Abstract
Cannabidiol (CBD)-based oil preparations are becoming extremely popular, as CBD has been shown to have beneficial effects on human health. CBD-based oil preparations are not unambiguously regulated under the European legislation, as CBD is not considered as a controlled substance. This means that companies can produce and distribute CBD products derived from non-psychoactive hemp varieties, providing an easy access to this extremely advantageous cannabinoid. This leaves consumers with no legal quality guarantees. The objective of this project was to assess the quality of 14 CBD oils commercially available in European countries. An in-depth chemical profiling of cannabinoids, terpenes and oxidation products was conducted by means of GC-MS and HPLC-Q-Exactive-Orbitrap-MS in order to improve knowledge regarding the characteristics of CBD oils. Nine out of the 14 samples studied had concentrations that differed notably from the declared amount, while the remaining five preserved CBD within optimal limits. Our results highlighted a wide variability in cannabinoids profile that justifies the need for strict and standardized regulations. In addition, the terpenes fingerprint may serve as an indicator of the quality of hemp varieties, while the lipid oxidation products profile could contribute in evaluation of the stability of the oil used as milieu for CBD rich extracts.
Keywords: cannabidiol, CBD oil, terpenes, hemp seed oil, GC-MS, HPLC-Q-Exactive-Orbitrap-MS
1. Introduction
Cannabidiol (CBD) and tetrahydrocannabinol (THC) are the most common cannabinoids in medical cannabis preparations [1]. The are both responsible for a variety of pharmacological actions that can have remarkable applications, but unlike THC, CBD does not possess any psychoactive effects [1]. Several studies suggest that CBD can be effective in treating epilepsy and other neuropsychiatric disorders, including anxiety and schizophrenia [2,3,4]. CBD may also be effective in treating post-traumatic stress disorder and may have anxiolytic, antipsychotic, antiemetic and anti-inflammatory properties [5,6,7]. This plethora of pharmacological activities has led to rapid changes in the cultural, social and political legal viewpoints regarding the utilization of cannabis-based preparations [8]. Although there is still a complicated legal milieu that calls for caution, it is undeniable that there is an enormous interest from consumers/patients in the utilization of CBD dietary supplements. This has created an exploding industry of CBD products in Europe and around the world. “CBD enriched oils”, obtained from extraction of different Cannabis sativa L. chemotypes with high content of CBD, are the most popular products used [9,10,11,12].
Since CBD, in contrast to THC, is not a controlled substance in the European Union [13] several companies produce and distribute CBD-based products obtained from inflorescences of industrial hemp varieties. However, due to the lack of specific regulations, no analytical controls are mandatory for CBD-based products, leaving consumers with no legal protection or guarantees about the composition and quality of the product they are acquiring. Currently, CBD-based products are not subject to any obligatory testing or basic regulatory framework to determine the indication area, daily dosage, route of administration, maximum recommended daily dose, packaging, shelf life and stability. Exceptions are galenical “CBD oil” prepared by pharmacists following medical prescriptions in several European Union countries such as Germany, Italy and Holland. The German Drug Codex (DAC), which is published by the Federal Union of German Associations of Pharmacists (ABDA) and functions as a supplementary book to the Pharmacopoeia, suggests a preparation of 5% CBD in medium chain triglycerides oil also indicating detailed analytical controls of galenic preparations [14].
In Italy medical cannabis represents a multifaceted reality [9,10,11,12]. At present Dutch Bedrocan varieties (Bedrocan, Bediol, Bedica and Bedrolite as representative) [15] and the new strain FM2 produced by Military Pharmaceutical Chemical Works of Florence, Italy (authorized in November 2015 by a Ministerial Decree) can be prescribed to treat a wide range of pathological conditions [16]. Indeed. Italian galenic pharmacies are authorized to prepare precise cannabis doses for vaping, herbal teas, resins, micronized capsules and oils. The oil preparation has received considerable attention since it is easy to adjust the individual administration dose required throughout the treatment period, and due to the enhanced bioavailability of its active compounds [9,10,11,12]. Among abovementioned strains, Bedrolite with CBD and THC contents of 9% and <1%, respectively, is frequently used for the preparation of galenic “CBD-based oil”. Anyway, pharmacies are also allowed to distribute CBD oils obtained from hemp, but declared as additives or aromatic preparations, if produced in Italy or designed as dietary supplement if imported from other European countries.
Cannabis sativa L. has been cultivated throughout the world for industrial and medical purposes. The European Union permits the cultivation of plants for hemp products based on the THC content being less than 0.2%. EU Regulation 1307/2013 [17] states that hemp farmers are required to use seeds of cannabis varieties included in the European Union catalogue. In general, specialized extraction procedures, among which the most common is supercritical CO2 extraction, are used to draw out an extract rich in CBD from the cannabis to obtain CBD oil formulations [18,19]. This product also contains other biological active compounds such as omega-3 fatty acids, vitamins, terpenes, flavonoids and other phytocannabinoids like cannabichromene (CBC), cannabigerol (CBG), cannabinol (CBN) and cannabidivarian (CBCV) [10,11,12].
Among non-cannabinoids compounds, special attention must be paid to terpenes that represent the largest group (more than 100 different molecules) of cannabis phytochemicals [20,21,22,23]. Monoterpenes, diterpenes, triterpenes and sesquiterpenes are important components present in the cannabis resin responsible for its unique aromatic properties. Due to their ability to easily cross cell membranes and the blood-brain barrier, they can also influence the medicinal quality of different cannabis chemotypes [24]. Several therapeutic approaches based on the combined use of cannabinoids and terpenes have been developed recently. Particularly, treatment of sleeping disorders and social anxiety by adding caryophyllene, linalool and myrcene to CBD/THC extracts gave encouraging results [25]. In addition, differences between the pharmaceutical properties of diverse Cannabis sativa L. varieties have been attributed to strict interactions, defined as ‘entourage effects’, between cannabinoids and terpenes as a result of synergic action [25]. Recently Pagano et al. [26] investigated the different effect of a pure CBD preparation versus a standardized Cannabis sativa extract with the same concentration of cannabidiol (CBD) in the remission of mucosal inflammation in a mouse models of colitis. The author reveled that under the same experimental conditions, pure CBD just partially ameliorated colitis, while Cannabis sativa L. extract almost entirely reduced the injuries. These findings sustain the rationale of the ‘entourage effect’ achievable by combining CBD with other minor Cannabis constituents.
The quality of Cannabis macerated oils has already been investigated in previous research demonstrating the importance of selecting correct preparation methods and conditions as well as studying the evolution of major and minor compounds (cannabinoid and terpenes) during storage in order to define the ideal shelf-life and management guidelines (storage temperature) [12]. Oxidation products derived from fatty acid degradation during the storage period of macerated oils are critical for overall formulation stability [27,28]. Galenic preparations are usually prepared by using pharmacopeia grade olive oil (FU) to minimise the formation of large quantities of aldehydes and ketones that can also influence the digestibility of the macerated oil [9,12,29].
Since the production of CBD-based oils as dietary supplements has increased rapidly, and since they are frequently used for therapeutic purposes, the main scope of this study was to assess the overall quality of 14 CBD oil preparations produced in different European countries and purchased on the Internet and highlight possible criticisms. Moreover, a Bedrolite macerated oil prepared as a galenic product was used as a reference therapeutic formulation. In order to define and increase knowledge about the characteristic of CBD oils, an in-depth chemical profiling of cannabinoids, terpenes and oxidation compounds by means of GC-MS and HPLC-Q-Exactive-Orbitrap-MS analytical platforms was presented herein.
2. Results and Discussion
2.1. Cannabinoids Content
Current ambiguous all-purpose regulations allow huge variations in the quality and safety of the CBD-based preparations available on the market and clear labelling regarding the exact concentration of CBD is not yet mandatory. Our results demonstrate that CBD concentrations were not always in accordance with producer information (Table 1). As a matter of fact, nine out of 14 tested samples presented concentrations that differed notably from the declared amount, while the remaining five preserved CBD levels within optimal limits (the variation was less than 10%). Our analysis also revealed that two preparations (particularly oils 8 and 10) exhibited higher levels of CBD than those specified by producers, while in another two (samples Oil_3 and Oil_14) the CBD content was far inferior to the stated values. In one sample, the theoretical CBD concentration was not indicated on the label and therefore values obtained could not be compared to the producer’s statement. Taken together, the results highlighted the extreme variability of the commercialised CBD oil preparations, justifying the need for stricter regulations/controls. Precise information regarding the composition of each lot that is available on the market is crucial for consumers who have to be able to properly adapt the recommended dose to the available/purchased preparation [9]. These results are in agreement with those obtained from a preliminary study toward the labeling accuracy of cannabidiol extracts preparations from products available on the US market. In the tested products, 26% contained less CBD than labeled, which could negate any potential clinical response [30]. The over labeling of CBD products in the study was similar in magnitude to levels that triggered warning letters to 14 businesses in 2015–2016 from the US Food and Drug Administration suggesting that there is a continued need for federal and state regulatory agencies to take steps to ensure label accuracy of these consumer products.
Table 1.
Cannabinoid content (expressed as % w/w and in μg/g) in investigated CBD oils (average ± S.D., n = 2).
| (% w/w) | Cannabinoids Content (μg/g) | |||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Samples Code | Deviation from Declared CBD Percentage | Declared CBD 1 | Revealed CBD 2 | CBD | THC | CBN | CBG | CBDA | THCA | CBGA | ||||||||
| Average | ±SD | Average | ±SD | Average | ±SD | Average | ±SD | Average | ±SD | Average | ±SD | Average | ±SD | |||||
| Oil_1 3 | 9.00 | 0.9 | 0.89 | 8143 | 170.2 | 232 | 4.9 | 14 | 0.3 | <0.01 | / | 884 | 18.8 | 123 | 2.6 | 7 | 0.1 | |
| Oil_2 | 8.49 | 4 | 3.66 | 36,567 | 257.3 | 1908 | 13.5 | 208 | 1.5 | 716 | 5.1 | 42 | 0.3 | 1 | 0.0 | 12 | 0.1 | |
| Oil_3 | 21.21 | 1 | 0.79 | 3247 | 241.7 | 148 | 10.5 | 40 | 2.8 | 16 | 1.1 | 5282 | 373.5 | 191 | 13.5 | 693 | 49.0 | |
| Oil_4 | 15.29 | 5 | 4.24 | 42,352 | 2395.8 | 0.01 | 0.0 | 3 | 0.2 | <0.01 | / | 6 | 0.3 | 196 | 11.1 | 19 | 1.1 | |
| Oil_5 | 10.53 | 4 | 4.42 | 43,509 | 3076.6 | 533 | 37.7 | 69 | 4.9 | <0.01 | / | 802 | 56.7 | 17 | 1.2 | 27 | 1.9 | |
| Oil_6 | 4.44 | 3 | 2.87 | 28,536 | 1008.9 | 3546 | 125.4 | 481 | 17.0 | <0.01 | / | 152 | 5.4 | 29 | 1.0 | 8 | 0.3 | |
| Oil_7 | 8.27 | 4 | 4.33 | 42,601 | 1807.4 | 526 | 22.3 | 65 | 2.8 | <0.01 | / | 804 | 34.1 | 12 | 0.5 | 26 | 1.1 | |
| Oil_8 | 35.41 | 3 | 4.06 | 39,962 | 3108.3 | 695 | 54.1 | 62 | 4.8 | <0.01 | / | 753 | 58.6 | 47 | 3.7 | 22 | 1.7 | |
| Oil_9 | 7.63 | 3 | 3.23 | 32,212 | 683.3 | 1607 | 34.1 | 345 | 7.3 | 23 | 0.5 | 88 | 1.9 | 25 | 0.5 | 6 | 0.1 | |
| Oil_10 | 23.89 | 4 | 4.96 | 48,879 | 1036.9 | 557 | 11.8 | 79 | 1.7 | <0.01 | / | 774 | 16.4 | 58 | 1.2 | 23 | 0.5 | |
| Oil_11 | / | / | 0.24 | 1875 | 68.9 | 36 | 1.3 | 7 | 0.3 | <0.01 | / | 634 | 23.3 | 32 | 1.2 | 18 | 0.7 | |
| Oil_12 | 19.28 | 2 | 1.61 | 12,758 | 180.4 | 494 | 7.0 | 188 | 2.7 | 6 | 0.1 | 3862 | 54.6 | 107 | 1.5 | 97 | 1.4 | |
| Oil_13 | 36.20 | 4 | 2.55 | 24,444 | 2419.8 | 568 | 56.2 | 1105 | 109.4 | 624 | 61.8 | 1229 | 121.7 | 27 | 2.7 | 22 | 2.2 | |
| Oil_14 | 38.14 | 5 | 3.09 | 23,186 | 655.8 | 524 | 14.8 | 67 | 1.9 | 460 | 13.0 | 8828 | 249.7 | 358 | 10.1 | 216 | 6.1 | |
| Oil_15 | 24.33 | 3 | 2.27 | 22,692 | 320.9 | <0.01 | / | <0.01 | / | 5687 | 80.4 | 9 | 0.1 | <0.01 | / | 4 | 0.1 | |
1 CBD declared on labels, 2 CBDtot (sum of CBD +0.877 × CBDA); 3 Bedrolite oil extract prepared as galenic product—detailed description of the method and its suitability was given previously by Calvi et al., 2018 [12].
