Abstract
Objectives:
This study had 2 objectives: First, to explore the gender-related differences in emotional processing (EP) and theory of mind—both cognitive (CToM) and affective (AToM)—in patients with schizophrenia and in a control group of healthy subjects; and, second, to examine, from a gender perspective, the possible association between EP and CToM in the AToM performance.
Methods:
Forty patients with schizophrenia/schizoaffective disorder were recruited and matched by gender, age and years of education with 40 healthy subjects. EP was measured by the pictures of facial affect (POFA) test. CToM was measured using first- and second-order false-belief (FB) stories. AToM was measured by the reading the mind in the eyes test (RMET). Group and gender differences in CToM were analysed using the X2 test, whereas EP and AToM were analysed using the non-parametric Mann–Whitney U Test and a general linear model. Results were adjusted by intelligence quotient and negative symptomatology.
Results:
Patients with schizophrenia underperformed against healthy subjects in the POFA test, second-order FB, and RMET, but not in first-order FB. No significant gender differences were found. However, there was a trend showing that females outperformed males in the POFA (P = 0.056). Group (P < 0.001), POFA (P < 0.001) and second-order FB (P = 0.022) were the best factors predicting RMET performance (adjusted R2 = 0.584).
Conclusions:
Our results suggest that the illness is the main factor related to the deficit in social cognition, except for the basic aspects of the CToM that were unimpaired in most patients. Nevertheless, the influence of female gender in EP should not be neglected in any group. Finally, the hierarchal interaction between these domains is discussed.
Keywords: gender differences, social cognition, schizophrenia, emotion recognition, theory of mind, empathy.
Abstract
Objectifs:
Premièrement, explorer les différences liées au sexe du processus émotionnel (PE) et de la théorie de l’esprit, à la fois cognitive (TdEC) et affective (TdEA), chez les patients souffrant de schizophrénie et dans un groupe témoin de sujets en santé. Deuxièmement, examiner du point de vue du sexe l’association possible entre le PE et la TdEC dans le rendement de la TdEA.
Méthodes:
Quarante patients souffrant d’un trouble schizophrène/schizo-affectif ont été recrutés et appariés selon le sexe, l’âge, et les années de scolarité à 40 sujets en santé. Le PE a été mesuré à l’aide des images de l’expression faciale (IEF). La TdEC a été mesurée à l’aide des histoires de fausse croyance (FC) de premier ordre et de deuxième ordre. La TdEA a été mesurée par le test de lecture de l’état d’esprit dans les yeux (TLEEY). Les différences entre les groupes et les sexes dans la TdEC ont été analysées par le test chi carré, alors que le PE et la TdEA ont été analysés à l’aide du test U de Mann-Whitney non paramétrique et d’un modèle linéaire général. Les résultats ont été ajustés selon le quotient intellectuel et la symptomatologie négative.
Résultats:
Les patients ont réussi moins bien que les sujets en santé aux IEF, aux FC de deuxième ordre, et au TLEEY, mais pas aux FC de premier ordre. Aucune différence liée au sexe significative n’a été constatée. Cependant, une tendance des femmes à réussir mieux que les hommes aux IEF (p = 0,056) a été observée. Le groupe (p < 0,001), les IEF (p < 0,001) et les FC de deuxième ordre (p = 0,022) étaient les meilleurs facteurs pour prédire le rendement au TLEEY (R2 ajusté = 0,584).
Conclusions:
Nos résultats suggèrent que la maladie est le principal facteur lié au déficit de cognition sociale, excepté pour les aspects de base de la TdEC qui étaient intacts chez la plupart des patients. Néanmoins, l’influence du sexe féminin sur le PE ne devrait pas être négligée dans aucun groupe. Finalement, l’interaction hiérarchique entre ces domaines est discutée.
Introduction
Social cognition generally refers to the mental operations underlying social interaction,1,2 with emotional processing and theory of mind (ToM) being the most explored domains. Facial recognition of basic emotions is one of the most extended procedures to explore emotional processing,3 whereas ToM, the ability to infer and attribute mental states, comprises both cognitive and affective components of mentalization.4 Cognitive ToM is characterised by the requirement of a systematic cognitive process to understand thoughts and beliefs. Affective ToM is considered to engage an additional empathic and emotional approach to understand feelings.5,6 It is worth noting that, although inherently related, there is some evidence to suggest that both emotional processing and ToM components act partially independently. Moreover, studies exploring the neuroanatomical correlates of mentalization in healthy people and patients with schizophrenia support the existence of a dissociated, yet interacting, prefrontal network between both ToM processes.7–9 Interestingly, an overlap of activation between emotional processing and affective ToM neuronal networks has been described.10
In healthy people, men and women show differences in social cognition11,12 as well as in the social brain.13 In terms of emotional processing, women recognize facial expressions of basic emotions more accurately and faster than men. However, it is unclear whether this happens for all emotions, in all circumstances, and regardless of the gender of the expresser.14 There is mixed evidence regarding ToM; although, evidence points to differences in the strategies men and women use when processing social information, with women considered as stronger empathizers and men stronger systemizers.15 Furthermore, women seem to engage more emotional brain areas during social cognition tasks. Therefore, although there does appear to be gender-related differences in cognitive ToM, the advantage women have in mentalizing may only be more obvious for affective ToM.5 Nevertheless, we have not found any study that specifically explored the relationship between gender and ToM—both cognitive and affective—within healthy subjects.
