Abstract
Enaminones, 4-methyl-1-[4-(piperazin/morpholin-1-yl) phenyl] pent-2-en-1-one (IIa–b) were synthesized by refluxing 1-[4-(piperazin/morpholin-1-yl) phenyl] ethan-1-one (Ia–b) with dimethylformamide dimethylacetal (DMF–DMA) without any solvent. The three dimensional structure of enaminone (IIb) containing morpholine moiety was confirmed by single crystal X-ray crystallography. Finally, the dihydropyrimidinone derivatives (1–20) were obtained by reacting enaminones (IIa–b) with urea and different substituted benzaldehydes in the presence of glacial acetic acid. Dihydropyrimidinone derivatives containing piperazine/morpholine moiety were synthesized in a good yield by means of simple and efficient method.
Keywords: dihydropyrimidinone derivatives, morpholine, piperazine, Biginelli synthesis
1. Introduction
Pyrimidines scaffold have played a significant role in the area of medicinal chemistry [1]. Pyrimidines are important moieties because of their potential biological activities such as antitumor, antiviral, and antibacterial agents [2,3]. Dihydropyridines are the most potent calcium channel modulators available for the treatment of various cardiovascular diseases [4]. Dihydropyrimidines, also known as Biginelli’s compounds, display various pharmacological activities [5]. Dihydropyrimidinone compounds were first synthesized by Pietro Biginelli. The process comprised of reacting numerous aldehydes with urea and a beta-keto ester to give a tetrahydropyrimidinone. Substituted dihydropyrimidinone compounds show interesting biological properties. Some of the analogs of dihydropyrimidine compounds are antitumor agents [6]. Dihydropyrimidinones have emerged as calcium channel blockers and antihypertensive agents [7]. These compounds exhibit a broad range of pharmacological activities, such as antibacterial, antitumor, antiviral, and anti-inflammatory [8].
Piperazine moiety contains two nitrogen atoms at two opposite positions of a six-membered heterocyclic ring. Polar nitrogen atoms increase the favorable interactions of piperazine with macromolecules. It has the ability to cross the blood brain barrier (BBB) due to its lipophilic nature, and is useful in various diseases, such as Alzheimer’s disease, psychosis, and depression. Many potent marketed drugs like fluphenazine, cinnarizine, flunarizine, lomerizine, ciprofloxacin, indinavir, etc., have a piperazine moiety (Figure 1). Piperazine derivatives have shown significant pharmacological activities, such as anti-tuberculosis, anti-inflammatory, antiviral, as Central Nervous System (CNS) agents, anticancer, as cardioprotective agents, and antidiabetic [9,10,11,12,13,14,15,16,17,18,19,20,21,22,23,24,25,26,27,28].
Figure 1.
Marketed drugs containing piperazine moiety.
Morpholine is an organic moiety containing nitrogen and oxygen in a heterocyclic six-membered ring, and is considered as an important building block in the field of medicinal chemistry. The linezolid antibiotic having a morpholine moiety is commercially available as antimicrobial agent. Timolol, moclobemide, emorfazone (anti-inflammatory drug and analgesic), phenadoxone (heptalgin, opioid analgesic), antidepressants reboxetine and gefitinib, fenpropimorph (fungicide) and antibacterial drugs finafloxacin and levofloxacin contain a morpholine moiety (Figure 2). Morpholine derivatives are very much essential in the drug discovery process. Morpholine scaffolds are important, due to their variety of pharmacological activities [29,30,31,32,33,34,35].
Figure 2.
Marketed drugs containing morpholine moiety.
The literature review suggested that molecules possessing these important scaffolds (piperazine/morpholine and dihydropyrimidinone) may have significant therapeutic activity. In the present disclosure, a series of novel piperazine/morpholine dihydropyrimidinone hybrids were prepared and analyzed by spectral data.
2. Results and Discussion
As shown in (Scheme 1), enaminones, 4-methyl-1-[4-(piperazin/morpholin-1-yl) phenyl] pent-2-en-1-one (IIa–b) were synthesized by refluxing 1-[4-(piperazin/morpholin-1-yl) phenyl] ethan-1-one (Ia–b) with dimethylformamide dimethylacetal (DMF–DMA), without solvent for 10 h. To prepare the final dihydropyrimidinone derivatives, a mixture of substituted benzaldehyde (0.01 mol) III, enaminones (IIa/IIb) (0.01 mol), urea (0.01 mol) IV, and glacial acetic acid (10 mL) was heated on a heating mantle under refluxing condition for 3 h. The precipitates of compounds (1–20) were collected by vacuum filtration. The product was washed several times with water, and recrystallized from glacial acetic acid and ethanol mixture. 1H NMR spectrum of (IIa) displayed two singlets at δH 2.89, 3.12 ppm due to the N,N-dimethyl protons and two doublets at δH 5.80–5.82 and 7.63–7.65 ppm (d, J = 14 Hz) due to the ethylenic protons, in addition to the two doublets at the region δH 7.0 ppm (2H, d, aromatic) and δH 7.82 ppm (2H, d, aromatic). The protons of piperazine moiety appears at δH 3.40 (4H, singlet) and 3.52 (4H, singlet). 1H NMR spectrum of (IIb) displayed two singlets at δH 2.90, 3.12 ppm due to the N,N-dimethyl protons and two doublets at δH 5.80–5.82 and 7.63–7.65 ppm (d, J = 14 Hz) due to the ethylenic protons, in addition to the two doublets at the region δH 6.94 ppm (2H, d, aromatic) and δH 7.82 ppm (2H, d, aromatic). The protons of morpholine moiety appears at δH 3.21 (4H, singlet) and 3.74 (4H, singlet). The three-dimensional structure of enaminone (IIb) was confirmed by single crystal X-ray. The coupling constant (J = 14 Hz) for the ethylenic protons indicated that the enaminones existed in the E-configuration. Single crystal X-ray crystallography also confirmed the E-configuration of the enaminone [36].
Scheme 1.
Reaction scheme for the synthesis of dihydropyrimidinone derivatives (1–20).
| Comp. | X | R |
|---|---|---|
| 1 | NH | C6H5– |
| 2 | NH | 2-NO2–C6H4– |
| 3 | NH | 4-NO2–C6H4– |
| 4 | NH | 3-NO2–C6H4– |
| 5 | NH | 4-Cl–C6H4– |
| 6 | NH | 2-OCH3–C6H4– |
| 7 | NH | 4-OH–C6H4– |
| 8 | NH | 3-OH–C6H4– |
| 9 | NH | 3-OCH3–C6H4– |
| 10 | NH | 4-OC2H5–C6H4– |
| 11 | O | C6H5– |
| 12 | O | 2-NO2–C6H4– |
| 13 | O | 4-NO2–C6H4– |
| 14 | O | 3-NO2–C6H4– |
| 15 | O | 4-Cl–C6H4– |
| 16 | O | 2-OCH3–C6H4– |
| 17 | O | 4-OH–C6H4– |
| 18 | O | 3-OH–C6H4– |
| 19 | O | 3-OCH3–C6H4– |
| 20 | O | 4-OC2H5–C6H4– |
Compounds (1–20) presented the D2O exchangeable broad singlet at δH 6.71–8.52 ppm and δH 9.00–9.42 ppm corresponding to the two NH protons. The eight protons (4×CH2) of piperazine moiety were observed as singlet of four protons at δH 2.00–2.09, and another singlet of four protons at δH 3.20–3.41 ppm. The eight protons of morpholine moiety were observed as triplets at δH 3.20–3.22 ppm with coupling constant (J = 4.7 Hz) for four protons and another triplet at δH 3.72–3.82 with coupling constant (J = 4.6 Hz) for four protons. The H-4 and =CH protons of dihydropyrimidinone moiety were observed at δH 5.32–6.08 and 7.79–8.24 ppm, respectively [37,38,39]. 13C NMR spectra confirmed the presence of all carbon atoms of compounds (1–20).