Although CBD is a principal constituent of the examined cannabis oil extracts, the original plant is only capable of producing its acid form, cannabidiolic acid (CBDA). Decarboxylation of CBDA catalysed by thermal exposure during extraction conditions leads to the conversion of CBDA to the CBD as the corresponding decarboxylated (neutral) counterpart. Therefore, the determination of CBDA is important in order to evaluate the CBDA decarboxylation rate and effectiveness of the reaction during the extraction process [31]. Interestingly, looking through the web sites of the CBD oil producers enrolled in this survey, it can be found that some of them published an analytical report in which only the total CBD content as the sum of CBD + (0.877 CBDA) is reported. This is quite problematic as the biological effects of the neutral and acidic forms are remarkably different [5]. Generally, expressing the CBD content as a sum of the acidic and neutral forms is conditioned by the analytical method applied. Concretely, it occurs when gas chromatography (GC), one of the most commonly used analytical platforms for cannabinoid analysis, is used [31]. It involves the heating of the sample at high temperature in the injector prior to the chromatographic separation that leads inevitably to the decarboxylation of the cannabinoid acids. Therefore, the analytical result is the sum of the acid and neutral forms. The GC method is still officially employed by the authorities for the determination of cannabinoids, but obviously is unsuitable. A few research groups continue to suggest that an accurate cannabinoid profile should be evaluated by determining the acid and neutral forms separately [12,31]. Results obtain in this study confirms this necessity. Employing the LC-HRMS technology, we were able to distinguish the acidic form from neutral CBD, and to examine the wide concentration range. As can be seen in Table 1, in the majority of the samples the CBDA concentration was found to be negligible compared to the amount of CBD (for example, samples Oil_4 and Oil_15). On the contrary, there were a few samples with a significant amount of CBDA. A striking example is Oil_3, in which the CBDA content exceeded CBD, and only the sum of both forms justified the CBD percentage declared by the producer. Furthermore, it is evident that the label concentration of CBD in Oil_12 is reached only when the sum of both forms is considered, bearing in mind the significant amount of CBDA.
Nevertheless, all producers underline that their manufacturing methods yield the so called full spectrum extract, which means that hemp extracts contain different phyto-cannabinoids, including THC, CBN, CBG, THCA, CBGA and others, depending from cannabis strain and extraction method. In order to achieve full-spectrum in a hemp extract, the profile of bioactive compounds that a plant flower contains must be transferred into the extract itself without compromising any aspect of the profile.
In comparison with previous works available on cannabis oil [9,10,11,31] we employed a HRMS method that provided more complete information regarding the cannabinoids profile and amount in the oil composition. Actually, besides CBD as a principal cannabinoid, we were able to detect and to quantify the six most significant cannabinoids, including the essential ones (THC, THCA and CBDA) along with quantification of CBN, CBG and CBGA. The obtained results clearly show that 12 out of 14 samples contained THC which is attention-grabbing because of its potential intoxicating activity. The THC content showed the considerable variability in the analysed samples, but was mainly at the levels describable as low (0.2%) [17]. Only one among all THC-positive sample (Oil_6) contained a considerable amount of THC (0.35%), which is matter of concern because the manufacturer declared the product to be THC-free. This result highlights the importance of also specifying the amount of THC or any another intoxicating cannabinoid present in commercialised CBD oils.
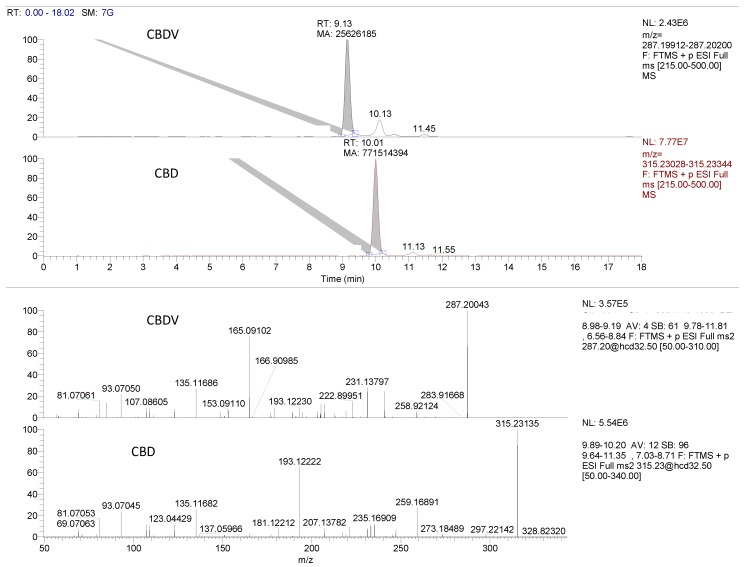
CBN was quantifiable in the vast majority of samples (except Oil_14). Its detection is of great importance as it is not considered to be a natural cannabinoid but rather an artefact formed by THC oxidation during plant aging, by use of an inadequate extraction procedure or inappropriate storage conditions [32]. Therefore, its determination may assist in the evaluation of the quality of CBD oils with regards to the raw plant material used, extraction method applied and storage. For example, in the sample Oil_13 the quantity of the CBN was more than twice the amount of THC. Considering CBN as a degradation product of THC, it would be better to think through the sum of THC+CBN as a relevant parameter for the evaluation of initial THC concentration in the oil extract. In addition, CBN, though much less psychoactive than THC, express sedative effects [33,34] which is why its content should be indicated on the label along with THC. It is well known that THC derives from the decarboxylation of tetrahydrocannabinolic acid (THCA) [20], and this is the reason why the amount of THCA was quantified in this study. Our results did not reveal any significant presence of either THCA (Table 1) or cannabigerolic acid (CBGA) which is the precursor of the all other cannabinoid acids. CBGA gives by decarboxylation cannabigerol (CBG) that either was completely absent or present in minor quantities. The quantification of CBGA and CBG did not turn out to be imperative, but their presence could serve as a confirmation that the oil sample contains a natural, full spectrum cannabis extract. Furthermore, employing the retrospective analysis, several other minor “untargeted” compounds were detected by means of the Orbitrap (Thermo Fisher Scientific, San Jose, CA, USA) ® analyser. Among others (data not shown) it is important to highlight the persistent occurrence of CBDV in all analysed samples. Figure 1 shows the fragmentation pattern of CBDV and CBD. Bearing in mind that this compound has expressed significant physiological activity [33] and that accompanies the CBD as its analogue, it should be included in any quality evaluation of full spectrum CBD oil preparations. Besides, we noticed that when the hemp seed oil was used as matrix, the signal of CBDV augments notably, which means that maybe one portion of CBDV derives from hemp oil, not from flower extract [34].
Figure 1.
Retrospective data analysis reveals the occurrence of CBDV: full MS-dd-MS2 chromatogram and relative fragmentation pattern of parent ion (287.20048) obtained in dd-MS2 acquisition mode. For the comparison, the CBD signal and fragmentation pattern is also presented.
Bedrolite oil extract (Oil_1) obtained by a recently published procedure [12], is a defined galenic formulation that has been used for distinct therapeutic purposes. It was included in this study as a “reference material” from a well-defined starting material (cannabis plant variety) and made using a standardized/authorized preparation procedure. There are at least two reasons to use the cannabinoid profile of Bedrolite oil extract as a reference point in the evaluation of CBD-rich hemp oils. Firstly, it can be considered as a full-spectrum extract that preserves the natural ratios of cannabinoids, any impurities that can compromise the experiments should be absent. Secondly, many consumers tend to replace galenic oil preparations (such Bedrolite oil extract) with CBD-rich hemp oil extract, due to the fact that a medical prescription is required for the former. Our study revealed that Bedrolite oil extract contains 0.8% of CBD. This is in agreement with theoretical percentage (0.9%) that should be found in the Bedrolite oil extract: the inflorescence contains 9% of CBD and the dilution ratio during the extraction is 1:10. However, as regards the cannabinoids profile (Table 1), it is evident that the quantities of CBDA, THC, THCA and CBGA are inferior compared with CBD-rich hemp oil extracts. These data are of great importance as they highlight the reduced concentration of all cannabinoids in Bedrolite oil extract compared to CBD hemp oil extract.
The reasons for all the abovementioned variations between examined samples are numerous and multiple. The final composition of CBD-rich hemp oil extracts depends on the chemotype and quality of the industrial hemp used, but it is also conditioned by the extraction method applied. Unfortunately, not all producers indicate the extraction method used. Only four declared the use of supercritical CO2 fluid extraction, which is shown to be the method of choice in that the low temperature and inert atmosphere results in higher CBD yields [18,19]. However, the main drawback of this technology is its high cost, and it is reasonable to assume that solvent extraction is also used for the inexpensive industrial processing. However, it is questionable if this is a correct choice for a product for human consumption because residual solvents (typically hexane, ethanol, isopropyl alcohol, toluene, benzene, xylene and acetone) may contaminate the final product [32]. Without having complete information on the methods of CBD oils preparation, we investigated the occurrence of the most frequently used extraction solvents as solvent residues. Our analysis revealed the sporadic incidence of acetone (Table 2, ketones section) that is more probably present as a lipid oxidation product rather than as a true residual solvent. Nevertheless, the presence of some volatile compounds that might be considered as problematic impurities from solvents residues was detected (Table 2, miscellaneous section). Namely, the samples Oil_4 and Oil_6 showed the presence of 1,3-dimethylbenzene while in the sample Oil_3, 1,2,4-trimethylbenzene was detected. Those aromatic compounds were not present in galenic preparation (Oil_1).
Table 2.
Volatile compounds profile extracted by using HS-SPME and GC/MS from CBD oils samples.