Social cognition in patients with schizophrenia, on the other hand, has been widely explored,16 with patients showing moderate-to-severe deficits in emotional processing17,18 and ToM,19,20 as well as altered activation in several regions of the social brain.21 These deficits have been observed in patients with chronic schizophrenia, a first-episode of psychosis, those with an ultra-high risk (UHR) for psychosis, and first-degree unaffected relatives.22–26 Moreover, social cognition, and ToM in particular, are associated with poor work functioning, independent living, and social networks,27 having a greater predictive value than other neurocognitive domains.28 Along these lines, higher levels of negative symptoms are related to poor cognitive functioning, which may also contribute to the functional deficits described in schizophrenia.29,30
Currently, little is known about social cognition and gender in schizophrenia. According to Scholten et al.,31 emotional processing is disproportionately altered in men relative to women. Erol et al.32 found similar results, showing better performance for females, in a pattern that closely resembled performance by healthy subjects. In contrast, Vaskinn et al.33 failed to find any gender-related difference in visual-emotion perception. However, they observed that male patients performed much worse than their female counterparts on an auditory-emotion perception task. On the other hand, Kohler et al.19 found that the impact of gender in emotion perception was isolated to healthy subjects, with illness superseding any gender-related difference in schizophrenia. To our knowledge, only Abu-Akel and Bo34 have specifically explored the relationship between gender and specific aspects of ToM, with women showing superior mentalizing abilities. Interestingly, when comparing both processes, female patients with schizophrenia were better able to attribute and understand affective mental states.
A recurrent limitation in the literature is the over-representation of male patients in cohorts, and this discrepancy may compromise the generalizability of the current knowledge about social cognition in females.34 Therefore, given the multiple differences between men and women in the course and symptoms of schizophrenia—such as a more benign disease in females35,36—the assessment of gender-related differences in emotional processing and ToM is strongly required.
Considering previous evidence, we hypothesised that women with and without schizophrenia will outperform men in the affective ToM tasks, but not in the cognitive ToM tasks. In addition, the better affective ToM abilities of women will be directly related to their advantage in emotional processing skills.
The main objective of this pilot study was to examine the possible gender-related differences in 2 specific aspects of social cognition—emotional processing and ToM, both cognitive and affective—in a group of patients recently diagnosed with schizophrenia and in a control group of healthy subjects. As a secondary objective, we attempted to determine, from a gender perspective, the possible association between emotional processing and cognitive ToM in affective ToM performance.
Methods
Participants and Procedure
Forty patients (20 males, 20 females) meeting the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition criteria for schizophrenia/schizoaffective disorder were recruited from the Mental Health Department of the Parc Taulí University Hospital (Sabadell, Barcelona), and matched by gender, age and years of education with 40 healthy subjects (20 males, 20 females). Patients were included if they met the following inclusion criteria: 1) less than 5 y of onset of the disorder; 2) no changes in antipsychotic medication during the month prior to the study recruitment; and 3) clinical stability, defined as being an outpatient for at least 4 weeks prior to the study, a score of less than 4 in the items P1 (delusions), P2 (conceptual disorganization), and P3 (hallucinatory behaviour) of the Positive and Negative Syndrome Scale (PANSS),37 and a score less than 4 in the Calgary Depression Scale.38 Patients were excluded if they had an 1) intelligence quotient (IQ) less than or equal to 70, (2) a history of brain damage, or (3) were abusing substances (except nicotine and caffeine) for at least 12 mo before study enrolment. Healthy subjects were recruited among the students of an adult education centre. They were included if they were aged between 18 and 64 y, and they were excluded if had 1) an IQ less than or equal to 70; (2) a history of brain damage; (3) a history of psychiatric disorder, including substance abuse (except nicotine and caffeine) for at least 12 mo before study enrolment; and (4) family history of severe mental disease. All subjects had reached the age of 18 y, were informed about the characteristics of the study, accepted to participate voluntarily, and gave written informed consent. The study was approved by the local Ethics Committee (Ref. 2015534).
An expert psychiatrist interviewed each patient using the Structured Clinical Interview for the DSM-IV Axis I Disorders39 for diagnosis, reviewed the medical records, confirmed the inclusion and exclusion criteria, administered the clinical scales, and controlled the pharmacological treatment. Two expert neuropsychologists administered and corrected the cognitive evaluation. Another neuropsychologist interviewed all healthy subjects, confirmed the inclusion and exclusion criteria, and administered and corrected the cognitive evaluation.
Social Cognition Tasks
Three aspects of social cognition were assessed: emotional processing, cognitive ToM, and affective ToM. A higher score meant better social cognition abilities.
