationary phase in TSB had a higher staphyloxanthin content than those grown in other media. TSB- and MHB-grown cells were most pigmented after 12–24 h of growth whereas the serum-grown cells had similar pigmentation over the entire growth period (Table 2). In summary, pigmentation varied considerably with growth media and over the growth phases in a given medium, and, in general, the cells having a higher proportion of BCFAs had a higher staphyloxanthin content.
Table 2.
Carotenoid content 1 (OD465/mg of dry cell mass) of Pig1 grown in various media.
| Medium | Log 2 | 12 h | 24 h | 48 h |
|---|---|---|---|---|
| TSB | 0.017 ± 0.0006 | 0.127 ± 0.0121 | 0.132 ± 0.0081 | 0.110 ± 0.0075 |
| MHB | 0.051 ± 0.0029 | 0.086 ± 0.0029 | 0.063 ± 0.0012 | 0.041 ± 0.0040 |
| Serum | 0.036 ± 0.0029 | 0.039 ± 0.0052 | 0.038 ± 0.0023 | 0.047 ± 0.0058 |
1 Standard error of the mean (SEM) in the carotenoid content was determined from at least three independent experiments; 2 OD600 ~0.8.
2.3. The Carotenoid-Deficient Strains Had Essentially Similar Proportions of Fatty Acids to the Pigmented Strains
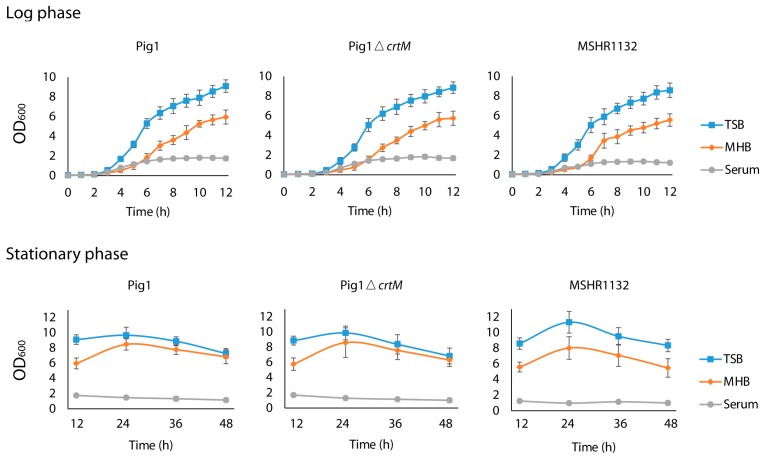
The lack of pigmentation had no effect in the overall growth patterns of S. aureus strains grown in various media (Figure 1). To understand whether the lack of pigmentation affects the fatty acid composition in S. aureus the fatty acid composition was determined in the TSB-, MHB and serum-grown carotenoid-deficient mutant (Pig1ΔcrtM) and S. argenteus. The proportion of fatty acids of Pig1ΔcrtM grown in different media at both log and stationary phases was very similar to those observed in the parent strain (Table 1 and Table 3). Strain MSHR1132 also had a similar fatty acid composition compared to the other two strains (Table 1, Table 3 and Table 4); one difference was the presence of linoleic acid (C18:2Δ9, 12) when grown in serum at both log (7%) and stationary (10%) phases whereas in the other serum-grown strains this fatty acid was present only at the stationary phase (Pig1, 12%; Pig1ΔcrtM, 11%) (Supplementary Materials Table S2). Collectively, the overall similarities of the fatty acid composition in the S. aureus strains were unaffected by the loss of ability to produce staphyloxanthin.
Figure 1.
Staphyloxanthin production does not affect growth patterns of pigmented and non-pigmented S. aureus grown in various media. The strains were grown in TSB, MHB and serum and optical density (OD600) was measured at intervals. Error bars indicate standard deviation on the mean OD600 values determined from three independent growth experiments for each strain.
Table 3.
Fatty acid composition (%) 1 of a carotenoid-deficient strain Pig1ΔcrtM grown in various media.
| Fatty Acids | Log 2 | Stationary 3 | ||||
|---|---|---|---|---|---|---|
| TSB | MHB | Serum | TSB | MHB | Serum | |
| SCSFAs | 49.9 ± 0.06 | 19.6 ± 0.53 | 45.2 ± 0.07 | 24.5 ± 0.25 | 6.0 ± 0.03 | 45.6 ± 0.04 |
| BCFAs | 49.9 ± 0.13 | 80.4 ± 0.53 | 15.5 ± 0.33 | 75.5 ± 0.25 | 94.0 ± 0.02 | 18.1 ± 0.08 |
| SCUFAs | nd | nd | 39.3 ± 0.26 | nd | nd | 36.4 ± 0.01 |
1 Standard error of the mean (SEM) in the % of each fatty acid was determined from two independent fatty acid analysis experiments; 2 OD600 ~0.8; 3 12 h. nd, not detected.
Table 4.
Fatty acid composition (%) 1 of a naturally carotenoid-deficient strain MSHR1132 (S. argenteus) grown in various media.
| Fatty Acids | Log 2 | Stationary 3 | ||||
|---|---|---|---|---|---|---|
| TSB | MHB | Serum | TSB | MHB | Serum | |
| SCSFAs | 41.1 ± 0.86 | 17.5 ± 1.44 | 49.2 ± 0.19 | 20.9 ± 0.08 | 6.9 ± 0.04 | 49.8 ± 0.04 |
| BCFAs | 58.5 ± 1.05 | 82.2 ± 1.41 | 14.7 ± 0.04 | 79.1 ± 0.08 | 93.1 ± 0.03 | 12.9 ± 0.04 |
| SCUFAs | nd | nd | 36.2 ± 0.22 | nd | nd | 37.3 ± 0.01 |
1 Standard error of the mean (SEM) in the % of each fatty acid was determined from two independent fatty acid analysis experiments; 2 OD600 ~0.8; 3 12 h. nd, not detected.
2.4. Alteration in Fatty Acid Composition Significantly Affects Membrane Fluidity in Staphyloxanthin-Lacking S. aureus
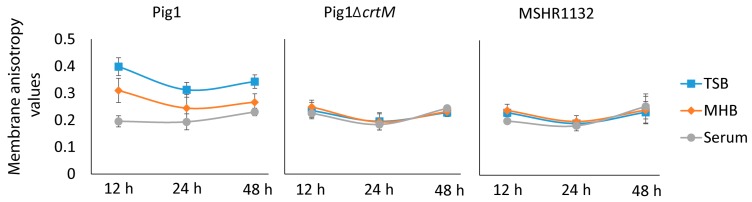
To understand how the loss of staphyloxanthin affects membrane biophysical properties in S. aureus we determined membrane anisotropies of strains Pig1, Pig1ΔcrtM and MHSR1132 grown in TSB, MHB and serum. A membrane with lower anisotropy value indicates higher fluidity. As shown in Table 5, the Pig1 cells grown in TSB and MHB at log phase had similar anisotropy values (0.315 ± 0.025 and 0.318 ± 0.035 respectively) while the cells grown in serum had significantly more fluid membranes (0.212 ± 0.017). The MHB-grown Pig1 cells had higher staphyloxanthin content as well as a higher proportion of membrane fluidizing BCFAs. On the other hand, the carotenoid lacking mutant (Pig1ΔcrtM) had significantly higher membrane fluidity (TSB, 0.260 ± 0.033; MHB, 0.202 ± 0.032; serum, 0.159 ± 0.007) compared to its similarly grown pigmented parent strain. The MHB-grown carotenoid-deficient cells had significantly higher membrane fluidity than those grown in TSB. Additionally, the naturally carotenoid-lacking strain MSHR1132 (S. argenteus) had significantly higher membrane fluidities (TSB, 0.254 ± 0.024; MHB, 0.239 ± 0.010; serum, 0.135 ± 0.016) compared to the Pig1 strain. On the other hand, the Pig1 strain grown in TSB at stationary phase had higher staphyloxanthin content (see Table 2) and, thus, had correspondingly higher membrane rigidity (lower fluidity) than it did when grown in other media (Figure 2). The carotenoid-lacking strains grown in all media had highly fluid membranes (Figure 2). These observations also support the idea that staphyloxanthin content corresponds to increased membrane rigidity, and this relationship prevails over the entire growth period. In summary, staphyloxanthins play a major role in maintenance of membrane viscosity in S. aureus and alteration of fatty acid composition significantly affects membrane fluidity in the non-pigmenting strains.
