Significance
Our paper provides an approach for the durable deployment of anti-HIV agents in the developing world. We developed a transgenic rice line expressing three microbicidal proteins (the HIV-neutralizing antibody 2G12 and the lectins griffithsin and cyanovirin-N). Simultaneous expression in the same plant allows the crude seed extract to be used directly as a topical microbicide cocktail, avoiding the costs of multiple downstream processes. This groundbreaking strategy is realistically the only way that microbicidal cocktails can be manufactured at a cost low enough for the developing world, where HIV prophylaxis is most in demand.
Keywords: HIV combination microbicides, plant-made pharmaceuticals, Oryza sativa, gp120 binding, rice globulins
Abstract
The transmission of HIV can be prevented by the application of neutralizing monoclonal antibodies and lectins. Traditional recombinant protein manufacturing platforms lack sufficient capacity and are too expensive for developing countries, which suffer the greatest disease burden. Plants offer an inexpensive and scalable alternative manufacturing platform that can produce multiple components in a single plant, which is important because multiple components are required to avoid the rapid emergence of HIV-1 strains resistant to single microbicides. Furthermore, crude extracts can be used directly for prophylaxis to avoid the massive costs of downstream processing and purification. We investigated whether rice could simultaneously produce three functional HIV-neutralizing proteins (the monoclonal antibody 2G12, and the lectins griffithsin and cyanovirin-N). Preliminary in vitro tests showed that the cocktail of three proteins bound to gp120 and achieved HIV-1 neutralization. Remarkably, when we mixed the components with crude extracts of wild-type rice endosperm, we observed enhanced binding to gp120 in vitro and synergistic neutralization when all three components were present. Extracts of transgenic plants expressing all three proteins also showed enhanced in vitro binding to gp120 and synergistic HIV-1 neutralization. Fractionation of the rice extracts suggested that the enhanced gp120 binding was dependent on rice proteins, primarily the globulin fraction. Therefore, the production of HIV-1 microbicides in rice may not only reduce costs compared to traditional platforms but may also provide functional benefits in terms of microbicidal potency.
HIV-1 infection rates are declining, but there were still 2.1 million new cases in 2015 (1, 2). The virus continues to spread because there are no effective vaccines, and preexposure prophylaxis remains largely reliant on barrier methods or the oral administration of tenofovir/emtricitabine (3–5). HIV-1 entry into susceptible cells begins when the viral surface glycoprotein gp120 engages CD4 on the surface of lymphocytes, followed by its binding to coreceptor CCR5 or CXCR4; then the transmembrane subunit gp41 mediates membrane fusion (6). Molecules that bind to gp120/gp41 could therefore act as HIV entry inhibitors and may be suitable as topical microbicides, representing a subset of preexposure prophylaxis strategies (7). Many different entry inhibitors have been tested in vitro, in animal studies, and in human clinical trials, including broadly neutralizing monoclonal antibodies, such as 2G12, and lectins, such as griffithsin (GRFT) and cyanovirin-N (CV-N) (8–11). These show low nanomolar to picomolar IC50 values against all tested HIV-1 clades in vitro and in animal models (12–16).
The deployment of antibodies and lectins as HIV-1 entry inhibitors requires large-scale inexpensive production. Several HIV-neutralizing antibodies (including 2G12) and lectins (including GRFT and CV-N) have been produced as recombinant proteins in mammalian cells (17, 18) and microbial systems (19–25), respectively, but these are expensive because the products must be extensively purified (26). Furthermore, an effective microbicide requires three or more components targeting different epitopes to ensure broad coverage of HIV strains and to prevent the emergence of “escape mutants” (27), adding even further to the production costs. Plants could address these issues by allowing the inexpensive production of multiple HIV-1 entry inhibitors in the same tissue followed by the application of crude extracts directly to avoid purification costs (28, 29). Cereal seeds are likely to be the most suitable platform for the production of microbicides in developing countries because the cultivation infrastructure is in place (30, 31), and cereal seeds have “generally regarded as safe” (GRAS) status so the crude extracts would be considered safe for direct application (32). This minimal processing concept has already been demonstrated for other proteins (33, 34), and the economic viability of production processes has been confirmed, even when downstream processing is included (35, 36).
We have previously expressed 2G12, GRFT, and CV-N individually in rice with maximum yields comparable to the same components (and other HIV-neutralizing antibodies) produced in other plants (37–45). Before attempting to express all three components in the same line, we tested the activity of the components reconstituted in wild-type rice endosperm extracts compared with a control mixture reconstituted in PBS. We then generated transgenic rice plants expressing all three components to investigate the neutralization activity of the crude extracts against different strains of HIV. We tested the simultaneous expression of one antibody and two lectins because multiple antibodies expressed in the same cell can assemble as nonfunctional heterologous heavy/light chain combinations (46).
Results
In Vitro Analysis of Combinatorial gp120 Binding and HIV Neutralization.
Before generating transgenic plants expressing 2G12, GRFT, and CV-N, the three pure recombinant proteins were prepared as a dilution series in PBS, and ELISAs were carried out using the individual components, all three pairwise combinations, and the triple combination to determine whether there was evidence of interdependent binding, mutual inhibition, or cooperative binding. We used two different versions of gp120 to ensure that our results were not dependent on a specific HIV strain.
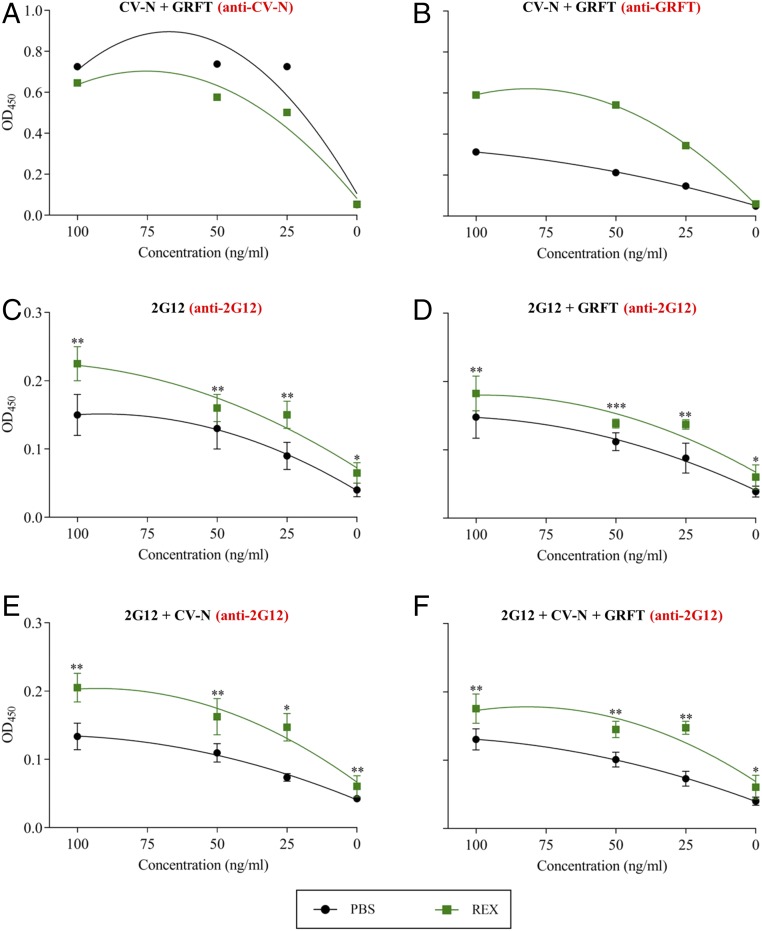
When different concentrations of GRFT and CV-N were combined in a matrix and gp120 binding was detected using either an anti-GRFT or anti–CV-N polyclonal antiserum, there was no evidence that either component influenced the ability of the other to bind gp120, as shown by the clear concentration-dependent binding of the primary lectin regardless of the concentration of the competing lectin (SI Appendix, Fig. S1). However, when we repeated the ELISAs by reconstituting the components in extracts of wild-type rice endosperm rather than PBS, we observed a small inhibitory effect on the binding of CV-N, with slightly lower OD values in the presence of rice extract (Fig. 1A), but a remarkable increase in the binding activity of GRFT, with the OD values doubling in the presence of the rice extract (Fig. 1B). The pH of the extract was the same as that of PBS, so we ruled out pH-dependent effects on electrostatic interactions. The ELISA experiments suggested that GRFT binding to gp120 was enhanced by the rice extract, but there was no enhancement of the interaction between CV-N and gp120. Additional ELISA experiments were carried out to test the binding of 2G12 alone or in the presence of different concentrations of single lectins and both lectins. Again, we observed the expected concentration-dependent binding of 2G12 in PBS, and the presence of either individual lectin or both lectins had no effect at any of the concentrations we tested, including both lectins at increasing concentrations in concert or in opposing gradients (SI Appendix, Fig. S1). As with GRFT, however, 2G12 binding activity increased when PBS was replaced with the rice extract (Fig. 1C) in the presence of individual lectins (Fig. 1 D and E) and both lectins (Fig. 1F). Overall, these experiments indicated that components in the crude rice endosperm extract doubled the gp120-binding activity of 2G12 and GRFT compared with the same components at the same concentrations in PBS but had no effect on CV-N.
