Significance
Securing the stable delivery of ecosystem services related to plant biomass (e.g., food, carbon sequestration, and soil fertility) is a pressing issue under ongoing climate change. Biodiversity increases ecosystem stability, but climate change may alter this positive relationship. We coupled a field survey of plant diversity conducted in drylands worldwide with remote sensing estimates of primary productivity to show a strong climate dependency of the biodiversity–ecosystem stability relationship. Our findings suggest that land management should be adapted to the aridity conditions if we aim to secure stable plant production. For instance, promoting higher species richness may represent a simple yet effective strategy to stabilize plant biomass over time in the face of the increasing aridity forecasted for drylands worldwide.
Keywords: aridity, NDVI, plant functional traits, species richness, temporal stability
Abstract
The insurance hypothesis, stating that biodiversity can increase ecosystem stability, has received wide research and political attention. Recent experiments suggest that climate change can impact how plant diversity influences ecosystem stability, but most evidence of the biodiversity–stability relationship obtained to date comes from local studies performed under a limited set of climatic conditions. Here, we investigate how climate mediates the relationships between plant (taxonomical and functional) diversity and ecosystem stability across the globe. To do so, we coupled 14 years of temporal remote sensing measurements of plant biomass with field surveys of diversity in 123 dryland ecosystems from all continents except Antarctica. Across a wide range of climatic and soil conditions, plant species pools, and locations, we were able to explain 73% of variation in ecosystem stability, measured as the ratio of the temporal mean biomass to the SD. The positive role of plant diversity on ecosystem stability was as important as that of climatic and soil factors. However, we also found a strong climate dependency of the biodiversity–ecosystem stability relationship across our global aridity gradient. Our findings suggest that the diversity of leaf traits may drive ecosystem stability at low aridity levels, whereas species richness may have a greater stabilizing role under the most arid conditions evaluated. Our study highlights that to minimize variations in the temporal delivery of ecosystem services related to plant biomass, functional and taxonomic plant diversity should be particularly promoted under low and high aridity conditions, respectively.
The stability of plant community biomass over time, defined as the ratio of the temporal mean to the SD (1), is a fundamental ecosystem property (2). Securing the stable delivery of ecosystem services related to plant biomass (e.g., food, forage, carbon sequestration, soil fertility) is a pressing socioecological issue under ongoing climate change. Accordingly, the positive relationship between biodiversity and ecosystem stability predicted by the insurance hypothesis (3), also referred to as portfolio effects (4), has gained ample research attention and guided global political efforts [e.g., Intergovernmental Science-Policy Platform on Biodiversity and Ecosystem Services (5)].
Most evidence of the biodiversity–stability relationship comes from local-scale experiments, where the species included are randomly selected from a small pool and stability is evaluated under a limited set of environmental conditions (6–8, but also refer to refs. 9–11). Ecosystem stability may respond to climate change in complex and multidimensional ways (12), and recent experiments suggest that changing climatic conditions impact the stabilizing role of plant diversity (8, 13, 14). For instance, decreases in local plant species richness with drought were related to similar reductions in ecosystem stability across 12 multiyear experiments at Cedar Creek (8). To assess how climate change impacts ecosystem stability, local-scale experiments and modeling efforts should be complemented with the assessment of the biodiversity–stability relationship in “real-world” ecosystems located across a wide range of climatic conditions and species pools.
Beyond species richness, the functional identity and diversity of dominant species may also influence ecosystem stability (7, 15). Dominant plant species may affect ecosystem stability if they are well adapted to environmental fluctuations in the availability of resources (7). For instance, Mediterranean vegetation is often dominated by medium-height plant species with a low growth rate and specific leaf area (SLA) that are resistant to climatic fluctuations (16, 17). The dominance of a photosynthetic pathway is also important (13), as C4 species have higher water-use efficiency than C3 species (18), and their productivity may show higher stability, particularly in water-limited systems. Alternatively, plant functional diversity (i.e., the dispersion of functional trait values within the plant community) has been shown to positively impact ecosystem stability in European forests (19) and grasslands (20) via species complementarity in resource use, and increasing functional diversity can promote ecosystem resistance to aridity in Mediterranean drylands (16). Evaluating the interplay between climatic conditions and multiple facets of plant diversity may thus shed light on the ultimate determinants of stability in terrestrial ecosystems worldwide.
Monitoring ecosystem stability at regional and global scales requires large-scale measurements of plant biomass over time. Satellite-based time series of aboveground biomass [e.g., normalized difference vegetation index (NDVI)] have been used to infer ecosystem stability at large spatial and temporal scales (21–23). To assess the role of biodiversity as a driver of ecosystem stability across large environmental gradients, field measurements of multiple facets of plant diversity are also needed. Regional studies at the European scale have already evaluated the biodiversity–stability relationship by coupling NDVI measurements with field vegetation surveys (21, 22). Evaluating this relationship across global environmental gradients can provide general insights to help land managers by making ecosystem stability predictable under different climate change scenarios (11). Despite this, such a global assessment has not yet been performed.