Table 5.
Membrane anisotropy values 1 of S. aureus strains grown to log phase 2 in various media.
| Strains | TSB | MHB | Serum |
|---|---|---|---|
| Pig1 | 0.315 ± 0.025 | 0.318 ± 0.035 | 0.212 ± 0.017 |
| Pig1ΔcrtM | 0.260 ± 0.033 | 0.202 ± 0.032 | 0.159 ± 0.007 |
| S. argenteus | 0.254 ± 0.024 | 0.239 ± 0.010 | 0.135 ± 0.016 |
1 Standard error of the mean (SEM) in the anisotropy values was determined from at least three independent experiments. Statistical significance (Student’s paired t-test, two tail) values (p): Pig1TSBvs.MHB 0.387; Pig1TSBvs.Serum 0.0001; Pig1ΔcrtMTSBvs.MHB 0.003; Pig1ΔcrtMTSBvs.Serum 0.017; S. argenteusTSBvs.MHB 0.189; S. argenteusTSBvs.Serum 0.041; TSBPig1vs.Pig1ΔcrtM 0.023; TSBPig1vs.S.argenteus 0.023; 2 OD600 ~0.8.
Figure 2.
Staphyloxanthin in the pigmented S. aureus helps cells maintain membrane fluidity over stationary phase too. Membrane anisotropy values of the stationary phase cells were determined using a membrane intercalating fluorescent dye (DPH) as described for the log phase cells. Note that anisotropy values do not have unit. Error bars indicate standard deviation from mean anisotropy values obtained from at least three independent experiments.
2.5. Expression of the Genes for Staphyloxanthin and Fatty Acid Biosynthesis Do Not Appear to Be Co-Regulated
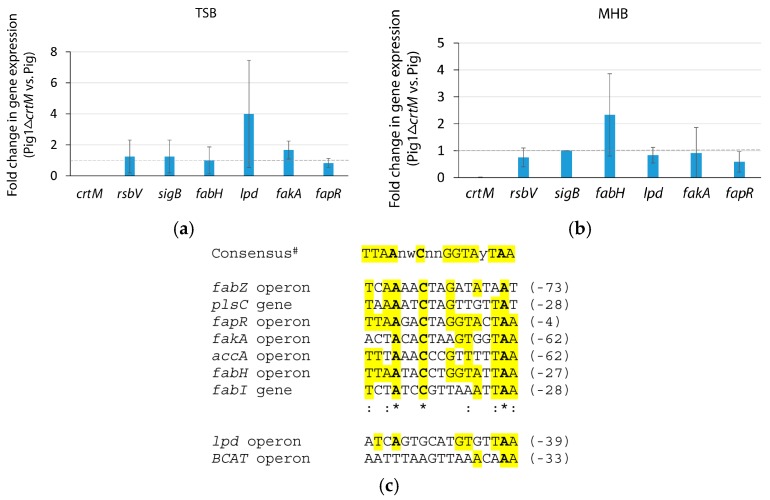
To understand whether there was cellular co-regulation of fatty acid biosynthesis and staphyloxanthin in S. aureus the level of expression of the genes encoding key enzymes involved in the biosynthesis and regulation of both pathways were analyzed by qRT-PCR assays. As expected, the transcript of the crtM gene in strain Pig1ΔcrtM was barely detected (Figure 3A,B). Strain Pig1ΔcrtM had a nearly similar level of expression of rsbV (positive regulator of sigB), sigB (regulator of crtOPQMN operon), fabH (initiation of fatty acid biosynthesis), fakA (fatty acid uptake) and fapR (negative regulator of fatty acid biosynthesis) genes when grown in TSB compared to that of its parent strain (Pig1) (Figure 3A). On the other hand, rsbV, sigB and fakA were similarly expressed while fapR was slightly downregulated and fabH was slightly upregulated in the MHB-grown mutant (Figure 3B). The relative differences in the fapR and fabH levels may indicate the MHB-grown mutant has slightly increased activity in fatty acid biosynthesis. However, in totality, the similar level of expression of most of the key genes for fatty acid and staphyloxanthin biosynthesis and their regulation in the pigmented and non-pigmented strains indicate that the pathways appear to be independently regulated. Notably, the expression of lpd (BCFA biosynthesis) in the TSB-grown mutant strain was markedly elevated (Figure 3A) while in MHB it was relatively unchanged (Figure 3B). In silico study of S. aureus NCTC8325 revealed that the operator sequence of the lpd-operon and BCAT gene (initial recruitment of branched-chain amino acids for BCFA biosynthesis) are unlikely be regulated by FapR (Figure 3C). Since the proportion of BCFAs in the staphyloxanthin-lacking mutant is similar to that of the pigmenting parent strain grown in a given medium the reason for the relative differences of the expression of lpd gene in TSB-grown strains is not clear.
Figure 3.
Transcription of fatty acid biosynthesis genes is typically unaffected by the loss of staphyloxanthin production in S. aureus. (a) Fold change in gene expression in Pig1ΔcrtM compared to that in Pig1 grown in TSB; (b) Fold change in gene expression in Pig1ΔcrtM compared to that in Pig1 grown in MHB; (c) FapR recognition sequence (operator sequence) in the gene or operon involved in fatty acid biosynthesis and regulation in S. aureus NCTC 8325. The transcriptional level of each gene was normalized to that of 16SrRNA. The dashed line in (a,b) indicates similar level of gene expression in the Pig1 pair strains. fabZ operon, SAOUHSC_02337–02336; plsC, SAOUHSC_01837; fapR operon, SAOUHSC_01196–01199; fakA operon, SAOUHSC_01192–01193; accA operon, SAOUHSC_01809–01808; fabH operon, SAOUHSC_00920–00921; fabI, SAOUHSC_00947; lpd operon, SAOUHSC_01617–SAOUHSC_01615–01611; BCAT, SAOUHSC_00536–00537. The number in parentheses after each operator sequence in (c) indicates the last nucleotide in the given sequence. # [30].