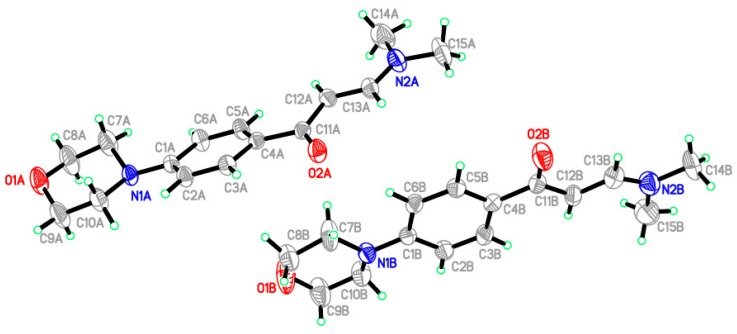
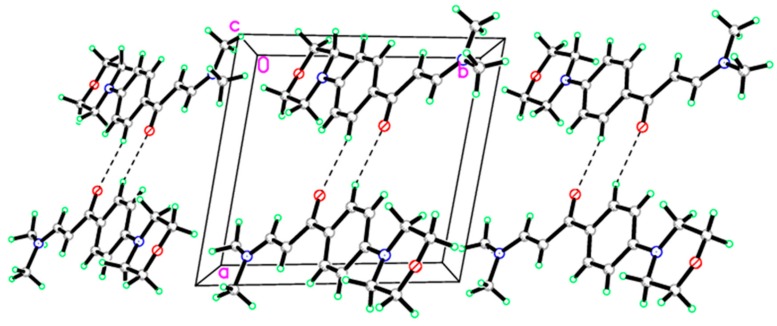
Mass spectral data confirmed the molecular weight of compounds. All the compounds presented molecular ion peak respective to their molecular weights. The experimental part contains the detailed spectral results of 1H NMR, 13C NMR spectra, and mass spectra. The information regarding the crystallographic data and refinement of the compound (IIb), C15H20N2O2 are summarized in Table 1. The selected bond angles and bond lengths are listed in Table 2. Two independent molecules were found in the asymmetric unit as shown in Figure 3. All the bond lengths and angles were in normal ranges as reported [40]. The molecules were linked via two intermolecular hydrogen bonds in the crystal packing (Figure 4, Table 3).
Table 1.
Experimental details.
| Crystal Data | |
|---|---|
| Chemical formula | C15H20N2O2 |
| Mr | 260.33 |
| Crystal system, space group | Triclinic, P-1 |
| Temperature (K) | 293 |
| a, b, c (Å) | 9.5268 (7), 10.2914 (8), 15.3140 (11) |
| α β γ (°) | 104.458 (3), 97.224 (3), 97.984 (3) |
| V (Å3) | 1419.51 (18) |
| Z | 4 |
| Radiation type | Mo Kα |
| µ (mm−1) | 0.08 |
| Crystal size (mm) | 0.61 × 0.31 × 0.28 |
| Data collection | |
| Diffractometer | Bruker APEX-II D8 venture diffractometer |
| Absorption correction | Multi-scan SADABS Bruker 2014 |
| Tmin, Tmax | 0.952, 0.977 |
| No. of measured, independent and observed [I > 2σ(I)] reflections | 27345, 5007, 2799 |
| Rint | 0.102 |
| Refinement | |
| R[F2 > 2σ(F2)], wR(F2), S | 0.075, 0.239, 1.04 |
| No. of reflections | 5007 |
| No. of parameters | 348 |
| No. of restraints | 0 |
| H-atom treatment | H atoms treated by a mixture of independent and constrained refinement |
| Δρmax, Δρmin (e Å−3) | 0.34, −0.32 |
Table 2.
Selected geometric parameters (Å).
| O1A—C8A | 1.393 (5) | N2A—C13A | 1.326 (4) |
| O1A—C9A | 1.399 (5) | N2A—C14A | 1.429 (5) |
| O2A—C11A | 1.230 (4) | N2A—C15A | 1.443 (4) |
| O1B—C9B | 1.327 (6) | N1B—C1B | 1.402 (4) |
| O1B—C8B | 1.369 (5) | N1B—C7B | 1.406 (5) |
| O2B—C11B | 1.233 (5) | N1B—C10B | 1.427 (5) |
| N1A—C10A | 1.460 (4) | N2B—C13B | 1.332 (5) |
| N1A—C1A | 1.403 (4) | N2B—C14B | 1.440 (4) |
| N1A—C7A | 1.447 (5) | N2B—C15B | 1.453 (6) |
| C8A—O1A—C9A | 110.1 (3) | N1A—C7A—C8A | 111.8 (3) |
| C8B—O1B—C9B | 117.7 (3) | O1A—C8A—C7A | 113.1 (3) |
| C1A—N1A—C10A | 117.1 (2) | O1A—C9A—C10A | 112.6 (3) |
| C7A—N1A—C10A | 111.9 (3) | N1A—C10A—C9A | 111.4 (3) |
| C1A—N1A—C7A | 117.5 (2) | O2A—C11A—C4A | 118.5 (3) |
| C13A—N2A—C15A | 121.8 (3) | O2A—C11A—C12A | 123.1 (3) |
| C14A—N2A—C15A | 116.7 (3) | N2A—C13A—C12A | 127.7 (3) |
| C13A—N2A—C14A | 121.5 (3) | N1B—C1B—C2B | 121.2 (3) |
| C1B—N1B—C7B | 118.9 (3) | N1B—C1B—C6B | 122.0 (3) |
| C1B—N1B—C10B | 119.3 (3) | N1B—C7B—C8B | 115.9 (3) |
| C7B—N1B—C10B | 117.4 (3) | O1B—C8B—C7B | 116.9 (4) |
| C13B—N2B—C14B | 122.6 (3) | O1B—C9B—C10B | 119.2 (4) |
| C13B—N2B—C15B | 121.2 (3) | N1B—C10B—C9B | 115.8 (3) |
| C14B—N2B—C15B | 115.9 (3) | O2B—C11B—C4B | 119.0 (3) |
| N1A—C1A—C2A | 122.5 (3) | O2B—C11B—C12B | 121.6 (3) |
| N1A—C1A—C6A | 120.4 (3) | N2B—C13B—C12B | 128.1 (4) |
Figure 3.
ORTEP diagram of the enaminone (IIb) containing morpholine moiety. Displacement ellipsoids are plotted at the 40% probability level for non-H atoms.
Figure 4.
Molecular packing of enaminone (IIb) viewed hydrogen bonds which are drawn as dashed lines along a axis.
Table 3.
Hydrogen-bond geometry (Å).
| D—H···A | D—H | H···A | D···A | D—H···A |
|---|---|---|---|---|
| C5B—H5BA···O2Bi | 0.930 | 2.5100 | 3.391 (4) | 158.00 |
| C13A—H13A···O2Bi | 0.930 | 2.5900 | 3.451 (4) | 154.00 |
| C13B—H13B···O2Ai | 0.930 | 2.5800 | 3.418 (4) | 151.00 |
| C15A—H15A···O2Bi | 0.960 | 2.5100 | 3.375 (5) | 149.00 |
| Symmetry code: (i) –x + 1, −y + 1, −z. | ||||
The mechanism involves the acid-catalyzed formation of iminium ion intermediate from the substituted benzaldehydes and urea. Reaction of iminium ion by enaminone of piperzine/morpholine produces ureidenone, which cyclizes to form hexahydropyrimidine. Elimination of N(CH3)2 group from hexahydropyrimidine in presence of glacial acetic acid produces final dihydropyrimidinone derivatives containing piperazine/morpholine moiety (Scheme 2).
Scheme 2.
