Significance
Following hemorrhage in damaged tissues, hemoglobin induces macrophages (Mϕs) possessing ability to protect against tissue inflammation. Hemorrhage-appearing mucosa is observed in patients with inflammatory bowel disease. However, heme-mediated modulation of intestinal Mϕ activity remains poorly understood. Here, we provide evidence that Spi-C induced by heme is a key molecule for providing noninflammatory gene expression patterns of intestinal CX3CR1high Mϕs. We found that the Spic deficiency in intestinal Mϕs resulted in increased sensitivity to dextran sodium sulfate-induced colitis. Heme-mediated Spi-C inhibited a subset of LPS-induced genes such as Il6 and Il1a by intestinal CX3CR1high Mϕs through inhibition of IRF5-NF-κB p65 complex formation. These results reveal a mechanism modulating the noninflammatory phenotype of intestinal Mϕs and may help identify targets for therapy of intestinal inflammation.
Keywords: Spi-C, intestinal macrophage, inflammation, heme, IRF5
Abstract
The local environment is crucial for shaping the identities of tissue-resident macrophages (Mϕs). When hemorrhage occurs in damaged tissues, hemoglobin induces differentiation of anti-inflammatory Mϕs with reparative function. Mucosal bleeding is one of the pathological features of inflammatory bowel diseases. However, the heme-mediated mechanism modulating activation of intestinal innate immune cells remains poorly understood. Here, we show that heme regulates gut homeostasis through induction of Spi-C in intestinal CX3CR1high Mϕs. Intestinal CX3CR1high Mϕs highly expressed Spi-C in a heme-dependent manner, and myeloid lineage-specific Spic-deficient (Lyz2-cre; Spicflox/flox) mice showed severe intestinal inflammation with an increased number of Th17 cells during dextran sodium sulfate-induced colitis. Spi-C down-regulated the expression of a subset of Toll-like receptor (TLR)-inducible genes in intestinal CX3CR1high Mϕs to prevent colitis. LPS-induced production of IL-6 and IL-1α, but not IL-10 and TNF-α, by large intestinal Mϕs from Lyz2-cre; Spicflox/flox mice was markedly enhanced. The interaction of Spi-C with IRF5 was linked to disruption of the IRF5-NF-κB p65 complex formation, thereby abrogating recruitment of IRF5 and NF-κB p65 to the Il6 and Il1a promoters. Collectively, these results demonstrate that heme-mediated Spi-C is a key molecule for the noninflammatory signature of intestinal Mϕs by suppressing the induction of a subset of TLR-inducible genes through binding to IRF5.
Tissue-resident macrophages (Mϕs) play a key role in innate immune surveillance against tissue-invading pathogens. In addition, they have been characterized as anti-inflammatory Mϕs responsible for maintaining tissue homeostasis and resolving inflammatory responses (1). For example, intestinal resident CX3CR1high Mϕs derived from Ly6Chigh CCR2+ monocytes after birth (2, 3) mediate innate immune defense against intestinal microorganisms by exerting high phagocytic activity (4). To maintain mucosal tolerance, noninflammatory gene expression patterns in intestinal CX3CR1high Mϕs are precisely controlled through IL-10 receptor signaling-dependent mechanisms (5, 6). Intestinal CX3CR1high Mϕs in Il10ra-deficient mice displayed the elevated expression of Toll-like receptor (TLR)-dependent proinflammatory mediators such as Il12b, Il23a, and Nos2, while a subset of TLR-inducible genes such as Il6 and Tnf was unchanged (5). Accordingly, large intestinal CX3CR1high Mϕs from Il10−/− mice did not show increased IL-6 production (7). Thus, the activity of intestinal CX3CR1high Mϕs might be modulated by an unknown IL-10–independent mechanism.
In addition to precursor heterogeneity, local environmental factors are implicated in the diversity of tissue-resident Mϕs by inducing tissue-specific transcription factors as well as epigenetic modifications, leading to unique transcriptional profiles (8, 9). When hemorrhage occurs in damaged tissues, hemoglobin provides either an anti-inflammatory feature or a restorative function for infiltrating Mϕs through a scavenger receptor such as CD163 (10–15). Among the colonoscopic features, bleeding within the mucosa is commonly seen in patients with ulcerative colitis that is a form of inflammatory bowel disease (16). However, whether heme evokes the noninflammatory profiles in Mϕs, thereby contributing to mucosal healing, is unclear.
Spi-C, which belongs to the Spi subfamily of Ets transcription factors including PU.1, Spi-B, and Spi-D (17), was reported to regulate the differentiation of splenic red pulp Mϕs (RPMϕs) and F4/80+ VCAM+ bone marrow Mϕs (BMMϕs) (18, 19) and the development of B lymphocytes (20–22). In addition, erythrocyte-derived heme is a tissue factor that drives the expression of Spic during the development of RPMϕs and F4/80+ VCAM+ BMMϕs (19). However, the function of Spi-C in the regulation of innate immune responses remains poorly understood.
In this study, we identified the mechanism by which heme-mediated Spi-C negatively regulates a subset of TLR-inducible genes in intestinal Mϕs through disruption of IRF5-NF-κB p65 complex formation to provide noninflammatory profiles for intestinal Mϕs, thereby maintaining intestinal homeostasis.
Results
Expression of Spi-C in Intestinal CX3CR1high Mϕs.