2.6. Glucose and Acetate Affect Staphyloxanthin Production in S. aureus
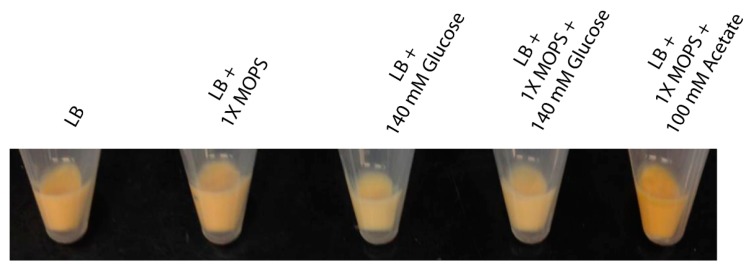
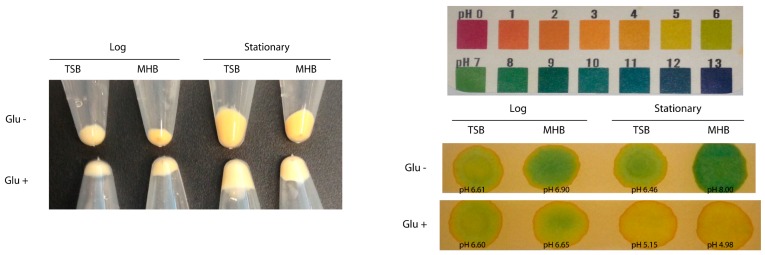
TSB but not MHB contains free glucose, hence, the availability of glucose in the growth media might play a role in alteration of pigmentation. To investigate how glucose affects staphyloxanthin production in S. aureus, the change in pigmentation in strain Pig1 was studied by growing it in LB broth pH 7.2 (that lacks carbohydrates) supplemented with 140 mM glucose. Since glucose catabolism yields acetate [31], acetate (100 mM) containing LB broth was also included in this study. To avoid alteration of pH by acetate, acetate was supplemented in LB containing MOPS buffer (pH 7.2). Compared to the staphyloxanthin content in the plain LB-grown cells (log, 0.033 ± 0.007; stationary, 0.060 ± 0.007) a significantly reduced pigmentation was observed in LB broths to which glucose had been supplemented (0.021 ± 0.007 and 0.033 ± 0.004 respectively) (Table 6). Note that MOPS buffer had no effect on pigmentation. Exponentially growing cells in acetate-supplemented LB broth had a slightly higher staphyloxanthin content (0.038 ± 0.005) than in plain LB. A significantly higher pigmentation was observed in cells grown at stationary phase in acetate-containing LB (0.071 ± 0.004) (Table 6). The cell pellets obtained from stationary phase LB cultures had a visible difference in the depth of yellow color (Figure 4). In summary, the pigmentation in S. aureus decreases with the presence of glucose and increases with presence of acetate in the growth media.
Table 6.
Staphyloxanthin content 1 (OD465/mg of dry cell mass) in Pig1 grown in various LB broths.
| Growth Conditions | Log 2 | Stationary 3 |
|---|---|---|
| LB | 0.03276 ± 0.0074 | 0.06030 ± 0.0072 |
| LB-MOPS | 0.03120 ± 0.0066 | 0.05814 ± 0.0073 |
| LB-Glucose | 0.02101 ± 0.0068 | 0.03297 ± 0.0042 |
| LB-MOPS-Glucose | 0.02040 ± 0.0061 | 0.03457 ± 0.0039 |
| LB-MOPS-Acetate | 0.03755 ± 0.0050 | 0.07133 ± 0.0040 |
1 Standard error of the mean (SEM) in the carotenoid content was determined from at least three independent experiments. Statistical significance (Student’s paired t-test, two tail) values (p): LogLBvs.LB-MOPS 0.083; LogLBvs.LB-Glucose 0.001; LogLBvs.LB-MOPS-Glucose 0.001; LogLB-Glucosevs.LB-MOPS-Glucose 0.455; LogLBvs.LB-MOPS-Acetate 0.109; StationaryLBvs.LB-MOPS 0.221; StationaryLBvs.LB-Glucose 0.0007; StationaryLBvs.LB-MOPS-Glucose 0.001; StationaryLB-Glucosevs.LB-MOPS-Glucose 0.146; StationaryLBvs.LB-MOPS-Acetate 0.019; 2 OD600 ~0.8. 3 12 h.
Figure 4.
Visible differences in carotenoid content in the Pig1 cells grown in various LB broths. Strain Pig1 was grown for 12 h in LB broths with or without glucose or acetate. The cell pellets were collected and photographed.
2.7. Availability of Glucose and Acetate in the Growth Medium Lowers BCFAs and Increases SCSFAs in S. aureus
To understand how a carbon source affects fatty acid composition in a pigmented S. aureus strain strain Pig1 was grown in LB or LB supplemented with glucose or acetate and fatty acid composition was determined. Fatty acid analysis was done for the stationary phase cells that had significant differences in pigment production (see Table 6). As shown in Table 7, the LB-grown cells had 91% BCFAs and 9% SCSFAs. Addition of glucose in LB decreased the proportion of BCFAs to 63% and increased SCSFAs to 37%. Addition of acetate in the medium similarly decreased BCFA contents (63%) and increased SCSFAs (27%). These observations indicate that the carbon sources might be the major factors in determining the proportion of BCFAs and SCSFAs in S. aureus.
Table 7.
Fatty acid composition (%) 1 in S. aureus Pig1 grown at stationary phase 2 in glucose or acetate containing LB broths.
| Fatty Acids | LB | LB-MOPS-Glucose | LB-MOPS-Acetate |
|---|---|---|---|
| SCSFA | 9.2 ± 0.30 | 38.0 ± 1.00 | 27.1 ± 0.04 |
| BCFA | 90.8 ± 0.28 | 61.9 ± 0.95 | 62.8 ± 0.15 |
| Unknown | nd | nd | 10.2 ± 0.11 |
1 Standard error of the mean (SEM) in the % of each fatty acid was determined from two independent fatty acid analysis experiments; 2 Fatty acid analysis was done for the stationary phase (12 h grown) cells that had significant differences in pigment production (see Table 6). nd, not detected.
2.8. Glucose and Acetate also Affect Pigmentation in Other S. aureus Strains
To determine whether pigmentation in other S. aureus strains was affected by glucose or acetate, methicillin-susceptible (MSSA) strains (SH1000, laboratory derived MSSA; Newman, clinical isolate MSSA) and methicillin-resistant (MRSA) strains (COL, homogeneously resistant MRSA; MW2, heterogeneously resistant MRSA) were included in this study. The strains were grown in LB broth without or with glucose or acetate until stationary phase (12 h). Similar to the effects on pigmentation in strain Pig1, the cell pellets for all the strains (viz., SH1000, Newman, COL and MW2) obtained from the glucose-supplemented cultures had decreased pigmentation while those from acetate-supplemented cultures had increased pigmentation (Figure 5). This observation indicates that the effect of glucose and acetate on pigmentation extends to other pigmented S. aureus strains.
Figure 5.
Pigmentation among various S. aureus strains grown in glucose or acetate added medium. Strains SH1000, Newman, COL and MW2 were grown for 12 h in LB broths without or with glucose or acetate. Cell pellets were collected and photographed.
