Abstract
A parotid gland abscess is uncommon and if not responding to conservative management, requires surgical intervention. However, surgery is invasive with the risk of complicating facial nerve damage and possible poor cosmetic outcome. We present a case of a parotid gland abscess in association with an underlying Warthin’s tumour requiring percutaneous drainage, as patient co-morbidity precluded a safe surgical approach. Percutaneous drainage was aided by a contrast-enhanced ultrasound examination, which permitted delineation of the fluid aspects of the collection from the underlying tumour and allowed successful percutaneous ultrasound-guided aspiration without complication.
Keywords: Ultrasound, contrast-enhanced ultrasound, ENT, parotid gland, SonoVue, drainage, salivary gland, abscess
Introduction
The parotid gland is a superficial salivary gland, which contains a number of intraglandular and periglandular lymph nodes draining from various sites including the forehead, external auditory canal and cheek. This, in combination with ascending infection via Stensen’s duct, may result in either a bacterial or viral infection (parotitis). Parotitis is not uncommon with known risk factors including ductal dysfunction, immunosuppression, dehydration, poor oral hygiene or dental infections, or a pre-existing Warthin’s tumour.1,2 Parotid gland abscess is an uncommon sequelae of parotitis: clinical presentation is usually with inflammatory features of pain, swelling and palpable mass.2–4 Mass effect symptoms may result in painful jaw movement including mastication and swallowing. The anatomy of the parotid gland lends to serious complications of infection such as a facial nerve palsy, retropharyngeal extension, osteomyelitis of the jaw or systemic sepsis.1,3,4 Conventional treatment for a parotid abscess is conservative; however, failure to respond will necessitate surgical incision and drainage.1–4 A surgical approach may be undesirable because of the need for several long incisions along the distribution of the facial nerve to identify and drain multiple collections, increasing complications and resultant cosmetic scars.5,6 Imaging-guided percutaneous drainage provides an alternative for the relief of symptoms by decreasing the size of the abscess through a minimal incision, under local anaesthetic in an outpatient setting. This decrease in collection size may then result in susceptibility to antibiotic therapy.
Warthin’s tumours are relatively common benign salivary gland tumours comprised of lymphoid tissue, which predominantly occur in the 5th–6th decade. The vast majority occur in the tail of the parotid gland and are bilateral in up to 20% of cases. On ultrasound, Warthin’s tumours classically are round or lobulated with cystic and solid elements, or may be entirely cystic. On Doppler imaging, they may show increased vascular signal. Greyscale appearances are similar to other causes of a mass including a haematoma or abscess, varying only in degrees of internal echogenicity, but critically these lesions are avascular in nature. Contrast-enhanced ultrasound (CEUS) is commonly used in assessing focal liver lesions, but CEUS is now firmly established in a variety of other applications such as renal, testicular, and vascular as well as in paediatric applications.7 Recently, Huang et al. demonstrated the utility of CEUS in interventional procedures.8 The spatial and temporal resolution allows for clear delineation of vascular and non-vascular areas within a target lesion, and the use of CEUS has now been recommended to guide drainage for aiding biopsy of viable tissue in a partially necrotic lesion.9
We present a patient with a Warthin’s tumour and a co-existent parotid abscess, who was a poor anaesthetic and surgical candidate, and instead underwent a successful CEUS-guided percutaneous drainage without complication.
Case presentation
A 51-year-old man presented with a painful, red and warm swelling at the angle of his left jaw which had gradually worsened over a week. He was generally unwell, lethargic and had decreased appetite. He had no further symptoms relating to the ear, nose or throat. On examination, there was a 7 × 7 cm warm, tender, erythematous swelling overlying the left parotid gland which was mildly fluctuant and not discharging. Cranial nerve examination and all eye movement were normal. There was no obvious dental abnormality. Blood tests showed neutrophilia with a raised C-reactive protein (CRP). The international normalised ratio (INR) was 2.1.
The patient’s past medical history included bilateral Warthin’s tumours which were under investigation. In addition, there was a history of ischaemic heart disease, and a current left-ventricular thrombus treated with anticoagulants (Warfarin) with a target INR of 3–4.