Here, we evaluated whether greater plant diversity is associated with greater ecosystem stability at a global scale, and whether climate mediates such a relationship. To do that, we coupled a field survey of plant diversity conducted in 123 dryland ecosystems from six continents with remote sensing estimates of productivity and climate data. Ecosystem stability is of major importance in dynamic systems such as drylands, where the key resource (water) strongly fluctuates over time (24). Drylands cover about 45% of the Earth’s land surface (25), and increases in aridity forecasted with climate change may jeopardize the provision of ecosystem services related to plant biomass (26). We investigated a key suite of spatial, climatic, soil, and plant diversity variables that we hypothesize might modulate ecosystem stability and its two components (mean temporal plant biomass and SD) globally. Specifically, we evaluated the interactive effects of aridity and plant (taxonomic and functional) diversity on the stability of dryland ecosystems using multiple regression models, and explored the direct and indirect aridity pathways with structural equation modeling.
Results
Predictors of Ecosystem Stability and Its Components.
The best model explaining ecosystem stability included all predictors (model 6′ in SI Appendix, Table S1). Within the selected Akaike Information Criterion threshold (ΔAICc ≤ 2), we only found two alternative models for model 6′ (SI Appendix, Table S2), which explained a high proportion of the variance in ecosystem stability (R2 = 0.73). Abiotic factors (climate and soil) and plant diversity metrics were responsible for 56.1% and 43.9% of the explained variance in ecosystem stability, respectively (Fig. 1A).
Fig. 1.
Relative effects of multiple predictors of ecosystem stability (A) and its two components, temporal mean NDVI (B) and SD of NDVI (C). The averaged parameter estimates (standardized regression coefficients) of the model predictors are shown with their associated 95% confidence intervals along with the relative importance of each predictor, expressed as the percentage of explained variance. The graph represents the best model selected based on the AICc (model 6′ in SI Appendix, Table S1). The relative effect of the predictors, and their interactions, can be simply calculated as the ratio between the parameter estimate of the predictor and the sum of all parameter estimates, and it is expressed as a percentage. Abiotic factors include climate and soil. Aridity is defined as 1 − AI, where the AI is the ratio of precipitation to potential evapotranspiration. H, adult plant height; Inter Rain, interannual rainfall variability; Inter Temp, interannual temperature variability; Intra Rain, intraannual rainfall variability. *P < 0.05; **P < 0.01; ***P < 0.001.
Aridity did not impact stability directly but interacted with the facets of plant diversity evaluated (Fig. 1A). We observed significant interactions between aridity and both species richness and the variance of SLA. The effect of species richness on ecosystem stability shifted from slightly negative at low aridity levels to markedly positive under high aridity conditions (Fig. 2A). The effect of the variance of SLA on stability shifted from positive to negative as aridity increased (Fig. 2B). We found a quadratic effect of mean height on ecosystem stability (Fig. 1A), indicating that plant communities dominated by medium-sized species were more stable. Mean SLA and the relative abundance of C4 species were not selected in the best models (SI Appendix, Table S1), suggesting that they did not impact stability. Interannual rainfall variability showed a quadratic relationship with stability, whereas we found a strong destabilizing effect of intraannual rainfall variability. Soil pH had a quadratic effect upon this variable centered at pH 7, and sand content was positively correlated with stability.
Fig. 2.
Predicted interactive effects between species (Sp.) richness and aridity (A) and between variance in SLA and aridity (B) on ecosystem stability. (C) Predicted interactive effect between Sp. richness and aridity on the temporal mean NDVI. (D) Predicted interactive effect between the variance of SLA and aridity on the SD of NDVI. The effects of interactions are represented using the standardized parameter estimates shown in Fig. 1. In each panel, the predicted stability value for the two interactive factors is shown, with all other standardized parameter estimates being fixed at their mean value. The color of the predicted planes changes from blue (low ecosystem stability) to red (high ecosystem stability).
When addressing the components of ecosystem stability [i.e., the temporal mean of the NDVI and its SD (SD of NDVI); SI Appendix, Fig. S1], we found that the proportion of explained variation by plant diversity predictors remained similar to that observed when analyzing stability (mean NDVI = 50.2% and SD of NDVI = 56.9%; Fig. 1 B and C). Aridity increased the SD of NDVI but did not affect mean NDVI directly. We found a significant species richness × aridity interaction and an effect of the variance of SLA when analyzing mean NDVI. The interaction between the variance of SLA and aridity was significant for the SD of NDVI. Species richness was related to higher mean NDVI values at the driest end of the aridity gradient (Fig. 2C). The variance of SLA decreased the SD of NDVI at low aridity sites but increased it at high aridity sites (Fig. 2D).
Indirect Effects of Aridity on Ecosystem Stability.
