Abstract
Monohexosylceramides (CMHs) are highly conserved fungal glycosphingolipids playing a role in several cellular processes such as growth, differentiation and morphological transition. In this study, we report the isolation, purification and chemical characterization of CMHs from Rhizopus stolonifer and R. microspores. Using positive ion mode ESI-MS, two major ion species were observed at m/z 750 and m/z 766, respectively. Both ion species consisted of a glucose/galactose residue attached to a ceramide moiety containing 9-methyl-4,8-sphingadienine with an amidic linkage to a hydroxylated C16:0 fatty acid. The antimicrobial activity of CMH was evaluated against Gram positive and Gram negative bacteria using the agar diffusion assay. CMH from both Rhizopus species inhibited the growth of Bacillus terrae, Micrococcus luteus (M. luteus) and Pseudomonas stutzeri (P. stutzeri) with a MIC50 of 6.25, 6.25 and 3.13 mg/mL, respectively. The bactericidal effect was detected only for M. luteus and P. stutzeri, with MBC values of 25 and 6.25 mg/mL, respectively. Furthermore, the action of CMH on the biofilm produced by methicillin-resistant Staphylococcus aureus (MRSA) was analyzed using 12.5 and 25 mg/mL of CMH from R. microsporus. Total biofilm biomass, biofilm matrix and viability of the cells that form the biofilm structure were evaluated. CMH from R. microsporus was able to inhibit the MRSA biofilm formation in all parameters tested.
Keywords: monohexosylceramides, Rhizopus, biofilm, antibacterial activities
1. Introduction
Fungi of the order Mucorales are commonly found in soil as saprophytes and decomposing organic matter all around the world. In Brazil these fungi are widely found in the northeastern region in arid and semi-arid ecosystems such as the Caatinga, which is an exclusively Brazilian domain in the semi-arid region of Brazil [1,2]. Some species have been described as agents of systemic infections in humans, especially in immunocompromised patients [3,4]. Rhizopus species are frequently and amply distributed in soils of the Caatinga in the Brazilian Northeast [2]. Little was known about the cell wall glycoconjugates of the Rhizopus species and, consequently, their specific functions in the fungal cell and in their interaction with the environment.
Ceramide monohexosides (CMHs) are highly conserved fungal glycosphingolipids with structural modifications that include different sites of unsaturation as well as fatty acid residues of varying length in their ceramide moieties [5]. CMHs play diverse roles in fungal cell processes, such as growth and morphological transition in Cryptococcus neoformans, Pseudallescheria boydii, Candida albicans, Aspergillus fumigatus and Collectotrichum gloeosporioides [6,7,8,9,10]. In addition, CMH are important in promoting alkaline tolerance in vitro due to the involvement of CMH in the regulation of membrane fungal lipid domains that lead to the redistribution of CMH in the membrane in an alkaline environment [11,12,13]. CMH also interact with defensins isolated from insects and plants [14]. Studies on CMH with antimicrobial activity have been performed by isolating them from plants and microorganisms, such as fungi, where they play a role in the microbial growth [15,16,17,18].
Bacterial infectious diseases are highly prevalent in tropical and developing countries such as Brazil, affecting mainly hospitalized and/or immunosuppressed individuals [19,20,21]. Staphylococcus aureus is one of the most frequently found bacteria and is the etiological agent of nosocomial infections that is associated with the production of biofilm on medical implants. Furthermore, it possesses a strong capacity to acquire resistance resulting in high rates of therapeutic failure [22,23]. In addition, several environmental bacteria, such as Pseudomonas stutzeri and Micrococcus luteus, have emerged as opportunistic pathogens due to the increase in the number of immunosuppressed individuals, either by immunosuppressive treatment after transplantation, HIV infection or cancer [24,25,26,27].
In this context, the goal of this work was to characterize CMHs from two species of Rhizopus, R. stolonifer and R. microsporus, and to evaluate the ability of these highly conserved fungal glycosphingolipids to either inhibit the growth or to kill four different bacterial species. The influence of both Rhizopus CMHs in methicillin-resistant S. aureus (MRSA) biofilm formation was also studied.
2. Results
2.1. CMH Purification and Chemical Analysis
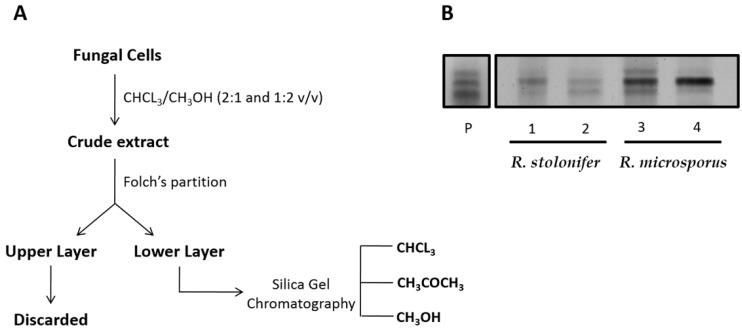
The purification steps of CMH from the Rhizopus species grown in different media are shown in Figure 1A. The Folch lower layer containing neutral glycolipids was fractionated by silica gel chromatography and the fractions monitored by HPTLC on silica plates developed with chloroform: methanol: 2 M NH4OH (40:10:1 v/v). The spots were stained by spraying with orcinol-sulfuric acid reagent (Figure 1B).
Figure 1.
(A) Isolation and purification of glycosphingolipids from Rhizopus species. (B) High performance thin layer chromatography (HPTLC) of neutral glycosphingolipid fractions by silica gel column chromatography. P. CMH standard. Lane 1: Methanol fraction from R. stolonifer grown in potato dextrose broth (PDB). Lane 2: Methanol fraction from R. stolonifer grown in yeast extract-peptone-dextrose growth medium (YEPD). Lane 3: Methanol fraction from R. microsporus grown in PDB. Lane 4: Methanol fraction from R. microspores grown in YEPD. Solvent system: Chloroform/methanol/2 M NH4OH (40:10:1 v/v/v). Detection: iodine vapor and orcinol-sulfuric acid reagent.
