Abstract
B-Flow imaging directly displays the flowing intravascular echoes during real-time gray-scale ultrasound without using Doppler techniques. The objective of our study was to evaluate the feasibility of B-Flow imaging in the placenta and to evaluate the artifacts seen on B-Flow imaging. After IRB approval, 36 women (17 normal and 19 high risk women) were enrolled in our study. B-Flow images were acquired on GE LOGIC E9 machine. Retrospective analysis of the B-Flow and cine capture images was performed for artifacts and for vessels visualized. Pregnant women enrolled in the study ranged from 19 to 43 years of age with an average age of 31.7 years. Gestational age varied from 17 weeks and five days to 36 weeks and three days with an average of 26 weeks and three days. From a total of 161 B-Flow images reviewed by one researcher, 15 images were acceptable with no evidence of artifact. The remainder of the images had some artifact in them. For the 36 women with color Doppler and B-Flow images reviewed by the two independent blinded reviewers, a total of 144 reads were obtained. More small horizontal (p = 0.046) and small vertical running vessels (p < 0.001) were identified with B-Flow than color Doppler images. B-Flow is capable of showing perfusion in a human placenta; however, recognizing artifacts and modifying the image acquisition to reduce artifacts is necessary with this new technique to acquire the best images for interpretation.
Keywords: B-Flow, Doppler, placenta, pregnancy
Introduction
Preeclampsia (PE) is a major cause of maternal and fetal morbidity and mortality1 and is thought to be predominantly as the consequence of impaired placentation. PE and intrauterine growth restriction (IUGR) affect 4–7% of all pregnancies and are thought to be related to chronic uteroplacental hypoperfusion.2 Human placentation is associated with important uterine vascular remodeling that permits a large increase in uterine blood flow of up to 600 ml/min during pregnancy.3 Evaluation of uteroplacental vascular modification during pregnancy using noninvasive methods such as ultrasound could be clinically important in order to predict development of PE or IUGR later in pregnancy.4,5
After implantation, trophoblast cells induce vascular remodeling, which begins first in the endometrium and then in adjacent myometrium. This can be detected by Doppler imaging.6–8 Until recently, the most promising method to screen for PE/IUGR was uterine artery and umbilical artery Doppler velocimetry by 2D pulsed Doppler. Published data suggest that impaired placental perfusion, which is associated with development of PE/IUGR, could be reflected in an increased uterine artery pulsatility index and increased umbilical artery resistive index.9–14 This approach, however, has some major disadvantages: Doppler velocimetry of the uterine or umbilical arteries is an indirect assessment of the placenta, and its sensitivity is too poor for the study of slow flow within the placenta itself.15 The very low flow seen in the placental vasculature may be visible by decreasing the pulse repetition frequency in color Doppler; however, this would lead to aliasing artifact and this would limit adequate visualization of the flow in the placenta on color Doppler. B-Flow imaging has been very sensitive to slow flow and may potentially be able to show the slow flow in the placenta easily.
New technology has enabled the quantitative analysis of blood flow in an organ using ultrasound. B-Flow imaging was introduced by General Electric Medical Systems during the late 1990s. It is a technique of displaying flow information without using Doppler technology. It directly displays the flowing intravascular echoes during real-time gray-scale ultrasound. Flow information is derived by digitally encoding the outgoing ultrasound beam, then decoding and filtering the returning beam so as to amplify echoes generated by the particulate constituents of flowing blood.16 The real-time B-Flow imaging appearance of blood flow consists of mobile intravascular echoes that simulate a conventional contrast angiogram, similar to the appearance of sonographic IV contrast agent. The technique is relatively simple to learn and operate with fewer parameters to manipulate than color Doppler ultrasound and is particularly sensitive to blood flow due to the imaging technique which is not flow direction specific. This can lead to better visualization of slow flow seen in the placental vasculature, even though quantification is not available at present; it will provide a subjective assessment of the flow in the placenta.
Previous studies using B-Flow imaging have shown that B-Flow imaging is very sensitive to flow since it is not dependent on Doppler phenomenon and is not direction sensitive and hence we postulate that B-Flow would be better able to visualize and characterize the perfusion within the placenta.16 However since B-Flow imaging is very sensitive to flow, it can also produce flash artifacts due to tissue motion which should be recognized and taken into consideration when evaluating the blood flow in an organ. The objective of our study was to evaluate the feasibility of B-Flow imaging in the placenta and to evaluate the artifacts seen on B-Flow imaging of the placenta. The B-Flow images are also compared to color Doppler images of the placenta acquired at the same time. Our study is unique since we believe that B-Flow imaging has not been used in placental imaging previously and artifacts in B-Flow imaging have not been discussed in prior articles.
