Abstract
Allergic rhinitis is serious public health problems and one of the most common chronic diseases worldwide. We aimed to assess the cost-effectiveness of clinically relevant treatment options for allergic rhinitis using evidence-based literature. In addition, we aimed to develop recommendations for allergic rhinitis treatment based on health economic facts. We searched MEDLINE via PubMed from 2009 to 2014 to identify all therapeutic options described in the current literature and selected randomized controlled trials that used a symptom score, had at least one placebo control group and used adult patients. We analyzed the side effects and the number of cases in which treatment was discontinued for each treatment option. Local antihistamines were the most cost-effective local therapy and are recommended due to the low number of complications. Regarding systemic therapies, although the use of oral steroids is indeed significantly cost-effective, this treatment was found to be associated with strong side effects. Sublingual immunotherapy was identified as the most cost-effective immunotherapy and exhibits a good side-effect profile. Overall, local therapy with antihistamines was found to be the most cost-effective option of all therapies. This study showed that there are only minor differences between sublingual and subcutaneous immunotherapy. Based on our results, we recommend the use of an international, uniform nasal symptom score to facilitate the comparison of clinical trials on allergic rhinitis in the future.
Keywords: Allergic rhinitis, cost-effectiveness, side effects, symptom score
Introduction
Allergic rhinitis (AR) is a serious public health problem worldwide and one of the most common chronic diseases that affects the daily life of patients, causes severe symptoms and major disabilities.1,2 AR occurs in people from all countries, ethnicities and age groups.1 In some countries, it has been estimated that over 50% of the young adult population suffers from AR.1 Nevertheless, AR is continually increasing in several countries, primarily in countries with a low or medium prevalence.1 AR is often underestimated from the health economic perspective because its treatment is associated with avoidable, low direct costs. However, the direct costs of AR are not apparent, and there may be substantial indirect costs.3 The total cost (direct, indirect and intangible) of AR in Germany was 240 million € in 2000.4
Several studies have determined or estimated the financial burden of AR in different countries. The efficacies of different therapeutic options for AR have also been compared. However, there is a lack of health economic studies evaluating the therapeutic options for AR in terms of their effectiveness and costs. Therefore, we aimed to assess the cost-effectiveness of clinically relevant treatment options for AR using evidence-based literature, the uniform German Value Scale 2014 of otolaryngologists,5 and German pharmacy prices.6 In addition, we created a health economics based recommendation for the treatment of AR.
Materials and methods
A literature search was performed using MEDLINE via PubMed to identify relevant articles in English published from 2009 to 2014 that were appropriate for the comparison of the effectiveness of different treatment options (systemic and local). If any article in this period did not fulfill the inclusion criteria, we considered studies from 2000 to 2009. If we did not find an appropriate study, we searched for the most recent publications before these periods that reported treatment options.
We used several search terms, and the keywords were matched to database-specific indexing terms. We used the operators AND and OR to link keywords with different and similar meanings.
Selection criteria
Studies were included in the present analysis if they met the following inclusion criteria:
A randomized controlled trial,
Must contain at least one placebo control group,
Must use a symptom score,
All AR patients enrolled in study must be adult (⩾18 years).
Studies involving region/country-specific allergens (e.g. Texas mountain cedar pollen or Japanese cedar pollen) were excluded.
Two raters independently applied the inclusion and exclusion criteria and extracted the therapeutic options (intervention groups) and results of the symptom scores from the final set of eligible studies by screening the full-text articles. Any discrepancies were resolved by discussion. Because the highest quality study for each treatment option should be used, we initially used the Oxford scale to assess the quality of the studies.7 The study that achieved the highest score for a particular treatment option was used. In addition, the quality of studies was assessed using the CONSORT statement (CONsolidated Standards of Reporting Trials).8 After the examination, if no difference was observed in the quality of the studies between trials, the study with the most relevance to the cost-effectiveness analysis was selected.
Each individual symptom score was examined closely for scaling and characterization after the highest methodological evidence was selected for each therapeutic option. To compare the therapeutic options, we needed a uniform method to scale the scores. Because the most common scale used in the selected studies was a 4-point scale (0–3),9–15 we used this scale for effectiveness with the following coding: 0 = no symptoms, 1 = mild symptoms, 2 = moderate symptoms and 3 = severe symptoms. We converted all other scales, such as 6-point scales or percentages, to this 4-point scale (Online Appendix 1).