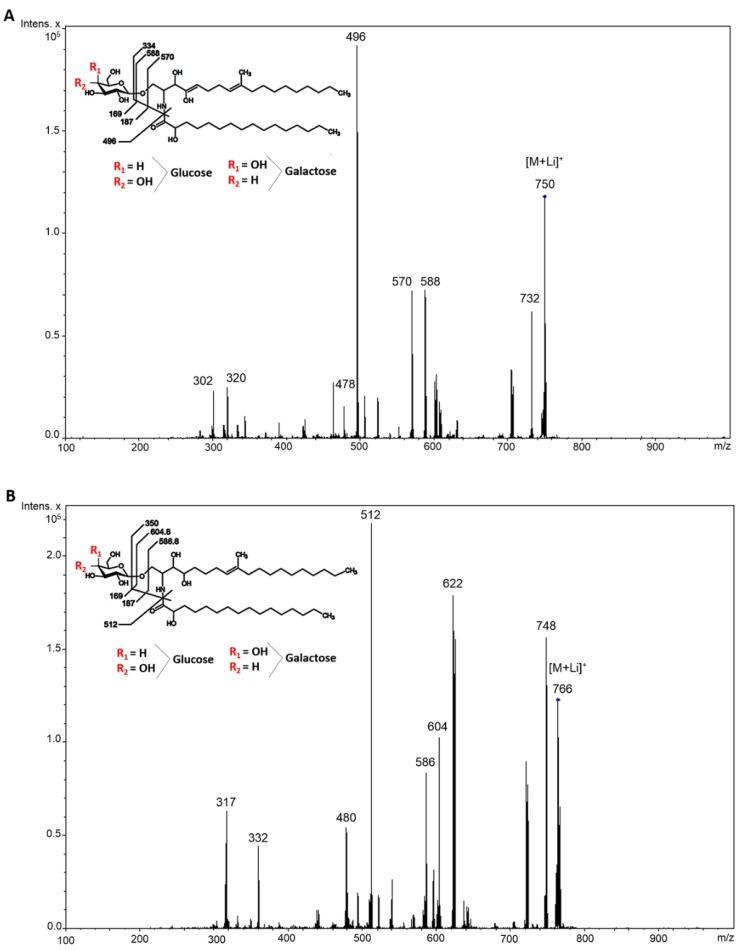
An ESI-MS analysis (positive mode) was performed to elucidate the chemical structure of purified CMHs from Rhizopus species. Lithiated molecular ions were observed at m/z 750 and m/z 766. A major lithiated ion species at m/z 750 was observed in R. microsporus grown in PDB and YEPD media and in R. stolonifer grown in YEPD medium, respectively. When submitted to the ESI-MS/MS analysis, these major peaks exhibited a fragment at m/z 732 (loss of water) and daughter ions at m/z 588 and m/z 570 (loss of water). The loss of 162 mass units common to the CMHs analyzed corresponds to the loss of a hexose residue (glucose or galactose) (Figure 2A). The prominent ion at m/z 496 suggests the presence of an extra hydroxyl group in the long chain base [9]. CMH from R. stolonifer grown in PDB medium presents a major lithiated species at m/z 766. The loss of 162 units generated daughter ions at m/z 622 [M-hexose + Li+] and m/z 604 [M-hexose-H2O+ Li+] corresponding to the ceramide monolithiated ion (Figure 2B). According to these results, we concluded that the glycosphingolipid structures consisted of a hexose, long-chain bases (4-OH-9-methyl-octadeca-4,8-sphingadienine and 4-OH-9-methyl-eicosa-4,8-sphingadienine) and hydroxylated C16:0 fatty acids. After hydrolysis with 3 M TFA, the constituent monosaccharides were identified by GC to be glucose and galactose in a ratio 1:1 from both Rhizopus species studied.
Figure 2.
ESI-MS [M + Li] analysis of GlcCer/GalCer from Rhizopus species. (A) CMH from R. microsporus grown in PDB and YEPD, and CMH from R. stolonifer grown in YEPD. Major ions species at m/z 750. (B) CMH from R. stolonifer grown in PDB. Major ions species at m/z 766.
2.2. Antibacterial Activity Evaluation
The antibacterial activity of CMHs from Rhizopus species was evaluated against six different species of indicator bacteria using the agar diffusion assay (Table 1 and Figure S1). In this assay, the lower layer from Folch partition and CMH from both species (50 mg/mL–10 µL), were able to inhibit the growth of B. terrae, M. luteus and P. stutzeri, but there was no inhibition of other bacteria tested (B. cepacea, E. coli, S. aureus and MRSA-S. aureus). The lower layer from Folch partition containing the neutral lipids from the crude lipid extract shows lower inhibition when compared with the purified CMH.
Table 1.
Antimicrobial Activity of CMHs from Rhizopus species by Agar Diffusion Assay. Lipid samples (Folch lower layer and CMH) from R. stolonifer and R. microsporus grown in PDB were dissolved in chloroform/methanol (2:1 v/v) at a concentration of 50 mg/mL (10 µL). The negative control consisted of 10 µL of chloroform/methanol (2:1 v/v).
