Abstract
Inflammatory bowel disease (IBD) is a common disease characterized by chronic inflammation in gastrointestinal tracts, which is primarily treated by administering anti-inflammatory and immunosuppressive drugs that inhibit the burden of intestinal inflammation and improve disease-related symptoms. However, the established therapeutic strategy has limited therapeutic efficacy and adverse drug reactions. Therefore, new disease-targeting drug-delivery strategies to develop more effective treatments are urgent. This review provides an overview of the drug-targeting strategies that can be used to treat IBD, and our recent attempts on the colon-specific delivery system (Pae-SME-CSC) with a paeonol-loaded self-microemulsion (Pae-SMEDDS) are introduced.
Keywords: targeted drug-delivery strategies, treatment, inflammatory bowel disease
1. Introduction
Inflammatory bowel disease (IBD) includes two major types of disease: Crohn’s disease (CD) and ulcerative colitis (UC) [1,2], which are chronic recurrent inflammatory diseases of the gastrointestinal tract involving the large intestine or colon [3]. UC and CD are considered different conditions; however, they share some common clinical features, such as cycles of relapse and remitting mucosal inflammation [4]. For UC, the inflammation is restricted to the colon and rectum continuously, with some cases even reaching the whole colon. Ulcerative colitis is one of the most common causes of colorectal cancer. Carcinogenesis is related to the time limit and extent of lesions of ulcerative colitis. The longer the disease course, the greater the chance of carcinogenesis in active cases with more extensive lesions. The incidence of ulcerated colorectal cancer is significantly higher than that of the general population. In the process of inflammatory hyperplasia, inflammatory or pseudopolyps are often formed and cancer occurs. However, it takes a long time and the cancerous rate of this colonic polyp is low. The occurrence of cancerous changes in the karyotype is more common in the undifferentiated type, with a higher degree of malignancy and a poorer prognosis [5]. CD affects any region of the GI tract, with the terminal ileum and the colon commonly affected, and the inflammation is generally noncontinuous [6,7]. The exact cause of IBD is uncertain, but some factors have been suggested, such as immunological, genetic and environmental [8].
The cause and cure for IBD are yet to be discovered, and therapeutic strategies are aimed towards attaining and maintaining remission from inflammatory episodes. Intestinal mucosa of patients with IBD has been previously reported and is characterized by overproduction of reactive oxygen species (ROS) and an imbalance of important antioxidants, leading to oxidative damage. Self-sustaining cycles of oxidant production may amplify inflammation and mucosal injury [7,8]. Thus, the main goal of IBD treatment is to prevent frequent recurrence of inflammation and maintain remission. Nonenzymatic therapies include drugs classified as aminosalicylate, corticosteroids, immunosuppressive agents and biological agents. Corticosteroids such as synthetic prednisone are still the most effective treatment in activated stage of IBD. These steroids act on the immune response in a wide range of ways, and patients often have long-term dependence on these drugs. 5-aminosalicylic acid (5-ASA) preparations such as mesalamine and the like are widely used for remission treatment. Chemotherapy can be administered by using azathioprine (AZA), its metabolite 6-mercaptopurine (6-MP) and methotrexate. Antibiotics such as metronidazole or ciprofloxacin are used in IBD to treat intestinal infections caused by high bacterial load (Bacteroides, Clostridium difficile). Although many patients are successfully treated with conventional medications (most relief), two-thirds require surgery. Further treatment strategies for IBD that target long-term treatment of lymphocytes and inflammatory cytokines have been designed and under investigation. Traditionally, drugs mediating these desired effects, such as salicylic acid, glucocorticoid, immunosuppressive agents, immune modulators, and other conventional drugs [9], are usually administered in high doses and/or systemically, leading to significant adverse events. Therefore, the prevention and reduction of drug-related side effects are highly challenging in IBD treatment [10].
The oral dosage form is characterized by low production cost, easy handling for the patient, accurate dose, and excellent stability and storage. In contrast, the oral route is affected by changes in intestinal absorption, metabolism of the intestinal cells, and usually the liver is encountered in the portal circulation, thereby passing the first-pass effect. In general, the oral route is the most desirable and acceptable route for administering drugs in the treatment of IBD [11]. After oral formulations are administered, the dosage forms release the active ingredients into the intestinal lumen where they are absorbed by the gastrointestinal mucosa. Finally, the active ingredient reaches the systemic circulation and is distributed throughout the body. However, systemic adverse drug reactions may also occur and may affect the quality of life of patients. The marked differences in the intestinal tract environment of the gastrointestinal tract and the differences between the healthy and inflamed intestinal regions have facilitated the development of pharmaceutical technologies that specifically deliver the active compounds to the inflamed intestinal regions. Oral drug-delivery systems for the treatment of IBD have been developed and allow more or less effective delivery of drugs to the site of disease. Based on the persistent large intestine in UC, most oral dosage forms are used to treat the colon. Therefore, it is much more difficult to treat discrete areas of inflammation in the CD by using an oral drug-delivery system [12].
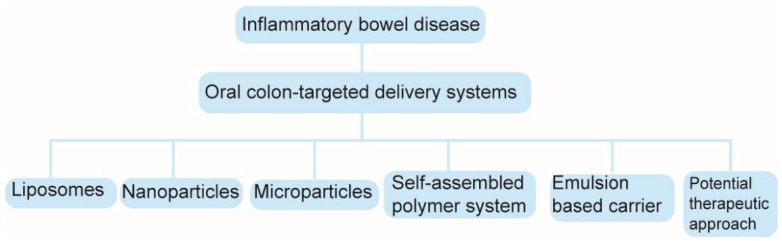
Targeting preparations could improve pharmacological effects and reduce adverse reactions [13]. Targeted drug delivery can be divided based on carriers. These include liposomes, microparticles, nanoparticles and emulsions. Among recent techniques used for colon-specific delivery, micro- and nanoparticles are well known for achieving site specificity and increasing drug stability via encapsulation [14]. The main objective of targeted drug strategy is to target the maximum concentration of active agents in inflamed intestinal tissues by using selective delivery to achieve therapeutic efficacy, while simultaneously reducing adverse effects. In addition, such a targeted delivery system must meet the conditions for complete biodegradation and high biocompatibility without pro-inflammatory properties [15]. Nanotherapeutics [16,17], therapeutic targets [18] and colon-targeted oral drug-delivery systems [19] for inflammatory bowel disease have been reviewed. In our review, new targeting preparations in IBD therapy are systematically reviewed. The structural diagram of these oral colon-targeted delivery systems is shown in Figure 1. This paper provides a valuable pharmaceutical strategy for studying the formula and preparation technology of oral colon-targeted delivery systems that are suitable for treating IBD.
Figure 1.
Structural diagram of oral colon-targeted delivery systems in IBD.