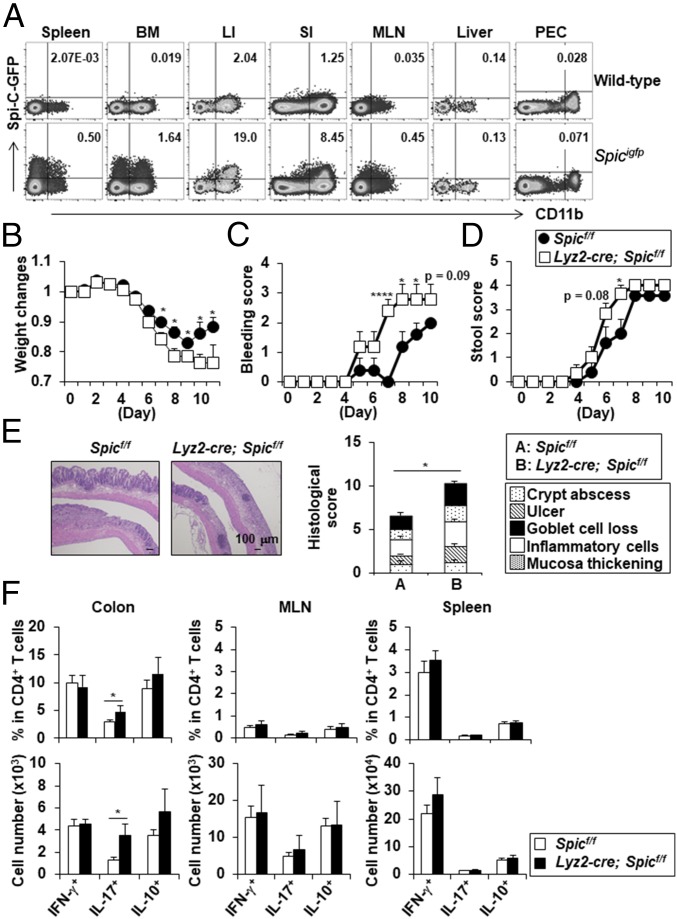
Unlike other tissue-resident Mϕs, transcription factors that regulate the differentiation and function of CX3CR1high Mϕs in the large intestine are poorly understood. Thus, to identify transcription factors specifically expressed in CX3CR1high Mϕs among large intestinal innate myeloid cells, we comprehensively analyzed gene expression profiles in CX3CR1high Mϕs, CX3CR1− CD11b+ CD11c+ cells, CD11b− CD11chigh DCs, and CD11b+ CD11c− cells (SI Appendix, Table S1). Among four subsets, CX3CR1high Mϕs highly expressed Spic. Spicigfp/igfp mice showed that a subpopulation of CD11b+ innate myeloid cells in the colon, small intestine, and mesenteric lymph nodes (MLN) expressed Spi-C, as did those in the spleen and bone marrow (19) (Fig. 1A). In the colon, Spi-C–expressing CD11b+ CD11c+/− cells highly expressed CX3CR1 in both a steady state and a dextran sodium sulfate (DSS)-induced inflammatory state (SI Appendix, Fig. S1), suggesting that Spi-C is selectively expressed in CX3CR1high Mϕs among large intestinal innate myeloid cells.
Fig. 1.
Lyz2-cre; Spicflox/flox mice showed exacerbated intestinal inflammation following DSS administration. (A) Flow cytometric plots of Spi-C-EGFP– and CD11b-expressing cells. The cells from the spleen, bone marrow (BM), large intestine (LI), small intestine (SI), mesenteric lymph nodes (MLN), liver, and peritoneal cavity (PEC) from wild-type and SpicIRES-GFP/GFP mice were stained with anti-CD45 antibody, anti-CD11b antibody, and 7-AAD. All data are representative of two independent experiments. (B−F) Spicflox/flox and Lyz2-cre; Spicflox/flox mice were administered with 1.5% DSS for 7 d. (B) Weight changes of Spicflox/flox (n = 9) and Lyz2-cre; Spicflox/flox (n = 8) mice. *P < 0.05. (C and D) Bleeding score (C) and stool score (D) of mice administered with 1.5% DSS for 10 d (n = 5 per group). *P < 0.05, ****P < 0.001. Mean ± SEM from two independent experiments are shown. (E) H&E staining and histopathological score of Spicflox/flox (n = 9) and Lyz2-cre; Spicflox/flox (n = 8) mice. *P < 0.05. (F) The frequency (Upper) and number (Bottom) of IFN-γ–, IL-17–, and IL-10–producing CD4+ T cells from Spicflox/flox (n = 7) and Lyz2-cre; Spicflox/flox (n = 4) mice. *P < 0.05. All graphs show mean ± SEM from two independent experiments.
Exacerbated Colitis in Myeloid Lineage Cell-Specific Spi-C–Deficient Mice.
To assess the physiological role of Spi-C in intestinal CX3CR1high Mϕs, myeloid lineage cell-specific Spi-C–deficient (Lyz2-cre; Spicflox/flox) mice were generated (SI Appendix, Fig. S2 A–C). The expression of Spic mRNA was markedly reduced in large intestinal CX3CR1high Mϕs in Lyz2-cre; Spicflox/flox mice compared with Spicflox/flox mice (SI Appendix, Fig. S2D). However, the frequency of CX3CR1high Mϕs was not altered in the large intestinal lamina propria (SI Appendix, Fig. S2E). In the steady state, there was no difference in IL-17–, IFN-γ–, and IL-10–producing CD4+ T cells as well as in Foxp3+ Treg cells in the colon, MLN, and spleen between Spicflox/flox and Lyz2-cre; Spicflox/flox mice (SI Appendix, Fig. S2 F and G). In the spleen of Lyz2-cre; Spicflox/flox mice, the number of RPMϕs was normal (SI Appendix, Fig. S3A). In addition, Spic expression was not reduced in RPMϕs isolated from Lyz2-cre; Spicflox/flox mice (SI Appendix, Fig. S3B). This might be due to the lower expression level of Lyz2 in RPMϕs than intestinal in CX3CR1high Mϕs (SI Appendix, Fig. S3C).
We investigated whether Spi-C in CX3CR1high Mϕs regulates intestinal inflammation by orally administering 1.5% DSS. Compared with Spicflox/flox mice, Lyz2-cre; Spicflox/flox mice showed greater weight loss, more profound bleeding in the stools, slightly increased pasty stools, and more severe intestinal pathologies (Fig. 1 B–E). In addition, the number of IL-17–producing CD4+ T cells was increased in the colon, but not in the MLN and spleen of Lyz2-cre; Spicflox/flox mice following DSS administration compared with Spicflox/flox mice (Fig. 1F). These findings indicate that Spi-C in intestinal CX3CR1high Mϕs plays an important role in the suppression of intestinal inflammation.
Attenuation of DSS-Induced Colitis by Heme Is Spi-C–Dependent.