Fig. 1.
ELISA experiments to determine gp120-binding activity. (A and B) GRFT and CV-N reconstituted in PBS or crude rice endosperm extract (REX) detected with a CV-N–specific antibody (A) or a GRFT-specific antibody (B). (C–F) 2G12 detected with an Ig-specific antibody: 2G12 in PBS or REX alone (C); 2G12 + GRFT (D); 2G12 + CV-N (E); and 2G12 + GRFT and CV-N (F). In all panels, the concentrations on the x axis refer to all components in the assay, i.e., all components were present at the same concentrations in all assays. This was appropriate because competition experiments established that the components do not interfere with each other’s ability to bind gp120 at any of the tested concentrations (SI Appendix, Fig. S1). Data are presented as a fitted quadratic regression model to compare the different curve parameters and establish significant differences between PBS and REX. No statistical differences were found for A and B (P > 0.05). Asterisks represent statistically significant differences (ANOVA) at different concentrations of the three components (***P < 0.001, **P < 0.01, *P < 0.05). Error bars indicate the SEM from three replicates.
We investigated the functional impact of the enhanced binding promoted by rice extracts by conducting TZM-bl cell HIV-neutralization assays. Recombinant GRFT, CV-N, and 2G12 were tested against the laboratory-adapted NL4.3-pseudotyped virus. All three components showed neutralizing activity when reconstituted in PBS, with individual IC50 values of 0.29, 1.6, and 795 ng/mL, respectively. The IC50 value of GRFT presented alone (0.29 ng/mL) did not change significantly when copresented with CV-N (0.23 ng/mL), 2G12 (0.31 ng/mL), or both CV-N and 2G12 (0.22 ng/mL). However, when the three components were reconstituted in the endosperm extract, we observed similar IC50 values for GRFT alone or in a double combination with either of the other components, but the IC50 value of GRFT in the triple combination fell by ∼50%, suggesting that the rice endosperm extract promotes synergistic activity among the three components to increase the potency of GRFT (Table 1).
Table 1.
GRFT-neutralization activity in rice endosperm extract in the presence or absence of CV-N and/or 2G12
| Components | GRFT IC50, ng/mL |
| GRFT only | 1.15 ± 0.02 |
| GRFT + CV-N | 0.83 ± 0.17 |
| GRFT + 2G12 | 0.96 ± 0.04 |
| GRFT + CV-N + 2G12 | 0.47 ± 0.11 |
The NL4.3 laboratory-adapted pseudovirus was tested for neutralization using recombinant proteins reconstituted in wild-type rice endosperm extract. The GRFT IC50 value was calculated alone or in the presence of a constant concentration of CV-N, 2G12, or both, corresponding to their IC30 values: 1 ng/mL for CV-N and 100 ng/mL for 2G12. Values are expressed in nanograms per milliliter and represent the average value with SEs from two independent experiments performed in duplicate.
Transgenic Plants and Expression Analysis.
Having established that the interactions between 2G12, GRFT, and CV-N differ according to whether they are reconstituted in PBS or rice endosperm extract, we generated transgenic lines expressing all three proteins to characterize the behavior of the components in this context. Rice embryos were cotransformed with four constructs containing the coding sequences for the 2G12 heavy and light chains, GRFT, and CV-N, each controlled by endosperm-specific promoters (37, 40, 44). A fifth construct containing the selectable marker hpt was controlled by the constitutive Cauliflower mosaic virus 35S promoter (47). Embryo-derived callus was selected on hygromycin-supplemented medium, and 20 independent transformants were regenerated. Leaves and T1 seeds from these plants were analyzed by PCR to confirm the presence of the transgenes, and the T1 seed extracts were analyzed by sandwich ELISA to confirm the presence of correctly assembled 2G12 and the two lectins.
Nineteen transgenic lines were fertile, eight of which expressed a single microbicidal component (Table 2). Eight lines expressed two components, with all three possible pairwise combinations represented, and three further lines were recovered expressing all three components simultaneously. The double- and triple-component transgenic lines were renamed lines 1–11 for simplicity (Table 2). The analysis of individual and combinatorial expression revealed no specific relationship between the presence of each transgene and their expression levels, which made it possible to identify lines expressing any combination of the proteins at adequate levels for further analysis, even among a small population. T1 seeds from the single, double, and triple transgenic lines were propagated to produce T1 populations, and T2 homozygous seeds were used for more detailed analysis.
Table 2.
The 19 transgenic rice lines and the expression levels of each component
| Microbicide component(s) | Transgenic line | 2G12 concentration, μg/g dry seed weight | CV-N concentration, μg/g dry seed weight | GRFT concentration, μg/g dry seed weight |
| 2G12 | 2G12-1 | 14.4 | ||
| 2G12 | 2G12-2 | 11.6 | ||
| 2G12 | 2G12-3 | 14.8 | ||
| CV-N | CV-N-1 | 10 | ||
| CV-N | CV-N-2 | 2.4 | ||
| CV-N | CV-N-3 | 0.8 | ||
| GRFT | GRFT-1 | 32 | ||
| GRFT | GRFT-2 | 26 | ||
| CV-N + GRFT | 1 | 4.4 | 37.6 | |
| CV-N + GRFT | 2 | 2 | 1.6 | |
| CV-N + GRFT | 3 | 0.4 | 3.2 | |
| 2G12 + CV-N | 4 | 11.6 | 0.8 | |
| 2G12 + CV-N | 5 | 2 | 0.8 | |
| 2G12 + CV-N | 6 | 12 | 0.8 | |
| 2G12 + GRFT | 7 | 4 | 17.6 | |
| 2G12 + GRFT | 8 | 14.8 | 12.8 | |
| 2G12 + CV-N + GRFT | 9 | 16.4 | 0.4 | 2.8 |
| 2G12 + CV-N + GRFT | 10 | 9.6 | 0.8 | 8.4 |
| 2G12 + CV-N + GRFT | 11 | 17.2 | 1.2 | 6.4 |
| Wild type | Wild type |
Empty cells indicate that the corresponding component was not present.
Crude Extracts from the T2 Transgenic Seeds Show gp120-Binding and Synergistic HIV-1–Neutralization Activity in Vitro.