Clinically a parotid abscess was assumed and considered to be within the Warthin’s tumour of the left parotid gland. Initial treatment was with intravenous antibiotics (Co-Amoxiclav and Metronidazole) but symptomology and biochemical markers showed no improvement over three days. The patient was also managed symptomatically with analgesia and antiemetics. Anticoagulation was continued, as the left ventricular thrombus was deemed life-threatening if stopped. Surgery was essentially precluded by the level of anticoagulation needed and the presence of the left ventricular thrombus presenting high anaesthetic risk. Conservative management was continued.
The patient underwent ultrasound using Logiq E9 (GE Healthcare) using a 9 MHz linear transducer. Optimised greyscale imaging showed a large, well circumscribed heterogeneous mass in the tail of the left parotid gland measuring 5.6 × 3.7 cm, without a significant fluid component (Figure 1). Colour Doppler ultrasound showed minimal vascularity traversing the lesion (Figure 1). A 4.8 mL bolus of SonoVue™ (Bracco, Milan) was administered as an intravenous bolus, and continuous imaging using low mechanical index contrast specific mode was performed. The Warthin’s tumour was rapidly hyper-enhancing with late venous phase mild washout being observed (Figure 2). Surrounding the enhancing tumour were avascular non-enhancing pockets with thin septae and perilesional hyper-enhancement (Figure 2). The abscess could be clearly delineated from the tumour. Using a dual screen greyscale and CEUS imaging, an 18 gauge cannula was positioned percutaneously into the dominant aspects of the collection. This was achieved using two percutaneous punctures (Figure 3). Approximately 20 ml of blood and pus was aspirated. Inflamed tissue and small pockets of fluid remained, but the abscess cavity size decreased substantially and no fluid element remained. There were no complications.
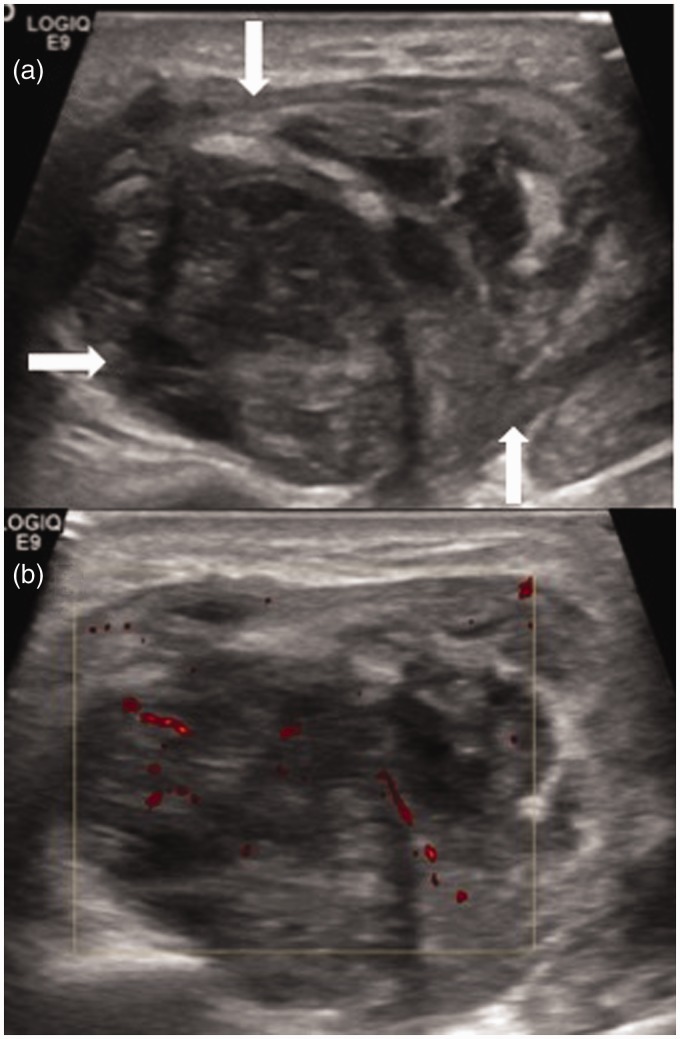
Figure 1.