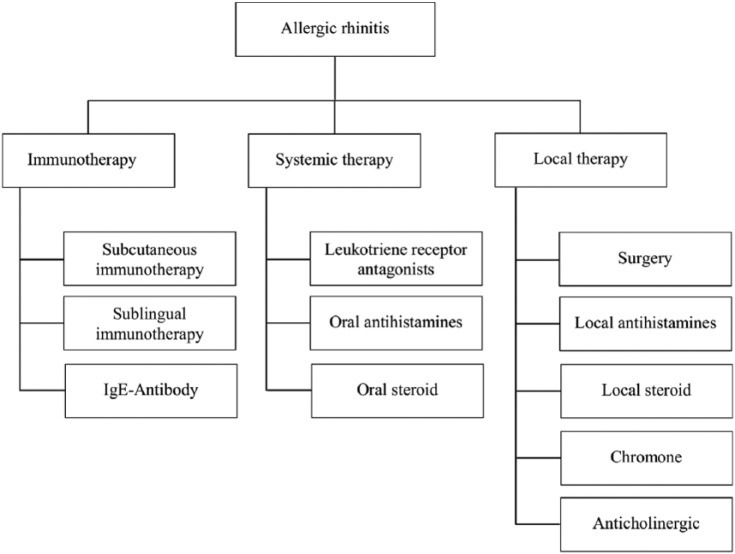
The therapeutic options were divided into three new groups according to the type of application or the therapeutic aim for the cost-effectiveness comparisons: “immunotherapies,” “systemic therapies,” and “local therapies” (Figure 1).
Figure 1.
Distribution of treatment options for allergic rhinitis.
Costs and cost-effectiveness
The direct costs of each medical therapy were identified for the economic evaluation of the different treatments. We identified the medications used to treat AR (substances) as well as the dosage and method of administration. Subsequently, the current German pharmacy selling prices were determined according to drug price regulation (WEBAPO® Infosystem—Lauer Fischer Taxe® Dr Mack-Straße 95, 90762 Fürth)6 of the individual drugs from 1 January 2015. The nasal spray that included ipratropium bromide (anticholinergic) could not be found in the WEBAPO database because this spray was not licensed at the time of inquiry in Germany. Therefore, the anticholinergic therapies were excluded from the economic evaluation. In one study, the patients were treated surgically using kalium titanyl phosphate (KTP) laser to control the perennial AR symptoms in minimally invasive fashion. In this study, the anterior one-fourth of the inferior turbinate was removed with a KTP laser on one side; the other side was untreated and acted as a control.16 Because the drug therapies were considered an out-patient treatment, we used only the costs of the ambulatory based on the Uniform Value Scale (“Einheitlicher Bewertungsmaßstab”—EBM) system.5 Furthermore, we identified additional medical services, laboratory tests, or other therapies in the selected studies and calculated the costs in the same way (according to EBM). All costs are reported in Euros for currency year 2015. The costs were not discounted, as they were based on the current medication price.
To compare the effectiveness and costs of the therapeutic options, we also determined the costs scaled by 33% or based on a score of 1.0 for each therapeutic option. For example, if a therapy had a cost of “Y” and a score of 0.5 points, we assumed that the cost would be multiplied by 2 (1.0/0.5 = 2) (Y × 2) to obtain the cost for an improvement of 1.0 points. According to this principle, an amount in Euros was determined for each treatment, which represents 33% of the cost of the symptom scores.
Sensitivity analysis
We also performed 10% sensitivity analyses to assess the uncertainty of the outcomes. The three main treatment groups (immunotherapy, oral therapy and local therapy) were considered individually in the sensitivity analyses. The score-value of the most cost-effective therapy in each group was reduced by 10%, and the score-value of the other therapies was increased by 10%.17 Subsequently, these score-values were assigned to costs, and a comparable value was calculated for the improvement of the score to 1.0. Thus, it should be determined whether inaccuracies in the score-values (e.g. distortion through conversions and incorrect documentation) would lead to a change in the result.
Side effects and discontinuations
The risks and complications associated with the treatments were critically discussed in the context of the cost-effectiveness results. Therefore, the side effects and number of treatment discontinuations (drop-outs) were identified for each treatment option. Both sets of information were, as much as possible, taken directly from the selected studies.