Mechanism of the reaction for the synthesis of dihydropyrimidinone derivatives (1–20).
3. Material and Methods
3.1. Chemistry
All the solvents were purchased from Merck (Kenilworth, NJ, USA). To check the purity of compounds, thin layer chromatography (TLC), was performed on silica gel 60 F254 coated plates (Merck). For performing FTIR, Perkin Elmer (Waltham, MA, USA) FT-IR spectrophotometer was used. Melting points were measured by Gallenkamp melting point apparatus. 1H and 13C NMR were recorded in Bruker (Billerica, MA, USA) NMR 500/700 MHz and 125/176 MHz spectrophotometer. The samples were run in DMSO-d6 with tetramethyl silane (TMS) as an internal standard. Molecular weights of compounds were determined in mass spectroscopy. The elemental analysis of compounds was performed by CHN Elementar (Analysensysteme GmbH, Langenselbold, Germany). The compound (IIb) was obtained as single crystal by reported method. Data were collected on a Bruker APEX-II D8 Venture area diffractometer. SHELXT was used to solve structure [41,42]. CCDC 1532829 contains the supplementary crystallographic data for the compound (IIb). These data can be obtained free of charge via http://www.ccdc.cam.ac.uk/conts/retrieving.html (or from the CCDC, 12 Union Road, Cambridge CB2 1EZ, UK; Fax: +44 1223 336033; E-mail: deposit@ccdc.cam.ac.uk).
3.2. Synthesis of 3-(dimethylamino)-1-(4-(piperazin-1-yl) phenyl)prop-2-en-1-one (IIa)
A mixture of 1-[4-(piperazin-1-yl) phenyl]ethan-1-one (I) (0.02 mol) and dimethylformamide dimethylacetal (DMF–DMA) (II) (0.023 mol) was refluxed for 10 h without solvent, then, the reaction mixture was left to cool slowly at room temperature. Diethyl ether was added to reaction mixture. The precipitate was obtained and filtration was performed under vacuum. The product was washed with cold diethyl ether. The product so obtained was recrystallized from absolute ethanol. Yield: 92%; m.p.: 105–107 °C; IR (KBr): νmax/cm−1: 1658 (C=O), 1541 (C=C), 1115 (C–O); 1H NMR (700 MHz, DMSO-d6) δ ppm: 8.10 (1H, s, NH), 7.82 (2H, d, J = 14 Hz, Ar–H), 7.63–7.65 (1H, d, J = 14 Hz, =CH), 7.0 (2H, d, J = 7 Hz, Ar–H) 5.80–5.82 (1H, d, J = 14 Hz, =CH), 3.52 (4H, s, piperazine), 3.40 (4H, s, piperazine), 3.12 (3H, s, N–CH3), 2.89 (3H, s, N–CH3); 13C NMR (176.0 MHz, DMSO-d6): δ = 26.2, 44.6, 46.8, 48.0, 91.0, 114.1, 127.5, 129.2, 130.5, 153.7, 154.0, 161.4, 185.1, 196.1; MS: m/z = 259.16 [M]+; Analysis: for C15H21N3O, calcd. C 69.47, H 8.16, N 16.20%; found C 69.20, H 8.14, N 16.14%.
3.3. Synthesis of 3-(dimethylamino)-1-(4-morpholinophenyl)prop-2-en-1-one (IIb)
Yield: 90%; m.p.: 210–212 °C; IR (KBr): νmax/cm−1: 1640 (C=O), 1540 (C=C), 1111 (C–O); 1H NMR (700 MHz, DMSO-d6) δ ppm: 7.82 (2H, d, J = 7 Hz, Ar–H), 7.63–7.65 (1H, d, J = 14 Hz, =CH), 6.94 (2H, d, J = 7 Hz, Ar–H) 5.80–5.82 (1H, d, J = 14 Hz, =CH), 3.74 (4H, s, morpholine), 3.21 (4H, s, morpholine), 3.12 (3H, s, N–CH3), 2.90 (3H, s, N–CH3); 13C NMR (176.0 MHz, DMSO-d6): δ = 26.6, 47.2, 47.8, 66.42, 91.1, 113.7, 130.5, 130.7, 153.7, 185.1, 196.1; MS: m/z = 260.1 [M]+; Analysis: for C15H20N2O2, calcd. C 69.20, H 7.74, N 10.76%; found C 69.22, H 7.72, N 10.70%.
3.4. General Synthesis of 4-(substituted phenyl)-5-[4-(piperazin/morpholin-1-yl)benzoyl]-3,4-dihydropyrimidin-2(1H)-one (1–20)
A mixture of enaminones, (2E)-4-methyl-1-[4-(piperazin/morpholin-1-yl) phenyl] pent-2-en-1-one (0.01 mol), differently substituted benzaldehydes (0.01 mol), urea (0.01 mol), and glacial acetic acid (10 mL) were refluxed for 3 h on a heating mantle. The reaction mixture was precipitated by pouring into the cold water. The products were obtained by vacuum filtration. The final products were recrystallized from glacial acetic acid and ethanol.
4-Phenyl-5-[4-(piperazin-1-yl)benzoyl]-3,4-dihydropyrimidin-2(1H)-one (1): Yield: 75%; m.p.: 150–152 °C; 1H NMR (500 MHz, DMSO-d6): δ = 9.41 (1H, s, NH, D2O exch), 8.50 (1H, s, NH, D2O exch), 8.0 (1H, s, =CH), 6.90–7.80 (9H, m, Ar–H), 6.0 (1H, s, H-4), 3.40 (2H, s, CH2 piperazine), 3.31 (2H, s, CH2 piperazine), 2.06 (2H, s, CH2 piperazine), 2.0 (2H, s, CH2 piperazine), 1.80 (1H, s, NH, D2O exch); 13C NMR (125.76 MHz, DMSO-d6): δ = 44.6 (CH2), 47.0 (CH2), 48.0 (CH), 50.10 (CH2), 65.5 (CH2), 111.5, 113.0, 114.0, 114.2, 124.4, 130.5, 134.1, 138.7, 148.0, 149.0, 151.0, 161.1, 168.0, 190.40 (C=O), 207.0 (C=O) MS: m/z = 362.42 [M]+; Analysis: for C21H22N4O2, calcd. C 69.59, H 6.12, N 15.46%; found C 69.32, H 6.10, N 15.40%.
4-(2-Nitrophenyl)-5-[4-(piperazin-1-yl)benzoyl]-3,4-dihydropyrimidin-2(1H)-one (2): Yield: 70%; m.p.: 170–172 °C; 1H NMR (500 MHz, DMSO-d6): δ = 9.40 (1H, s, NH, D2O exch), 8.52 (1H, s, NH, D2O exch), 8.04 (1H, s, =CH), 6.89–7.88 (8H, m, Ar–H), 6.07 (1H, s, H-4), 3.41 (2H, s, CH2 piperazine), 3.32 (2H, s, CH2 piperazine), 2.06 (2H, s, CH2 piperazine), 2.0 (2H, s, CH2 piperazine), 1.79 (1H, s, NH, D2O exch); 13C NMR (125.76 MHz, DMSO-d6): δ = 44.7 (CH2), 47.2 (CH2), 48.4 (CH), 50.11 (CH2), 65.4 (CH2), 111.7, 113.6, 114.1, 114.5, 124.4, 130.5, 134.1, 138.7, 148.3, 149.1, 151.2, 161.5, 168.9, 190.40 (C=O), 207.0 (C=O); MS: m/z = 407.40 [M]+; Analysis: for C21H21N5O4, calcd. C 61.91, H 5.20, N 17.19%; found C 62.15, H 5.22, N 17.12%.
