Significance
In mammals, each embryo forms both male and female reproductive tract progenitor tissues. Anti-Müllerian hormone (AMH) secreted by fetal testes acts on mesenchyme cells adjacent to Müllerian duct (MD) epithelium, the progenitor tissue of female reproductive tract, to induce MD epithelial regression. While AMH and early AMH signaling components are elucidated, downstream gene networks directing this process are largely unknown. A global nonbiased approach using whole-transcriptome sequencing of fetal MD mesenchymal cells identified 82 factors as potential target genes of AMH including Osterix (Osx). Our findings provide in vivo evidence that Osx is an AMH-induced gene that regulates MD regression. Identification of Osx may provide key insights into gene-regulatory networks underlying MD regression, male sex differentiation, and mesenchyme–epithelial interactions.
Keywords: Osterix, Müllerian duct regression, reproductive tract development, anti-Müllerian hormone, sex differentiation
Abstract
In mammals, the developing reproductive tract primordium of male and female fetuses consists of the Wolffian duct and the Müllerian duct (MD), two epithelial tube pairs surrounded by mesenchyme. During male development, mesenchyme–epithelia interactions mediate MD regression to prevent its development into a uterus, oviduct, and upper vagina. It is well established that transforming growth factor-β family member anti-Müllerian hormone (AMH) secreted from the fetal testis and its type 1 and 2 receptors expressed in MD mesenchyme regulate MD regression. However, little is known about the molecular network regulating downstream actions of AMH signaling. To identify potential AMH-induced genes and regulatory networks controlling MD regression in a global nonbiased manner, we examined transcriptome differences in MD mesenchyme between males (AMH signaling on) and females (AMH signaling off) by RNA-seq analysis of purified fetal MD mesenchymal cells. This analysis found 82 genes up-regulated in males during MD regression and identified Osterix (Osx)/Sp7, a key transcriptional regulator of osteoblast differentiation and bone formation, as a downstream effector of AMH signaling during MD regression. Osx/OSX was expressed in a male-specific pattern in MD mesenchyme during MD regression. OSX expression was lost in mutant males without AMH signaling. In addition, transgenic mice ectopically expressing human AMH in females induced a male pattern of Osx expression. Together, these results indicate that AMH signaling is necessary and sufficient for Osx expression in the MD mesenchyme. In addition, MD regression was delayed in Osx-null males, identifying Osx as a factor that regulates MD regression.
In mammals, the Wolffian ducts (WDs) differentiate into the male epididymides, vas deferens, and seminal vesicles, whereas the Müllerian ducts (MDs) develop into the female oviducts, uterus, and upper vagina. Reproductive tract differentiation in amniotes is unique because initially both WD and MD are generated in the embryo independent of genetic sex. Sex-specific signaling results in loss of the WD in females and regression of the MD in males (1, 2). MD regression requires transforming growth factor-β family member anti-Müllerian hormone (AMH) secreted from the Sertoli cells of the fetal testis and its type 1 and 2 receptors present in MD mesenchyme (3–7). Following AMH binding, AMH type 2 receptor (AMHR2) recruits a type 1 receptor into a heteromeric complex. Within the complex, AMHR2 transphosphorylates and activates the type 1 receptor kinase. This activation results in the phosphorylation of an R-Smad and formation of an R-SMAD/SMAD-4 complex that translocates into the nucleus to transcriptionally activate AMH signaling pathway target genes. AMH type 1 receptors ACVR1 and BMPR1A, and AMH R-Smad effectors (SMAD1, SMAD5, and SMAD8) function redundantly for MD regression and are shared with the bone morphogenetic protein (BMP) pathway (8, 9).
While the upstream components (AMH, type 1 and 2 receptors, and R-Smads) of AMH signaling are well known, the downstream transcriptional effectors of AMH signaling are still largely unidentified. To date, only the WNT pathway effector β-catenin (Ctnnb1) has been shown to be required for AMH-induced MD regression in vivo (10). The requirement of Ctnnb1 for MD regression suggests WNT signaling is important for the downstream actions of AMH during MD regression in males. However, candidate gene approaches have failed to identify an individual WNT, WNT effector, or WNT regulator whose in vivo loss blocks MD regression (10–12).
Because of the limited success of candidate gene approaches in uncovering AMH signaling effectors, in the current study we undertook a nonbiased global approach using next-generation transcriptome sequencing. To elucidate potential gene networks and novel AMH signaling targets, we used RNA-seq analysis of yellow fluorescent protein (YFP)-positive MD mesenchymal cells flow sorted from embryonic day 14.5 (E14.5) Amhr2Cre/+; R26Ryfp/+ reproductive tracts to identify transcriptome differences between males and females during regression. This analysis identified Osterix (Osx)/Sp7 as a downstream effector of AMH signaling during MD regression. Our results indicate that AMH signaling is necessary and sufficient for Osx expression in the MD mesenchyme and Osx regulates the timing of MD regression.
Results
Transcriptome Analysis Identifies Candidate Genes Up-Regulated in Male MD Mesenchyme During Regression.