Emotional processing was measured by the Pictures Of Facial Affect (POFA) test.3 This task contains 60 black and white photographs of male and female faces with 6 multiple choice answers (happiness, sadness, anger, fear, disgust, and surprise). Participants were asked to recognize basic emotions through facial expression. All answers were scored by 0 (wrong) or 1 (correct). The total achievable score was 60. Internal consistency (Cronbach’s alpha) for the total score was 0.810.40
Cognitive ToM was measured using 4 false-belief (FB) stories. The “Sally & Anne” and “The Box of Chocolate” stories were applied to assess first-order cognitive ToM abilities, and “The Burglar” and “The Ice-Cream Van” for second-order cognitive ToM abilities.41,42 Stories were read aloud by the examiner and participants were asked to listen and answer a cognitive ToM question and a control question. All questions were scored as 0 (wrong) or 1 (correct), and 2 dichotomous variables were created to measure the failure/no failure in cognitive ToM; one for the 2 first-order FB stories and another one for the 2 second-order FB stories. Variables were categorised as 1 (no-failure) when the subject scored 1 in both cognitive ToM and control questions of the 2 same-order stories, and 0 (failure) when the subject scored 0 in both cognitive ToM questions and 1 in both control questions of the 2 same-order stories. Thus, the total achievable score was 0 (wrong) or 1 (correct) for each cognitive ToM order. Despite being one of the classical tasks in assessing ToM in patients with autism spectrum disorder, there is no data on the validity and reliability of these stories. However, in patients with schizophrenia, Mazza et al.43 have previously demonstrated the usefulness of these stories in detecting impairments in mentalization. In addition, a strict definition of failure/no failure was used, and all participants adjusted to this categorization.44,45
Affective ToM was measured by the Spanish version of the Reading the Mind in the Eyes Test (RMET).46,47 This task contains 36 photographs of male and female eyes with 4 multiple choice answers, including thoughts and feelings. Participants were asked to infer the mental states through the gaze. All answers were scored by 0 (wrong) or 1 (correct). The total achievable score was 36. Internal consistency (Cronbach’s alpha) for the total score was 0.704.48
Intelligence Quotient Assessment
IQ in patients with schizophrenia was obtained using an abbreviated form of the Wechsler Adult Intelligence Scale (WAIS-III), including Information, Block design, Arithmetic and Digit symbol subtests.49 IQ in healthy subjects was estimated using the Vocabulary subtest of the WAIS-III,50 which is a good measure of the general intelligence (r = 0.83; P = 0.05).51 A higher score meant better intellectual functioning.
Socio-demographic and Clinical Variables
Socio-demographic variables, such as manual dominance, current occupation, and civil status were also collected. Severity of clinical symptoms was rated with the PANSS.37 A lower score meant better clinical status.
Statistical Analysis
Socio-demographic and clinical variables were analysed using Pearson’s Chi-square (X2) test for qualitative variables and Student’s t-test (t) for quantitative variables.
The X2 test was used to study differences by group (healthy subjects-patients) and gender (males-females) in first- and second-order cognitive ToM (measured by the FB stories). Due to the lack of homoscedasticity in the POFA and RMET measures, group differences in emotional processing and affective ToM were analysed using the non-parametric Mann–Whitney U test. In parallel, differences in both variables were also analysed using a General Linear Model of repeated measures considering group and gender as the 2 conditions of the analysis. For these analyses, the POFA total score was used as a global measure of emotional processing skills.
Finally, a multiple linear regression analysis was conducted to study the interaction of illness, gender, emotional processing, and cognitive ToM in the affective ToM performance (measured by the RMET). The factors were group, gender, POFA total score and second-order FB stories. The model was adjusted for IQ and negative symptoms (measured by the PANSS_Negative).
Results
Socio-demographic and Clinical Variables
There were no significant group differences between healthy subjects and patients with schizophrenia for any socio-demographic variable, including age (mean [SD] 29.7 [6.4] v. 29.3 [6.7]; P = 0.609), years of education (13.1 [2.7] v. 13.2 [3.6]; P = 0.617), manual dominance (right-handed: 87.5% v. 90%, left-handed: 12.5% v. 7.5%, ambidextrous: 0% v. 2.5%; P = 0.649), and civil status (unmarried: 57.5% v. 70%, married: 42.5% v. 27.5%, divorced: 0% v. 2.5%; P = 0.250). Only IQ (101.9 [9.3] v. 84.7 [13.9]; P < 0.001) and current occupation (working: 79.5% v. 12.8%, studying: 12.8% v. 28.2%, and other: 7.7% v. 59%; P < 0.001) were significantly different. The socio-demographic and clinical characteristics between males and females in both groups are shown in Table 1.
Table 1.
Socio-Demographic and Clinical Characteristics of the Sample Organized by Group and Gender.