4-(4-Nitrophenyl)-5-[4-(piperazin-1-yl)benzoyl]-3,4-dihydropyrimidin-2(1H)-one (3): Yield: 75%; m.p.: 175–177 °C; 1H NMR (500 MHz, DMSO-d6): δ = 9.41 (1H, s, NH, D2O exch), 8.28 (1H, s, =CH), 6.93–7.58 (8H, m, Ar–H), 7.93 (1H, s, NH, D2O exch), 5.55 (1H, s, H-4), 3.30 (2H, s, CH2 piperazine), 3.23 (2H, s, CH2 piperazine), 2.07 (2H, s, CH2 piperazine), 2.0 (2H, s, CH2 piperazine), 1.85 (1H, s, NH, D2O exch); 13C NMR (125.76 MHz, DMSO-d6): δ = 44.7 (CH2), 45.5 (CH2), 47.5 (CH), 48.0 (CH2), 53.9 (CH2), 118.8, 114.1, 124.3, 128.3, 130.6, 147.2, 151.6, 153.1, 161.3, 168.8, 190.4 (C=O), 207.0 (C=O); MS: m/z = 407.42 [M]+; Analysis: for C21H21N5O4, calcd. C 61.91, H 5.20, N 17.19%; found C 62.10, H 5.23, N 17.13%.
4-(3-Nitrophenyl)-5-[4-(piperazin-1-yl)benzoyl]-3,4-dihydropyrimidin-2(1H)-one (4): Yield: 75%; m.p.: 180–182 °C; 1H NMR (500 MHz, DMSO-d6): δ = 9.42 (1H, s, NH, D2O exch), 8.23 (1H, s, =CH), 6.93–7.65 (8H, m, Ar–H), 7.95 (1H, s, NH, D2O exch), 5.58 (1H, s, H-4), 3.31 (2H, s, CH2 piperazine), 3.23 (2H, s, CH2 piperazine), 2.07 (2H, s, CH2 piperazine), 2.01 (2H, s, CH2 piperazine), 1.87 (1H, s, NH, D2O exch); 13C NMR (125.76 MHz, DMSO-d6): δ = 44.7 (CH2), 47.2 (CH2), 48.0 (CH), 53.8 (CH2), 65.4 (CH2), 111.8, 114.1, 121.6, 122.9, 128.3, 130.3, 131.3, 133.7, 146.7, 148.2, 151.6, 161.3, 168.8, 190.5 (C=O), 207.0 (C=O); MS: m/z = 407.42 [M]+; Analysis: for C21H21N5O4, calcd. C 61.91, H 5.20, N 17.19%; found C 60.67, H 5.22, N 17.10%.
4-(4-Chlorophenyl)-5-[4-(piperazin-1-yl)benzoyl]-3,4-dihydropyrimidin-2(1H)-one (5): Yield: 75%; m.p.: 160–162 °C; 1H NMR (500 MHz, DMSO-d6): δ = 9.32 (1H, s, NH, D2O exch), 8.50 (1H, s, NH, D2O exch), 8.23 (1H, s, =CH), 6.95–8.11 (8H, m, Ar–H), 5.46 (1H, s, H-4), 3.31 (2H, s, CH2 piperazine), 3.23 (2H, s, CH2 piperazine), 2.09 (2H, s, CH2 piperazine), 2.04 (2H, s, CH2 piperazine), 1.90 (1H, s, NH, D2O exch); 13C NMR (125.76 MHz, DMSO-d6): δ = 44.7 (CH2), 47.3 (CH2), 48.4 (CH), 53.6 (CH2), 65.4 (CH2), 112.5, 114.1, 115.6, 116.0, 128.5, 140.0, 143.6, 148.1, 151.8, 153.1, 156.7, 161.3, 168.7, 190.5 (C=O), 207.0 (C=O); MS: m/z = 396.87 [M]+; Analysis: for C21H21ClN4O2, calcd. C 63.55, H 5.33, N 14.12%; found C 63.31, H 5.34, N 14.17%.
4-(2-Methoxyphenyl)-5-[4-(piperazin-1-yl)benzoyl]-3,4-dihydropyrimidin-2(1H)-one (6): Yield: 75%; m.p.: 120–122 °C; 1H NMR (500 MHz, DMSO-d6): δ = 9.22 (1H, s, NH, D2O exch), 8.51 (1H, s, NH, D2O exch), 8.13 (1H, s, =CH), 6.93–7.98 (8H, m, Ar–H), 5.77 (1H, s, H-4), 3.82 (3H, s, OCH3), 3.41 (2H, s, CH2 piperazine), 3.24 (2H, s, CH2 piperazine), 2.09 (2H, s, CH2 piperazine), 2.04 (2H, s, CH2 piperazine), 1.93 (1H, s, NH, D2O exch); 13C NMR (125.76 MHz, DMSO-d6): δ = 44.6 (CH2), 46.7 (CH2), 49.6 (CH), 55.9 (CH2), 56.1 (CH2), 111.1, 114.2, 120.6, 130.5, 131.0, 137.3, 154.0, 157.3, 158.5, 161.4, 168.8, 172.6, 187.1, 190.5, 196.1 (C=O), 207.0 (C=O); MS: m/z = 392.45 [M]+; Analysis: for C22H24N4O3, calcd. C 67.33, H 6.16, N 14.28%; found C 67.58, H 6.14, N 14.23%.
4-(4-Hydroxyphenyl)-5-[4-(piperazin-1-yl)benzoyl]-3,4-dihydropyrimidin-2(1H)-one (7): Yield: 75%; m.p.: 210–212 °C; 1H NMR (500 MHz, DMSO-d6): δ = 9.16 (1H, s, OH, D2O exch), 9.0 (1H, s, NH, D2O exch), 8.51 (1H, s, NH, D2O exch), 8.17 (1H, s, =CH), 6.71–8.08 (8H, m, Ar–H), 5.33 (1H, s, H-4), 3.35 (2H, s, CH2 piperazine), 3.20 (2H, s, CH2 piperazine), 2.07 (2H, s, CH2 piperazine), 2.01 (2H, s, CH2 piperazine), 1.82 (1H, s, NH, D2O exch); 13C NMR (125.76 MHz, DMSO-d6): δ = 44.7 (CH2), 48.1 (CH2), 49.2 (CH), 53.5 (CH2), 65.4 (CH2), 115.4, 116.0, 128.0, 129.6, 130.3, 151.7, 152.0, 153.0, 155.8, 156.4, 157.1, 159.1, 161.3, 168.8, 190.7 (C=O), 207.0 (C=O); MS: m/z = 378.42 [M]+; Analysis: for C21H22N4O3, calcd. C 66.65, H 5.86, N 14.81%; found C 66.40, H 5.84, N 14.86%.
4-(3-Hydroxyphenyl)-5-[4-(piperazin-1-yl)benzoyl]-3,4-dihydropyrimidin-2(1H)-one (8): Yield: 75%; m.p.: 158–160 °C; 1H NMR (500 MHz, DMSO-d6): δ = 9.8 (1H, s, OH, D2O exch), 9.10 (1H, s, NH, D2O exch), 8.04 (1H, s, =CH), 6.82–7.77 (8H, m, Ar–H), 6.71 (1H, s, NH, D2O exch), 5.32 (1H, s, H-4), 3.30 (2H, s, CH2 piperazine), 3.20 (2H, s, CH2 piperazine), 2.04 (2H, s, CH2 piperazine), 2.0 (2H, s, CH2 piperazine), 1.86 (1H, s, NH, D2O exch); 13C NMR (125.76 MHz, DMSO-d6): δ = 44.6 (CH2), 46.7 (CH2), 48.0 (CH), 53.8 (CH2), 56.5 (CH2), 115.5, 117.3, 120.1, 122.9, 128.6, 130.5, 136.7, 139.5, 143.0, 146.1, 152.1, 153.1, 154.0, 157.8, 161.4, 168.8, 172.6, 187.0, 190.6, 196.6 (C=O), 207.0 (C=O); MS: m/z = 378.42 [M]+; Analysis: for C21H22N4O3, calcd. C 66.65, H 5.86, N 14.81%; found C 66.39, H 5.83, N 14.85%.