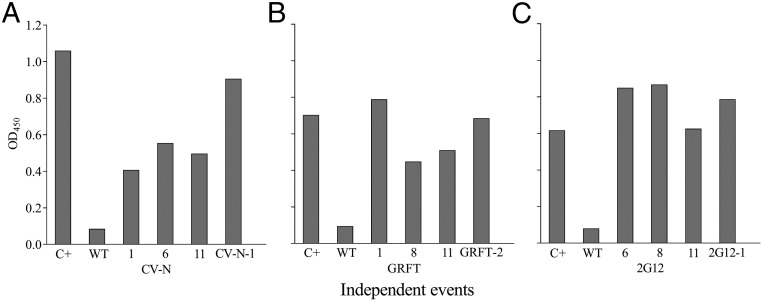
Crude extracts from the T2 seeds showed gp120 IIIB-binding activity in vitro by ELISA. To ensure that the assay was calibrated correctly and that the three proteins were correctly folded and able to bind their target in the context of rice endosperm extracts, we compared recombinant proteins with extracts from the transgenic lines expressing the individual components and extracts from the 11 double and triple transgenic lines. We observed gp120 binding in all cases (Fig. 2 and SI Appendix, Fig. S2).
Fig. 2.
Binding activity of crude extracts from representative transgenic lines containing OSCV-N, OSGRFT, and OS2G12 alone or in combination with gp120. (A) Binding activity of CV-N. (B) Binding activity of GRFT. (C) Binding activity of 2G12. Numbers on the x axis refer to the transgenic lines listed in Table 2. C+, ECCV-N, ECGRFT, or OS2G12 positive controls (starting concentration 250 ng/mL) (wild-type endosperm extracts were used as a negative control); EC, E. coli; OS, rice (O. sativa).
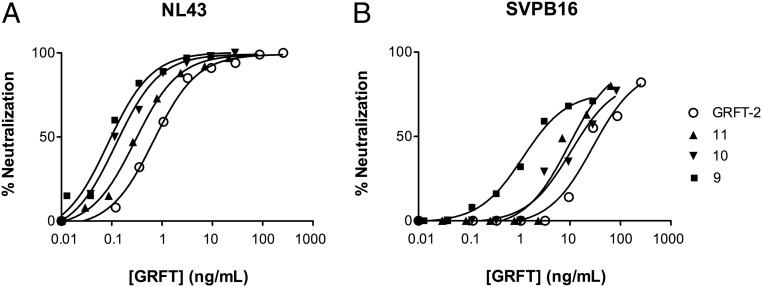
The same extracts were tested in the TZM-bl cell HIV-neutralization assays using the NL4.3-pseudotyped virus. The extracts from T2 seeds expressing single components achieved HIV-1 neutralization with titers of up to >104 [reciprocal dilution to a median infective dose (ID50)]. The best apparent IC50 values for each component were >119, 2.3, and 0.7 ng/mL for 2G12, CV-N, and GRFT, respectively. Combinations of 2G12 and CV-N were no more effective than the corresponding single-component extracts, but all combinations containing GRFT showed high neutralization activity. Remarkably, we observed substantially lower apparent IC50 values when all three components were present. The lowest overall IC50 values were observed in extracts from the triple transgenic lines 9, 10, and 11, with IC50 ranges of 0.14–0.77 ng/mL for 2G12, 0.01–0.05 ng/mL for CV-N, and 0.09–0.29 ng/mL for GRFT (Table 3). Assuming that GRFT achieved the highest neutralizing activity (Table 3, single transgenic lines), we compared the apparent IC50 values for GRFT in single, double, and triple transgenic lines, revealing significantly lower values for the extracts containing all three components than for the extracts containing single and double components (Fig. 3A), suggesting synergistic interactions similar to those observed in the reconstitution experiments described above. To confirm this observation, we mixed similar concentrations of CV-N or 2G12 single extracts with a GRFT extract and calculated the GRFT apparent IC50 values. Again, dose–response curves shifted in the presence of all three components, and apparent IC50 values in the triple combination were lower than for single components or double combinations (Fig. 3B). These data suggest that the components may interact in pairwise combinations but more profound synergy occurs in the triple combination. We therefore compared the extracts from all three triple transgenic lines against the single transgenic line expressing GRFT in TZM-bl cell HIV-neutralization assays using the laboratory-adapted NL4.3 and the primary isolate SVPB16 pseudotyped viruses. In both cases, we observed lower apparent IC50 values for GRFT in the triple transgenic lines than in the single transgenic line, indicating that synergy occurred between the microbicide components to increase the efficacy of the combinatorial microbicide at lower concentrations, particularly against a primary HIV-1 isolate (Fig. 4).
Table 3.
The NL4.3 laboratory-adapted pseudovirus was neutralized by seed extracts from each transgenic line
| Line | Crude ID50 (reciprocal dilution) | Apparent IC50 (ng/mL) for individual components | ||
| 2G12 | CV-N | GRFT | ||
| 2G12-1 | <25 | >144 | ||
| 2G12-2 | <25 | >119 | ||
| 2G12-3 | <25 | >148 | ||
| CV-N-1 | <25 | >220 | ||
| CV-N-2 | 234 | 2.50 | ||
| CV-N-3 | 86 | 2.30 | ||
| GRFT-1 | 11,376 | 0.70 | ||
| GRFT-2 | 4,808 | 1.35 | ||
| 1 | 8,892 | 0.12 | 1.06 | |
| 2 | 755 | 0.66 | 0.53 | |
| 3 | — | — | — | — |
| 4 | <25 | >116 | >8 | |
| 5 | <25 | >20 | >8 | |
| 6 | <25 | >120 | >8 | |
| 7 | 4,178 | 0.24 | 0.24 | |
| 8 | 1,734 | 2.13 | 1.85 | |
| 9 | 7,482 | 0.55 | 0.01 | 0.09 |
| 10 | 17,061 | 0.14 | 0.01 | 0.12 |
| 11 | 5,585 | 0.77 | 0.05 | 0.29 |
| Wild type | <25 | |||
Values represent the ID50 for each extract (reciprocal dilution) and apparent IC50 (ng/mL) for each component. The sample from line 3 was compromised in transit and was not tested.
Fig. 3.
When GRFT is combined with 2G12 and CV-N in transgenic plants, the neutralization potency of GRFT is enhanced. (A) Plant extracts containing GRFT alone, GRFT + 2G12, GRFT + CV-N, or GRFT + 2G12 + CV-N were tested against NL4.3 pseudotyped virus to evaluate GRFT-mediated neutralization. The bar graph shows the mean GRFT IC50 from two or three different extracts for each composition (biological replicates), each tested in duplicate (technical replicates). The asterisk denotes significant differences (*P < 0.05) between triple extracts and single/double extracts (Mann–Whitney U test). (B) GRFT dose–response curve against NL4.3-pseudotyped virus. One plant extract containing only GRFT was serially diluted to evaluate its neutralization potency alone or in the presence of 0.2 μg/mL 2G12, CV-N, or both from individual extracts.
Fig. 4.
Comparison of neutralization IC50 values for GRFT in single or triple extracts for the laboratory-adapted pseudotyped virus strain NL4.3 (A) and the primary isolate SVP16 (B). In both cases, the triple extracts show enhanced activity. Numbered symbols refer to the triple-component transgenic lines listed in Tables 2 and 3.
Fractionation of Rice Endosperm to Identify Potentiating Components.