| Rhizopus Species | Bacteria Species | Inhibition (+) or No Inhibition (−) | ||
|---|---|---|---|---|
| Control | Folch Lower Layer | CMH | ||
| R. stolonifer | B. cepacea | − | − | − |
| B. terrea | − | ± | + | |
| E. coli | − | − | − | |
| M. luteus | − | ± | + | |
| S. aureus | − | − | − | |
| S. aureus MRSA | − | − | − | |
| P. stutzeri | − | + | + | |
| R. microsporus | B. cepacea | − | − | − |
| B. terrea | − | + | ± | |
| E. coli | − | − | − | |
| M. luteus | − | ± | ± | |
| S. aureus | − | − | − | |
| S. aureus MRSA | − | − | − | |
| P. stutzeri | − | + | + | |
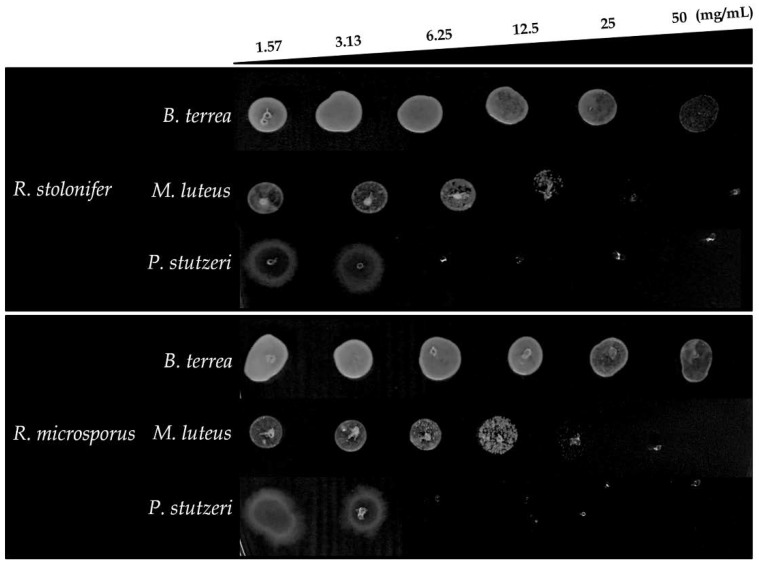
To confirm the results obtained in the agar diffusion assay, the Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) values of CMH were determined against B. terrae, M. luteus and P. stutzeri (Table 2). CMHs from both species showed considerable inhibitory activity, with MIC50 of 6.25 and 3.13 mg/mL for M. luteus and P. stutzeri, respectively. CMH from R. stolonifer with MIC50 values of 6.25 mg/mL against B. terrae and 3.13 mg/mL for CMH from R. microsporus. P. stutzeri was most sensitive to CMH, resulting in the lowest MIC50 and MBC values of 6.25 mg/mL, followed by M. luteus with MIC50 of 6.25 and MBC of 25 mg/mL. However, CMHs did not show antimicrobial activity against B. terrae (MBC > 50 mg/mL) (Figure 3). Metabolic activity of the bacteria was measured using XTT-assay for all concentration tested (1.57 to 50 mg/mL). CMHs from R. stolonifer and R. microsporus were able to reduce the viability more than 50% at the concentration of 6.25 mg/mL. Streptomycin/Penicillin were used as standard references for activity against B. terrae, M. luteus and P. stutzeri.
Table 2.
Minimum Inhibitory Concentration (MIC) and Minimum Bactericidal Concentration (MBC) values from CMH of R. stolonifer and R. microsporus grown in PDB media. MIC values from Streptomycin/Penicillin as a drug control.
| CMH Fraction | Bacteria | MIC50 | MBC |
|---|---|---|---|
| R. stolonifer | B. terrea | 6.25 mg/mL | >50 mg/mL |
| M. luteus | 6.25 mg/mL | 25 mg/mL | |
| P. stutzeri | 3.13 mg/mL | 6.25 mg/mL | |
| R. microsporus | B. terrea | 3.13 mg/mL | >50 mg/mL |
| M. luteus | 6.25 mg/mL | 25 mg/mL | |
| P. stutzeri | 3.12 mg/mL | 6.25 mg/mL | |
| Streptomycin/Penicillin | B. terrea | 0.004 mg/mL | - |
| M. luteus | 0.004 mg/mL | - | |
| P. stutzeri | 0.004 mg/mL | - |
MIC50 Minimal Inhibitory Concentration; MBC Minimal Bactericidal Concentration.
Figure 3.
Minimal Bactericidal Concentration (MBC) of CMHs from Rhizopus species against B. terrea, M. luteus and P. stutzeri analyzed by dot spot technique.
2.3. Evaluation of Anti-Biofilm Activity
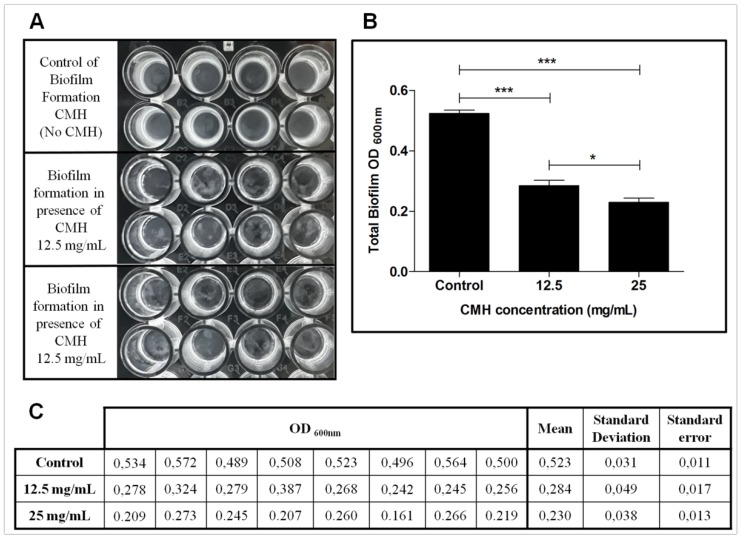
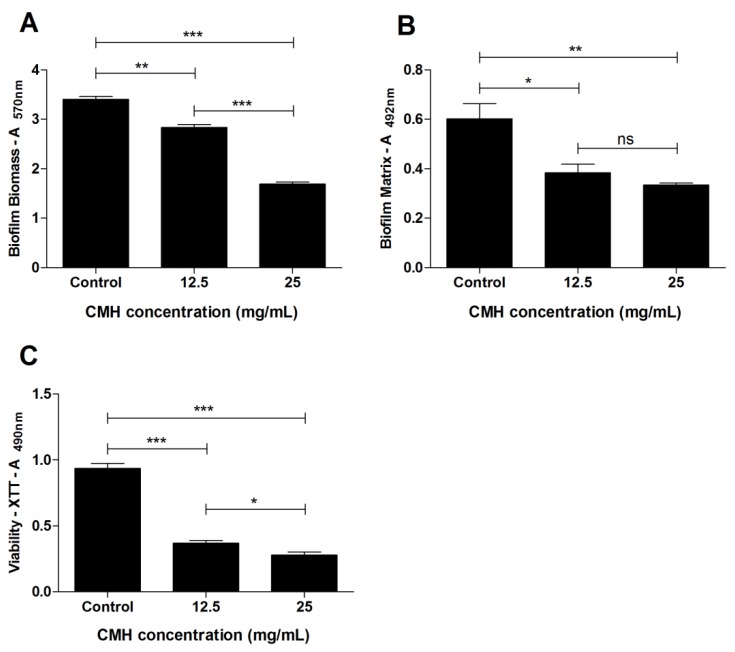
The effect of CMH (m/z 750) from R. microsporus on the biofilm produced by methicillin resistant S. aureus (MRSA) was analyzed using two different concentrations (12.5 and 25 mg/mL). CMH was not able to inhibit the growth of MRSA, however it could interfere with biofilm formation. Therefore, S. aureus (MRSA) was chosen as indicator bacterium due to its multi-drug resistance and ability to form biofilm. Representative biofilms formed by MRSA-S. aureus are shown in Figure 4A and the absorbance was determined at 600 nm (Figure 4B). Absorbance values, mean, standard deviation and standard error are represented in Figure 4C. Biofilm production on polystyrene microplates was also analyzed by correlating the results of three different analyses: The total biofilm biomass (including biofilm structure depleted of planktonic cells) was evaluated using crystal violet assay (Figure 5A, Table S1). The production of extracellular matrix was, evaluated by safranin assay (Figure 5B, Table S1), and the metabolic activity of the biofilm was quantified using XTT-assay (Figure 5C, Table S1). CMH from R. microsporus was able to reduce the total biofilm biomass in both concentrations tested. The biofilm matrix was also reduced and the metabolic activity of the biofilm was drastically decreased. Although CMH was not able to kill MRSA, it was could capably interfere on the biofilm structure formation.