| Oil Samples | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | ||||||||||
| Matrix | FU Oil | Hemp Seed Oil | Olive Oil | MCT Oil | Olive Oil | Hemp Seed Oil | Olive Oil | Hemp Seed Oil | ||||||||||
| RI a | R.T b | Compound |
Average
c
μg/g |
SD
(±) |
Average c
μg/g |
SD
(±) |
Average c
μg/g |
SD
(±) |
Average c
μg/g |
SD
(±) |
Average c
μg/g |
SD
(±) |
Average c
μg/g |
SD
(±) |
Average c
μg/g |
SD
(±) |
Average c
μg/g |
SD
(±) |
| Alcohols | ||||||||||||||||||
| 831 | 20.63 | 1-Hexanol | 2.08 | 0.14 | 5.15 | 0.71 | n.d. | - | n.d. | - | 2.55 | 0.16 | 8.10 | 0.12 | 2.58 | 0.03 | 11.52 | 0.37 |
| 868 | 21.43 | 3-Hexen-1-ol | 0.66 | 0.07 | 0.67 | 0.11 | n.d. | - | 0.55 | 0.02 | 1.47 | 0.08 | 1.46 | 0.04 | 1.76 | 0.05 | n.d. | - |
| 849 | 22.02 | 2-Hexen-1-ol | n.d. | - | n.d. | - | n.d. | - | n.d. | - | 0.77 | 0.11 | n.d. | - | 1.10 | - | n.d. | - |
| 969 | 23.07 | 1-Octen-3-ol | n.d. | - | 3.90 | 0.47 | n.d. | - | n.d. | - | 0.73 | 0.15 | 1.73 | 0.03 | 1.09 | 0.51 | n.d. | - |
| 960 | 23.17 | 1-Heptanol | n.d. | - | 0.83 | 0.13 | n.d. | - | n.d. | - | 0.50 | 0.01 | 2.49 | 0.10 | n.d. | - | n.d. | - |
| 1059 | 25.48 | 1-Octanol | n.d. | - | n.d. | - | 1.29 | 0.11 | n.d. | - | 0.59 | 0.03 | 5.23 | 0.63 | n.d. | - | n.d. | - |
| 1068 | 27.98 | 3,3,6-Trimethyl-1,5-heptadien-4-ol | 2.39 | 0.46 | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - |
| 1036 | 31.59 | α-Toluenol | 0.42 | 0.03 | 2.74 | 0.59 | n.d. | - | n.d. | - | 2.36 | 0.16 | 3.96 | 0.47 | 3.22 | 0.23 | n.d. | - |
| 1136 | 32.06 | Benzeneethanol | 0.51 | 0.04 | 1.39 | 0.32 | n.d. | - | n.d. | - | 2.73 | 0.16 | 5.05 | 0.58 | 3.43 | 0.22 | 0.76 | 0.01 |
| Total | 6.06 | 14.68 | 1.29 | 0.55 | 11.70 | 28.01 | 13.18 | 12.28 | ||||||||||
| Aldehydes | ||||||||||||||||||
| 508 | 2.31 | Propanal | n.d. | - | n.d. | - | 0.77 | 0.03 | n.d. | - | 0.96 | 0.04 | 1.10 | 0.08 | 0.97 | 0.05 | n.d. | - |
| 574 | 3.23 | 2-Methyl-2-propenal | n.d. | - | n.d. | - | 0.61 | 0.03 | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - |
| 643 | 3.74 | 2-Methyl-butanal | 1.73 | 0.02 | 0.56 | 0.02 | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - |
| 643 | 3.83 | 3-Methyl-butanal | 0.90 | 0.16 | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - |
| 785 | 10.12 | Hexanal | 0.70 | 0.02 | 5.57 | 0.14 | 10.15 | 0.29 | n.d. | - | 2.97 | 0.17 | 6.89 | 0.01 | 2.60 | 0.15 | 3.42 | 0.03 |
| 905 | 15.05 | Heptanal | 0.76 | 0.03 | n.d. | - | 1.03 | 0.03 | n.d. | - | n.d. | - | n.d. | - | 0.67 | 0.10 | 0.62 | 0.03 |
| 814 | 16.24 | 2-Hexenal | n.d. | - | n.d. | - | n.d. | - | n.d. | - | 1.19 | 0.12 | n.d. | - | 1.41 | 0.03 | n.d. | - |
| 1005 | 18.74 | Octanal | n.d. | - | n.d. | - | 2.47 | 0.02 | n.d. | - | 2.03 | 0.23 | 17.40 | 0.16 | 1.87 | 0.03 | n.d. | - |
| 913 | 19.72 | 2-Heptenal | n.d. | - | 3.10 | 0.05 | 2.61 | 0.22 | n.d. | - | 1.86 | 0.09 | 6.44 | 0.41 | 1.59 | 0.03 | 1.22 | 0.05 |
| 1104 | 21.67 | Nonanal | 0.73 | 0.16 | n.d. | - | 2.63 | 0.30 | n.d. | - | n.d. | - | 4.92 | 0.03 | 1.07 | - | n.d. | - |
| 1013 | 22.53 | 2-Octenal | n.d. | - | n.d. | - | 0.68 | 0.05 | n.d. | - | n.d. | - | 2.07 | 0.08 | n.d. | - | 0.61 | 0.02 |
| 921 | 24.01 | 2,4-Heptadienal | n.d. | - | 1.27 | 0.05 | 0.74 | 0.14 | n.d. | - | n.d. | - | 7.97 | 0.34 | n.d. | - | n.d. | - |
| 982 | 24.77 | Benzaldehyde | n.d. | - | n.d. | - | n.d. | - | n.d. | - | 1.86 | 0.01 | 25.84 | 0.45 | n.d. | - | n.d. | - |
| 1174 | 28.04 | 3,7-Dimethyl-2,6-octadienal | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | 44.49 | 4.40 | n.d. | - | n.d. | - |
| Total | 4.82 | 10.50 | 21.68 | n.d. | 10.87 | 117.14 | 10.19 | 5.88 | ||||||||||
| Esters | ||||||||||||||||||
| 487 | 2.63 | Acetic acid-methyl ester | 0.78 | 0.02 | 0.72 | 0.04 | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - |
| 586 | 3.33 | Acetic acid-ethyl ester | n.d. | - | 8.70 | 0.12 | 302.74 | 12.32 | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - |
| 686 | 5.35 | Acetic acid-propyl ester | n.d. | - | n.d. | - | 1.45 | 0.07 | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - |
| 721 | 6.77 | Acetic acid-2-methyl-propyl ester | n.d. | - | n.d. | - | 5.39 | 0.06 | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - |
| 785 | 9.76 | Acetic acid-buthyl ester | n.d. | - | n.d. | - | 1.17 | 0.11 | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - |
| 820 | 12.27 | 1-Butanol-3-methyl acetate | n.d. | - | n.d. | - | 7.46 | 0.17 | n.d. | - | n.d. | - | 16.98 | 0.21 | n.d. | - | n.d. | - |
| 992 | 19.62 | 3-Hexen-1-ol-acetate | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | 0.64 | 0.02 | n.d. | - |
| 1183 | 22.24 | Butanoic acid-hexyl ester | n.d. | - | n.d. | - | n.d. | - | 0.60 | 0.02 | n.d. | - | n.d. | - | n.d. | - | n.d. | - |
| Total | 0.78 | 9.42 | 318.21 | 0.60 | n.d. | 16.98 | 0.64 | n.d. | ||||||||||
| Ketones | ||||||||||||||||||
| 455 | 2.50 | 2-Propanone | 1.76 | 0.13 | 3.50 | 0.47 | 8.56 | 0.57 | n.d. | - | 2.46 | 0.51 | 5.03 | 0.65 | 1.78 | 0.14 | 0.96 | 0.01 |
| 1161 | 13.19 | 1-(1,3-dimethyl-3-cyclohexen-1-yl)-Ethanone | n.d. | - | 1.89 | 0.18 | n.d. | - | n.d. | - | n.d. | - | 2.07 | 0.15 | n.d. | - | 0.90 | 0.06 |
| 853 | 14.89 | 2-Heptanone | n.d. | - | 3.32 | 0.09 | 0.71 | 0.02 | n.d. | - | 2.69 | 0.16 | n.d. | - | 2.74 | 0.06 | 1.00 | 0.06 |
| 952 | 18.57 | 2-Octanone | n.d. | - | 4.63 | 0.64 | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - |
| 960 | 19.99 | 6-Octen-2-one | n.d. | - | 0.86 | 0.11 | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - |
| 987 | 20.17 | 6-Methyl-5 hepten-2 one | 1.25 | 0.04 | 8.97 | 1.20 | 10.55 | 0.42 | n.d. | - | 2.28 | 0.11 | 17.04 | 0.14 | 1.63 | 0.00 | 2.26 | 0.13 |
| 960 | 21.96 | 3-Octen-2-one | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - |
| 962 | 22.51 | Ketone | 0.69 | 0.02 | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - |
| 968 | 24.67 | 3,5-Octadien-2-one | n.d. | - | n.d. | - | 4.94 | 0.41 | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - |
| Total | 3.70 | 23.17 | 24.77 | n.d. | 7.44 | 24.14 | 6.15 | 5.12 | ||||||||||
| Terpenes | ||||||||||||||||||
| 939 | 6.67 | α-Pinene | 14.25 | 0.55 | 21.47 | 0.38 | n.d. | - | 73.23 | 9.63 | 112.95 | 1.47 | 5883.13 | 22.05 | 119.09 | 0.23 | 52.84 | 1.11 |
| 932 | 7.03 | α-Thujene | 0.98 | 0.17 | 1.18 | 0.08 | n.d. | - | 0.79 | 0.08 | 2.86 | 0.19 | 44.54 | 1.08 | 3.24 | 0.10 | n.d. | - |
| 961 | 8.67 | Camphene | n.d. | - | n.d. | - | n.d. | - | 0.94 | 0.27 | 1.57 | 0.09 | 65.97 | 3.91 | 1.60 | 0.01 | 0.95 | 0.03 |
| 989 | 10.75 | β-Pinene | 4.15 | 0.05 | 4.45 | 0.08 | n.d. | - | 23.36 | 4.71 | 35.65 | 2.06 | 625.28 | 4.35 | 37.08 | 0.48 | 17.91 | 0.28 |
| 985 | 11.66 | Sabinene | n.d. | - | 1.26 | 0.07 | n.d. | - | n.d. | - | 1.66 | 0.01 | 98.17 | 0.06 | 1.53 | 0.08 | n.d. | - |
| 879 | 11.74 | 2,4(10)-Thujadien | n.d. | - | 0.85 | 0.00 | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - |
| 1017 | 12.99 | δ-3-Carene | 1.73 | 0.03 | 0.61 | 0.09 | n.d. | - | 7.42 | 1.33 | 50.22 | 1.18 | 4.69 | 0.31 | 51.31 | 2.68 | 6.17 | 0.50 |
| 1015 | 13.81 | α-Phellandrene | 3.70 | 0.14 | 3.90 | 0.09 | n.d. | - | 2.91 | 0.65 | 6.78 | 0.01 | 4.97 | 0.04 | 8.06 | 0.18 | 1.85 | 0.09 |
| 991 | 14.24 | β-Myrcene | 108.13 | 0.09 | 34.63 | 1.00 | 9.14 | 0.48 | 419.53 | 33.32 | 389.37 | 20.60 | 2908.00 | 60.01 | 419.01 | 6.04 | 189.05 | 1.74 |
| 1026 | 14.44 | α-Terpinene | 3.60 | 0.38 | 10.01 | 0.56 | n.d. | - | 2.44 | 0.07 | 5.51 | 0.17 | 34.87 | 3.09 | 6.82 | 0.12 | 1.74 | 0.01 |
| 1038 | 15.31 | Limonene | 6.05 | 0.01 | 8.03 | 0.44 | 1.60 | 0.07 | 65.17 | 7.36 | 23.67 | 1.33 | 8841.42 | 171.34 | 20.17 | 0.26 | 23.97 | 0.34 |
| 1045 | 15.49 | Eucalyptol | 3.67 | 0.01 | 5.60 | 0.35 | 7.15 | 0.26 | 2.65 | 0.13 | 4.18 | 0.27 | 13.66 | 1.60 | 3.09 | 0.07 | 8.62 | 0.12 |
| 946 | 15.61 | β-Phellandrene | 7.16 | 0.14 | 4.80 | 0.52 | n.d. | - | 8.40 | 0.80 | 19.02 | 1.05 | 61.90 | 4.71 | 20.05 | 0.16 | 7.38 | 0.22 |
| 976 | 17.05 | Cis-ocimene | 0.48 | 0.02 | 2.17 | 0.17 | 0.48 | 0.00 | 16.55 | 1.07 | 21.17 | 0.88 | 7.13 | 0.24 | 19.24 | 0.23 | 5.50 | 0.11 |
| 1066 | 17.15 | γ-Terpinene | 5.38 | 0.01 | 5.84 | 0.58 | 0.59 | 0.06 | 2.11 | 0.09 | 4.80 | 0.23 | 499.58 | 9.26 | 4.61 | 0.08 | 2.48 | 0.04 |
| 1000 | 17.24 | Terpene | n.d. | - | n.d. | - | 2.48 | 0.24 | n.d. | - | 2.59 | 0.02 | 17.49 | 0.05 | 1.82 | 0.04 | n.d. | - |
| 1029 | 17.60 | β-Ocimene | 21.75 | 1.08 | 7.55 | 0.53 | 9.96 | 0.48 | 194.00 | 21.13 | 192.39 | 5.19 | 22.82 | 0.17 | 213.15 | 1.26 | 42.00 | 1.72 |
| 1034 | 18.01 | p-Cymene | 3.13 | 0.11 | 8.53 | 0.97 | 0.81 | 0.01 | 2.98 | 0.33 | 12.37 | 0.05 | 144.55 | 1.92 | 10.02 | 0.15 | 5.62 | 0.09 |
| 1094 | 18.43 | α-Terpinolene | 62.12 | 0.67 | 7.48 | 0.80 | n.d. | - | 111.31 | 14.58 | 265.95 | 16.97 | 33.73 | 0.11 | 297.20 | 3.11 | 73.16 | 3.13 |
| 1177 | 22.74 | Para-cymenyl | 13.97 | 0.88 | 11.28 | 0.16 | n.d. | - | 3.30 | 0.82 | 64.48 | 5.97 | 134.94 | 1.32 | 49.21 | 0.39 | 4.70 | 0.24 |
| 1136 | 23.01 | Terpene | n.d. | - | n.d. | - | n.d. | - | 1.55 | 0.36 | 1.39 | 0.11 | n.d. | - | 1.43 | 0.05 | n.d. | - |
| 1083 | 23.35 | 4,8-Epoxy-p-menth-1-ene | 1.30 | 0.08 | 2.08 | 0.23 | n.d. | - | 2.22 | 0.03 | 6.44 | 0.16 | 3.08 | 0.03 | 5.20 | 0.60 | 1.32 | 0.01 |