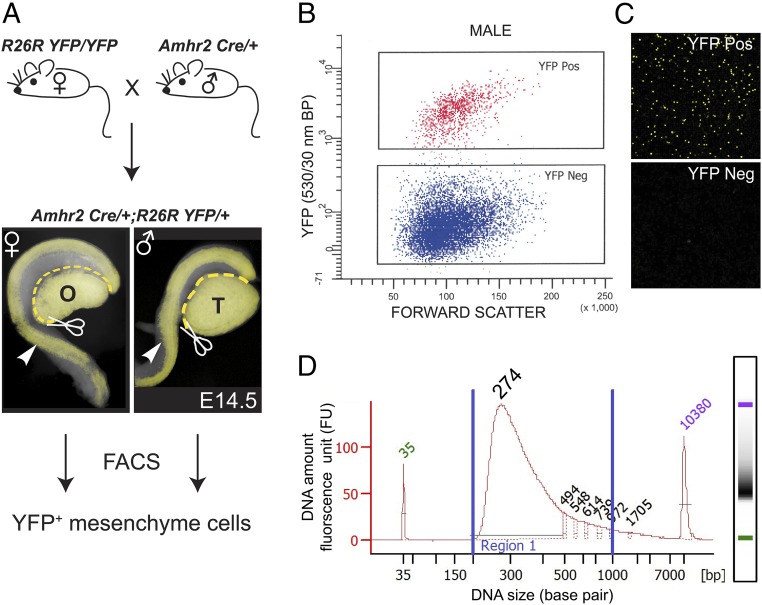
During embryogenesis, Amhr2 is expressed in the MD mesenchyme of male and female fetuses, but Amh is only expressed in males. Thus, the AMH signaling pathway is only active in male fetuses. To isolate male and female MD mesenchymal cells, Amhr2-Cre mice, which express Cre recombinase in the MD mesenchyme and somatic cells of the gonad, were bred to R26R-YFP reporter mice (SI Appendix, Fig. S1). E14.5 was chosen for this analysis because AMH has been expressed for 2 d in males, there are changes in the MD mesenchyme and MD epithelium, but no breaks or gaps in the MD have occurred. Mice carrying the Cre and YFP alleles were identified by fluorescence, and sex was determined by gross morphology of the gonad using a dissecting microscope. The mesonephros/gonad complex was isolated, and the gonads were removed. Two or more mesonephroi were pooled and digested in trypsin, and the resulting single-cell suspensions were then sorted for YFP+ cells using FACS (Fig. 1 A–C). cDNA libraries were generated from three biological replicates each for male and female. Indexed libraries were pooled and sequenced on the Illumina HiSeq 2000 platform (Fig. 1D). Approximately 20 million paired-end 76-bp reads were obtained for each library, of which ∼70% mapped to at least one location in the mouse reference genome build NCBIM37. Mapped mouse RNA-seq data were then subjected to differential expression analysis, using Cufflinks (13) and DESeq (14). The list of differentially expressed genes was generated based on a false discovery rate of <0.05 and a logtwofold change of ≥2. Expression levels (mean of counts normalized to sequencing depth) of differentially up-regulated genes in male and female samples are listed in SI Appendix, Table S1. Genes with significant differential expression were then further parsed by removing genes with expression levels below those for Amh in males, a gene known to be expressed at low levels in MD mesenchyme (mean counts, <195; SI Appendix, Table S1) (15). These analyses revealed 82 genes that were significantly up-regulated in males versus females during MD regression (Table 1). Ingenuity Pathway Analysis (IPA) predicted both BMP2 and BMP4 as upstream activators (SI Appendix, Fig. S2). This is consistent with activation by the AMH signaling pathway because it shares components of the BMP signaling pathway, including type 1 receptors and R-SMADs. In the IPA analysis, we identified the BMP-induced gene, Osx, as a male-up-regulated gene (SI Appendix, Fig. S3). Our focus on Osx was bolstered because it was expressed in the MD of male but not female mouse fetuses (https://www.gudmap.org/). This suggested Osx as a potential downstream effector of AMH signaling and led us to analyze Osx expression and function during MD regression.
Fig. 1.
A transcriptome screen for male-specific genes expressed in Amhr2-expressing Müllerian duct (MD) mesenchyme. (A) Breeding scheme to isolate Amhr2-marked MD mesenchyme cells from E14.5 male and female embryos. The Amhr2-Cre allele is expressed in MD mesenchyme cells and somatic cells of the gonad. Mesonephroi containing the MD (arrowheads) and WDs were isolated by removing the gonads. Subsequently, YFP+ MD mesenchyme cells were isolated by FACS. (B) Representative plot forward scatter versus YFP emission (530/30-nm bandpass filter) from five male Amhr2Cre/+; R26RYFP/+ mesenophros pairs to isolate YFP+ cells. (C) Representative confocal image of sorted cell fractions. (D) High-sensitivity DNA chip analysis of index pooled cDNA libraries for RNA-seq. O, ovary; T, testis. (Magnification: A, 40×; C, 200×.)
Table 1.
Genes up-regulated in male MD mesenchyme
| Gene | Logtwofold change | Gene | Logtwofold change | Gene | Logtwofold change | Gene | Logtwofold change | Gene | Logtwofold change | Gene | Logtwofold change |
| Ankrd63 | 8.92 | Slc22a3 | 5.24 | Dbc1 | 4.14 | Smad6 | 3.56 | Trim9 | 2.98 | Col9a3 | 2.67 |
| Wif1 | 8.41 | Ednrb | 5.10 | Ntsr1 | 4.08 | P4ha3 | 3.43 | Fam181b | 2.96 | Nog | 2.57 |
| Syt1 | 7.57 | Syt6 | 5.09 | Enpp1 | 4.01 | Grin3a | 3.42 | Fam151a | 2.96 | Bmper | 2.55 |
| Galnt5 | 7.14 | Ablim3 | 4.61 | Bean1 | 4.00 | Acot11 | 3.38 | St3gal5 | 2.93 | Stmn2 | 2.55 |
| Sp6 | 6.84 | Fbxo2 | 4.59 | Pi16 | 3.94 | Galnt14 | 3.37 | Lrrc55 | 2.92 | Hey1 | 2.54 |
| Dlx5 | 6.69 | Olfm2 | 4.58 | Smad9 | 3.92 | Ntf5 | 3.23 | Id4 | 2.91 | Olfml3 | 2.54 |
| Gm11532 | 6.57 | Col15a1 | 4.52 | Adam12 | 3.92 | Rap1gap2 | 3.21 | Plcb2 | 2.86 | Rgs2 | 2.53 |
| Msx2 | 6.51 | Dgkk | 4.52 | Shisa3 | 3.82 | Slc24a3 | 3.20 | Draxin | 2.86 | Tagln | 2.50 |
| Gata5 | 6.45 | Tac2 | 4.49 | Kazald1 | 3.80 | Gdf10 | 3.19 | Me1 | 2.82 | Gngt2 | 2.49 |
| Smpd3 | 5.96 | Lor | 4.42 | Epas1 | 3.78 | Wnk4 | 3.16 | Shisa2 | 2.78 | Bambi | 2.47 |
| Ndufa4l2 | 5.57 | H2-T23 | 4.38 | Tgfb1 | 3.71 | Msx1 | 3.16 | Atp1b1 | 2.76 | Pid1 | 2.43 |
| Aplnr | 5.53 | Col13a1 | 4.34 | Ntrk3 | 3.68 | Cux2 | 3.06 | Ramp2 | 2.73 | Sox9 | 2.43 |
| Cadps | 5.43 | Prss35 | 4.33 | Igfbp7 | 3.68 | Ecm1 | 3.00 | Acta2 | 2.73 | ||
| Ltbp2 | 5.35 | Fgfr3 | 4.23 | Acer2 | 3.65 | Osx | 2.99 | Unc5b | 2.71 |
Osterix Is Expressed Only in Male MD Mesenchyme During AMH-Induced MD Regression.