2.9. Acetate Consumption Determines the Extent of Pigment Production in S. aureus
S. aureus containing higher proportions of BCFAs also had higher levels of pigmentation in general (see Table 1 and Table 2). However, the cells having higher staphyloxanthin content did not always have a higher proportion of BCFAs (see Table 6 and Table 7 for LB-MOPS-Acetate grown cells; and Table 1 and Table 2 for 12 h-grown cells in TSB and MHB). These data suggest that staphyloxanthin and BCFAs levels are not always related and that glucose catabolism could be important in determining the relative amount of BCFAs and staphyloxanthin in the cell membrane. Exponentially-growing S. aureus consumes glucose converting it into acetyl-CoA that is secreted as acetate into the growth medium [31]. With the depletion of glucose during stationary phase the cells consume acetate from the growth medium, converting it into acetyl-CoA that feeds into the TCA cycle [31,32]. To demonstrate whether a change in pigmentation in S. aureus was due to consumption of acetate, acetate concentration in the culture supernatants in glucose-supplemented or plain TSB and MHB broths were determined. As shown in Table 8, S. aureus grown in glucose-supplemented MHB had higher acetate concentration in the supernatants collected from both log (5.17 ± 0.98 mM) and stationary (27.07 ± 1.63 mM) phase cells than those in plain MHB (3.09 ± 0.64 mM and 7.33 ± 1.02 mM respectively). Further, the cells grown in the glucose-supplemented MHB had correspondingly lower staphyloxanthin production (log, 0.035 ± 0.001; stationary, 0.031 ± 0.005) than cells in unsupplemented MHB (log, 0.051 ± 0.003; stationary, 0.086 ± 0.003) (Table 8). Similar relationships of lower acetate consumption and concomitantly lower pigmentation were observed in cells grown in glucose-supplemented TSB (Table 8). Similarly, the effect of glucose on acetate consumption was observed in the TSB-grown cells. Since TSB contains free glucose (14 mM), there was no obvious difference in acetate consumption and pigmentation in the glucose-supplemented broth during log phase growth (OD600 ~0.8). However, the effects of glucose-supplementation (140 mM) on acetate consumption and pigmentation were pronounced in the cells grown at stationary phase (OD600 ~8.0). The pigmentation in the cell pellets obtained from various growth conditions is shown in Figure 6A. Additionally, the corresponding pH in the culture supernatants is shown in Figure 6B. In summary, acetate consumption over the growth phases largely determines pigment production in S. aureus.
Table 8.
Medium acetate 1 concentration and staphyloxanthin production in S. aureus strain Pig1.
| [Acetate] 2, mM | Staphyloxanthin 3 | |||
|---|---|---|---|---|
| Log | Stationary | Log | Stationary | |
| TSB | 8.50 ± 0.99 | 19.38 ± 2.31 | 0.017 ± 0.0006 | 0.127 ± 0.0121 |
| TSB-Glucose | 8.07 ± 1.42 | 25.82 ± 1.85 | 0.018 ± 0.0023 | 0.033 ± 0.0012 |
| MHB | 3.09 ± 0.64 | 7.33 ± 1.02 | 0.051 ± 0.0029 | 0.086 ± 0.0029 |
| MHB-Glucose | 5.17 ± 0.98 | 27.07 ± 1.63 | 0.035 ± 0.0006 | 0.031 ± 0.0046 |
1 Standard error of the mean (SEM) in the acetate concentration was determined from three independent experiments; 2 Acetate concentration was determined in the supernatants of log (OD600 ~0.8) and stationary phase (12 h; TSB, OD600 ~8.0; MHB, OD600 ~6.0) cultures; 3 OD465 per mg dry weight of cell mass.
Figure 6.
Effect of glucose in S. aureus Pig1 grown in TSB and MHB. (a) Pigmentation in cell pellets of strain Pig1 grown with or without glucose in the growth media. The strain was grown in plain TSB or MHB and glucose-added TSB or MHB at log phase (OD600 ~0.8) and at stationary (12 h; TSB, OD600 ~8.0; MHB, OD600 ~6.0). Cell pellets from 5 mL cultures were collected, washed, and photographed; (b) pH change in the culture supernatants. pH change in the corresponding culture supernatants was measured using a pH meter and pH paper (pHydrion Insta-Check, Micro Essential laboratory, B’klyn, NY). Five microliter of each supernatant was put on the pH paper. Upper panel shows the reference color for a given pH value.
3. Discussion
Fatty acids in phospholipids play major roles in determining membrane biophysical properties. S. aureus synthesizes and alters the balance of membrane fluidizing BCFAs (mostly anteiso C15:0 and anteiso C17:0) and rigidifying SCSFAs under different growth conditions. The bacterium can take up SCUFAs from environment and incorporate them into phospholipids [2,6]. Many S. aureus strains produce a characteristic membrane component, staphyloxanthin, which is a triterpenoid pigment with an anteiso C15:0 at the C6-position of the glucose residue in glycosyl-4,4′-diaponeurosporenoate [10,12,13,33,34]. The pigment has been shown to provide rigidity in the membrane [2,7,8,9,10,11]. Here, we grew S. aureus strains Pig1 (pigmented), Pig1ΔcrtM (non-pigmented), and S. argenteus (a close relative of S. aureus, non-pigmented) in various culture media and analyzed the relationship between fatty acid composition and pigmentation. We observed that production of staphyloxanthin does not influence the fatty acid composition but helps maintain membrane fluidity. Notably, fatty acid composition and pigmentation in the bacterium were markedly affected by supplementation of glucose or acetate in the growth medium. We believe that staphyloxanthin production and fatty acid composition are regulated metabolically.
The relationship of fatty acid composition and staphyloxanthin production is complex. As observed previously [2] in two different strains of S. aureus, here we observed that S. aureus Pig1 grown in MHB at log phase had higher BCFAs and higher pigmentation than those grown in TSB. Further, serum-grown cells had a large proportion of SCUFAs and higher pigmentation than the TSB-grown cells. Additionally, we observed that the 12 h-grown cells in TSB and MHB had higher proportion of BCFAs and were more pigmented than the respective log phase cells indicating a direct relationship of BCFAs and pigmentation in S. aureus. However, despite having lower BCFAs, the TSB-grown cells at 12 h had markedly higher pigmentation than those grown in MHB. Further, despite having a substantial proportion of SCUFAs the serum-grown cells at 12 h had least pigmentation. Additionally, acetate-supplementation in culture broths increased pigmentation and decreased BCFAs in S. aureus. These observations indicate that there may not always be a direct relationship of fatty acid composition and pigmentation in S. aureus. We observed that the fatty acid composition in pigmented and non-pigmented strains were similar. Further, the genes for biosynthesis and regulation of fatty acids and staphyloxanthin in the strains were appear to be independently regulated. Taken together our observation suggest that the relationship of fatty acid composition and pigmentation in S. aureus is complex.
S. aureus acetate metabolism determines the extent of its pigment production. S. aureus continuously secretes acetate in glucose-containing medium and consumes acetate when glucose is exhausted [31]. We observed that supplementation of media with glucose decreased pigmentation while acetate-supplementation increased pigmentation. Further, glucose-supplementation increased acetate concentration in the culture supernatants even at stationary phase where the cells preferentially consume acetate otherwise. Since glucose catabolism yields acetyl-CoA and the excess acetyl-CoA is converted into acetate, which is continuously secreted out of cells [31], the secretion of the precursor molecule might reduce pigmentation in the cells in glucose-supplemented media. On the other hand, supplementation of acetate in the media might provide surplus acetyl CoA in the cells leading to higher pigment production. Hence, these observations indicate that acetate consumption directly correlates to pigmentation in S. aureus.