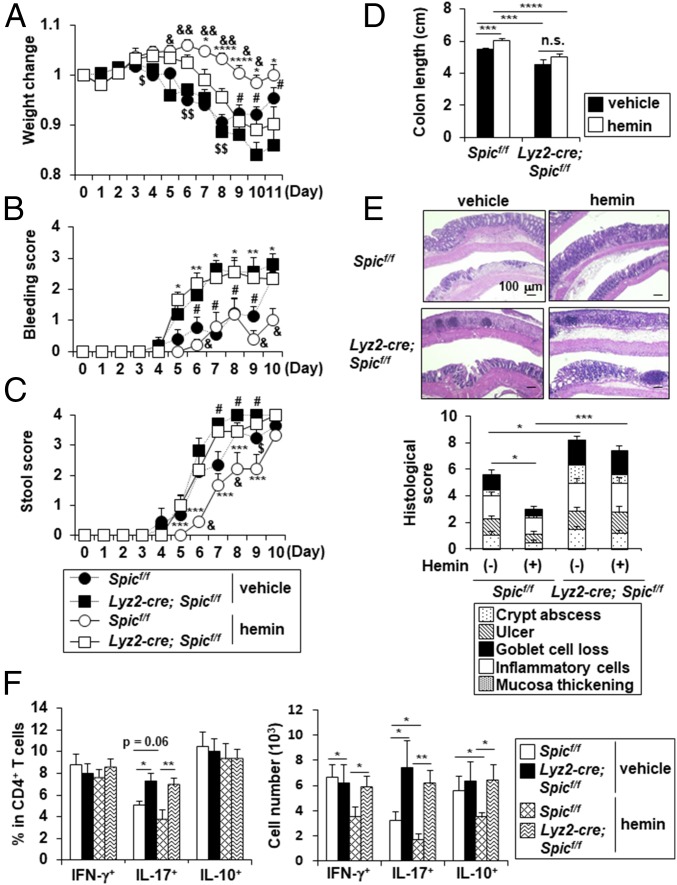
A previous study identified heme as an inducer of Spic expression in Mϕs (19). Heme-associated iron was present in the intestinal lamina propria as well as the spleen in both a steady state and a DSS-induced inflammatory condition (SI Appendix, Fig. S4A). In addition, hemoglobin and iron were detected in the intestinal lumen in the steady state, and their concentrations were increased after DSS administration (SI Appendix, Fig. S4B), thus indicating that intestinal Mϕs have a chance to be exposed to heme in the intestine. Therefore, we examined whether the induction of Spic expression in CX3CR1high Mϕs by heme mediates the suppression of intestinal inflammation. The i.p. injection of hemin (a Fe3+ oxidization product of heme) up-regulated Spic expression as well as iron/heme metabolism-related genes such as Hmox1, Slc40a1, and Blvrb in large intestinal CD11b+ CD11c+ cells (SI Appendix, Fig. S4C). Among CD11b+ innate myeloid cells in the large intestinal lamina propria of hemin-injected mice, CX3CR1high Mϕs most highly expressed Spic (SI Appendix, Fig. S4D). We next examined the therapeutic effect of heme on DSS-induced colitis in Spicflox/flox and Lyz2-cre; Spicflox/flox mice (Fig. 2). Without hemin pretreatment, Lyz2-cre; Spicflox/flox mice exhibited severe clinical parameters such as weight loss, stool bleeding, stool consistency, and colon shortening (Fig. 2 A–D), which was associated with worsened colonic histopathology (Fig. 2E), compared with those in Spicflox/flox mice. In Spicflox/flox mice, hemin treatment reduced all of the clinical parameters with a marked amelioration of intestinal pathology, as reported previously (23). In contrast, Lyz2-cre; Spicflox/flox mice suffered from profound weight loss with severe stool bleeding and shortening of the colon even after hemin treatment, although a partial suppression of the weight loss and amelioration of stool consistency were observed. Moreover, histological analysis did not show a hemin-mediated remediation of pathological changes in Lyz2-cre; Spicflox/flox mice. In accordance with the reduced severity of colitis in the colons of Spicflox/flox mice, but not of Lyz2-cre; Spicflox/flox mice, the number of colitogenic Th1 and Th17 cells after DSS administration was markedly decreased by hemin pretreatment (Fig. 2F). Without pretreatment with hemin, production of IL-6 and IL-17 in the colon was slightly increased in Lyz2-cre; Spicflox/flox mice compared with that in Spicflox/flox mice (SI Appendix, Fig. S5). In Spicflox/flox mice pretreated with hemin, production of proinflammatory cytokines including IL-6, IL-17, IFN-γ, and IL-1α in the colon was greatly reduced compared with that in untreated Spicflox/flox mice. In contrast, Lyz2-cre; Spicflox/flox mice did not show the hemin-mediated suppression of IL-17, IFN-γ, and IL-1α production in the colons, although a modest reduction of IL-6 production was induced. These findings suggest that Spi-C is required for the heme-dependent suppression of intestinal inflammation.
Fig. 2.
Minimal therapeutic effects of hemin on symptoms of DSS-induced colitis in Lyz2-cre; Spicflox/flox mice. Spicflox/flox and Lyz2-cre; Spicflox/flox mice intraperitoneally injected with 1 mg hemin or vehicle on days −2 and −1 of DSS administration and administered with 1.5% DSS for 7 d (n = 8–9 per group) (A, E, and F) or 11 d (n = 5–8 per group) (B–D). (A) Weight changes. *P < 0.05, ****P < 0.001, #P < 0.05, $P < 0.05, $$P < 0.01, &P < 0.05, &&P < 2 × 10−5. (B and C) Bleeding score (B) and stool score (C). *P < 0.05, **P < 0.01, ***P < 0.005, #P < 0.05, $P < 0.05, &P < 0.05, *P denotes significance between heme-treated control and Spic mutant mice, #P denotes significance between vehicle-injected control and Spic mutant mice, $P denotes significance between vehicle-injected and heme-injected Spic mutant mice, and &P denotes significance between vehicle-injected and heme-injected control mice (A–C). (D) Colon length. ***P < 0.005, ****P < 0.001. (E) Representative colon sections (Upper) and histological score (Bottom) of the colons. *P < 0.05, ***P < 0.005. (F) Frequency (Left) and number (Right) of IFN-γ–, IL-17–, and IL-10–producing CD4+ T cells. *P < 0.05, **P < 0.01.
Decreased Expression of a Subset of TLR-Dependent Genes by Heme-Inducible Spi-C.
