Significance
Many physiological events in insects are controlled by both juvenile hormone (JH) and 20-hydroxyecdysone (20E). The presence of JH sometimes alters the nature of the 20E action, but the underlying mechanisms have not been fully elucidated. In Aedes aegypti mosquitoes, four protein isoforms are generated by the taiman gene, which plays an indispensable role in the action of both JH and 20E. Our study indicates that different hormone responses demand distinct Taiman isoforms. Moreover, JH controls the formation of the Taiman isoforms that are specifically required for 20E-regulated gene expression after blood feeding. Therefore, this study discovers a previously unknown mode of JH action and provides insight into how JH influences cellular responses to 20E in insects.
Keywords: insect hormone, RNA splicing, signal transduction, gene expression
Abstract
Juvenile hormone (JH) regulates many aspects of insect development and reproduction. In some processes, JH plays a critical role in defining the action of the steroid hormone 20-hydroxyecdysone (20E). In Aedes aegypti mosquitoes, JH prepares newly emerged female adults to become competent to synthesize vitellogenin in response to 20E after blood ingestion. The molecular basis of this competence is still not well understood. Here, we report that JH regulates pre-mRNA splicing of the taiman gene, which encodes a key transcriptional regulator required for both JH- and 20E-controlled gene expression. JH stimulated the production of the Taiman isoforms A/B, while reducing the levels of the isoforms C/D, in the fat body after adult eclosion. The appearance of the A/B isoforms in maturing mosquitoes was accompanied by acquisition of the competence to respond to 20E. Depletion of the A/B isoforms, by inhibiting the alternative splicing or by isoform-specific RNA interference, considerably diminished the 20E-induced gene expression after a blood meal and substantially impaired oocyte development. In accordance with this observation, further studies indicated that in the presence of 20E, the Taiman A/B isoforms showed much stronger interactions with the 20E receptor complex than the Taiman C/D isoforms. In contrast, all four isoforms displayed similar capabilities of forming active JH receptor complexes with the methoprene-tolerant protein (Met). This study suggested that JH confers the competence to newly emerged female mosquitoes by regulating mRNA splicing to generate the Taiman isoforms that are essential for the vitellogenic 20E response.
The development and reproduction of many insects, such as beetles, flies, and mosquitoes, are essentially governed by the steroid 20-hydroxyecdysone (20E) and the sesquiterpenoid juvenile hormone (JH) (1). An important role of JH in these processes is to shape the stage-specific 20E responses. During postembryonic development in holometabolous insects, 20E initiates each molt and JH preserves the “status quo,” preventing metamorphosis (2). In immature larvae, the presence of JH suppresses the expression of metamorphosis-initiation genes and 20E can only induce the larval-larval molt (3–5). After larvae acquire competence to undergo metamorphosis, the JH titer drops substantially, relieving the suppression of the program-switching action of 20E (6). As a result, 20E induces larval-pupal and pupal-adult molts in the absence of JH (7).
In dipterans, both 20E and JH play vital roles in female reproduction (8). Female adults of most mosquito species require blood meals for egg maturation, a critical process that involves the massive synthesis of vitellogenin (Vg) and other yolk protein precursors (YPPs) in the fat body, as well as the deposition of YPPs in the developing oocytes (8). Synthesis and secretion of JH in female adults increases rapidly after adult emergence (9, 10). JH regulates many aspects of the development that occurs after eclosion in the dengue fever mosquito, Aedes aegypti, stimulating the growth of primary follicles and priming the mosquitoes for vitellogenesis (11). Blood feeding then triggers a decline of JH and an increase in the 20E titers. While the synthesis of YPPs in the fat body is directed by 20E after blood ingestion, JH is essential for the fat body of the newly emerged mosquitoes to become competent for the 20E response during mosquito egg development (11, 12). The molecular basis of the competence is still not well understood (13).
Recent studies have established that JH acts through its receptor methoprene-tolerant (Met) (14–16). Met belongs to the basic helix–loop–helix (bHLH)/Per-Arnt-Sim (PAS) domain family of transcription factors. After binding of JH, Met protein recruits Taiman (Tai), another bHLH/PAS domain-containing protein, to form an active receptor complex, leading to the transcriptional activation of JH response genes (15, 17–19). In this well-characterized JH action, Tai serves as an obligatory DNA-binding partner of Met (20). More recently, we have reported that DNA binding of the Met–Tai complex to the JH response genes is modulated by phosphorylation of both Met and Tai in female A. aegypti mosquitoes (21). JH regulates the phosphorylation via a cell membrane-initiated signal pathway involving receptor tyrosine kinases (RTKs), phospholipase C (PLC), and protein kinase C (PKC) (21, 22).
Tai is also an important player in the 20E regulatory cascade. It serves as a transcriptional coactivator of the functional 20E receptor, which is a heterodimer of the ecdysone receptor (EcR) and Ultraspiracle (USP) (23, 24). Ligand-dependent binding of Tai to EcR/USP is crucial for the ecdysone-regulated border cell migration during Drosophila oogenesis (24). In A. aegypti, Tai is required for the expression of Vg after blood ingestion and is recruited to the Vg promoter via protein interaction with EcR/USP (25). Isoforms of Tai have been found in the German cockroach Blattella germanica (26), the red flour beetle Tribolium castaneum (26), the migratory locust Locusta migratoria (27), and the oriental fruit fly Bactrocera dorsalis (28). The Tai isoforms in each species differ at the C termini of the proteins, presumably due to alternative splicing. Depletion of individual Tai isoforms has indicated that each isoform has a distinct contribution to the antimetamorphic action of JH in B. germanica and B. dorsalis (26, 28). In L. migratoria, the JH-regulated ovarian development and oocyte maturation rely heavily on a single Tai isoform containing an insertion of a histidine- and proline-rich motif (27).
In light of these findings, we start to investigate whether different Tai isoforms are required to mediate the signaling of JH and 20E. We report here a JH-regulated developmental shift toward specific alternatively spliced Tai isoforms in the fat body of adult female A. aegypti. After adult emergence, JH stimulates alternative splicing of taiman pre-mRNA via a signaling pathway containing RTK, phosphatidylinositol-3-kinase (PI3K), and protein kinase B (PKB/Akt). This leads to the production of Tai isoforms A and B in the previtellogenic mosquitoes; the appearance of the isoforms A and B coincides with the acquisition of the competence for the vitellogenic 20E response in the fat body. The Tai isoforms A and B are required by the 20E regulatory cascade during the blood meal-initiated egg maturation. By implicating JH in the alternative splicing of taiman, this study uncovers a link between JH and 20E signaling in mosquito reproduction.
Results
JH Activates the Phosphorylation of Pre-mRNA Splicing Factors in Newly Emerged Mosquitoes.
To elucidate the signaling pathway of JH, we performed a proteomic study to identify JH-induced protein phosphorylation. The A. aegypti Aag2 cells were cultured in growth medium containing l-lysine and l-arginine labeled with stable heavy isotopes of nitrogen (15N) and carbon (13C). Mass spectrometry-based quantitative analyses revealed dozens of proteins showing increased phosphorylation after the Aag2 cells were treated with JH. Among those proteins were several serine/arginine-rich (pre-mRNA) splicing factors (SRSFs) and a protein kinase specific for the SRSFs (SI Appendix, Table S1). The activity and subcellular localization of SRSFs are extensively modified in metazoans by phosphorylation of the C-terminal serine/arginine-rich domains in response to different cellular conditions and signals, affecting constitutive and alternative splicing of pre-mRNA (29). The enhanced phosphorylation of SRSFs in the JH-treated Aag2 cells prompted us to determine whether JH action involves alternative splicing of specific pre-mRNAs.
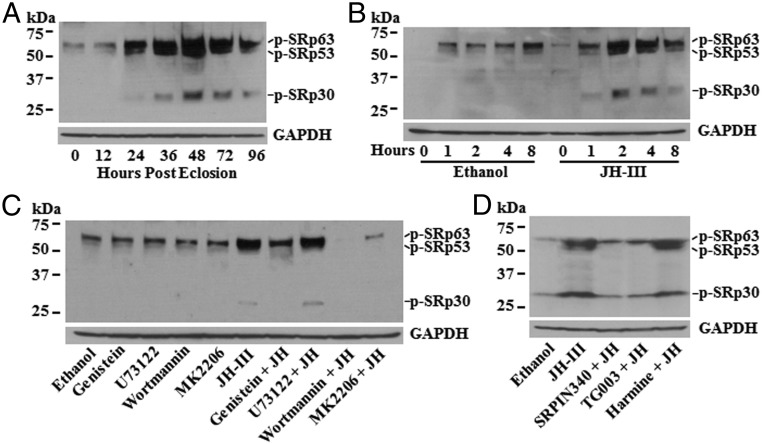
We first examined the phosphorylation status of SRSFs in the fat body of previtellogenic female mosquitoes. Immunoblotting was conducted using an antibody that recognizes the phosphoepitope in multiple members of the SRSF family (30). Phosphorylation of SRSF proteins underwent significant changes during the previtellogenic stage. In newly emerged adult mosquitoes, when the JH concentration was at the basal level, two weak protein bands were detected, with sizes of ∼63 kDa and ∼53 kDa (Fig. 1A). At 24 h posteclosion (PE), when the JH titers were substantially elevated, the phosphorylation of these two SRSF proteins increased considerably, and a smaller protein (SRp30) became detectable at this stage. Signals from the phosphorylated SRp63, SRp53, and SRp30 reached their peaks at 48 h PE and then weakened slightly thereafter (Fig. 1A). The changes in the protein phosphorylation implied that the activity of A. aegypti SRSFs was developmentally regulated in the previtellogenic stage.