| 1164 | 23.48 | Linalool oxide | n.d. | - | n.d. | - | n.d. | - | n.d. | - | 1.11 | 0.03 | 17.95 | 0.94 | 1.51 | 0.01 | n.d. | - |
| 1221 | 23.68 | α-Ylangene | n.d. | - | 1.09 | 0.23 | n.d. | - | n.d. | - | 0.52 | 0.00 | 2.19 | 0.09 | n.d. | - | 1.18 | 0.07 |
| 1082 | 25.29 | β-Linalool | 5.52 | 0.81 | 2.22 | 0.32 | 10.55 | 1.27 | 0.82 | 0.01 | 5.19 | 0.01 | 1471.75 | 15.90 | 5.36 | 0.37 | n.d. | - |
| 1494 | 25.75 | γ-Caryophyllene | n.d. | - | 5.87 | 1.68 | 2.02 | 0.24 | 1.03 | 0.06 | 1.81 | 0.05 | 30.72 | 1.10 | 1.32 | 0.04 | 4.40 | 0.17 |
| 1430 | 26.03 | α-Bergamotene | 2.42 | 0.21 | 11.89 | 3.95 | 1.75 | 0.08 | 2.21 | 0.19 | 2.31 | 0.02 | 40.96 | 0.21 | 1.01 | 0.08 | 9.44 | 0.07 |
| 1456 | 26.12 | α-Guaiene | 2.46 | 0.05 | n.d. | - | 7.19 | 0.57 | n.d. | - | n.d. | - | 5.20 | 0.18 | n.d. | - | n.d. | - |
| 1494 | 26.23 | Trans-caryophyllene | 17.34 | 1.81 | 159.92 | 49.08 | 90.68 | 7.15 | 40.67 | 3.44 | 48.77 | 1.93 | 425.63 | 7.84 | 32.06 | 4.48 | 110.41 | 1.33 |
| Terpenes | ||||||||||||||||||
| 1209 | 26.40 | 4-Terpineol | 2.32 | 0.23 | 3.34 | 0.62 | 0.55 | 0.06 | n.d. | - | 2.80 | 0.24 | 15.34 | 1.66 | 1.71 | 0.18 | n.d. | - |
| 1440 | 26.59 | Sesquiterpene | n.d. | - | 3.03 | 0.70 | 1.37 | 0.18 | n.d. | - | 1.64 | 0.07 | 17.73 | 0.11 | n.d. | - | 1.13 | 0.10 |
| 1386 | 27.21 | Sesquiterpene | n.d. | - | 6.09 | 2.17 | n.d. | - | n.d. | - | 0.58 | 0.05 | 12.62 | 0.59 | n.d. | - | 7.01 | 0.26 |
| 1131 | 27.47 | Trans-pinocarveol | n.d. | - | 9.69 | 1.69 | n.d. | - | n.d. | - | 3.04 | 0.07 | 10.99 | 0.49 | 2.30 | 0.08 | 5.12 | 0.08 |
| 1482 | 27.73 | α-Humulene | 6.28 | 0.44 | 45.95 | 15.88 | 21.61 | 1.49 | 10.13 | 1.54 | 9.62 | 0.69 | 117.14 | 3.27 | 5.94 | 0.88 | 32.25 | 1.15 |
| 1189 | 28.13 | 1,8-Menthadien-4-ol | 6.23 | 1.02 | 21.18 | 4.16 | n.d. | - | 2.19 | 0.43 | 19.68 | 0.44 | 44.91 | 2.39 | 12.61 | 0.58 | 6.86 | 0.41 |
| 1209 | 28.32 | α-Terpineol | 2.79 | 0.43 | 3.31 | 0.85 | 1.13 | 0.02 | n.d. | - | 2.90 | 0.24 | 14.35 | 1.10 | 1.83 | 0.04 | 0.63 | 0.07 |
| 1189 | 28.40 | Borneol | 0.52 | 0.04 | 2.78 | 0.74 | n.d. | - | n.d. | - | 0.64 | 0.04 | 1.59 | 2.25 | n.d. | - | n.d. | - |
| 1490 | 28.65 | δ-Guaiene | 1.56 | 0.01 | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - |
| 1519 | 28.72 | β-Selinene | 0.85 | 0.01 | 11.64 | 4.28 | 1.65 | 0.02 | 0.96 | 0.19 | 1.46 | 0.13 | 41.02 | 1.77 | 0.85 | 0.17 | 8.15 | 0.36 |
| 1522 | 28.82 | α-Selinene | 1.04 | 0.01 | 7.51 | 2.69 | 1.06 | 0.14 | n.d. | - | 1.04 | 0.06 | 26.41 | 1.64 | n.d. | - | 4.51 | 0.20 |
| 1474 | 28.98 | Sesquiterpene | n.d. | - | 1.88 | 0.58 | n.d. | - | n.d. | - | n.d. | - | 53.97 | 6.99 | n.d. | - | 0.92 | 0.02 |
| 1190 | 29.03 | Carvone | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - |
| 1507 | 29.86 | Selina-3,7(11)-diene | n.d. | - | 6.79 | 2.41 | n.d. | - | 0.96 | 0.31 | 1.85 | 0.06 | 25.33 | 2.61 | n.d. | - | 3.78 | 0.21 |
| 1191 | 30.13 | Myrtenol | n.d. | - | 1.49 | 0.26 | n.d. | - | n.d. | - | 0.94 | 0.04 | 3.18 | 0.40 | n.d. | - | 0.80 | 0.03 |
| 1284 | 31.17 | Cuminol | 4.00 | 0.64 | 7.44 | 1.98 | n.d. | - | 0.54 | 0.07 | 7.87 | 0.61 | 30.26 | 3.84 | 7.02 | 0.08 | 1.46 | 0.10 |
| 1322 | 33.41 | Humulene oxide | n.d. | - | 2.22 | 0.92 | 0.72 | 0.13 | n.d. | - | n.d. | - | n.d. | - | n.d. | - | 1.36 | 0.14 |
| 1419 | 34.11 | Sesquiterpene | n.d. | - | n.d. | - | 1.54 | 0.03 | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - |
| 1392 | 34.86 | Eugenol | n.d. | - | n.d. | - | n.d. | - | n.d. | - | 0.82 | 0.00 | 1.12 | 0.05 | 1.17 | 0.02 | n.d. | - |
| Total | 314.90 | 457.03 | 174.05 | 1000.37 | 1339.56 | 21860.3 | 1367.63 | 644.70 | ||||||||||
| Miscellaneous | ||||||||||||||||||
| 906 | 11.03 | 3,3,6-Trimethyl-1,5-heptadiene | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | 11.87 | 0.33 | n.d. | - | n.d. | - |
| 907 | 12.72 | 1,3-Dimethyl-benzene | n.d. | - | 0.96 | 0.08 | n.d. | - | 2.17 | 0.38 | n.d. | - | 1.41 | 0.02 | n.d. | - | n.d. | - |
| 1040 | 16.87 | 2-Pentyl-furan | n.d. | - | 1.82 | 0.20 | n.d. | - | n.d. | - | 0.24 | 0.01 | 3.94 | 0.10 | n.d. | - | 1.99 | 0.07 |
| 1020 | 18.31 | 1,2,4,-Trimethyl-benzene | n.d. | - | n.d. | - | 1.35 | 0.38 | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - |
| 894 | 19.54 | 2,5-Dimethyl-pyrazine | 1.03 | 0.05 | n.d. | - | n.d. | - | n.d. | - | n.d. | - | 1.23 | 0.04 | n.d. | - | n.d. | - |
| 891 | 19.74 | 2,6-Dimethyl-pyrazine | 0.82 | 0.03 | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - |
| 1176 | 20.72 | 1,4-Bis (1-methylethyl)-benzene | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | 1.81 | 0.01 | n.d. | - | n.d. | - |
| 985 | 21.89 | 2,6-Dimethyl-2,6-octadiene | n.d. | - | n.d. | - | 3.25 | 0.14 | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - |
| 1081 | 22.10 | Diethyl carbitol | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - |
| 1039 | 22.36 | 1,3,5-Trimethylenecycloheptane | n.d. | - | 1.07 | 0.26 | n.d. | - | 0.80 | 0.12 | 1.52 | 0.00 | 2.62 | 0.06 | 1.70 | 0.02 | n.d. | - |
| 986 | 28.45 | 5-Ethyldihydro-2(3H)-furanone | n.d. | - | 1.92 | 0.14 | n.d. | - | n.d. | - | 3.89 | 0.41 | 27.39 | 2.75 | 6.63 | 0.41 | 0.96 | 0.15 |
| 1190 | 30.85 | 1-Methoxy-4(1-propenyl)-benzene | n.d. | - | n.d. | - | n.d. | - | 3.22 | 0.07 | n.d. | - | n.d. | - | n.d. | - | n.d. | - |
| Total | 1.85 | 7.19 | 4.60 | 6.19 | 8.38 | 54.23 | 10.93 | 2.95 | ||||||||||
| Oil Samples | 9 | 10 | 11 | 12 | 13 | 14 | 15 | |||||||||||
| Matrix | Hemp Seed Oil | Olive Oil | Hemp Seed Oil | Hemp Seed Oil | Olive Oil | Hemp Seed Oil | Hemp Seed Oil | |||||||||||
| RI a | R.T b | Compound |
Average c
μg/g |
SD
(±) |
Average c
μg/g |
SD
(±) |
Average c
μg/g |
SD
(±) |
Average c
μg/g |
SD
(±) |
Average c
μg/g |
SD
(±) |
Average c
μg/g |
SD
(±) |
Averagec
μg/g |
SD
(±) |
||
| Alcohols | ||||||||||||||||||
| 831 | 20.63 | 1-Hexanol | 6.19 | 0.32 | 2.66 | 0.08 | 2.00 | 0.14 | 4.37 | 0.20 | 0.67 | 0.02 | 3.52 | 0.23 | 9.67 | 0.16 | ||
| 868 | 21.43 | 3-Hexen-1-ol | n.d. | - | 1.81 | 0.03 | n.d. | - | n.d. | - | 0.65 | 0.01 | 0.86 | 0.06 | n.d. | - | ||
| 849 | 22.02 | 2-Hexen-1-ol | n.d. | - | 1.15 | 0.05 | n.d. | - | 0.62 | 0.07 | 0.63 | 0.00 | n.d. | - | n.d. | - | ||
| 969 | 23.07 | 1-Octen-3-ol | 1.08 | 0.03 | 0.79 | 0.01 | 1.26 | 0.13 | 1.23 | 0.07 | n.d. | - | 1.35 | 0.00 | n.d. | - | ||
| 960 | 23.17 | 1-Heptanol | n.d. | - | n.d. | - | n.d. | - | 0.72 | 0.04 | n.d. | - | n.d. | - | n.d. | - | ||
| 1059 | 25.48 | 1-Octanol | n.d. | - | 0.58 | 0.11 | n.d. | - | n.d. | - | n.d. | - | 2.30 | 0.25 | n.d. | - | ||
| 1068 | 27.98 | 3,3,6-Trimethyl-1,5-heptadien-4-ol | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | 1.56 | 0.10 | n.d. | - | ||
| 1036 | 31.59 | α-Toluenol | n.d. | - | 3.03 | 0.35 | n.d. | - | 1.39 | 0.15 | n.d. | - | 2.08 | 0.61 | n.d. | - | ||
| 1136 | 32.06 | Benzeneethanol | 0.62 | 0.03 | 3.07 | 0.62 | n.d. | - | 1.25 | 0.01 | 0.87 | 0.13 | 2.72 | 0.98 | n.d. | - | ||
| Total | 7.89 | 13.09 | 3.26 | 9.57 | 2.82 | 14.38 | 9.67 | |||||||||||
| Aldehydes | ||||||||||||||||||
| 508 | 2.31 | Propanal | n.d. | - | 1.04 | 0.00 | n.d. | - | n.d. | - | 0.97 | 0.02 | 0.62 | 0.10 | n.d. | - | ||
| 574 | 3.23 | 2-Methyl-2-propenal | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | ||
| 643 | 3.74 | 2-Methyl-butanal | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | ||
| 643 | 3.83 | 3-Methyl-butanal | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | ||
| 785 | 10.12 | Hexanal | 6.35 | 0.40 | 2.53 | 0.01 | 10.51 | 0.36 | 7.04 | 0.29 | 3.18 | 0.36 | 2.60 | 0.18 | 1.80 | 0.12 | ||
| 905 | 15.05 | Heptanal | n.d. | - | 0.63 | 0.03 | 0.46 | 0.02 | n.d. | - | n.d. | - | n.d. | - | n.d. | - | ||
| 814 | 16.24 | 2-Hexenal | 1.23 | 0.06 | 1.41 | 0.03 | 1.02 | 0.02 | 0.53 | 0.75 | 11.50 | 0.40 | 0.82 | 0.04 | n.d. | - | ||
| 1005 | 18.74 | Octanal | n.d. | - | 1.92 | 0.07 | n.d. | - | 1.93 | 0.32 | n.d. | - | n.d. | - | n.d. | - | ||
| 913 | 19.72 | 2-Heptenal | 1.17 | 0.05 | 1.72 | 0.09 | 5.21 | 0.57 | 8.29 | 0.77 | 1.54 | 0.00 | 5.44 | 0.12 | 1.11 | 0.02 | ||
| 1104 | 21.67 | Nonanal | n.d. | - | 1.20 | 0.14 | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | ||
| 1013 | 22.53 | 2-Octenal | 1.49 | 0.02 | n.d. | - | n.d. | - | 0.95 | 0.00 | n.d. | - | n.d. | - | n.d. | - | ||
| 921 | 24.01 | 2,4-Heptadienal | 8.05 | 0.11 | n.d. | - | 2.31 | 0.19 | 1.37 | 0.20 | n.d. | - | 1.24 | 0.02 | n.d. | - | ||
| 982 | 24.77 | Benzaldehyde | 1.45 | 0.18 | n.d. | - | 1.90 | 1.67 | 1.55 | 0.31 | 0.43 | 0.10 | 8.46 | 3.09 | n.d. | - | ||
| 1174 | 28.04 | 3,7-Dimethyl-2,6-octadienal | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | 1.30 | 0.07 | n.d. | - | ||
| Total | 19.73 | 10.43 | 21.41 | 21.66 | 17.61 | 20.48 | 2.92 | |||||||||||
| 487 | 2.63 | Acetic acid-methyl ester | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | ||
| 586 | 3.33 | Acetic acid-ethyl ester | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | ||
| 686 | 5.35 | Acetic acid-propyl ester | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | ||
| 721 | 6.77 | Acetic acid-2-methyl-propyl ester | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | ||
| 785 | 9.76 | Acetic acid-buthyl ester | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | ||