4-(3-Methoxyphenyl)-5-[4-(piperazin-1-yl)benzoyl]-3,4-dihydropyrimidin-2(1H)-one (9): Yield: 70%; m.p.: 118–120 °C; 1H NMR (500 MHz, DMSO-d6): δ = 9.28 (1H, s, NH, D2O exch), 8.50 (1H, s, NH, D2O exch), 8.24 (1H, s, =CH), 6.88–8.11 (8H, m, Ar–H), 5.47 (1H, s, H-4), 3.73 (3H, s, OCH3), 3.33 (2H, s, CH2 piperazine), 3.20 (2H, s, CH2 piperazine), 2.09 (2H, s, CH2 piperazine), 2.04 (2H, s, CH2 piperazine), 1.92 (1H, s, NH, D2O exch); 13C NMR (125.76 MHz, DMSO-d6): δ = 44.6 (CH2), 46.8 (CH2), 47.3 (CH), 55.4 (CH2), 65.4 (CH2), 112.7, 114.1, 118.9, 130.5, 136.8, 139.9, 142.8, 146.1, 151.7, 152.0, 153.1, 154.0, 159.7, 160.1, 161.4, 172.7, 187.0, 190.6, 196.1 (C=O), 207.0 (C=O); MS: m/z = 392.45 [M]+; Analysis: for C22H24N4O3, calcd. C 67.33, H 6.16, N 14.28%; found C 67.57, H 6.14, N 14.23%.
4-(3-Ethoxyphenyl)-5-[4-(piperazin-1-yl)benzoyl]-3,4-dihydropyrimidin-2(1H)-one (10): Yield: 65%; m.p.: 88–90 °C; 1H NMR (500 MHz, DMSO-d6): δ = 9.21 (1H, s, NH, D2O exch), 8.52 (1H, s, NH, D2O exch), 8.20 (1H, s, =CH), 6.87–8.11 (8H, m, Ar–H), 5.40 (1H, s, H-4), 4.0 (2H, q, OCH2), 3.33 (2H, s, CH2 piperazine), 3.20 (2H, s, CH2 piperazine), 2.09 (2H, s, CH2 piperazine), 2.08 (2H, s, CH2 piperazine), 1.92 (1H, s, NH, D2O exch), 1.35 (3H, t, CH3); 13C NMR (125.76 MHz, DMSO-d6): δ = 15.0 (CH3), 44.6 (OCH2), 46.8 (CH2), 47.3 (CH2), 48.0 (CH), 48.5 (CH2), 63.7 (CH2), 114.2, 115.1, 128.0, 130.5, 130.9, 131.0, 153.9, 158.2, 160.8, 161.4, 172.5, 186.9, 190.6, 191.7, 196.1 (C=O), 207.0 (C=O); MS: m/z = 406.43 [M]+; Analysis: for C23H26N4O3, calcd. C 67.96, H 6.45, N 13.78%; found C 67.70, H 4.46, N 13.73%.
5-[4-(Morpholin-4-yl)benzoyl]-4-phenyl-3,4-dihydropyrimidin-2(1H)-one (11): Yield: 70%; m.p.: 258–260 °C; 1H NMR (500 MHz, DMSO-d6): δ = 9.21 (1H, s, NH, D2O exch), 7.79 (1H, s, =CH), 7.09–7.45 (6H, m, Ar–H), 7.01 (1H, s, NH, D2O exch), 6.95 (3H, m, Ar–H), 5.44 (1H, s, H-4), 3.74 (4H, t, J = 4.6 Hz, 2×CH2 morpholine), 3.22 (4H, t, J = 4.8 Hz, 2×CH2 morpholine); 13C NMR (125.76 MHz, DMSO-d6): δ = 47.6 (CH2), 47.7 (CH2), 54.0 (CH), 66.35 (CH2), 66.37 (CH2), 112.9, 113.81, 113.84, 126.8, 127.8, 128.5, 128.9, 130.4, 130.5, 139.8, 144.6, 151.9, 153.3, 153.5, 190.6 (C=O), 194.0 (C=O); MS: m/z = 363.42 [M]+; Analysis: for C21H21N3O3, calcd. C 69.41, H 5.82, N 11.56%; found C 69.58, H 5.80, N 11.59%.
5-[4-(Morpholin-4-yl)benzoyl]-4-(2-nitrophenyl)-3,4-dihydropyrimidin-2(1H)-one (12): Yield: 75%; m.p.: 198–200 °C; 1H NMR (500 MHz, DMSO-d6): δ = 9.42 (1H, d, NH, D2O exch), 8.07 (1H, s, =CH), 7.06–7.91 (8H, m, Ar–H), 6.93 (1H, s, NH, D2O exch), 6.08 (1H, s, H-4), 3.73 (4H, t, J = 4.6 Hz, 2×CH2 morpholine), 3.21 (4H, t, J = 4.7 Hz, 2×CH2 morpholine); 13C NMR (125.76 MHz, DMSO-d6): δ = 47.0 (CH2), 47.6 (CH2), 50.0 (CH), 66.2 (CH2), 66.3 (CH2), 111.7, 113.4, 124.4, 128.1, 129.2, 130.0, 132.6, 134.3, 138.7, 140.8, 148.3, 151.1, 153.5, 190.3 (C=O), 192.8 (C=O); MS: m/z = 408.43 [M]+; Analysis: for C21H20N4O5, calcd. C 61.76, H 4.94, N 13.72%; found C 61.90, H 4.92, N 13.77%.
5-[4-(Morpholin-4-yl)benzoyl]-4-(4-nitrophenyl)-3,4-dihydropyrimidin-2(1H)-one (13): Yield: 70%; m.p.: 202–204 °C; 1H NMR (500 MHz, DMSO-d6): δ = 9.42 (1H, d, NH, D2O exch), 8.23 (1H, s, =CH), 7.43–7.92 (6H, m, Ar–H), 7.09 (1H, s, NH, D2O exch), 6.94 (2H, d, J = 8.9 Hz, Ar–H), 5.57 (1H, s, H-4), 3.72 (4H, t, J = 4.6 Hz, 2×CH2 morpholine), 3.21 (4H, t, J = 4.7 Hz, 2×CH2 morpholine); 13C NMR (125.76 MHz, DMSO-d6): δ = 47.60 (CH2), 46.67 (CH2), 53.9 (CH), 66.2 (CH2), 66.3 (CH2), 111.8, 113.8, 124.3, 128.2, 128.3, 130.5, 134.0, 138.0, 140.7, 147.2, 151.7, 151.8, 153.6, 190.5 (C=O), 192.0 (C=O); MS: m/z = 408.42 [M]+; Analysis: for C21H20N4O5, calcd. C 61.76, H 4.94, N 13.72%; found C 61.90, H 4.92, N 13.76%.
5-[4-(Morpholin-4-yl)benzoyl]-4-(3-nitrophenyl)-3,4-dihydropyrimidin-2(1H)-one (14): Yield: 70%; m.p.: 205–207 °C; 1H NMR (500 MHz, DMSO-d6): δ = 9.40 (1H, d, NH, D2O exch), 8.15 (1H, s, =CH), 7.45–7.95 (6H, m, Ar–H), 7.12 (1H, s, NH, D2O exch), 6.95 (2H, d, J = 8.9 Hz, Ar–H), 5.59 (1H, s, H-4), 3.72 (4H, t, J = 4.6 Hz, 2×CH2 morpholine), 3.22 (4H, t, J = 4.7 Hz, 2×CH2 morpholine); 13C NMR (125.76 MHz, DMSO-d6): δ = 47.6 (2×CH2), 53.7 (CH), 66.3 (2×CH2), 111.8, 113.8, 121.6, 122.9, 128.2, 130.5, 130.7, 133.7, 140.8, 146.7, 148.2, 151.6, 153.6, 190.5 (C=O), 192.0 (C=O); MS: m/z = 408.41 [M]+; Analysis: for C21H20N4O5, calcd. C 61.76, H 4.94, N 13.72%; found C61.89, H 4.91, N 13.75%.