Figure 4.
Effect of CMH on MRSA-S. aureus biofilm formation. (A) Representative biofilm formation at different concentration of CMH (12.5 and 25 mg/mL) and control. (B) Quantification of total biofilm formed in presence or absence of CMH. Absorbance determined at 600 nm. (C) CMH effect on MRSA biofilm formation determined by absorbance measurement at 600 nm. Mean, standard deviation and standard error are represented. Statistical differences (* p < 0.05, ** p < 0.001, *** p < 0.0001) are represented by asterisks (ns = not significant).
Figure 5.
Effect of CMH on MRSA-S. aureus biofilm formation, after 24 h of growth. (A) Total biomass of the biofilm was quantified by crystal violet assay. (B) The biofilm matrix was quantified by safranin assay. (C) Metabolic activity of the cells inside the biofilm was detected by XTT-reduction assay. Statistical differences (* p < 0.05, ** p < 0.001, *** p < 0.0001) are represented by asterisks (ns = not significant) ((A) = absorbance). Control represent MRSA growth in absence of CMH. Values represent the mean ± S.D. of three independent experiments performed in triplicate.
3. Discussion
Glycosphingolipids are membrane lipids distributed among all eukaryotic cells. They are highly enriched in the plasma membrane and are present in membrane microdomains together with sterols and proteins. Glycosylceramides are the main neutral glycosphingolipids expressed in fungal pathogens [7,28,29], and may have glucose and galactose as glycan motifs [30]. Glycosphingolipids play an important role in relevant cellular functions such as cell growth, cell adhesion and motility, carbohydrate-carbohydrate interactions and also act as potential antifungal agents [31,32]. In this work, CMHs obtained from R. stolonifer and R. microsporus grown in PDB or YEPD medium had their chemical structures elucidated by electrospray ionization mass spectrometry (ESI-MS) and by gas chromatography (GC). In this work, the role of CMH in inhibiting the growth of several bacterial species or the biofilm formation in methicillin-resistant S. aureus (MRSA) was also studied.
The major glycosphingolipids detected in Rhizopus species grown in PDB and YEPD are N-2′-hydroxyhexadecanoyl-1-O-β-d-glucopyranosyl-4-OH-9-methyl-octadeca-4,8-sphingadienine and N-2′-hydroxyhexadecanoyl-1-O-β-d-galactopyranosyl-4-OH-9-methyl-octadeca-4,8-sphingadienine with a ratio of 1:1. The main difference between R. stolonifer CMHs grown in PDB medium and the other Rhizopus species described above is the presence of 4-OH-9-methyl-eicosa-4,8-sphingadienine as the main long chain base. To our knowledge, this is the first time that different CMHs structures were identified in R. stolonifer grown in different culture media. CMHs with ceramide mono-, di-, tri-, tetra- and pentasaccharides have been found in other Zygomycetes species as Mucor hiemalis [33].
Currently, many clinically important and emerging pathogens are resistant to all or almost all antibiotics tested. This is a serious public health problem with great medical and social dimensions. The indiscriminate use of antibiotics has greatly increased the number of different bacterial species resistant to antimicrobials, which are normally used in clinical settings [34]. Therefore, there is an urgency for studies looking for the discovery of new antimicrobial agents.
In this context, CMHs isolated from R. stolonifer and R. microsporus grown in PDB were tested against both pathogenic and opportunistic bacteria, such as B. cepacea, B. terrae and E. coli (Gram negative bacteria), and M. luteus, P. stutzeri and S. aureus (Gram positive bacteria). In the agar diffusion assay, 50 mg/mL of the Folch lower phase (containing neutral lipids) or purified CMH from Rhizopus species showed antimicrobial activity against B. terrae, M. luteus and P. stutzeri. The growth of B. cepacea, E. coli, S. aureus and MRSA was not affected by either the Folch lower phase or by CMH. The best antimicrobial potential was observed for CMH from both fungi, with MIC50 values of 3.13 mg/mL against P. stutzeri, followed by M. luteus with MIC50 6.25 mg/mL. The MIC values for CMH from R. stolonifer against B. terrae was 6.25 mg/mL and from R. microsporus was 3.13 mg/mL. The CMHs from both fungi were able to decrease the viability of the three indicator bacteria tested. However, the bactericidal activity of CMH was only detected against M. luteus and P. stutzeri, with MBC values of 25 and 6.25 mg/mL, respectively. Previous work has shown that glycosphingolipids, which are amphipathic molecules, may present several biological activities, such as antimicrobial and biosurfactant actions [35].