Materials and methods
Institutional Review Board (IRB) approval from the Human Subjects Division in our institution was obtained. Through the Maternal Infant Care Clinic, pregnant women with no fetal anomalies detected on their routine prenatal ultrasound were enrolled to participate in the study. Written informed consent was obtained from the women after explaining the study. One of the inclusion criteria included the ability to visualize majority of the placenta if the placenta was posterior in location. Basal metabolic index (BMI) was not included as an exclusion criteria in our study since the color Doppler images were compared to B-Flow images as an internal comparison and patients over a wide spectrum of BMI were included in our study.
Images were acquired on the GE LOGIQ E9 machine (General Electric Healthcare, Wauwatosa, WI, USA) by our sonographers (three in number who were experienced in acquiring B-Flow images) utilizing a curvilinear (1–6 MHz) probe to obtain the data. Routine B-mode and color Doppler images of the placenta were acquired, optimized for visualization of the flow in the placenta without causing bleeding artifacts from over gain. Then B-Flow images were obtained of the placenta and the cord origin through multiple areas in the placenta. Protocol for scanning the placenta with B-Flow included documenting the cord origin and the complete placenta as seen in one plane—if the placenta did not fit into one image, three images were acquired, one lateral to cord origin on both sides and one on the cord origin. Still images and cine clips of the B-Flow images were saved for every location. Any vascular abnormalities were documented as well. Cine capture images were formatted from cine clips which involve accumulating multiframe images (Figure 1, movie 1). Data were stored for off-line analysis and online analysis.
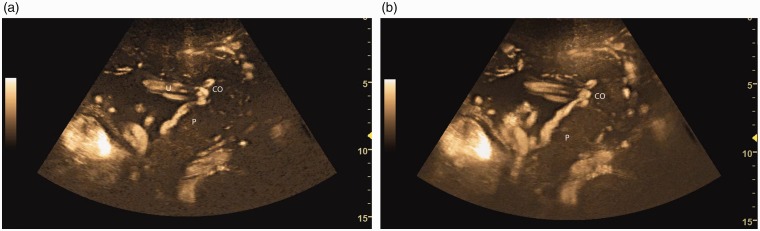
Figure 1.
B-flow image (a) through the placenta. The placenta (P) appears as dark against the brighter appearing vessels including the umbilical cord (U) and cord origin (CO). Cine capture (b) image which is created by accumulating multiple images into one frame, overlapping and combining all the images together, shows better visualization of the placenta along with the surrounding vasculature. Note retroplacental vessels seen as a conglomerate of vasculature (arrows).
Retrospective analysis of the B-Flow and cine capture images for artifacts was performed by one researcher (JT—15 years’ experience as a sonographer) blinded to the pregnant woman’s information and with experience in B-Flow imaging. B-Flow images and corresponding paired color Doppler images (Figure 2(a) and (b)) were also saved on a network protected drive after randomization of all the images. Randomization was done by giving each image an independent number and not pairing the B-Flow and color Doppler images. A standard form for analysis of the B-Flow and color Doppler images was created in REDCap. REDCap is a secure web application for building and managing online surveys and databases. Two independent reviewers, one Radiologist and another Maternal Fetal Medicine Specialist with greater than 10 and 5 years’ experience, respectively, in reading obstetric ultrasound—blinded to the clinical data analyzed by the B-Flow images. They were asked to characterize the vasculature seen on B-Flow and color Doppler into three categories. Small vessels were defined as <5 mm, medium as between 5 and 10 mm, and large vessels as >10 mm (Figure 3). Since blooming artifact (bleeding of color information outside the vessel wall due to incorrect gain setting) does not happen with B-Flow imaging as it does with color Doppler images, this approach was thought to be feasible. They were also asked to define the orientation of the vessels as horizontal or vertical.
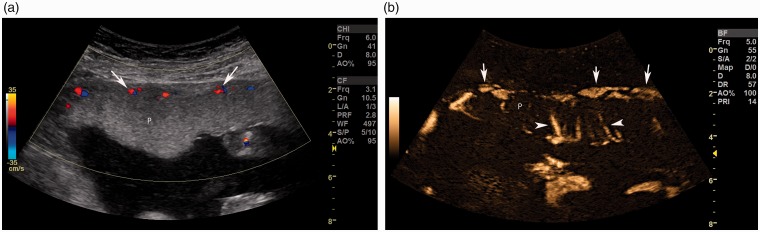
Figure 2.