Fig. 1.
JH regulates the phosphorylation of mosquito SRSF proteins in the fat body after eclosion. (A) Phosphorylation of SRSFs in female adults at 0, 12, 24, 36, 48, and 72 h PE was detected by immunoblotting. Equal amounts of proteins were loaded on SDS/PAGE and were blotted with an antibody (Clone 1H4; EMD Millipore) that binds to a phosphoepitope in SR proteins (p-SR). Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) was used as a loading control. (B) Phosphorylation of SRSFs was regulated by JH in the fat body of newly emerged mosquitoes. Fat bodies were isolated from female mosquitoes at 30 min PE and were cultured in vitro in the presence of JH-III (1 μM) for 0, 1, 2, 4, and 8 h. The phosphorylation of SRSFs was monitored by immunoblotting. (C) RTK/PI3K/Akt pathway was required for the JH-regulated SRSF phosphorylation. In vitro-cultured fat bodies were preincubated with genistein, U73122, wortmannin, and MK2206 (the specific inhibitors of RTK, PLC, PI3K, and Akt, respectively) for 1 h, followed by incubation with JH-III (1 μM) for 4 h. Equal amounts of proteins from each treatment were used for immunoblotting analysis. (D) SRPK and Clk were primarily responsible for the JH-regulated phosphorylation of SRSFs. SRPIN340, TG003, and harmine were used in the pretreatment to inactivate SRPK, Clk, and DYRK, respectively. The phosphorylation of SRSFs in the cultured fat bodies was assessed by immunoblotting after incubation with JH-III.
To investigate whether JH in the adult mosquitoes affects the phosphorylation of SRSFs, fat bodies were collected from female mosquitoes immediately after eclosion and were cultured in the medium containing JH-III or the solvent ethanol. Compared with the ethanol control, JH considerably increased the phosphorylation levels of SRp63, SRp53, and SRp30. The maximal phosphorylation was detected at 2 h after the addition of JH-III, and the enhanced phosphorylation was maintained for over 4 h (Fig. 1B). The result suggested that the phosphorylation of SRSFs in the previtellogenic fat body is induced by the rising JH titers after eclosion.
The RTK/Akt Pathway Is Required for the JH-Induced Phosphorylation of SRSFs.
In metazoan cells, regulation of alternative splicing is often mediated by the PI3K/Akt pathway (31). PI3K and Akt are activated downstream from RTKs upon ligand binding. Activated Akt, in turn, modulates the activity and/or localization of the SRSF-specific kinases, such as the serine/arginine-rich protein kinase (SRPK) and the Cdc2-like kinase (Clk), leading to the phosphorylation of SRSFs (31). To determine whether the JH-induced phosphorylation of SRSFs requires the PI3K/Akt pathway or the RTK/PLC pathway that modulates the phosphorylation of the JH receptor Met (21), fat bodies isolated from newly emerged female mosquitoes were cultured in vitro with JH-III and various inhibitors that are specific for RTKs or their downstream pathway components. Compared with the ethanol control, JH-III markedly stimulated the phosphorylation of SRp63, SRp53, and SRp30 (Fig. 1C). Inhibition of RTKs with genistein evidently diminished the phosphorylation of the three proteins in the presence of JH-III. Similarly, when PI3K and Akt were suppressed by wortmannin and MK2206, respectively, JH-III treatment failed to induce the phosphorylation of SRp63, SRp53, and SRp30 (Fig. 1C). Conversely, while PLC is required for the JH-triggered phosphorylation of Met and Tai, the PLC inhibitor U73122 did not block the JH-induced phosphorylation of these SRSF proteins (Fig. 1C).
SRPK, Clk, and the dual-specificity tyrosine phosphorylation-regulated kinases (DYRKs) are known for directly phosphorylating SRSFs in mammals (31). In the cultured fat body, the JH-induced phosphorylation of SRp63, SRp53, and SRp30 was substantially dampened by inactivation of either SRPK or Clk with the cognate inhibitors SRPIN340 and TG003, respectively (Fig. 1D). Suppression of the DYRK activity by harmine, in contrast, did not affect the JH-induced phosphorylation of the three SRSFs. Taken together, these results suggested that JH regulates the phosphorylation of SRSFs via the RTK/PI3K/Akt pathway and that SRPK and Clk are likely the major downstream protein kinases that phosphorylate the SRSFs in the fat body of A. aegypti.
JH Regulates Alternative Splicing of the taiman Gene.
The changes in phosphorylation of SRSFs in response to JH suggested that JH plays a regulatory role in splicing of pre-mRNAs. To identify the mosquito genes that undergo alternative splicing after JH treatment, we performed RNA deep sequencing to analyze mRNAs extracted from the cultured fat bodies that were collected upon eclosion and incubated with JH. Besides the identification of transcriptional up-regulation of genes like AaKr-h1, AaHairy, AAEL002576, and AAEL002619 (Dataset S1), this analysis revealed 162 mosquito genes that were significantly regulated by differential splicing after incubation with JH for 3 h, compared with the ethanol control (SI Appendix, Fig. S1 and Dataset S2). These genes were categorized into 23 functional groups based on the evolutionary genealogy of genes: Nonsupervised Orthologous Groups (eggNOG) database and were enriched in signal transduction mechanisms. The list included a GATA factor gene (AAEL010222) that produces two isoforms in the fat body of female mosquitoes, driving opposite transcriptional regulation of Vg (32–34).
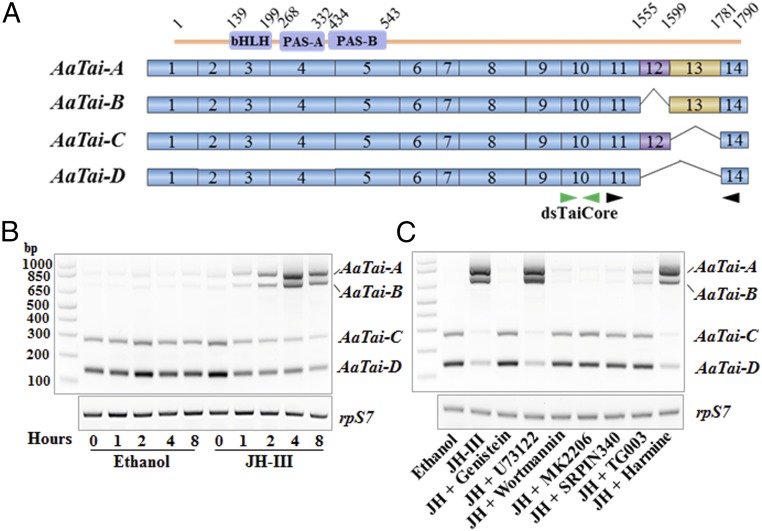
Interestingly, we found that JH regulated RNA splicing of the A. aegypti taiman gene (AaTai) in this experiment. Among the 14 exons of AaTai, exon 12 (E12) and E13 were differentially spliced in adult female mosquitoes, resulting in four isoforms differing only at the C-terminal regions (GenBank accession nos. MH187550, MH187551, MH187552, and MH187553) (Fig. 2A). AaTai-A includes both E12 and E13, AaTai-B and AaTai-C omit one of the two exons, and AaTai-D lacks both E12 and E13 (Fig. 2A). Our RNA-sequencing (RNA-Seq) analysis showed that inclusion of E13 was significantly increased after the JH treatment (Dataset S2). To validate this result, the AaTai isoforms in the cultured fat bodies were examined using semiquantitative RT-PCR (Fig. 2B). A pair of primers were designed to anneal to E11 and E14; the four isoforms were discerned by different sizes of the RT-PCR products. DNA fragments of 820 bp, 682 bp, 274 bp, and 136 bp on the gel were cloned for Sanger sequencing, and the results confirmed that they indeed represented the isoform-specific regions of AaTai isoforms A, B, C, and D, respectively (Fig. 2B). In the fat body of newly emerged mosquitoes, AaTai-C and AaTai-D were the predominant AaTai isoforms, while the transcripts of AaTai-A and AaTai-B were not detectable. When the fat body was cultured in vitro with JH-III, the mRNA levels of AaTai-A and AaTai-B increased considerably in a dose-dependent manner (SI Appendix, Fig. S2), while the abundance of the AaTai-C and AaTai-D mRNA decreased accordingly. At 4 h after addition of JH-III, AaTai-A and AaTai-B became the main isoforms in terms of mRNA levels (Fig. 2B). Incubation with ethanol, however, did not lead to the appearance of the AaTai-A and AaTai-B mRNA. This experiment confirmed that JH regulated alternative splicing of AaTai by promoting the inclusion of E13.
Fig. 2.