An important survival strategy of microorganisms is the formation of biofilm, which is considered an adaptation to hostile environmental conditions [36]. Biofilms can be defined as a community of cells attached to a substrate embedded in a matrix of extracellular polymeric substances. Inside the structure of the biofilm, channels are formed that allow the passage of water, oxygen and nutrients [37]. The biofilm produced by S. aureus is an important virulence factor associated with many localized infections. In this work, although CMH did not inhibit the growth of MRSA, we investigated whether it would be able to inhibit the formation or disaggregation of the biofilm structure formed by MRSA. The biofilm produced by MRSA in the presence or absence of CMH was evaluated by three parameters: Total biofilm biomass, biofilm matrix and viability of the biofilm-forming cells. The results showed that both CMH concentrations (12.5 and 25 mg/mL) were able to influence all tested parameters. Biosurfactants isolated from Lactobacillus jensenii and L. rhamnosus showed anti-biofilm activities against clinical Multidrug Resistant (MDR) strains of Acinetobacter baumannii, E. coli, and S. aureus (MRSA) at the concentrations of 25 and 50 mg/mL [38]. However, the chemical structure of these molecules has not been elucidated.
CMH could inhibit MRSA adhesion which represents the initial stage of biofilm formation [35]. However, studies are needed to investigate possible synergism between CMHs from Rhizopus species and the antibiotics used routinely for the treatment of MRSA infections.
4. Material and Methods
4.1. Microorganisms and Culture Conditions
Rhizopus stolonifer UPC1300 and Rhizopus microsporus var. microsporus UPC1304, isolated from the Brazilian Caatinga area, were supplied by the Culture Collection (RENNORFUN) from the Catholic University of Pernambuco, Recife, Brazil. Strains were maintained in Sabouraud agar solid medium under refrigeration at 4 °C. Cells were inoculated in Erlenmeyer flasks containing 200 mL of potato dextrose broth (PDB) and/or YEPD (glucose 2%, peptone 2%, yeast extract 1%), and incubated at room temperature for 7 days with orbital shaking (pre-inoculum). Cultures (200 mL) were then transferred to the same medium (3 L) and incubated for a further 7 days at the same temperature with shaking. At the end, the mycelium was filtered, washed with distilled water, and stored at −20 °C.
4.2. Extraction and Purification of CMH from R. stolonifer and R. microsporus
Total lipids from intact hyphae of R. stolonifer and R. microsporus grown in PDB or YEPD were successively extracted at room temperature using chloroform/methanol at ratios 2:1 and 1:2 (v/v), as described [7]. The crude lipid extracts were partitioned with chloroform/methanol/ 0.45% KCl (8:4:3 v/v) as described by Folch et al. (1957) [39]. The Folch lower layer containing neutral lipids, glycosphingolipids and phospholipids was fractionated on a silica gel column which was sequentially eluted with chloroform, acetone and then methanol [12]. The recovery of glycosphingolipids by elution with methanol was monitored by thin-layer chromatography (TLC), on silica gel plates developed with chloroform/methanol/2 M ammonium hydroxide, 40:10:1 (v/v). The spots were visualized with iodine and by charring with orcinol/H2SO4.
4.3. Sugar Analysis
Glycosphingolipids were hydrolyzed with 3 M trifluoroacetic acid at 100 °C for 3 h and the released monosaccharides were characterized by HPTLC developed with n-butanol/acetone/water (4:5:1 v/v) and visualized by spraying with orcinol-sulfuric acid reagent. The monosaccharides were converted to their alditol acetate derivatives and quantified by GC using the chromatograph GC-2010 Plus-Shimadzu, with a SH-RTX-5 capillary column (30 m, 0.25 mm ID, 0.25 Um df), programmed for an initial isothermal period of 10 min at 190 °C, and subsequent temperature increase of 2 °C/min, until 210 °C were reached [40,41].
4.4. ESI-MS Analysis
CMHs were analyzed by electrospray ionization (ESI-MS) in positive (ESI+) ion mode, using an ESI-ion Trap instrument (Model Amazon SL, Bruker, Germany). CMHs were diluted in chloroform/methanol/water (5:4:1 v/v), containing 1 mM lithium chloride and analyzed via direct injection using a microsyringe pump (Hamilton) [42]. Nitrogen was used as nebulizer and carrier gas.
4.5. Antimicrobial Assay
4.5.1. Bacterial Strains
Burkholderia cepacea (American Type Culture Collection, ATCC 25416), B. terrea BS001 (Microbial Ecology culture collection, University of Groningen, Groningen, The Netherlands), Escherichia coli (American Type Culture Collection, ATCC11229), Micrococcus luteus (American Type Culture Collection, ATCC4698) Methicillin Resistant Staphylococcus aureus-MRSA (American Type Culture Collection, ATCC9393), Pseudomonas stutzeri R55 (Laboratory of Molecular Microbial Ecology and Microbial Diversity Culture Collection, Instituto de Microbiologia Paulo de Góes, Universidade Federal do Rio de Janeiro/UFRJ). Bacteria were grown at 37 °C in Luria-Bertani (LB) broth (peptone 10 g/L, yeast extract 5 g/L and NaCl 5 g/L).
4.5.2. Antimicrobial Activity Assay
Antimicrobial activity of CMH [50 mg/mL in chloroform/methanol (2:1 v/v)] was determined against all bacterial strains by the agar diffusion method with modifications. Briefly, 10 µL of CMH (50 mg/mL) were spotted on a Petri dish containing LB agar medium. After evaporation of the organic solvents, an inoculum of each bacterial strain containing approximately 1.5 × 108 colony forming units (CFU/mL) prepared in Luria-Bertani broth medium was spread out on the plates, and the plates were incubated for 24 h at 37 °C. A positive control with inoculum without CMH was included. Growth inhibition zones were evidenced using MTT (Thiazolyl Blue Tetrazolium Bromide—Sigma Aldrich, St. Louis, MO, USA) [43].
4.5.3. Determination of MIC and MBC
MIC was evaluated by the dilution method in LB broth. CMH concentrations ranging from 15.7 mg/mL to 50 mg/mL were tested and 10 μL of the bacterial suspension containing 1.5 × 108 CFU/mL (0.1 OD600 nm) were added to each well and incubated at 37 °C for 24 h. DMSO was used as control (5% in LB broth medium) and streptomycin/penicilin was used as a reference compound (8–0.015 µg/mL). After 24 h, the plates were read at 600 nm to evaluate the MIC50. After MICs reading, MBC (minimal bactericidal concentration) was determined by subculturing an aliquot of 10 µL from each well that showed complete growth inhibition in LB agar medium without addition of CMH and the bacterial growth was evaluated for the MBC determination. After 24 h, the MBC values were defined as the lowest concentration of CMH able of eliminate bacteria [44]. The experiments were carried out in duplicates. Following the MIC assays, cell viability was evaluated using XTT reduction assay (XTT sodium salt—0.5 mg/mL—Sigma Aldrich, St. Louis, MO, USA) [45].