| Healthy Subjects | Patients with Schizophrenia | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Males | Females | X2 | T | P | Males | Females | X2 | T | P | |
| N | 20 | 20 | 20 | 20 | ||||||
| Age (y), mean (SD) | 29.0 (5.8) | 30.5 (7.1) | 0.58 | 0.450 | 28.6 (5.6) | 30.0 (7.9) | 2.51 | 0.524 | ||
| Education (y), mean (SD) | 13.1 (2.5) | 13.1 (3.0) | 1.51 | 0.954 | 13.0 (2.6) | 13.5 (4.4) | 0.54 | 0.667 | ||
| IQ, mean (SD) | 101.0 (9.5) | 102.9 (9.1) | 0.01 | 0.522 | 84.9 (13.9) | 84.5 (13.9) | 0.01 | 0.930 | ||
| Manual dominance, N (%) | 2.06 | 0.151 | 4.44 | 0.108 | ||||||
| Right-handed | 16 (80) | 19 (95) | 16 (80) | 20 (100) | ||||||
| Left-handed | 4 (20) | 1 (5) | 3 (15) | 0 (0) | ||||||
| Ambidextrous | 0 (0) | 0 (0) | 1 (5) | 0 (0) | ||||||
| Current occupation, N (%) | 3.70 | 0.157 | 0.29 | 0.865 | ||||||
| Working | 14 (70) | 18 (90) | 3 (15) | 2 (10) | ||||||
| Studying | 3 (15) | 2 (10) | 5 (25) | 6 (30) | ||||||
| Other (unemployed, pensioner) | 3 (15) | 0 (0) | 12 (60) | 12 (60) | ||||||
| Civil status, N (%) | 0.10 | 0.749 | 2.39 | 0.303 | ||||||
| Unmarried | 11 (55) | 12 (60) | 16 (80) | 12 (60) | ||||||
| Married | 9 (45) | 8 (40) | 4 (20) | 7 (35) | ||||||
| Divorced | 0 (0) | 0 (0) | 0 (0) | 1 (5) | ||||||
| Diagnosis, N (%) | 0.12 | 0.723 | ||||||||
| Schizophrenia | 15 (75) | 14 (70) | ||||||||
| Schizoaffective disorder | 5 (25) | 6 (30) | ||||||||
| Illness duration (y), mean (SD) | 2.4 (2.0) | 3.1 (2.0) | 0.01 | 0.342 | ||||||
| PANSS, mean (SD) | ||||||||||
| Total score | 54.7 (11.6) | 51.5 (11.5) | 0.18 | 0.396 | ||||||
| Positive | 9.9 (2.5) | 9.5 (1.9) | 1.58 | 0.610 | ||||||
| Negative | 18.3 (5.3) | 16.4 (4.9) | 0.30 | 0.249 | ||||||
| General | 26.4 (7.1) | 25.6 (5.4) | 2.71 | 0.669 | ||||||
N, number; SD, standard deviation; IQ, intelligence quotient; PANSS, Positive and Negative Syndrome Scale.
Social Cognition Variables
There were significant performance differences between healthy subjects and patients with schizophrenia in second-order FB stories (38 correct v. 29 correct; P = 0.006), POFA (52.45 [3.62] v. 44.45 [6.43]; P < 0.001) and RMET (28.95 [3.22] v. 22.35 [4.18]; p<0.001), but not in first-order FB stories (37 correct v. 35 correct; P = 0.456).
There were no significant performance differences between males and females in first- and second-order FB stories in any group (see Table 2).
Table 2.
Performance Differences in First- and Second-Order FB Stories (Cognitive ToM) between Males and Females in Both Groups.
| Healthy Subjects | X2 | P | Patients with Schizophrenia | X2 | P | |||
|---|---|---|---|---|---|---|---|---|
| Males | Females | Males | Females | |||||
| N | 20 | 20 | 20 | 20 | ||||
| First-order FB stories, N correct | 18 |
19 |
0.36 |
0.548 |
16 |
19 |
2.06 |
0.151 |
| Second-order FB stories, N correct | 18 | 20 | 2.10 | 0.147 | 16 | 13 | 1.13 | 0.288 |
N, number; FB, false-belief; ToM, theory of mind.
Means, main effects, and interactions of emotional processing and affective ToM variables are shown in Table 3. The main effects of group were observed in both the POFA and RMET. The main effect of gender was close to being statistically significant in the POFA but not in RMET. No significant group by gender interaction was observed in any variable. Performance graphs for the POFA and RMET are included (see Figures 1 and 2).
Table 3.
Performance Differences in POFA (Emotional Processing) and RMET (Affective ToM) between Males and Females in Both Groups.
| Males | Females | Group | Gender | Interaction | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Healthy Subjects | Patients with Schizophrenia | Healthy Subjects | Patients with Schizophrenia | F | P | F | P | F | P | |
| N | 20 | 20 | 20 | 20 | ||||||
| POFA, mean (SD)a | 51.35 (4.0) | 43.10 (7.5) | 53.55 (2.9) | 45.80 (5.0) | 58.76 | <0.001 | 3.89 | 0.056 | 0.06 | 0.812 |
| RMET, mean (SD)a | 28.05 (2.4) | 21.75 (4.3) | 29.85 (3.7) | 22.95 (4.1) | 69.26 | <0.001 | 3.04 | 0.089 | 0.14 | 0.707 |
N, number; SD, standard deviation; POFA, Pictures of Facial Affect; RMET, Reading the Mind in the Eyes Test; ToM, theory of mind. Significant main effects (P < 0.05): amain effect of group; bmain effect of gender; cinteraction effect group × gender.
Figure 1.
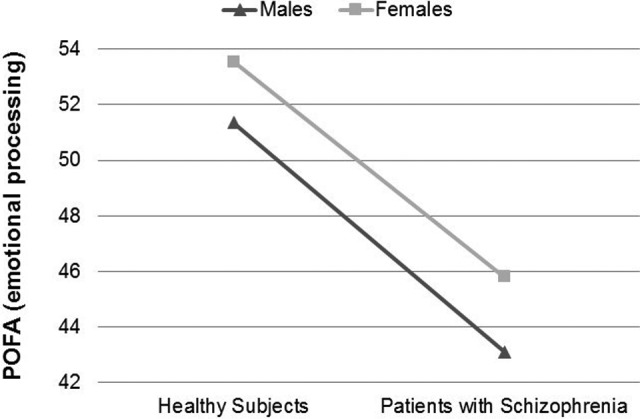
Performance differences in “Pictures Of Facial Affect” (POFA) test between males and females in both groups.