| 820 | 12.27 | 1-Butanol-3-methyl acetate | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | ||
| 992 | 19.62 | 3-Hexen-1-ol-acetate | n.d. | - | 0.66 | 0.01 | n.d. | - | n.d. | - | 0.82 | 0.08 | n.d. | - | n.d. | - | ||
| 1183 | 22.24 | Butanoic acid-hexyl ester | n.d. | - | 0.53 | 0.04 | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | ||
| Total | n.d. | 1.19 | n.d. | n.d. | 0.82 | n.d. | n.d. | |||||||||||
| Ketones | ||||||||||||||||||
| 455 | 2.50 | 2-Propanone | 0.63 | 0.04 | 2.20 | 0.24 | 0.54 | 0.05 | 2.11 | 0.02 | 0.78 | 0.15 | 2.41 | 0.45 | n.d. | - | ||
| 1161 | 13.19 | 1-(1,3-dimethyl-3-cyclohexen-1-yl)-Ethanone | n.d. | - | n.d. | - | n.d. | - | 2.75 | 0.05 | n.d. | - | 0.89 | 0.05 | n.d. | - | ||
| 853 | 14.89 | 2-Heptanone | n.d. | - | 2.70 | 0.03 | 1.05 | 0.04 | 1.32 | 0.02 | n.d. | - | 1.45 | 0.36 | n.d. | - | ||
| 952 | 18.57 | 2-Octanone | n.d. | - | n.d. | - | 5.08 | 0.42 | 0.55 | 0.03 | n.d. | - | n.d. | - | n.d. | - | ||
| 960 | 19.99 | 6-Octen-2-one | n.d. | - | n.d. | - | 0.80 | 0.04 | n.d. | - | n.d. | - | n.d. | - | n.d. | - | ||
| 987 | 20.17 | 6-Methyl-5 hepten-2 one | 0.73 | 0.10 | 1.65 | 0.06 | 0.53 | 0.03 | 6.00 | 0.58 | 0.76 | 0.04 | 6.50 | 0.28 | n.d. | - | ||
| 960 | 21.96 | 3-Octen-2-one | n.d. | - | n.d. | - | 0.57 | 0.05 | n.d. | - | n.d. | - | n.d. | - | n.d. | - | ||
| 962 | 22.51 | Ketone | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | 0.71 | 0.01 | n.d. | - | ||
| 968 | 24.67 | 3,5-Octadien-2-one | 0.59 | 0.07 | n.d. | - | 1.44 | 0.17 | 1.44 | 0.13 | n.d. | - | 1.39 | 0.20 | n.d. | - | ||
| Total | 1.94 | 6.55 | 10.01 | 14.16 | 1.54 | 13.35 | n.d. | |||||||||||
| Terpenes | ||||||||||||||||||
| 939 | 6.67 | α-Pinene | 1.35 | 0.06 | 120.21 | 0.54 | 4.27 | 0.25 | 147.07 | 1.23 | n.d. | - | 94.47 | 3.96 | 2.27 | 0.07 | ||
| 932 | 7.03 | α-Thujene | n.d. | - | 2.86 | 0.13 | n.d. | - | 9.40 | 0.18 | n.d. | - | 2.16 | 0.11 | n.d. | - | ||
| 961 | 8.67 | Camphene | n.d. | - | 1.69 | 0.15 | n.d. | - | 3.04 | 0.02 | n.d. | - | 7.45 | 0.26 | n.d. | - | ||
| 989 | 10.75 | β-Pinene | 0.70 | 0.00 | 37.67 | 1.33 | 1.30 | 0.14 | 33.28 | 0.36 | n.d. | - | 21.91 | 0.34 | 0.87 | 0.01 | ||
| 985 | 11.66 | Sabinene | n.d. | - | 1.64 | 0.13 | n.d. | - | 0.71 | 0.05 | n.d. | - | n.d. | - | n.d. | - | ||
| 879 | 11.74 | 2,4(10)-Thujadien | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | ||
| 1017 | 12.99 | δ-3-Carene | n.d. | - | 48.70 | 4.87 | n.d. | - | 3.82 | 0.10 | n.d. | - | 6.63 | 0.99 | n.d. | - | ||
| 1015 | 13.81 | α-Phellandrene | n.d. | - | 7.37 | 0.11 | n.d. | - | 2.59 | 0.40 | n.d. | - | 3.70 | 0.60 | n.d. | - | ||
| 991 | 14.24 | β-Myrcene | 13.88 | 2.66 | 429.64 | 5.70 | 10.12 | 0.33 | 344.78 | 5.22 | n.d. | - | 74.69 | 5.07 | 10.65 | 0.30 | ||
| 1026 | 14.44 | α-Terpinene | n.d. | - | 4.29 | 0.26 | n.d. | - | 2.35 | 0.19 | n.d. | - | 2.95 | 0.49 | n.d. | - | ||
| 1038 | 15.31 | Limonene | 2.38 | 0.50 | 20.56 | 0.29 | 2.40 | 0.15 | 50.09 | 1.95 | n.d. | - | 23.56 | 1.63 | 5.53 | 0.46 | ||
| 1045 | 15.49 | Eucalyptol | n.d. | - | 3.13 | 0.02 | 0.41 | 0.06 | 15.41 | 0.72 | 0.56 | 0.09 | 17.91 | 0.72 | n.d. | - | ||
| 946 | 15.61 | β-Phellandrene | n.d. | - | 20.96 | 0.14 | n.d. | - | 7.16 | 0.27 | n.d. | - | 13.75 | 0.51 | n.d. | - | ||
| 976 | 17.05 | Cis-ocimene | n.d. | - | 19.76 | 0.13 | n.d. | - | 5.45 | 0.45 | n.d. | - | 7.22 | 0.43 | n.d. | - | ||
| 1066 | 17.15 | γ-Terpinene | n.d. | - | 4.77 | 0.03 | n.d. | - | 7.17 | 0.43 | n.d. | - | 4.59 | 0.24 | n.d. | - | ||
| Terpenes | ||||||||||||||||||
| 1000 | 17.24 | Terpene | 0.87 | 0.01 | 1.88 | 0.14 | n.d. | - | n.d. | - | n.d. | - | 1.30 | 0.17 | n.d. | - | ||
| 1029 | 17.60 | β-Ocimene | 4.44 | 0.07 | 217.33 | 2.36 | 2.42 | 0.28 | 40.93 | 2.46 | 0.88 | 0.01 | 24.18 | 1.39 | 2.91 | 0.27 | ||
| 1034 | 18.01 | p-Cymene | n.d. | - | 10.17 | 0.39 | 0.66 | 0.05 | 43.02 | 2.52 | 0.66 | 0.27 | 13.51 | 0.89 | n.d. | - | ||
| 1094 | 18.43 | α-Terpinolene | 3.55 | 0.19 | 249.23 | 0.57 | 0.76 | 0.07 | 52.02 | 3.15 | n.d. | - | 16.01 | 1.06 | 1.00 | 0.07 | ||
| 1177 | 22.74 | Para-cymenyl | 1.81 | 0.11 | 47.64 | 0.55 | 0.56 | 0.20 | 7.84 | 0.51 | 1.07 | 0.02 | 7.16 | 0.93 | n.d. | - | ||
| 1136 | 23.01 | Terpene | n.d. | - | 1.67 | 0.11 | n.d. | - | 0.53 | 0.06 | n.d. | - | n.d. | - | n.d. | - | ||
| 1083 | 23.35 | 4,8-Epoxy-p-menth-1-ene | n.d. | - | 5.65 | 0.01 | n.d. | - | 2.49 | 0.35 | n.d. | - | n.d. | - | n.d. | - | ||
| 1164 | 23.48 | Linalool oxide | 0.70 | 0.01 | 1.37 | 0.40 | n.d. | - | 2.96 | 0.25 | n.d. | - | n.d. | - | n.d. | - | ||
| 1221 | 23.68 | α-Ylangene | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | ||
| 1082 | 25.29 | β-Linalool | n.d. | - | 6.35 | 0.07 | n.d. | - | 3.98 | 0.45 | n.d. | - | 4.90 | 0.14 | n.d. | - | ||
| 1494 | 25.75 | γ-Caryophyllene | 8.60 | 1.21 | 1.63 | 0.09 | 0.57 | 0.00 | 5.30 | 0.59 | 1.23 | 0.05 | 8.86 | 1.07 | n.d. | - | ||
| 1430 | 26.03 | α-Bergamotene | 6.78 | 1.09 | 1.06 | 0.24 | 0.53 | 0.03 | 11.82 | 1.57 | 2.94 | 0.11 | 19.89 | 3.59 | n.d. | - | ||
| 1456 | 26.12 | α-Guaiene | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | 1.08 | 0.19 | n.d. | - | ||
| 1494 | 26.23 | Trans-caryophyllene | 78.99 | 10.43 | 39.18 | 2.69 | 4.72 | 0.14 | 92.75 | 9.99 | 15.78 | 0.51 | 228.18 | 33.12 | 1.62 | 0.28 | ||
| 1209 | 26.40 | 4-Terpineol | n.d. | - | 2.07 | 0.13 | n.d. | - | 3.74 | 0.68 | n.d. | - | 2.46 | 0.32 | n.d. | - | ||
| 1440 | 26.59 | Sesquiterpene | n.d. | - | n.d. | - | n.d. | - | 2.50 | 0.02 | n.d. | - | 2.80 | 1.76 | n.d. | - | ||
| 1386 | 27.21 | Sesquiterpene | 1.73 | 0.24 | n.d. | - | n.d. | - | 6.03 | 0.75 | 1.56 | 0.03 | 7.09 | 1.10 | n.d. | - | ||
| 1131 | 27.47 | Trans-pinocarveol | n.d. | - | 2.74 | 0.03 | n.d. | - | 8.40 | 0.80 | 0.85 | 0.10 | 2.41 | 0.17 | n.d. | - | ||
| 1482 | 27.73 | α-Humulene | 31.19 | 5.31 | 7.35 | 0.58 | 1.28 | 0.04 | 27.08 | 3.69 | 5.27 | 0.05 | 68.14 | 12.59 | n.d. | - | ||
| 1189 | 28.13 | 1,8-Menthadien-4-ol | 2.13 | 0.13 | 15.23 | 1.04 | 1.44 | 0.81 | 12.22 | 1.05 | 2.44 | 0.08 | 19.06 | 0.12 | n.d. | - | ||
| 1209 | 28.32 | α-Terpineol | n.d. | - | 2.13 | 0.19 | n.d. | - | 3.26 | 0.03 | 0.76 | 0.12 | 3.59 | 1.20 | n.d. | - | ||
| 1189 | 28.40 | Borneol | n.d. | - | n.d. | - | n.d. | - | 1.13 | 0.09 | n.d. | - | n.d. | - | n.d. | - | ||
| 1490 | 28.65 | δ-Guaiene | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | ||
| 1519 | 28.72 | β-Selinene | 6.81 | 1.46 | 0.90 | 0.15 | n.d. | - | 8.70 | 1.17 | 3.03 | 0.03 | 13.72 | 2.84 | n.d. | - | ||
| 1522 | 28.82 | α-Selinene | 3.53 | 0.59 | 1.05 | 0.12 | n.d. | - | 5.44 | 0.74 | 1.81 | 0.05 | 9.48 | 1.82 | n.d. | - | ||
| 1474 | 28.98 | Sesquiterpene | 1.60 | 0.24 | n.d. | - | n.d. | - | 1.01 | 0.15 | 0.58 | 0.04 | 2.13 | 0.41 | n.d. | - | ||
| 1190 | 29.03 | Carvone | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | 0.79 | 0.14 | ||
| 1507 | 29.86 | Selina-3,7(11)-diene | 5.95 | 1.25 | 1.88 | 0.33 | n.d. | - | n.d. | - | 1.86 | 0.03 | 6.99 | 1.18 | n.d. | - | ||
| 1191 | 30.13 | Myrtenol | n.d. | - | n.d. | - | n.d. | - | 1.23 | 0.16 | n.d. | - | n.d. | - | n.d. | - | ||
| 1284 | 31.17 | Cuminol | 1.51 | 0.26 | 8.56 | 1.41 | n.d. | - | 3.13 | 0.07 | 0.97 | 0.11 | 6.46 | 1.79 | n.d. | - | ||
| 1322 | 33.41 | Humulene oxide | 1.71 | 0.47 | n.d. | - | n.d. | - | 1.54 | 0.32 | 0.92 | 0.05 | 2.44 | 0.37 | n.d. | - | ||
| 1419 | 34.11 | Sesquiterpene | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | ||
| 1392 | 34.86 | Eugenol | 0.76 | 0.15 | 1.18 | 0.14 | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | ||
| Total | 180.97 | 1349.52 | 31.43 | 981.37 | 43.15 | 752.82 | 25.64 | |||||||||||
| Miscellaneous | ||||||||||||||||||
| 906 | 11.03 | 3,3,6-Trimethyl-1,5-heptadiene | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | ||
| 907 | 12.72 | 1,3-Dimethyl-benzene | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | ||
| 1040 | 16.87 | 2-Pentyl-furan | 3.01 | 0.27 | n.d. | - | 0.56 | 0.04 | n.d. | - | n.d. | - | 1.26 | 0.12 | n.d. | - | ||
| 1020 | 18.31 | 1,2,4,-Trimethyl-benzene | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | ||
| 894 | 19.54 | 2,5-Dimethyl-pyrazine | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | ||
| 891 | 19.74 | 2,6-Dimethyl-pyrazine | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | ||
| 1176 | 20.72 | 1,4-Bis (1-methylethyl)-benzene | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | ||
| 985 | 21.89 | 2,6-Dimethyl-2,6-octadiene | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | ||
| 1081 | 22.10 | Diethyl carbitol | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | 0.91 | 0.23 | ||
| 1039 | 22.36 | 1,3,5-Trimethylenecycloheptane | n.d. | - | 1.54 | 0.00 | n.d. | - | n.d. | - | n.d. | - | 1.15 | 0.06 | n.d. | - | ||
| 986 | 28.45 | 5-Ethyldihydro-2(3H)-furanone | 0.82 | 0.04 | 5.86 | 1.44 | 0.73 | 0.19 | 1.26 | 0.10 | n.d. | - | 5.40 | 1.36 | n.d. | - | ||
| 1190 | 30.85 | 1-Methoxy-4(1-propenyl)-benzene | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | n.d. | - | ||
RI a: retention index calculated on a Rtx-Wax (30 m × 0.25 mm × 0.25 μm f.t.); RT b: retention time (min); Average c: mean value (n = 3); Data are expressed in μg/g SD d: Standard deviatio ; n.d.: not detected.