Results
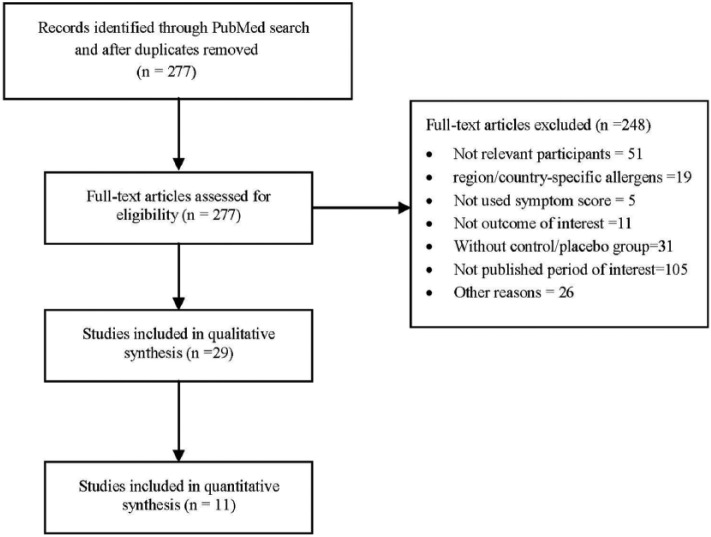
The titles and abstracts were screened after the duplicates were removed, resulting in 277 potentially relevant articles. Based on the screening of the full-text articles, 29 articles fulfilled the inclusion criteria. A flow diagram of the search and selection strategy is shown in Figure 2. The quality of these studies was assessed using the Oxford scale and CONSORT statement, and the highest quality study associated with each of the 11 treatment options was selected.9–16,18–20 An overview of the symptom score-value for the medical therapies compared with the placebo is provided in Table 1.
Figure 2.
PRISMA flow diagram for the review process to select studies.
Table 1.
Description of the studies according to the quality criteria.
| Study ID | Study design | Patients (n) | Intervention groups | Results | Difference in the symptom scores |
|---|---|---|---|---|---|
| Subcutaneous immunotherapy | |||||
| Du Buske et al.10 | Randomized, double-blind, placebo-controlled | 1028 | a) Grass MATA MPL (300 SU in injection 1; 800 SU in injection 2;
2000 SU in injections 3 and 4 (n = 514) b) Placebo (n = 514) |
ITT population: was reduced by 13.6% with Grass MATA MPL compared with placebo (p = 0.0038); complete data population: 24.3% (p = 0.0031) | −0.41 |
| Sublingual immunotherapy | |||||
| Nelson et al.11 | Randomized, double-blind, placebo-controlled | 439 | a) Timothy Grass AIT treatment (75,000 standardized quality
tablet) for 16 weeks before the anticipated start of the GPS and
continuing throughout the 2009 GPS (n = 213) b) Placebo (n = 225) |
Significant improvement following the Grass AIT treatment (18%, p = 0.02) during the GPS | −0.45 |
| IgE-Antibody | |||||
| Ädelroth et al.9 | Randomized, double-blind, placebo-controlled, parallel-group | 251 | a) 300 mg of rhumAb-E25 2/3 times (n = 164) b) Placebo (n = 86) |
RhumAb-E25 was similar at baseline and throughout the treatment, but it increased in the placebo group (p < 0.001) | −0.21 |
| Leukotriene receptor antagonists | |||||
| Ciebiada et al.13 | Randomized, double-blind, placebo-controlled, crossover | 40 | Group A: (n = 20) a) Montelukast 10 mg/day b) Levocetrizine 5 mg/day c) Combination of both d) Placebo Group B: (n = 20) a) Montelukast 10 mg/day b) Desloratadine 5 mg/day c) Combination of both d) Placebo |
Montelukast significantly improved nasal symptoms during the first 24 h | −0.65 |
| Oral antihistamines | |||||
| Marmouz et al.12 | Randomized, double-blind, placebo-controlled | 308 | a) Rupatadine 10 mg/day for 4 weeks (n = 68) b) Rupatadine 20 mg/day for 4 weeks (n = 65) c) Cetirizine 10 mg/day for 4 weeks (n = 66) d) Placebo (n = 70) |
Morning score: significant reductions in the 10 and 20 mg rupatadine groups compared with placebo; cetirizine 10 mg not significant; evening score: significant reductions in the 10 and 20 mg rupatadine groups and in the 10 mg cetirizine compared with placebo | a) −0.23 b) −0.31 c) −0.23 |
| Oral steroid | |||||
| Hissaria et al.18 | Randomized, double-blind, placebo-controlled | 40 | a) Prednisolone 50 mg/day for 14 days (n = 20) b) Placebo (n = 20) |
Nasal-specific RSOM (31-item Rhinosinusitis Outcome Measure)
scores (6 parameters) showed significant improvement only in the
group treated with prednisolone (p < 0.001); a significantly
greater reduction in the symptom score was observed in the