2. Pharmaceutical Strategies
2.1. Liposomes
Liposomes are double-layer vesicle structures based on phospholipids that are enclosed in aqueous volumes. Liposomes exhibit highly compatible phospholipid vesicles that are capable of carrying hydrophilic (aqueous core) and lipophilic (in lipid bilayer) medicaments due to their amphoteric properties. A lipophilic drug is incorporated in the liposome at the same time the lipid membrane is formed, and the hydrophilic drug is dissolved in the aqueous medium. The lipid membrane is hydrated by continuous rotation, extrusion, sonication or other ways to form vesicles. These systems are designed using a controlled delivery system, but the system must be targeted and protected from changes in pH [20]. Liposomes, which are biodegradable and essentially nontoxic vehicles, can encapsulate both hydrophilic and hydrophobic materials [21]. The use of liposomes has been shown to selectively target inflamed tissues, with the disruption of the intestinal barrier function at the site of inflammation, allowing accumulation of particulate delivery carriers. Liposomes can also be modified to enhance binding and cellular uptake to diseased tissue with the use of cationic lipids or attachment of targeting ligands [22].
L. Li and his colleagues evaluated the preclinical antitumor activity of liposomal curcumin in colorectal cancer. In this study, the efficacy of liposomal curcumin with standard chemotherapeutic agents (oxaliplatin) was compared. Different ratios of total lipid to curcumin (w/w) were evaluated from 10:1 to 4:1. The optimized 10:1 ratio was selected based on the test to determine the optimal encapsulation efficiency of curcumin by the liposomes. In-vitro studies with curcumin liposomes and dose-dependent growth inhibition were observed, and apoptosis in LoVo and Colo205 human colorectal cancer cell lines was observed. Synergy was also observed in in-vitro LoVo cells with a 4:1 ratio of liposomal curcumin and oxaliplatin. In-vivo studies also showed significant tumor growth inhibition in Colo205 and LoVo cells, and the growth inhibition observed with liposome curcumin was higher in Colo205 cells than oxaliplatin. Antiangiogenic effects are seen when tumors from animals are treated with liposomal curcumin. By immunohistochemical observation of CD31, the expression of vascular endothelial growth factor and interleukin-8 was attenuated. Therefore, curcumin liposomes exhibit better in-vitro and in-vivo activity in colorectal cancer [23,24].
2.2. Nanoparticles
Granular carriers are small carrier-based lipids and polymer matrix systems, where the drug is dispersed or dissolved in a lipid or polymer matrix. These systems are called nanoparticles or microparticles, based on size. Nanoparticles (NPs) in nanoscale units consist of two types: solid lipid nanoparticles and nanostructured lipid particles. Microparticles, on the other hand, are usually micron-sized microspheres. In order to increase the efficacy of IBD treatment, these particle systems can be further modified by coating or encapsulating with alginate beads. This matrix system prevents rapid drug release and promotes controlled release by reducing mobility of the drug molecules bound to the solid matrix. The release of the drug in the matrix system is further influenced by matrix composition and particle structure [25]. NPs, with a diameter of 10–1000 nm, are drug-loaded particles that are prepared by taking natural polymers or synthetic chemicals as carriers. Drugs can be embedded or dissolved in NPs and adsorbed or coupled on their surface. Encapsulating drugs within NPs can improve the solubility and pharmacokinetics of drugs and, in some cases, enable further clinical development of new chemical entities that have stalled because of poor pharmacokinetic properties [26].
Solid lipid NPs (SLNs) are beneficial in terms of drug protection and prevention of degradation. As a result of the homogenization of SLNs, increase in entrapment efficiency and initial release results in an increase in the bioavailability of the encapsulated drug. Due to the slow degradation of the lipid matrix, SLNs have unique properties, such as micro size with high surface area, high drug-loading capacity, and extended drug release. These systems are typically prepared by applying high-pressure homogenate and ultrasonic treatment of molten lipids. Some NP carriers for the treatment of IBD are based on chitosan, poly (lactic-co-glycolic acid) (PLGA), Eudragit P-4135F, which is a new pH-sensitive polymer, and silica NPs [27]. Nanostructured lipid carriers (NLCs) are part of the nanometer linear system. These systems are mixed with solid and liquid lipids. Liquid lipids provide flexibility in the carrier system, allowing for improved drug loading. The concept behind its preparation is that the solid lipid crystals with a higher melting point form a lipid core, and then the liquid lipid forms an outer layer containing a higher amount of lipophilic drug. Advantages of this structure include providing oxidation and hydrolytic stability [28]. Table 1 shows the formula, preparation method and biological activity of different nanoparticles in treating UC.
Table 1.
Studies on the formula, preparation method and biological activity of different nanoparticles in treating ulcerative colitis (UC).
| Categories | Carrier Materials | Pharmaceutical Ingredients | Preparation Methods | Biological Activity | Ref. |
|---|---|---|---|---|---|
| Nanoparticles | Polymethacrylate (Eudragit RL) | Clodronate | Modified solvent-displacement method | Confirmed therapeutic benefit of ClNP in vivo | [29] |
| Nanoparticles | Poly (lactic acid) poly (ethylene glycol) block copolymer (PLA-PEG) | TNFα siRNA | Double emulsion/solvent evaporation | Powerful and efficient nanosized tools for delivering siRNAs into colonic macrophages | [30] |
| Nanoparticles | --- | Lipids, proteins, microRNAs (miRNAs), and ginger bioactive constituents (6-gingerol and 6-shogaol) | Derived from edible ginger | Improve inflammatory bowel disease (IBD) prevention and treatment with an added benefit of overcoming limitations such as potential toxicity and limited production scale | [31] |
| Nanoparticles | Polymeric mixtures of poly (lactic-co-glycolic) acid (PLGA) | Budesonide | Oil/water (O/W) emulsion-evaporation technique | An efficient delivery system for targeted drug delivery to the inflamed intestinal mucosa | [32] |
| Nanoparticles | PLGA 50:50 | Betamethasone | Oil-in-water solvent-evaporation method (simple oil/water emulsification technique) | Stable targeting moiety in the gastrointestinal tract | [33] |
| Nanoparticles | Eudragit FS30D, Eudragit RS100 | Budesonide | Oil-in-water emulsion method | An effective oral colon-targeted delivery system for colitis therapy | [34] |
| Nanocapsules | Eudragit S100 | Prednisolone | Nanoprecipitation method | Provide effective way of treatment of colonic disease | [35] |
| Nanoparticles | Polymeric mixtures of poly PLGA and a pH-sensitive methacrylate copolymer | Budesonide | An adaption of the modified spontaneous emulsification solvent diffusion method | Useful for colon-specific delivery in inflammatory bowel disease | [36] |
| Nanoparticles | PLGA | Budesonide | Oil-in-water (O/W) emulsion technique | Targeted drug delivery to the inflamed intestinal mucosa | [37] |