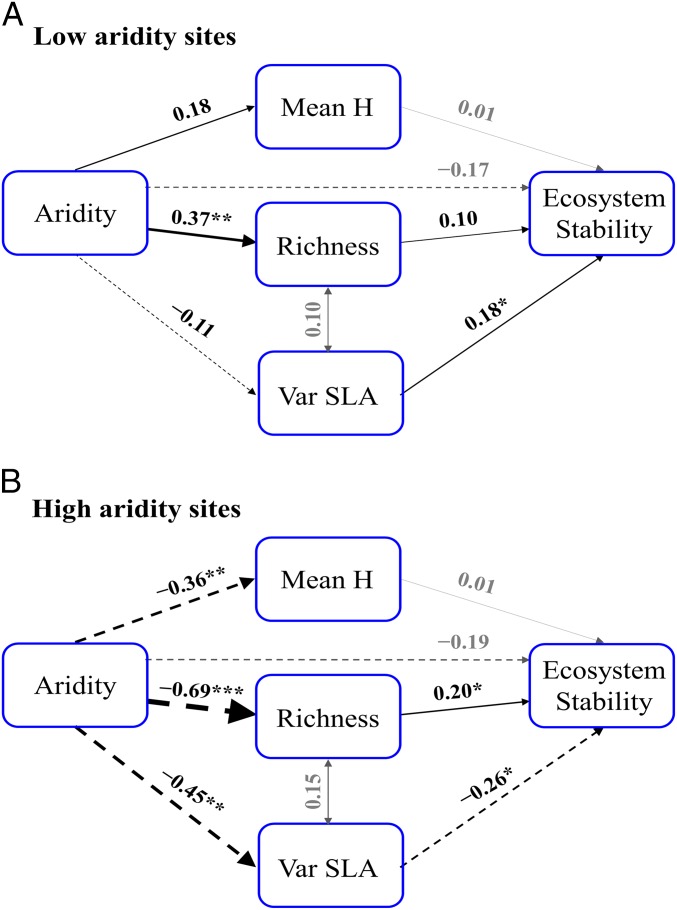
We focused on the direct and indirect linkages between aridity, plant diversity (mean H, species richness, and variance of SLA), and stability. We first removed the influence of the spatial, climatic, and soil variables on ecosystem stability and saved the residuals. The residual variance in stability was then fed into the structural equation models (SEMs). Our multigroup analysis revealed significant and contrasted indirect relationships between aridity, plant diversity, and ecosystem stability at low (<0.6) and high (>0.6) aridity levels (Fig. 3 and SI Appendix, Table S3).
Fig. 3.
Relationships between aridity, facets of plant diversity, and residual variance of ecosystem stability in sites with low (A; <0.6) and high (B; >0.6) aridity levels. Before feeding into the SEM, the residual variance of ecosystem stability was calculated by accounting for spatial, climatic, and soil variables. Such residual variance represents 46% and 29% of the original ecosystem stability in the low and high aridity sites, respectively. The weight of the arrows indicates the strength of the causal relationship, supplemented by a path coefficient. Double-headed arrows indicate correlations. Black arrows indicate relationships that are statistically different (P < 0.05) between the two aridity levels (statistical details are provided in SI Appendix, Table S3). Gray arrows indicate relationships that are not statistically different between the two aridity levels. Continuous and dashed arrows indicate positive and negative relationships, respectively. Goodness-of-fit tests for multigroup comparisons: χ2 = 4.43, P = 0.359; GFI = 0.976, RMSEA = 0.029, P = 0.556. *P < 0.05; **P < 0.01; ***P < 0.001. R2 values for residual variance of ecosystem stability are 0.07 in the low aridity sites and 0.11 in the high aridity sites (n = 61 and n = 62 in the low and high aridity models, respectively). Var, variance.
At low aridity sites, greater aridity significantly increased species richness. However, richness was not related to ecosystem stability. Only the variance of SLA, independent of aridity, was positively related to stability (Fig. 3A). At high aridity sites, species richness and the variance of SLA were positively and negatively associated, respectively, with ecosystem stability (Fig. 3B). Under these harsh environmental conditions, aridity may indirectly offset the stabilizing role of richness, as we found a strong negative link between aridity and richness (r = −0.69, P < 0.001). On the other hand, by reducing the variance of SLA, aridity may also indirectly increase stability, as we found a negative relationship between the variance of SLA and ecosystem stability (r = −0.26, P = 0.040).
Discussion
Our results indicate that spatial, climatic, soil, and plant diversity variables can explain up to 73% of the variation in ecosystem stability in global drylands. Previous large spatial-scale studies using remote sensing approaches to assess ecosystem stability only considered climatic variables and/or taxonomic diversity, explaining 29% (21), 16% (22), and 29–33% (27) of the observed variation in ecosystem stability. The multiple plant diversity facets considered in our study were as important as climatic and soil features, suggesting that the stabilizing role of biodiversity is conspicuous in real-world ecosystems. We observed a shift in the relative importance of plant diversity facets that are positively associated with ecosystem stability in low (diversity of leaf functional traits) and high (species richness) aridity sites. This result may suggest a strong climatic dependency of the biodiversity–stability relationship, extending the results found in local-scale experiments conducted in grasslands (6, 8, 10).
At the lowest end of the aridity gradient evaluated, the variance of SLA was positively related to mean NDVI, as indicated by the multiple regression models (Fig. 1B). The variance of SLA also affected ecosystem stability via its negative link with the SD of NDVI (Fig. 2D), representing a truly stabilizing role of leaf trait diversity (4). This functional diversity effect may indicate that a higher diversity in plant resource-use strategies, evaluated with the variance of SLA (15), promotes a higher asynchrony in the responses of species to environmental fluctuations (e.g., between evergreen and deciduous shrubs). The variance of functional trait values within the community is a major predictor of ecosystem functioning in global drylands (28) and determines its responses to aridity (16). Our study generalizes the importance of functional diversity for maintaining the stability of drylands under low aridity conditions, as found in central European forests (19) and grasslands (20).