actively treated group (p < 0.001) here: “History of atopy” >38 of 40 patients >95% |
−1.59 |
| Surgery | |||||
| Kunachak et al.16 | Randomized, placebo-controlled | 58 | a) One side of the nose KTP laser at 12 watts; total energy per
side ranging from 121 to 440 J (mean = 252) b) Other side = Placebo |
The mean improvement on the treated side was 71 ± 17.8% and on the control side was 38.4 ± 29.4%; the difference was significant (p < 0.001) | −0.98 |
| Local antihistamines | |||||
| Patel et al.14 | Randomized, double-blind, placebo-controlled, parallel-group | 425 | a) Olopatadine hydrochloride nasal spray 665 µg b) Mometasone furoate monohydrate 50 µg c) Placebo |
Olopatadine was superior to the placebo in reducing the symptom score within 30 min after dosing and maintained superiority for at least 12 h (p < 0.05) | −0.48 |
| Local steroid | |||||
| Bende et al.15 | Randomized, placebo-controlled, parallel-group | 438 | a) Budesonide 256 μg/day for 4 weeks (n = 107) b) Budesonide 128 μg/day for 4 weeks (n = 110) c) Mometasone furoate 200 μg/day for 4 weeks (n = 106) d) Placebo (n = 114) |
All three active treatments reduced the nasal index score compared with the placebo; no significant difference was found between the treatments | a) −0.33 b) −0.31 c) −0.28 |
| Chromone | |||||
| Cohan et al.19 | Randomized, double-blind, placebo-controlled, crossover | 34 | a) 4% solution of cromolyn sodium (n = 17) b) Placebo (n = 17) |
Many patients exhibited greater improvement with the drug than the placebo (p < 0.005) | −0.72 |
| Anticholinergic | |||||
| Meltzer et al.20 | Randomized, double-blind, placebo-controlled, parallel-group | 123 | a) ipratropium bromide nasal spray (21 µg) b) ipratropium bromide nasal spray (42 µg) c) Placebo |
Significant reduction in the rhinorrhea severity score following treatment with 42 μg of ipratropium bromide nasal spray | a) −0.12 b) −0.48 |
MATA: Modified Allergen Tyrosine Adsorbate; MPL: monophosphoryl lipid A; SU: standardized units; ITT: intention to treat; AIT: allergy immunotherapy tablet; GPS: grass pollen season; IgE: immunoglobulin E; rhumAb-E25: recombinant humanized murine antibody-E25; RSOM: rhinosinusitis outcome measure; KTP: kalium titanyl phosphate.
To determine the most effective therapy, we compared the most effective therapies in each of the main treatment groups together. The oral steroid therapy had the highest score of all the therapies (−1.59), and surgical therapy (−0.98; local therapy) achieved the second-best score. Although sublingual immunotherapy showed the best effectiveness of all the immunotherapies, the score (0.45) showed that sublingual immunotherapy was not as effective as the second most effective local and oral therapy (chromones and leukotriene receptor antagonists).
The most favorable treatment was local therapy with antihistamines (0.91 €). Treatments with local steroids (3.36 € and 3.64 €) and oral antihistamines (4.20 €) were the next most favorable treatments. The immunotherapies were the most expensive treatments. However, a large price difference in the immunotherapies was observed. The cost of the most favorable immunotherapy (666.23 €; sublingual immunotherapy) was 20-fold higher than that of the most expensive oral and local therapies (32.48 €; rupatadine, 20 mg). Table 2 shows the direct costs of the therapies.
Table 2.