| Nanostructure lipid carriers (NLCs) | Precirol ATO®5, Miglyol 812 | Budesonide | High-pressure homogenization | A targeted drug-delivery system for IBD treatment | [38] |
| Nanoparticles | Trimethylchitosan (TMC) Eudragit® S100 PLGA, PEG-PLGA and PEG-PCL | Ovalbumin (OVA) | Water-in-oil-in-water solvent-evaporation method, ionic complexation/gelation method | The highest accumulation of ovalbumin (OVA) in inflamed colon | [10] |
| Nanoparticles | PLA | CD98 Fab′-bearing quantum dots (QDs) | A modified oil-in-water (O/W) emulsion solvent-evaporation technique | Active colitis-targeted delivery | [39] |
| Nanoparticles | EC | Betamethasone | Emulsification solvent-evaporation technique | A significantly higher mitigating effect | [40] |
| Nanoparticles | Eudragit RL PO | Silybin | Solvent-evaporation emulsification technique | Reduced TNF-a, IL-6 and MPO activity significantly | [41] |
| Nanoparticles | Enzyme-sensitive azo-polyurethane and pH-sensitive methacrylate copolymer | Budesonide | A quasiemulsion solvent diffusion with some modifcations | An effective and safe colon-targeted delivery system for colitis therapy | [42] |
| Nanoparticles | Novel pH-sensitive hydrolyzed polyacrylamide-grafted xanthan gum (PAAm-g-XG) | Curcumin | A modified version of the solvent-evaporation cross-linking technique | Suitable for colon targeting | [43] |
| Silica nanoparticles (SiNPs) | Silica | 5-Amino salicylic acid (5ASA) | --- | Combine advantages from selective drug targeting and prodrugs | [44] |
| Nanoparticles | Eudragit S100 (EU S100) | 5-Aminosalicylic acid (5-ASA) | Supercritical fluids (SEDS) technique | 5-ASA was imbedded into EU S100 in an amorphous state after SEDS processing and the SEDS process did not induce degradation of 5-ASA | [45] |
| Nanoparticles | Oxidation-responsive b-cyclodextrin material (OxbCD) | Tempol (Tpl) | A modified nanoprecipitation/self-assembly method | Reduce ulcerative colitis in mice effectively | [46] |
| Nanoparticles | EudragitR S100 | Curcumin–celecoxib combination | Emulsion solvent-evaporation technique | More efficacious than nanoparticles of either drugs or drug suspension | [47] |
| Nanovesicles | Hydrogenated soy phosphatidylcholine-coating polyethylene glycol-containing vesicles with chitosan and nutriose | Quercetin | --- | A marked amelioration of symptoms of 2,4,6-trinitrobenzenesulfonic acid-induced colitis | [48] |
2.3. Microparticles
Microparticles (MPs) used in IBD therapy often range from 1–150 µm in diameter and are designed to target inflamed intestinal tissues and/or to be internalized by immune cells. The most common methods for MP fabrication include the complex coacervation method. This involves the emulsion solvent-evaporation approach, spray-drying process, and solvent-extraction method. MPs can be divided into noncoated and coated forms. Noncoated MPs can be characterized as a system that encapsulates the drug directly into polymers [49]. Table 2 shows the formula, preparation method and biological activity of different microparticles in treating UC.
Table 2.
Studies on the formula, preparation method and biological activity of different microparticles in treating UC.
| Categories | Carrier Materials | Loaded-Ingredients | Preparation Methods | Biological Activity | Ref. |
|---|---|---|---|---|---|
| Microsphere | Chitosan-alginate | Icariin | Emulsification-internal gelation technique | Exert the colon-protective effects through reducing the inflammatory response | [50] |
| Microsphere | Eudragit S100 liquid paraffin | Metronidazole | Emulsification solvent-evaporation method | Enhance drug entrapment, and effect the drug release | [51] |
| Microsphere | PLGA microsphere | Glucagon-like peptide-2 | Solid-in-oil-in-water (S/O/W) method | Resistant to degradation and decreased the severity of dextran sulfate sodium (DSS)-induced ulcerative colitis | [52] |
| Microspheric vehicle | Microspheric vehicle formed by cationic konjac glucomannan (cKGM), phytagel | An antisense oligonucleotide against TNF-α | Water-in-oil (W/O) emulsion method | Significantly decreased the local level of TNF-α and alleviated the symptoms of colitis in the mice | [53] |
| Microsphere | pH-triggered Eudragit-coated chitosan microspheres | Curcumin | Emulsion crosslinking method followed by coating with Eudragit S-100 | A promising system for pH-dependent delivery of drug to colon in ulcerative colitis | [54] |
| Microsphere | The enzyme diamine oxidase (DAO) in CaCMS/alginate microspheres | The enzyme diamine oxidase (DAO) | --- | A procedure able to afford protection of the entrapped enzyme against gastrointestinal degradation | [55] |
| Microsphere | Colon-targeted microspheres which were compressed into tablets using the enzyme-dependent polymer (pectin) as coat | The nonsteroidal anti-inflammatory bumadizone calcium dihydrate | Quasi-emulsion solvent-diffusion method | Achieved significant decrease in myeloperoxidase activity and inflammation with delayed Tmax (4 h) and lower Cmax (2700 ng/mL) when compared to marketed product | [56] |
| Microsphere | Hydrogel microspheres of chitosan grafted with vinyl polymers | 5-Aminosalicylic acid (5-ASA) | Water-in-oil (W/O) emulsification method | Exhibited better therapeutic effects in comparison to 5ASA plain drug solution in oral administration | [57] |
| Microsphere | Chitosan microspheres | 5-ASA and camylofine dihydrochloride | Emulsion method followed by enteric coating with Eudragit® S-100 | Specific delivery of drug to the colon and reduce symptoms of ulcerative colitis | [58] |
| Microsphere | Eudragit L100 (EuL)-coated chitosan (Ch)–succinyl-prednisolone (SP) conjugate microspheres (Ch SP-MS/EuL) | Prednisolone (PD) | --- | Enhanced effectiveness of PD and reduced toxic side effects of PD greatly | [59] |
| Microsphere | Budesonide (BUD) guar gum microspheres | Budesonide (BUD) | Emulsion crosslinking technique | Prolong the acting time of BUD in vivo | [60] |
| Microsphere | Chitosan microparticles | Mesalamine | Emulsion chemical crosslinking technique | Maintain the drug concentration within target ranges for a long period of time | [61] |
| Microparticle | Kafrin microparticles | Prednisolone | A phase-separation method | The majority of the loaded prednisolone was not released in in-vitro conditions simulating the upper gastrointestinal tract | [62] |
| Microparticle | N-Succinyl-chitosan (SucCH) microparticle | 5-ASA | Spray-drying method | Improved efficacy in the healing of induced colitis in rats | [63] |
| Microsphere | pH-sensitive microspheres using Eudragit P4135F | Low-molecular-weight heparins (LMWH) | A double emulsion technique with either solvent extraction or evaporation | Exhibited a particle size adapted to the needs of inflammatory bowel disease therapy, an efficient LMWH encapsulation, and a pH-controlled drug release | [64] |
| Microparticle | Poly-ε-caprolactone (PCL) celecoxib-loaded microparticles | Celecoxib | Solvent-diffusion technique | Enhanced the bioavailability and extended the duration of drug-plasma concentration in rats | [65] |
2.4. Self-Assembled Polymer System
Self-assembled polymer systems consist of natural and synthetic polymers, which are oriented in specific shapes or forms, or swell in the presence of water or any suitable specific polar solvent system. These systems are commonly used in antifungal and topical forms to treat ulcers and cancers.