4-(4-Chlorophenyl)-5-[4-(morpholin-4-yl)benzoyl]-3,4-dihydropyrimidin-2(1H)-one (15): Yield: 80%; m.p.: 288–290 °C; 1H NMR (500 MHz, DMSO-d6): δ = 9.25 (1H, d, NH, D2O exch), 7.81 (1H, s, =CH), 7.33–7.45 (6H, m, Ar–H), 7.02 (1H, s, NH, D2O exch), 6.95 (2H, d, J = 8.5 Hz, Ar–H), 5.43 (1H, s, H-4), 3.73 (4H, t, J = 4.6 Hz, 2×CH2 morpholine), 3.22 (4H, t, J = 4.7 Hz, 2×CH2 morpholine); 13C NMR (125.76 MHz, DMSO-d6): δ = 47.6 (2×CH2), 53.6 (CH), 66.3 (2×CH2), 112.5, 113.8, 128.4, 128.8, 128.9, 130.5, 132.3, 140.1, 143.6, 151.8, 153.5, 190.6 (C=O), 192.0 (C=O); MS: m/z = 397.86 [M]+; Analysis: for C21H20ClN3O3, calcd. C 63.40, H 5.07, N 10.56%; found C 63.65, H 5.08, N 10.59%.
4-(2-Methoxyphenyl)-5-[4-(morpholin-4-yl)benzoyl]-3,4-dihydropyrimidin-2(1H)-one (16): Yield: 80%; m.p.: 178–180 °C; 1H NMR (500 MHz, DMSO-d6): δ = 9.22 (1H, s, NH, D2O exch), 7.81 (1H, s, =CH), 7.20–7.50 (5H, m, Ar–H), 7.09 (1H, s, NH, D2O exch), 6.89–7.01 (3H, m, Ar–H), 5.75 (1H, s, H-4), 3.82 (4H, t, J = 4.7 Hz, 2×CH2 morpholine), 3.21 (4H, t, J = 4.7 Hz, 2×CH2 morpholine); 13C NMR (125.76 MHz, DMSO-d6): δ = 47.6 (2×CH2), 49.6 (OCH3), 55.9 (CH), 66.2 (CH2), 66.3 (CH2), 111.5, 117.7, 113.8, 120.7, 127.9, 128.7, 129.3, 130.5, 131.3, 140.4, 152.2, 153.5, 157.3, 190.5 (C=O), 192.0 (C=O); MS: m/z = 393.41 [M]+; Analysis: for C22H23N3O4, calcd. C 67.16, H 5.89, N 10.68%; found C 66.89, H 5.87, N 10.64%.
4-(4-Hydroxyphenyl)-5-[4-(morpholin-4-yl)benzoyl]-3,4-dihydropyrimidin-2(1H)-one (17): Yield: 60%; m.p.: 118–120 °C; 1H NMR (500 MHz, DMSO-d6): δ = 9.14 (1H, s, NH, D2O exch), 9.01 (1H, s, OH), 8.08 (1H, s, =CH), 7.43–7.77 (4H, m, Ar–H), 7.07 (1H, s, NH, D2O exch), 6.70–7.05 (4H, m, Ar–H), 5.35 (1H, s, H-4), 3.74 (4H, t, J = 4.6 Hz, 2×CH2 morpholine), 3.21 (4H, t, J = 4.6 Hz, 2×CH2 morpholine); 13C NMR (125.76 MHz, DMSO-d6): δ = 47.0 (CH2), 47.6 (CH2), 53.5 (CH), 66.2 (CH2), 66.4 (CH2), 113.4, 113.8, 115.5, 128.0, 128.6, 130.5, 132.6, 135.2, 139.3, 152.0, 153.5, 154.5, 154.7, 190.7 (C=O), 191.8 (C=O); MS: m/z = 379.41 [M]+; Analysis: for C21H21N3O4, calcd. C 66.48, H 5.58, N 11.08%; found C 66.72, H 5.60, N 11.04%.
4-(3-Hydroxyphenyl)-5-[4-(morpholin-4-yl)benzoyl]-3,4-dihydropyrimidin-2(1H)-one (18): Yield: 60%; m.p.: 120–122 °C; 1H NMR (500 MHz, DMSO-d6): δ = 9.19 (1H, s, NH, D2O exch), 9.01 (1H, s, OH), 8.08 (1H, s, =CH), 7.43–7.77 (4H, m, Ar–H), 7.06 (1H, s, NH, D2O exch), 6.63–7.01 (4H, m, Ar–H), 5.37 (1H, s, H-4), 3.72 (4H, t, J = 4.6 Hz, 2×CH2 morpholine), 3.20 (4H, t, J = 4.6 Hz, 2×CH2 morpholine); 13C NMR (125.76 MHz, DMSO-d6): δ = 48.3 (CH2), 48.5 (CH2), 55.2 (CH), 66.7 (CH2), 67.6 (CH2), 114.6, 115.0, 131.2, 131.8, 138.9, 140.1, 140.8, 147.4, 153.4, 154.6, 154.8, 155.8, 159.2, 192.0 (C=O), 194.0 (C=O); MS: m/z = 379.42 [M]+; Analysis: for C21H21N3O4, calcd. C 66.48, H 5.58, N 11.08%; found C 66.63, H 5.59, N 11.12%.
4-(3-Methoxyhenyl)-5-[4-(morpholin-4-yl)benzoyl]-3,4-dihydropyrimidin-2(1H)-one (19): Yield: 60%; m.p.: 170–172 °C; 1H NMR (500 MHz, DMSO-d6): δ = 9.23 (1H, d, NH, D2O exch), 8.09 (1H, s, =CH), 7.24–7.79 (4H, m, Ar–H), 6.87 (1H, s, NH, D2O exch), 6.91–7.04 (4H, m, Ar–H), 5.45 (1H, s, H-4), 3.83 (3H, s, OCH3), 3.73 (4H, t, J = 4.6 Hz, 2×CH2 morpholine), 3.22 (4H, t, J = 4.6 Hz, 2×CH2 morpholine); 13C NMR (125.76 MHz, DMSO-d6): δ = 47.0 (CH2), 47.6 (CH2), 53.9 (OCH3), 55.4 (CH), 66.2 (CH2), 66.3 (CH2), 112.77, 112.79, 112.93, 113.8, 118.9, 128.5, 130.1, 130.5, 139.8, 146.1, 152.0, 153.5, 159.7, 190.7 (C=O), 192.0 (C=O); MS: m/z = 393.40 [M]+; Analysis: for C22H23N3O4, calcd. C 67.16, H 5.89, N 10.68%; found C 66.87, H 5.91, N 10.66%.
4-(4-Ethoxyphenyl)-5-[4-(morpholin-4-yl)benzoyl]-3,4-dihydropyrimidin-2(1H)-one (20): Yield: 60%; m.p.: 200–202 °C; 1H NMR (500 MHz, DMSO-d6): δ = 9.16 (1H, s, NH, D2O exch), 8.08 (1H, s, =CH), 6.86–7.77 (8H, m, Ar–H), 6.78 (1H, s, NH, D2O exch), 5.38 (1H, s, H-4), 3.97 (2H, q, J = 6.9 Hz, OCH2), 3.73 (4H, t, J = 4.6 Hz, 2×CH2 morpholine), 3.21 (4H, t, J = 4.6 Hz, 2×CH2 morpholine), 1.27 (3H, t, J = 6.9 Hz, CH3); 13C NMR (125.76 MHz, DMSO-d6): δ = 18.0 (CH3), 47.0 (CH2), 47.7 (CH2), 53.4 (OCH2), 63.2 (CH2), 66.3 (CH2), 113.2, 114.2, 125.8, 128.0, 129.5, 132.7, 136.7, 138.8, 139.5, 151.9, 153.3, 154.6, 157.1, 158.2, 190.7 (C=O), 192.8 (C=O); MS: m/z = 407.42 [M]+; Analysis: for C23H25N3O4, calcd. C 67.80, H 6.18, N 10.31%; found C 66.88, H 6.20, N 10.35%.