As aridity increased, the link between the variance of SLA and stability shifted from positive to negative (Fig. 2B). Aridity could act as a strong environmental filter in drylands, selecting well-adapted species within a narrow range of leaf trait values (29). Under these harsh environmental conditions, communities with a high variance of SLA may reflect the replacement of stress-tolerant evergreen species by competitive summer deciduous plants that avoid drought via leaf shedding (29), increasing the SD of plant biomass over time (Fig. 2D). Interestingly, our results also indicated that species richness can buffer the temporal stability of plant biomass under the most arid conditions (Fig. 2A). The aridity × richness interaction was significant (P < 0.01) when addressing the mean NDVI, but not when looking at the SD of NDVI (Fig. 1 B and C). These results may point to overyielding, instead of statistical averaging, as the driving mechanism of the observed relationship between species richness and ecosystem stability (1, 3, 4, 30). The opposite effects of richness and variance of SLA on ecosystem stability found at high aridity conditions suggest that increasing the number of species with similar SLA values may maximize the stabilizing role of species richness on ecosystem stability, a signature for a positive functional redundancy–ecosystem stability relationship (31, 32).
We further explored the direct and indirect relationships between aridity, plant diversity, and stability using the multigroup analysis feature of structural equation modeling (Fig. 3). We found a significant shift in the main pathways mediating such relationships when comparing sites with low (<0.6) vs. high (>0.6) aridity levels (Fig. 3). This analysis revealed that although aridity was not directly related to ecosystem stability, it impacted stability via changes in the variance of SLA and species richness at high aridity sites (Fig. 3B). These indirect linkages are especially relevant for the management of ecosystem stability in drylands due to the increased aridity forecasted with climate change in these areas (27). On one side, increasing species loss with aridity may alter stability via lower mean NDVI. At the same time, the strong environmental filtering selecting for species adapted to aridity (e.g., low variance of SLA) may reduce the SD of NDVI, and consequently enhance ecosystem stability. By decoupling the effects of taxonomic and functional diversity on contrasted components of ecosystem stability, our study may help to identify effective management strategies that could promote drylands stability under climate change via overyielding (1, 30) and buffering effects (3, 4).
The aridity level (0.6) selected to split the network of sites represents the lower boundary of a transition zone (0.6–0.8) between semiarid and arid conditions (33), where a discontinuity in net primary production (34), plant–plant interactions (35), and ecosystem functioning (36) has been observed. Despite this evidence about a potential discontinuity at aridity levels of 0.6–0.8 in drylands worldwide, our results should be considered as exploratory because they are based on observational data and the comparison of specific groups (sites with aridity level < 0.6 vs. sites with aridity level > 0.6).
A counterview of the biodiversity–stability relationship states that dominant species play a major role in driving ecosystem stability (7). The dominance of particular species was associated with ecosystem stability across the global network of drylands studied, as indicated by the significant quadratic effect of mean plant height. Medium-sized plant communities, such as those dominated by stress-tolerant shrubs in Mediterranean regions (e.g., Rosmarinus spp.), were more stable. Plant communities dominated by species with high SLA, such as drought-deciduous species in North American hot deserts (e.g., Encelia spp.), were related to higher aboveground biomass in our study. Nevertheless, this biomass was also more variable over time (higher SD of NDVI), offsetting any potential stabilizing effect from mean SLA. Our results also indicated that plant communities with a higher relative abundance of C4 species were related to increased mean NDVI under high aridity levels. These results agree with the high water-use efficiency typically found in C4 species, which allows them to maintain high photosynthetic and productivity rates under these harsh climatic conditions (18). However, the relative abundance of C4 species was not related to ecosystem stability, which does not support recent experimental findings indicating that C4 species can help to maintain stable plant community biomass over time in dry conditions (13).
Intra- and interannual climatic variability significantly impacted ecosystem stability in our study. Intraannual rainfall variability, which is crucial for plant phenology (37), played a destabilizing role via reduced mean NDVI (Fig. 1B and SI Appendix, Fig. S2). Dryland sites with high intraannual rainfall variability (i.e., higher rainfall seasonality) may have lower mean plant biomass. Seasonal rainfall events can lead to high water losses through runoff (38), decreasing soil water availability and, ultimately, plant growth (39). Instead, interannual rainfall variability showed a quadratic relationship with ecosystem stability, particularly with mean NDVI (Fig. 1B and SI Appendix, Fig. S3). This nonlinear relationship may suggest that once a threshold in climatic conditions is surpassed (e.g., prolonged droughts), ecosystem stability is drastically reduced, indicating a shift toward a degraded ecosystem state (40).
Our global study using observational data across a wide range of climatic conditions contrasts with the general approach addressing the biodiversity–stability relationship using local experiments and/or time series measurements of plant diversity and biomass (6–10). Although these studies have explicitly investigated the plant diversity mechanisms determining ecosystem stability, they have not addressed such relationships on a global scale (but refer to ref. 11). Our study suggests that a snapshot field survey of perennial vegetation across a large number of ∼0.1-km2 plots can be used to infer midterm (2000–2013) patterns of satellite-based plant biomass across a global range of climatic conditions. This upscaling supports recent results from global grasslands, where the synchrony of local plant communities is an important component of the temporal stability at larger spatial scales (11). Future progress in large-scale estimations of ecosystem stability can be glimpsed by simultaneously evaluating temporal changes in plant biomass, with shifts in plant species and functional diversity, using new satellite sensors. For example, Sentinel-2, with a high spatial resolution (pixel size of 10 m × 10 m) and a short revisit period (5 d), represents a promising avenue (41), but the temporal range covered (2015–present) is still too limited to evaluate dynamic processes in natural ecosystems.