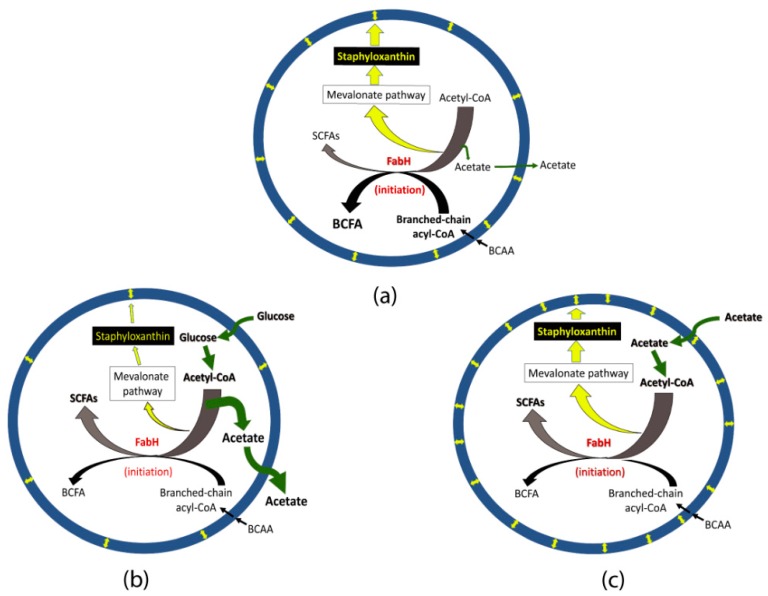
Acetate metabolism may affect initiation of fatty acid biosynthesis leading to alteration in BCFA and staphyloxanthin production. As explained above, increased BCFAs is usually associated with increased pigmentation in glucose-lacking media (Figure 7a); however, an increased pigmentation may not result in increased BCFAs. This notion is supported by an observation that a S. aureus pdh mutant impaired in acetyl-CoA production had both significantly reduced staphyloxanthin and significantly higher BCFAs (80%; wild type, 50%) [29]. Similarly, a bkd mutant had significantly higher pigmentation but had significantly lower BCFAs (31%) [29]. Since, BCFA synthesis requires branched-chain acyl-CoA (precursors synthesized by the BKD complex) and fatty acid biosynthesis initiation enzyme (FabH) prefers branched-chain acyl-CoA to acetyl-CoA [35], the scarcity of acetyl-CoA in the pdh mutant might be a condition where the FabH enzyme synthesizes more BCFAs. On the other hand, a decreased synthesis of BCFAs in the bkd mutant might result in FabH enzyme utilizing more acetyl-CoA in the initiation step leading to increased SCSFAs than in the parent strain. Based on this assumption of acetyl-CoA consumption in a bkd mutant, a continuous production (cellular availability) of acetyl-CoA in glucose-supplemented broths might affect FabH activity leading to increased SCSFAs (Figure 7b). Similarly, a continuous uptake of acetate and subsequent availability of surplus acetyl-CoA could affect FabH activity leading to increased SCSFAs in the acetate-supplemented medium (Figure 7c).
Figure 7.
Proposed model on alterations in fatty acid composition and staphyloxanthin in S. aureus. Upper model (a) shows that higher proportion of BCFAs is achieved with higher affinity of FabH (initiation of fatty acid biosynthesis) for branched-chain acyl-CoA leaving abundant amounts of acetyl-CoA that can overflow towards staphyloxanthin production when S. aureus grown in glucose-lacking media such as LB or MHB. Lower left model (b) shows that glucose-supplementation in the growth medium causes production of excess acetyl-CoA molecules that are mostly converted into acetate and secreted outside. Note that the continuous supply of acetyl-CoA may favor an increased proportion of SCSFAs compared to cells grown without glucose-supplementation; Lower right model (c) shows that acetate-supplementation increases acetyl-CoA pool in the cytoplasm and, thus, increased the production of staphyloxanthin and SCSFAs. BCAA, branched-chain amino acids (valine, leucine, isoleucine). Note that acetyl-CoA is also used to make malonyl-CoA to be used in fatty acid elongation. The elongation process is the same for both SCSFA and BCFA synthesis utilizing malonyl-CoA biosynthesized from acetyl-CoA. The yellow double-arrowheads represent staphyloxanthin molecules in the membrane (thick blue circles).
An increased flow of acetyl CoA causes a higher pigmentation in S. aureus Pig1. The notion of increased flow of acetyl-CoA towards the mevalonate pathway leading to increased pigmentation is corroborated by the findings of addition of fatty acids or mevalonate in the growth medium [28], blocking polyprenyl synthetase [24] and inactivation of the TCA cycle [17] increase pigmentation in S. aureus. Inactivation of the TCA cycle provides more acetyl-CoA in the cytoplasmic pool that can be used in the mevalonate pathway [17]. Notably, the mevalonate pathway also yields undecaprenyl phosphate that is required to synthesize peptidoglycan, teichoic acid, and capsule [36] and menaquinone. Interestingly, most (~90%) of the undecaprenyl phosphate is converted and stored as an inactive form, undecaprenol, which is recycled back to undecaprenyl phosphate when necessary [36,37]. Therefore, we believe that consumption of most of the surplus acetyl-CoA by the mevalonate pathway is committed to staphyloxanthin production.
On the other hand, a highly pigmented bkd mutant had an unaltered level of crtM transcript compared to the parent strain indicating an unaltered SigB (that regulates the crt operon) activity in the mutant [29]. Hence, an increased flow of acetyl-CoA towards the mevalonate pathway could be responsible for increased pigmentation in the bkd mutant [29]. In this study, we supplemented broths with glucose (140 mM) and acetate (100 mM) to alter the carbon flow in a clinical isolate Pig1. A decreased pigmentation with glucose-supplementation and an increased pigmentation with acetate-supplementation was observed in both log- and stationary-phase cells indicating that the alterations in pigmentation might not be influenced by SigB (the activity of which changes over growth cycle, [38,39,40]). Taken altogether, an increased flow of carbon to the mevalonate pathway might cause an increase in pigmentation in S. aureus Pig1 having higher proportion of BCFAs.
4. Materials and Methods
S. aureus strains and growth media. A markedly yellow pigmented clinical S. aureus isolate (Pig1) and its non-pigmented crtM-knockout mutant (Pig1ΔcrtM) [14], kindly supplied by Dr. George Liu, University of California San Diego, La Jolla, CA, and a naturally crt-operon lacking clinical S. argenteus strain (Deutsche Sammlung von Mikroorganismen und Zellkulturen (DSMZ), Leibniz-Institut, Germany [19,20]) were used in this study (Table 9). Other S. aureus strains used were SH1000 [41], Newman [42], COL [43] and MW2 [44] (Table 9). The growth media (culture to volume ratio of 1:5) used were BactoTM Tryptic soy broth (TSB; Becton, Dickinson and Company, Baltimore, MD, USA), BactoTM Mueller-Hinton broth (MHB; Becton, Dickinson and Company, MD), DifcoTM Luria-Bertani (LB, Miller; Becton, Dickinson and Company) medium and whole human serum (BioreclammationIVT, New York, NY, USA). Human serum was heated to 56 °C for 30 min before using.
Table 9.