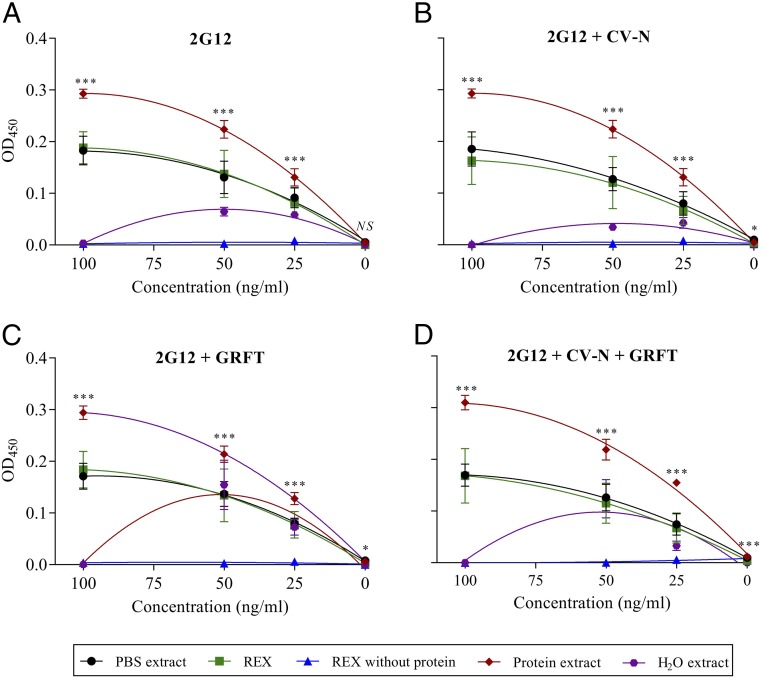
Having discovered that a crude extract of rice endosperm can increase the gp120-binding activity of GRFT and 2G12 but not CV-N, we fractionated the extract into broad components to determine the source of the active principle, using 2G12 as a case study. We prepared a total protein extract and protein-depleted extract and found that only the protein extract increased the binding activity of 2G12, whereas the depleted extract was similar in performance to the PBS control, indicating the source of the activity was proteinaceous (Fig. 5). We therefore prepared the rice endosperm extract again using the traditional approach to distinguish among different classes of seed-storage proteins: the albumins (water extract), globulins (PBS extract), glutelins (alkaline extract), and prolamins (ethanolic extract). ELISAs with these fractions (used directly without buffer exchange), in addition to the reconstituted PBS control, the protein extract, the protein-depleted extract, and the original complete extract, provided strong evidence that the active principle segregates primarily with the globulin fraction of the endosperm (Fig. 5).
Fig. 5.
Competition ELISA experiments. (A) 2G12 at different concentrations in different extracts of rice endosperm, detected with an Ig-specific antibody. (B) As above with different concentrations of CV-N. (C) As above with different concentrations of GRFT. (D) As above with different concentrations of CV-N + GRFT. In all four panels, the signals are corrected for background (equivalent extraction solvent without components). Data are presented as a fitted quadratic regression model to compare the different curve parameters and establish significant differences among extracts at different concentrations of the three components. The curve comparison revealed no significant difference between the original rice endosperm extract (REX) in PBS (P > 0.05) and the modified PBS extract prepared using the protocol recommended for the isolation of globulins (PBS), indicating the two extracts were functionally equivalent. Asterisks represent statistically significant differences (ANOVA) at different concentrations of the three components (***P < 0.001; **P < 0.01; *P < 0.05; NS, nonsignificant). Error bars indicate the SEM from three replicates.
Discussion
Cereal plants such as rice expressing multiple HIV-neutralizing proteins in GRAS-status seeds offer an inexpensive platform for the production of microbicidal mixtures with the added benefit that dry seeds can be stored indefinitely under ambient conditions (48, 49). Rice has been developed as a production platform for proteins that can be administered in minimally processed seed extracts, such as oral vaccines and topical prophylactics, without the need to remove toxic metabolites or endotoxins (33, 34, 49–53). Although there are many reports of plants expressing multiple recombinant enzymes for metabolic engineering (54, 55), the only previous reports of multiple microbicidal components have involved fusion proteins such as b12/CV-N (45) and CV-N/12pi (56). The expression of mixtures of individual proteins is potentially more versatile because the relative quantities can be varied, and different combinations can be produced by crossing transgenic lines. However, the components must remain functional when coexpressed, so it is necessary to check for interactions such as antagonistic or synergistic binding and neutralization.
Our ELISA experiments revealed little evidence for interference among the three components: the OD values for GRFT bound to gp120 did not change in the presence of 0–250 ng/mL CV-N, and vice versa. When we added 2G12 to the samples, we observed the same OD values for 2G12 bound to gp120 regardless of the presence or absence of either or both lectins in the same concentration ranges as above. In the context of CV-N and 2G12, CV-N pretreatment has been shown to prevent 2G12 binding to gp120, but 2G12 pretreatment does not prevent subsequent CV-N binding (57). Although CV-N can block 2G12 binding to gp120, it does not interfere with other neutralizing or nonneutralizing monoclonal antibodies binding to the same protein, e.g., F5 and b12 (58). It is likely that 2G12 does not prevent CV-N binding to gp120, because there are multiple CV-N–binding sites, and only some of them overlap the 2G12 epitope (58). Indeed, 2G12 binds an only partially characterized, glycosylation-dependent epitope comprising elements from the C2, C3, C4, and V4 domains of gp120 (59). In the crystal structure of the 2G12–gp120 core (HXB2c) from a laboratory isolate of HIV-1, a large fraction of the predicted accessible surface of gp120 in the trimer was composed of variable, heavily glycosylated core-and-loop structures that surround the CD4- and coreceptor-binding regions (60–62). The 2G12 epitope overlaps the stem of the V3 loop and the V4 variable region and is characterized by high-mannose glycans (61). Also, 2G12 is unique in recognizing this epitope, although the epitope is conserved across viral isolates. The gp120 crystal structure indicates that there is a large, heavily glycosylated, immunologically silent domain (60–62). It is possible that, in addition to binding at or near the 2G12 epitope, CV-N might bind other glycosylated domains in this immunologically silent region. Both CV-N and 2G12 bind terminal αMan-(1, 2)-αMan residues on gp120. However, CV-N seems to bind a single N-linked glycan, whereas 2G12 interacts with two glycans simultaneously (63, 64). Our data suggest that the simultaneous presentation of CV-N and 2G12 does not result in any reciprocal cross-blocking between these components.
When we repeated the ELISA experiments using extracts from wild-type rice endosperm instead of PBS at the same pH, we observed a significant increase in the gp120-binding activity of GRFT and 2G12 (both alone and in mixtures) but no equivalent increase in the gp120-binding activity of CV-N. These experiments indicated that a soluble component of the rice endosperm can enhance the binding of 2G12 and GRFT (but not CV-N) to gp120. Fractionation revealed that the principle responsible for enhanced 2G12–gp120 and GRFT–gp120 binding separated with the proteinaceous component, and further fractionation into the four principal protein classes in the endosperm revealed that the globulin fraction retained most of the activity. The extraction of glutelins and prolamins requires 0.1 M NaOH and 70% ethanol as solvents, respectively, both of which could interfere with 2G12–gp120 binding, but albumins are extracted in water, and we did not observe any interference in control experiments, suggesting that the restriction to globulins is genuine and not an artifact of the extraction method. The mechanism of enhanced 2G12–gp120 and GRFT–gp120 binding is unclear, but possibilities include an impact on the oligomerization state of the components, molecular crowding affecting glycan-based binding sites, or induced fitting as previously described for CV-N (65–67).