Hydrogels are crosslinked networks of hydrophobic polymers that are physically or chemically linked to each other. The system is expanded by absorbing large amounts of water, and the drug is released by swelling or by degradation of the polymer after swelling. The intestinal mucosal region acts as a hydrogel that provides effective control of release of the drug into the inflammatory site. Hydrogels can be classified as macroporous, microporous, or nonporous according to pore size formed by the entanglement of the polymer. The pore size of macroporous hydrogels is 0.1–1 μm. The microporous water gel pore is 100–1000 Å, and the nonporous hydrogel pore is 10–100 Å [66].
In our previous study, we provided evidence for paeonol as a novel therapeutic agent in the treatment of UC, which was isolated from Cynanchum paniculatum (Bge.) Kitag. or Aaeoina suffruticosa Andr. in traditional Chinese medicine [67]. We also developed satisfactory paeonol coating tablets with pH-time-delayed controlled release in the colon [68,69]. Moreover, we prepared the colon-specific delivery system (Pae-SME-CSC) with paeonol-loaded self-microemulsion (Pae-SMEDDS), and evaluated its properties in vitro and in vivo, especially the anti-inflammatory effects on UC rats. It indicated that the developed Pae-SME-CSC was suitable for colon-specific drug delivery [70].
2.5. Emulsion-Based Carrier
These carriers are formed by the dispersion of two or more immiscible liquids stabilized by a surfactant or an emulsifier. The emulsifier causes the liquid to disperse evenly into the continuous liquid medium and produce a physical exclusion between the droplets to avoid coalescence by coating the droplets and lowering the interfacial tension [71].
Microemulsions are thermodynamically stable isotropic dispersions. The biphasic immiscible liquid is stabilized by the interfacial membrane of the surfactant molecule bound to the co-surfactant. The relative concentration of these three components can be estimated by constructing a ternary phase diagram. The components are oil in water (o/w) or water in oil (w/o), in the range of 5–100 nm. Microemulsions (o/w and w/o) improve the oral bioavailability of drugs. These have additional formulation advantages, thermodynamic stability, easy sterilization by filtration, small droplet size, and high surface area, which provide increased surface area for absorption and delivery of drug molecules. Release from the microemulsion is controlled by the interaction between the drug surfactant and the distribution of the drug between the oil phase and the aqueous phase. These systems can be highly developed for the treatment of internal inflammatory diseases [72].
Nanoemulsions are a uniform population of particle droplets consisting of long-term thermodynamically stable lipids and surfactants. These droplets are usually composed of lipid monolayer surrounding the liquid lipid core. Nanoemulsions are prepared by high-pressure homogenization, which results in the formation of droplets of uniform size. Using nanoemulsions as a carrier system has already been studied for broad-spectrum antimicrobial activity against microorganisms. In-vivo studies reveal its efficacy for treating vaginal, fungal and respiratory infections against the skin and mucous membranes. Moreover, this may be effective in treating gastritis [73].
2.6. Potential Therapeutic Approach
The complement system is widely considered to protect the host from invading microorganisms. However, previous studies examining the activation of the complement system have shown that it may play a detrimental role in the pathogenesis of many inflammatory and immune diseases. Complement activation products include complement components (C) 3a, C4a, C5a and C5b-9, and membrane-attack complexes. The complement activation product complement component 5a (C5a) is a potent inflammatory peptide with a broad spectrum of functions. In-vivo and in-vitro studies have demonstrated that C5a plays an important role in inflammation. Li Zhiping studied the role of C5a in IBD using an experimental mouse colitis model. Colitis was induced in mice using 2,4,6-trinitrobenzene sulphonic acid (TNBS), followed by administration of C5a aptamer by intraperitoneal injection. The clinical signs of the disease, the histopathological analysis of the colon and the level of inflammatory components were examined. Symptoms of colitis, including altered behavior, weight loss, colon damage and increased inflammatory cytokines, attenuated after treatment of mice with TNBS-induced colitis containing C5a aptamers. By phenotypic observation, histological examination and levels of inflammatory cytokines demonstrated that aptamer-treated mice exhibited significant colitis-attenuating effects compared to untreated mice. Colitis is characterized by an imbalance between proinflammatory and anti-inflammatory media. Current research results show that C5a may play a key role in IBD inflammation [74].
3. Pharmacokinetic Studies
Pharmacokinetics is used to evaluate pharmaceutical preparations for slow-release drug-delivery systems. First, studying pharmacokinetic properties allows us to understand the absorption, distribution, metabolism and excretion characteristics of drugs in the body. Related pharmacokinetic parameters, combined with the physical and chemical properties of drugs, pharmacodynamic properties and clinical needs, help determine drug preparation’s necessity [75]. During drug preparation into a sustained-release drug-delivery system, pharmacokinetic principles are used to design dosage form, dose, release mode, release time, and other factors. In addition, pharmacokinetic studies are used to evaluate and monitor whether the system achieves the desired effect of sustained drug release. In recent years, the development of modern instrumental analysis technology brought new technologies for pharmacokinetic studies. These include liquid chromatography, ion-selective electrode method, gas chromatography, mass spectrometry, and application of tandem mass spectrometry in the detection of drug concentration in chemical drugs by modern instrumental analysis methods [76].
Ye Liu applied HPLC analysis using a Dikma Diamonsil C18 on a Shimadzu LC-20A HPLC system with an ultraviolet detector at room temperature. The wavelength of the ultraviolet detector was 245 nm. Water and ethanol (57:43 v/v) were used as the mobile phase at a flow rate of 1 mL/min. The prolongation of the half-life (t1/2), enhanced residence time (mean residence time, MRT), and decreased total clearance (CL) indicated that BUD microspheres could prolong the acting time of BUD in vivo [60]. Srinivas Mutalik prepared novel pH-sensitive hydrolyzed polyacrylamide-grafted xanthan gum (PAAm-g-XG) nanoparticles (NPs) loaded with curcumin for colonic delivery. Curcumin was better absorbed systemically in nanoparticulate form with increased Cmax (3-fold) and AUC (2.5-fold) than when delivered as free curcumin [43]. Hiraku Onishi prepared and evaluated simple Eudragit S100 microparticles loaded with prednisolone (ES-MP) and Eudragit S100-coated chitosan-succinyl-prednisolone conjugate microparticles (Ch-MP/ES) in vitro. It was demonstrated that Ch-MP/ES could enhance the efficacy of PD and reduce the toxic side-effect of PD, while ES-MP could hardly improve the effects of PD. Only Ch-MP/ES significantly changed in-vitro and in-vivo characteristics and was found to improve PD’s in-vivo function [77].