2.2. Volatile Fingerprint: Terpene Profile and Secondary Lipid’s Oxidation Products
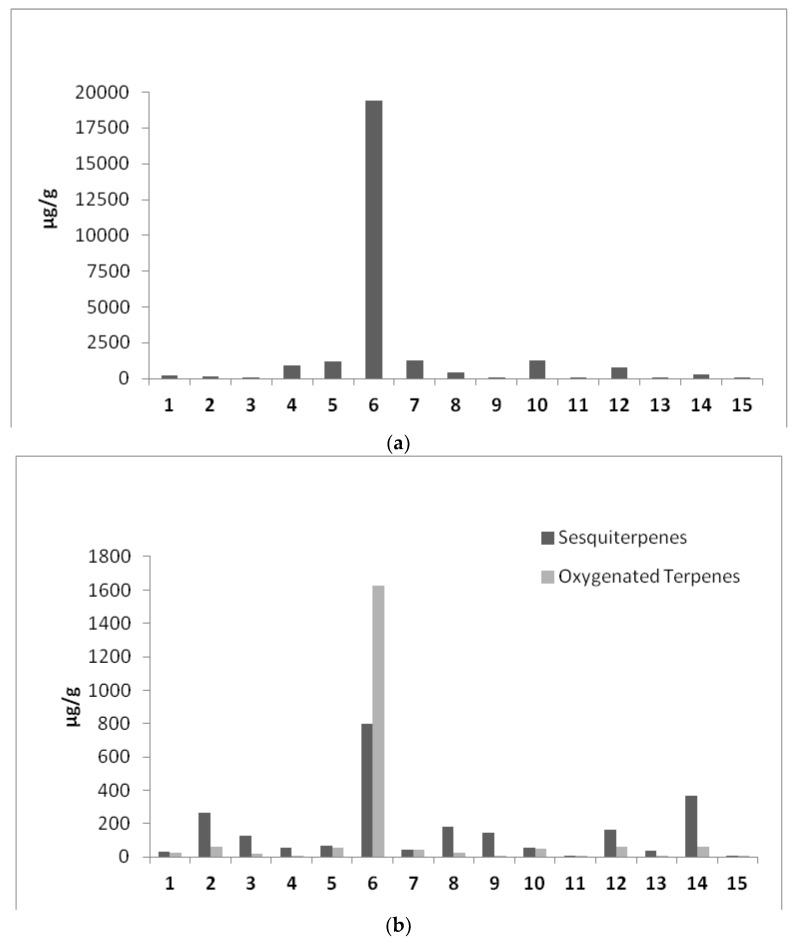
Terpenes and cannabinoids share biosynthetic pathways and, in fact, cannabinoids are terpenophenolic compounds. In Cannabis plants, terpenes are secreted and stored together with cannabinoids in glandular trichomes. Considering this fact also in relation to recent evidence of the synergic action of terpenes and cannabinoids (“entourage effect”), a comprehensive survey of terpenes is fundamental for the evaluation of cannabis oil preparations as dietary supplement with therapeutic applications. Complete data concerning the terpenes profile are summarized and reported in Table 2. Overall, up to 110 volatile compounds composed the volatile fingerprint, including 48 terpenes that are further divided into classes as presented in Figure 2. The sample Oil_6 contained an extremely high amount of terpenes compared with all other samples. α-Pinene, β-myrcene and limonene are the most concentrated terpenes in this preparation, which points toward the extremely efficient extraction method applied. Samples 5, 12 and 14 contained a distinct number of various terpenes, although in far lower concentration compared to sample Oil_6. Apparently, these formulations were obtained by an extraction process able to preserve naturally occurring terpenes profile from initial Cannabis sativa plants, as their terpene profile is in accordance with those already published in literature [12,21,22,23,24,35]. Similarly, Bedrolite oil extract (Oil_1) contains various terpene structures, reflecting the initial plant profiling. A particular profile is observed for the sample Oil_4 that showed a different terpene fingerprint compared to the other oils as it predominantly contains monoterpene subclass molecules. It has previously been demonstrated how the preparation method used for the production of cannabis extracts is able to affect the presence of different terpenes [18,19], and this is most probably reason of such a specific terpene profile found in Oil_4.
Figure 2.
Terpenes classes quantified in CBD based oils preparations (expressed as μg/g IS equivalents) (a) Mono/di/tri Terpenes (b) sesquiterpenes and oxygenated terpenes.
β-Myrcene and limonene accompanied by β-ocimene and trans-caryophyllene were found in all samples, but in a much reduced amounts compared to sample 6, and their concentration differed greatly from sample to sample. α-Pinene and β-pinene were identified in the majority of samples, excluding samples Oil_3 and Oil_13. This remains unclear considering that the two pinenes are quite balanced within the different Cannabis varieties representing around the 10% of the terpenes group and not exceeding 15–20% [20]. The occurrence of α-terpinolene in all samples (except Oil_2 and Oil_13), might be important as this compound was suggested as a genetic marker for distinguishing two important gene pools for breeding low-THC varieties [35,36]. Sample Oil_14 was particularly rich in trans-caryophyllene followed by α-humulene. The dominance of those two sesquiterpenes over the other terpenes detected in this preparation may indicate the geographic provenience of the starting Cannabis sativa material, and as a matter of fact, the producer specified the mountain region where the plant was cultivated.
It is also important to notice that sample Oil_15 was almost completely deprived of a terpene fraction aside from the traces of the main three mentioned above. This might indicate an inefficient/inadequate processing of the starting materials or even artificial addition of CBD to the oil matrix, as some essential cannabinoids were also missing (Table 1). In addition, extremely low terpene content was established for samples 11 and 13, while the remaining samples had total terpene contents in the range between 174 and 1367 μg/g, with pronounced variation in composition from sample to sample. Nevertheless, qualitative and quantitative differences observed in the chemical profiles of terpene fractions are conditioned by many factors such as: hemp variety, cultivation and environmental conditions harvest time and post-harvest conditions, storage and drying of raw plants, extraction procedure applied, matrix used and finally storage of the oil formulation.
Besides hydrocarbon terpenes, oxygenated terpenoids such as linalool and α-terpineol, were found in some preparations (Table 2, Figure 2) with a notably high concentration again in sample Oil_6. Those compounds correspond to secondary photooxidation products of the initial terpenes. In the presence of light and singlet oxygen, terpenes are also known to undergo photooxidation leading to the formation of allylic hydroperoxides [35].
In addition to the terpene compound profiles that accounted for more than 90% of the detected volatile constituents of the oils, it was possible to note the presence of other organic compounds commonly found in natural extracts such as esters, alcohols, aldehydes and ketones (Table 2). Only a few low chain esters could actually be identified, with ethyl acetate dominating in sample Oil_3. Its presence is most likely to be due to the preparation method and could also be considered indicative of potential adulteration.
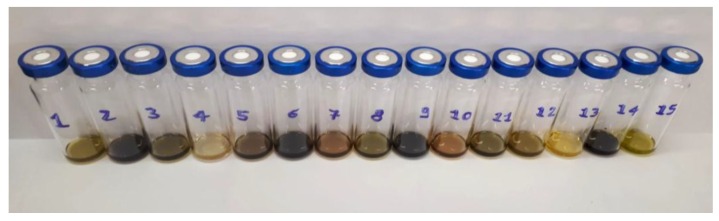
On the another hand, the detection of aldehydes and ketones suggests the initiation of lipid peroxidation of polyunsaturated fatty acids (PUFA) in the oils used as a matrix, as demonstrated in our previous work concerning the observed trends of these compounds during storage of macerated Cannabis-derived oils [12]. It is well documented that peroxidation of PUFA leads to the formation of a well-defined series of aldehydes and ketones such as nonenal, hexanal and pentanal, 2-heptenal, especially during storage. The rate of formation of lipid oxidation products depends strictly on several factors, among which the most important are the preparation method temperature, fatty acid composition of the oil in which Cannabis extract was dissolved and the storage conditions (storage temperature) as recently demonstrated in a study [12]. These parameters are crucial to define the ultimate characteristics of the final products as evidenced also by the color of the samples (Figure 3). Other volatile decomposition compounds frequently encountered include 2-hexenal, 2-octenal, 2,4-nonadienal, 4,5-dihydroxydecenal [37], some of which also appeared to be present in some of our samples.
Figure 3.
Different colors observed in CBD-based oil products.
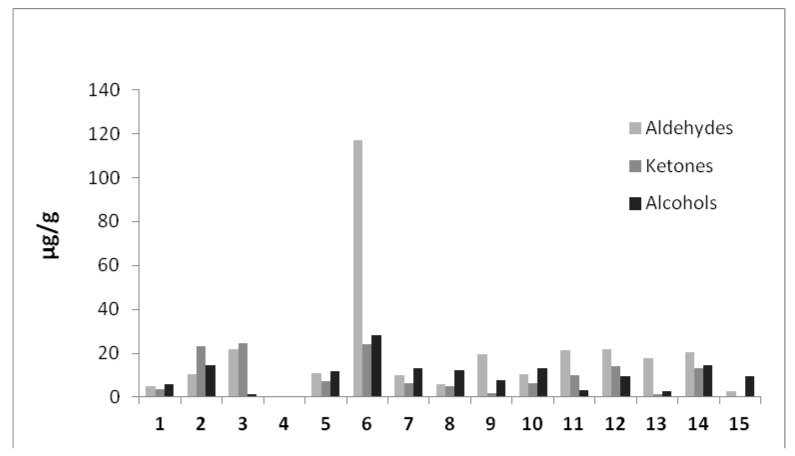
For the CBD oils analyzed in this study, tree different oil typologies were used: medium chain triglycerides (MCT oil, one sample), olive oil (six samples) and hemp seed oil (eight samples). It is worth emphasizing that sample Oil_4 was almost completely deprived of lipid oxidation products (Table 2, Figure 4). This preparation was the only one prepared in MCT oil, which means that this kind of matrix is less susceptible to oxidative degradation than the olive or hemp seed oils declared as matrices for other preparations enrolled herein
Figure 4.
Lipid oxidation products quantified in CBD based oils preparations (expressed as μg/g SI equivalents).
As far as olive oil is concerned, is often used by producers as it has a strong nutritional potential, being rich in the polyunsaturated fatty acids. Moreover, FU oil (pharmaceutical grade olive oil) is used for the preparation of CBD galenic formulations [9,12,29] as it was performed for Bedrolite oil extract.