Direct medical costs and cost-effectiveness analysis.
| Study ID | Treatment option | Active substance | Direct medical costs (€) | Costs (€) to improve score to 1.0 |
|---|---|---|---|---|
| Du Buske et al.10 | Subcutaneous immunotherapy | Grass MATA MPL standardized units | 750.06 | 1830.15 |
| Nelson et al.11 | Sublingual immunotherapy | 2800 bioequivalent allergen units of standardized Timothy Grass AIT treatment | 666.23 | 1479.03 |
| Ädelroth et al.9 | IgE-Antibody | 300 mg of recombinant humanized murine antibody (rhumAb-E25) | a) 2 Inj: 1,998.04 B) 3 Inj: 2,997.06 |
a) 9510.67 b) 14,266.01 |
| Ciebiada et al.13 | Leukotriene receptor antagonists | Montelukast 10 mg | 20.58 | 31.69 |
| Marmouz et al.12 | Oral antihistamines | a) Rupatadine 10 mg b) Rupatadine 20 mg c) Cetirizine 10 mg |
a) 16.24 b) 32.48 c) 4.20 |
a) 70.64 b) 104.91 c) 18.27 |
| Hissaria et al.18 | Oral steroid | Prednisolone 50 mg | 7.70 | 4.85 |
| Kunachak et al.16 | Surgery | KTP laser (12 watts) in a continuous noncontact mode; total energy:121–440 J | 111.53 | 113.76 |
| Patel et al.14 | Local antihistamines | 2 sprays per nostril of: 0.1% azelastine hydrochloride solution (137 µg/spray) | 0.91 | 1.98 |
| Bende et al.15 | Local steroid | a) BANS 256 µg b) BANS 128 µg c) MF 200 µg |
a) 7.28 b) 3.64 c) 3.36 |
a) 21.77 b) 11.54 c) 12.00 |
| Cohan et al.19 | Chromone | 4% solution of cromolyn sodium: 0.13 ml per spray | 9.88 | 13.73 |
| Meltzer et al.20 | Anticholinergic | Ipatropium bromide 21 µg Ipatropium bromide 42 µg |
– | – |
MATA: Modified Allergen Tyrosine Adsorbate; MPL: monophosphoryl lipid A; AIT: allergy immunotherapy tablet; Inj: injections; KTP: kalium titanyl phosphate; BANS: budesonide aqueous nasal spray.
Cost-effectiveness analysis
Immunotherapy
The analyses of the various immunotherapies showed that IgE-antibody therapy was clearly the least cost-effective treatment (9510.67 € for two injections and 14,266.01 € for three injections). The costs of two and three injections of this therapy were 5-fold higher than the costs of the two other therapies. Although the cost difference between the sublingual (1479.03 €) and subcutaneous (1830.15 €) immunotherapies was much lower, it still amounted to 351.12 €.
Oral therapy
The most cost-effective therapy in this group was oral steroids. Although the therapy with leukotriene receptor antagonists showed a high degree of effectiveness, it was not very cost-effective due to its very high costs.
Local therapy
Local antihistamines were the most cost-effective therapies due to their very low cost and good therapeutic effects. Surgery was the least cost-effective therapy, although it had the best therapeutic effect. Although the chromone therapy was rather effective, it was much less cost-effective because of its high cost.
Total
The local therapy with antihistamines was the most cost-effective therapy of all the therapies. However, oral therapy with steroids had the best therapeutic effect and the second-best cost-effectiveness among the other therapies. Immunotherapies showed the least cost-effectiveness (Table 2).
Sensitivity analysis
Table 3 shows the results of the 10% sensitivity analysis for each therapy. Immunotherapy approached the costs of the sublingual and subcutaneous therapies, but sublingual immunotherapy remained the most cost-effective treatment. The difference between the IgE-antibody therapy and the two other therapies was clear, and there was no change in the results even after the 10% sensitivity analysis was performed. The results of the 10% sensitivity analysis showed that the cost difference between oral steroid therapy as the most cost-effective therapy and the other two therapies in the oral therapy group was not changed. The local antihistamines were the most cost-effective local therapy. The 10% sensitivity analysis did not change the results in the local and oral therapy groups.
Table 3.