(a) B-mode ultrasound of the left parotid gland showing a large heterogeneous mass (arrows) in a patient with known Warthin’s tumour. (b) Colour Doppler ultrasound demonstrated minimal vascularity.
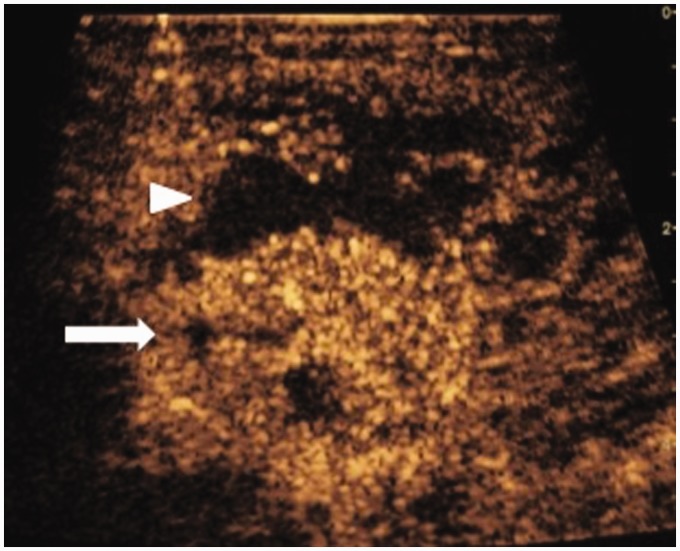
Figure 2.
Contrast-enhanced ultrasound (CEUS) specific mode following 4.8 ml SonoVue injected intravenously. The hypervascular Warthin’s tumour is clearly delineated (arrow) from the avascular abscess (arrowhead).
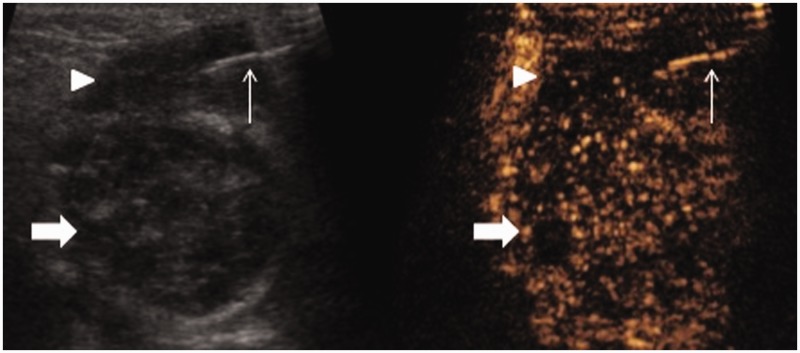
Figure 3.
Simultaneous B-mode and Contrast-enhanced ultrasound (CEUS) ultrasound. An 18 g needle (thin arrow) was percutaneously advanced into the avascular fluid component of the lesion (arrowhead) and pus was drained. The Warthin’s tumour remains distant from the site of intervention and now shows relative washout in the late phase (thick arrow).
On the following day, there was a noticeable clinical improvement in symptoms and cosmetic appearance. The patient was commenced on oral antibiotics and discharged. Fourteen days post-discharge, the patient’s condition had further improved, with the swelling decreased and no longer affected swallowing or mastication.
Discussion
We have demonstrated the utility of CEUS to differentiate neoplastic tissue from avascular, abscess formation within the parotid gland. The ability to differentiate fluid elements from vascular elements allowed accurate and effective percutaneous drainage in a compromised surgical candidate. CEUS was shown to be both a useful diagnostic tool and have the ability to guide real time intervention, employing excellent spatial resolution coupled with real time imaging.