Description of the strains used in this study.
| Strains | Description | References |
|---|---|---|
| S. aureus Pig1 | Markedly pigmented clinical isolate | [14] |
| S. aureus Pig1ΔcrtM | crtM-knockout construct of S. aureus Pig1 | [14] |
| S. argenteus | Naturally crt-operon lacking clinical strain. Closely related to S. aureus. Also referred to as S. aureus MSHR1132 | [19,20] |
| S. aureus SH1000 | A laboratory-derived S. aureus strain NCTC 8325-4 by complementing rsbU+ from S. aureus Newman | [41] |
| S. aureus Newman | Clinical isolate sensitive to methicillin | [42] |
| S. aureus COL | Hospital-associated homogeneous methicillin-resistant strain | [43] |
| S. aureus MW2 | Community-associated heterogeneous methicillin-resistant strain | [44] |
Determination of growth patterns of S. aureus strains grown in TSB, MHB and human serum. Overnight grown strains Pig1, Pig1ΔcrtM and MHSR1132 were inoculated into TSB, MHB and serum, grown for 48 h and growth was monitored by measuring turbidity (OD600) at intervals.
Determination of fatty acid compositions in S. aureus strains grown in TSB, MHB and human serum. Strains Pig1, Pig1ΔcrtM and MHSR1132 were grown to an optical density (OD600) of about 0.8 in TSB, MHB and serum, were harvested (3000× g for 5 min), washed with cold water, and the pellets were sent as frozen sample with overnight delivery for fatty acid methyl ester (FAME) analysis at MIDI, Inc. (Newark, Delaware, USA). At MIDI, fatty acids were extracted and transesterified to fatty acid methyl esters, which were then separated and identified by gas-chromatography [45].
Determination of staphyloxanthin production in S. aureus strains grown in TSB, MHB and human serum. Staphyloxanthin content in the S. aureus cells was determined as described previously [23,46]. Briefly, TSB-, MHB- and serum-grown Pig1 cells were pelleted (3000× g for 5 min at 4 °C) at OD600 of ~0.8, and then at 12 h, 24 h and 48 h. Each pellet was washed with water and resuspended in 0.8 mL of 100% methanol and incubated at 65 °C for 10 min with vortexing every three minutes. OD465 was measured for each methanol extract and the staphyloxanthin-content was determined by dividing OD465 by cell mass (OD600 1.0 corresponds to 0.39 mg dry wt./mL; Bieber and Wilkinson, 1984). Three independent assays were performed for each category.s
Determination of membrane fluidity of S. aureus strains grown in TSB, MHB and human serum. The membrane fluidities of the strains Pig1, Pig1ΔcrtM and MHSR1132 were determined using 1,6-diphenyl-1,3,5-hexatriene (DPH, Sigma-Aldrich, St. Louis, MO, USA) as described previously [47] with some modifications. Briefly, the strains were grown in TSB, MHB and serum, pelleted, washed, resuspended in phosphate buffered saline (PBS, pH 7.5) to an OD600 of ~0.8, DPH (5 µM) was added and the suspension was incubated at 30 °C in a water bath. All steps involving DPH were carried out in the dark. Fluorescence polarization emitted by the fluorophore was measured using a PTIModel Quanta Master-4 Scanning Spectrofluorometer at an excitation wavelength of 360 nm and an emission wavelength of 430 nm. The experiments were performed with three separate fresh batch cultures of cells grown to early-exponential phase, 12, 24 and 48 h.
Determination of expression of genes for staphyloxanthin and fatty acid biosynthesis and their regulation. The level of expression of the genes encoding key enzymes for staphyloxanthin and fatty acid biosynthesis and their regulation were determined by quantitative Real-time Polymerase Chain Reaction (qRT-PCR). Briefly, overnight grown cultures of the strains Pig1 and Pig1ΔcrtM were inoculated in TSB or MHB, grown to OD600 of ~0.8, harvested, and total RNA was extracted and purified using a RNAeasy Mini Kit (Qiagen). cDNA was prepared from 300 ng of each RNA using the High Capacity RNA-to-cDNA kit (Applied Biosystems, Foster City, CA, USA). qRT-PCR reactions were conducted using the DyNAmo Flash SYGR Green Kit (Thermo Scientific, Waltham, MA, USA) with gene-specific primers (Supplementary Materials Table S1) along with for 16SrRNA as an internal control of gene expression. PCR was run using the ABI 7300 Real-Time PCR system. The levels of expression of the genes were calculated by the Comparative Ct method. The reactions were run in triplicates for each set and each experiment was repeated with three independent biological replicates.
Determination of staphyloxanthin in Pig1 grown in glucose or acetate containing LB. Overnight grown culture was inoculated into (i) LB; (ii) LB with MOPS [3-(N-morpholino)propanesulfonic acid] buffer, pH 7.5; (iii) LB with 140 mM glucose; (iv) LB with MOPS and 140 mM glucose; and (v) LB with MOPS and 100 mM acetate. The cells were grown at 37 °C, harvested at 3 h and 12 h, and staphyloxanthin content was determined as described previously. Each experiment was repeated at least three times.
Determination of acetate content in culture supernatants. Culture supernatants were collected from strain Pig1 grown in TSB, MHB, 140 mM glucose-supplemented TSB and MHB, and harvested (10,000× g for 10 min at 4 °C) at ~3 h (OD600 ~0.8) and at 12 h. The supernatants were filtered through 0.2 µ filter (Millipore) and acetate concentration was determined with an acetate standard calibration curve prepared using an Acetic Acid Assay Kit (Megazyme International Ireland, Wicklow, Ireland). Additionally, staphyloxanthin content in the cell pellets were determined. Each experiment was repeated three times.
5. Conclusions
Membrane biophysical properties are largely determined by the fatty acid composition of the cellular phospholipids. S. aureus produces BCFAs and SCSFAs and alters the balance of these fatty acids in response to environmental conditions. S. aureus also produces the hallmark membrane pigment, staphyloxanthin. Here we studied various aspects of fatty acid composition and pigmentation in pigmented and non-pigmented strains. We observed that the relationship of fatty acid composition and pigmentation in S. aureus is a complex phenomenon in which the availability of glucose in growth medium reduces both pigmentation and BCFAs while the availability of acetate increases pigmentation but decreases BCFAs. Our data suggests that the higher the production of BCFAs the higher there is acetate flux towards staphyloxanthin production through the mevalonate pathway. We believe that the overall relationship of fatty acid composition and pigmentation is largely regulated by carbon flow in S. aureus.
Acknowledgments
This work was supported in part by R21AI135351 to B.J.W. and R15GM061583 to C.G.
Supplementary Materials
The following are available online, Table S1: Primer sequences used in qRT-PCR assays. Table S2: Fatty acid profiles of S. aureus grown in various media.