Figure 2.
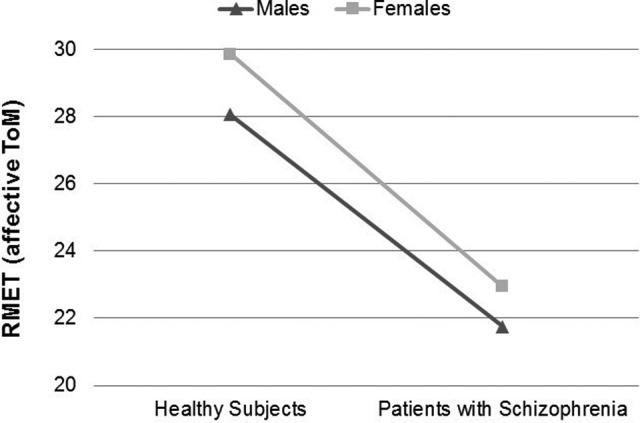
Performance differences in “Reading the Mind in the Eyes Test” (RMET) between males and females in both groups.
The regression model, adjusted by IQ and negative symptoms, obtained an adjusted R 2 = 0.584. First-order FB stories were not included in the final model due to the lack of significant differences previously found between both groups. Gender was not significant (B, 0.828; 95% CI, −0.65 to 2.30; P = 0.267), but group (B, 3.864; 95%CI, 2.00 to 5.72; P < 0.001), POFA (B, 0.299; 95% CI, 0.15 to 0.44; P < 0.001) and second-order FB stories (B, −2.423; 95%CI, −4.48 to −0.36; P = 0.022) were significant factors for the RMET performance.
Discussion
The main objective of this pilot study was to analyse the role of gender in the social cognition performance of patients with schizophrenia and healthy subjects. Our preliminary results suggest that the illness is the main factor related to both emotional processing and ToM deficit. However, although being a woman was not significant as a protective factor, the influence of female gender in emotional processing should not be neglected in any group.
Like that described in previous studies, we found that patients with schizophrenia performed worse than healthy subjects in emotional processing, second-order cognitive ToM and affective ToM.17–20 However, the performance in first-order cognitive ToM was unimpaired. According to Janssen et al.,52 first-order mentalizing tasks involve less complex cognitive ToM skills than second-order mentalizing tasks, so these abilities could be preserved in most patients. Interestingly, our results suggest that patients in the early stages of schizophrenia may present with a similar ToM profile to chronic patients,53 which is consistent with previous studies describing the stability of cognitive deficits throughout the course of the disease.22,23,25
Unlike other studies describing performance differences between males and females with schizophrenia in emotional processing and ToM,31,32,34 our results suggest that the effect of the illness may impose itself upon any gender-related difference, which is in accordance with the meta-analysis by Kohler et al.17 It is worth noting that these authors also found that gender-related differences in emotion perception are restricted to healthy people. However, our results do not support this statement. In any case, the trend showing that females with and without schizophrenia outperform males in emotional processing should not be ignored.
One of the main findings of the present study is how affective ToM abilities are determined by performance in other domains of social cognition and the existence of a schizophrenic disorder. In our study, a diagnosis of schizophrenia, POFA total score, and performance in the second-order FB stories were the best factors for predicting the ability to attribute and understand the affective mental states of others, as measured by the RMET. In contrast, IQ, negative symptoms, and gender were not significantly related to task performance. The final model explained at least 58% of the variance in the RMET score, suggesting a medium effect size influence of the illness, emotional processing skills, and cognitive ToM abilities in the affective ToM performance. In that regard, our results are in line with the findings of Mier et al.10 and the review by Mitchell and Philips,54 who describe the existence of shared neuronal networks between emotional processing and ToM, both cognitive and affective.
Although it is unclear the exact point at which social cognition deficits occur or fail to develop in the course of schizophrenia, alterations in emotional processing and ToM have been described in subjects who are UHR for psychosis and in first-degree unaffected relatives, and may be considered possible trait markers of most psychotic disorders.24–26 Thus, our results strengthen the notion that the disease plays a central role in social cognition impairment, along with the hierarchical interaction among its different domains. Nowadays, most models agree with the idea that social cognition domains are often linked at multiple levels. Specifically, the empathy model of Shamay-Tsoory et al.55 postulates that cognitive ToM is a prerequisite for affective ToM, which also requires intact emotional empathy processing (the ability to “share in” the internal mental states and emotions of others5). Meanwhile, Sebastian et al.6 argue that emotion perception and recognition may be necessary but not sufficient for empathy processes, which contributes to a successful affective ToM. What this suggests, is that affective ToM may result from the combination of 2 processes: an automatic non-cognitive process related to emotional processing and other aspects of empathy (such as emotional contagion and emotional priming5), and a higher-level conscious and integrative process related to cognitive ToM. Thus, our finding that emotional processing and cognitive ToM predict the affective ToM performance is consistent with previous studies.5,6,10,34,54
Along the same lines, the trend toward better emotional processing skills in female subjects may suggest that women, somehow, could perform better in affective ToM. However, due to the lack of significance in the RMET performance between males and females, an alternative explanation is that men and women employ distinct strategies when processing social information, rather than there being clear superior mentalizing skills in females. Therefore, we hypothesize that women may use more emotional strategies to perform slightly better in the affective ToM task.15 In that regard, 2 findings must be taken into account: First, that females seem to engage more emotional brain areas than males during social cognition5; and second, as reported by Mitchell and Philips, that an integrated decoding of emotions and intentions is likely to occur when performing the RMET, indicating a possible overlap between the functioning of the 2 concepts.54
Several limitations in the present study should be considered. First, although strict matching between healthy subjects and patients with schizophrenia has been used, the sample size could have reduced the significance of some results. Second, executive functions were not evaluated, and we were therefore unable to describe their relationship with second-order cognitive ToM, as per previous studies.45 Third, using non-ecological tasks could have limited the test sensitivity when trying to detect subtle differences in social cognition between males and females. Finally, the sample included subjects with schizophrenia and schizoaffective disorder. Although Kohler et al.17 found no cognitive differences between patients with these psychiatric conditions, all of these aspects should be considered when interpreting the results.