JH regulates the splicing of A. aegypti taiman. (A) Scheme of the AaTai isoforms. The constitutive exons are shown as solid blue boxes. E12 (purple) and E13 (orange) are alternatively spliced in the isoforms. The bHLH, PAS-A, and PAS-B domains are located in the N-terminal end of AaTai proteins. Green arrows point to the region where dsRNA was designed to knock down all AaTai isoforms. Black arrows indicate the locations of the primers used in RT-PCR. (B) JH induces the inclusion of E13 to produce the isoforms AaTai-A and AaTai-B. Fat bodies from female mosquitoes at 30 min PE were cultured in vitro in the presence of JH-III (1 μM). The mRNA levels of individual AaTai isoforms were assessed using RT-PCR with the primers shown in A. The RpS7 gene was used as an internal control. (C) RTK/PI3K/Akt pathway is required for the JH-regulated alternative splicing of AaTai. In vitro-cultured fat bodies were preincubated with genistein, U73122, wortmannin, MK2206, SRPIN340, TG003, or harmine for 1 h, followed by incubation with JH-III (1 μM) for 4 h.
Next, we examined whether the effect of JH on the splicing of AaTai pre-mRNA relied on the JH-induced phosphorylation of SRSFs. In the cultured fat bodies, we blocked the RTK/PI3K/Akt pathway using specific inhibitors for RTK, PI3K, Akt, SRPK, or Clk and then stimulated the fat bodies with JH-III. Inactivation of any of these five proteins essentially aborted the JH-induced increase in AaTai-A and AaTai-B mRNA and the decline of AaTai-C and AaTai-D transcripts (Fig. 2C). PLC and DYRK, which were not required for the JH-induced phosphorylation of SRp63, SRp53, and SRp30 in the fat body, did not influence the splicing of AaTai in the presence of JH-III when inactivated by U73122 and harmine, respectively (Fig. 2C). Therefore, the results clearly indicated that the RTK/PI3K/Akt pathway transduces the JH signal through SRPK and Clk to induce the alternative splicing of AaTai, stimulating the production of the E13-containing isoforms AaTai-A and AaTai-B.
The Appearance of AaTai Isoforms A and B in the Previtellogenic Fat Body Correlates with the Competence of the 20E Response.
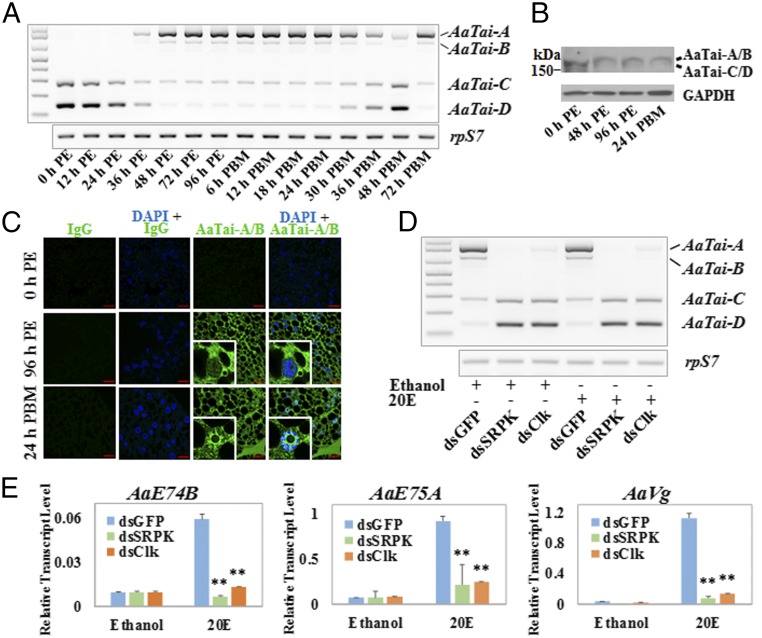
To gain insights into the roles of individual AaTai isoforms, we used RT-PCR to obtain the expression profiles of the AaTai isoforms in the fat body of adult female mosquitoes in the first gonotrophic cycle (Fig. 3A). During the first 24 h after adult emergence, AaTai-C and AaTai-D were the only two isoforms detected at the mRNA level, with AaTai-D being the more abundant one. Transcripts of AaTai-A and AaTai-B emerged at 36 h PE, while the expression of AaTai-C and AaTai-D showed a marked decrease. By 48 h PE, AaTai-D mRNA declined further to undetectable levels; AaTai-A took over and became the predominant isoform. The isoform composition remained unchanged from 48 h PE to 30 h post-blood meal (PBM). A decline of AaTai-A and AaTai-B transcripts started at 30 h PBM, accompanied by a rise in the expression of AaTai-C and AaTai-D. At that time, the 20E titers normally drop back to the basal level and the robust synthesis of YPPs comes to an end (11). At 48 h PBM, the AaTai-A mRNA was barely detectable and AaTai-D became the main isoform, reminiscent of the isoform composition in the newly emerged mosquitoes (0 h PE). Later, when female mosquitoes oviposit at about 72 h PBM and prepare for the next gonadotrophic cycle, the AaTai-A and AaTai-B mRNA increased in abundance, while AaTai-C and AaTai-D became nearly undetectable (Fig. 3A). Western blot analysis confirmed the alteration of AaTai isoforms in the fat body after eclosion, although we could not distinguish AaTai-A from AaTai-B and AaTai-C from AaTai-D because of their close molecular masses (Fig. 3B). To detect the isoforms A and B, we generated an antibody specific for the peptide encoded by the E13 (AaTaiE13). Whole-mount immunohistochemistry with this antibody verified that the AaTai isoforms A/B were absent in the fat body at eclosion but appeared in mature adult mosquitoes at 96 h PE and 24 h PBM (Fig. 3C). The A/B isoforms were predominantly located in the cytoplasm at 96 h PE and largely accumulated in the nucleus after blood feeding, consistent with a previous experiment using another AaTai antibody that recognizes the common regions of AaTai isoforms (25).
Fig. 3.
Expression of AaTai-A and AaTai-B in the previtellogenic fat body coincides with the acquisition of the competence for the 20E response. (A) Expression profiles of the AaTai isoforms in the fat body of adult female mosquitoes were examined by RT-PCR at the indicated time points. (B) AaTai isoform proteins were detected by immunoblotting with an antibody for the common regions of all isoforms. The predicted molecular masses of isoforms A–D are 191 kDa, 186 kDa, 175 kDa, and 170 kDa, respectively. (C) Visualization of AaTai-A and AaTai-B in the fat body by whole-mount immunohistochemistry with an antibody for AaTaiE13. Nonspecific rabbit IgG was used as a negative control. Nuclei of the fat body cells were stained blue with 4′,6-diamidino-2-phenylindole (DAPI). The red bar denotes 20 μm. (D) In vitro culture of the fat body dissected from the RNAi mosquitoes that failed to form AaTai-A and AaTai-B. DsRNAs for AaSRPK and AaClk were injected into newly emerged mosquitoes, and the dsRNA corresponding to the GFP gene (dsGFP) was used as a control. Fat bodies were isolated at 96 h PE from the RNAi mosquitoes and cultured in the presence of 20E (1 μM) or ethanol for 8 h. The mRNA transcripts of AaTai-A and AaTai-B in the cultured fat bodies were examined by RT-PCR. (E) Expression of the 20E-response genes AaE74B, AaE75A, and AaVg in the cultured fat bodies, where the formation of AaTai-A and AaTai-B was blocked by the depletion of AaSRPK or AaClk. The qRT-PCR results are the mean ± SD of three independent experiments. Statistical significance is shown (**P < 0.01).
In A. aegypti, the competence for the vitellogenic response to 20E is acquired between 36 h PE and 48 h PE (13). The appearance of AaTai-A and AaTai-B mRNA in the fat body seemed to coincide with the acquisition of the competence, prompting us to determine whether these two isoforms were critical for the 20E-regulated gene expression. We injected double-stranded RNA (dsRNA) into newly emerged female mosquitoes to independently knock down the expression of AaSRPK and AaClk, the two protein kinases that were crucial for the JH-induced phosphorylation of SRp63, SRp53, and SRp30. While AaTai-A was the major isoform at 96 h PE in the control mosquitoes, depletion of either AaSRPK or AaClk completely thwarted the generation of AaTai-A/B and increased the amounts of AaTai-C/D instead (Fig. 3D and SI Appendix, Fig. S3). Fat bodies were collected from the RNAi mosquitoes at 96 h PE and cultured in vitro in the presence of 20E. The mRNA levels of 20E response genes, including the ecdysteroid-induced early genes AaE74B and AaE75A and the vitellogenin gene AaVg, were measured after the 20E treatment. In the cultured fat body of the dsGFP-injected mosquitoes, 20E led to a substantial increase in the transcripts of these three genes (P < 0.01) (Fig. 3E). However, this induction was largely inhibited in the fat body if AaTai-A and AaTai-B were depleted due to the knockdown of AaSRPK and AaClk, confirming that the JH-triggered production of AaTai-A and AaTai-B was linked to the competence for the 20E response.
Blocking the Formation of AaTai-A and AaTai-B Inhibits the 20E Response and Impairs Oocyte Development After Blood Ingestion.