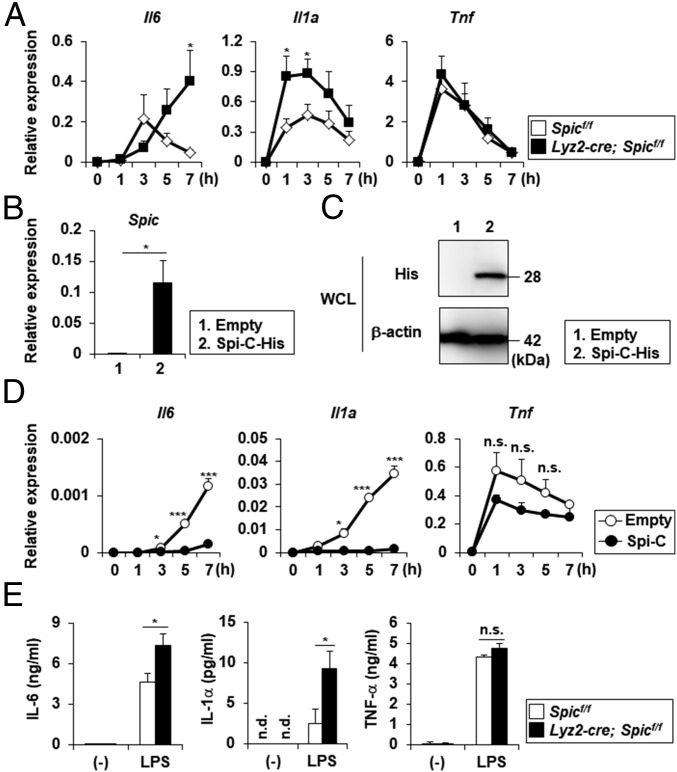
To determine how Spi-C controls the function of intestinal CX3CR1high Mϕs, we investigated the overall gene expression patterns in BMDMϕs prepared from Spicflox/flox and Lyz2-cre; Spicflox/flox mice. BMDMϕs were stimulated with or without LPS following hemin treatment and used for RNA-seq analysis. In Spic−/− BMDMϕs stimulated with LPS, 85 genes were up-regulated compared with LPS-stimulated Spicflox/flox BMDMϕs (SI Appendix, Table S2). We focused on proinflammatory cytokine genes such as Il6 and Il1a among these genes because they were reported to promote intestinal inflammation (24–28). To confirm the RNA-seq results, we analyzed the LPS-induced expression of Il6, Il1a, and Tnf in hemin-pretreated Mϕs derived from peripheral blood monocytes (PB-MO) isolated from Spicflox/flox and Lyz2-cre; Spicflox/flox mice using quantitative RT-PCR (Fig. 3A). The expression of Il6 and Il1a, but not Tnf, was markedly increased in Spic−/− PB-MOMϕs compared with control cells (Fig. 3A). To confirm the effects of Spi-C on LPS-inducible gene expression, we generated RAW264.7 cells stably expressing Spi-C (Fig. 3 B and C) and analyzed the expression of Il6, Il1a, and Tnf (Fig. 3D). Spi-C–expressing RAW264.7 cells showed a marked decrease in the expression of Il6 and Il1a, but not of Tnf, following LPS stimulation. In accordance with the mRNA expression patterns, the production of IL-6 and IL-1α, but not of TNF-α, by hemin-treated Spic−/− PB-MOMϕs in response to LPS was augmented compared with control cells (Fig. 3E). These findings indicate that the heme-mediated expression of Spi-C negatively regulates the expression of a subset of LPS-inducible genes.
Fig. 3.
Expression of a subset of LPS-inducible genes up-regulated in Spic−/− Mϕs. (A) PB-MOMϕs prepared from control and Spic mutant mice were stimulated with LPS following pretreatment with hemin for 18 h and analyzed for the expression of Il6, Il1a, and Tnf. Graphs show the mean ± SEM of three independent experiments. *P < 0.05. (B) Expression of Spic in RAW264.7 cells stably expressing Spi-C-His and control cells. Graphs show the mean ± SD. Data are representative of four independent experiments. *P < 0.05. (C) Western blot analysis with anti-His and anti–β-actin antibodies using Spi-C-His stably expressing RAW264.7 cells and control cells. Data are representative of two independent experiments. (D) RAW264.7 cells stably expressing Spi-C-His and control cells stimulated with LPS were analyzed for the expression of Il6, Il1a, and Tnf. Graphs show the mean ± SD. Data are representative of two independent experiments. *P < 0.05, ***P < 0.005. n.s., not significant. (E) Production of IL-6, IL-1α, and TNF-α in response to LPS by hemin-pretreated PB-MOMϕs. Graphs show the mean ± SEM from four independent experiments. *P < 0.05. n.d., not detected; n.s., not significant.
Enhanced Production of IL-6 and IL-1α by Spic−/− Intestinal CX3CR1high Mϕs.
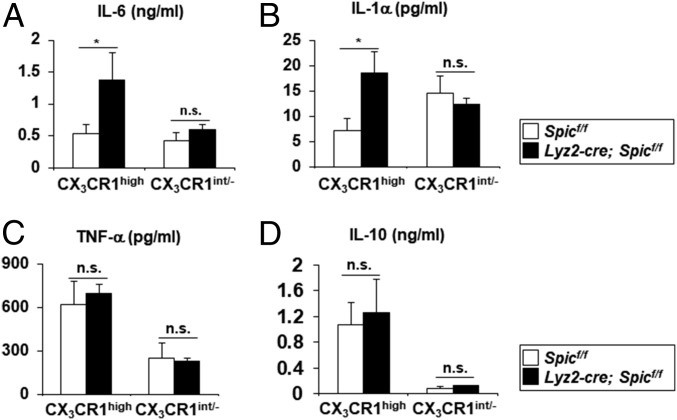
We analyzed the role of Spi-C in the regulation of TLR-induced proinflammatory cytokine production by intestinal myeloid cells. Among intestinal CD11b+ innate myeloid cells, CX3CR1high Mϕs and CX3CR1int/- cells from the large intestinal lamina propria were cultured in the presence of LPS and analyzed for the production of IL-6, IL-1α, TNF-α, and IL-10 (Fig. 4). The Spi-C deficiency in intestinal CX3CR1high Mϕs led to increased production of IL-6 and IL-1α, but not of TNF-α and IL-10, while there was no difference in the production of all cytokines by CX3CR1int/- cells from control or mutant mice, indicating that Spi-C suppresses a subset of TLR4-inducible genes including Il6 and Il1a in intestinal CX3CR1high Mϕs.
Fig. 4.
Increased production of IL-6 and IL-1α by intestinal CX3CR1high Mϕs of Lyz2-cre; Spicflox/flox mice. (A–D) LPS-induced production of IL-6 (A), IL-1α (B), TNF-α (C), and IL-10 (D) by large intestinal CX3CR1high Mϕs and CX3CR1intermediate (int)/- cells. Mean values ± SEM from four independent experiments are shown. n.s., not significant. *P < 0.05.
Dietary Iron Mediates the Induction of Spi-C in Intestinal Innate Myeloid Cells.