4. Conclusions
IBD is a chronic disease that has an immunization period when the disease is not active. Hence, most patients need to maintain drug therapy to relieve symptoms and shorten the number and severity of seizures [78]. Treatment of IBD is minimal, but some drugs can reduce the severity of inflammation, increase the duration of remission, and reduce the risk of more serious health problems, such as colorectal cancer. Years of research have demonstrated the suitability of the colon as an absorption site, especially in GI diseases [79]. Conventional drug-delivery systems rely primarily on several nonstable parameters in the gastrointestinal tract, such as changes in pH and local enzyme-induced drug release. New drug-delivery systems, particularly multiparticle systems such as microspheres and nanoparticles, exhibit higher drug-delivery capability due to their specific accumulation, spread and long-term retention in the target inflammatory tissue compared with conventional single-unit modes [80]. Unfortunately, the problem associated with the nanoparticle process is it exhibits altered physical and chemical properties and potential risk of causing possible toxicity compared with its larger counterpart. Abiotic nanoparticle carriers can alter normal cellular activity and cause cytotoxicity because the particles cause wrinkling to the cell membrane, cytoskeleton rearrangement, and phagocytosis leading to their entry into phagocytic cells [81]. Thus, biotechnology systems with fewer side effects may have great potential in future IBD treatments. A highly biological approach in IBD treatment with a drug-delivery system does not alter normal cellular function. This may be the best method to achieve satisfactory effects in IBD therapy. Although studies on the effects of new multiparticle systems in the human gastrointestinal tract during IBD treatment are limited and need further exploration, we see that these systems will be used in combination with new biological agents in the near future to achieve maximum targeted drug efficacy at lower drug doses and side effects due to their superior advantages, such as sustainability and controlled release.
Author Contributions
B.W. and T.Z. contributed to the conception of the review; Y.G. contributed significantly to complete manuscript preparation; S.Z., Y.P. and B.X. contributed to the constructive discussions. All authors read and approved the review.
Funding
This research was funded by grants from Program of Shanghai Committee of Science and Technology (Grant No. 17401902300), Program of Shanghai Academic/Technology Research Leader (Grant No. 18XD1403700) and the National Scientific and Technological Major Special Project of China (Grant No. 2017ZX09301068).
Conflicts of Interest
The authors declare there is no conflict of interest.
References
- 1.Podolsky D.K. Inflammatory Bowel Disease. N. Engl. J. Med. 2002;347:417–429. doi: 10.1056/NEJMra020831. [DOI] [PubMed] [Google Scholar]
- 2.Li F.F., Wu G.L., Zheng H.X., Wang L., Zhao Z.B. Synthesis, colon-targeted studies and pharmacological evaluation of an anti-ulcerative colitis drug 4-Aminosalicylic acid-beta-O-glucoside. Eur. J. Med. Chem. 2016;108:486–494. doi: 10.1016/j.ejmech.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 3.Zigra P.I., Maipa V.E., Alamanos Y.P. Probiotics and remission of ulcerative colitis: A systematic review. Neth. J. Med. 2007;65:411–418. [PubMed] [Google Scholar]
- 4.Nimmons D. Elderly patients and inflammatory bowel disease. World J. Gastrointest. Pharmacol. Ther. 2016;7:51. doi: 10.4292/wjgpt.v7.i1.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yashiro M. Ulcerative colitis-associated colorectal cancer. World J. Gastroenterol. 2014;20:16389–16397. doi: 10.3748/wjg.v20.i44.16389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sartor R.B. Genetics and environmental interactions shape the intestinal microbiome to promote inflammatory bowel disease versus mucosal homeostasis. Gastroenterology. 2010;139:1816–1819. doi: 10.1053/j.gastro.2010.10.036. [DOI] [PubMed] [Google Scholar]
- 7.Di Sabatino A., Biancheri P., Rovedatti L., Macdonald T.T., Corazza G.R. Recent advances in understanding ulcerative colitis. Intern. Emerg. Med. 2012;7:103–111. doi: 10.1007/s11739-011-0719-z. [DOI] [PubMed] [Google Scholar]
- 8.Xavier R.J., Podolsky D.K. Unravelling the pathogenesis of inflammatory bowel disease. Nature. 2007;448:427–434. doi: 10.1038/nature06005. [DOI] [PubMed] [Google Scholar]
- 9.Carter M.J., Lobo A.J., Travis S.P., IBD Section of the British Society of Gastroenterology Guidelines for the management of inflammatory bowel disease in adults. Gut. 2004;53(Suppl. 5):V1–V16. doi: 10.1136/gut.2004.043372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dahan A., Amidon G.L., Zimmermann E.M. Drug targeting strategies for the treatment of inflammatory bowel disease: A mechanistic update. Expert Rev. Clin. Immunol. 2010;6:543–550. doi: 10.1586/eci.10.30. [DOI] [PubMed] [Google Scholar]
- 11.Coco R., Plapied L., Pourcelle V., Jerome C., Brayden D.J., Schneider Y.J., Preat V. Drug delivery to inflamed colon by nanoparticles: Comparison of different strategies. Int. J. Pharm. 2013;440:3–12. doi: 10.1016/j.ijpharm.2012.07.017. [DOI] [PubMed] [Google Scholar]
- 12.Lautenschlager C., Schmidt C., Fischer D., Stallmach A. Drug delivery strategies in the therapy of inflammatory bowel disease. Adv. Drug Deliv. Rev. 2014;71:58–76. doi: 10.1016/j.addr.2013.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Li D.C., Zhong X.K., Zeng Z.P., Jiang J.G., Li L., Zhao M.M., Yang X.Q., Chen J., Zhang B.S., Zhao Q.Z., et al. Application of targeted drug delivery system in Chinese medicine. J. Control. Release. 2009;138:103–112. doi: 10.1016/j.jconrel.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 14.Collnot E.M., Ali H., Lehr C.M. Nano- and microparticulate drug carriers for targeting of the inflamed intestinal mucosa. J. Control. Release. 2012;161:235–246. doi: 10.1016/j.jconrel.2012.01.028. [DOI] [PubMed] [Google Scholar]