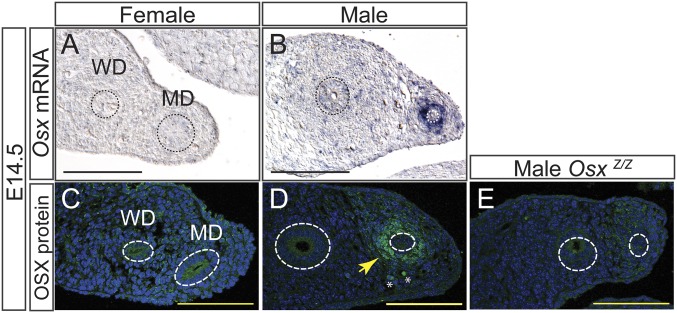
OSTERIX (OSX) is a C2H2-type zinc finger transcription factor first cloned in a screen of C2C12 cells induced by BMP2 to differentiate into osteoblasts. Osx is required for osteoblast differentiation and bone formation (16). However, in this study, we identify a role for Osx during reproductive tract development. Using in situ hybridization we found that Osx transcripts are localized specifically in the male MD mesenchyme at E14.5 (Fig. 2 A and B). To determine whether the OSX protein followed a similar dynamic, we assayed OSX via immunofluorescence and found that OSX is expressed only in male MD mesenchyme at E14.5, indicating a sexually dimorphic expression pattern (Fig. 2 C–E). This analysis was consistent with the GUDMAP database (https://www.gudmap.org) wherein Osx expression in the MD has a male-specific pattern (17).
Fig. 2.
Male-specific expression of Osterix in the mouse MD mesenchyme. (A–D) In situ hybridization for Osx transcripts (A and B) and immunofluorescence for OSX (C–E) on paraffin sections of mesonephroi at E14.5. MD and WD epithelium outlined with a dashed line. No staining is observed in OsxZ/Z male controls (E). White asterisk indicates nonspecific autofluorescence. Yellow arrow points to OSX-positive MD mesenchyme cells (D). MD, Müllerian duct; WD, Wolffian duct. (Scale bar: 100 µm.)
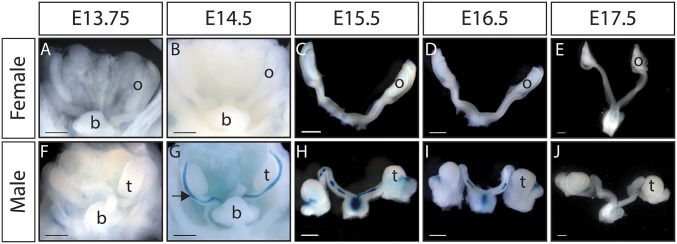
Previously, we generated an Osx-lacZ (Z)-null allele in mice that originally demonstrated that Osx is required for osteoblast differentiation (16). Osx-lacZ expresses β-galactosidase from the endogenous Osx locus and was used to temporally and spatially map expression of Osx during MD regression. Expression of OsxZ/+ begins at around E13.75 in male MD mesenchyme and robust expression throughout the length of the MD is present by E14.5 (Fig. 3 F and G). This temporal expression pattern is consistent with activation of Osx expression by the AMH signaling pathway. While Amh transcripts are present in the testis as early as E11.5 in mouse, initiation of AMH signaling in the MD follows expression of Amhr2 in the MD mesenchyme beginning at ∼E13.25 (18, 19). No expression is detected in the female reproductive tract at any time points examined (Fig. 3 A–E). In males, OsxZ/+ expression is subsequently lost as the MD regresses (Fig. 3 H–J). The expression patterns of endogenous OSX and Osx-lacZ suggested that Osx expression in the MD mesenchyme is mediated by the AMH signaling pathway.
Fig. 3.
Osterix-lacZ (Osx-Z) expression is detectable in MD mesenchyme during regression in males but absent in females. (A–J) Whole-mount β-galactosidase staining of OsxZ/+ female and male reproductive tracts. (A–E) No expression is detected in female embryos from E13.75 to E17.5. (F and G) Osx-lacZ is expressed in male embryos beginning at E13.75 and by E14.5 is expressed in the mesenchyme throughout the length of the MD. (H–J) As the MD regresses, Osx-lacZ expression is also lost. b, bladder; o, ovary; t, testis; (Scale bar: 500 µm.)
AMH Signaling Is Necessary and Sufficient for OSX/Osterix Expression in MD Mesenchyme.
To determine whether AMH signaling is required for Osx expression in the male MD mesenchyme, we examined OSX expression in E14.5 Amhr2Z/Cre males. Amhr2Z/Cre is a functional Amhr2-null genotype and therefore lacks AMH signaling, leading to female reproductive tract development in mutant males (18, 20). While OSX protein can be detected in the MD mesenchyme of wild-type males at E14.5, no detectable OSX is present in Amhr2Z/Cre males (Fig. 4 A and B). The ability of AMH signaling alone to activate Osx expression was tested using our MT-hAMH transgenic mouse model. The MT-hAMH mouse broadly expresses human AMH, causing MD regression in females (5). Osx-lacZ is not expressed in E14.5 OsxZ/+ females but is induced in the MD mesenchyme of OsxZ/+; MT-hAMH females (Fig. 4 C–F). These results indicate that activation of the AMH signaling pathway is necessary and sufficient to initiate expression of Osx/OSX in the MD mesenchyme during reproductive tract differentiation.