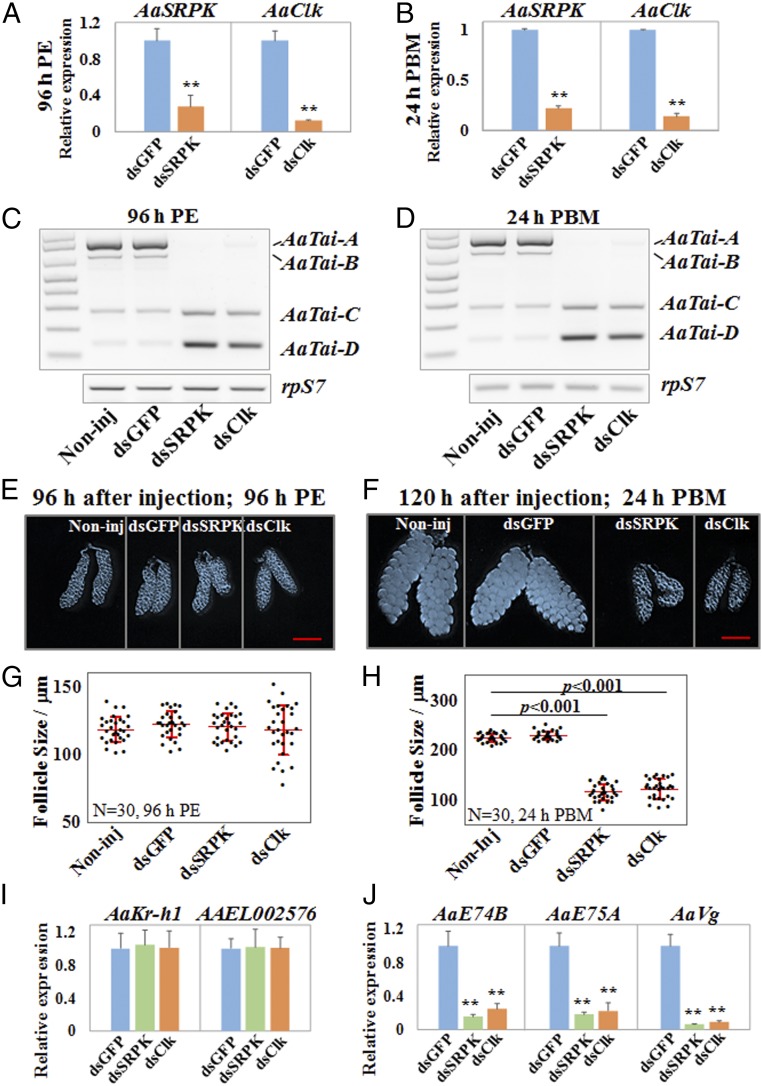
We next examined whether impeding the JH-induced alternative splicing of AaTai affected egg maturation in adult female mosquitoes. DsRNAs for AaSRPK and AaClk were injected into newly emerged female adults. The mRNA levels of AaSRPK and AaClk were reduced by more than 70% at 96 h PE and 24 h PBM, compared with the control mosquitoes (Fig. 4 A and B). The depletion of either AaSRPK or AaClk completely shut down production of the AaTai isoforms A and B at 96 h PE and 24 h PBM, causing the cells to produce primarily the AaTai isoforms C and D (Fig. 4 C and D). The growth of the primary follicles in the previtellogenic stage seemed normal in the AaSRPK RNAi and AaClk RNAi mosquitoes, as the average length of the follicles in these mosquitoes was comparable (P > 0.05) to those in the control mosquitoes at 96 h PE (Fig. 4 E and G). Mosquitoes injected with dsRNAs were then provided with a blood meal to initiate egg maturation. At 24 h PBM, oocyte development was halted in the AaSRPK- or AaClk-depleted mosquitoes; the follicles failed to increase in size after blood feeding, compared with the control groups (Fig. 4 F and H).
Fig. 4.
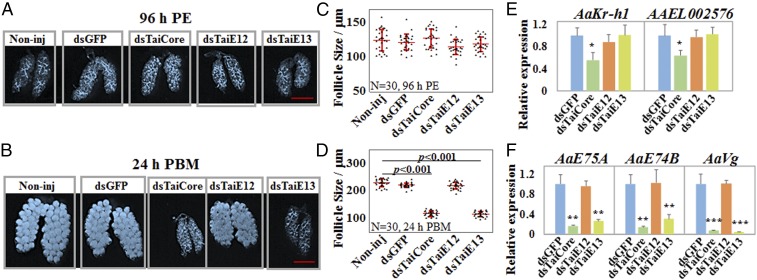
Blocking the formation of AaTai-A and AaTai-B by the knockdown of AaSRPK or AaClk arrests ovarian development. DsRNAs for AaSRPK and AaClk were injected into female mosquitoes within 30 min PE. DsRNA for the GFP gene (dsGFP) was used as a control. The knockdown of AaSRPK and AaClk was validated at 96 h PE (A) and 24 h PBM (B) using qRT-PCR. The results are presented as the mean ± SD of three independent experiments (**P < 0.01). The expression of AaTai isoforms was examined using RT-PCR at 96 h PE (C) and 24 h PBM (D). Noninj, noninjected. (E and F) Images of representative ovaries that were isolated from the Noninj and dsRNA-injected female mosquitoes at 96 h PE and 24 h PBM. (Scale bars: 500 µm.) (G and H) Average lengths of primary follicles at 96 h PE and 24 h PBM. The sizes were measured using the Leica Application Suite (v4.5). Thirty individuals in each group were used, and the results are shown as the mean ± SD. (I) Expression of the JH response genes AaKr-h1 and AAEL002576 was examined by qRT-PCR in the fat body at 96 h PE. qRT-PCR results are presented as a fold change compared with the dsGFP-injected sample (P > 0.05). (J) Expression of the 20E response genes AaE74B, AaE75A, and AaVg was measured by qRT-PCR at 24 h PBM. Statistical significance is shown (**P < 0.01).
Since AaTai plays important roles in the JH signaling in the previtellogenic stage and in the 20E response after blood ingestion (17, 25), we investigated whether the blocked formation of AaTai-A and AaTai-B, caused by the knockdown of AaSRPK or AaClk, affects gene expression in the JH and 20E signaling pathways. At 96 h PE, the transcript levels of two JH response genes, AaKr-h1 and AAEL002576, were not significantly different (P > 0.05) in the fat body between the control mosquitoes and the AaSRPK RNAi or AaClk RNAi mosquitoes (Fig. 4I). We also examined the expression of several 20E response genes (AaE74B, AaE75A, and AaVg) in the fat body at 24 h PBM. Importantly, the knockdown of AaSRPK or AaClk led to a drastic reduction of the mRNA levels of the three 20E-controlled genes, compared with the dsGFP-injected control (P < 0.01) (Fig. 4J).
To validate those observations, we performed a separate RNAi experiment using dsRNAs that specifically targeted the common region (TaiCore), E12 (TaiE12), or E13 (TaiE13) of AaTai. Injection of dsTaiCore led to a substantial decrease in the expression of the AaTai isoforms A, B, and C in the fat body at 96 h PE and 24 h PBM (SI Appendix, Fig. S4). RNAi induced by dsTaiE12 reduced the mRNA amounts of AaTai-A and AaTai-C but had no marked effect on the expression of AaTai-B at both 96 h PE and 24 h PBM. In the mosquitoes injected with dsTaiE13, the mRNA levels of AaTai-A and AaTai-B were considerably lower than those in the dsGFP-injected mosquitoes (SI Appendix, Fig. S4). As determined by the lengths of primary follicles at 96 h PE, injection of dsTaiCore, dsTaiE12, and dsTaiE13 seemed to have no manifest impact on the previtellogenic growth of primary follicles (P > 0.05), compared with the control groups (Fig. 5 A and C). At 24 h PBM, silencing the expression of all AaTai isoforms by dsTaiCore completely arrested the egg maturation (Fig. 5 B and D). Similarly, when the expression of AaTai-A and AaTai-B was knocked down by dsTaiE13, oocyte development in these mosquitoes was largely suppressed (Fig. 5 B and D). Therefore, RNAi-mediated knockdown of AaTai-A and AaTai-B essentially phenocopied the depletion of AaSRPK and AaClk in the egg maturation in adult female mosquitoes. Notably, in the dsTaiE12-injected female mosquitoes, the sizes of the developing oocytes at 24 h PBM were similar to those in the control mosquitoes (Fig. 5 B and D). Since dsTaiE12 selectively reduced the mRNA levels of AaTai-A but did not alter the expression of AaTai-B (SI Appendix, Fig. S4), the different phenotypes caused by the injection of dsTaiE12 and dsTaiE13 suggested that the egg maturation after a blood meal requires at least one AaTai isoform harboring the E13.
Fig. 5.
RNAi-mediated knockdown of AaTai-A and AaTai-B impedes ovarian development and ecdysteroid response. DsRNAs for the AaTai common region (dsTaiCore), AaTai-E12 (dsTaiE12), and AaTai-E13 (dsTaiE13) were injected into adult female mosquitoes within 30 min PE. DsGFP was used as a control. (A and B) Ovaries isolated from each group of mosquitoes at 96 h PE and 24 h PBM. Non-inj, noninjected. (Scale bars: 500 µm.) (C and D) Average lengths of primary follicles were measured at 96 h PE and 24 h PBM. Thirty individuals were measured for each group, and the results are presented as the mean ± SD. (E) Expression of the JH response genes AaKr-h1 and AAEL002576 in the fat body at 96 h PE. qRT-PCR results are presented as a fold change compared with the dsGFP-injected sample. Results are the mean ± SD of three independent experiments. (F) Expression of AaE74B, AaE75A, and AaVg at 24 h PBM. qRT-PCR results are the mean ± SD of three independent experiments. Statistical significance between the groups is shown (*P < 0.05; **P < 0.01; ***P < 0.001).