- 15.Nidhi, Rashid M., Kaur V., Hallan S.S., Sharma S., Mishra N. Microparticles as controlled drug delivery carrier for the treatment of ulcerative colitis: A brief review. Saudi Pharm. J. SPJ. 2016;24:458–472. doi: 10.1016/j.jsps.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen Q., Xiao B., Merlin D. Nanotherapeutics for the treatment of inflammatory bowel disease. Expert Rev. Gastroenterol. Hepatol. 2017;11:495–497. doi: 10.1080/17474124.2017.1309282. [DOI] [PubMed] [Google Scholar]
- 17.Si X.-Y., Merlin D., Xiao B. Recent advances in orally administered cell-specific nanotherapeutics for inflammatory bowel disease. World J. Gastroenterol. 2016;22:7718–7726. doi: 10.3748/wjg.v22.i34.7718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Neurath M.F. Current and emerging therapeutic targets for IBD. Nat. Rev. Gastroenterol. Hepatol. 2017;14:269–278. doi: 10.1038/nrgastro.2016.208. [DOI] [PubMed] [Google Scholar]
- 19.Amidon S., Brown J.E., Dave V.S. Colon-targeted oral drug delivery systems: Design trends and approaches. AAPS PharmSciTech. 2015;16:731–741. doi: 10.1208/s12249-015-0350-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gupta A.S., Kshirsagar S.J., Bhalekar M.R., Saldanha T. Design and development of liposomes for colon targeted drug delivery. J. Drug Target. 2013;21:146–160. doi: 10.3109/1061186X.2012.734311. [DOI] [PubMed] [Google Scholar]
- 21.Zhang J.X., Wang K., Mao Z.F., Fan X., Jiang D.L., Chen M., Cui L., Sun K., Dang S.C. Application of liposomes in drug development--focus on gastroenterological targets. Int. J. Nanomed. 2013;8:1325–1334. doi: 10.2147/IJN.S42153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jubeh T.T., Nadler-Milbauer M., Barenholz Y., Rubinstein A. Local treatment of experimental colitis in the rat by negatively charged liposomes of catalase, TMN and SOD. J. Drug Target. 2006;14:155–163. doi: 10.1080/10611860600648429. [DOI] [PubMed] [Google Scholar]
- 23.Li L., Ahmed B., Mehta K., Kurzrock R. Liposomal curcumin with and without oxaliplatin: Effects on cell growth, apoptosis, and angiogenesis in colorectal cancer. Mol. Cancer Ther. 2007;6:1276–1282. doi: 10.1158/1535-7163.MCT-06-0556. [DOI] [PubMed] [Google Scholar]
- 24.Terse P., Mallya R. Importance of Colon Targeted Drug Delivery Systems in Herbal Medicines. Int. J. Pharm. Sci. Res. 2017;8:4513–4524. [Google Scholar]
- 25.Manju Rawat S., Kusum P., Deependra S. Lipid Matrix Systems with Emphasis on Lipid Microspheres: Potent Carriers for Transcutaneous Delivery of Bioactives. Curr. Drug Deliv. 2012;9:243–254. doi: 10.2174/156720112800389124. [DOI] [PubMed] [Google Scholar]
- 26.Takedatsu H., Mitsuyama K., Torimura T. Nanomedicine and drug delivery strategies for treatment of inflammatory bowel disease. World J. Gastroenterol. 2015;21:11343–11352. doi: 10.3748/wjg.v21.i40.11343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin C.H., Chen C.H., Lin Z.C., Fang J.Y. Recent advances in oral delivery of drugs and bioactive natural products using solid lipid nanoparticles as the carriers. J. Food Drug Anal. 2017;25:219–234. doi: 10.1016/j.jfda.2017.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Poonia N., Kharb R., Lather V., Pandita D. Nanostructured lipid carriers: Versatile oral delivery vehicle. Future Sci. OA. 2016;2:Fso135. doi: 10.4155/fsoa-2016-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Niebel W., Walkenbach K., Beduneau A., Pellequer Y., Lamprecht A. Nanoparticle-based clodronate delivery mitigates murine experimental colitis. J. Control. Release. 2012;160:659–665. doi: 10.1016/j.jconrel.2012.03.004. [DOI] [PubMed] [Google Scholar]
- 30.Laroui H., Viennois E., Xiao B., Canup B.S., Geem D., Denning T.L., Merlin D. Fab’-bearing siRNA TNFalpha-loaded nanoparticles targeted to colonic macrophages offer an effective therapy for experimental colitis. J. Control. Release. 2014;186:41–53. doi: 10.1016/j.jconrel.2014.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang M., Viennois E., Prasad M., Zhang Y., Wang L., Zhang Z., Han M.K., Xiao B., Xu C., Srinivasan S., et al. Edible ginger-derived nanoparticles: A novel therapeutic approach for the prevention and treatment of inflammatory bowel disease and colitis-associated cancer. Biomaterials. 2016;101:321–340. doi: 10.1016/j.biomaterials.2016.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ali H., Weigmann B., Collnot E.M., Khan S.A., Windbergs M., Lehr C.M. Budesonide Loaded PLGA Nanoparticles for Targeting the Inflamed Intestinal Mucosa--Pharmaceutical Characterization and Fluorescence Imaging. Pharm. Res. 2016;33:1085–1092. doi: 10.1007/s11095-015-1852-6. [DOI] [PubMed] [Google Scholar]
- 33.Moulari B., Beduneau A., Pellequer Y., Lamprecht A. Lectin-decorated nanoparticles enhance binding to the inflamed tissue in experimental colitis. J. Control. Release. 2014;188:9–17. doi: 10.1016/j.jconrel.2014.05.046. [DOI] [PubMed] [Google Scholar]
- 34.Naeem M., Choi M., Cao J., Lee Y., Ikram M., Yoon S., Lee J., Moon H.R., Kim M.S., Jung Y., et al. Colon-targeted delivery of budesonide using dual pH- and time-dependent polymeric nanoparticles for colitis therapy. Drug Des. Dev. Ther. 2015;9:3789–3799. doi: 10.2147/DDDT.S88672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kshirsagar S.J., Bhalekar M.R., Patel J.N., Mohapatra S.K., Shewale N.S. Preparation and characterization of nanocapsules for colon-targeted drug delivery system. Pharm. Dev. Technol. 2012;17:607–613. doi: 10.3109/10837450.2011.557732. [DOI] [PubMed] [Google Scholar]
- 36.Makhlof A., Tozuka Y., Takeuchi H. pH-Sensitive nanospheres for colon-specific drug delivery in experimentally induced colitis rat model. Eur. J. Pharm. Biopharm. 2009;72:1–8. doi: 10.1016/j.ejpb.2008.12.013. [DOI] [PubMed] [Google Scholar]
- 37.Ali H., Weigmann B., Neurath M.F., Collnot E.M., Windbergs M., Lehr C.M. Budesonide loaded nanoparticles with pH-sensitive coating for improved mucosal targeting in mouse models of inflammatory bowel diseases. J. Control. Release. 2014;183:167–177. doi: 10.1016/j.jconrel.2014.03.039. [DOI] [PubMed] [Google Scholar]
- 38.Beloqui A., Coco R., Alhouayek M., Solinis M.A., Rodriguez-Gascon A., Muccioli G.G., Preat V. Budesonide-loaded nanostructured lipid carriers reduce inflammation in murine DSS-induced colitis. Int. J. Pharm. 2013;454:775–783. doi: 10.1016/j.ijpharm.2013.05.017. [DOI] [PubMed] [Google Scholar]