Fig. 4.
AMH signaling is necessary and sufficient for OSX expression in the MD mesenchyme. (A and B) Immunofluorescence for OSX on paraffin sections of mesonephroi at E14.5. OSX is not expressed in Amhr2Z/Cre males, indicating AMH signaling is required for OSX expression in the male MD. (C–F) β-Galactosidase staining of OsxZ/+ and OsxZ/+; Mt-hAMH female reproductive tracts. Activation of the AMH signaling pathway in the female reproductive tract is sufficient to induce expression of Osx-lacZ in the MD. MD and WD epithelium outlined with a dashed line. b, bladder; MD, Müllerian duct; o, ovary; WD, Wolffian duct. [Scale bars: 100 µm (A, B, E, and F); 500 µm (C and D).]
Osx Expression Is Downstream of AMH-Effector β-Catenin in MD Mesenchyme.
β-Catenin functions downstream of AMH signaling and is required to regulate MD regression during male sex differentiation (10). However, the downstream molecular targets of β-catenin in the MD mesenchyme are unknown. Osx expression is up-regulated by activated β-catenin in osteoblasts, cementoblasts, and long bones (21, 22). In addition, the proximal promoter of Osx can be activated by β-catenin in in vitro luciferase assays, and β-catenin directly binds the first intron of Osx in osteoblasts (21, 23). Therefore, we wanted to determine whether Osx is a downstream target of β-catenin in the MD mesenchyme and possibly a contributing factor to the complete block in MD regression observed in males with MD mesenchyme knockout of β-catenin. Reproductive tracts of Amhr2Cre/+; Ctnnb1flox/flox male embryos lack expression of β-catenin in the MD mesenchyme and were examined at E14.5 for OSX expression by immunofluorescence. Amhr2Cre/+; Ctnnbflox/flox male embryos expressed OSX protein in the MD mesenchyme at E14.5 (Fig. 5 A and B). Loss of β-catenin expression in the MD mesenchyme in Amhr2Cre/+; Ctnnbflox/flox males was confirmed by immunofluorescence (Fig. 5 C and D). Because immunofluorescence is not quantitative, we performed qPCR to detect changes in Osx transcript levels in Amhr2Cre/+; Ctnnbflox/flox E14.5 male mesonephroi. These results showed a significant 61% reduction in Osx transcript levels in Amhr2Cre/+; Ctnnbflox/flox males in comparison of Ctnnbflox/flox controls (Fig. 5E). Specificity of the primers for the Osx transcript was confirmed by analysis of OsxZ/Z and OsxZ/+ E14.5 male mesonephroi. As expected, no Osx transcripts were detected in OsxZ/Z samples and transcripts were reduced by ∼50% in OsxZ/+ males (Fig. 5E). Together, the presence of OSX protein and reduced Osx transcripts in the MD mesenchyme suggests that Osx is downstream of Ctnnb1 but there are other transcriptional activation inputs. Given that we established Osx is a downstream target of AMH signaling and AMH-effector β-catenin, we next analyzed the influence of Osx loss on MD regression in vivo.
Fig. 5.
β-Catenin regulates Osx expression in the MD mesenchyme. (A and B) Immunofluorescence for OSX on paraffin sections of mesonephroi at E14.5. OSX is expressed in Amhr2Cre/+; Ctnnb1flox/flox males, indicating OSX expression is regulated by other factors in addition to β-catenin in the male MD. (C and D) Immunofluorescence for β-catenin. Amhr2Cre/+; Ctnnb1flox/flox E14.5 males have significant reduction of β-catenin staining in MD mesenchyme compared with controls. Yellow arrow points to MD mesenchyme. MD and WD epithelium outlined with a dashed line. CK8 staining included as a marker for MD epithelium. White asterisks indicate nonspecific autofluorescent cells. MD, Müllerian duct; WD, Wolffian duct. (Scale bar: 100 µm.) (E) Osx mRNA in whole E14.5 mesonephroi in Ctnnb1flox/flox, Amhr2Cre/+, Ctnnb1flox/flox, OsxZ/+ and OsxZ/Z was quantified by qPCR. There was a statistically significant reduction in Osx expression between Ctnnb1flox/flox, Amhr2Cre/+; Ctnnb1flox/flox and OsxZ/+ as determined by one-way ANOVA [F(2,9) = 6.993, P = 0.0147] followed by Tukey’s post hoc test. Osx expression in Amhr2Cre/+; Ctnnb1flox/flox, and OsxZ/+ mesonephroi is significantly reduced in comparison with Ctnnb1flox/flox controls (P values, 0.018 and 0.039, respectively). As expected, Osx transcript was not detected in OsxZ/Z mesenophroi. Graph is representative of three independent assays. Samples were normalized to Actb. Error bars ± SEM of fold change. n = 4 biological replicates. N.D., not detected; ns, not significant.
MD Regression Is Delayed in Osx-Null Male Mice.