Conclusions
Our results indicate that a positive link between plant diversity and ecosystem stability is conspicuous across a global network of dryland ecosystems. The magnitude of this relationship is similar to that found between stability and abiotic features (climate and soil). However, the pathways behind the biodiversity–stability relationship are dependent on climate, since aridity modulates the stabilizing role of plant diversity. Our results may suggest that the diversity of leaf functional traits (e.g., SLA) promotes stability at low aridity levels, whereas plant richness and higher redundancy in leaf traits play such a stabilizing role at high aridity levels. Forecasting the temporal delivery of ecosystem services related to plant biomass is key for the subsistence of over 38% of the world’s human population inhabiting drylands (26). Our study suggests that different facets of plant diversity should be promoted to increase ecosystem stability in low and high aridity sites.
Methods
Study Sites.
We studied 123 dryland ecosystems located in 13 countries, including sites of the major vegetation types found in drylands (e.g., Mediterranean shrublands, African savannas, the Eurasian steppe, Australian open woodlands, North American deserts). These sites are a subset of the sites used by Maestre et al. (42), and they comprise a good representation of the soil and climatic conditions currently found in drylands worldwide, with aridity conditions ranging from arid to dry-subhumid and mean annual temperature and precipitation varying from −0.1 to 26.5 °C and 101 to 1,218 mm, respectively.
Field Plant Diversity Measurements.
Field surveys took place between June 2006 and January 2011. The cover of perennial species at each site was measured by using 80 quadrats of 2.25 m2 established along four 30-m-long transects at each site. The sum of the cover for each species was used as a proxy of species abundance. Species richness at each site was estimated as the number of perennial plant species found within these 80 quadrats. We also gathered mean values per species of functional (adult plant height and SLA) and physiological (relative abundance of C4 species) traits from the TRY database (43). Using trait (from databases) and cover (from the field survey) data, we calculated the community-weighted mean (functional identity) and variance (functional dispersion) for each trait separately as follows:
| [1] |
| [2] |
where Pi and Ti are the relative abundance and the trait value of species i in community j, respectively, and n is the total number of species in a given community. More details on these calculations are provided in SI Appendix. The photosynthetic pathway was assigned to each species, and the dominance of C4 species was calculated as the proportion of this pathway (estimated using the cover of each species measured in the field) within each surveyed community.
Satellite-Based Measurements of Aboveground Biomass.
We used the NDVI as our proxy of aboveground plant biomass (21–23). The NDVI provides a global measure of the “greenness” of vegetation across the Earth’s landscapes for a given composite period (44). NDVI data for each site were acquired using the MOD13Q1 product from the Moderate Resolution Imaging Spectroradiometer aboard NASA’s Terra satellites (daac.ornl.gov), which provides data 23 times per year (every 16 d) with a pixel size of 250 m × 250 m. These data are geometrically and atmospherically corrected, and they include a reliability index of data quality based on the environmental conditions under which the data were recorded. At each site, we calculated the annual NDVI for each year between 2000 and 2013. To do so, we averaged the 23 values placed between the date of the mean minimum NDVI (date n) to the date n − 1 of the following year at each site. This approach allowed us to account for the different annual cycles of vegetation growth across our network of sites. We also tested whether the field areas surveyed were sufficiently homogeneous to avoid spatial scale mismatch between field (30 m × 30 m) and remote sensing (250 m × 250 m) data (SI Appendix). Using the 14 annual NDVIs, we calculated ecosystem temporal stability as the ratio of the annual mean NDVI calculated from 2000 to 2013 (mean NDVI) to the SD of the annual NDVI (SD of NDVI) over that period.
Spatial, Climatic, and Soil Variables.
We gathered annual climatic conditions (potential evapotranspiration, mean air temperature, and total precipitation) from the same period of ecosystem stability measurements (2000–2013) using FetchClimate (45). From these variables, we calculated the aridity index (AI = precipitation/potential evapotranspiration), which is widely used to define the degree of aridity experienced by drylands worldwide (33). To facilitate the interpretation of results, we used 1 − AI to define aridity conditions in our analyses such that higher aridity values indicate drier conditions (42, 46). Aridity was highly correlated with total precipitation in our dataset (r = −0.84). Climate variability was assessed with two indices: (i) interannual rainfall variability (SD of total annual precipitation), which is typically used when assessing the climatic drivers of global terrestrial net primary production (47), and (ii) intraannual rainfall variability (coefficient of variation of monthly precipitation), which is a major determinant of the functional structure and diversity of plant communities in drylands worldwide (29). We summarized soil parameters at each site using sand content and pH, because they play key roles in the availability of water and nutrients (46) and are major drivers of plant diversity (29) and ecosystem functioning (28) in drylands. Briefly, soil variables were measured from soil samples (7.5-cm depth) collected under the canopy of the dominant perennial plants and in open areas devoid of vascular vegetation, as described by Maestre et al. (42) and in SI Appendix.
Identifying the Best Set of Predictors for Ecosystem Stability and Its Components.
We used multiple regression models to assess the joint effects of spatial, climatic, soil, species richness, functional diversity, and C4 on ecosystem stability, as well as on its two components (mean NDVI and SD of NDVI). This separate analysis can help to identify the potential pathways by which plant diversity may drive ecosystem stability: (i) a positive diversity–mean NDVI relationship supports the complementarity hypothesis, where higher plant diversity enhances mean biomass via overyielding (1, 30), and (ii) a positive diversity–SD NDVI relationship supports the buffering effect hypothesis, which predicts a decrease in the SD of plant biomass as diversity increases via asynchrony and/or statistical averaging of species’ responses to environmental fluctuations (4, 30, 48).