We also evaluated how the depletion of AaTai-A/B affected transcription of the JH and 20E response genes. At 96 h PE, down-regulation of AaTai-A/B by dsTaiE13 did not alter the expression of AaKr-h1 and AAEL002576, compared with the dsGFP-injected control (Fig. 5E). Conversely, the knockdown of AaTai-A/B by dsTaiE13 at 24 h PBM significantly reduced the transcript levels of AaE74B, AaE75A, and AaVg (P < 0.01) (Fig. 5F). We thus concluded that the presence of AaTai-A/B in mature female adults is essential for the expression of 20E response genes and for the blood meal-induced egg maturation.
AaTai-A and AaTai-B Potentiate the Transcriptional Activation by the Ecdysteroid Receptor Complex.
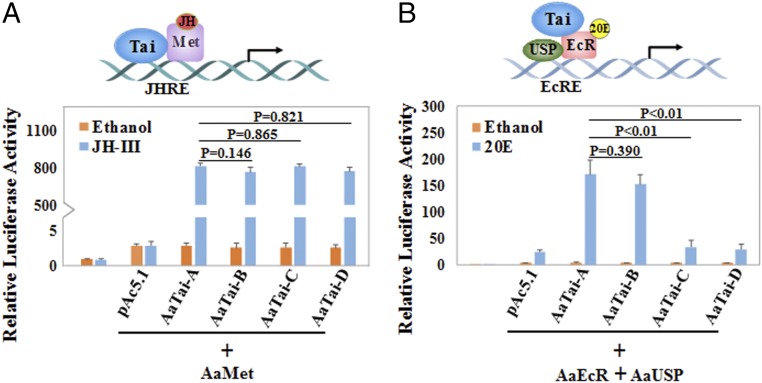
Our RNAi experiments suggested that AaTai-A and AaTai-B have a unique function in the 20E response and cannot be substituted with AaTai-C/AaTai-D. By contrast, either AaTai-A/AaTai-B or AaTai-C/AaTai-D seemed to be sufficient for the JH-induced gene expression in the previtellogenic mosquitoes. To determine the role of each AaTai isoform in the JH-regulated gene expression, we performed a transient transfection assay using the 4× JH response element (JHRE)-Luc reporter construct. Drosophila L57 cells were transfected with the reporter construct and the expression vectors for AaMet and each isoform of AaTai. All four AaTai isoforms were expressed in comparable amounts in the transfected cells (SI Appendix, Fig. S5A). AaMet alone was unable to activate the reporter gene in response to JH, but coexpression of AaMet with any AaTai isoform substantially enhanced the expression of the reporter when JH was present (Fig. 6A). The transactivation activities of the AaMet–AaTai complex were quite similar for the four AaTai isoforms (P > 0.05).
Fig. 6.
AaTai-A and AaTai-B potentiate transcriptional activation by the AaEcR–AaUSP complex in response to 20E. (A) Drosophila L57 cells were transfected with the 4× JHRE-Luc reporter construct and the expression plasmids of AaMet and individual AaTai isoforms. A Renilla luciferase reporter construct was cotransfected in each well. Transfected cells were treated with JH-III (1 μM) or ethanol and then were lysed for measuring the luciferase activity. (B) L57 cells were transfected with the hsp27-EcRE-Luc reporter construct and the expression plasmids of AaEcR, AaUSP, and each AaTai isoform. Transfected cells were incubated with 20E (1 μM) or ethanol. Results are presented as the ratio of firefly/Renilla luciferase activity and are reported as the mean ± SD of three independent experiments.
A separate transfection assay was carried out using the hsp27-ecdysone response element (EcRE)-Luc reporter construct to compare the function of the AaTai isoforms in the 20E-regulated gene expression. L57 cells were transfected by this reporter plasmid, the expression vectors for AaEcR and AaUSP, and the expression vectors for each AaTai isoform. Protein levels of AaEcR, AaUSP, and AaTai isoforms were comparable among the transfected cells after the 20E treatment (SI Appendix, Fig. S5B). A significant activation of the luciferase reporter gene was detected in the cells overexpressing AaEcR and AaUSP after 20E stimulation, compared with the ethanol treatment (5.5-fold; P < 0.01) (Fig. 6B). When AaTai-A or AaTai-B was coexpressed with AaEcR and AaUSP, the luciferase reporter activity was 43.1-fold and 39.8-fold stronger (P < 0.01), respectively, in response to 20E than to the ethanol control. Overexpression of either AaTai-C or AaTai-D did not significantly augment the 20E activation (P > 0.05) (Fig. 6B). These results indicated that robust transcriptional activation induced by 20E requires at least one of the two isoforms AaTai-A and AaTai-B.
AaTai Isoforms Have Distinct Binding Affinities to the Ecdysteroid Receptor Complex.
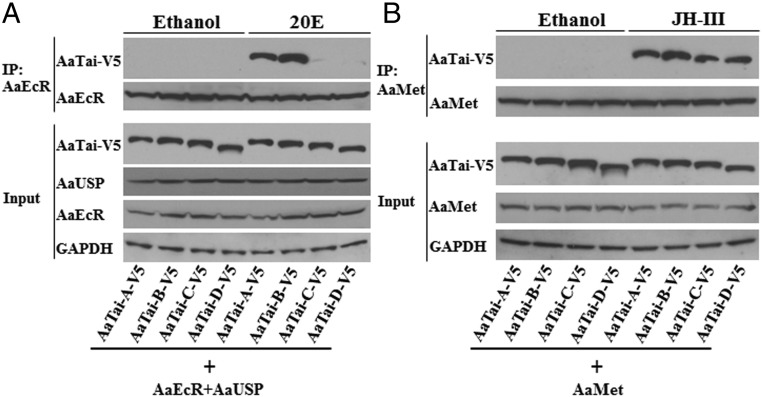
Tai is recruited to the 20E-regulated promoters via the protein interaction with EcR/USP. In light of the different contribution of individual AaTai isoforms to the 20E-regulated gene expression, we compared the protein interactions between the AaTai isoforms and AaEcR/AaUSP. In Drosophila L57 cells, V5-tagged AaTai isoforms were overexpressed together with AaEcR and AaUSP. The protein interactions were evaluated using coimmunoprecipitation assays. When the transfected cells were treated with ethanol, none of the AaTai isoforms was precipitated with AaEcR (Fig. 7A). When the transfected cells were treated with 20E, AaTai-A and AaTai-B in the cellular extracts were readily pulled down by the antibody of AaEcR, while only trace amounts of AaTai-C and AaTai-D were precipitated with AaEcR (Fig. 7A), indicating that the 20E-dependent binding of AaTai-A or AaTai-B to AaEcR/AaUSP was much stronger than that of AaTai-C or AaTai-D. To validate the specificity of the detected interactions, we performed similar experiments using L57 cells that only overexpressed AaTai-A and AaUSP. Results indeed showed that in the absence of AaEcR, AaTai-A was not precipitated by the AaEcR antibody following the 20E treatment (SI Appendix, Fig. S6A).
Fig. 7.
Binding of individual AaTai isoforms to the AaEcR–AaUSP complex and AaMet. (A) Protein interaction between AaTai and AaEcR/AaUSP. Drosophila L57 cells were transfected with the expression plasmids for the V5-tagged AaTai isoforms, together with the expression vectors for AaEcR and AaUSP. The transfected cells were treated with 20E (1 μM) or ethanol. AaEcR in the cell extracts was precipitated by anti-AaEcR antibody, and the pellets were analyzed by immunoblotting using anti-V5 antibody. Input [10% of the amount of cell lysate used for the immunoprecipitation (IP)] was analyzed using anti-V5, anti-AaEcR, anti-AaUSP, and anti-GAPDH antibodies. (B) Coimmunoprecipitation of AaTai isoforms with AaMet. Drosophila L57 cells were transfected with the expression plasmids for the V5-tagged AaTai isoforms and for AaMet. The transfected cells were treated with JH-III (1 μM) or ethanol. Immunoprecipitation was performed using an anti-AaMet antibody, and Western blotting was performed using anti-V5 antibody. Input was analyzed using anti-V5, anti-AaMet, and anti-GAPDH antibodies.
The AaTai–AaMet interaction was also investigated using similar coimmunoprecipitation assays. In the presence of JH, each AaTai isoform was detected at comparable levels in the pellets precipitated by the AaMet antibody (Fig. 7B and SI Appendix, Fig. S6B), indicating that the four AaTai isoforms have a similar capability of forming the JH receptor complex with AaMet. Together, the results thus suggested that the isoform-specific structures at the C-terminal portion of AaTai do not affect the binding of AaTai to AaMet, but favor the interaction between AaEcR/AaUSP and AaTai-A or AaTai-B in the presence of 20E.
Discussion
Transcriptional regulation of JH target genes has been shown to be mediated by Met and Tai in many insects (16, 18, 19, 35). Here, we report a previously unknown regulatory mechanism of gene expression in response to JH. For a subset of genes in mosquitoes, JH does not alter their overall abundance but stimulates alternative splicing of pre-mRNA to generate different protein isoforms. In particular, taiman, a key player in both JH and 20E signaling pathways, was found to generate functionally different isoforms in response to the rising JH titers in the newly emerged mosquitoes. Our study clearly demonstrates that distinct Tai isoforms are involved in the JH signaling and the 20E response. This JH action requires a pathway containing RTK, PI3K, and Akt, which modulates the phosphorylation of pre-mRNA splicing factors, such as SRp63, SRp53, and SRp30. We have previously reported that the RTK-PLC pathway transduces JH signal through the cell membrane and converges on the Met/Tai receptor complex in the nuclei (21, 22). The present study suggests that the RTK family is required for the JH-induced alternative splicing of AaTai, but PLC is not likely involved. PI3K-Akt probably represents another branch downstream of RTKs to transduce the signal across the cell membrane. It is unclear at this stage whether different RTK members are required for the transcriptional and posttranscriptional regulation initiated by JH.