Osx-null mice die at birth with severe skeletal defects (16). However, MD regression is complete in males at the time of birth. To determine whether Osx is required for MD regression, we examined OsxZ/Z embryos at E15.5, E16.5, E17.5, and E18.5. At E15.5, the MD of OsxZ/+ and OsxZ/Z males are intact. At E15.5, the OsxZ/+ MD is thinner than the OsxZ/Z MD, indicating a delay in MD regression in the OsxZ/Z males (Fig. 6 A and E). At E16.5, the MD of OsxZ/+ males have large gaps. However, the MD of OsxZ/Z males retain longer lengths of intact MD and fewer gaps are observed (Fig. 6 B and F). At E17.5, longer MD tissue remnants are observed near the testis in OsxZ/Z males in comparison with OsxZ/+ males (Fig. 6 C and G). At E18.5, OsxZ/Z males have a normal pattern of MD regression and the delay in MD regression defects is resolved (Fig. 6 D and H). Histological analysis of regressing Müllerian system in OsxZ/+ and OsxZ/Z males showed that the thick stained regions included MD epithelium, whereas the thin stained regions lacked MD epithelium (SI Appendix, Fig. S4). Other developmental delays or growth defects were not evident by gross examination of Osx-null males (SI Appendix, Fig. S5). Furthermore, testis morphology and AMH expression were comparable between Osx-null mutants and wild type at E13.5 (SI Appendix, Fig. S6). This ∼24-h delay in MD regression was observed in all Osx-null mutant males examined (n = 3). This suggests that Osx contributes to MD regression. Osx has well-established roles in osteoblast, odontoblast, and cementoblast differentiation (16, 24). However, this is evidence for Osx function in sex differentiation.
Fig. 6.
Progression of MD regression is delayed in OSXZ/Z-null mice. (A–F) Whole-mount β-galactosidase staining of OsxZ/+ (A–D) and OsxZ/Z (E–H) male reproductive tracts. (A and E) E15.5 OsxZ/Z males have intact MDs that appear thicker than OsxZ/+. (B and F) E16.5 OsxZ/Z males have longer lengths of MD remnants in comparison with OsxZ/+ males. (C and G) E17.5 MD tissue remnants are observed near the testis in OsxZ/Z males. (D and H) E18.5 OsxZ/Z regression defects are resolved and appear similar to OsxZ/+ males. (Scale bar: 500 µm.) Three or more of each genotype were observed for each developmental time point.
Discussion
We report on an AMH-induced mesenchyme gene, Osx, as a regulator of MD epithelial regression identified using a global unbiased approach. The identification of Osx in this process adds a key link to the gene expression network that may underlie MD regression. During development in skeletal bone and tooth, Osx has been demonstrated to be a key mesenchymal factor necessary for cell fate decisions in the differentiation of specialized cells (16, 25). Our study implicates Osx as a factor in sex differentiation. In addition to its role in eliminating the MDs in male embryos, AMH is expressed by postnatal ovarian granulosa cells to regulate folliculogenesis (26). Intriguingly, AMH and its type II receptor gene have also been shown to regulate gonadal sex determination in certain species of fishes (27). Osx is not expressed in fetal or adult gonads. This suggests that AMH-induced gene regulatory networks are diverse in different tissues with distinct functions. Insights gained into the gene-regulatory networks underlying MD regression, a temporally and spatially defined mesenchyme–epithelial signaling event, may be applied more broadly to further understand mesenchyme–epithelial signaling in other developmental processes and disease.
Osx is an important factor in bone homeostasis in adults and has roles in degradation of cartilage matrix by directly transcriptionally activating matrix metalloproteinase, Mmp13 (28). Furthermore, in metastatic breast cancer, modulation of Osx levels results in a corresponding change in MMP-2 promoter activity (29). Two important processes in MD regression are the breakdown of the basement membrane and apoptosis of MD epithelial cells. Secreted MMPs function to degrade extracellular matrix and also cleave signaling molecules to control multiple cellular processes including differentiation and apoptosis (30). Mmp2 has been identified as a downstream target of AMH signaling and is expressed in a male-specific pattern in the MD during regression. Knockdown of Mmp2 using a morpholino and MMP inhibitors block MD regression in in vitro organ culture experiments (31). However, Mmp2-null male mice regress the MD, suggesting other MMPs may compensate for loss of MMP-2 (31). Perhaps Osx may be contributing to MD regression by regulating MMP levels. However, Mmp13 is not expressed in our dataset, and there are no differences in Mmp2 levels in Osx-null males compared with controls (SI Appendix, Fig. S7). Thus, it appears that these Mmp genes are not OSX targets to mediate MD regression.
The AMH signaling pathway shares type 1 receptors, ACVRI and BMPR1A, and downstream SMADs with the BMP pathway. This suggests that AMH-induced expression of Osx in the reproductive tract may be regulated by similar mechanisms as its regulation by BMP signaling in bone and tooth differentiation. During osteoblast differentiation, BMP signaling induces Osx expression by activation of Runx2 and also via independent mechanisms (32). In our study, Runx2 transcript is not differentially expressed between male and female E14.5 MD mesenchyme, suggesting in male reproductive tract differentiation Osx expression is independent of Runx2. In addition to BMP signaling, activated β-CATENIN up-regulates Osx expression in osteoblasts, cementoblasts, and long bones (21, 22). In our previous study, we found that β-catenin in the MD mesenchyme is required for AMH-induced regression of the MD. Intriguingly, we found that OSX protein was expressed in β-catenin conditional knockout male mesonephroi but Osx transcript levels were significantly reduced compared with controls. Because β-catenin conditional knockout mice have complete retention of the MD and Osx-null males only show a delay in MD regression, we interpret this to mean that Ctnnb1 is upstream of Osx but that there are other transcriptional activation inputs. Of note, the chromosomal location of the Osx gene is within 88 kb of the Amhr2 gene, and this linkage is conserved in mammals. This implies the potential for shared enhancer/regulatory elements for MD mesenchyme expression.
Interestingly, Park et al. (12) performed a related study but used microarrays to determine transcript profiles of whole E14.5 mesonephroi from wild-type and Amhr2 mutant males to identify AMH-induced genes. Our study (MD mesenchymal cells only) and the Park study (whole mesonephroi) resulted in very similar lists of genes up-regulated in male MD (12). This suggests gene activation that mediates MD regression is primarily occurring in the MD mesenchyme.
Loss of OSX protein results in a ∼24-h delay in the progression of MD regression. The spatial pattern of MD regression in Osx-null male embryos was comparable to what we observed in heterozygous littermates with gaps apparent throughout the MD by E16.5. However, the length of remaining MD was increased in the absence of Osx at E16.5 and E17.5. By E18.5, the delay in MD regression was resolved. This suggests that OSX may play an important role in the timing of initiation of MD regression. It is possible OSX is an early activator of molecular signals necessary for clearance of MD cells by apoptosis or other mechanisms during regression. After a brief delay due to the loss of initial activation by OSX, these clearance signals are then activated by other as-of-yet unidentified AMH-induced factors.