The ELISA data were consistent with the in vitro neutralization assays, in which we observed the synergistic effect of rice extracts against different HIV-1 strains (including a primary isolate) and when using different forms of gp120 originating from diverse HIV-1 isolates, suggesting that the effect mediated by rice extracts could be broadly effective. We observed no apparent antagonism or synergy among the three components reconstituted in PBS, but there was a small additive effect when CV-N and GRFT were combined. In contrast, when the same components were reconstituted in rice endosperm extract, the latter effect was repressed, although additive effects of 2G12 and CV-N were still observed in a triple formulation. A previous study with three components, i.e., CV-N, GRFT, and scytovirin (SVN) all purified from Escherichia coli and then mixed (68), provided evidence for antagonism, but by replacing SVN with 2G12 we demonstrated no interaction in PBS and synergy in rice endosperm extracts, at least in terms of the potency of GRFT. Synergy between 2G12 and GRFT was previously reported when the components were produced separately and mixed (2G12 was produced in CHO cells and GRFT in Nicotiana benthamiana) (69), but we did not observe this binary synergy even in the rice extracts, perhaps because of the different production and purification strategies. Synergistic neutralization activity was observed only when all three of our microbicidal components were present in rice endosperm extracts.
Having established that all three components were active in reconstituted extracts, we generated transgenic lines expressing the same proteins. The simultaneous expression of up to three microbicidal components makes it very important to characterize transgenic lines in terms of transgene integration and expression to ensure there is a sufficient dose of each product in the extracts to achieve full microbicidal activity. Our panel of 19 transgenic lines included examples of all possible combinations of transgenes (single, double, and triple) which were passed as intact units through to at least the T2 generation, confirming that the transgenes supplied on different vectors integrated at a single locus and there was no bias for the integration of particular sequences (70, 71). There was no interdependence among the transgenes in terms of expression levels; e.g., the triple transgenic line 11 expressed the highest level of 2G12 among all the lines we tested (17.2 μg/g) but expressed comparatively low levels of CV-N (1.2 μg/g) and moderate levels of GRFT (6.4 μg/g). The absence of a correlation between different transgene expression levels in the same lines suggests that the ideal relative dose of each microbicide could be achieved by screening an appropriate number of primary transformants. Our success rate of ∼15% triple transformants suggests that screening for ideal combinatorial doses would require only a relatively small number of lines, which is achievable in the case of rice due to the high efficiency of the transformation process (54, 71, 72). Another important result from the analysis of the double/triple transgenic lines is that the simultaneous expression of multiple proteins does not appear to attract a penalty in terms of achievable expression levels.
We tested the extracts of the transgenic plants in the TZM-bl cell HIV-neutralization assays to confirm that each component was active against HIV-1 in the context of the transgenic rice endosperm extract. Given the concentrations of active components defined in the ELISA experiments, the HIV-1–neutralization assays were also anticipated to reveal any interactions between the components that might affect their overall activity, e.g., synergy or antagonism. In agreement with our data, the reported IC50 for the inhibition of HIV-1 strain NL4.3 by 2G12 is 0.98 μg/mL and by CV-N is 0.78 μg/mL (73). We observed a lower IC50 value for two extracts and no activity for the third one. For GRFT, the IC50 values were consistent and clearly lower in extracts containing both 2G12 and CV-N but not in extracts containing GRFT plus one of the other components. Our results therefore showed that the triple combination of GRFT with 2G12 and CV-N enhances the neutralization potency of GRFT both in transgenic plants and when the same components are reconstituted in rice endosperm extracts. These data indicate there is a synergistic effect solely when the triple combination of components is present in rice extract, i.e., GRFT is more active against HIV-1 at a lower concentration than anticipated from the concentrations and neutralization activities in the single transgenic lines.
Materials and Methods
ELISA.
The specific antigen-binding activity of each protein was determined by coating the wells of ELISA plates with 100 ng recombinant gp120 IIIB or gp120 W61D (MRC Centralized Facility for AIDS Reagents). After washing with PBS + 0.1% Tween-20 (PBST) and blocking with 2.5% BSA in PBST, serial dilutions of rice 2G12, GRFT, or CV-N were added. The presence of bound 2G12 was detected using an HRP-conjugated sheep anti-human κ chain antiserum (The Binding Site) diluted 1:1,000. GRFT and CV-N were detected using primary rabbit anti-GRFT and anti–CV-N polyclonal antisera (The Binding Site) and a secondary HRP-conjugated anti-rabbit IgG antibody (The Binding Site), each diluted 1:1,000. For the recapitulation experiments, each ELISA was carried out three times with 2G12 and lectins diluted in PBS and another three times with 2G12 and lectins diluted in wild-type rice endosperm extract (prepared by extraction in PBS). The three components were tested alone, in all three pairwise combinations, and as a mixture of all three components at different concentrations.
The yield of soluble, correctly folded protein in transgenic endosperm tissue was confirmed by grinding seeds in three volumes of PBS and centrifuging twice at 13,000 × g for 10 min at 4 °C to remove debris. The wells of ELISA plates were coated with 100 ng recombinant gp120 IIIB or gp120 W61D. After washing and blocking as above, serial dilutions of each seed extract were added, and the three proteins were detected using the antibodies described above. Dilution series of standards produced in CHO cells (2G12) or E. coli (GRFT and CV-N) were used to construct a calibration curve to calculate the concentration of each component in plant extracts. The final concentration (in micrograms per gram) of each protein in the extract was calculated as the product of the OD450 value, taking the dilution factors into consideration, and the amount of buffer (120 μL) divided by the weight of the seeds (30 mg).
HIV-Neutralization Assays.
HIV-1 pseudoviruses (NL4.3 and SVPB16 isolates) were generated by the cotransfection of 293T cells with Env-expressing plasmids and the PSG3 vector as previously described (74, 75). The supernatants were harvested 24 h posttransfection and were passed through a 0.45-μm filter; the viral stocks were frozen at −80 °C. Cell-free HIV neutralization was tested using a standard TZM-bl–based assay as described in SI Appendix, Supplementary Methods (74, 75).
Transformation and Recovery of Transgenic Plants.
Seven-day-old mature rice zygotic embryos (Oryza sativa cv. Nipponbare) were transferred to osmotic medium [4.4 g/L Murashige and Skoog (MS) powder supplemented with 0.3 g/L casein hydrolysate, 0.5 g/L proline, 72.8 g/L mannitol, and 30 g/L sucrose] 4 h before bombardment with 10-mg gold particles coated with the four constructs carrying the microbicidal transgenes (SI Appendix, Supplementary Methods) and the selectable marker hpt (76–79). We used a 6:6:3:3:1 ratio with the 2G12 heavy and light chains represented at twice the molar ratio of the lectins to ensure the recovery of plants expressing all four transgenes and the hpt marker gene as the minority component. The embryos were returned to osmotic medium for 12 h before selection on MS medium (4.4 g/L MS powder, 0.3 g/L casein, 0.5 g/L proline, and 30 g/L sucrose) supplemented with 50 mg/L hygromycin and 2.5 mg/L 2,4-dichlorophenoxyacetic acid in the dark for 2–3 wk. Transgenic plantlets were regenerated and hardened off in soil. Plants were grown in the greenhouse or growth chamber at 28/20 °C day/night temperature with a 10-h photoperiod and 60–90% relative humidity for the first 50 d, followed by maintenance at 21/18 °C day/night temperature with a 16-h photoperiod thereafter in a growth chamber.
Endosperm Fractionation.
Protein fractions were prepared by the overnight precipitation of rice endosperm extracts with ammonium sulfate (80) followed by centrifugation at 4,500 × g for 30 min. The protein pellet was resuspended in PBS, and the supernatant was used as the protein-free fraction. The seed protein fractions were prepared as previously described (81, 82) by mixing 2 g of rice flour with 10 mL of solvent for 90 min followed by centrifugation at 14,000 × g for 15 min at 4 °C. Albumins were extracted with water, globulins with PBS, glutelins with 0.1 M NaOH, and prolamins with 70% ethanol. The supernatant in each case was used directly in the ELISA. The supernatants were tested for their impact on gp120–2G12 binding; water, PBS, and 70% ethanol preserved the interaction, but no binding was observed in 0.1 M NaOH.