Color Doppler (a) image shows perfusion (arrow) in the placenta (P) seen predominantly in the retroplacental area with a smaller number of vessels seen compared to B-flow image. B-Flow image (b) in the same part of the placenta (P) shows perfusion in the retroplacental area (arrows) and also in the placenta seen as vertical medium-sized vessels (arrowhead).
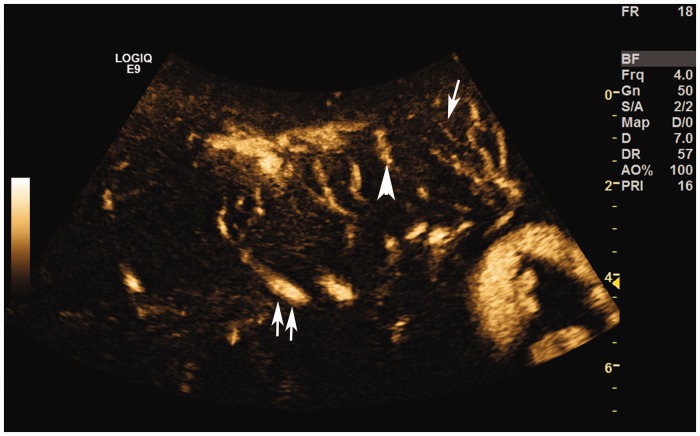
Figure 3.
B-Flow image showing small (arrow), medium (arrowhead), and large (double arrow) vessels in the placenta.
Descriptive statistics for the vascularity visualized on the color Doppler and B-Flow were calculated as the number (percentage) of each vascularity category for each orientation (horizontal and vertical vessels) and size of vessels (small, medium, and large). The reads of the two readers were combined in the analysis. Vascularity categories were compared between the color Doppler and B-Flow images using the Wilcoxon signed-rank test, clustered by patient to account for nonindependence of the two reads of each patient. P-values were adjusted for multiple comparisons using Holm’s procedure, a more powerful alternative to the Bonferroni correction.17 All statistical calculations were conducted with the statistical computing language R (version 3.1.1; R Foundation for Statistical Computing, Vienna, Austria).
Results
Thirty-six pregnant women were enrolled in this study, 17 normal women and 19 high-risk women. Women enrolled in the study ranged from 19 years to 43 years of age with an average age of 31.7 years. Gestational age varied from 17 weeks and five days to 36 weeks and three days with an average of 26 weeks and three days. Of the 19 high-risk women, three were referred for IUGR, seven had diabetes, and 11 had hypertension or PE. Nine women were lost to follow-up since they were either referred to our clinic for consult only (four women), desired homebirth (three women), or relocated (two women). Of the nine women lost to follow-up, five were from the normal group and four were from the high-risk group. Hence our study included 12 normal pregnant women and 15 high-risk pregnant women. Fourteen women had placentas located predominantly along the posterior uterus with the rest having placentas located either anterior, fundal, or on the lateral aspect of the uterus. Thirteen fetuses were delivered via a normal spontaneous vaginal delivery while 14 women underwent a cesarean section. Average birth weight of the fetuses delivered was 3315 g and were between the first and 100th percentile of birth weight with an average birth weight of 52nd percentile. Average in low-risk women was 3454 g and average in high-risk women was 3268 g.
A total of 161 B-Flow images were captured (72 images were captured in normal women and 89 images in high-risk women). In the B-Flow images, 15 images (9.32 %) showed no evidence of artifact. The remainder of the images had some artifact in them as shown in Table 1. This was decided with a binary method—if an artifact was present or not and we did not have a method to grade the severity of the artifact. A total of 138 cine capture images were recorded (60 in normal women and 78 in high-risk women). In the cine capture images, 22 images (15.9%) were good with no evidence of artifact. The remainder of the images had some artifact in them as shown in Table 1. Most of the images with artifacts, however, were acceptable for interpretation to evaluate the number of vessels seen within the placenta.
Table 1.