The MD regression defects described have included complete and partial retention of MD-derived tissues in postnatal and adult males and delayed regression that resolves at later embryonic stages. Factors whose loss results in no regression of the MD include Wnt7a (required for Amhr2 expression), Amh, Amhr2, Bmpr1a/Acvr1, Smad1, -5, -8, and β-catenin. With the exception of β-catenin, all of these genes function in the initial activation of the AMH signaling pathway (2). Partial retention of MD-derived tissues has been associated with reductions in levels of AMH hormone and AMHR2, leading to AMH signaling below a threshold level needed for complete MD regression (33, 34). Similarly, mice with lags in testis development and consequent delays in AMH secretion also exhibit deferred MD regression (35). Partial retention of MD remnants was also observed in conditional knockout (CKO) in the MD mesenchyme of the TGF-β signaling pathway common Smad (co-Smad) Smad4. The defect in MD regression in the CKO Smad4 males is hypothesized to be caused by an observed reduction in β-catenin (36). Interestingly, constitutive activation of β-catenin in the MD mesenchyme resulted in a similar partial retention of MD tissues in adult males (37). The delay in MD regression in the Osx-null males is independent of AMH signaling levels.
Numerous studies have examined the consequences of gene mutations on MD regression in postnatal or adult males and considered any mutants without retained uterine tissue to have “normal” MD regression. This approach works well to identify factors required for activation of AMH signaling during MD regression such as Wnt7a, Amh, and Amhr2 or early downstream effectors in the signaling cascade such as SMADs 1, 5, and 8 (2). However, monitoring mutant males for retained MD-derived tissues after MD regression is complete does not identify potential changes in the timing or process of MD regression. By examining Osx-null mutant males at early time points during MD regression, we were able to identify a potential role for Osx in the initiation of MD regression that would have been missed if we had only examined newborn males. Considering the results of this study, it may be of benefit to revisit candidate factors without postnatally retained uterine tissue at earlier time points during MD regression.
Materials and Methods
Mice.
Ctnnb1flox/flox mice were obtained from The Jackson Laboratory and maintained on a C57BL/6J × 129 × 1/SvJ × 129S1/Sv mixed background (36). Amhr2tm3(Cre)Bhr (Amhr2-Cre; ref. 20), Gt(ROSA)26Sortm1(EYFP)Cos (R26R-YFP; ref. 38), and Amhr2tm2Bhr (Amhr2-lacZ; ref. 18) were maintained on a C57BL/6J × 129/SvEv mixed background. Sp7tm1Crm (Osx-lacZ; ref. 16) and Mt-hAMHtg/+ (Mt-hAMH; ref. 7) were maintained on a C57BL/6J congenic background. Amhr2-Cre, R26R-YFP, Amhr2-lacZ, Osx-lacZ, Ctnnb1flox/flox, and Mt-hAMHtg/+ mice were genotyped as previously described (7, 8, 16, 18, 38, 39). The Institutional Animal Care and Use Committee of the University of Texas MD Anderson Cancer Center approved all animal procedures. Experiments were performed in agreement with the principles and procedures outlined in the NIH Guide for the Care and Use of Laboratory Animals (40).
Isolation of MD Mesenchyme RNA.
Total RNA was isolated from FACS-sorted YFP+ MD mesenchymal cells from E14.5 Amhr2Cre/+; R26Ryfp/+ mesonephroi using TRI reagent RT-LS (Molecular Research Center) following the manufacturer’s recommended protocol with the following modifications: PelletPaint (EMD Millipore) was used and an additional ethanol precipitation was performed. RNA integrity number greater than or equal to 8 was considered acceptable.
Generation of RNA-Seq cDNA Libraries.
cDNA libraries were generated using the TruSeq, version 2, kit (Illumina) following the manufacturer’s recommended protocol from three biological replicates each of male and female. Each replicate consisted of ≥100 ng of total RNA isolated from pooled YFP+ MD mesenchyme cells from two to seven embryos. Individual library quality was assessed by high-sensitivity DNA Bioanalyzer chip (Aligent), and the concentration of each library was determined using the Qubit picogreen assay (Life Technologies). Equal quantities of each indexed library were pooled and sequenced on the Illumina HiSeq 2000 platform to generate paired-end 76-bp reads. Raw data files for the RNA-seq analysis have been deposited in the GEO database with the accession number GSE116157.
Bioinformatic Analysis of Transcriptomes.
RNA-seq data were mapped to the mouse genome with the bioinformatics tools Tophat and Bowtie using reference genome build NCBIM37 (13). Ensembl annotation for the mouse genome was downloaded from the iGenome website (support.illumina.com/sequencing/sequencing_software/igenome.ilmn). Mapped RNA-seq data were then subject to differential expression analysis. Two bioinformatics tools were used for the analysis: Cufflink (13) and DESeq (14). Cufflink uses mapped reads to generate a parsimonious set of transcripts, and then estimates the abundance of the transcripts (41). DESeq utilizes the raw counts of mapped reads as data input and uses the negative binomial distribution model (14). Using both methods, a list of genes with significant differential expression was obtained. To study the possible functions related to the changes of gene expression, pathway analysis was performed using IPA tool from Ingenuity Systems (https://www.ingenuity.com/) (SI Appendix, Methods).
In Situ Hybridization.
Embryos and reproductive tissues from neonates or adults were processed and section in situ hybridization was performed as previously described (10). Osx RNA probe was generated from plasmid pBSOsxBP containing a 560-bp fragment of mouse Osx (Osx transcript NM_1330458.4 base pairs 106–672) (16).
X-Gal Staining.
Embryos were collected at stages E13.5–E18.5. Lower body trunks with urogenital tissues were isolated and processed for X-gal staining to visualize lacZ expression as described (18). Following β-galactosidase staining, reproductive tracts were embedded in paraffin, sectioned at 10 µm, and counterstained with nuclear fast red and/or eosin Y (Sigma Aldrich).