Statistical Analysis.
A randomized complete ANOVA was applied to each component (2G12, CV-N, and GRFT) at each concentration, and statistically significant differences between rice endosperm extracts and PBS, between different transgenic plant lines, and between different endosperm fractions, were determined, using Tukey’s test for multiple comparisons, or the Mann–Whitney U test for pairwise comparisons, in JMP-PRO v12.0.1 (SAS Institute Inc.). Statistical significance was expressed as follows: ***P < 0.001; **P < 0.01; *P < 0.05. A nonlinear regression function was also used to determine the performance of each component at different concentrations, a quadratic nonlinear regression curve was fitted, and statistically significant differences were determined by comparing the fitted curve parameters. Model fitting and parameter estimations were carried out using GraphPad Prism v7.03 (GraphPad Software, Inc.).
Supplementary Material
Acknowledgments
We thank Ms. Jennifer Wilson for technical assistance with live-virus anti-HIV assays. We received funding from Spanish Ministry of Science and Innovation Grant BIO2014-54426-P; Spanish Ministry of Economy, Industry and Competitiveness Grant AGL2017-85377-R; the European Fund for Economic and Regional Development; Generalitat de Catalunya Grant 2017 SGR 828 to the Agricultural Biotechnology and Bioeconomy Unit; and European Union Farma-Factory Grant Agreement 774078, H2020-BB-2016-2017/H2020-BB-2017-1. This project was funded in whole or in part with federal funds from the National Cancer Institute (NCI), NIH, under Contract HHSN26120080001E and was supported in part by the Intramural Research Program of the NIH, NCI, Center for Cancer Research, and Instituto de Salud Carlos III project PI14/01307. The EVA648 W61D and gp120 0607 IIIB were provided by the Medical Research Center Centralized Facility for AIDS reagents. The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does the mention of trade names, commercial products, or organizations imply endorsement by the US government.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1806022115/-/DCSupplemental.
References
- 1.GBD 2015 HIV Collaborators Estimates of global, regional, and national incidence, prevalence, and mortality of HIV, 1980–2015: The global burden of disease study 2015. Lancet HIV. 2016;3:e361–e387. doi: 10.1016/S2352-3018(16)30087-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.UNAIDS 2016 Fact sheet–Latest statistics on the status of the AIDS epidemic. Available at www.unaids.org/en/resources/fact-sheet. Accessed July 15, 2017.
- 3.Ramessar K, Sabalza M, Miralpeix B, Capell T, Christou P. Can microbicides turn the tide against HIV? Curr Pharm Des. 2010;16:468–485. doi: 10.2174/138161210790232202. [DOI] [PubMed] [Google Scholar]
- 4.Shattock RJ, Rosenberg Z. Microbicides: Topical prevention against HIV. Cold Spring Harb Perspect Med. 2012;2:a007385. doi: 10.1101/cshperspect.a007385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pialoux G, et al. Pre-exposure prophylaxis: A useful tool to prevent human immunodeficiency virus infection? Clin Microbiol Infect. 2016;22:757–767. doi: 10.1016/j.cmi.2016.08.029. [DOI] [PubMed] [Google Scholar]
- 6.Haase AT. Early events in sexual transmission of HIV and SIV and opportunities for interventions. Annu Rev Med. 2011;62:127–139. doi: 10.1146/annurev-med-080709-124959. [DOI] [PubMed] [Google Scholar]
- 7.McGowan I. Microbicides: A new frontier in HIV prevention. Biologicals. 2006;34:241–255. doi: 10.1016/j.biologicals.2006.08.002. [DOI] [PubMed] [Google Scholar]
- 8.McCoy LE, Weiss RA. Neutralizing antibodies to HIV-1 induced by immunization. J Exp Med. 2013;210:209–223. doi: 10.1084/jem.20121827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mascola JR, Haynes BF. HIV-1 neutralizing antibodies: Understanding nature’s pathways. Immunol Rev. 2013;254:225–244. doi: 10.1111/imr.12075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Caskey M, et al. Viraemia suppressed in HIV-1-infected humans by broadly neutralizing antibody 3BNC117. Nature. 2015;522:487–491. doi: 10.1038/nature14411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bar KJ, et al. Effect of HIV antibody VRC01 on viral rebound after treatment interruption. N Engl J Med. 2016;375:2037–2050. doi: 10.1056/NEJMoa1608243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mori T, et al. Isolation and characterization of griffithsin, a novel HIV-inactivating protein, from the red alga Griffithsia sp. J Biol Chem. 2005;280:9345–9353. doi: 10.1074/jbc.M411122200. [DOI] [PubMed] [Google Scholar]
- 13.Lagenaur LA, et al. Prevention of vaginal SHIV transmission in macaques by a live recombinant Lactobacillus. Mucosal Immunol. 2011;4:648–657. doi: 10.1038/mi.2011.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Balzarini J. Targeting the glycans of gp120: A novel approach aimed at the Achilles heel of HIV. Lancet Infect Dis. 2005;5:726–731. doi: 10.1016/S1473-3099(05)70271-1. [DOI] [PubMed] [Google Scholar]
- 15.O’Keefe BR, et al. Broad-spectrum in vitro activity and in vivo efficacy of the antiviral protein griffithsin against emerging viruses of the family Coronaviridae. J Virol. 2010;84:2511–2521. doi: 10.1128/JVI.02322-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Emau P, et al. Griffithsin, a potent HIV entry inhibitor, is an excellent candidate for anti-HIV microbicide. J Med Primatol. 2007;36:244–253. doi: 10.1111/j.1600-0684.2007.00242.x. [DOI] [PubMed] [Google Scholar]
- 17.Hofmann-Lehmann R, et al. Postnatal passive immunization of neonatal macaques with a triple combination of human monoclonal antibodies against oral simian-human immunodeficiency virus challenge. J Virol. 2001;75:7470–7480. doi: 10.1128/JVI.75.16.7470-7480.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stiegler G, et al. Antiviral activity of the neutralizing antibodies 2F5 and 2G12 in asymptomatic HIV-1-infected humans: A phase I evaluation. AIDS. 2002;16:2019–2025. doi: 10.1097/00002030-200210180-00006. [DOI] [PubMed] [Google Scholar]
- 19.Giomarelli B, et al. Recombinant production of anti-HIV protein, griffithsin, by auto-induction in a fermentor culture. Protein Expr Purif. 2006;47:194–202. doi: 10.1016/j.pep.2005.10.014. [DOI] [PubMed] [Google Scholar]
- 20.Gao X, et al. Soluble cytoplasmic expression, rapid purification, and characterization of cyanovirin-N as a His-SUMO fusion. Appl Microbiol Biotechnol. 2010;85:1051–1060. doi: 10.1007/s00253-009-2078-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Colleluori DM, et al. Expression, purification, and characterization of recombinant cyanovirin-N for vaginal anti-HIV microbicide development. Protein Expr Purif. 2005;39:229–236. doi: 10.1016/j.pep.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Boyd MR, et al. Discovery of cyanovirin-N, a novel human immunodeficiency virus-inactivating protein that binds viral surface envelope glycoprotein gp120: Potential applications to microbicide development. Antimicrob Agents Chemother. 1997;41:1521–1530. doi: 10.1128/aac.41.7.1521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Giomarelli B, et al. The microbicide cyanovirin-N expressed on the surface of commensal bacterium Streptococcus gordonii captures HIV-1. AIDS. 2002;16:1351–1356. doi: 10.1097/00002030-200207050-00006. [DOI] [PubMed] [Google Scholar]