4. Conclusions
In conclusion, novel dihydropyrimidinone derivatives (1–20) containing piperazine and morpholine moieties were synthesized efficiently in good yield with a simple method consisting of three components in a single pot. The starting material, enaminones, 4-methyl-1-[4-(piperazin/morpholin-1-yl) phenyl] pent-2-en-1-one (IIa–b) were synthesized by reacting 4-methyl-1-[4-(piperazin/morpholin-1-yl) phenyl] pent-2-en-1-one (Ia–b) with dimethylformamide dimethylacetal (DMF–DMA) without solvent. The E -configuration of the enaminone was confirmed by the single crystal X-ray crystallography.
Author Contributions
Data curation, A.M.N.; Formal analysis, H.A.G.; Investigation, M.A.B.; Project administration, M.A.A.-O.
Funding
The authors would like to extend their sincere appreciation to the Deanship of Scientific Research at King Saud University for funding this research group no. (RG 1435–006).
Conflicts of Interest
The authors declare no conflict of interests.
Footnotes
Sample Availability: Samples of the compounds (1–20) with 99% purity are available from authors.
References
- 1.Folkers K., Harwood H.J., Johnson T.B. Researches on pyrimidines. cxxx. Synthesis of 2-keto-1,2,3,4-tetrahydropyrimidines. J. Am. Chem. Soc. 1932;54:3751–3758. doi: 10.1021/ja01348a040. [DOI] [Google Scholar]
- 2.Atwal K.S., Ahmed S.Z., Bird J.E., Delaney C.L., Dickinson K.E., Ferrara F.N., Hedberg A., Miller A.V., Moreland S., O’Reilly B.C., et al. Dihydropyrimidine angiotensin II receptor antagonists. Dihydropyrimidine calcium channel blockers. 3. 3-Carbamoyl-4-aryl-1,2,3,4-tetrahydro-6-methyl-5-pyrimidinecarboxylic acid esters as orally effective antihypertensive agents. J. Med. Chem. 1992;35:4751–4763. doi: 10.1021/jm00103a014. [DOI] [PubMed] [Google Scholar]
- 3.Rovnyak G.C., Kimball S.D., Beyer B., Cucinotta G., DiMarco J.D., Gougoutas J., Hedberg A., Malley M., McCarthy J.P., Zhang R., et al. Calcium entry blockers and activators: Conformational and structural determinants of dihydropyrimidine calcium channel modulators. J. Med. Chem. 1995;38:119–129. doi: 10.1021/jm00001a017. [DOI] [PubMed] [Google Scholar]
- 4.Rana K., Kaur B., Kumar B. Synthesis and antihypertensive activity of some dihydropyrimidines. Ind. J. Chem. 2004;43:1553–1557. doi: 10.1002/chin.200445173. [DOI] [Google Scholar]
- 5.Beena K.P., Suresh R., Rajasekaran A., Manna P.K. DihydroPyrimidinones-A Versatile Scaffold with Diverse Biological Activity. J. Pharm. Sci. Res. 2016;8:741–746. [Google Scholar]
- 6.Bhat M.A., Al-Dhfyan A., Al-Omar M.A. Targeting Cancer Stem Cells with Novel 4-(4-2-substitutedphenyl)-5-(3,4,5-trimethoxy/3,4-dimethoxy)-3benzoyl-3,4-ihydropyrimidine-2(1H)-one/thiones. Molecules. 2016;21:1746. doi: 10.3390/molecules21121746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Atwal K.S., Swanson B.N., Unger S.E., Floyd D.M., Moreland S., Hedberg A., O’Reilly B.C. Dihydropyrimidine calcium channel blockers. 3. 3-Carbamoyl-4-aryl-1,2,3,4-tetrahydro-6-methyl-5-pyrimidinecarboxylic acid esters as orally effective antihypertensive agents. J. Med. Chem. 1991;34:806–811. doi: 10.1021/jm00106a048. [DOI] [PubMed] [Google Scholar]
- 8.Kappe C.O. 100 Years of the Biginelli dihydropyridine synthesis. Tetrahedron. 1993;49:6937–6963. doi: 10.1016/S0040-4020(01)87971-0. [DOI] [Google Scholar]
- 9.Al-Ghorbani M., Bushra B.A., Zabiulla M.S.V., Mamatha S.V., Khanum S.A. Piperazine and morpholine: Synthetic preview and pharmaceutical applications. J. Chem. Pharm. Res. 2015;7:281–301. doi: 10.5958/0974-360X.2015.00100.6. [DOI] [Google Scholar]
- 10.Patel R.V., Park S.W. An evolving role of piperazine moieties in drug design and discovery. Mini-Rev. Med. Chem. 2013;13:1579–1601. doi: 10.2174/13895575113139990073. [DOI] [PubMed] [Google Scholar]
- 11.Lopez-Rodriguez M.L., Ayala D., Benhamu B., Morcillo M.J., Viso A. Arylpiperazine derivatives acting at 5-HT(1A) receptors. Curr. Med. Chem. 2002;9:443–469. doi: 10.2174/0929867023371030. [DOI] [PubMed] [Google Scholar]
- 12.Lacivita E., Leopoldo M., De Giorgio P., Berardi F., Perrone R. Determination of 1-aryl-4-propylpiperazine pKa values: The substituent on aryl modulates basicity. Bioorg. Med. Chem. 2009;17:1339–1344. doi: 10.1016/j.bmc.2008.12.015. [DOI] [PubMed] [Google Scholar]
- 13.Haupt A., Pohlki F., Drescher K., Wicke K., Unger L., Relo A., Bespalov A., Vogg B., Backfisch G., Delzer J., et al. N-Phenyl-(piperazinyl or homopiperazinyl)-benzenesulfonamide or Benzenesulfonyl-phenyl-(piperazine or homopiperazine) Compounds Suitable for Treating Disorders that Respond to Modulation of the Serotonin 5-HT-6 Receptor. WO 2010125134 A1. 2010 Nov 4;
- 14.Freire T.R.V., Mannochio D.S.R.E. Compounds and Pharmaceutical Compositions for Treating Disorders Associated with the 5-HT1a and 5-HT2a Receptors. WO 2012037634 A1. 2012 Mar 29;
- 15.Højer A.M., Drewes P.G., Kateb J. New Compositions of 1-[2-(2,4-dimethyl-phenylsulfanyl)-phenyl]piperazine. WO 2011023194 A2. 2011 Mar 3;
- 16.Ito N., Sasaki H., Tai K., Shinohara T. Heterocyclic Compounds for Treating or Preventing Disorders Caused by Reduced Neurotransmission of Serotonin, Norephnephrine or Dopamine. WO 2012036253 A1. 2012 Mar 22;
- 17.Bang-Andersen B., Mork A., Moore N., Stensbol T. 1-[2-(2,4-Dimethylphenylsulfanyl)-phenyl]piperazine as a Compound with Combined Serotonin Reuptake, 5-HT3 and 5-HT1a Activity for the Treatment of Cognitive Impairment. 20140248355 A1. U.S. Patent. 2014 Sep 4;
- 18.Zhang Y., Jin C., Zhou R. Preparation of Pyrimidinyl Piperazine Derivatives as Selective Serotonin Reuptake Inhibitors for the Treatment and Prevention of CNS Disorders. WO 2015014256 A1. 2015 Feb 5;