We built six competing models, which consider an increasing level of biological complexity, to identify the set of predictors that provide the most parsimonious model for explaining the variation in ecosystem stability: (i) an “abiotic” model (includes aridity, intra- and interannual rainfall variability, interannual temperature variability, sand content, and soil pH), (ii) a “species richness” model (the abiotic model plus species richness); (iii) a “C4” model (the abiotic model plus the relative abundance of C4); (iv) a “C4 and species richness” model (the abiotic model plus species richness and C4); (v) a “functional traits” model (the abiotic model plus the community-weighted mean and variance for SLA and height), and (vi) a “full” model (includes all predictors). We ran each model with and without accounting for an interactive effect of the plant variables used (species richness, relative abundance of C4, mean and variance of SLA, and height) and aridity. We also considered quadratic terms for climatic variables, plant community-weighted mean traits, and pH because these variables have been observed to affect ecosystem functioning in drylands in a nonlinear way (34, 46). We included the elevation, latitude, and longitude of the study sites to account for the spatial structure of our dataset (29, 42).
We first used a backward stepwise regression procedure using the software JMP 11 (SAS Institute) to select, between all models, the best-fitting models that minimized the AICc. For models i–vi, we started with the full model and subsequently removed the variables that impacted the AICc the most. Second, and using the best model (from models i–vi), we performed a model-averaging procedure based on the AICc (ΔAICc < 2) to determine parameter coefficients for the best final set of predictors of ecosystem stability. This procedure was performed using the function dredge in the R package Multi-Model Inference (MuMIn) (49). Model residuals were inspected for constant variance and normality. All predictors and response variables were standardized before analyses, using the Z-score to interpret parameter estimates on a comparable scale. Predictors were log-transformed when necessary before Z-score transformation to meet the assumptions of the tests used. To evaluate the relative importance of the predictors as drivers of ecosystem stability, we calculated the relative effect of the parameter estimates for each of the predictors compared with the effect of all parameter estimates in the model. The following four identifiable variance fractions were examined: (i) abiotic variables, (ii) species richness, (iii) relative abundance of C4 species, and (iv) functional trait diversity.
Comparing the Direct and Indirect Relationships Between Climate, Plant Diversity, and Stability at Low vs. High Aridity Levels.
We used multigroup analysis of SEMs to assess whether different indirect pathways (e.g., species richness, leaf functional trait diversity) drive the climate–biodiversity–stability relationship at low vs. high aridity levels. This approach is recommended when addressing the biotic drivers of stability, as these ecosystems are highly dynamic in response to water availability (40). To do so, we split the network of sites into two groups: aridity level < 0.6 (low aridity) and aridity level > 0.6 (high aridity). Although data splitting can impact the performance of linear models, this is a common practice in multigroup analysis of SEM when a model structure based on biological foundation is compared among different groups (50). A discontinuity in net primary production, plant–plant interactions, and ecosystem functioning has been found at the transition zone between semiarid and arid conditions [aridity values of 0.6–0.8 (34–36)]. Thus, the selected 0.6 aridity level fell within this range and allowed us to select an equal number of sites on both sides (61 and 62 sites in the low and high aridity groups, respectively), which is important for multigroup analysis when sample size is below 100 (51).
We focused the multigroup analysis of SEM on the diversity–stability relationship and reduced the number of variables included in the model as recommended for small sample sizes (50). First, the variances in H and mean SLA were excluded from the model because their interaction with aridity was not found significant in the multiple regression models (Fig. 1). Second, we removed the effects of spatial, climatic (except aridity), and soil variables on ecosystem stability. To do that, we fitted a multiple regression model using these variables as predictors and saved the residuals. Thus, the residual variance in ecosystem stability accounted for the effects of spatial, climate, and soil, and was selected as our response variable in the SEM. We then hypothesized a path diagram where a set of plant diversity facets (richness, variance of SLA, and mean height) mediated the effects of aridity on the residual variance in ecosystem stability. The multigroup analysis tested whether path coefficients differ significantly between the high and low aridity models. Path coefficients were obtained using maximum likelihood estimation. We used χ2, root mean square error of approximation, and goodness-of-fit index as goodness-of-fit tests. All SEM analyses were performed with AMOS 22.0 (Amos Development Co.).
Supplementary Material
Acknowledgments
We thank Yoann Le Bagousse-Pinguet, Samantha Travers, Xavier Morin, and Miguel Berdugo for providing comments on previous versions of the manuscript. We appreciate the use of data from the TRY initiative on plant traits. This work was funded by the European Research Council (ERC) under the European Community’s Seventh Framework Programme (FP7/2007-2013)/ERC Grant Agreements 242658 (BIOCOM) and 647038 (BIODESERT). P.G.-P. was funded by the Spanish Ministry of Economy and Competitiveness (Grant IJCI-2014-20058). N.G. was supported by the AgreenSkills+ Fellowship Programme, which has received funding from the European Union’s Seventh Framework Programme under Grant Agreement FP7-609398 (AgreenSkills+ contract).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in Figshare Digital Repository, https://figshare.com/s/beb37e3f3180959ab480.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1800425115/-/DCSupplemental.