- 24.Liu X, et al. Engineered vaginal lactobacillus strain for mucosal delivery of the human immunodeficiency virus inhibitor cyanovirin-N. Antimicrob Agents Chemother. 2006;50:3250–3259. doi: 10.1128/AAC.00493-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mori T, et al. Functional homologs of cyanovirin-N amenable to mass production in prokaryotic and eukaryotic hosts. Protein Expr Purif. 2002;26:42–49. doi: 10.1016/s1046-5928(02)00513-2. [DOI] [PubMed] [Google Scholar]
- 26.Buyel JF, Twyman RM, Fischer R. Extraction and downstream processing of plant-derived recombinant proteins. Biotechnol Adv. 2015;33:902–913. doi: 10.1016/j.biotechadv.2015.04.010. [DOI] [PubMed] [Google Scholar]
- 27.Garg AB, Nuttall J, Romano J. The future of HIV microbicides: Challenges and opportunities. Antivir Chem Chemother. 2009;19:143–150. doi: 10.1177/095632020901900401. [DOI] [PubMed] [Google Scholar]
- 28.Tusé D, Tu T, McDonald KA. Manufacturing economics of plant-made biologics: Case studies in therapeutic and industrial enzymes. BioMed Res Int. 2014;2014:256135. doi: 10.1155/2014/256135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ma JK, et al. Regulatory approval and phase-I clinical trial of a monoclonal antibody produced in tobacco. Plant Biotechnol J. 2015;13:1106–1120. doi: 10.1111/pbi.12416. [DOI] [PubMed] [Google Scholar]
- 30.Lotter-Stark HC, Rybicki EP, Chikwamba RK. Plant made anti-HIV microbicides–A field of opportunity. Biotechnol Adv. 2012;30:1614–1626. doi: 10.1016/j.biotechadv.2012.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ramessar K, et al. Cost-effective production of a vaginal protein microbicide to prevent HIV transmission. Proc Natl Acad Sci USA. 2008;105:3727–3732. doi: 10.1073/pnas.0708841104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stöger E, Fischer R, Moloney M, Ma JK. Plant molecular pharming for the treatment of chronic and infectious diseases. Annu Rev Plant Biol. 2014;65:743–768. doi: 10.1146/annurev-arplant-050213-035850. [DOI] [PubMed] [Google Scholar]
- 33.Ning T, et al. Oral administration of recombinant human granulocyte-macrophage colony stimulating factor expressed in rice endosperm can increase leukocytes in mice. Biotechnol Lett. 2008;30:1679–1686. doi: 10.1007/s10529-008-9717-2. [DOI] [PubMed] [Google Scholar]
- 34.Xie T, et al. A biologically active rhIGF-1 fusion accumulated in transgenic rice seeds can reduce blood glucose in diabetic mice via oral delivery. Peptides. 2008;29:1862–1870. doi: 10.1016/j.peptides.2008.07.014. [DOI] [PubMed] [Google Scholar]
- 35.Evangelista RL, Kusnadi AR, Howard JA, Nikolov ZL. Process and economic evaluation of the extraction and purification of recombinant beta-glucuronidase from transgenic corn. Biotechnol Prog. 1998;14:607–614. doi: 10.1021/bp980047c. [DOI] [PubMed] [Google Scholar]
- 36.Nandi S, et al. Process development and economic evaluation of recombinant human lactoferrin expressed in rice grain. Transgenic Res. 2005;14:237–249. doi: 10.1007/s11248-004-8120-6. [DOI] [PubMed] [Google Scholar]
- 37.Vamvaka E, et al. Rice endosperm produces an underglycosylated and potent form of the HIV-neutralizing monoclonal antibody 2G12. Plant Biotechnol J. 2016;14:97–108. doi: 10.1111/pbi.12360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rademacher T, et al. Recombinant antibody 2G12 produced in maize endosperm efficiently neutralizes HIV-1 and contains predominantly single-GlcNAc N-glycans. Plant Biotechnol J. 2008;6:189–201. doi: 10.1111/j.1467-7652.2007.00306.x. [DOI] [PubMed] [Google Scholar]
- 39.O’Keefe BR, et al. Scaleable manufacture of HIV-1 entry inhibitor griffithsin and validation of its safety and efficacy as a topical microbicide component. Proc Natl Acad Sci USA. 2009;106:6099–6104. doi: 10.1073/pnas.0901506106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vamvaka E, et al. Rice endosperm is cost-effective for the production of recombinant griffithsin with potent activity against HIV. Plant Biotechnol J. 2016;14:1427–1437. doi: 10.1111/pbi.12507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sexton A, et al. Transgenic plant production of Cyanovirin-N, an HIV microbicide. FASEB J. 2006;20:356–358. doi: 10.1096/fj.05-4742fje. [DOI] [PubMed] [Google Scholar]
- 42.Drake PMW, de Moraes Madeira L, Szeto TH, Ma JKC. Transformation of Althaea officinalis L. by Agrobacterium rhizogenes for the production of transgenic roots expressing the anti-HIV microbicide cyanovirin-N. Transgenic Res. 2013;22:1225–1229. doi: 10.1007/s11248-013-9730-7. [DOI] [PubMed] [Google Scholar]
- 43.O’Keefe BR, et al. Engineering soya bean seeds as a scalable platform to produce cyanovirin-N, a non-ARV microbicide against HIV. Plant Biotechnol J. 2015;13:884–892. doi: 10.1111/pbi.12309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vamvaka E, et al. Cyanovirin-N produced in rice endosperm offers effective pre-exposure prophylaxis against HIV-1BaL infection in vitro. Plant Cell Rep. 2016;35:1309–1319. doi: 10.1007/s00299-016-1963-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Sexton A, Harman S, Shattock RJ, Ma JK. Design, expression, and characterization of a multivalent, combination HIV microbicide. FASEB J. 2009;23:3590–3600. doi: 10.1096/fj.09-131995. [DOI] [PubMed] [Google Scholar]
- 46.Smith W, Jarrett AL, Beattie RE, Corvalan JR. Immunoglobulins secreted by a hybrid-hybridoma: Analysis of chain assemblies. Hybridoma. 1992;11:87–98. doi: 10.1089/hyb.1992.11.87. [DOI] [PubMed] [Google Scholar]
- 47.Christou P, Ford TL. The impact of selection parameters on the phenotype and genotype of transgenic rice callus and plants. Transgenic Res. 1995;4:44–51. [Google Scholar]
- 48.Ramessar K, Capell T, Christou P. Molecular pharming in cereal crops. Phytochem Rev. 2008;7:579–592. [Google Scholar]
- 49.Sabalza M, Vamvaka E, Christou P, Capell T. Seeds as a production system for molecular pharming applications: Status and prospects. Curr Pharm Des. 2013;19:5543–5552. doi: 10.2174/1381612811319310009. [DOI] [PubMed] [Google Scholar]
- 50.He Y, et al. Large-scale production of functional human serum albumin from transgenic rice seeds. Proc Natl Acad Sci USA. 2011;108:19078–19083. doi: 10.1073/pnas.1109736108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhang L, et al. Expression and characterization of recombinant human alpha-antitrypsin in transgenic rice seed. J Biotechnol. 2012;164:300–308. doi: 10.1016/j.jbiotec.2013.01.008. [DOI] [PubMed] [Google Scholar]
- 52.Obembe OO, Popoola JO, Leelavathi S, Reddy SV. Advances in plant molecular farming. Biotechnol Adv. 2011;29:210–222. doi: 10.1016/j.biotechadv.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 53.Arfi ZA, Hellwig S, Drossard J, Fischer R, Buyel JF. Polyclonal antibodies for the specific detection of tobacco host cell proteins can be generated more efficiently following RuBisCO depletion and the removal of endotoxins. Biotechnol J. 2016;11:507–518. doi: 10.1002/biot.201500271. [DOI] [PubMed] [Google Scholar]