We also did not include a measure of functionality, which could also be a limitation. However, previous reviews and meta-analysis have clearly shown that social cognition, particularly ToM, is a well-established predictor of psychosocial functioning in patients with schizophrenia,28 with females outperforming males in most outcome measures.35,36 In that regard, it has been suggested that gender-related differences in social cognition may account for these differences.34 Moreover, two e-neurocognitive rehabilitation platforms have proven efficacious in improving emotional processing and ToM deficits in patients with schizophrenia.56,57 Thus, in terms of clinical implications, if our results are replicated with larger sample sizes, rehabilitation modules based on specific-gender profiles could be implemented in clinical and research contexts to test the efficacy of improving social cognition from a gender perspective, together with the impact in patitents functionality.
Finally, we can make the following conclusions. First, the effect of the illness may be the main factor related to the social cognition deficit in patients with schizophrenia, except for the basic aspects of cognitive ToM, which was unimpaired in most patients. However, the effect of gender in some specific aspects of social cognition, particularly emotional processing, should not be neglected. Second, we believe that our results align with previous studies describing different strategies for men and women when dealing with emotional information. Third, aside from a diagnosis of schizophrenia, emotional processing and cognitive ToM seem to be the best factors for predicting affective ToM performance, and this may indicate a dual-process relationship between 2 domains. Nonetheless, further studies with larger samples and including other psychiatric illnesses (e.g., unipolar depression, bipolar disorder) and more complex and ecological tasks (e.g., morphed faces, videotaped tasks)58 are needed to confirm our preliminary results in patients with schizophrenia and healthy control subjects.
Acknowledgments
We thank Rebeca García and Carla Hernández for their collaboration in the sample recruitment and psychiatric diagnosis. We also appreciate the collaboration of the Antaviana Mental Health Day Center (Sabadell, Barcelona) for helping us with the assessments of patients with schizophrenia, and the Morera Pomar Adult Education Center (Badalona, Barcelona) and Judith Joseph for helping us with the assessment of the healthy subjects. Finally, we thank the contribution of Joan Carles Oliva for the design of the statistical analysis.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
ClinicalTrials.gov Identifier: https://clinicaltrials.gov/ct2/show/NCT02575209
Funding: The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Green MF, Penn DL, Bentall R, et al. Social cognition in schizophrenia: an NIMH workshop on definitions, assessment, and research opportunities. Schizophr Bull. 2008;34(6):1211–1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Penn D, Sanna L, Roberts L. Social cognition in schizophrenia: an overview. Schizophr Bull. 2008;34(3):408–411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ekman P, Friesen WC. Pictures of facial affect. Palo Alto (CA): Consulting Psychologist Press; 1976. [Google Scholar]
- 4. Abu-Akel A, Shamay-Tsoory S. Neuroanatomical and neurochemical bases of theory of mind. Neuropsychologia. 2011;49(11):2971–2984. [DOI] [PubMed] [Google Scholar]
- 5. Christov-Moore L, Simpson EA, Coudé G, et al. Empathy: gender effects in brain and behavior. Neurosci Biobehav Rev. 2014;46(Pt 4):604–627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sebastian CL, Fontaine NMG, Bird G, et al. Neural processing associated with cognitive and affective theory of mind in adolescents and adults. Scan. 2012;7(1):53–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kalbe E, Schlegel M, Sack AT, et al. Dissociating cognitive from affective theory of mind: a TMS study. Cortex. 2010;46(6):769–780. [DOI] [PubMed] [Google Scholar]
- 8. Shamay-Tsoory SG, Aharon-Peretz J, Levkovitz Y. The neuroanatomical basis of affective mentalizing in schizophrenia: comparison of patients with schizophrenia and patients with localized prefrontal lesions. Schizophr Res. 2007;90(1-3):274–283. [DOI] [PubMed] [Google Scholar]
- 9. Shamay-Tsoory SG, Shur S, Barcai-Goodman L, et al. Dissociation of cognitive from affective components of theory of mind in schizophrenia. Psychiatry Res. 2007;149(1-3):11–23. [DOI] [PubMed] [Google Scholar]
- 10. Mier D, Lis S, Neuthe K, et al. The involvement of emotion recognition in affective theory of mind. Psychophysiology. 2010;47(6):1028–1039. [DOI] [PubMed] [Google Scholar]