Author Contributions
B.J.W., C.G. and K.B.T. conceived and designed the experiments; K.B.T. performed the experiments; B.J.W., C.G. and K.B.T. analyzed the data; B.J.W. and C.G. contributed reagents/materials/analysis tools; B.J.W., C.G. and K.B.T. wrote the paper.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References
- 1.Zhang Y.-M., Rock C.O. Membrane lipid homeostasis in bacteria. Nat. Rev. Microbiol. 2008;6:222–233. doi: 10.1038/nrmicro1839. [DOI] [PubMed] [Google Scholar]
- 2.Sen S., Sirobhushanam S., Johnson S.R., Song Y., Tefft R., Gatto C., Wilkinson B.J. Growth-environment dependent modulation of Staphylococcus aureus branched-chain to straight-chain fatty acid ratio and incorporation of unsaturated fatty acids. PLoS ONE. 2016;11:e0165300. doi: 10.1371/journal.pone.0165300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kaneda T. Iso- and anteiso-fatty acids in bacteria: Biosynthesis, function, and taxonomic significance. Microbiol. Rev. 1991;55:288–302. doi: 10.1128/mr.55.2.288-302.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willecke K., Pardee A.B. Fatty acid-requiring mutant of Bacillus subtilis defective in branched chain α-keto acid dehydrogenase. J. Biol. Chem. 1971;246:5264–5272. [PubMed] [Google Scholar]
- 5.Legendre S., Letellier L., Shechter E. Influence of lipids with branched-chain fatty acids on the physical, morphological and functional properties of Escherichia coli cytoplasmic membrane. Biochim. Biophys. Acta Biomembr. 1980 doi: 10.1016/0005-2736(80)90328-4. [DOI] [PubMed] [Google Scholar]
- 6.Parsons J.B., Frank M.W., Jackson P., Subramanian C., Rock C.O. Incorporation of extracellular fatty acids by a fatty acid kinase-dependent pathway in Staphylococcus aureus. Mol. Microbiol. 2014;92:234–245. doi: 10.1111/mmi.12556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wisniewska A., Widomska J., Subczynski W.K. Carotenoid-membrane interactions in liposomes: Effect of dipolar, monopolar, and nonpolar carotenoids. Acta Biochim. Pol. 2006;53:475–484. [PubMed] [Google Scholar]
- 8.Mishra N.N., Liu G.Y., Yeaman M.R., Nast C.C., Proctor R.A., McKinnell J., Bayer A.S. Carotenoid-related alteration of cell membrane fluidity impacts Staphylococcus aureus susceptibility to host defense peptides. Antimicrob. Agents Chemother. 2011;55:526–531. doi: 10.1128/AAC.00680-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chamberlain N.R., Mehrtens B.G., Xiong Z., Kapral F.A., Boardman J.L., Rearick J.I. Correlation of carotenoid production, decreased membrane fluidity, and resistance to oleic acid killing in Staphylococcus aureus 18Z. Infect. Immun. 1991;59:4332–4337. doi: 10.1128/iai.59.12.4332-4337.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor R.F. Bacterial triterpenoids. Microbiol. Rev. 1984;48:181–198. doi: 10.1128/mr.48.3.181-198.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kossakowska-Zwierucho M., Kaźmierkiewicz R., Bielawski K.P., Nakonieczna J. Factors determining Staphylococcus aureus susceptibility to photoantimicrobial chemotherapy: RsbU activity, staphyloxanthin level, and membrane fluidity. Front. Microbiol. 2016;7:1141. doi: 10.3389/fmicb.2016.01141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marshall J.H., Wilmoth G.J. Pigments of Staphylococcus aureus, a series of triterpenoid carotenoids. J. Bacteriol. 1981;147:900–913. doi: 10.1128/jb.147.3.900-913.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Marshall J.H., Wilmoth G.J. Proposed pathway of triterpenoid carotenoid biosynthesis in Staphylococcus aureus: Evidence from a study of mutants. J. Bacteriol. 1981;147:914–919. doi: 10.1128/jb.147.3.914-919.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Liu G.Y., Essex A., Buchanan J.T., Datta V., Hoffman H.M., Bastian J.F., Fierer J., Nizet V. Staphylococcus aureus golden pigment impairs neutrophil killing and promotes virulence through its antioxidant activity. J. Exp. Med. 2005;202:209–215. doi: 10.1084/jem.20050846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Clauditz A., Resch A., Wieland K.P., Peschel A., Götz F. Staphyloxanthin plays a role in the fitness of Staphylococcus aureus and its ability to cope with oxidative stress. Infect. Immun. 2006;74:4950–4953. doi: 10.1128/IAI.00204-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu C.-I., Liu G.Y., Song Y., Yin F., Hensler M.E., Jeng W.-Y., Nizet V., Eric A.H.-J.W., Oldfield E. A cholesterol biosynthesis inhibitor blocks Staphylococcus aureus virulence. Science. 2008;319:1391–1394. doi: 10.1126/science.1153018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lan L., Cheng A., Dunman P.M., Missiakas D., He C. Golden pigment production and virulence gene expression are affected by metabolisms in Staphylococcus aureus. J. Bacteriol. 2010;192:3068–3077. doi: 10.1128/JB.00928-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kaiser J.C., Sen S., Sinha A., Wilkinson B.J., Heinrichs D.E. The role of two branched-chain amino acid transporters in Staphylococcus aureus growth, membrane fatty acid composition and virulence. Mol. Microbiol. 2016;102:850–864. doi: 10.1111/mmi.13495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tong S.Y.C., Schaumburg F., Ellington M.J., Corander J., Pichon B., Leendertz F., Bentley S.D., Parkhill J., Holt D.C., Peters G., et al. Novel staphylococcal species that form part of a Staphylococcus aureus-related complex: The non-pigmented Staphylococcus argenteus sp. nov. and the non-human primate-associated Staphylococcus schweitzeri sp. nov. Int. J. Syst. Evol. Microbiol. 2015;65:15–22. doi: 10.1099/ijs.0.062752-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Holt D.C., Holden M.T.G., Tong S.Y.C., Castillo-Ramirez S., Clarke L., Quail M.A., Currie B.J., Parkhill J., Bentley S.D., Feil E.J., et al. A very early-branching Staphylococcus aureus lineage lacking the carotenoid pigment staphyloxanthin. Genome Biol. Evol. 2011;3:881–895. doi: 10.1093/gbe/evr078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kullik I., Giachino P., Fuchs T. Deletion of the alternative sigma factor SigB in Staphylococcus aureus reveals its function as a global regulator of virulence genes. J. Bacteriol. 1998;180:4814–4820. doi: 10.1128/jb.180.18.4814-4820.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katzif S., Lee E.-H., Law A.B., Tzeng Y.-L., Shafer W.M. CspA regulates pigment production in Staphylococcus aureus through a SigB-dependent mechanism. J. Bacteriol. 2005;187:8181–8184. doi: 10.1128/JB.187.23.8181-8184.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hall J.W., Yang J., Guo H., Ji Y. The Staphylococcus aureus AirSR two-component system mediates reactive oxygen species resistance via transcriptional regulation of staphyloxanthin production. Infect. Immun. 2017;85:e00838-16. doi: 10.1128/IAI.00838-16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Fey P.D., Endres J.L., Yajjala V.K., Widhelm T.J., Boissy R.J., Bose J.L., Bayles K.W. A genetic resource for rapid and comprehensive phenotype screening of nonessential Staphylococcus aureus genes. MBio. 2013;4:e00537-12. doi: 10.1128/mBio.00537-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu Y., Wu N., Dong J., Gao Y., Zhang X., Shao N., Yang G. SsrA (tmRNA) acts as an antisense RNA to regulate Staphylococcus aureus pigment synthesis by base pairing with crtMN mRNA. FEBS Lett. 2010;584:4325–4329. doi: 10.1016/j.febslet.2010.09.024. [DOI] [PubMed] [Google Scholar]