Contrast-enhanced ultrasound has been growing in popularity since entering in clinical practice 20 years ago and is used in over 50 countries worldwide10,11 recently gaining FDA (Food and Drug Administration) approval in the USA for liver lesions in both adults and paediatrics.12 The technique uses a ‘microbubble’ ultrasound contrast agents (UCAs) made up of microscopic bubbles of sulphur hexafluoride gas in a lipid-based shell, measuring 3–5 µm (approximately the size of a red blood cell), which oscillate at low acoustic pressures.7,11 Due to the size, UCAs are purely intravascular, unlike contrast agents used for computed tomography (CT) or magnetic resonance (MR) imaging.10 This results in high-resolution images and exquisite vascularity at the microcirculatory level.11,13 Multiparametric ultrasound allows repeatability of vascular and parenchymal assessment without the risk of nephrotoxicity,13,14 an excellent safety profile,15 and no risk of radiation exposure.
Warthin’s tumours constitute 5–12% of benign parotid lesions13 and sonographically are commonly rounded, well defined and hypoechoic with microcystic development.16 However, there remains a large overlap in the B-mode appearances of benign and malignant parotid lesions.13,17 The application of UCAs establishes the sensitivity and specificity to a level 100−93% when prospectively assessing Warthin’s tumours.18 Contrast-enhanced ultrasound allows the cultivation of qualitative data.13,16,18 The uptake pattern of Warthin’s tumours is a hyper-vascular lesion,13,18 and studies suggest that Time To Peak (TTP) uptake in Warthin’s tumours is much shorter than that of other benign and malignant lesions13,18 with the mean normalised TTP being 0.62 seconds.18 Washout is a feature of these tumours as illustrated with the present case.
Haematoma and abscess may cause diagnostic dilemma with greyscale appearances manifesting differently depending on the age of the abnormality. In the subacute period, haematoma may appear heterogeneous with microcystic development as it progresses to liquefication. By distinction, haematomas and abscesses are avascular structures, which have been shown on CEUS in the testis to conform to this description.19 Definition of vascular from avascular regions within a tumour or collections allow intervention to be guided, both drainage or biopsy.8 An UCA was essential for the successful guidance of needle aspiration to delineate areas of abscess formation from the underlying tumour in the present case, particularly in light of the ongoing anticoagulation. CEUS achieves excellent spatial resolution and crucially determine the presence of vascularity allowing the procedure to be undertaken with real-time imaging and continuous feedback on-screen.8,11,17,20
The properties of UCAs mean that CEUS lends itself well to use in interventional radiology, by providing a truly intravascular agent and assessment at a microvascular level allowing clear delineation of abnormal tissue when a UCA is administered. Interventional guided procedures may be guided by improved lesion identification or delineation of viable components thereby assisting diagnostic or therapeutic procedures (e.g. biopsy, ablation, drainage, follow-up).8 Assessment of macrovasculature also can be performed to delineate luminal abnormality such as in angiographic intervention and is of particular use in the iodine sensitive population.8
In conclusion, we have presented a patient where CEUS has been safely employed to successfully drain a parotid abscess with symptomatic relief and avoidance of high-risk surgery. CEUS-guided intervention allows real-time images with excellent spatial and temporal resolution in a portable setting.
Learning points
CEUS reliably delineates vascular and avascular tissues, such as abscess and neoplasia.
CEUS-guided drainage offers a potentially safer alternative to drainage than conventional greyscale US.
CEUS gives greater confidence in accurate drainage in coagulopathic patients.
CEUS drainage of salivary gland collections can offer a preferable alternative to surgical intervention.
Acknowledgement
N/A.
Declaration of Conflicting Interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Ethics approval
Written permission was obtained from the patient for the publication of this case report.
Guarantor
PSS.
Contributors
N/A.