- 11. Gur RC, Richard J, Calkins ME, et al. Age group and sex differences in performance on a computerized neurocognitive battery in children age 8–21. Neuropsychology. 2012;26(2):251–265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gur RC, Richard J, Hughett P, et al. A cognitive neuroscience-based computerized battery for efficient measurement of individual differences: standardization and initial construct validation. J Neurosci Methods. 2010;187(2):254–262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ingalhalikar M, Smith A, Parker D, et al. Sex differences in the structural connectome of the human brain. Proc Natl Acad Sci USA. 2014;111(2):823–828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Kret ME, De Gelder B. A review on sex differences in processing emotional signals. Neuropsychologia. 2012;50(7):1211–1221. [DOI] [PubMed] [Google Scholar]
- 15. Baron-Cohen S, Kinickmeyer RC, Belmonte MK. Sex differences in the brain: implications for explaining autism. Science. 2005;310(5749):819–823. [DOI] [PubMed] [Google Scholar]
- 16. Savla GN, Vella L, Armstrong CC, et al. Deficits in domains of social cognition in schizophrenia: a meta-analysis of the empirical evidence. Schizophr Bull. 2012;39(5):979–992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kohler CG, Walker JB, Martin A, et al. Facial emotion perception in schizophrenia: a meta-analytic review. Schizophr Bull. 2010;36(5):1009–1019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chan RC, Li H, Cheung EF, et al. Impaired facial emotion perception in schizophrenia: a meta-analysis. Psychiatry Res. 2010;178(2):381–390. [DOI] [PubMed] [Google Scholar]
- 19. Bora E, Yucel M, Pantelis C. Theory of mind impairment in schizophrenia: meta-analysis. Schizophr Res. 2009;109(1-3):1–9. [DOI] [PubMed] [Google Scholar]
- 20. Brüne M. “Theory of Mind” in schizophrenia: a review of the literature. Schizophr Bull. 2005;31(1):21–42. [DOI] [PubMed] [Google Scholar]
- 21. Grady CL, Keightley ML. Studies of altered social cognition in neuropsychiatric disorders using functional neuroimaging. Can J Psychiatry. 2002;47(4):327–336. [DOI] [PubMed] [Google Scholar]
- 22. Bora E, Murray RM. Meta-analysis of cognitive deficits in ultra-high risk to psychosis and first-episode psychosis: do the cognitive deficits progress over, or after, the onset of psychosis? Schizophr Bull. 2014;40(4):744–755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Green MF, Bearden CE, Cannon TD, et al. Social cognition in schizophrenia, part 1: performance across phase of illness. Schizophr Bull. 2012;38(4):854–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Lavoie MA, Plana I, Lavroix JB, et al. Social cognition in first-degree relatives of people with schizophrenia: a meta-analysis. Psychiatry Res. 2013;209(2):129–135. [DOI] [PubMed] [Google Scholar]
- 25. Bora E, Pantelis C. Theory of mind impairments in first-episode psychosis, individuals at ultra-high risk for psychosis and in first-degree relatives of schizophrenia: systematic review and meta-analysis. Schizophr Res. 2013;144(1-3):31–36. [DOI] [PubMed] [Google Scholar]
- 26. Lincoln SH, Norkett EM, Frost KH, et al. A developmental perspective on social-cognition difficulties in youth at clinical high risk for psychosis. Harv Rev Psychiatry. 2017;25(1):4–14. [DOI] [PubMed] [Google Scholar]
- 27. Horan WP, Green MF, Degroot M, et al. Social cognition in schizophrenia, part 2: 12-month stability and prediction of functional outcome in first-episode patients. Schizophr Bull. 2012;38(4):856–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fett AJ, Viechtbauer W, Dominguez M, et al. The relationship between neurocognition and social cognition with functional outcomes in schizophrenia: a meta-analysis. Neurosci Biobehav Rev. 2011;35(3):573–588. [DOI] [PubMed] [Google Scholar]
- 29. Fervaha G, Foussias G, Agid O, et al. Impact of primary negative symptoms on functional outcomes in schizophrenia. Eur Psychiatry. 2014;29(7):449–455. [DOI] [PubMed] [Google Scholar]
- 30. Bliksted V, Videbech P, Fagerlund B, et al. The effect of positive symptoms on social cognition in first-episode schizophrenia is modified by the presence of negative symptoms. Neuropsychology. 2017;31(2):209–219. [DOI] [PubMed] [Google Scholar]
- 31. Scholten MRM, Aleman A, Montagne B, et al. Schizophrenia and processing of facial emotions: sex matters. Schizophr Res. 2005;78(1):61–67. [DOI] [PubMed] [Google Scholar]
- 32. Erol A, Putgul G, Kosger F, et al. Facial emotion recognition in schizophrenia: the impact of gender. Psychiatry Investig. 2013;10(1):69–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vaskinn A, Sundet K, Friis S, et al. The effect of gender on emotion perception in schizophrenia and bipolar disorder. Acta Psychiatr Scand. 2007;116(4):263–270. [DOI] [PubMed] [Google Scholar]
- 34. Abu-Akel A, Bo S. Superior mentalizing abilities of female patients with schizophrenia. Psychiatry Res. 2013;210(3):794–799. [DOI] [PubMed] [Google Scholar]