- 26.Hartmann T., Baronian G., Nippe N., Voss M., Schulthess B., Wolz C., Eisenbeis J., Schmidt-Hohagen K., Gaupp R., Sunderkötter C., et al. The catabolite control protein E (CcpE) affects virulence determinant production and pathogenesis of Staphylococcus aureus. J. Biol. Chem. 2014;289:29701–29711. doi: 10.1074/jbc.M114.584979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ding Y., Liu X., Chen F., Di H., Xu B., Zhou L., Deng X., Wu M., Yang C.-G., Lan L. Metabolic sensor governing bacterial virulence in Staphylococcus aureus. Proc. Natl. Acad. Sci. USA. 2014;111:E4981–E4990. doi: 10.1073/pnas.1411077111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willis A., O’Connor J., Smith J. Colonial pigmentation of Staphylococcus aureus. J. Pathol. Bacteriol. 1966;92:97–106. doi: 10.1002/path.1700920112. [DOI] [PubMed] [Google Scholar]
- 29.Singh V.K., Sirobhushanam S., Ring R.P., Singh S., Gatto C., Wilkinson B.J. Role of pyruvate dehydrogenase and branched-chain α-keto acid dehydrogenase in branched-chain membrane fatty acid levels and associated functions in Staphylococcus aureus. J. Med. Microbiol. 2018;67:570–578. doi: 10.1099/jmm.0.000707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schujman G.E., Guerin M., Buschiazzo A., Schaeffer F., Llarrull L.I., Reh G., Vila A.J., Alzari P.M., de Mendoza D. Structural basis of lipid biosynthesis regulation in Gram-positive bacteria. EMBO J. 2006;25:4074–4083. doi: 10.1038/sj.emboj.7601284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Somerville G.A., Saïd-salim B., Wickman J.M., Raffel S.J., Kreiswirth B.N., Musser J.M. Correlation of acetate catabolism and growth yield in Staphylococcus aureus: Implications for host-pathogen interactions. Infect. Immun. 2003;71:4724–4732. doi: 10.1128/IAI.71.8.4724-4732.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Somerville G.A., Chaussee M.S., Dorward D.W., Reitzer L.J., Musser J.M. Staphylococcus aureus aconitase inactivation unexpectedly inhibits post-exponential-phase growth and enhances stationary-phase survival. Infect. Immun. 2002;70:6373–6382. doi: 10.1128/IAI.70.11.6373-6382.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Pelz A., Wieland K.P., Putzbach K., Hentschel P., Albert K., Götz F. Structure and biosynthesis of staphyloxanthin from Staphylococcus aureus. J. Biol. Chem. 2005;280:32493–32498. doi: 10.1074/jbc.M505070200. [DOI] [PubMed] [Google Scholar]
- 34.Wieland B., Feil C., Gloria-maercker E., Thumm G., Lechner M., Bravo J.-M., Poralla K., Gotz F. Genetic and biochemical analyses of the biosynthesis of the yellow carotenoid 4,4’-diaponeurosporene of Staphylococcus aureus. J. Bacteriol. 1994;176:7719–7726. doi: 10.1128/jb.176.24.7719-7726.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Choi K.-H., Heath R.J., Rock C.O. β-Kketoacyl-acyl carrier protein synthase III (FabH) is a determining factor in branched-chain fatty acid biosynthesis. J. Bacteriol. 2000;182:365–370. doi: 10.1128/JB.182.2.365-370.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bouhss A., Trunkfield A.E., Bugg T.D.H., Mengin-Lecreulx D. The biosynthesis of peptidoglycan lipid-linked intermediates. FEMS Microbiol. Rev. 2008;32:208–233. doi: 10.1111/j.1574-6976.2007.00089.x. [DOI] [PubMed] [Google Scholar]
- 37.Higashi Y., Stro J.L., Sweeley C.C. Biosynthesis of the peptidoglycan of bacterial cell walls. XXI. Isolation of free C55-isoprenoid alcohol and of lipid intermediates in peptidoglycan synthesis from Staphylococcus aureus. J. Biol. Chem. 1970;245:3697–3702. [PubMed] [Google Scholar]
- 38.Bischoff M., Entenza J.M., Giachino P. Influence of a functional sigB operon on the global regulators sar and agr in Staphylococcus aureus. J. Bacteriol. 2001;183:5171–5179. doi: 10.1128/JB.183.17.5171-5179.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Senn M.M., Giachino P., Homerova D., Steinhuber A., Strassner J., Kormanec J., Flückiger U., Berger-Bächi B., Bischoff M. Molecular analysis and organization of the sigmaB operon in Staphylococcus aureus. J. Bacteriol. 2005;187:8006–8019. doi: 10.1128/JB.187.23.8006-8019.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Donegan N.P., Cheung A.L. Regulation of the mazEF toxin-antitoxin module in Staphylococcus aureus and its impact on sigB expression. J. Bacteriol. 2009;191:2795–2805. doi: 10.1128/JB.01713-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Horsburgh M.J., Aish J.L., White I.J., Shaw L., Lithgow J.K., Foster S.J. SigmaB modulates virulence determinant expression and stress resistance: Characterization of a functional rsbU strain derived from Staphylococcus aureus 8325-4. J. Bacteriol. 2002;184:5457–5467. doi: 10.1128/JB.184.19.5457-5467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duthie E.S., Lorenz L.L. Staphylococcal coagulase: Mode of action and antigenicity. J. Gen. Microbiol. 1952;6:95–107. doi: 10.1099/00221287-6-1-2-95. [DOI] [PubMed] [Google Scholar]
- 43.Gill S.R., Fouts D.E., Archer G.L., Mongodin E.F., DeBoy R.T., Ravel J., Paulsen I.T., Kolonay J.F., Brinkac L., Beanan M., et al. Insights on evolution of virulence and resistance from the complete genome analysis of an early methicillin-resistant Staphylococcus aureus strain and a biofilm-producing methicillin-resistant Staphylococcus epidermidis strain. J. Bacteriol. 2005;187:2426–2438. doi: 10.1128/JB.187.7.2426-2438.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Baba T., Takeuchi F., Kuroda M., Yuzawa H., Aoki K.I., Oguchi A., Nagai Y., Iwama N., Asano K., Naimi T., et al. Genome and virulence determinants of high virulence community-acquired MRSA. Lancet. 2002;359:1819–1827. doi: 10.1016/S0140-6736(02)08713-5. [DOI] [PubMed] [Google Scholar]
- 45.Kunitsky C., Osterhout G., Sasser M. In: Identification of Microorganisms Using Fatty Acid Methyl Ester (FAME) Analysis and the MIDI Sherlock Microbial Identification System. Miller M.J., editor. Volume III. Davis Healthcare International Publishing, LLC; Baltimore, MD, USA: 2006. pp. 1–18. [Google Scholar]
- 46.Davis A.O., O’Leary J.O., Muthaiyan A., Langevin M.J., Delgado A., Abalos A.T., Fajardo A.R., Marek J., Wilkinson B.J., Gustafson J.E. Characterization of Staphylococcus aureus mutants expressing reduced susceptibility to common house-cleaners. J. Appl. Microbiol. 2005;98:364–372. doi: 10.1111/j.1365-2672.2004.02460.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Singh V.K., Hattangady D.S., Giotis E.S., Singh A.K., Chamberlain N.R., Stuart M.K., Wilkinson B.J. Insertional inactivation of branched-chain α-keto acid dehydrogenase in Staphylococcus aureus leads to decreased branched-chain membrane fatty acid content and increased susceptibility to certain stresses. Appl. Environ. Microbiol. 2008;74:5882–5890. doi: 10.1128/AEM.00882-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.