Sensitivity analysis.
| Study ID | Treatment option | Costs (€) to improve score to 1.0 | Original score | Score (−) 10% | Score (+) 10% | Costs (€) to improve score to 1.0 after ± 10% score change |
|---|---|---|---|---|---|---|
| Du Buske et al.10 | Subcutaneous immunotherapy | 1830.15 | −0.41 | – | −0.45 | 1665.13 |
| Nelson et al.11 | Sublingual immunotherapy | 1479.03 | −0.45 | −0.41 | – | 1625.60 |
| Ädelroth et al.9 | IgE-Antibody | a) 2 Inj: 9,510.67 b) 3 Inj: 14,266.01 |
−0.21 | – | −0.23 | a) 8694.47 b) 13,037.21 |
| Ciebiada et al.13 | Leukotriene receptor antagonists | 31.69 | −0.65 | – | −0.72 | 28.61 |
| Marmouz et al.12 | Oral antihistamines | 18.27 | −0.23 | – | −0.25 | 16.80 |
| Hissaria et al.18 | Oral steroid | 4.85 | −1.59 | −1.43 | – | 5.39 |
| Kunachak et al.16 | Surgery | 113.76 | −0.98 | – | −1.08 | 103.72 |
| Patel et al.14 | Local antihistamines | 1.98 | −0.48 | −0.43 | – | 2.12 |
| Bende et al.15 | Local steroid | 11.54 | −0.315 | – | −0.35 | 10.41 |
| Cohan et al.10 | Chromone | 13.73 | −0.72 | – | −0.79 | 12.55 |
| Meltzer et al.20 | Anticholinergic | – | −0.15 | – | – | – |
Inj: Injections.
In summary, this analysis showed that the results of the sublingual and subcutaneous immunotherapies were similar, but the slight variations in the symptom scores persisted. The two other forms of therapy (systemic and local therapy) included oral steroid and local antihistamines which were found to be significantly more cost-effective, and no significant convergence of the results was found even after the 10% sensitivity analysis was performed.
Side effects
We also examined the side effects of the therapies in each group.
Immunotherapy
Sublingual immunotherapy had a slight advantage over subcutaneous immunotherapy and a significant advantage over IgE-antibody therapy with respect to side effects. Overall, the sublingual immunotherapy was very well tolerated. There was a relatively small difference in the frequency of side effects between the therapy and placebo groups. Itching and impairment of sensation were reported more than all the other side effects associated with sublingual immunotherapy. When comparing the number of withdrawals due to side effects, there was a small difference between the treatment groups (11/8 patients). In contrast, the ratio of side effects from the subcutaneous treatment was somewhat different. In fact, 70.2% of the patients in the therapy group and 47% of the patients in the placebo group experienced at least one adverse event. The number of patients who withdrew from subcutaneous treatment was higher (24/12 patients) than the number who withdrew from sublingual therapy. The side effects associated with subcutaneous injections are well known (e.g. erythema). However, IgE-antibody therapy showed the best side-effect profile. There was no significant difference in side effects between the therapy group (37% of patients) and the placebo group (36% of patients). Regarding treatment discontinuation, more placebo patients discontinued treatment (3/8 patients, mainly due to an unsatisfactory therapeutic effect).
Sublingual immunotherapy is considered the method of choice due to its cost-effectiveness and better side-effect profile than subcutaneous immunotherapy. Although the IgE-antibody therapy has the best side-effect profile, it is not recommended due to its very poor efficacy and high cost.
Oral therapy
Steroid therapy was significantly more cost-effective than treatments with antihistamines (cetirizine) and leukotriene receptor antagonists in the oral therapy groups. However, there was a substantial difference in the side effects between the treatment and placebo groups (28/7 patients). Insomnia, mood disturbances, increased appetite, and headaches occur almost exclusively in this treatment group. However, there were no significant differences in the number of treatment discontinuations, but treatment with oral antihistamines had a significantly better side-effect profile. According to the study, there was no significant difference in the number of side effects. A significant increase in sleepiness was reported by only the patients in the treatment group. However, this side effect occurred primarily in patients treated with rupatadine (first-generation antihistamine). However, in patients treated with the more cost-effective drug cetirizine (second-generation antihistamine), the difference compared with the placebo group was significantly reduced. There was no significant difference between treatment groups with respect to treatment discontinuation. Although the leukotriene receptor therapy showed the overall worst cost-effectiveness, it exhibited a very good side-effect profile. The incidence of side effects in the treatment group (11.5% of patients) and the placebo group (8.6% of patients) was comparable, and the number of patients who discontinued treatment was only slightly different (5/1 patient). Overall, the oral steroids were the most cost-effective, but they had significantly more side effects than the other two forms of therapy.