List of artifacts and optimization issues seen in the B-Flow images and B-Flow cine captures. One image or cine capture may have multiple artifacts in it. Actual number of images are quoted below
| Type of artifact | B-Flow image | B-Flow cine capture |
|---|---|---|
| Pulse repetition interval (PRI) set inappropriately | 2 | 4 |
| Flash | 89 | 48 |
| Respiratory motion | 27 | 37 |
| Arterial motion | 24 | 5 |
| Fetal motion | 67 | 20 |
| Over gain | 3 | 3 |
| Under gain | 50 | 9 |
| Dark ray lines | 7 | 7 |
| Poor penetration | 51 | 49 |
| Too zoomed image | 17 | 10 |
Pulse repetition interval (PRI) is the time interval between each pulse. PRI was decreased to suppress flash artifact; however, decreasing PRI led to loss of slow flow and small vasculature information. PRI was changed on individual cases to provide the best imaging in the placenta without suppression of flow information. Flash artifact (movie 2) was seen due to sudden movement and was registered on B-Flow images due to its sensitivity. Motion can degrade images and the different types of motion seen while imaging the pregnant woman included maternal respiratory motion (seen as up and down movement of the tissues correlating with the respiratory rate), arterial pulsation motion due to proximity of large vessel to the tissues which was typically seen in close of the placental insertion site due to arterial pulsation seen in the umbilical arteries, and fetal motion which is seen as random motion and displacement of the placental tissue due to fetal parts moving in the amniotic fluid and compressing the placenta. Motion from maternal respiration, arterial pulsation in the mother or fetus, and fetal motion (movie 3) caused artifacts in the images. Even quiet respiration lead to “streaking” of vessels, and therefore, women were asked to hold their breath during acquisition of B-Flow images. Over and under gain was seen as low or high background information, respectively, and this was thought to interfere with adequate interpretation of the images. Over gain led to accentuation of the flow information; however, there was loss of the tissue information and vice versa with under gain of B-Flow images. Poor penetration led to loss of information from the deeper locations (Figure 4). Too zoomed images were seen as too much magnification in the image with inability to clearly see adjacent tissues around the placenta. Too much zooming of images led to loss of perspective and orientation due to tissue suppression in B-Flow images. Ray lines could be dark or bright and were due to electrical interference (movie 4).
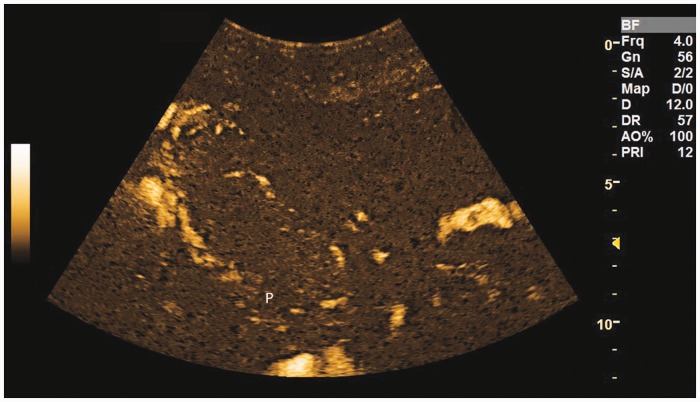
Figure 4.
Poor penetration artifact. Note the lack of information from the deeper part of this posterior placenta (P) which is due to lack of penetration. Placenta has been outlined on this image in white line.
For the 36 women with color Doppler and B-Flow images reviewed by the two independent blinded reviewers, a total of 144 reads were obtained. More small horizontal (p = 0.046) and small vertical running vessels (p < 0.001) were identified with B-Flow than color Doppler images (Table 2), based on the combination of two readers, with p-values adjusted for multiple comparisons. No other size or orientation of vessel was significantly different between B-Flow and color Doppler images.
Table 2.