We next attempted to identify factors driving Spic expression in intestinal innate myeloid cells. Heme-mediated degradation of the transcription repressor Bach1 elicits the expression of Spic as well as Hmox1, Slc40a1, and Blvrb (19, 29). Previous studies have shown that a reduced intake of dietary iron decreased hemoglobin levels in vivo (30, 31). Thus, we examined the effect of dietary iron on Spic expression in large intestinal CX3CR1high Mϕs. C57BL/6J mice fed with an AIN93G (control) or iron-reduced (ΔFe) diet were analyzed for the expression of Spic in intestinal CX3CR1high Mϕs. Mice fed with the ΔFe diet had a lower concentration of blood hemoglobin compared with the control diet (SI Appendix, Fig. S6A). In addition, ΔFe-diet–fed mice exhibited a decreased concentration of iron in the serum, spleen, and colon (SI Appendix, Fig. S6B). In this context, expression of Spic, Hmox1, Slc40a1, and Blvrb was reduced in CX3CR1high Mϕs isolated from the colon of mice given the ΔFe diet (SI Appendix, Fig. S6C). Bacterial composition of feces analyzed by 16S rRNA gene sequencing was not altered at the phylum level between mice fed with the AIN93G and ΔFe diet, although a minor Proteobacteria population was slightly increased in the iron deficiency (SI Appendix, Fig. S6D). Thus, the iron deficiency did not alter the bacterial composition at the phylum level in the colon, indicating that the reduced Spic expression in the ΔFe-diet–fed mice was not induced by the altered composition of microbiota. To assess the impact of the decline in iron/hemoglobin levels by the ΔFe diet on the TLR-dependent production of proinflammatory cytokines by intestinal CX3CR1high Mϕs, we compared the expression of Il6 and Tnf in CX3CR1high Mϕs stimulated with or without LPS (SI Appendix, Fig. S6 E–G). In large intestinal CX3CR1high Mϕs from ΔFe-diet–fed mice, the LPS-induced expression of Il6, but not of Tnf, was markedly increased (SI Appendix, Fig. S6E). Accordingly, IL-6 production in response to LPS by CX3CR1high Mϕs was enhanced in mice fed with the ΔFe diet compared with the control diet, whereas TNF-α was normally produced (SI Appendix, Fig. S6F). In contrast, expression of Il6 was not increased by the ΔFe diet in Lyz2-cre; Spicflox/flox mice (SI Appendix, Fig. S6G). Administration of hemin reduced Il6 expression in CX3CR1high Mϕs of ΔFe-diet–fed Spicflox/flox mice, but not Lyz2-cre; Spicflox/flox mice (SI Appendix, Fig. S6G). Thus, the reduced Spic expression caused by the iron deficiency is associated with the enhanced Il6 expression in CX3CR1high Mϕs.
Spi-C–Mediated Repression of a Subset of LPS-Inducible Genes by Association with IRF5.
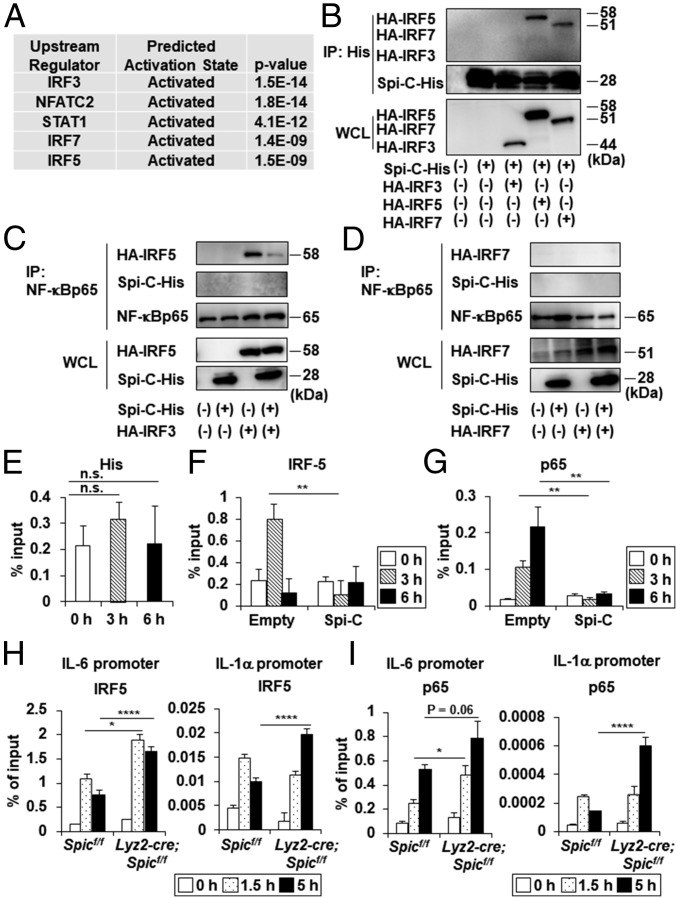
Because dietary iron-mediated Spi-C expression is implicated in the down-regulation of Il6 expression in CX3CR1high Mϕs, we examined how Spi-C impacts on the transcriptional initiation of its target genes in Mϕs. Ingenuity Pathway Analysis displayed the upstream regulators of 85 genes up-regulated in Spic−/− BMMϕs (SI Appendix, Table S2). Among the top five predicted upstream regulators, the IRF family members such as IRF3, IRF7, and IRF5 were included (Fig. 5A). Therefore, we analyzed the association of Spi-C with IRF3, IRF5, and IRF7 by a coimmunoprecipitation assay (Fig. 5B). Among the three molecules, Spi-C interacted with IRF5 and IRF7, but not IRF3. A recent study demonstrated that IRF5-NF-κB p65 complex formation is indispensable for driving the expression of a subset of LPS-inducible genes (32). Therefore, we examined whether Spi-C altered the association of IRF5 and NF-κB p65 (Fig. 5C). NF-κB p65 bound to IRF5 as previously reported (32), but not to Spi-C. However, in the presence of Spi-C, formation of the IRF5-NF-κB p65 complex was markedly reduced. In contrast to IRF5, IRF7 did not bind to NF-κB p65 (Fig. 5D). IRF5 recruitment to the NF-κB–binding site in the promoter of its target genes is guided by NF-κB p65 (32). Therefore, to investigate the effects of the Spi-C–mediated disruption of IRF5-NF-κB p65 complex formation on transcriptional initiation of Il6 in response to LPS, we analyzed the recruitment of NF-κB p65 and IRF5 to the NF-κB site of the Il6 promoter in the RAW264.7 cells stably expressing Spi-C-His used in Figs. 3 and 5 E–G. Chromatin immunoprecipitation assay showed that Spi-C did not bind to the Il6 promoter even after LPS stimulation (Fig. 5E). In control cells, the LPS-dependent recruitment of IRF5 and NF-κB p65 was observed at the NF-κB site of the Il6 promoter (Fig. 5 F and G). However, neither IRF5 nor NF-κB p65 was recruited to the Il6 promoter of Spi-C–expressing RAW264.7 cells (Fig. 5 F and G). We analyzed the LPS-induced recruitment of IRF5 and NF-κB p65 to the promoters of Spi-C target genes in Spic−/− BMDMϕ (Fig. 5 H and I). BMDMϕ prepared from Spicflox/flox and Lyz2-cre; Spicflox/flox mice was pretreated with hemin for 18 h and stimulated with LPS. In Spic−/− BMMϕ, the recruitment of IRF5 to the Il6 promoter was increased after 1.5 and 5 h of LPS stimulation compared with control cells (Fig. 5H). In addition, elevated IRF5 recruitment to the Il1a promoter was observed 5 h after LPS stimulation in Spic−/− cells (Fig. 5H), and significantly enhanced NF-κB p65 recruitment to the promoters of Il6 and Il1a was found in Spic−/− BMMϕ relative to that found in control cells (Fig. 5I). Furthermore, the recruitment of IRF5 to the promoters of Il6 and Il1a, but not of Tnf, in large intestinal CX3CR1high Mϕs was increased in Lyz2-cre; Spicflox/flox mice relative to that in Spicflox/flox mice during DSS-induced colitis (SI Appendix, Fig. S7). These findings suggest that Spi-C suppresses the expression of a subset of LPS-inducible genes such as Il6 and Il1a by disrupting formation of the IRF5-NF-κB p65 complex through direct binding to IRF5.