A total of 162 genes were found to be primarily regulated at pre-mRNA splicing by JH in the fat body of newly emerged mosquitoes, implying that this JH-regulated RNA splicing has a profound impact on mosquito physiology and egg maturation. Among these genes is a GATA factor gene that plays an important role in coordinating the expression of Vg in female mosquitoes (33). The AaGATA gene produces two isoforms that both recognize the GATA motifs present in the upstream regulatory region of Vg: GATAa has a single zinc finger motif and activates the expression of Vg after blood feeding, and GATAr consists of two zinc finger motifs and acts as a repressor to inhibit the expression of Vg in the late previtellogenic stage (32–34). Our data suggest that JH induces the alternative splicing of two mutually exclusive exons to stimulate the synthesis of the repressive isoform GATAr and reduce the abundance of GATAa in the newly emerged mosquitoes. Therefore, it seems that JH regulates RNA splicing of the AaGATA and AaTai genes to maintain Vg in a silent state in the previtellogenic stage while keeping it poised for subsequent expression upon taking a blood meal.
Tai isoforms, derived from alternative splicing, have been found in other insects. Individual isoforms seem to have distinct contributions to the JH action in metamorphosis and adult reproduction (26–28). The alternatively included or skipped exons are called insertions and deletions (INDELs) in those studies. Two INDELs are located in the C terminus of the Tai protein in the German cockroach B. germanica, giving rise to four Tai isoforms (26). The BgTai isoforms containing INDEL-1 seemed to play a more prominent role in mediating the antimetamorphic function of JH than the isoforms lacking INDEL-1. Similarly, the two Tai isoforms in L. migratoria differ at INDEL-1. The one with INDEL-1 is more potent in transducing the vitellogenic JH signal than the other isoform (27). The alternatively spliced E12 in AaTai matches exactly to INDEL-1 found in the Tai proteins in B. germanica, D. melanogaster, L. migratoria, and T. castaneum (SI Appendix, Fig. S7), suggesting that alternative splicing of E12 is evolutionarily conserved in insects. In our study, depletion of the isoforms that contain E12 (AaTai-A and AaTai-C) in the newly emerged mosquitoes did not markedly affect the JH- or 20E-controlled gene expression or egg maturation. It is still possible that there is a unique demand for AaTai-A and AaTai-C in other biological events in mosquitoes.
The 3′ end of AaTai E13 is homologous to the INDEL-2 found in BgTai. In the previtellogenic A. aegypti mosquitoes, JH regulates the splicing of E13, but not E12, in AaTai, leading to an increased expression of the isoforms A and B. While all four isoforms readily partner with AaMet to drive the JH-regulated gene expression as revealed in the RNAi experiments and the reporter assays, only AaTai-A and AaTai-B were able to effectively regulate the gene expression in response to 20E. The two shorter isoforms, AaTai-C and AaTai-D, failed to substitute for AaTai-A and AaTai-B in mediating the 20E response. Consistent with these observations, our immunoprecipitation experiments have shown that all four AaTai isoforms bind to AaMet equally well in a JH-dependent manner, while AaTai-C and AaTai-D display a much weaker interaction with AaEcR/AaUSP than AaTai-A and AaTai-B. The observed differential binding may stem from how Tai uses distinct domains to interact with Met and EcR/USP. In the presence of JH, Tai is the DNA-binding partner of Met. The N-terminal bHLH-PAS domain of Tai is required and sufficient for its binding to Met (15, 17, 20). Therefore, the different C-terminal regions of the Tai isoforms reasonably have no significant impact on the interaction between Tai and Met. When 20E is present, the central region of Tai that harbors the LXXLL repeats is essential for its interaction with the EcR/USP heterodimer, according to the previous result of a modified two-hybrid assay (25). The immunoprecipitation experiment in the present study suggested that the C-terminal region of Tai also plays an important role in the interaction between Tai and EcR/USP. Our current hypothesis is that the peptide encoded by E13 either provides an additional interface for the protein interaction or helps Tai assume a conformation that facilitates its binding to EcR/USP. Sequence alignment revealed that the N-terminal end of the peptide encoded by E13 was highly conserved in the Tai proteins of A. mellifera, B. germanica, D. melanogaster, L. migratoria, and T. castaneum (SI Appendix, Fig. S7). The 13-amino acid peptide (TSEHVRQELRAVV) in the most conserved region is able to form an α-helix. Whether this putative α-helix facilitates the contacts between AaTai and AaEcR/AaUSP remains to be determined.
In this study, we have demonstrated that JH regulates pre-mRNA splicing of the AaTai gene to generate the isoforms AaTai-A and AaTai-B, which are potent coactivators of the ecdysteroid receptor complex during vitellogenesis. In the newly emerged mosquitoes, the default splicing only produces the isoforms AaTai-C and AaTai-D. In response to the JH titers after eclosion, the isoforms AaTai-A and AaTai-B appear at 36 h PE and are the predominant isoforms from 48 h PE to 30 h PBM. This JH action confers a competence on mosquito reproductive tissues to the vitellogenic 20E response. AaTai is expressed in the fat body, gut, and ovaries of adult female mosquitoes (25). Blocking the JH-controlled production of AaTai A/B probably compromises the 20E responses in multiple tissues, collectively leading to the arrest of ovarian development and, consequently, the termination of egg production. Since substantial amounts of AaTai-A and AaTai-B transcripts exist at 30–36 h PBM, long after the rapid decline of JH concentrations upon blood ingestion, JH may act as the signal to initiate the alternative splicing of AaTai, and other factors are likely required to maintain the alternative splicing. Nevertheless, this study reveals a different mode of JH action and uncovers an important layer in the cross-talk between the JH and 20E signaling pathways. We are going to determine if JH regulates the splicing of AaTai in the larval stages of mosquitoes. Whether the alternative splicing of Tai by JH occurs in other insects also merits further investigation.
Materials and Methods
Mosquito Rearing and Fat Body Culture.
A. aegypti mosquitoes of the Liverpool strain were maintained at 28 °C and 60–70% relative humidity, with a 14-h/10-h day/night light cycle. Mosquito larvae were fed on pulverized fish food (Tetra), and adults were provided with a 10% sucrose solution. At 4–6 d PE, female mosquitoes were fed on anesthetized rats to initiate egg production. All dissections were performed in Aedes physiological saline (36). Fat bodies were isolated from either newly emerged mosquitoes or mature mosquitoes and cultured in a complete medium as described previously (21, 37). JH-III (Sigma–Aldrich) and 20E (Cell Signaling Technologies) were dissolved in ethanol. In the experiment in which inhibitors were used, fat bodies were preincubated with the inhibitors for 1 h before the addition of hormones. Final concentrations of inhibitors in all tissue culture studies were as follows: genistein (MP Biomedicals), 20 μM; harmine (Sigma–Aldrich), 30 μM; MK2206 (Selleck Chemicals), 5 μM; SRPIN340 (Sigma–Aldrich), 10 μM; TG003 (Sigma–Aldrich), 5 μM; U73122 (EMD Millipore), 1 μM; and wortmannin (Sigma–Aldrich), 1 μM.
Stable Isotope Labeling with Amino Acids in Cell Culture and Quantitative Proteomic Analysis.
A. aegypti mosquito Aag2 cells were maintained at 28 °C in Schneider’s Drosophila medium (Sigma–Aldrich) supplemented with 10% (vol/vol) FBS (Atlanta Biologicals). “Heavy” and “light” media were prepared by adding 13C615N4 l-arginine and 13C615N2 l-lysine (Thermo Fisher Scientific) or the corresponding nonlabeled amino acids, respectively. To ensure the efficient incorporation of the isotopes, cells were cultured in heavy medium at 28 °C for seven replication cycles. Subsequently, the heavy and light cells were incubated with JH-III (1 μM) and ethanol, respectively, for 30 min. Equal amounts of proteins from both cell extracts were mixed together. After trypsin digestion, the peptide mix was used for phosphopeptide isolation by titanium dioxide enrichment. Liquid chromatography-tandem mass spectrometry was performed on a Thermo Scientific LTQ Orbitrap Elite mass spectrometer equipped with a Waters nanoACQUITY UPLC system. Matrix Science’s Mascot Distiller (www.matrixscience.com/) and the Mascot search algorithm (38) were used for database searching against Aaeg_Uniprot_v2.fasta. Phosphormodification at serine, threonine, and tyrosine was chosen for the search. Mass spectrometry analysis was carried out by the Keck Biotechnology Resource Laboratory at Yale University.
Preparation of Illumina mRNA Library.