Frequencies of vessels as detected by color Doppler and B-Flow US. Both readers combined
| Imaging |
||||
|---|---|---|---|---|
| Variable | Number of vessels | Doppler number (%) | B-Flow number (%) | P-valuea |
| Horizontal vessels | ||||
| Small | 0 | 17 (23.6) | 6 (8.3) | 0.046 |
| 1–5 | 39 (54.2) | 40 (55.6) | ||
| 6–10 | 11 (15.3) | 21 (29.2) | ||
| >10 | 5 (6.9) | 5 (6.9) | ||
| Medium | 0 | 6 (8.3) | 6 (8.3) | 0.49 |
| 1–5 | 50 (69.4) | 39 (54.2) | ||
| 6–10 | 13 (18.1) | 24 (33.3) | ||
| >10 | 3 (4.2) | 3 (4.2) | ||
| Large | 0 | 38 (52.8) | 43 (59.7) | 0.68 |
| 1–5 | 31 (43.1) | 28 (38.9) | ||
| 6–10 | 3 (4.2) | 1 (1.4) | ||
| >10 | 0 (0.0) | 0 (0.0) | ||
| Vertical vessels | ||||
| Small | 0 | 35 (48.6) | 10 (13.9) | <0.001 |
| 1–5 | 36 (50.0) | 51 (70.8) | ||
| 6–10 | 1 (1.4) | 8 (11.1) | ||
| >10 | 0 (0.0) | 3 (4.2) | ||
| Medium | 0 | 15 (20.8) | 6 (8.3) | 0.90 |
| 1–5 | 52 (72.2) | 61 (84.7) | ||
| 6–10 | 5 (6.9) | 4 (5.6) | ||
| >10 | 0 (0.0) | 1 (1.4) | ||
| Large | 0 | 43 (59.7) | 49 (68.1) | 0.68 |
| 1–5 | 29 (40.3) | 23 (31.9) | ||
| 6–10 | 0 (0.0) | 0 (0.0) | ||
| >10 | 0 (0.0) | 0 (0.0) |
Values are no. (%) unless otherwise specified.
Test for average difference between readers using a permutation test based on the Wilcoxon signed-rank test. Adjusted for multiple comparison’s using Holm’s procedure.
Discussion
To our knowledge this is the first study evaluating the feasibility of B-Flow in assessing blood flow in the placenta. Color and Spectral Doppler imaging (Doppler flow imaging (DFI)) has become the most widely used noninvasive method of evaluating placental vascularity. Commonly used parameters like umbilical artery and uterine artery DFI provide an estimate of the flow in these vessels but not directly in the placenta.15 It is a known fact that very slow flow may not produce a Doppler signal of a detectable magnitude and hence the accuracy of these DFI techniques is low within the placenta, which contains multiple small vessels with slow flow.18 B-Flow is an underutilized technique that utilizes a unique signal processing algorithm for visualization of blood flow data. B-Flow was introduced in the 1990s though it has not been used routinely and only a few papers can be found in the literature.
B-Flow imaging has been used previously to show better visualization of the femoral arteries in infants before catheter angiography.19 Groth et al.20 also showed that B-Flow imaging was equal or better at vessel delineation compared to standard Doppler imaging methods to evaluate the basal cerebral arteries. Other studies have shown capability of B-Flow to visualize intravascular flow in various arteries.21,22 In terms of perfusion, Andrioli and Valcavi23 have shown in their study in thyroid nodules that B-Flow imaging was better able to suppress unwanted signals in thyroid nodules and boost weak signals from blood vessels to show the completeness of ablation in these nodules. Matsumoto et al.24 in their study showed that B-Flow detected hepatic tumor blood flow with sensitivity as high as that of color Doppler.
Our study has shown that B-Flow imaging is very sensitive to imaging blood flow in the placenta. A larger number of small (less than 5 mm) vessels were seen on B-Flow images compared to the color Doppler images. This was also seen in the study performed by Hançerlioğulları et al. on torsed ovaries in rabbits. They found that B-Flow allowed for a better assessment of blood flow in ovarian torsion than color Doppler ultrasound. They noted a decreased reperfusion in the ovary at 1 hour measured by B-Flow, which was more sensitive than that measured by Doppler US. The flow equalized by the end of 2 hours in both techniques.25 Andrioli and Valcavi23 in their study mention that B-Flow imaging would be helpful in evaluating viable tissue in women post thermal ablation of thyroid nodules due to the advantage of B-Flow being able to detect small vessels. Evaluating the number and pattern of smaller vessels in the placenta may potentially help in earlier detection of placental disease associated with PE or IUGR.