- 54.Naqvi S, et al. When more is better: Multigene engineering in plants. Trends Plant Sci. 2010;15:48–56. doi: 10.1016/j.tplants.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 55.Zorrilla-López U, et al. Engineering metabolic pathways in plants by multigene transformation. Int J Dev Biol. 2013;57:565–576. doi: 10.1387/ijdb.130162pc. [DOI] [PubMed] [Google Scholar]
- 56.McFadden K, et al. A recombinant allosteric lectin antagonist of HIV-1 envelope gp120 interactions. Proteins. 2007;67:617–629. doi: 10.1002/prot.21295. [DOI] [PubMed] [Google Scholar]
- 57.Mariner JM, McMahon JB, O’Keefe BR, Nagashima K, Boyd MR. The HIV-inactivating protein, cyanovirin-N, does not block gp120-mediated virus-to-cell binding. Biochem Biophys Res Commun. 1998;248:841–845. doi: 10.1006/bbrc.1998.9060. [DOI] [PubMed] [Google Scholar]
- 58.Esser MT, et al. Cyanovirin-N binds to gp120 to interfere with CD4-dependent human immunodeficiency virus type 1 virion binding, fusion, and infectivity but does not affect the CD4 binding site on gp120 or soluble CD4-induced conformational changes in gp120. J Virol. 1999;73:4360–4371. doi: 10.1128/jvi.73.5.4360-4371.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Trkola A, et al. Human monoclonal antibody 2G12 defines a distinctive neutralization epitope on the gp120 glycoprotein of human immunodeficiency virus type 1. J Virol. 1996;70:1100–1108. doi: 10.1128/jvi.70.2.1100-1108.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kwong PD, et al. Structure of an HIV gp120 envelope glycoprotein in complex with the CD4 receptor and a neutralizing human antibody. Nature. 1998;393:648–659. doi: 10.1038/31405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Wyatt R, et al. The antigenic structure of the HIV gp120 envelope glycoprotein. Nature. 1998;393:705–711. doi: 10.1038/31514. [DOI] [PubMed] [Google Scholar]
- 62.Wyatt R, Sodroski J. The HIV-1 envelope glycoproteins: Fusogens, antigens, and immunogens. Science. 1998;280:1884–1888. doi: 10.1126/science.280.5371.1884. [DOI] [PubMed] [Google Scholar]
- 63.Calarese DA, et al. Antibody domain exchange is an immunological solution to carbohydrate cluster recognition. Science. 2003;300:2065–2071. doi: 10.1126/science.1083182. [DOI] [PubMed] [Google Scholar]
- 64.Chang LC, Bewley CA. Potent inhibition of HIV-1 fusion by cyanovirin-N requires only a single high affinity carbohydrate binding site: Characterization of low affinity carbohydrate binding site knockout mutants. J Mol Biol. 2002;318:1–8. doi: 10.1016/S0022-2836(02)00045-1. [DOI] [PubMed] [Google Scholar]
- 65.Liu Y, et al. Multivalent interactions with gp120 are required for the anti-HIV activity of cyanovirin. Biopolymers. 2009;92:194–200. doi: 10.1002/bip.21173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Matei E, et al. Anti-HIV activity of defective cyanovirin-N mutants is restored by dimerization. J Biol Chem. 2010;285:13057–13065. doi: 10.1074/jbc.M109.094938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Barrientos LG, Matei E, Lasala F, Delgado R, Gronenborn AM. Dissecting carbohydrate-cyanovirin-N binding by structure-guided mutagenesis: Functional implications for viral entry inhibition. Protein Eng Des Sel. 2006;19:525–535. doi: 10.1093/protein/gzl040. [DOI] [PubMed] [Google Scholar]
- 68.Alexandre KB, et al. The lectins griffithsin, cyanovirin-N and scytovirin inhibit HIV-1 binding to the DC-SIGN receptor and transfer to CD4(+) cells. Virology. 2012;423:175–186. doi: 10.1016/j.virol.2011.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Férir G, et al. Combinations of griffithsin with other carbohydrate-binding agents demonstrate superior activity against HIV type 1, HIV type 2, and selected carbohydrate-binding agent-resistant HIV type 1 strains. AIDS Res Hum Retroviruses. 2012;28:1513–1523. doi: 10.1089/aid.2012.0026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Altpeter F, et al. Particle bombardment and the genetic enhancement of crops: Myths and realities. Mol Breed. 2005;15:305–327. [Google Scholar]
- 71.Kohli A, et al. Transgene integration, organization and interaction in plants. Plant Mol Biol. 2003;52:247–258. doi: 10.1023/a:1023941407376. [DOI] [PubMed] [Google Scholar]
- 72.Maqbool SB, Christou P. Multiple traits of agronomic importance in transgenic indica rice plants: Analysis of transgene integration patterns, expression levels and stability. Mol Breed. 1999;5:471–480. [Google Scholar]
- 73.Huskens D, Van Laethem K, Vermeire K, Balzarini J, Schols D. Resistance of HIV-1 to the broadly HIV-1-neutralizing, anti-carbohydrate antibody 2G12. Virology. 2007;360:294–304. doi: 10.1016/j.virol.2006.10.027. [DOI] [PubMed] [Google Scholar]
- 74.Sánchez-Palomino S, et al. A cell-to-cell HIV transfer assay identifies humoral responses with broad neutralization activity. Vaccine. 2011;29:5250–5259. doi: 10.1016/j.vaccine.2011.05.016. [DOI] [PubMed] [Google Scholar]
- 75.Li M, et al. Human immunodeficiency virus type 1 env clones from acute and early subtype B infections for standardized assessments of vaccine-elicited neutralizing antibodies. J Virol. 2005;79:10108–10125. doi: 10.1128/JVI.79.16.10108-10125.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Naqvi S, et al. Transgenic multivitamin corn through biofortification of endosperm with three vitamins representing three distinct metabolic pathways. Proc Natl Acad Sci USA. 2009;106:7762–7767. doi: 10.1073/pnas.0901412106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Sudhakar D, et al. An efficient rice transformation system utilizing mature seed-derived explants and a portable, inexpensive particle bombardment device. Transgenic Res. 1998;7:289–294. [Google Scholar]
- 78.Valdez M, Cabrera-Ponce JL, Sudhakar D, Herrera-Estrella L, Christou P. Transgenic central American, west African and Asian elite rice varieties resulting from particle bombardment of foreign DNA into mature seed-derived explants utilizing three different bombardment devices. Ann Bot. 1998;82:795–801. [Google Scholar]
- 79.Christou P, Ford TL, Kofron M. Genotype-independent stable transformation of rice (Oryza sativa) plants. Bio/Technology. 1991;9:957–962. [Google Scholar]
- 80.Burgess RR. Protein precipitation techniques. Methods Enzymol. 2009;463:331–342. doi: 10.1016/S0076-6879(09)63020-2. [DOI] [PubMed] [Google Scholar]
- 81.Lang GH, Kagiya Y, Ohnishi-Kameyama M, Kitta K. Evaluation of extraction solutions for biochemical analyses of the proteins in rice grains. Biosci Biotechnol Biochem. 2013;77:126–131. doi: 10.1271/bbb.120617. [DOI] [PubMed] [Google Scholar]
- 82.Balindong JL, Liu L, Ward RM, Barkla BJ, Waters DLE. Optimisation and standardisation of extraction and HPLC analysis of rice grain protein. J Cereal Sci. 2016;72:124–130. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.