References
- 1.Tilman D, Reich PB, Knops JMH. Biodiversity and ecosystem stability in a decade-long grassland experiment. Nature. 2006;441:629–632. doi: 10.1038/nature04742. [DOI] [PubMed] [Google Scholar]
- 2.Pimm SL. The complexity and stability of ecosystems. Nature. 1984;307:321–326. [Google Scholar]
- 3.Yachi S, Loreau M. Biodiversity and ecosystem productivity in a fluctuating environment: The insurance hypothesis. Proc Natl Acad Sci USA. 1999;96:1463–1468. doi: 10.1073/pnas.96.4.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Doak DF, et al. The statistical inevitability of stability-diversity relationships in community ecology. Am Nat. 1998;151:264–276. doi: 10.1086/286117. [DOI] [PubMed] [Google Scholar]
- 5.Díaz S, et al. The IPBES conceptual framework–Connecting nature and people. Curr Opin Environ Sustain. 2015;14:1–16. [Google Scholar]
- 6.Isbell FI, Polley HW, Wilsey BJ. Biodiversity, productivity and the temporal stability of productivity: Patterns and processes. Ecol Lett. 2009;12:443–451. doi: 10.1111/j.1461-0248.2009.01299.x. [DOI] [PubMed] [Google Scholar]
- 7.Grman E, Lau JA, Schoolmaster DR, Jr, Gross KL. Mechanisms contributing to stability in ecosystem function depend on the environmental context. Ecol Lett. 2010;13:1400–1410. doi: 10.1111/j.1461-0248.2010.01533.x. [DOI] [PubMed] [Google Scholar]
- 8.Hautier Y, et al. Plant ecology. Anthropogenic environmental changes affect ecosystem stability via biodiversity. Science. 2015;348:336–340. doi: 10.1126/science.aaa1788. [DOI] [PubMed] [Google Scholar]
- 9.Jucker T, Bouriaud O, Avacaritei D, Coomes DA. Stabilizing effects of diversity on aboveground wood production in forest ecosystems: Linking patterns and processes. Ecol Lett. 2014;17:1560–1569. doi: 10.1111/ele.12382. [DOI] [PubMed] [Google Scholar]
- 10.Hallett LM, et al. Biotic mechanisms of community stability shift along a precipitation gradient. Ecology. 2014;95:1693–1700. doi: 10.1890/13-0895.1. [DOI] [PubMed] [Google Scholar]
- 11.Wilcox KR, et al. Asynchrony among local communities stabilises ecosystem function of metacommunities. Ecol Lett. 2017;20:1534–1545. doi: 10.1111/ele.12861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Donohue I, et al. Navigating the complexity of ecological stability. Ecol Lett. 2016;19:1172–1185. doi: 10.1111/ele.12648. [DOI] [PubMed] [Google Scholar]
- 13.Shi Z, et al. Dual mechanisms regulate ecosystem stability under decade-long warming and hay harvest. Nat Commun. 2016;7:11973. doi: 10.1038/ncomms11973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma Z, et al. Climate warming reduces the temporal stability of plant community biomass production. Nat Commun. 2017;8:15378. doi: 10.1038/ncomms15378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Díaz S, et al. Incorporating plant functional diversity effects in ecosystem service assessments. Proc Natl Acad Sci USA. 2007;104:20684–20689. doi: 10.1073/pnas.0704716104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Valencia E, et al. Functional diversity enhances the resistance of ecosystem multifunctionality to aridity in Mediterranean drylands. New Phytol. 2015;206:660–671. doi: 10.1111/nph.13268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Soliveres S, et al. Functional traits determine plant co-occurrence more than environment or evolutionary relatedness in global drylands. Perspect Plant Ecol Syst. 2014;16:164–173. doi: 10.1016/j.ppees.2014.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Way DA, Katul GG, Manzoni S, Vico G. Increasing water use efficiency along the C3 to C4 evolutionary pathway: A stomatal optimization perspective. J Exp Bot. 2014;65:3683–3693. doi: 10.1093/jxb/eru205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Morin X, Fahse L, de Mazancourt C, Scherer-Lorenzen M, Bugmann H. Temporal stability in forest productivity increases with tree diversity due to asynchrony in species dynamics. Ecol Lett. 2014;17:1526–1535. doi: 10.1111/ele.12357. [DOI] [PubMed] [Google Scholar]
- 20.Fischer FM, et al. Plant species richness and functional traits affect community stability after a flood event. Philos Trans R Soc Lond B Biol Sci. 2016;371:2015027. doi: 10.1098/rstb.2015.0276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van Rooijen NM, et al. Plant species diversity mediates ecosystem stability of natural dune grasslands in response to drought. Ecosystems. 2015;18:1383–1394. [Google Scholar]
- 22.Oehri J, Schmid B, Schaepman-Strub G, Niklaus PA. Biodiversity promotes primary productivity and growing season lengthening at the landscape scale. Proc Natl Acad Sci USA. 2017;114:10160–10165. doi: 10.1073/pnas.1703928114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wang S, et al. An invariability-area relationship sheds new light on the spatial scaling of ecological stability. Nat Commun. 2017;8:15211. doi: 10.1038/ncomms15211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gillson L, Hoffman MT. Ecology. Rangeland ecology in a changing world. Science. 2007;315:53–54. doi: 10.1126/science.1136577. [DOI] [PubMed] [Google Scholar]