Local therapy
All three pharmaceutical therapies (local antihistamines, topical steroids and chromones) were well tolerated and showed similar side-effect profiles. There were no significant differences in the number of side effects or in the number of treatment discontinuations between the treatment and placebo groups for all three treatments. The patients who underwent surgery did not show relevant post-operative pain or bleeding. None of the patients who underwent surgery discontinued treatment, and only five patients were lost to follow-up. As all therapies have similar side-effect profiles, the most cost-effective therapy, local antihistamines, is recommended.
Discussion
We assessed the cost-effectiveness of clinical therapeutic options for AR and also evaluated the side effects of these therapies. This study showed that only the comparison between the causal and symptomatic therapies could be performed for all AR therapeutic options. Of the symptomatic therapies (local and oral), which had fewer side effects and similarly good effectiveness, the local therapies are preferable from a health economic perspective. Thus, treatment with local antihistamines or topical steroids should be regarded as the method of choice, and sublingual therapy is the first choice among the causal therapies.
Only a small number of publications have focused specifically on the cost-effectiveness of the therapeutic options for AR. The study by Lange et al.21 evaluated three different local therapies (local steroids, chromones and local antihistamines) regarding their effectiveness and cost-effectiveness, and it was methodologically similar to our study. However, they showed that the local steroids (mometasone nasal spray) were significantly more effective and cost-effective than the local antihistamines (levocabastine; second-best result) and chromones (cromolyn sodium; third-best result). This result may be due to a different definition of treatment success. However, this result is in accordance with the results of the critical evaluation of local therapies in cost-effectiveness analyses.
There are several limitations to this study. We searched MEDLINE via PubMed and used several keywords matched to database-specific indexing terms; thus, certain publication of therapeutic options of AR may have been overlooked. However, we selected a study with highest quality for each treatment option, but the selection of only one study for each treatment option may have influenced our results. We did not identify a study on treatment with oral steroids that met all the criteria for inclusion in the literature search, likely because oral steroids are a relatively old medication, widely used at a time when the quality requirements for drug trials were not as high as they are today. We found only studies in which the patients were treated with nasal polyps and chronic rhinosinusitis. Because the study by Hissaria et al.18 had the largest number of participants with an allergy (95%) and also met all the inclusion and exclusion criteria, the study was used for the cost-effectiveness comparison in this study.
Conclusion
The selected studies used very different symptom scores with different scales and a different number of rated symptoms. Because there is no uniform international standard for symptom scores in diseases of the nose and sinuses, a standard uniform nasal symptom score would enable researchers to better compare the results of different treatment options.
Supplemental Material
Supplemental material, Appendix_1 for Cost-effectiveness of allergic rhinitis treatment: An exploratory study by Jan Titulaer, Habibollah Arefian, Michael Hartmann, Mustafa Z Younis and Orlando Guntinas-Lichius in SAGE Open Medicine
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD: Mustafa Z Younis  https://orcid.org/0000-0001-8448-808X
https://orcid.org/0000-0001-8448-808X
References
- 1. Bousquet J, Khaltaev N, Cruz AA, et al. Allergic Rhinitis and its Impact on Asthma (ARIA) 2008 update (in collaboration with the World Health Organization, GA(2)LEN and AllerGen). Allergy 2008; 63(Suppl 86): 8–160. [DOI] [PubMed] [Google Scholar]
- 2. Konig V, Mosges R. A model for the determination of pollen count using Google search queries for patients suffering from allergic rhinitis. J Allergy 2014; 2014: 381983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Bousquet J, Van Cauwenberge P, Khaltaev N. Allergic rhinitis and its impact on asthma. J Allergy Clin Immunol 2001; 108: S147–S334. [DOI] [PubMed] [Google Scholar]
- 4. Bachert C, Borchard U, Wedi B, et al. Allergische rhinokonjunktivitis leitlinie der DGAI in abstimmung mit der DDG. J Dtsch Dermatol Ges 2006; 4: 264–275. [DOI] [PubMed] [Google Scholar]
- 5. Bundesvereinigung KK. Einheitlicher Bewertungsmaßstab (EBM). Stand: 4. Quartal 2014, http://www.kbv.de/html/arztgruppen_ebm.php#content2403,EBM-2009-Archiv(Zip-Datei),enthält4.Quartal2013bis4.Quartal2016 (accessed 02 Aug 2018).
- 6. Taxe LF. WEBAPO Lauer Taxe, 2015, https://www.lauer-fischer.de/LF/Seiten/WEBAPO-InfoSystem/WEBAPO-Infosystem.aspx (accessed 2 August 2018).