- 39.Xiao B., Yang Y., Viennois E., Zhang Y., Ayyadurai S., Baker M., Laroui H., Merlin D. Glycoprotein CD98 as a receptor for colitis-targeted delivery of nanoparticles. R. Soc. Chem. 2014;2:1499–1508. doi: 10.1039/c3tb21564d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wachsmann P., Moulari B., Beduneau A., Pellequer Y., Lamprecht A. Surfactant-dependence of nanoparticle treatment in murine experimental colitis. J. Control. Release. 2013;172:62–68. doi: 10.1016/j.jconrel.2013.07.031. [DOI] [PubMed] [Google Scholar]
- 41.Varshosaz J., Minaiyan M., Khaleghi N. Eudragit nanoparticles loaded with silybin: A detailed study of preparation, freeze-drying condition and in vitro/in vivo evaluation. J. Microencapsul. 2015;32:211–223. doi: 10.3109/02652048.2014.995728. [DOI] [PubMed] [Google Scholar]
- 42.Naeem M., Cao J., Choi M., Kim W.S., Moon H.R., Lee B.L., Kim M.S., Jung Y., Yoo J.W. Enhanced therapeutic efficacy of budesonide in experimental colitis with enzyme/pH dual-sensitive polymeric nanoparticles. Int. J. Nanomed. 2015;10:4565–4580. doi: 10.2147/IJN.S87816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mutalik S., Suthar N.A., Managuli R.S., Shetty P.K., Avadhani K., Kalthur G., Kulkarni R.V., Thomas R. Development and performance evaluation of novel nanoparticles of a grafted copolymer loaded with curcumin. Int. J. Biol. Macromol. 2016;86:709–720. doi: 10.1016/j.ijbiomac.2015.11.092. [DOI] [PubMed] [Google Scholar]
- 44.Moulari B., Pertuit D., Pellequer Y., Lamprecht A. The targeting of surface modified silica nanoparticles to inflamed tissue in experimental colitis. Biomaterials. 2008;29:4554–4560. doi: 10.1016/j.biomaterials.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 45.Hu D., Liu L., Chen W., Li S., Zhao Y. A novel preparation method for 5-aminosalicylic acid loaded Eudragit S100 nanoparticles. Int. J. Mol. Sci. 2012;13:6454–6468. doi: 10.3390/ijms13056454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Zhang Q., Tao H., Lin Y., Hu Y., An H., Zhang D., Feng S., Hu H., Wang R., Li X., et al. A superoxide dismutase/catalase mimetic nanomedicine for targeted therapy of inflammatory bowel disease. Biomaterials. 2016;105:206–221. doi: 10.1016/j.biomaterials.2016.08.010. [DOI] [PubMed] [Google Scholar]
- 47.Gugulothu D., Kulkarni A., Patravale V., Dandekar P. pH-sensitive nanoparticles of curcumin-celecoxib combination: Evaluating drug synergy in ulcerative colitis model. J. Pharm. Sci. 2014;103:687–696. doi: 10.1002/jps.23828. [DOI] [PubMed] [Google Scholar]
- 48.Castangia I., Nacher A., Caddeo C., Merino V., Diez-Sales O., Catalan-Latorre A., Fernandez-Busquets X., Fadda A.M., Manconi M. Therapeutic efficacy of quercetin enzyme-responsive nanovesicles for the treatment of experimental colitis in rats. Acta Biomater. 2015;13:216–227. doi: 10.1016/j.actbio.2014.11.017. [DOI] [PubMed] [Google Scholar]
- 49.Kim J., Schlesinger E.B., Desai T.A. Nanostructured materials for ocular delivery: Nanodesign for enhanced bioadhesion, transepithelial permeability and sustained delivery. Ther. Deliv. 2015;6:1365–1376. doi: 10.4155/tde.15.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang Q.S., Wang G.F., Zhou J., Gao L.N., Cui Y.L. Colon targeted oral drug delivery system based on chitosan/alginate microspheres loaded with icariin in the treatment of ulcerative colitis. Int. J. Pharm. 2016;515:176–185. doi: 10.1016/j.ijpharm.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 51.Awasthi R., Kumar M. Development of Metronidazole-Loaded Colon-Targeted Microparticulate Drug Delivery System. Polym. Med. 2015;45:57–65. doi: 10.17219/pim/60583. [DOI] [PubMed] [Google Scholar]
- 52.Wu J., Qi K., Xu Z., Wan J. Glucagon-like peptide-2-loaded microspheres as treatment for ulcerative colitis in the murine model. J. Microencapsul. 2015;32:598–607. doi: 10.3109/02652048.2015.1065923. [DOI] [PubMed] [Google Scholar]
- 53.Huang Z., Gan J., Jia L., Guo G., Wang C., Zang Y., Ding Z., Chen J., Zhang J., Dong L. An orally administrated nucleotide-delivery vehicle targeting colonic macrophages for the treatment of inflammatory bowel disease. Biomaterials. 2015;48:26–36. doi: 10.1016/j.biomaterials.2015.01.013. [DOI] [PubMed] [Google Scholar]
- 54.Sareen R., Jain N., Rajkumari A., Dhar K.L. pH triggered delivery of curcumin from Eudragit-coated chitosan microspheres for inflammatory bowel disease: Characterization and pharmacodynamic evaluation. Drug Deliv. 2016;23:55–62. doi: 10.3109/10717544.2014.903534. [DOI] [PubMed] [Google Scholar]
- 55.Blemur L., Le T.C., Marcocci L., Pietrangeli P., Mateescu M.A. Carboxymethyl starch/alginate microspheres containing diamine oxidase for intestinal targeting. Biotechnol. Appl. Biochem. 2016;63:344–353. doi: 10.1002/bab.1369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Nour S.A., Abdelmalak N.S., Naguib M.J. Bumadizone calcium dihydrate microspheres compressed tablets for colon targeting: Formulation, optimization and in vivo evaluation in rabbits. Drug Deliv. 2015;22:286–297. doi: 10.3109/10717544.2014.889779. [DOI] [PubMed] [Google Scholar]
- 57.Jain S.K., Jain A., Gupta Y., Jain A., Khare P., Kannandasan M. Targeted delivery of 5-ASA to colon using chitosan hydrogel microspheres. J. Drug Deliv. Sci. Technol. 2008;18:315–321. doi: 10.1016/S1773-2247(08)50062-1. [DOI] [Google Scholar]
- 58.Dubey R., Dubey R., Omrey P., Vyas S.P., Jain S.K. Development and characterization of colon specific drug delivery system bearing 5-ASA and Camylofine dihydrochloride for the treatment of ulcerative colitis. J. Drug Target. 2010;18:589–601. doi: 10.3109/10611860903572933. [DOI] [PubMed] [Google Scholar]
- 59.Onishi H., Oosegi T., Machida Y. Efficacy and toxicity of Eudragit-coated chitosan-succinyl-prednisolone conjugate microspheres using rats with 2,4,6-trinitrobenzenesulfonic acid-induced colitis. Int. J. Pharm. 2008;358:296–302. doi: 10.1016/j.ijpharm.2008.02.015. [DOI] [PubMed] [Google Scholar]