Fig. 5.
Spi-C suppresses the expression of a subset of TLR4-dependent genes by interacting with IRF5. (A) Enrichment analysis of the upstream transcriptional regulators of genes elevated in Spic−/− BMDMϕs. (B–D) Coimmunoprecipitation (IP) assay with anti-His (B) and anti–NF-κB p65 (C and D) antibodies for immunoprecipitation and the indicated antibodies for immunoblotting. (E–G) ChIP assay for the Il6 promoter of the NF-κB–binding site was performed with anti-His (E), anti-IRF5 (F), and anti–NF-κB p65 (G) antibodies. All graphs show the mean ± SD. Data are representative of two independent experiments. **P < 0.01. n.s., not significant. (H and I) ChIP assay for the NF-κB site of the Il6 promoter and Il1a promoter was performed with anti-IRF5 (H) and anti–NF-κB p65 (I) antibodies using control and Spic−/− BMDMϕs stimulated with LPS following pretreatment with hemin for 18 h. Graphs show the means ± SD. Data are representative of two independent experiments. *P < 0.05, ****P < 0.001.
Discussion
In this study, we showed that induction of Spi-C by the dietary iron-mediated heme in intestinal CX3CR1high Mϕs negatively regulates the transcription of a subset of TLR-inducible genes through IRF5 binding and subsequent disruption of IRF5-NF-κB p65 complex formation, leading to the prevention of intestinal inflammation.
We demonstrated that Spi-C is expressed in CX3CR1high Mϕs residing in the large intestine and small intestine. Although a previous study reported that a lack of Spic resulted in the absence of splenic RPMϕs (18), intestinal CX3CR1high Mϕs developed normally in Lyz2-cre; Spicflox/flox mice. Thus, it would be important to determine if the reason for this discrepancy is associated with either a differential expression level of Spic or distinct progenitors between RPMϕs and intestinal CX3CR1high Mϕs in the future.
In the colon of hemin-injected mice, Spic expression was increased in CX3CR1high Mϕ compared with that in Ly6C+ monocytes, which give rise to CX3CR1high Mϕs in the intestinal lamina propria, indicating that the induction of Spi-C in CX3CR1high Mϕs by hemin might take place locally in the intestine. Intestinal lamina propria CX3CR1high Mϕs extend their dendrites into the lumen, where hemoglobin and iron are present even in the steady state. In addition, CX3CR1high Mϕs express CD163 (33, 34), which scavenges hemoglobin (35). Therefore, endocytosis of the luminal hemoglobin-haptoglobin complex through CD163 may induce the expression of Spic in intestinal CX3CR1high Mϕs. In the current study, we verified that systemic heme supplementation greatly remedied DSS-induced colitis in Spicflox/flox mice and suppressed Il6 expression in CX3CR1high Mϕs in ΔFe-diet–fed mice. In Lyz2-cre; Spicflox/flox mice, hemin treatment partially prevented severe weight loss during the early phase of DSS-induced colitis and only modestly suppressed expression of Il6 in ΔFe-diet–fed Lyz2-cre; Spicflox/flox mice. Therefore, systemic heme repletion might abrogate intestinal inflammation in at least two ways: a Spi-C–dependent mechanism and a heme oxynase-1–dependent mechanism as previously reported (23).
Previous studies have defined that the hemoglobin-mediated modulation of Mϕ phenotypes is implicated in reduction of tissue injury in the brain (10–12, 36). Mucosal bleeding is a common symptom of inflammatory bowel diseases such as ulcerative colitis. Therefore, it would be interesting to analyze whether induction of SPI-C in human intestinal Mϕs by local hemolysis is implicated in their acquisition of noninflammatory features, thereby associating them with mucosal healing.
The LPS-mediated recruitment of IRF5 to the NF-κB–binding site in the promoters of its target genes was aided by NF-κB p65 (32). However, whether IRF5 mediates the persistent occupancy of NF-κB p65 in these promoters is unclear. In the present study, we observed a reduced recruitment of NF-κB p65 as well as of IRF5 to the Il6 promoter in Spi-C stably expressing RAW264.7 cells stimulated with LPS. In addition, the recruitment of NF-κB p65 and IRF5 to the promoters of Il6 and Il1a was enhanced in Spic−/− BMMϕs. These findings suggest that IRF5 may regulate NF-κB p65 occupancy at the promoters of its target genes. Therefore, it is important to analyze whether the LPS-induced recruitment of NF-κB p65 to the promoters of IRF5 target genes is altered in Irf5−/− Mϕs. TNF-α is an IRF5-dependent gene as are IL-6 and IL-1α (32). However, the IRF5 recruitment to the Tnf promoter remained normal in Spic−/− large intestinal CX3CR1high Mϕs, suggesting that proper IRF5 occupancy at the Tnf promoter is controlled by a Spi-C–independent mechanism. Therefore, it would be important to determine why Spi-C is selectively required for modulation of a subset of IRF5-dependent genes in the future.