- 7. Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1–12. [DOI] [PubMed] [Google Scholar]
- 8. Antes G. The new CONSORT statement. BMJ 2010; 340: c1432. [DOI] [PubMed] [Google Scholar]
- 9. Adelroth E, Rak S, Haahtela T, et al. Recombinant humanized mAb-E25, an anti-IgE mAb, in birch pollen-induced seasonal allergic rhinitis. J Allergy Clin Immunol 2000; 106: 253–259. [DOI] [PubMed] [Google Scholar]
- 10. Du Buske LM, Frew AJ, Horak F, et al. Ultrashort-specific immunotherapy successfully treats seasonal allergic rhinoconjunctivitis to grass pollen. Allergy Asthma Proc 2011; 32: 239–247. [DOI] [PubMed] [Google Scholar]
- 11. Nelson HS, Nolte H, Creticos P, et al. Efficacy and safety of Timothy grass allergy immunotherapy tablet treatment in North American adults. J Allergy Clin Immunol 2011; 127: 72–80.e1–e2. [DOI] [PubMed] [Google Scholar]
- 12. Marmouz F, Giralt J, Izquierdo I. Morning and evening efficacy evaluation of rupatadine (10 and 20 mg), compared with cetirizine 10 mg in perennial allergic rhinitis: a randomized, double-blind, placebo-controlled trial. J Asthma Allergy 2011; 4: 27–35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ciebiada M, Gorska-Ciebiada M, Barylski M, et al. Use of montelukast alone or in combination with desloratadine or levocetirizine in patients with persistent allergic rhinitis. Am J Rhinol Allergy 2011; 25: e1–e6. [DOI] [PubMed] [Google Scholar]
- 14. Patel D, Garadi R, Brubaker M, et al. Onset and duration of action of nasal sprays in seasonal allergic rhinitis patients: olopatadine hydrochloride versus mometasone furoate monohydrate. Allergy Asthma Proc 2007; 28: 592–599. [DOI] [PubMed] [Google Scholar]
- 15. Bende M, Carrillo T, Vona I, et al. A randomized comparison of the effects of budesonide and mometasone furoate aqueous nasal sprays on nasal peak flow rate and symptoms in perennial allergic rhinitis. Ann Allergy Asthma Immunol 2002; 88: 617–623. [DOI] [PubMed] [Google Scholar]
- 16. Kunachak S, Kulapaditharom B, Prakunhungsit S. Minimally invasive KTP laser treatment of perennial allergic rhinitis: a preliminary report. J Otolaryngol 2000; 29: 139–143. [PubMed] [Google Scholar]
- 17. Schöffski O, Von Der Schulenburg JMG. Gesundheitsökonomische Evaluationen. Berlin: Springer, 2011. [Google Scholar]
- 18. Hissaria P, Smith W, Wormald PJ, et al. Short course of systemic corticosteroids in sinonasal polyposis: a double-blind, randomized, placebo-controlled trial with evaluation of outcome measures. J Allergy Clin Immunol 2006; 118: 128–133. [DOI] [PubMed] [Google Scholar]
- 19. Cohan RH, Bloom FL, Rhoades RB, et al. Treatment of perennial allergic rhinitis with cromolyn sodium. Double-blind study on 34 adult patients. J Allergy Clin Immunol 1976; 58: 121–128. [DOI] [PubMed] [Google Scholar]
- 20. Meltzer EO, Orgel HA, Bronsky EA, et al. Ipratropium bromide aqueous nasal spray for patients with perennial allergic rhinitis: a study of its effect on their symptoms, quality of life, and nasal cytology. J Allergy Clin Immunol 1992; 90: 242–249. [DOI] [PubMed] [Google Scholar]
- 21. Lange B, Lukat KF, Rettig K, et al. Efficacy, cost-effectiveness, and tolerability of mometasone furoate, levocabastine, and disodium cromoglycate nasal sprays in the treatment of seasonal allergic rhinitis. Ann Allergy Asthma Immunol 2005; 95: 272–282. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, Appendix_1 for Cost-effectiveness of allergic rhinitis treatment: An exploratory study by Jan Titulaer, Habibollah Arefian, Michael Hartmann, Mustafa Z Younis and Orlando Guntinas-Lichius in SAGE Open Medicine