- 60.Liu Y., Zhou H. Budesonide-loaded guar gum microspheres for colon delivery: Preparation, characterization and in vitro/in vivo evaluation. Int. J. Mol. Sci. 2015;16:2693–2704. doi: 10.3390/ijms16022693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Jin L., Ding Y.C., Zhang Y., Xu X.Q., Cao Q. A novel pH-enzyme-dependent mesalamine colon-specific delivery system. Drug Des. Dev. Ther. 2016;10:2021–2028. doi: 10.2147/DDDT.S107283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lau E.T.L., Johnson S.K., Stanley R.A., Mikkelsen D., Fang Z., Halley P.J., Steadman K.J. Preparation andIn VitroRelease of Drug-Loaded Microparticles for Oral Delivery Using Wholegrain Sorghum Kafirin Protein. Int. J. Polym. Sci. 2015;2015:343647. doi: 10.1155/2015/343647. [DOI] [Google Scholar]
- 63.Mura C., Nacher A., Merino V., Merino-Sanjuan M., Carda C., Ruiz A., Manconi M., Loy G., Fadda A.M., Diez-Sales O. N-Succinyl-chitosan systems for 5-aminosalicylic acid colon delivery: In vivo study with TNBS-induced colitis model in rats. Int. J. Pharm. 2011;416:145–154. doi: 10.1016/j.ijpharm.2011.06.025. [DOI] [PubMed] [Google Scholar]
- 64.Meissner Y., Ubrich N., El Ghazouani F., Maincent P., Lamprecht A. Low molecular weight heparin loaded pH-sensitive microparticles. Int. J. Pharm. 2007;335:147–153. doi: 10.1016/j.ijpharm.2006.11.014. [DOI] [PubMed] [Google Scholar]
- 65.Ghorab D.M., Amin M.M., Khowessah O.M., Tadros M.I. Colon-targeted celecoxib-loaded Eudragit(R) S100-coated poly-epsilon-caprolactone microparticles: Preparation, characterization and in vivo evaluation in rats. Drug Deliv. 2011;18:523–535. doi: 10.3109/10717544.2011.595841. [DOI] [PubMed] [Google Scholar]
- 66.Laroui H., Dalmasso G., Nguyen H.T., Yan Y., Sitaraman S.V., Merlin D. Drug-loaded nanoparticles targeted to the colon with polysaccharide hydrogel reduce colitis in a mouse model. Gastroenterology. 2010;138:843–853. doi: 10.1053/j.gastro.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 67.Zong S.Y., Pu Y.Q., Xu B.L., Zhang T., Wang B. Study on the physicochemical properties and anti-inflammatory effects of paeonol in rats with TNBS-induced ulcerative colitis. Int. Immunopharmacol. 2017;42:32–38. doi: 10.1016/j.intimp.2016.11.010. [DOI] [PubMed] [Google Scholar]
- 68.Xu B.-L., Cai Y.-Y., Zhang Q., Pu Y.-Q., Wang B., Zhang T., Tao J.-S., Wu P.-Y., Shanghai University of Traditional Chinese Medicine. Minhang Branch of Shanghai Tumor Hospital Affiliated to Fudan University Study on coating p H-time process for colonal delayed-release Paeonol Tablets. Chin. Tradit. Pat. Med. 2015;37:4. [Google Scholar]
- 69.Xu B.L., Yu Z.S., Zhang T., Wang B., Tao J.S. Colon targeting of paeonol colonic targeting tablets in rats. J. Math. Med. 2015;28:2. [Google Scholar]
- 70.Zong S., Pu Y., Li S., Xu B., Zhang Y., Zhang T., Wang B. Beneficial anti-inflammatory effect of paeonol self-microemulsion-loaded colon-specific capsules on experimental ulcerative colitis rats. Artif. Cells Nanomed. Biotechnol. 2018:1–12. doi: 10.1080/21691401.2017.1423497. [DOI] [PubMed] [Google Scholar]
- 71.Hua S., Marks E., Schneider J.J., Keely S. Advances in oral nano-delivery systems for colon targeted drug delivery in inflammatory bowel disease: Selective targeting to diseased versus healthy tissue. Nanomed. Nanotechnol. Biol. Med. 2015;11:1117–1132. doi: 10.1016/j.nano.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 72.Sushama T., Adnan A., Farhan J.A., Roop K.K., Shadab A.P., Zeenat I.K. Microemulsions: A Novel Approach to Enhanced Drug Delivery. Recent Pat. Drug Deliv. Formul. 2008;2:238–257. doi: 10.2174/187221108786241679. [DOI] [PubMed] [Google Scholar]
- 73.Kim H.S., Mason T.G. Advances and challenges in the rheology of concentrated emulsions and nanoemulsions. Adv. Colloid Interface Sci. 2017;247(Suppl. C):397–412. doi: 10.1016/j.cis.2017.07.002. [DOI] [PubMed] [Google Scholar]
- 74.Li Z., Wang X., Chen M., Wang Y., Sun R., Qu H., Sun Y., Gao W., Li B., Dong X., et al. Effectiveness of C5a aptamers in a TNBS-induced colitis mouse model. Exp. Ther. Med. 2017;14:6119–6124. doi: 10.3892/etm.2017.5277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Onishi H., Machida Y. In Vitro and In Vivo Evaluation of Microparticulate Drug Delivery Systems Composed of Macromolecular Prodrugs. Molecules. 2008;13:2136–2155. doi: 10.3390/molecules13092136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Xin Y., Kawashima J., Weng W., Kwan E., Tarnowski T., Silverman J.A. Pharmacokinetics and Safety of Momelotinib in Subjects with Hepatic or Renal Impairment. J. Clin. Pharmacol. 2017;58:522–532. doi: 10.1002/jcph.1050. [DOI] [PubMed] [Google Scholar]
- 77.Onishi H., Kikuchi H. Comparison of Simple Eudragit Microparticles Loaded with Prednisolone and Eudragit-Coated Chitosan-Succinyl-Prednisolone Conjugate Microparticles: Part II. In Vivo Evaluation of Efficacy, Toxicity, and Biodisposition Characteristics. Int. J. Mol. Sci. 2015;16:26125–26136. doi: 10.3390/ijms161125949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Thakral N.K., Ray A.R., Jacobsen J., Bar-Shalom D., Eriksson A.H., Majumdar D.K. Colon targeting of fluticasone propionate inclusion complex: A novel approach in inflammatory bowel disease. J. Incl. Phenom. Macrocycl. Chem. 2012;75:175–184. doi: 10.1007/s10847-012-0159-z. [DOI] [Google Scholar]
- 79.Omwancha W., Kouba C., Yelamanchili S., Neau S.H. Colon-specific drug delivery using ethylcellulose and chitosan in the coat of compression-coated tablets. Drug Dev. Ind. Pharm. 2011;37:945–953. doi: 10.3109/03639045.2010.551773. [DOI] [PubMed] [Google Scholar]
- 80.Talaei F., Atyabi F., Azhdarzadeh M., Dinarvand R., Saadatzadeh A. Overcoming therapeutic obstacles in inflammatory bowel diseases: A comprehensive review on novel drug delivery strategies. Eur. J. Pharm. Sci. 2013;49:712–722. doi: 10.1016/j.ejps.2013.04.031. [DOI] [PubMed] [Google Scholar]
- 81.Papadakis K.A., Shaye O.A., Vasiliauskas E.A., Ippoliti A., Dubinsky M.C., Birt J., Paavola J., Lee S.K., Price J., Targan S.R., et al. Safety and Efficacy of Adalimumab (D2E7) in Crohn’s Disease Patients with an Attenuated Response to Infliximab. Am. J. Gastroenterol. 2005;100:75–79. doi: 10.1111/j.1572-0241.2005.40647.x. [DOI] [PubMed] [Google Scholar]