Regarding the hemp seed oil used as a carrier for dissolving the CBD extract (hence the term “CBD hemp oil”) some clarifications regarding this kind of preparation are indispensable because misconceptions that may confuse the final users of this preparations still exist. “CBD oil” expression is typically limited to extracts in oil of flowering buds and not stalks, fibers, or seeds of each Cannabis sativa L. variety. Hemp seeds do not contain any cannabinoids, but their contact with the resin secreted by the epidermal glands located on flowers, and leaves and/or a bad selection of the bracts of the perigonium can cause the appearance of some cannabinoids in hemp oil [34,38]. Therefore, any cannabinoids detected actually represent hemp seed oil contaminants. Their concentration is influenced by the hemp variety and by the seed cleaning process. Although the cannabinoid concentration in hemp seed oils is usually extremely low, it must be determined before oil commercialization [39]. Nevertheless, the seeds of industrial hemp plants have important uses in human nutrition [40,41] and this is reason why its oil is used as an adequate, naturally resembling matrix for CBD-enriched products. Hemp seed oils represent good sources of protein, and are rich in omega-3 and omega-6 fatty acids with an ideal n3/n6 PUFA nutrition ratio according to WHO guidelines [42,43]. It should be considered that hemp oil is rich in unsaturated fatty acids which are the components susceptible to oxidation phenomena during storage. Although hemp seed oil was shown to be more predisposed to peroxidation than olive oil [44], this study did not identify any significant differences between these two matrixes as far as on-going peroxidation was concerned. Nevertheless, the critical point is to assess stability during the storage period, that is not reported or available for any of the CBD preparations analysed here. This represents a fundamental issue since the formation of lipid oxidation products is related with the decreasing concentration of cannabinoids and terpenes, as well [12]. Therefore, the investigation of trends of compounds characterizing the formulations is essential to define the management conditions. Moreover, an adequate storage temperature would be useful to define the correct expiry date of the products as they are commercialized in EU as dietary supplements.
3. Materials and Methods
3.1. Materials
Fourteen samples of commercially available CBDs oil were purchased on the Internet between December 2017 and January 2018. The purchase was based on the main product brands available on the European market. Table 3 summarizes the main characteristics of the samples. Samples were kept at room temperature (as indicated by the manufacturers) before analyses and sample codes (Oils 2–15) were assigned to them in accordance with the order of acquisition. Bedrolite® oil olive extract (assigned as Oil_1) was obtained from a Bedrolite Bedrocan International, Postbus, CA, Veendam, Netherlands® medical Cannabis chemotype. Exhaustive analytical procedures were described in details in our recently published article [12].
Table 3.
Declared CBD content, oil matrix used and origin of the analysed CBD oil samples.
| Samples | CBD Declared Content (%, w/w) | Matrix | Origin | Extraction Method (When Indicated) |
|---|---|---|---|---|
| Oil_1 | / | Olive oil | Italy | Calvi et al., 2018 |
| Oil_2 | 4 | Hemp seed oil | Switzerland | ND * |
| Oil_3 | 1 | Olive oil | Switzerland | ND |
| Oil_4 | 5 | Caprylic/Capric Triglyceride (MCT) | Italy | ND |
| Oil_5 | 4 | Olive oil | Switzerland | CO2 supercritical |
| Oil_6 | 3 | Hemp oil | The Netherlands | CO2 supercritical |
| Oil_7 | 4 | Olive oil | Spain | ND |
| Oil_8 | 3 | Hemp seed oil | The Netherlands | ND |
| Oil_9 | 3 | Hemp seed oil | The Netherlands | ND |
| Oil_10 | 4 | Olive oil | The Netherlands | ND |
| Oil_11 | / | Hemp seed oil | Switzerland | ND |
| Oil_12 | 2 | Hemp seed oil | Switzerland | CO2 supercritical |
| Oil_13 | 4 | Olive oil | France | ND |
| Oil_14 | 5 | Hemp seed oil | Slovenia | CO2 supercritical |
| Oil_15 | 3 | Hemp seed oil | UK | ND |
* ND—not declared.
3.2. Chemical and Reagents
All HPLC or analytical grade chemicals were from Sigma (Sigma–Aldrich, St. Louis, MO, USA). Formic acid 98–100% was from Fluka (Sigma–Aldrich, St. Louis, MO, USA). Ultrapure water was obtained through a Milli-Q system (Millipore, Merck KGaA, Darmstadt, Germany). For head-space (HS) analysis, the SPME coating fiber (DVB/CAR/PDMS, 50/30 μm) was from Supelco (Bellefonte, PA, USA). Acetonitrile, 2-propanol, formic acid LC-MS grade were purchased from Carlo Erba (Milan, Italy). CBD, THC, CBN, CBG, CBNA, THCA, CBGA were purchased from Sigma Aldrich (Round Rock, TX, USA).
3.3. Terpenes GC-MS Analysis
One hundred mg of each oil sample were weighed and put into 20 mL glass vials along with 100 μL of the inyernal standard (IS, 4-nonylphenol, 2000 μg/mL in 2-propanol). Each vial was fitted with a cap equipped with a silicon/PTFE septum (Supelco). A temperature of 37 °C was selected as both as the extraction and equilibration temperature according to previous published research, in order to prevent possible matrix alterations ensuring the most efficient adsorption of volatile compounds onto the SPME fibre [15,16]. To keep the temperature constant during analysis, the vials were maintained in a cooling block (CTC Analytics, Zwingen, Switzerland). At the end of the sample equilibration time (30 min), a conditioned (60 min at 280 °C) SPME fiber was exposed to the headspace of the sample for 120 min using a CombiPAL system injector autosampler (CTC Analytics). All analytical parameters were already validated in our previous research [12].
Analyses were performed with a Trace GC Ultra coupled to a Trace DSQII quadrupole mass spectrometer (MS) (Thermo-Fisher Scientific, Waltham, MA, USA) equipped with an Rtx-Wax column (30 m × 0.25 mm i.d. × 0.25 μm film thickness) (Restek, Bellefonte, PA, USA). The oven temperature program was: from 35 °C, held for 8 min, to 60 °C at 4 °C/min, then from 60 to 160 °C at 6 °C/min and finally from 160 to 200 at 20 °C/min. Helium was the carrier gas, at a flow rate of 1 mL/min. Carry over and peaks originating from the fibres were regularly assessed by running blank samples. After each analysis fibres were immediately thermally desorbed in the GC injector for 5 min at 250 °C to prevent contamination. The MS was operated in electron impact (EI) ionisation mode at 70 eV. An alkanes mixture (C8-C22, Sigma R 8769) was run under the same chromatographic conditions as the samples to calculate the Kovats Retention Indices (RI) of the detected compounds. The mass spectra were obtained by using a mass selective detector, a multiplier voltage of 1456 V, and by collecting the data at rate of 1 scan/s over the m/z range of 35–350. Compounds were identified by comparing the retention times of the chromatographic peaks with those of authentic compounds analyzed under the same conditions when available, by comparing the Kovats retention indices with the literature data and through the National Institute of Standards and Technology (NIST) MS spectral database. The quantitative evaluation was performed using the internal standard procedure and the results were finally expressed as μg/g or mg/g IS equivalents of each volatile compounds. All analyses were done in triplicate.
3.4. Cannabinoids LC-Q-Exactive-Orbitrap-MS Analysis
The cannabinoids profile and content were evaluated by the procedure recently published by us [12]. Briefly, the oil samples for HPLC-Q-Exactive-Orbitrap-MS analysis were prepared by dissolving 100 mg of each oil in 10 mL of isopropanol. After adding the 1 μg/mL of IS, 10 μL of each sample were diluted in 890 μL of initial mobile phase from which 2 μL was injected. Chromatography was accomplished on an HPLC system (Thermo Fisher Scientific, San Jose, CA, USA) that was made up of a Surveyor MS quaternary pump with a degasser, a Surveyor AS autosampler with a column oven and a Rheodyne valve with a 20 μL loop. Analytical separation was carried out using a reverse-phase HPLC column 150 × 2 mm i.d., 4 μm, Synergi Hydro RP, with a 4 × 3 mm i.d. C18 guard column (Phenomenex, Torrance, CA, USA). The mobile phase used in the chromatographic separation consisted of a binary mixture of solvents A (0.1% aqueous formic acid) and B (acetonitrile). The gradient was initiated with 60% eluent A with a linear decrease up to 95% in 10 min. This condition was maintained for 4 min. The mobile phase was returned to initial conditions at 14 min, followed by a 6-min re-equilibration period (total run time: 20 min). The flow rate was 0.3 mL/min. The column and sample temperatures were 30 °C and 5 °C, respectively. The mass spectrometer Thermo Q-Exactive Plus (Thermo Scientific) was equipped with a heated electrospray ionisation (HESI) source. Capillary temperature and vaporiser temperature were set at 330 and 280 °C, respectively, while the electrospray voltage was adjusted at 3.50 kV (operating in both positive and negative mode). Sheath and auxiliary gas were 35 and 15 arbitrary units, with S lens RF level of 60. The mass spectrometer was controlled by the Xcalibur 3.0 software (Thermo Fisher Scientific). The exact mass of the compounds was calculated using Qualbrowser in Xcalibur 3.0 software. The FS-dd-MS2 (full scan data-dependent acquisition) in both positive and negative mode was used for both screening and quantification purposes. Resolving power of FS adjusted on 140,000 FWHM at m/z 200, with scan range of m/z 215–500. The automatic gain control (AGC) was set at 3 × 106, with an injection time of 200 ms. A targeted MS/MS (dd-MS2) analysis operated in both positive and negative mode at 35,000 FWHM (m/z 200). The AGC target was set to 2 × 105, with the maximum injection time of 100 ms. Fragmentation of precursors was optimised as two-stepped normalised collision energy (NCE) (25 and 40 eV). Detection was based on calculated exact mass of the protonated/deprotonated molecular ions, at least one corresponding fragment and on retention time of target compounds [12]. Extracted ion chromatograms (EICs) were obtained with an accuracy of 2 ppm m/z from total ion chromatogram (TIC) engaging the m/z corresponding to the molecular ions [M + H]+ 315,23145 for CBD and THC, 311,20020 for CBN, 317.24716 for CBG and 311.2024 for CBN. In ESI- the molecular ions [M−H]− considered were 357.2164 for CBDA and THCA, while CBGA was detected by 359,22269.
4. Conclusions
Taken together, the results presented in this study indicate the pronounced variability of CBD concentrations in commercialized CBD oil preparations. The differences found in the overall cannabinoids profiles accompanied with discrepancies revealed for the terpenes fingerprint justify the necessity to provide firmer regulation and control. Precise information regarding CBD oil composition is crucial for consumers, as individual doses throughout the administration period have to be adapted according to CBD bioavailability. This is of fundamental importance regarding consumer safety, as CBD oil preparations are also used in therapeutical purposes, regardless of the fact that they are registered as dietary supplements.
Acknowledgments
The authors want to acknowledge Carlo Privitera for his helpful suggestions and skills regarding the therapeutics applications of CBD oils formulations.
Author Contributions
Investigation, G.N.; Methodology, L.C. and S.P.; Project administration, L.G.; Validation, G.B.; Writing—original draft, R.P. and G.C.; Writing—review & editing, A.G.
Funding
The present paper is partially funded and realized within the project ITALIAN MOUNTAIN LAB, Ricerca e Innovazione per l’ambiente ed i Territori di Montagna—Progetto FISR Fondo integrativo speciale per la ricerca.
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds are not available from the authors.