Fat bodies from 30 newly emerged mosquitoes were cultured in vitro in the presence of ethanol or JH III (1 μM) for 3 h. Total RNA was isolated using Ambion TRIzol reagents (Life Technologies) and a Direct-zol RNA MiniPrep Plus kit (Zymo Research). mRNA was then purified using an Oligotex mRNA Midi Kit (Qiagen), and the quality was assessed with an Agilent 2100 Bioanalyzer (Agilent Technologies). Libraries were constructed using a NEBNext mRNA Library Prep Master Mix Set for Illumina (New England Biolabs) according to the manufacturer’s instructions. Six libraries in total were prepared, with three biological replicates per treatment.
Processing of Illumina RNA-Seq Data and Computational Analysis of Alternative Splicing.
The RNA libraries were sequenced on an Illumina high-output HiSeq platform, producing 100-bp paired-end reads. The RNA-Seq data have been deposited in the National Center for Biotechnology Information Sequence Read Archive database under accession number SRP136548. Reads were mapped to the genome (AaegL3) using STAR (39). An expanded transcript annotation was generated using the PASA pipeline with AaegL3.4 as a reference annotation (40, 41). Alternative splicing events were identified and quantified using rMATS (v3.2.5) through unpaired differential testing (42). Differential gene expression analysis was conducted using DESeq2 (43). Functional categories of the differentially spliced genes were determined using the dataset for insects: Nonsupervised Orthologous Groups (inNOG) and their proteins from the eggNOG database (v3.0) (44).
DsRNA-Induced Gene Silencing.
DsRNAs were synthesized using a MEGAscript RNAi kit (Thermo Fisher Scientific) (SI Appendix, Table S2). Microinjection was performed as described previously (13). Briefly, 1 μg of dsRNA was injected into the thorax of ice-anesthetized female mosquitoes 30 min after adult emergence. Mosquitoes were allowed to recover for 4 d; they were then dissected for RNA/protein extraction and fat body culture or were fed on anesthetized rats to initiate egg production.
RT-PCR.
Total RNA was isolated from cultured mosquito fat bodies using a TRIzol reagent and a Direct-zol RNA MiniPrep Plus kit. Genomic DNA contamination was completely removed by incubating the total RNA with DNase I (Thermo Fisher Scientific) for 15 min at room temperature. Three micrograms of the purified RNA was used for reverse transcription to synthesize cDNA. To amplify four different isoforms of AaTai, a pair of primers were designed in the common regions of the AaTai gene with the forward primer located at E11 and the reverse primer at E14 (SI Appendix, Table S2). Semiquantitative RT-PCR was performed with Platinum SuperFi DNA Polymerase (Thermo Fisher Scientific) on a T100 Thermal Cycler (Bio-Rad). The ribosomal protein S7 gene was used as an internal control. The PCR products were separated on a 1.75% agarose gel. The gel was stained with Gel-Red dye, and the image was visualized on a G:BOX F3 gel documentation system (Syngene). qRT-PCR was performed with GoTaq qPCR Master Mix (Promega) and gene-specific primers (SI Appendix, Table S2) on an ABI 7300 system (Applied Biosystems) following the manufacturer’s protocol. All assays were performed in triplicate. Transcript abundance was normalized to that of rpS7 and analyzed by the Student’s t test for significance.
Antibodies.
Antiphosphoepitope SR proteins antibody (Clone 1H4, MABE50) was purchased from EMD Millipore. Anti-V5 tag antibody was from Thermo Fisher Scientific. Production of AaMet and AaTai (common region) antibodies has been described previously (22). To generate antibodies for AaEcR and AaUSP, the full-length coding regions of AaEcRb and AaUSPb were cloned into a pGEX-6p-1 plasmid (GE Healthcare). Likewise, the E13 of AaTai was cloned into pGEX-6p-1 to generate antibodies that bind to the peptide encoded by AaTai E13. The plasmids were transformed into Escherichia coli BL21 competent cells. After purification using ÄKTA prime and a GSTrap FF column (GE Healthcare), the recombinant proteins were sent to Thermo Fisher Scientific for custom antibody production. Specific polyclonal antibodies were purified from each antiserum by antigen-affinity purification using an AminoLink Immobilization Kit (Thermo Fisher Scientific) following the manufacturer’s instructions.
Luciferase Reporter Assay.
Drosophila L57 cells were maintained at 26 °C in Schneider’s Drosophila medium (Life Technologies) supplemented with 5% (vol/vol) FBS. When the cells reached a density of 2 × 105 cells per square centimeter, at ∼80% confluency, they were transfected with plasmids as described by Li et al. (20). The full-length coding regions of four AaTai isoforms were cloned into pAc5.1/V5-His B plasmid using a NEBuilder HiFi DNA Assembly Cloning Kit (New England Biolabs) following the manufacturer’s instructions. The pCMA-AaMet, 4× JHRE1-Luc, pAc-AaEcRb, pAc-AaUSPb, and hsp27-EcRE-Luc plasmids have been described previously (17, 45). The Renilla luciferase construct pRL-CMV (Promega) was included in each transfection to normalize for variations in transfection efficiency. At 24 h after transfection, JH-III (1 μM) or 20E (1 μM) was added to the culture medium. Six hours later, the cells were harvested for luciferase activity measurement using a Dual-Luciferase Reporter Assay System (Promega). Results were presented as the ratio of firefly activity to Renilla luciferase activity.
Coimmunoprecipitation.
L57 cells growing in a six-well plate were transfected with 1 μg of each expression plasmid. Thirty-six hours after transfection, the cells were incubated with 20E or JH-III at 1 μM for an additional 6 h. Ethanol was used as a control. Cells from all treatments were washed with cold PBS and lysed in 1% Triton X-100, 20 mM Tris (pH 7.5), 50 mM NaCl, 0.1 mM EDTA, 1 mM NaF, 1 mM Na3VO4, and 2.5 mM Na4P2O7 supplemented with proteinase inhibitor mixture (EDTA-free; Thermo Fisher Scientific). The lysate was cleared by passing through a 0.45-μm filter microcentrifuge tube. Protein concentrations were determined by the bicinchoninic acid assay.
Ten micrograms of anti-AaEcR rabbit antibody and anti-AaMet goat antibody were incubated with Dynabeads Protein A and Protein G, respectively, at 4 °C for 1 h. The antibodies were then covalently cross-linked to the Dynabeads using Pierce BS3 cross-linker (Thermo Fisher Scientific). Ten percent of the lysate was set aside as input, and the rest of the lysate was incubated with the cross-linked Dynabeads–antibody complex at 4 °C for 4 h. The beads were washed with PBS containing 0.02% Tween-20. Proteins eluted from the beads and the inputs were analyzed by immunoblotting with V5 antibody and other antibodies.
Supplementary Material
Acknowledgments
We thank Jiyoung Lee (Interdisciplinary PhD Program in Genetics, Bioinformatics, and Computational Biology, Virginia Tech) for critical comments on bioinformatics analysis. We also acknowledge Megan Richardson for her detailed and helpful comments on the manuscript. This work was supported by NIH Grant R01 AI099250 (to J.Z.). Funding for this work was provided, in part, by the Virginia Agricultural Experiment Station and the Hatch Program (accession no. 1005118) of the National Institute of Food and Agriculture, US Department of Agriculture.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The RNA-seq data have been deposited in the National Center for Biotechnology Information Sequence Read Archive (accession no. SRP136548). The cDNA sequences for the taiman isoforms have been deposited in the GenBank database (accession nos. MH187550–MH187553).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1808146115/-/DCSupplemental.