B-Flow, however, is prone to multiple artifacts, most common of which was flash artifact (movie 2) seen in 89 of the 144 B-Flow images and 48 of the 138 cine capture images as noted in our study. This artifact is likely due to the sensitivity of B-Flow imaging to movement. Tola et al. studied carotid arteries using B-Flow imaging and found that excessive pulsation of the carotid artery led to movement of surrounding structures which hampered evaluation of the vessel wall in their study. They also found that the image evaluation was limited in some of their cases due to background flash artifact.26 Another artifact that we commonly encountered was poor depth penetration. We noticed that the placenta tissues located at a greater depth showed less number of vessels than when superficial parts of the placenta were imaged. This was similar to that seen with color Doppler images. We did not measure depth in our study; however, lower number of vessels were seen in tissues located at a depth with the same organ. Tola et al.26 also found that the sensitivity of B-Flow imaging is decreased with increasing depth because of strong dependence of B-Flow on signal intensity strength. Even with the presence of these artifacts in our placenta images, most of these images were interpretable as can happen with routine B-mode or color Doppler images as well. Recognition of these artifacts is important in order to avoid making mistakes in interpreting these images.
There are several limitations to our study including small number of subjects and thus placentas imaged. B-Flow is capable of showing a larger number of blood vessels in the placenta compared to color Doppler. The pattern of blood vessels in the placenta may be different in early compared to late gestational age placentas. In addition, the vessels may have a different pattern in PE compared to normal women; however, our study was a feasibility study and did not include enough number of women to study this problem. In addition, the evaluation of the pattern of blood vessels would be improved if quantification and 3D capability were present in B-Flow.
In our experience some of these artifacts can be avoided by using the correct technique and settings. For example, decreasing PRI leads to loss of slow flow and small vasculature information; however, it may need to be decreased to suppress flash artifact. Hence, PRI needs to be changed in individual cases to provide the best imaging in the placenta without suppression of flow information. Flash artifact can be avoided by repeating images when the fetus is relatively still. Breathhold may be helpful to avoid the motion artifacts from maternal breathing. Artifact from arterial pulsations is difficult to avoid and images can be acquired in the diastole during a cardiac cycle. Avoiding too much zoom to avoid the loss of perspective and orientation due to tissue suppression in B-Flow images may be helpful.
In conclusion, B-Flow is capable of showing perfusion in a human placenta. Recognizing artifacts and adaptively modifying the image acquisition are necessary with this new technique to acquire the best images for interpretation. Further studies with larger population size are needed to understand the utility of B-Flow imaging in women at risk for abnormal placental vasculature or perfusion.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) declared receipt of the following financial support for the research, authorship, and/or publication of this article: General Electric Healthcare.
Ethics approval
University of Washington Medical Center Human Subjects Department. IRB number 45374.
Guarantor
MKD.
Contributors
All authors.
References
- 1.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol 2009; 33: 130–130. [DOI] [PubMed] [Google Scholar]
- 2.Kaufmann P, Black S, Huppertz B. Endovascular trophoblast invasion: implications for the pathogenesis of intrauterine growth retardation and preeclampsia. Biol Reprod 2003; 69: 1–1. [DOI] [PubMed] [Google Scholar]
- 3.Burton GJ, Woods AW, Jauniaux E, et al. Rheological and physiological consequences of conversion of the maternal spiral arteries for uteroplacental blood flow during human pregnancy. Placenta 2009; 30: 473–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rolnik DL, Wright D, Poon LCY, et al. ASPRE trial: performance of screening for preterm pre-eclampsia. Ultrasound Obstet Gynecol 2017; 50: 492–495. [DOI] [PubMed] [Google Scholar]