References and Note
- 1.Leung L. Cannabis and its derivatives: Review of medical use. J. Am. Board Fam. Med. 2011;24:452–462. doi: 10.3122/jabfm.2011.04.100280. [DOI] [PubMed] [Google Scholar]
- 2.Flores-Sanchez I.J., Verpoorte R. Secondary metabolism in Cannabis. Phytochem. Rev. 2008;7:615–639. doi: 10.1007/s11101-008-9094-4. [DOI] [Google Scholar]
- 3.Rong C., Lee Y., Carmona N.E., Cha D.S., Ragguett R.M., Rosenblat J.D., Mansur R.B., Ho R.C., McIntyre R.S. Cannabidiol in medical marijuana: Research vistas and potential opportunities. Pharmacol. Res. 2017;121:213–218. doi: 10.1016/j.phrs.2017.05.005. [DOI] [PubMed] [Google Scholar]
- 4.Russo E.B. Cannabinoids in the management of difficult to treat pain. Ther. Clin. Risk Manag. 2008;4:245–259. doi: 10.2147/TCRM.S1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Whiting P.F., Wolff R.F., Deshpande S., Di Nisio M., Duffy S., Hernandez A.V., Keurentjes J.C., Lang S., Misso K., Ryder S., et al. Cannabinoids for medical use: A systematic review and meta-analysis. JAMA. 2015;313:2456–2473. doi: 10.1001/jama.2015.6358. [DOI] [PubMed] [Google Scholar]
- 6.Borgelt L.M., Franson K.L., Nussbaum A.M., Wang G.S. The pharmacologic and clinical effects of medical cannabis. Pharmacotherapy. 2013;33:195–209. doi: 10.1002/phar.1187. [DOI] [PubMed] [Google Scholar]
- 7.Pisanti S., Malfitano A.M., Ciaglia E., Lamberti A., Ranieri R., Cuomo G., Abate M., Faggiana G., Proto M.C., Fiore D., et al. Cannabidiol: State of the art and new challenges for therapeutic applications. Pharmacol. Ther. 2017;175:133–150. doi: 10.1016/j.pharmthera.2017.02.041. [DOI] [PubMed] [Google Scholar]
- 8.Mead A. The legal status of cannabis (marijuana) and cannabidiol (CBD) under U.S. law. Epilepsy Behav. 2017;70:288–291. doi: 10.1016/j.yebeh.2016.11.021. [DOI] [PubMed] [Google Scholar]
- 9.Carcieri C., Tomasello C., Simiele M., De Nicolò A., Avataneo V., Canzoneri L., Cusato J., Di Perri G., D’Avolio A. Cannabinoids concentration variability in cannabis olive oil galenic preparations. J. Pharm. Pharmacol. 2018;70:143–149. doi: 10.1111/jphp.12845. [DOI] [PubMed] [Google Scholar]
- 10.Pacifici R., Marchei E., Salvatore F., Guandalini L., Busardò F.P., Pichini S. Evaluation of cannabinoids concentration and stability in standardized preparations of cannabis tea and cannabis oil by ultra-high performance liquid chromatography tandem mass spectrometry. Clin. Chem. Lab. Med. 2017;55:1555–1563. doi: 10.1515/cclm-2016-1060. [DOI] [PubMed] [Google Scholar]
- 11.Citti C., Ciccarella G., Braghiroli D., Parenti C., Vandelli M.A., Cannazza G. Medicinal cannabis: Principal cannabinoids concentration and their stability evaluated by a high performance liquid chromatography coupled to diode array and quadrupole time of flight mass pectrometry method. J. Pharm. Biomed. Anal. 2016;128:201–209. doi: 10.1016/j.jpba.2016.05.033. [DOI] [PubMed] [Google Scholar]
- 12.Calvi L., Pentimalli D., Panseri S., Giupponi L., Gelmini F., Beretta G., Vitali D., Bruno M., Zilio E., Pavlovic R., et al. Comprehensive quality evaluation of medical Cannabis sativa L. inflorescence and macerated oils based on HS-SPME coupled to GC–MS and LC-HRMS (q-exactive orbitrap®) approach. J. Pharm. Biomed. Anal. 2018;150:208–219. doi: 10.1016/j.jpba.2017.11.073. [DOI] [PubMed] [Google Scholar]
- 13.Grotenhermen M.K.F., Lohmeyer D. THC Limits for Food: A Scientific Study. Nova-Institute; Hürth, Germany: 2015. [Google Scholar]
- 14.DAC/NRF. 2015/2. Cannabidiol. [(accessed on 1 May 2018)]; Available online: http://dacnrf.pharmazeutische-zeitung.de/index.php?id=557.
- 15.Hazekamp A. The medicinal power of cannabis [Cannabinoïden werken - Bewezen - pijnstillend: De medicinale kracht van cannabis] Pharm. Weekbl. 2007;142:38–41. [Google Scholar]
- 16.Website: Decreto 9 novembre 2015: Funzioni di Organismo statale per la cannabis previsto dagli articoli 23 e 28 della convenzione unica sugli stupefacenti del 1961, come modificata nel 1972. [(accessed on 1 May 2018)]; Available online: http://www.gazzettaufficiale.it/eli/id/2015/11/30/15A08888/sg;jsessionid=p1rnwNujUKlqQ5azhA%20Q95A__.ntc-as3-guri2a.
- 17.Regulation (EU) No 1307/2013 of the European Parliament and of the Council of 17 December 2013 establishing rules for direct payments to farmers under support schemes within the framework of the common agricultural policy and repealing Council Regulation (EC) No 637/2008 and Council Regulation (EC) No 73/2009; 2013.
- 18.Attard T.M., Bainier C., Reinaud M., Lanot A., McQueen-Mason S.J., Hunt A.J. Utilisation of supercritical fluids for the effective extraction of waxes and Cannabidiol (CBD) from hemp wastes. Ind. Crops Prod. 2018;112:38–46. doi: 10.1016/j.indcrop.2017.10.045. [DOI] [Google Scholar]
- 19.Sexton M., Shelton K., Haley P., West M. Evaluation of cannabinoid and terpenoid content: Cannabis flower compared to supercritical CO2 Concentrate. Planta Med. 2018;84:234–241. doi: 10.1055/s-0043-119361. [DOI] [PubMed] [Google Scholar]
- 20.Brenneisen R. Chemistry and Analysis of Phytocannabinoids and Other Cannabis Constituents. In: ElSohly M.A., editor. Marijuana and the Cannabinoids. Humana Press; New York, NY, USA: 2007. pp. 17–49. [Google Scholar]
- 21.Rothschild M., Bergström G., Wängberg S.A. Cannabis sativa: Volatile compounds from pollen and entire male and female plants of two variants, Northern Lights and Hawaian Indica. Bot. J. Linn. Soc. 2005;147:387–397. doi: 10.1111/j.1095-8339.2005.00417.x. [DOI] [Google Scholar]
- 22.Elzinga S., Fischedick R., Podkolinski J., Raber C. Cannabinoids and terpenes as chemotaxonomic markers in cannabis. Nat. Prod. Chem. Res. 2015;3:181. doi: 10.4172/2329-6836.1000181. [DOI] [Google Scholar]
- 23.Lewis M.A., Russo E.B., Smith K.M. Pharmacological Foundations of Cannabis Chemovars. Planta Med. 2018;84:225–233. doi: 10.1055/s-0043-122240. [DOI] [PubMed] [Google Scholar]
- 24.Aizpurua-Olaizola O., Soydaner U., Öztürk E., Schibano D., Simsir Y., Navarro P., Etxebarria N., Usobiaga A. Evolution of the Cannabinoid and Terpene Content during the Growth of Cannabis sativa Plants from Different Chemotypes. J. Nat. Prod. 2016;79:324–331. doi: 10.1021/acs.jnatprod.5b00949. [DOI] [PubMed] [Google Scholar]
- 25.Russo E.B. Taming THC: Potential cannabis synergy and phytocannabinoid-terpenoid entourage effects. Br. J. Pharmacol. 2011;163:1344–1364. doi: 10.1111/j.1476-5381.2011.01238.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pagano E., Capasso R., Piscitelli F., Romano B., Parisi O.A., Finizio S., Lauritano A., Di Marzo V., Izzo A.A., Borrelli F. An Orally Active Cannabis Extract with High Content in Cannabidiol attenuates Chemically-induced Intestinal Inflammation and Hypermotility in the Mouse. Front. Pharmacol. 2016;7:341. doi: 10.3389/fphar.2016.00341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Panseri S., Soncin S., Chiesa L.M., Biondi P.A. A Headspace Solid-Phase Microextraction Gas-Chromatographic Mass-Spectrometric Method (Hs-Spme-Gc/Ms) to Quantify Hexanal In Butter during Storage as Marker of Lipid Oxidation. Food Chem. 2011;127:886–889. doi: 10.1016/j.foodchem.2010.12.150. [DOI] [PubMed] [Google Scholar]
- 28.Raikos V., Konstantinidi V., Duthie G. Processing and storage effects on the oxidative stability of hemp (Cannabis sativa L.) oil-in-water emulsions. Int. J. Food Sci. Technol. 2015;50:2316–2322. doi: 10.1111/ijfs.12896. [DOI] [Google Scholar]
- 29.Casiraghi A., Roda G., Casagni E., Cristina C., Musazzi U.M., Franzè S., Rocco P., Giuliani C., Fico G., Minghetti P., et al. Extraction Method and Analysis of Cannabinoids in Cannabis Olive Oil Preparations. Planta Med. 2018;84:242–249. doi: 10.1055/s-0043-123074. [DOI] [PubMed] [Google Scholar]
- 30.Bonn-Miller M.O., Loflin M.J.E., Thomas B.F., Marcu J.P., Hyke T., Vandrey R. Labeling Accuracy of Cannabidiol Extracts Sold Online. JAMA. 2017;318:1708–1709. doi: 10.1001/jama.2017.11909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Citti C., Braghiroli D.M., Vandelli A., Cannazza G. Pharmaceutical and biomedical analysis of cannabinoids: A critical review. J. Pharm. Biomed. Anal. 2018;147:566–579. doi: 10.1016/j.jpba.2017.06.003. [DOI] [PubMed] [Google Scholar]
- 32.Romano L., Hazekamp A. Cannabis Oil: Chemical evaluation of an upcoming cannabis based medicine. Cannabinoids. 2013;1:1–11. [Google Scholar]
- 33.Iannotti F.A., Hill C.L., Leo A., Alhusaini A., Soubrane C., Mazzarella E., Russo E., Whalley B.J., Di Marzo V., Stephens G.J. Nonpsychotropic plant cannabinoids, cannabidivarin (CBDV) and cannabidiol (CBD), activate and desensitize transient receptor potential vanilloid 1 (TRPV1) channels in vitro: Potential for the treatment of neuronal hyperexcitability. ACS Chem. Neurosci. 2014;5:1131–1141. doi: 10.1021/cn5000524. [DOI] [PubMed] [Google Scholar]
- 34.Citti C., Pacchetti B., Vandelli M.A., Forni F., Cannazza G. Analysis of cannabinoids in commercial hemp seed oil and decarboxylation kinetics studies of cannabidiolic acid (CBDA) J. Pharm. Biomed. Anal. 2018;149:532–540. doi: 10.1016/j.jpba.2017.11.044. [DOI] [PubMed] [Google Scholar]
- 35.Marchini L.M., Charvoz C., Dujourdy L., Baldovini N., Filippi J.J. Multidimensional analysis of cannabis volatile constituents: Identification of 5,5-dimethyl-1-vinylbicyclo[2.1.1] hexane as a volatile marker of hashish, the resin of Cannabis sativa. J. Chromatogr. A. 2014;1370:200–215. doi: 10.1016/j.chroma.2014.10.045. [DOI] [PubMed] [Google Scholar]
- 36.Novak J., Zitterl-Eglseer K., Deans S.G., Franz C.M. Essential oils of different cultivars of Cannabis sativa L. and their antimicrobial activity. Flavour Fragr. J. 2001;16:259–262. doi: 10.1002/ffj.993. [DOI] [Google Scholar]
- 37.Guillén M.D., Goicoechea E. Toxic oxygenated alpha, beta-unsaturated aldehydes and their study in foods: A review. Crit. Rev. Food Sci. Nutr. 2008;48:119–136. doi: 10.1080/10408390601177613. [DOI] [PubMed] [Google Scholar]
- 38.Petrović M., Debeljak Ž., Kezić N., Džidara P. Relationship between cannabinoids content and composition of fatty acids in hempseed oils. Food Chem. 2015;170:218–225. doi: 10.1016/j.foodchem.2014.08.039. [DOI] [PubMed] [Google Scholar]
- 39.Sarmento L., Grotenhermen F., Kruse D. Scientifically Sound Guidelines for THC in Food. Nova-Institute; Hürth, Germany: 2015. [Google Scholar]
- 40.Callaway J.C. Hempseed as a nutritional resource: An overview. Euphytica. 2004;140:65–72. doi: 10.1007/s10681-004-4811-6. [DOI] [Google Scholar]
- 41.Callaway J.C., Pate D.W. Hempseed oil. In: Moreau R.A., Kamal-Eldin A., editors. Gourmet and Health-Promoting Specialty Oils. Academic Press and AOCS Press; Cambridge, MA, USA: 2009. pp. 185–213. [Google Scholar]
- 42.Da Porto C., Decorti D., Tubaro F. Fatty acid composition and oxidation stability of hemp (Cannabis sativa L.) seed oil extracted by supercritical carbon dioxide. Ind. Crops Prod. 2012;36:401–404. doi: 10.1016/j.indcrop.2011.09.015. [DOI] [Google Scholar]
- 43.Smeriglio A., Galati E.M., Monforte M.T., Lanuzza F., D’Angelo V., Circosta C. Polyphenolic Compounds and Antioxidant Activity of Cold-Pressed Seed Oil from Finola Cultivar of Cannabis sativa L. Phytother. Res. 2016;30:1298–1307. doi: 10.1002/ptr.5623. [DOI] [PubMed] [Google Scholar]
- 44.Sapino S., Carlotti M.E., Peira E., Gallarate M. Hemp-seed and olive oils: Their stability against oxidation and use in O/W emulsions. J. Cosmet. Sci. 2005;56:227–251. doi: 10.1111/j.1467-2494.2005.00290_2.x. [DOI] [PubMed] [Google Scholar]