- 25.Prăvălie R. Drylands extent and environmental issues. A global approach. Earth Sci Rev. 2016;161:259–278. [Google Scholar]
- 26.Reynolds JF, et al. Global desertification: Building a science for dryland development. Science. 2007;316:847–851. doi: 10.1126/science.1131634. [DOI] [PubMed] [Google Scholar]
- 27.Huang L, et al. Drought dominates the interannual variability in global terrestrial net primary production by controlling semi-arid ecosystems. Sci Rep. 2016;6:24639. doi: 10.1038/srep24639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gross N, et al. Functional trait diversity maximizes ecosystem multifunctionality. Nat Ecol Evol. 2017;1:0132. doi: 10.1038/s41559-017-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Le Bagousse-Pinguet Y, et al. Testing the environmental filtering concept in global drylands. J Ecol. 2017;105:1058–1069. doi: 10.1111/1365-2745.12735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Thibaut LM, Connolly SR. Understanding diversity-stability relationships: Towards a unified model of portfolio effects. Ecol Lett. 2013;16:140–150. doi: 10.1111/ele.12019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fonseca CR, Ganade G. Species functional redundancy, random extinctions and the stability of ecosystems. J Ecol. 2001;89:118–125. [Google Scholar]
- 32.Mayfield MM, et al. What does species richness tell us about functional trait diversity? Predictions and evidence for responses of species and functional trait diversity to land‐use change. Glob Ecol Biogeogr. 2010;19:423–431. [Google Scholar]
- 33.United Nations Environment Programme . World Atlas of Desertification. UNEP; London: 1993. [Google Scholar]
- 34.Berdugo M, Kéfi S, Soliveres S, Maestre FT. Plant spatial patterns identify alternative ecosystem multifunctionality states in global drylands. Nat Ecol Evol. 2017;1:3. doi: 10.1038/s41559-016-0003. [DOI] [PubMed] [Google Scholar]
- 35.Berdugo M, et al. Aridity preferences alter the relative importance of abiotic and biotic drivers on plant species abundance in global drylands. J Ecol. 2018 doi: 10.1111/1365-2745.13006. [DOI] [Google Scholar]
- 36.Wang C, et al. Aridity threshold in controlling ecosystem nitrogen cycling in arid and semi-arid grasslands. Nat Commun. 2014;5:4799. doi: 10.1038/ncomms5799. [DOI] [PubMed] [Google Scholar]
- 37.Gordo O, Sanz JJ. Impact of climate change on plant phenology in Mediterranean ecosystems. Glob Chang Biol. 2010;16:1082–1106. [Google Scholar]
- 38.Tietjen B, et al. Effects of climate change on the coupled dynamics of water and vegetation in drylands. Ecohydrology. 2010;3:226–237. [Google Scholar]
- 39.Guan K, et al. Continental-scale impacts of intra-seasonal rainfall variability on simulated ecosystem responses in Africa. Biogeosciences. 2014;11:6939–6954. [Google Scholar]
- 40.D’Odorico P, Laio F, Ridolfi L. Noise-induced stability in dryland plant ecosystems. Proc Natl Acad Sci USA. 2005;102:10819–10822. doi: 10.1073/pnas.0502884102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rocchini D, et al. Satellite remote sensing to monitor species diversity: Potential and pitfalls. Remote Sens Ecol Conserv. 2016;2:25–36. [Google Scholar]
- 42.Maestre FT, et al. Plant species richness and ecosystem multifunctionality in global drylands. Science. 2012;335:214–218. doi: 10.1126/science.1215442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kattge J, et al. TRY–A global database of plant traits. Glob Chang Biol. 2011;17:2905–2935. [Google Scholar]
- 44.Bastos A, Running SW, Gouveia C, Trigo RM. The global NPP dependence on ENSO: La Niña and the extraordinary year of 2011. J Geophys Res. 2013;118:1247–1255. [Google Scholar]
- 45.Grechka D, et al. Universal, easy access to geotemporal information: FetchClimate. Ecography. 2016;39:904–911. [Google Scholar]
- 46.Delgado-Baquerizo M, et al. Decoupling of soil nutrient cycles as a function of aridity in global drylands. Nature. 2013;502:672–676. doi: 10.1038/nature12670. [DOI] [PubMed] [Google Scholar]
- 47.Zhao M, Running SW. Drought-induced reduction in global terrestrial net primary production from 2000 through 2009. Science. 2010;329:940–943. doi: 10.1126/science.1192666. [DOI] [PubMed] [Google Scholar]
- 48.Loreau M, de Mazancourt C. Biodiversity and ecosystem stability: A synthesis of underlying mechanisms. Ecol Lett. 2013;16:106–115. doi: 10.1111/ele.12073. [DOI] [PubMed] [Google Scholar]
- 49.Bartoń K. 2013 MuMIn: Multi-Model Inference. R package v. 1. Available at CRAN.R-project.org/package=MuMIn.
- 50.Grace JB. Structural Equation Modeling and Natural Systems. Cambridge Univ Press; Cambridge, UK: 2006. [Google Scholar]
- 51.Hox JJ, Maas CJM. The accuracy of multilevel structural equation modeling with pseudobalanced groups and small samples. Struct Equ Modeling. 2001;8:157–174. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.