4.5.4. Effect of CMH on Inhibition of MRSA ATCC9393 Biofilm
50 μL of MRSA ATCC9393 suspension (0.1 OD600 nm) in Tryptic Soy Broth (TSB) supplemented with 1% glucose were mixed with 50 µL of CMH (12.5 and 25 mg/mL) from R. microspores and added to each well of a 96-well polystyrene plate. Culture medium with the bacterial suspension was used as negative control. After 24 h incubation at 37 °C, the formed biofilm was gently washed with sterile distilled water to remove planktonic cells, air-dried for 10 min and stained with 0.5% crystal violet for 10 min (total biofilm biomass) or 1% safranin for 10 min (biofilm matrix). The staining solutions were discarded and the biofilms were rinsed gently twice with sterile distilled water. The crystal violet impregnated in the biofilm was dissolved in 200 µL of ethanol (95%, v/v), and the colored solution was read at an absorbance of 595 nm using a spectrophotometer (Spectra MAX 340 Tunable; Molecular Devices Ltd., San Jose, CA, USA). Safranin was dissolved in water (100 µL), and the absorbance read at 492 nm. Viability analysis of the cells in the biofilm was performed by XTT reduction assay. Biofilms were washed with sterile distilled water, and 150 µL of XTT solution were added to each well. The XTT solution was prepared by dissolving 5 mg of XTT in 10 mL of water, followed by the addition of 400 μL Menadione solution (0.17 mg/mL in acetone). After incubation at 37 °C for 1 h under protection from light, the absorbance of the colored solution was measured at 490 nm.
4.6. Statistical Analysis
All statistical analyses were performed using GraphPad Prism 5.0 software (GraphPad, San Diego, CA, USA). A variance two-way ANOVA was performed using Tukey’s and Bonferroni’s comparisons tests to evaluate the biofilm formation.
Acknowledgments
This work was supported by grants from the Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq # 150072/2017-1/PDJ), Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ), Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES). We thank the Centro de Espectrometria de Massas de Biomoleculas (CEMBIO) from Universidade Federal do Rio de Janeiro for ESI-MS analysis. We thank Walter Oelemann for advice, encouragement and critical reading of the manuscript. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Supplementary Materials
The following are available, Figure S1: Antimicrobial Activity of CMHs from R. stolonifer and R. microsporus against a panel of Gram-positive and Gram-negative bacterium by agar disc diffusion method. CMH or Folch lower layer (Folch↓) were dissolved in chloroform/methanol (2:1 v/v) at a concentration of 50 mg/mL. Each spot contained 10 μL. The control spot contained 10 µL of chloroform/methanol (2:1 v/v). Growth inhibition zones were evidenced by MTT assay. Table S1: Effect of CMH on MRSA-S. aureus biofilm formation. Analyses were done correlating the results of total biofilm biomass (evaluated by crystal violet assay, A570 nm), production of extracellular matrix (evaluated by safranin assay, A492 nm) and metabolic activity (quantified by XTT-assay, A490 nm). Mean, standard deviation (SD) and standard error (SE) are also shown. (A = absorbance).
Author Contributions
Conceived and designed the experiments: E.R.V.; M.I.D.d.S.X.; E.B.-B. Performed the experiments: E.R.V.; M.I.D.d.S.X.; M.A.P.; D.S.A. Analyzed the data: M.I.D.d.S.X.; E.R.V.; M.A.P.; E.B.-B. Contributed reagents/materials/analysis tools: E.B.-B.; C.S.A.; D.S.A.; G.M.d.C.-T. Wrote the paper: M.I.D.d.S.X.; E.B.-B.; D.S.A.; G.M.d.C.-T.
Funding
This research was funded by Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) grant number #150072/2017-1/PDJ, Fundação de Amparo à Pesquisa do Estado do Rio de Janeiro (FAPERJ) and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES).
Conflicts of Interest
The authors declare no conflict of interest.
Footnotes
Sample Availability: Samples of the compounds monohexosylceramides (CMH) are available from the authors.
References
- 1.De Azevedo Santiago A.L.C.M., dos Santos P.J.P., Maia L.C. Mucorales from the semiarid of Pernambuco, Brazil. Braz. J. Microbiol. 2013;44:299–305. doi: 10.1590/S1517-83822013005000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lima D.X., De Azevedo Santiago A.L.C.M., De Souza-Motta C.M. Diversity of mucorales in natural and degraded semi-arid soils. Braz. J. Bot. 2016;39:1127–1133. doi: 10.1007/s40415-015-0156-8. [DOI] [Google Scholar]
- 3.Lackner M., Caramalho R., Lass-Flörl C. Laboratory diagnosis of mucormycosis: Current status and future perspectives. Future Microbiol. 2014;9:683–695. doi: 10.2217/fmb.14.23. [DOI] [PubMed] [Google Scholar]
- 4.Ribes J.A., Vanover-Sams C.L., Baker D.J. Zygomycetes in human disease. Clin. Microbiol. Rev. 2000;13:236–301. doi: 10.1128/CMR.13.2.236-301.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pinto M.R., Rodrigues M.L., Travassos L.R., Haido R.M.T., Wait R., Barreto-Bergter E. Characterization of glucosylceramides in Pseudallescheria boydii and their involvement in fungal differentiation. Glycobiology. 2002;12:251–260. doi: 10.1093/glycob/12.4.251. [DOI] [PubMed] [Google Scholar]
- 6.Barreto-Bergter E., Pinto M.R., Rodrigues M.L. Structure and biological functions of fungal cerebrosides. An. Acad. Bras. Ciênc. 2004;76:67–84. doi: 10.1590/S0001-37652004000100007. [DOI] [PubMed] [Google Scholar]