- 35. Morgan VA, Castle J, Jablensky AV. Do women express and experience psychosis differently from men? Epidemiological evidence from the Australian National Study of Low Prevalence (Psychotic) Disorders. Aust N Z J Psyciatry. 2008;42(1):74–82. [DOI] [PubMed] [Google Scholar]
- 36. Ochoa S, Usall J, Cobo J, et al. Gender differences in schizophrenia and first-episode psychosis: a comprehensive literature review. Schizophr Res Treatment. 2012;2012:1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Andreasen NC, Carpenter WT, Kane JM, et al. Remission in schizophrenia: proposed criteria and rationale for consensus. Am J Psychiatry. 2005;162(3):441–449. [DOI] [PubMed] [Google Scholar]
- 38. Addington D, Addington J, Schissel B. A depression rating scale for schizophrenics. Schizophr Res. 1990;3(4):247–251. [DOI] [PubMed] [Google Scholar]
- 39. American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed Washingtion (DC): American Psychiatric Association; 1994. [Google Scholar]
- 40. Young A, Perret D, Calder A, et al. Facial expressions of emotion - stimuli and tests (FEST) Psychology manual v1.0. Bury St Edmunds (GB): Thames valley company; 2002. [Google Scholar]
- 41. Baron-Cohen S. The autistic child’s theory of mind: a case of specific developmental delay. J Child Psychol Psyhiatry. 1989;30(2):285–287. [DOI] [PubMed] [Google Scholar]
- 42. Happé FG. An advanced test of theory of mind: understanding of story characters’ thoughts and feelings by able autistic, mentally handicapped, and normal children and adults. J Autism Dev Disord. 1994;24(2):129–154. [DOI] [PubMed] [Google Scholar]
- 43. Mazza M, de Riso A, Surian L, et al. Selective impairments of theory of mind in people with schizophrenia. Schizophr Res. 2001;47(2-3):299–308. [DOI] [PubMed] [Google Scholar]
- 44. Fernandez-Gonzalo S, Pousa E, Jodar M, et al. Influence of the neuropsychological functions in theory of mind in schizophrenia: the false-belief/deception paradigm. J Nerv Ment Dis. 2013;201(7):609–613. [DOI] [PubMed] [Google Scholar]
- 45. Fernandez-Gonzalo S, Jodar M, Pousa E, et al. Selective effect of neurocognition on different theory of mind domains in first-episode. J Nerv Ment Dis. 2014;202(8):576–58 2 [DOI] [PubMed] [Google Scholar]
- 46. Baron-Cohen S, Wheelwright S, Hill J, et al. The “Reading the Mind in the Eyes” test revised version: a study with normal adults, and adults with Asperger syndrome or high-functioning autism. J Child Psychol Psyhiatry. 2001;42(2):241–251. [PubMed] [Google Scholar]
- 47. Fernandez-Abascal EG, Cabello R, Fernandez-Berrocal P. Test-retest reliability of the “Reading the Mind in the Eyes” test: a one-year follow-up study. Mol Autism. 2013;4(1):33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Pinkham AE, Penn DL, Green MF, et al. Social cognition psychometric evaluation: results of the initial psychometric study. Schizophr Bull. 2016;42(2):494–504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Blyler CR, Gold JM, Iannone VN, et al. Short form of the WAIS-III for use with patients with schizophrenia. Schizophr Res. 2000;46(2-3):209–215. [DOI] [PubMed] [Google Scholar]
- 50. Wechsler D. Escala de inteligencia Wechsler para adultos (WAIS-III manual). Madrid (Spain): TEA; 1999. [Google Scholar]
- 51. Kaufman AS, Lichtenberger EO. Claves para la evaluación con el WAIS-III. Madrid, Spain: TEA; 2004. [Google Scholar]
- 52. Janssen I, Krabbendam L, Jolles J, et al. Alterations in theory of mind in patients with schizophrenia and non-psychotic relatives. Acta Psychiatr Scand. 2003;108(2):110–117. [DOI] [PubMed] [Google Scholar]
- 53. Mazza M, Pollice R, Pacitti F. New evidence in theory of mind deficits in subjects with chronic schizophrenia and first episode: correlation with symptoms, neurocognition and social functions. 2012;47(4):327–336. [DOI] [PubMed] [Google Scholar]
- 54. Mitchell RL, Phillips LH. The overlapping relationship between emotion perception and theory of mind. Neuropsychologia. 2015;70(4):1–10. [DOI] [PubMed] [Google Scholar]
- 55. Shamay-tsoory SG, Harari H, Aharon-peretz J, et al. The role of the orbitofrontal cortex in affective theory of mind deficits in criminal offenders with psychopathic tendencies. Cortex. 2010;46(5):668–677. [DOI] [PubMed] [Google Scholar]
- 56. Fernandez-Gonzalo S, Turon M, Jodar M, et al. A new computerized cognitive and social cognition training specifically designed for patients with schizophrenia/schizoaffective disorder in early stages of illness: a pilot study. Psychiatry Res. 2015;228(3):501–509. [DOI] [PubMed] [Google Scholar]
- 57. Vazquez-Campo M, Maroño Y, Lahera G, et al. e-Motional Training®: a pilot study on novel online training program on social cognition for patients with schizophrenia. Schizophr Res Cogn. 2016;12(4):10–17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Bediou B, Krolak-Salmon P, Saoud M, et al. Facial expression and sex recognition in schizophrenia and depression. Can J Psychiatry. 2005;50(9):525–533. [DOI] [PubMed] [Google Scholar]