Collectively, our results indicate that heme-induced Spi-C in intestinal Mϕs regulates the transcriptional initiation of a subset of TLR4-inducible genes including Il6 and Il1a to attenuate intestinal inflammation. Previous studies demonstrated that treatment with neutralizing IL-6 mAbs improved DSS-induced colitis (37, 38), whereas Il6−/− mice administrated with DSS showed severe intestinal pathology with reduced epithelial cell proliferation (39). Therefore, it appears that the IL-6–signaling pathway contributes to the pathogenesis of intestinal inflammation while exerting its homeostatic function by regulating intestinal epithelial integrity. In addition to IL-6, some of the Spi-C target genes such as IL-1α (26–28), TREM-1 (40), CD38 (41), and CXCL11 (42) have been reported to mediate intestinal inflammation. Thus, the development of the method that controls Spi-C expression in human intestinal Mϕs may represent a therapeutic approach for inflammatory bowel diseases.
Materials and Methods
Detailed information on the materials, methods, and associated references can be found in SI Appendix, SI Materials and Methods.
Mice.
C57BL/6J mice were purchased from Japan SLC. Spicigfp/igfp mice were generated as described previously (19). All of the mice were maintained under specific pathogen-free conditions. All animal experiments were conducted in accordance with the guidelines of the Animal Care and Use Committee of Osaka University.
ChIP Assay.
BMDMϕs stimulated with 100 ng/mL LPS for the indicated periods following pretreatment with 40 μM hemin for 18 h and intestinal CX3CR1high Mϕs were used for ChIP assay according to a previously described protocol (43). Il6- and Il1a-specific primers were designed to include the NF-κB–binding site.
Statistical Analysis.
Differences between the control and experimental groups were evaluated by Student’s t test. Differences where P < 0.05 were considered statistically significant.
Supplementary Material
Acknowledgments
We thank T. Kondo and Y. Magota for their technical assistance, C. Hidaka for secretarial assistance, and J. L. Croxford, PhD (Edanz Group: www.edanzediting.com/ac) for editing a draft of this manuscript. This work was supported by grants from the Ministry of Education, Culture, Sports, Science and Technology of Japan; the Japan Agency for Medical Research and Development (K.T.); a Grant-in-Aid for Young Scientists (B) (26860330); the Takeda Science Foundation; the Mochida Memorial Foundation for Medical and Pharmaceutical Research; the Japan Intractable Diseases (Nanbyo) Research Foundation; and the Astellas Foundation for Research on Metabolic Disorders (H.K.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession nos. GSE100804 and GSE100805).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1808426115/-/DCSupplemental.
References
- 1.Davies LC, Jenkins SJ, Allen JE, Taylor PR. Tissue-resident macrophages. Nat Immunol. 2013;14:986–995. doi: 10.1038/ni.2705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bain CC, et al. Constant replenishment from circulating monocytes maintains the macrophage pool in the intestine of adult mice. Nat Immunol. 2014;15:929–937. doi: 10.1038/ni.2967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yona S, et al. Fate mapping reveals origins and dynamics of monocytes and tissue macrophages under homeostasis. Immunity. 2013;38:79–91. doi: 10.1016/j.immuni.2012.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bain CC, et al. Resident and pro-inflammatory macrophages in the colon represent alternative context-dependent fates of the same Ly6Chi monocyte precursors. Mucosal Immunol. 2013;6:498–510. doi: 10.1038/mi.2012.89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zigmond E, et al. Macrophage-restricted interleukin-10 receptor deficiency, but not IL-10 deficiency, causes severe spontaneous colitis. Immunity. 2014;40:720–733. doi: 10.1016/j.immuni.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 6.Shouval DS, et al. Interleukin-10 receptor signaling in innate immune cells regulates mucosal immune tolerance and anti-inflammatory macrophage function. Immunity. 2014;40:706–719. doi: 10.1016/j.immuni.2014.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rivollier A, He J, Kole A, Valatas V, Kelsall BL. Inflammation switches the differentiation program of Ly6Chi monocytes from antiinflammatory macrophages to inflammatory dendritic cells in the colon. J Exp Med. 2012;209:139–155. doi: 10.1084/jem.20101387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lavin Y, Mortha A, Rahman A, Merad M. Regulation of macrophage development and function in peripheral tissues. Nat Rev Immunol. 2015;15:731–744. doi: 10.1038/nri3920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Amit I, Winter DR, Jung S. The role of the local environment and epigenetics in shaping macrophage identity and their effect on tissue homeostasis. Nat Immunol. 2016;17:18–25. doi: 10.1038/ni.3325. [DOI] [PubMed] [Google Scholar]
- 10.Chang CF, et al. Erythrocyte efferocytosis modulates macrophages towards recovery after intracerebral hemorrhage. J Clin Invest. 2018;128:607–624. doi: 10.1172/JCI95612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Min H, Jang YH, Cho IH, Yu SW, Lee SJ. Alternatively activated brain-infiltrating macrophages facilitate recovery from collagenase-induced intracerebral hemorrhage. Mol Brain. 2016;9:42. doi: 10.1186/s13041-016-0225-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gliem M, Schwaninger M, Jander S. Protective features of peripheral monocytes/macrophages in stroke. Biochim Biophys Acta. 2016;1862:329–338. doi: 10.1016/j.bbadis.2015.11.004. [DOI] [PubMed] [Google Scholar]
- 13.Boyle JJ, et al. Coronary intraplaque hemorrhage evokes a novel atheroprotective macrophage phenotype. Am J Pathol. 2009;174:1097–1108. doi: 10.2353/ajpath.2009.080431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Angele MK, et al. Hemorrhage decreases macrophage inflammatory protein 2 and interleukin-6 release: A possible mechanism for increased wound infection. Ann Surg. 1999;229:651–660; discussion 660–651. doi: 10.1097/00000658-199905000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Garton T, Keep RF, Hua Y, Xi G. CD163, a hemoglobin/haptoglobin scavenger receptor, after intracerebral hemorrhage: Functions in microglia/macrophages versus neurons. Transl Stroke Res. 2017;8:612–616. doi: 10.1007/s12975-017-0535-5. [DOI] [PubMed] [Google Scholar]