- 7.Barreto-Bergter E., Sassaki G.L., de Souza L.M. Structural analysis of fungal cerebrosides. Front. Microbiol. 2011;2:239. doi: 10.3389/fmicb.2011.00239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Da Silva A.F.C., Rodrigues M.L., Farias S.E., Almeida I.C., Pinto M.R., Barreto-Bergter E. Glucosylceramides in colletotrichum gloeosporioides are involved in the differentiation of conidia into mycelial cells. FEBS Lett. 2004;561:137–143. doi: 10.1016/S0014-5793(04)00156-5. [DOI] [PubMed] [Google Scholar]
- 9.Nimrichter L., Cerqueira M.D., Leitão E.A., Miranda K., Nakayasu E.S., Almeida S.R., Almeida I.C., Alviano C.S., Barreto-Bergter E., Rodrigues M.L. Structure, cellular distribution, antigenicity, and biological functions of Fonsecaea pedrosoi ceramide monohexosides. Infect. Immun. 2005;73:7860–7868. doi: 10.1128/IAI.73.12.7860-7868.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rodrigues M.L., Travassos L.R., Miranda K.R., Franzen A.J., Rozental S., de Souza W., Alviano C.S., Barreto-Bergter E. Human antibodies against a purified glucosylceramide from Cryptococcus neoformans inhibit cell budding and fungal growth. Infect. Immun. 2000;68:7049–7060. doi: 10.1128/IAI.68.12.7049-7060.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ramamoorthy V., Cahoon E.B., Thokala M., Kaur J., Li J., Shah D.M. Sphingolipid c-9 methyltransferases are important for growth and virulence but not for sensitivity to antifungal plant defensins in Fusarium graminearum. Eukaryot. Cell. 2009;8:217–229. doi: 10.1128/EC.00255-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rollin-Pinheiro R., Singh A., Barreto-Bergter E., Del Poeta M. Sphingolipids as targets for treatment of fungal infections. Future Med. Chem. 2016;8:1469–1484. doi: 10.4155/fmc-2016-0053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saito K., Takakuwa N., Ohnishi M., Oda Y. Presence of glucosylceramide in yeast and its relation to alkali tolerance of yeast. Appl. Microbiol. Biotechnol. 2006;71:515–521. doi: 10.1007/s00253-005-0187-3. [DOI] [PubMed] [Google Scholar]
- 14.Thevissen K., Warnecke D.C., François I.E.J.A., Leipelt M., Heinz E., Ott C., Zähringer U., Thomma B.P.H.J., Ferket K.K.A., Cammue B.P.A. Defensins from insects and plants interact with fungal glucosylceramides. J. Biol. Chem. 2004;279:3900–3905. doi: 10.1074/jbc.M311165200. [DOI] [PubMed] [Google Scholar]
- 15.Cateni F., Zilic J., Falsone G., Scialino G., Banfi E. New cerebrosides from Euphorbia peplis L.: Antimicrobial activity evaluation. Bioorgan. Med. Chem. Lett. 2003;13:4345–4350. doi: 10.1016/j.bmcl.2003.09.044. [DOI] [PubMed] [Google Scholar]
- 16.Derengowski L.S., De-Souza-Silva C., Braz S.V., Mello-De-Sousa T.M., Báo S.N., Kyaw C.M., Silva-Pereira I. Antimicrobial effect of farnesol, a Candida albicans quorum sensing molecule, on Paracoccidioides brasiliensis growth and morphogenesis. Ann. Clin. Microbiol. Antimicrob. 2009;8:13. doi: 10.1186/1476-0711-8-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Shu R.G., Wang F.W., Yang Y.M., Liu Y.X., Tan R.X. Antibacterial and xanthine oxidase inhibitory cerebrosides from Fusarium sp. IFB-121, and endophytic fungus in quercus variabilis. Lipids. 2004;39:667–673. doi: 10.1007/s11745-004-1280-9. [DOI] [PubMed] [Google Scholar]
- 18.Wu Z.-P., Chen Y., Xia B., Wang M., Dong Y.-F., Feng X. Two novel ceramides with a phytosphingolipid and a tertiary amide structure from Zephyranthes candida. Lipids. 2009;44:63–70. doi: 10.1007/s11745-008-3246-6. [DOI] [PubMed] [Google Scholar]
- 19.Ribeiro P.C.S., Monteiro A.S., Marques S.G., Monteiro S.G., Monteiro-Neto V., Coqueiro M.M.M., Marques A.C.G., de Jesus Gomes Turri R., Santos S.G., Bomfim M.R.Q. Phenotypic and molecular detection of the bla(KPC) gene in clinical isolates from inpatients at hospitals in São Luis, MA, Brazil. BMC Infect. Dis. 2016;16:737. doi: 10.1186/s12879-016-2072-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rossi Gonçalves I., Ferreira M.L., Araujo B.F., Campos P.A., Royer S., Batistão D.W.F., Souza L.P., Brito C.S., Urzedo J.E., Gontijo-Filho P.P., et al. Outbreaks of colistin-resistant and colistin-susceptible KPC-producing klebsiella pneumoniae in a brazilian intensive care unit. J. Hosp. Infect. 2016;94:322–329. doi: 10.1016/j.jhin.2016.08.019. [DOI] [PubMed] [Google Scholar]
- 21.Tognim M.C.B., Cardoso C.L. Nosocomial outbreaks in brazil: Can they be controlled? J. Hosp. Infect. 2016;94:320–321. doi: 10.1016/j.jhin.2016.10.002. [DOI] [PubMed] [Google Scholar]
- 22.Lima-e-Silva A.A., Silva-Filho R.G., Fernandes H.M.Z., Saramago C.S.M., Viana A.S., Souza M.J., Nogueira E.M. Sub-inhibitory concentrations of rifampicin strongly stimulated biofilm production in S. aureus. Open Microbiol. J. 2017;11:142–151. doi: 10.2174/1874285801711010142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rodríguez-Rojas A., Makarova O., Rolff J. Antimicrobials, stress and mutagenesis. PLoS Pathog. 2014;10:e1004445. doi: 10.1371/journal.ppat.1004445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ioannou A., Xenophontos E., Karatsi A., Petrides C., Kleridou M., Zintilis C. Insidious manifestation of pyogenic liver abscess caused by streptococcus intermedius and micrococcus luteus: A case report. Oxf. Med. Case Rep. 2016;2016:1–3. doi: 10.1093/omcr/omv071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lalucat J., Bennasar A., Bosch R., García-Valdés E., Palleroni N.J. Biology of Pseudomonas stutzeri. Microbiol. Mol. Biol. Rev. 2006;70:510–547. doi: 10.1128/MMBR.00047-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miltiadous G., Elisaf M. Native valve endocarditis due to micrococcus luteus: A case report and review of the literature. J. Med. Case Rep. 2011;5:251. doi: 10.1186/1752-1947-5-251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Miron D., Keness Y., Bor N., Spiegel R., Horowitz Y. Pseudomonas stutzeri knee arthritis in a child: Case report and review. J. Pediatr. Orthop. B. 2007;16:419–421. doi: 10.1097/BPB.0b013e3282f058bb. [DOI] [PubMed] [Google Scholar]