Immunofluorescence.
Immunofluorescence was performed as previously described (42). Primary antibodies and dilutions are found in SI Appendix, Table S2. At least three specimens of each genotype were analyzed for each staining.
qPCR.
Total RNA was extracted from whole mesonephroi using TRI reagent RT-LS (Molecular Research Center) and RNA Clean and Concentrator- 5 kit (Zymo Research). cDNA was generated from 50 ng of total RNA per biological replicate using the SuperScript II reverse transcriptase (RT) with supplied oligo(dT)12–18 primer (Invitrogen). qPCR was performed on cDNA using a 7900HT Thermocycler (Applied Biosystems) and iTAQ hotstart SYBR Green master mix (Bio-Rad). The fold change of the ΔCT normalized to Actb was used for analysis (2–∆∆Ct method). P values and statistical significance were determined by either ANOVA followed by Tukey’s post hoc analysis (three experimental groups of data) or by Welch’s paired t test (two experimental groups of data) using GraphPad Prism, version 7.00 for Windows (GraphPad Software; www.graphpad.com). Threshold P value was <0.05. For primer sequences, see SI Appendix, Table S3.
Supplementary Material
Acknowledgments
We thank Swathi Arur, Erin Lopez, Zer Vue, Shuo-Ting Yen, and Alejandro Elder Ontiveryos for helpful discussions. pBSOsxBP and OSX antibody were kindly provided by Benoit de Crombrugghe. We acknowledge Dr. Paulucci-Holthauzen at the Department of Genetics Microscopy Facility for training and support. This work was supported by NIH Grant HD030284 and the Ben F. Love Endowment (to R.R.B.). R.D.M. was supported by NIH T32 Grant CA009299 and a postdoctoral fellowship from Center for Stem Cell and Developmental Biology at University of Texas MD Anderson Cancer Center. We acknowledge NIH Shared Instrumentation Grant (1S10OD024976-01) for supporting the confocal microscope. Veterinary resources, flow cytometry, and DNA sequencing were supported by NIH Grant CA16672.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
Data deposition: The data reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, https://www.ncbi.nlm.nih.gov/geo (accession no. GSE116157).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1721793115/-/DCSupplemental.
References
- 1.Zhao F, et al. Elimination of the male reproductive tract in the female embryo is promoted by COUP-TFII in mice. Science. 2017;357:717–720. doi: 10.1126/science.aai9136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mullen RD, Behringer RR. Molecular genetics of Müllerian duct formation, regression and differentiation. Sex Dev. 2014;8:281–296. doi: 10.1159/000364935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Josso N, et al. Anti-Müllerian hormone: The Jost factor. Recent Prog Horm Res. 1993;48:1–59. doi: 10.1016/b978-0-12-571148-7.50005-1. [DOI] [PubMed] [Google Scholar]
- 4.Behringer RR. The in vivo roles of Müllerian-inhibiting substance. Curr Top Dev Biol. 1994;29:171–187. doi: 10.1016/s0070-2153(08)60550-5. [DOI] [PubMed] [Google Scholar]
- 5.Mishina Y, et al. Genetic analysis of the Müllerian-inhibiting substance signal transduction pathway in mammalian sexual differentiation. Genes Dev. 1996;10:2577–2587. doi: 10.1101/gad.10.20.2577. [DOI] [PubMed] [Google Scholar]
- 6.Picard JY, Cate RL, Racine C, Josso N. The persistent Müllerian duct syndrome: An update based upon a personal experience of 157 cases. Sex Dev. 2017;11:109–125. doi: 10.1159/000475516. [DOI] [PubMed] [Google Scholar]
- 7.Behringer RR, Cate RL, Froelick GJ, Palmiter RD, Brinster RL. Abnormal sexual development in transgenic mice chronically expressing Müllerian inhibiting substance. Nature. 1990;345:167–170. doi: 10.1038/345167a0. [DOI] [PubMed] [Google Scholar]
- 8.Jamin SP, Arango NA, Mishina Y, Behringer RR. Genetic studies of MIS signalling in sexual development. Novartis Found Symp. 2002;244:157–164; discussion 164–168, 203–206, 253–257. [PubMed] [Google Scholar]
- 9.Orvis GD, et al. Functional redundancy of TGF-beta family type I receptors and receptor-Smads in mediating anti-Mullerian hormone-induced Mullerian duct regression in the mouse. Biol Reprod. 2008;78:994–1001. doi: 10.1095/biolreprod.107.066605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kobayashi A, et al. β-Catenin is essential for Müllerian duct regression during male sexual differentiation. Development. 2011;138:1967–1975. doi: 10.1242/dev.056143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Cox S, et al. Sexually dimorphic expression of secreted frizzled-related (SFRP) genes in the developing mouse Müllerian duct. Mol Reprod Dev. 2006;73:1008–1016. doi: 10.1002/mrd.20507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Park JH, et al. Induction of WNT inhibitory factor 1 expression by Müllerian inhibiting substance/antiMullerian hormone in the Müllerian duct mesenchyme is linked to Müllerian duct regression. Dev Biol. 2014;386:227–236. doi: 10.1016/j.ydbio.2013.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trapnell C, et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat Protoc. 2012;7:562–578. doi: 10.1038/nprot.2012.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Anders S, Huber W. Differential expression analysis for sequence count data. Genome Biol. 2010;11:R106. doi: 10.1186/gb-2010-11-10-r106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Münsterberg A, Lovell-Badge R. Expression of the mouse anti-Müllerian hormone gene suggests a role in both male and female sexual differentiation. Development. 1991;113:613–624. doi: 10.1242/dev.113.2.613. [DOI] [PubMed] [Google Scholar]
- 16.Nakashima K, et al. The novel zinc finger-containing transcription factor Osterix is required for osteoblast differentiation and bone formation. Cell. 2002;108:17–29. doi: 10.1016/s0092-8674(01)00622-5. [DOI] [PubMed] [Google Scholar]