- 16.Paine ER. Colonoscopic evaluation in ulcerative colitis. Gastroenterol Rep (Oxf) 2014;2:161–168. doi: 10.1093/gastro/gou028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Carlsson R, Hjalmarsson A, Liberg D, Persson C, Leanderson T. Genomic structure of mouse SPI-C and genomic structure and expression pattern of human SPI-C. Gene. 2002;299:271–278. doi: 10.1016/s0378-1119(02)01078-8. [DOI] [PubMed] [Google Scholar]
- 18.Kohyama M, et al. Role for Spi-C in the development of red pulp macrophages and splenic iron homeostasis. Nature. 2009;457:318–321. doi: 10.1038/nature07472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Haldar M, et al. Heme-mediated SPI-C induction promotes monocyte differentiation into iron-recycling macrophages. Cell. 2014;156:1223–1234. doi: 10.1016/j.cell.2014.01.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bemark M, Mårtensson A, Liberg D, Leanderson T. Spi-C, a novel Ets protein that is temporally regulated during B lymphocyte development. J Biol Chem. 1999;274:10259–10267. doi: 10.1074/jbc.274.15.10259. [DOI] [PubMed] [Google Scholar]
- 21.Schweitzer BL, et al. Spi-C has opposing effects to PU.1 on gene expression in progenitor B cells. J Immunol. 2006;177:2195–2207. doi: 10.4049/jimmunol.177.4.2195. [DOI] [PubMed] [Google Scholar]
- 22.Li SK, Solomon LA, Fulkerson PC, DeKoter RP. Identification of a negative regulatory role for Spi-C in the murine B cell lineage. J Immunol. 2015;194:3798–3807. doi: 10.4049/jimmunol.1402432. [DOI] [PubMed] [Google Scholar]
- 23.Zhang L, et al. Heme oxygenase-1 ameliorates dextran sulfate sodium-induced acute murine colitis by regulating Th17/Treg cell balance. J Biol Chem. 2014;289:26847–26858. doi: 10.1074/jbc.M114.590554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Neurath MF. New targets for mucosal healing and therapy in inflammatory bowel diseases. Mucosal Immunol. 2014;7:6–19. doi: 10.1038/mi.2013.73. [DOI] [PubMed] [Google Scholar]
- 25.Neurath MF. Cytokines in inflammatory bowel disease. Nat Rev Immunol. 2014;14:329–342. doi: 10.1038/nri3661. [DOI] [PubMed] [Google Scholar]
- 26.Malik A, et al. IL-33 regulates the IgA-microbiota axis to restrain IL-1α-dependent colitis and tumorigenesis. J Clin Invest. 2016;126:4469–4481. doi: 10.1172/JCI88625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Scarpa M, et al. The epithelial danger signal IL-1α is a potent activator of fibroblasts and reactivator of intestinal inflammation. Am J Pathol. 2015;185:1624–1637. doi: 10.1016/j.ajpath.2015.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bersudsky M, et al. Non-redundant properties of IL-1α and IL-1β during acute colon inflammation in mice. Gut. 2014;63:598–609. doi: 10.1136/gutjnl-2012-303329. [DOI] [PubMed] [Google Scholar]
- 29.So AY, et al. Regulation of APC development, immune response, and autoimmunity by Bach1/HO-1 pathway in mice. Blood. 2012;120:2428–2437. doi: 10.1182/blood-2012-04-426247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bonaccorsi-Riani E, et al. Iron deficiency impairs intra-hepatic lymphocyte mediated immune response. PLoS One. 2015;10:e0136106. doi: 10.1371/journal.pone.0136106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Márquez-Ibarra A, et al. The effects of dietary iron and capsaicin on hemoglobin, blood glucose, insulin tolerance, cholesterol, and triglycerides, in healthy and diabetic wistar rats. PLoS One. 2016;11:e0152625. doi: 10.1371/journal.pone.0152625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Saliba DG, et al. IRF5:RelA interaction targets inflammatory genes in macrophages. Cell Rep. 2014;8:1308–1317. doi: 10.1016/j.celrep.2014.07.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Krause P, et al. IL-10-producing intestinal macrophages prevent excessive antibacterial innate immunity by limiting IL-23 synthesis. Nat Commun. 2015;6:7055. doi: 10.1038/ncomms8055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zigmond E, et al. Ly6C hi monocytes in the inflamed colon give rise to proinflammatory effector cells and migratory antigen-presenting cells. Immunity. 2012;37:1076–1090. doi: 10.1016/j.immuni.2012.08.026. [DOI] [PubMed] [Google Scholar]
- 35.Kristiansen M, et al. Identification of the haemoglobin scavenger receptor. Nature. 2001;409:198–201. doi: 10.1038/35051594. [DOI] [PubMed] [Google Scholar]
- 36.Finn AV, et al. Hemoglobin directs macrophage differentiation and prevents foam cell formation in human atherosclerotic plaques. J Am Coll Cardiol. 2012;59:166–177. doi: 10.1016/j.jacc.2011.10.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sommer J, et al. Interleukin-6, but not the interleukin-6 receptor plays a role in recovery from dextran sodium sulfate-induced colitis. Int J Mol Med. 2014;34:651–660. doi: 10.3892/ijmm.2014.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xiao YT, Yan WH, Cao Y, Yan JK, Cai W. Neutralization of IL-6 and TNF-α ameliorates intestinal permeability in DSS-induced colitis. Cytokine. 2016;83:189–192. doi: 10.1016/j.cyto.2016.04.012. [DOI] [PubMed] [Google Scholar]
- 39.Grivennikov S, et al. IL-6 and Stat3 are required for survival of intestinal epithelial cells and development of colitis-associated cancer. Cancer Cell. 2009;15:103–113. doi: 10.1016/j.ccr.2009.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Schenk M, Bouchon A, Seibold F, Mueller C. TREM-1–expressing intestinal macrophages crucially amplify chronic inflammation in experimental colitis and inflammatory bowel diseases. J Clin Invest. 2007;117:3097–3106. doi: 10.1172/JCI30602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Schneider M, et al. CD38 is expressed on inflammatory cells of the intestine and promotes intestinal inflammation. PLoS One. 2015;10:e0126007. doi: 10.1371/journal.pone.0126007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Liu Z, et al. Chemokine CXCL11 links microbial stimuli to intestinal inflammation. Clin Exp Immunol. 2011;164:396–406. doi: 10.1111/j.1365-2249.2011.04382.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kitada S, et al. BATF2 inhibits immunopathological Th17 responses by suppressing Il23a expression during Trypanosoma cruzi infection. J Exp Med. 2017;214:1313–1331. doi: 10.1084/jem.20161076. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.