- 5.Poon LC, Wright D, Rolnik DL, et al. Aspirin for evidence-based preeclampsia prevention trial: effect of aspirin in prevention of preterm preeclampsia in subgroups of women according to their characteristics and medical and obstetrical history. Am J Obstet Gynecol 2017; 217: 585.e1–585.e5. [DOI] [PubMed] [Google Scholar]
- 6.Jaffe R, Woods JR. Doppler velocimetry of intraplacental fetal vessels in the second trimester: improving the prediction of pregnancy complications in high-risk patients. Ultrasound Obstet Gynecol 1996; 8: 262–262. [DOI] [PubMed] [Google Scholar]
- 7.Jaffe R, Jauniaux E, Hustin J. Maternal circulation in the first-trimester human placenta – myth or reality? Am J Obstet Gynecol 1997; 176: 695–695. [DOI] [PubMed] [Google Scholar]
- 8.Jurkovic D, Jauniaux E, Kurjak A, et al. Transvaginal color Doppler assessment of the uteroplacental circulation in early pregnancy. Obstet Gynecol 1991; 77: 365–365. [PubMed] [Google Scholar]
- 9.Morel O, Pachy F, Chavatte-Palmer P, et al. Correlation between uteroplacental three-dimensional power Doppler indices and true uterine blood flow: evaluation in a pregnant sheep model. Ultrasound Obstet Gynecol 2010; 36: 635–635. [DOI] [PubMed] [Google Scholar]
- 10.Axt-Fliedner R, Schwarze A, Nelles I, et al. The value of uterine artery Doppler ultrasound in the prediction of severe complications in a risk population. Arch Gynecol Obstet 2005; 271: 53–53. [DOI] [PubMed] [Google Scholar]
- 11.Schwarze A, Nelles I, Krapp M, et al. Doppler ultrasound of the uterine artery in the prediction of severe complications during low-risk pregnancies. Arch Gynecol Obstet 2005; 271: 46–46. [DOI] [PubMed] [Google Scholar]
- 12.Baschat AA. Doppler application in the delivery timing of the preterm growth-restricted fetus: another step in the right direction. Ultrasound Obstet Gynecol 2004; 23: 111–111. [DOI] [PubMed] [Google Scholar]
- 13.Baschat AA, Güclü S, Kush ML, et al. Venous Doppler in the prediction of acid-base status of growth-restricted fetuses with elevated placental blood flow resistance. Am J Obstet Gynecol 2004; 191: 277–277. [DOI] [PubMed] [Google Scholar]
- 14.Peleg D, Kennedy CM, Hunter SK. Intrauterine growth restriction: identification and management. Am Fam Physician 1998; 58: 453–453. [PubMed] [Google Scholar]
- 15.Cnossen JS, Morris RK, ter Riet G, et al. Use of uterine artery Doppler ultrasonography to predict pre-eclampsia and intrauterine growth restriction: a systematic review and bivariable meta-analysis. CMAJ 2008; 178: 701–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wachsberg RH. B-flow imaging of the hepatic vasculature: correlation with color Doppler sonography. Am J Roentgenol 2007; 188: W522–W522. [DOI] [PubMed] [Google Scholar]
- 17.Holm S. A simple sequential rejective multiple test procedure. Scand J Stat 1979; 6: 65–70. [Google Scholar]
- 18.Kruskal JB, Newman PA, Sammons LG, et al. Optimizing Doppler and color flow US: application to hepatic sonography. Radiographics 2004; 24: 657–657. [DOI] [PubMed] [Google Scholar]
- 19.Groth M, Dammann E, Arndt F, et al. Comparison of B-Mode with B-flow sonography for the evaluation of femoral arteries in infants. Rofo 2017; 189: 1161–1161. [DOI] [PubMed] [Google Scholar]
- 20.Groth M, Ernst M, Deindl P, et al. B-flow sonography for evaluation of basal cerebral arteries in newborns. Clin Neuroradiol 2017; 27(Suppl 1): 1. 10.1007/s00062-017-0619-3. [DOI] [PubMed] [Google Scholar]
- 21.D’Abate F, de Bruin JL. Additional value of B-flow imaging in arterial wall calcifications. J Clin Ultrasound 2018; 46: 136–139. [DOI] [PubMed] [Google Scholar]
- 22.Avramovski P, Avramovska M, Sikole A. B-flow imaging estimation of carotid and femoral atherosclerotic plaques: vessel walls rheological damage or strong predictor of cardiovascular mortality in chronic dialysis patients. Int Urol Nephrol 2016; 48: 1713–1713. [DOI] [PubMed] [Google Scholar]
- 23.Andrioli M, Valcavi R. Ultrasound B-flow imaging in the evaluation of thermal ablation of thyroid nodules. Endocrine 2015; 48: 1013–1013. [DOI] [PubMed] [Google Scholar]
- 24.Matsumoto N, Ogawa M, Miura T, et al. B-flow imaging of vascular structure for the diagnosis of liver tumor. J Med Ultrason (2001) 2013; 40: 409–409. [DOI] [PubMed] [Google Scholar]
- 25.Hançerlioğulları K, Soyer T, Tosun A, et al. Is B-flow USG superior to color Doppler USG for evaluating blood flow patterns in ovarian torsion? J Pediatr Surg 2015; 50: 1156–1156. [DOI] [PubMed] [Google Scholar]
- 26.Tola M, Yurdakul M, Cumhur T. Combined use of color duplex ultrasonography and B-flow imaging for evaluation of patients with carotid artery stenosis. Am J Neuroradiol 2004; 25: 1856–1856. [PMC free article] [PubMed] [Google Scholar]