References
- 1.Vorrasi J, Zinberg G. Concomitant suppurative parotitis and condylar osteomyelitis. J Oral Maxillofac Surg 2017; 75: 543–549. [DOI] [PubMed]
- 2.Viselner G, van der Byl G, Maira A, et al. Parotid abscess: mini-pictorial essay. J Ultrasound 2013; 16: 11–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chi TH, Yuan CH, Chen HS. Parotid abscess: a retrospective study of 14 cases at a regional hospital in Taiwan. B-ENT 2014; 10: 315–318. [PubMed] [Google Scholar]
- 4.Tan VE, Goh BS. Parotid abscess: a five-year review – clinical presentation, diagnosis and management. J Laryngol Otol 2007; 121: 872–879. [DOI] [PubMed] [Google Scholar]
- 5.Graham SM, Hoffman HT, McCulloch TM, et al. Intra-operative ultrasound-guided drainage of parotid abscess. J Laryngol Otol 1998; 112: 1098–1100. [DOI] [PubMed] [Google Scholar]
- 6.Berman J, Myssiorek D, Reppucci A, et al. Sump catheter drainage of parotid abscess: an alternative to surgery. Ear Nose Throat J 1991; 70: 393–395. [PubMed] [Google Scholar]
- 7.Piscaglia F, Nolsøe C, Dietrich CF, et al. The EFSUMB guidelines and recommendations on the clinical practice of contrast enhanced ultrasound (CEUS): update 2011 on non-hepatic applications. Ultraschall Med 2012; 33: 33–59. [DOI] [PubMed] [Google Scholar]
- 8.Huang DY, Yusuf GT, Daneshi M, et al. Contrast-enhanced US-guided interventions: improving success rate and avoiding complications using us contrast agents. Radiographics 2017; 37: 652–664. [DOI] [PubMed] [Google Scholar]
- 9.Lorentzen T, Nolsøe CP, Ewertsen C, et al. EFSUMB guidelines on interventional ultrasound (INVUS), Part I. general aspects (long version). Ultraschall Med 2015; 36: E1–E14. [DOI] [PubMed] [Google Scholar]
- 10.Wilson SR, Burns PN. Microbubble-enhanced US in body imaging: what role? Radiology 2010; 257: 24–39. [DOI] [PubMed] [Google Scholar]
- 11.Cantisani V, Wilson SR. CEUS: where are we in 2015? Eur J Radiol 2015; 84: 1621–1622. [DOI] [PubMed] [Google Scholar]
- 12.Seitz K, Strobel D. A milestone: approval of CEUS for diagnostic liver imaging in adults and children in the USA. Ultraschall Med 2016; 37: 229–232. [DOI] [PubMed] [Google Scholar]
- 13.David E, Cantisani V, De Vincentiis M, et al. Contrast-enhanced ultrasound in the evaluation of parotid gland lesions: an update of the literature. Ultrasound 2016; 24: 104–110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yusuf GT, Sellars ME, Deganello A, et al. Retrospective analysis of the safety and cost implications of pediatric contrast-enhanced ultrasound at a single center. AJR Am J Roentgenol 2017; 208: 446–452. [DOI] [PubMed] [Google Scholar]
- 15.Piscaglia F, Bolondi L. The safety of Sonovue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol 2006; 32: 1369–1375. [DOI] [PubMed] [Google Scholar]
- 16.Kim J, Kim EK, Park CS, et al. Characteristic sonographic findings of Warthin's tumor in the parotid gland. J Clin Ultrasound 2004; 32: 78–81. [DOI] [PubMed] [Google Scholar]
- 17.Cantisani V, David E, Sidhu PS, et al. Parotid gland lesions: multiparametric ultrasound and MRI features. Ultraschall Med 2016; 37: 454–471. [DOI] [PubMed] [Google Scholar]
- 18.Knopf A, Mansour N, Chaker A, et al. Multimodal ultrasonographic characterisation of parotid gland lesions – a pilot study. Eur J Radiol 2012; 81: 3300–3305. [DOI] [PubMed] [Google Scholar]
- 19.Yusuf G, Konstantatou E, Sellars ME, et al. Multiparametric sonography of esticular hematomas: features on grayscale, color doppler, and contrast-enhanced sonography and strain elastography. J Ultrasound Med 2015; 34: 1319–1328. [DOI] [PubMed] [Google Scholar]
- 20.Yusuf GT, Sellars ME, Huang DY, et al. Cortical necrosis secondary to trauma in a child: contrast-enhanced ultrasound comparable to magnetic resonance imaging. Pediatr Radiol 2014; 44: 484–487. [DOI] [PubMed] [Google Scholar]