- 28.Guimarães L.L., Toledo M.S., Ferreira F.A.S., Straus A.H., Takahashi H.K. Structural diversity and biological significance of glycosphingolipids in pathogenic and opportunistic fungi. Front. Cell. Infect. Microbiol. 2014;4:138. doi: 10.3389/fcimb.2014.00138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Longo L.V.G., Nakayasu E.S., Gazos-Lopes F., Vallejo M.C., Matsuo A.L., Almeida I.C., Puccia R. Characterization of cell wall lipids from the pathogenic phase of Paracoccidioides brasiliensis cultivated in the presence or absence of human plasma. PLoS ONE. 2013;8:e63372. doi: 10.1371/journal.pone.0063372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Takahashi H.K., Toledo M.S., Suzuki E., Tagliari L., Straus A.H. Current relevance of fungal and trypanosomatid glycolipids and sphingolipids: Studies defining structures conspicuously absent in mammals. An. Acad. Bras. Ciênc. 2009;81:477–488. doi: 10.1590/S0001-37652009000300012. [DOI] [PubMed] [Google Scholar]
- 31.Hakomori S. Glycosynapses: Microdomains controlling carbohydrate-dependent cell adhesion and signaling. An. Acad. Bras. Ciênc. 2004;76:553–572. doi: 10.1590/S0001-37652004000300010. [DOI] [PubMed] [Google Scholar]
- 32.Hakomori S.-I. Structure and function of glycosphingolipids and sphingolipids: Recollections and future trends. Biochim. Biophys. Acta. 2008;1780:325–346. doi: 10.1016/j.bbagen.2007.08.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aoki K., Uchiyama R., Yamauchi S., Katayama T., Itonori S., Sugita M., Hada N., Yamada-Hada J., Takeda T., Kumagai H., et al. Newly discovered neutral glycosphingolipids in aureobasidin a-resistant zygomycetes: Identification of a novel family of gala-series glycolipids with core galα1-6galβ1-6galβ sequences. J. Biol. Chem. 2004;279:32028–32034. doi: 10.1074/jbc.M312918200. [DOI] [PubMed] [Google Scholar]
- 34.Weinstein R.A. Controlling antimicrobial resistance in hospitals: Infection control and use of antibiotics. Emerg. Infect. Dis. 2001;7:188–192. doi: 10.3201/eid0702.010206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cortés-Sánchez Ade J., Hernández-Sánchez H., Jaramillo-Flores M.E. Biological activity of glycolipids produced by microorganisms: New trends and possible therapeutic alternatives. Microbiol. Res. 2013;168:22–32. doi: 10.1016/j.micres.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 36.De la Fuente-Núñez C., Reffuveille F., Fernández L., Hancock R.E.W. Bacterial biofilm development as a multicellular adaptation: Antibiotic resistance and new therapeutic strategies. Curr. Opin. Microbiol. 2013;16:580–589. doi: 10.1016/j.mib.2013.06.013. [DOI] [PubMed] [Google Scholar]
- 37.Rabin N., Zheng Y., Opoku-Temeng C., Du Y., Bonsu E., Sintim H.O. Biofilm formation mechanisms and targets for developing antibiofilm agents. Future Med. Chem. 2015;7:493–512. doi: 10.4155/fmc.15.6. [DOI] [PubMed] [Google Scholar]
- 38.Sambanthamoorthy K., Feng X., Patel R., Patel S., Paranavitana C. Antimicrobial and antibiofilm potential of biosurfactants isolated from lactobacilli against multi-drug-resistant pathogens. BMC Microbiol. 2014;14:197. doi: 10.1186/1471-2180-14-197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Folch J., Lees M., Stanley G.H.S. A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 1957;226:497–509. [PubMed] [Google Scholar]
- 40.Leitão E.A., Bittencourt V.C.B., Haido R.M.T., Valente A.P., Peter-Katalinic J., Letzel M., de Souza L.M., Barreto-Bergter E. B-galactofuranose-containing O-linked oligosaccharides present in the cell wall peptidogalactomannan of aspergillus fumigatus contain immunodominant epitopes. Glycobiology. 2003;13:681–692. doi: 10.1093/glycob/cwg089. [DOI] [PubMed] [Google Scholar]
- 41.Sawardeker J.S., Sloneker J.H., Jeanes A. Quantitative determination of monosaccharides as their alditol acetates by gas liquid chromatography. Anal. Chem. 1965;37:1602–1604. doi: 10.1021/ac60231a048. [DOI] [Google Scholar]
- 42.Calixto R.O.R., Rollin-Pinheiro R., da Silva M.I.D., Liporagi-Lopes L.C., Vieira J.M., Sassaki G.L., Barreto-Bergter E. Structural analysis of glucosylceramides (GlcCer) from species of the pseudallescheria/scedosporium complex. Fungal Biol. 2016;120:166–172. doi: 10.1016/j.funbio.2015.05.007. [DOI] [PubMed] [Google Scholar]
- 43.Marinho P.R., Muricy G.R.S., Silva M.F.L., de Marval M.G., Laport M.S. Antibiotic-resistant bacteria inhibited by extracts and fractions from brazilian marine sponges. Rev. Bras. Farmacogn. 2010;20:267–275. doi: 10.1590/S0102-695X2010000200022. [DOI] [Google Scholar]
- 44.Hili P., Evans C.S., Veness R.G. Antimicrobial action of essential oils: The effect of dimethylsulphoxide on the activity of cinnamon oil. Lett. Appl. Microbiol. 1997;24:269–275. doi: 10.1046/j.1472-765X.1997.00073.x. [DOI] [PubMed] [Google Scholar]
- 45.Mosmann T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods. 1983;65:55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.