References
- 1.Nijhout HF. Insect Hormones. Princeton Univ Press; Princeton: 1994. [Google Scholar]
- 2.Riddiford LM. Cellular and molecular actions of juvenile-hormone. 1. General-considerations and premetamorphic actions. Adv Insect Physiol. 1994;24:213–274. [Google Scholar]
- 3.Muramatsu D, Kinjoh T, Shinoda T, Hiruma K. The role of 20-hydroxyecdysone and juvenile hormone in pupal commitment of the epidermis of the silkworm, Bombyx mori. Mech Dev. 2008;125:411–420. doi: 10.1016/j.mod.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 4.Ureña E, Manjón C, Franch-Marro X, Martín D. Transcription factor E93 specifies adult metamorphosis in hemimetabolous and holometabolous insects. Proc Natl Acad Sci USA. 2014;111:7024–7029. doi: 10.1073/pnas.1401478111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhou B, Hiruma K, Shinoda T, Riddiford LM. Juvenile hormone prevents ecdysteroid-induced expression of broad complex RNAs in the epidermis of the tobacco hornworm, Manduca sexta. Dev Biol. 1998;203:233–244. doi: 10.1006/dbio.1998.9059. [DOI] [PubMed] [Google Scholar]
- 6.Smykal V, et al. Importance of juvenile hormone signaling arises with competence of insect larvae to metamorphose. Dev Biol. 2014;390:221–230. doi: 10.1016/j.ydbio.2014.03.006. [DOI] [PubMed] [Google Scholar]
- 7.Jindra M, Palli SR, Riddiford LM. The juvenile hormone signaling pathway in insect development. Annu Rev Entomol. 2013;58:181–204. doi: 10.1146/annurev-ento-120811-153700. [DOI] [PubMed] [Google Scholar]
- 8.Roy S, Saha TT, Zou Z, Raikhel AS. Regulatory pathways controlling female insect reproduction. Annu Rev Entomol. 2018;63:489–511. doi: 10.1146/annurev-ento-020117-043258. [DOI] [PubMed] [Google Scholar]
- 9.Hernández-Martínez S, Rivera-Perez C, Nouzova M, Noriega FG. Coordinated changes in JH biosynthesis and JH hemolymph titers in Aedes aegypti mosquitoes. J Insect Physiol. 2015;72:22–27. doi: 10.1016/j.jinsphys.2014.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao B, et al. Determination of juvenile hormone titers by means of LC-MS/MS/MS and a juvenile hormone-responsive Gal4/UAS system in Aedes aegypti mosquitoes. Insect Biochem Mol Biol. 2016;77:69–77. doi: 10.1016/j.ibmb.2016.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raikhel A, Brown M, Belles X. Hormonal control of reproductive processes. In: Gilbert LI, Iatrou K, Gill SS, editors. Comprehensive Molecular Insect Science, Endocrinology. Vol 3. Elsevier; San Diego: 2005. pp. 433–491. [Google Scholar]
- 12.Flanagan TR, Hagedorn HH. Vitellogenin synthesis in mosquito–Role of juvenile hormone in development of responsiveness to ecdysone. Physiol Entomol. 1977;2:173–178. [Google Scholar]
- 13.Zhu J, Chen L, Raikhel AS. Posttranscriptional control of the competence factor betaFTZ-F1 by juvenile hormone in the mosquito Aedes aegypti. Proc Natl Acad Sci USA. 2003;100:13338–13343. doi: 10.1073/pnas.2234416100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ashok M, Turner C, Wilson TG. Insect juvenile hormone resistance gene homology with the bHLH-PAS family of transcriptional regulators. Proc Natl Acad Sci USA. 1998;95:2761–2766. doi: 10.1073/pnas.95.6.2761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Charles JP, et al. Ligand-binding properties of a juvenile hormone receptor, methoprene-tolerant. Proc Natl Acad Sci USA. 2011;108:21128–21133. doi: 10.1073/pnas.1116123109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jindra M, Uhlirova M, Charles JP, Smykal V, Hill RJ. Genetic evidence for function of the bHLH-PAS protein Gce/Met as a juvenile hormone receptor. PLoS Genet. 2015;11:e1005394. doi: 10.1371/journal.pgen.1005394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li M, Mead EA, Zhu J. Heterodimer of two bHLH-PAS proteins mediates juvenile hormone-induced gene expression. Proc Natl Acad Sci USA. 2011;108:638–643. doi: 10.1073/pnas.1013914108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Z, Xu J, Sheng Z, Sui Y, Palli SR. Steroid receptor co-activator is required for juvenile hormone signal transduction through a bHLH-PAS transcription factor, methoprene tolerant. J Biol Chem. 2011;286:8437–8447. doi: 10.1074/jbc.M110.191684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kayukawa T, et al. Transcriptional regulation of juvenile hormone-mediated induction of Krüppel homolog 1, a repressor of insect metamorphosis. Proc Natl Acad Sci USA. 2012;109:11729–11734. doi: 10.1073/pnas.1204951109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li M, et al. A steroid receptor coactivator acts as the DNA-binding partner of the methoprene-tolerant protein in regulating juvenile hormone response genes. Mol Cell Endocrinol. 2014;394:47–58. doi: 10.1016/j.mce.2014.06.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu P, Peng HJ, Zhu J. Juvenile hormone-activated phospholipase C pathway enhances transcriptional activation by the methoprene-tolerant protein. Proc Natl Acad Sci USA. 2015;112:E1871–E1879. doi: 10.1073/pnas.1423204112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ojani R, Liu P, Fu X, Zhu J. Protein kinase C modulates transcriptional activation by the juvenile hormone receptor methoprene-tolerant. Insect Biochem Mol Biol. 2016;70:44–52. doi: 10.1016/j.ibmb.2015.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yao TP, et al. Functional ecdysone receptor is the product of EcR and Ultraspiracle genes. Nature. 1993;366:476–479. doi: 10.1038/366476a0. [DOI] [PubMed] [Google Scholar]
- 24.Bai J, Uehara Y, Montell DJ. Regulation of invasive cell behavior by taiman, a Drosophila protein related to AIB1, a steroid receptor coactivator amplified in breast cancer. Cell. 2000;103:1047–1058. doi: 10.1016/s0092-8674(00)00208-7. [DOI] [PubMed] [Google Scholar]
- 25.Zhu J, Chen L, Sun G, Raikhel AS. The competence factor beta Ftz-F1 potentiates ecdysone receptor activity via recruiting a p160/SRC coactivator. Mol Cell Biol. 2006;26:9402–9412. doi: 10.1128/MCB.01318-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lozano J, Kayukawa T, Shinoda T, Belles X. A role for Taiman in insect metamorphosis. PLoS Genet. 2014;10:e1004769. doi: 10.1371/journal.pgen.1004769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wang Z, Yang L, Song J, Kang L, Zhou S. An isoform of Taiman that contains a PRD-repeat motif is indispensable for transducing the vitellogenic juvenile hormone signal in Locusta migratoria. Insect Biochem Mol Biol. 2017;82:31–40. doi: 10.1016/j.ibmb.2017.01.009. [DOI] [PubMed] [Google Scholar]
- 28.Liu H, et al. The alternative splicing of BdTai and its involvement in the development of Bactrocera dorsalis (Hendel) J Insect Physiol. 2017;101:132–141. doi: 10.1016/j.jinsphys.2017.07.012. [DOI] [PubMed] [Google Scholar]
- 29.Howard JM, Sanford JR. The RNAissance family: SR proteins as multifaceted regulators of gene expression. Wiley Interdiscip Rev RNA. 2015;6:93–110. doi: 10.1002/wrna.1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Neugebauer KM, Roth MB. Distribution of pre-mRNA splicing factors at sites of RNA polymerase II transcription. Genes Dev. 1997;11:1148–1159. doi: 10.1101/gad.11.9.1148. [DOI] [PubMed] [Google Scholar]
- 31.Zhou Z, Fu XD. Regulation of splicing by SR proteins and SR protein-specific kinases. Chromosoma. 2013;122:191–207. doi: 10.1007/s00412-013-0407-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Attardo GM, Higgs S, Klingler KA, Vanlandingham DL, Raikhel AS. RNA interference-mediated knockdown of a GATA factor reveals a link to anautogeny in the mosquito Aedes aegypti. Proc Natl Acad Sci USA. 2003;100:13374–13379. doi: 10.1073/pnas.2235649100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Martín D, Piulachs MD, Raikhel AS. A novel GATA factor transcriptionally represses yolk protein precursor genes in the mosquito Aedes aegypti via interaction with the CtBP corepressor. Mol Cell Biol. 2001;21:164–174. doi: 10.1128/MCB.21.1.164-174.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Park JH, Attardo GM, Hansen IA, Raikhel AS. GATA factor translation is the final downstream step in the amino acid/target-of-rapamycin-mediated vitellogenin gene expression in the anautogenous mosquito Aedes aegypti. J Biol Chem. 2006;281:11167–11176. doi: 10.1074/jbc.M601517200. [DOI] [PubMed] [Google Scholar]
- 35.Zou Z, et al. Juvenile hormone and its receptor, methoprene-tolerant, control the dynamics of mosquito gene expression. Proc Natl Acad Sci USA. 2013;110:E2173–E2181. doi: 10.1073/pnas.1305293110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hagedorn HH, et al. Postemergence growth of the ovarian follicles of Aedes aegypti. J Insect Physiol. 1977;23:203–206. doi: 10.1016/0022-1910(77)90030-0. [DOI] [PubMed] [Google Scholar]
- 37.Deitsch KW, Chen JS, Raikhel AS. Indirect control of yolk protein genes by 20-hydroxyecdysone in the fat body of the mosquito, Aedes aegypti. Insect Biochem Mol Biol. 1995;25:449–454. doi: 10.1016/0965-1748(94)00082-a. [DOI] [PubMed] [Google Scholar]
- 38.Hirosawa M, Hoshida M, Ishikawa M, Toya T. MASCOT: Multiple alignment system for protein sequences based on three-way dynamic programming. Comput Appl Biosci. 1993;9:161–167. doi: 10.1093/bioinformatics/9.2.161. [DOI] [PubMed] [Google Scholar]
- 39.Dobin A, et al. STAR: Ultrafast universal RNA-seq aligner. Bioinformatics. 2013;29:15–21. doi: 10.1093/bioinformatics/bts635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Campbell MA, Haas BJ, Hamilton JP, Mount SM, Buell CR. Comprehensive analysis of alternative splicing in rice and comparative analyses with Arabidopsis. BMC Genomics. 2006;7:327. doi: 10.1186/1471-2164-7-327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rhind N, et al. Comparative functional genomics of the fission yeasts. Science. 2011;332:930–936. doi: 10.1126/science.1203357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shen S, et al. rMATS: Robust and flexible detection of differential alternative splicing from replicate RNA-seq data. Proc Natl Acad Sci USA. 2014;111:E5593–E5601. doi: 10.1073/pnas.1419161111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Love MI, Huber W, Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Powell S, et al. eggNOG v3.0: Orthologous groups covering 1133 organisms at 41 different taxonomic ranges. Nucleic Acids Res. 2012;40:D284–D289. doi: 10.1093/nar/gkr1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang SF, Ayer S, Segraves WA, Williams DR, Raikhel AS. Molecular determinants of differential ligand sensitivities of insect ecdysteroid receptors. Mol Cell Biol. 2000;20:3870–3879. doi: 10.1128/mcb.20.11.3870-3879.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.