- 17.Harding SD, et al. The GUDMAP database—an online resource for genitourinary research. Development. 2011;138:2845–2853. doi: 10.1242/dev.063594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arango NA, et al. A mesenchymal perspective of Müllerian duct differentiation and regression in Amhr2-lacZ mice. Mol Reprod Dev. 2008;75:1154–1162. doi: 10.1002/mrd.20858. [DOI] [PubMed] [Google Scholar]
- 19.Hacker A, Capel B, Goodfellow P, Lovell-Badge R. Expression of Sry, the mouse sex determining gene. Development. 1995;121:1603–1614. doi: 10.1242/dev.121.6.1603. [DOI] [PubMed] [Google Scholar]
- 20.Jamin SP, Arango NA, Mishina Y, Hanks MC, Behringer RR. Requirement of Bmpr1a for Müllerian duct regression during male sexual development. Nat Genet. 2002;32:408–410. doi: 10.1038/ng1003. [DOI] [PubMed] [Google Scholar]
- 21.Bao Q, et al. Constitutive β-catenin activation in osteoblasts impairs terminal osteoblast differentiation and bone quality. Exp Cell Res. 2017;350:123–131. doi: 10.1016/j.yexcr.2016.11.013. [DOI] [PubMed] [Google Scholar]
- 22.Choi H, et al. A reciprocal interaction between β-catenin and Osterix in cementogenesis. Sci Rep. 2017;7:8160. doi: 10.1038/s41598-017-08607-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Felber K, Elks PM, Lecca M, Roehl HH. Expression of Osterix is regulated by FGF and Wnt/β-catenin signalling during osteoblast differentiation. PLoS One. 2015;10:e0144982. doi: 10.1371/journal.pone.0144982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He YD, et al. Site-specific function and regulation of Osterix in tooth root formation. Int Endod J. 2016;49:1124–1131. doi: 10.1111/iej.12585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takahashi A, Ono N, Ono W. The fate of Osterix-expressing mesenchymal cells in dental root formation and maintenance. Orthod Craniofac Res. 2017;20:39–43. doi: 10.1111/ocr.12167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Durlinger AL, et al. Control of primordial follicle recruitment by anti-Müllerian hormone in the mouse ovary. Endocrinology. 1999;140:5789–5796. doi: 10.1210/endo.140.12.7204. [DOI] [PubMed] [Google Scholar]
- 27.Pan Q, et al. Vertebrate sex-determining genes play musical chairs. C R Biol. 2016;339:258–262. doi: 10.1016/j.crvi.2016.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang C, Tang W, Li Y. Matrix metalloproteinase 13 (MMP13) is a direct target of osteoblast-specific transcription factor osterix (Osx) in osteoblasts. PLoS One. 2012;7:e50525. doi: 10.1371/journal.pone.0050525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dai QS, et al. Osterix transcriptional factor is involved in the metastasis of human breast cancers. Oncol Lett. 2015;10:1870–1874. doi: 10.3892/ol.2015.3448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nagase H, Visse R, Murphy G. Structure and function of matrix metalloproteinases and TIMPs. Cardiovasc Res. 2006;69:562–573. doi: 10.1016/j.cardiores.2005.12.002. [DOI] [PubMed] [Google Scholar]
- 31.Roberts LM, Visser JA, Ingraham HA. Involvement of a matrix metalloproteinase in MIS-induced cell death during urogenital development. Development. 2002;129:1487–1496. doi: 10.1242/dev.129.6.1487. [DOI] [PubMed] [Google Scholar]
- 32.Matsubara T, et al. BMP2 regulates Osterix through Msx2 and Runx2 during osteoblast differentiation. J Biol Chem. 2008;283:29119–29125. doi: 10.1074/jbc.M801774200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Migone FF, et al. Overactivation of hedgehog signaling in the developing Müllerian duct interferes with duct regression in males and causes subfertility. Reproduction. 2017;153:481–492. doi: 10.1530/REP-16-0562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Arango NA, Lovell-Badge R, Behringer RR. Targeted mutagenesis of the endogenous mouse Mis gene promoter: In vivo definition of genetic pathways of vertebrate sexual development. Cell. 1999;99:409–419. doi: 10.1016/s0092-8674(00)81527-5. [DOI] [PubMed] [Google Scholar]
- 35.Warr N, et al. Sfrp1 and Sfrp2 are required for normal male sexual development in mice. Dev Biol. 2009;326:273–284. doi: 10.1016/j.ydbio.2008.11.023. [DOI] [PubMed] [Google Scholar]
- 36.Petit FG, Deng C, Jamin SP. Partial Müllerian duct retention in Smad4 conditional mutant male mice. Int J Biol Sci. 2016;12:667–676. doi: 10.7150/ijbs.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tanwar PS, et al. Focal Mullerian duct retention in male mice with constitutively activated beta-catenin expression in the Mullerian duct mesenchyme. Proc Natl Acad Sci USA. 2010;107:16142–16147. doi: 10.1073/pnas.1011606107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Srinivas S, et al. Cre reporter strains produced by targeted insertion of EYFP and ECFP into the ROSA26 locus. BMC Dev Biol. 2001;1:4. doi: 10.1186/1471-213X-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brault V, et al. Inactivation of the beta-catenin gene by Wnt1-Cre-mediated deletion results in dramatic brain malformation and failure of craniofacial development. Development. 2001;128:1253–1264. doi: 10.1242/dev.128.8.1253. [DOI] [PubMed] [Google Scholar]
- 40.National Research Council . Guide for the Care and Use of Laboratory Animals. 8th Ed National Academies Press; Washington, DC: 2011. [Google Scholar]
- 41.Trapnell C, et al. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat Biotechnol. 2010;28:511–515. doi: 10.1038/nbt.1621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Stewart CA, et al. CTNNB1 in mesenchyme regulates epithelial cell differentiation during Müllerian duct and postnatal uterine development. Mol Endocrinol. 2013;27:1442–1454. doi: 10.1210/me.2012-1126. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.