- 19.Achanath R., Jose J., Rangaswamy C., Mandal S., Balaji S., KadavilpparampuIan M.A.M., Newington I.M. Preparation of Piperazine Derivatives as Imaging Agents. WO 2013041682 A1. 2013 Mar 28;
- 20.Hoenke C., Giovannini R., Lessel U., Rosenbrock H., Schmid B. Piperazine Derivatives and the Use of Thereof as a Medicament. WO 2015055698 A1. 2015 Apr 23;
- 21.Jin L., Yang R., Song R., Zhu H., Wu N., Yun L., Su R., Zhao R. Preparation of Novel Piperazine Derivatives as Dopamine D3 Receptor Ligands. 2014/0329831 A1. U.S. Patent. 2014 Nov 6;
- 22.Ganesh T., Sun A., Smith S.M., Lambeth J.D. Preparation of Piperazine Derivatives for Use as NADPH-Oxidase Inhibitors. WO 2012173952 A1. 2012 Dec 20;
- 23.Yeung K.S., Farkas M.E., Kadow J.F., Meanwell N.A., Taylor M., Johnston D., Coulter T., Wright J.J. Indole, Azaindole and Related Heterocyclic N-Substituted Piperazine Derivatives and Their Preparation and Use for the Treatment of HIV Infection. 8039486 B2. U.S. Patent. 2011 Oct 18;
- 24.Sofia M.J., Kakarla R., Liu J., Naduthambi D., Mosley R., Steuer H.M. Preparation of Piperazine Derivatives and Their Uses to Treat Viral Infections, Including Hepatitis C. 20120202794 A1. U.S. Patent. 2012 Aug 9;
- 25.Carniato D., Briand J.F., Gutmann M., Busnel O., Bougeret C., Deprez B., Jaillardon K. Preparation of Piperazine Derivatives Useful for the Treatment of Cancers, Especially Cancers Resistant to Chemotherapy. WO 2013098393 A1. 2013 Jul 4;
- 26.Wang S., Zhou H., Chen J., Aguilar A., Meagher J.L., Sun D., Yang C., Liu L., Bai L., McEachem D., et al. Synthesis of Piperazine Derivatives as Bcl-2/Bcl-xL Inhibitors. 20120189539 A1. U.S. Patent. 2012 Jul 26;
- 27.Csonka I., Grolmusz V., Répási J., Szabadka Z., Szabo A., Kertesz M. Piperazine Derivatives and Their Preparation and Use as Tuberculostatics. WO 2011089456 A1. 2011 Jul 28;
- 28.Keliher E.J., Reiner T., Weissleder R. Preparation of Substituted Piperazine Derivatives for Use in Targeting PARP-1 for Detection and Imaging of Cancer. WO 2012074840 A2. 2012 Jun 7;
- 29.Kato S., Morie T., Hino K., Kon T., Naruto S., Yoshida N., Karasawa T., Matsumoto J. Novel benzamides as selective and potent gastric prokinetic agents. 1. Synthesis and structure-activity relationships of N-[(2-Morpholinyl)alkyl]benzamides. J. Med. Chem. 1990;33:1406–1413. doi: 10.1021/jm00167a020. [DOI] [PubMed] [Google Scholar]
- 30.Chrysselis M.C., Rekka E.A., Kourounakis P.N. Hypocholesterolemic and hypolipidemic activity of some novel morpholine derivatives with antioxidant Activity. J. Med. Chem. 2000;43:609–612. doi: 10.1021/jm991039l. [DOI] [PubMed] [Google Scholar]
- 31.Hale J.J., Mills S.G., MacCoss M., Dorn C.P., Finke P.E., Budhu R.J., Reamer R.A., Huskey S.E., Luffer-Atlas D., Dean B.J., et al. Phosphorylated morpholine acetal human neurokinin-1 receptor antagonists as water-soluble prodrugs. J. Med. Chem. 2000;43:1234–1241. doi: 10.1021/jm990617v. [DOI] [PubMed] [Google Scholar]
- 32.Kuettel S., Zambon A., Kaiser M., Brun R., Scapozza L., Perozzo R. Synthesis and evaluation of antiparasitic activities of new 4-[5-(4-Phenoxyphenyl)-2H-pyrazol-3-yl]morpholine derivatives. J. Med. Chem. 2007;50:5833–5839. doi: 10.1021/jm700938n. [DOI] [PubMed] [Google Scholar]
- 33.Takaya M., Sato M., Terashima K., Tanizawa H., Maki Y. A new Nonsteroidal analgesic-anti-inflammatory Agent. Synthesis and activity of 4-Ethoxy-2-methyl-5-morpholino-3(2H)-pyridazinone and related compounds. J. Med. Chem. 1979;22:53–58. doi: 10.1021/jm00187a013. [DOI] [PubMed] [Google Scholar]
- 34.Araki K., Kuroda T., Uemori S., Moriguchi A., Ikeda Y., Hirayama F., Yokoyama Y., Iwao E., Yakushiji T. Quinolone antimicrobial agents substituted with morpholines at the 7-Position, synthesis and structure-activity relationships. J. Med. Chem. 1993;36:1356–1363. doi: 10.1021/jm00062a007. [DOI] [PubMed] [Google Scholar]
- 35.Lukas R.J., Muresan A.Z., Damaj M.I., Blough B.E., Huang X., Navarro H.A., Mascarella S.W., Eaton J.B., Marxer-Miller S.K., Carroll F.I. Synthesis and characterization of in vitro and in vivo profiles of hydroxybupropion analogues: Aids to smoking cessation. J. Med. Chem. 2010;53:4731–4748. doi: 10.1021/jm1003232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bhat M.A., Ahmed A.F., Wen Z.H., Al-Omar M.A., Abdel-Aziz H.A. Synthesis, anti-inflammatory and neuroprotective activity of pyrazole and pyrazolo[3,4-d]pyridazine bearing 3,4,5-trimethoxyphenyl. Med. Chem. Res. 2017;26:1557–1566. doi: 10.1007/s00044-017-1870-5. [DOI] [Google Scholar]
- 37.Bhat M.A., Al-Rashood K.A., Abdel-Aziz H.A. Unexpected configuration in stereoslective synthesis of some novel (1Z)-1-(morpholin-1-yl)-N2-arylamidrazones. Lett. Org. Chem. 2012;9:487–492. doi: 10.2174/157017812802139636. [DOI] [Google Scholar]
- 38.Bhat M.A., Al-Omar M.A., Naglah A.M. Synthesis and in vivo anti-ulcer evaluation of some novel piperidine linked dihydropyrimidinone derivatives. J. Enzym. Inhib. Med. Chem. 2018;33:978–988. doi: 10.1080/14756366.2018.1474212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Al-Dhfyan A., Bhat M.A. Method for Treating Cancer Using a Dihydropyrimidine Derivative. 9,119,856 B1. U.S. Patent. 2015 Sep 1;
- 40.Allen F.H., Kennard O., Watson D.G., Brammer L., Orpen A.G., Taylor R. Tables of bond lengths determined by X-ray and neutron diffraction. Part 1. Bond lengths in organic compounds. J. Chem. Soc. Perkins Trans. 1987;12:S1–S19. doi: 10.1039/p298700000s1. [DOI] [Google Scholar]
- 41.Sheldrick G.M. A short history of SHELX. Acta Crystallogr. A. 2008;64:112–122. doi: 10.1107/S0108767307043930. [DOI] [PubMed] [Google Scholar]
- 42.Sheldrick G.M. SHELXTL-PC. Siemens Analytical Instruments, Inc.; Madison, WI, USA: 1997. version 5.1. [Google Scholar]