Abstract
The recently discovered klotho proteins have roles in a diverse range of metabolic processes with the oldest protein, α-klotho, implicated in various cellular pathways in energy, glucose, and phosphate metabolism. Circulating soluble klotho (sKl), derived from membrane α-klotho cleavage, not only has effects on ion channels and insulin signaling pathways, but is inversely associated with mortality. Effects of physical exercise on sKl have not been well studied. The effect of a single high-intensity standardized exercise on sKl and serum phosphate (sPi) levels in healthy adults was investigated. A standard Bruce protocol treadmill exercise was undertaken by 10 fasting healthy volunteers. sKl, sPi, and blood glucose levels were measured in samples collected 1-week prior, immediately pre (T pre), 0 (T post), 30 (T 30), 240 (T 240) min, and 1-week after exercise. Median (interquartile range) age of participants was 47.5 (44–51) years; five (50%) were male. All study participants achieved at least 90% predicted maximum heart rate (MHR). sKl increased acutely after exercise (T pre median 448 pg/mL vs. T post median 576 pg/mL; p < 0.01). There was a nonsignificant sPi decline at T 30 (T pre 0.94 ± 0.12 mmol/L vs. T 30 0.83 ± 0.22 mmol/L). Exercise led to a reduction in blood glucose by T 240 with median glucose levels at T pre, T post, T 30, and T 240 of 6.0, 6.5, 6.3, and 5.7 mmol/L, respectively. In conclusion, a single high-intensity exercise session is associated with a transient increase in sKl, a delayed reduction in blood glucose, and a nonsignificant decrease in sPi levels in healthy adults. The evaluation of long-term effects of cardiovascular fitness programs on sKl and sPi in healthy individuals and disease cohorts are required to identify potential lifestyle modifications to help improve chronic disease management and long-term outcomes.
Keywords: Exercise, soluble klotho, phosphate
Introduction
Klotho was originally identified as an antiaging protein almost 20 years ago. Genetic studies identified the klotho (kl) gene highlighting that over-expression extended life span and deletion produced complex phenotypes in animals similar to human aging.1 The kl gene encodes a 1012 amino acid long protein, which is expressed in different tissues, although mostly in the kidney especially within the distal convoluted tubule.1,2 The gene product is commonly referred to as α-klotho, to differentiate it from two other subsequently discovered members of the klotho family: β-klotho and γ-klotho. While all three are single-pass transmembrane proteins of different lengths and share a substantial degree of homology,3 they appear to have different physiological actions and play a role in a diverse range of metabolic processes.1,4–8
α-Klotho exists in two forms—membrane-bound klotho (mKl) and soluble klotho (sKl). The actions of mKl and sKl differ, with mKl within the kidney predominantly involved in phosphate regulation and renal tubular phosphate reabsorption.6 Circulating sKl is the cleaved extracellular domain of mKl (approximately 130 kDa) and can act as a soluble paracrine or endocrine mediator.9–12 sKl has also been directly implicated in phosphate regulation.13 It displays enzymatic activity that may be important in regulating other ion channels (predominantly involving potassium and calcium transport).14–16 sKl has been implicated in peroxisome proliferator-activated receptor-γ (PPARγ) regulation and insulin antagonism and has been shown to be anti-fibrotic, possess antioxidant, and tumor-suppressive properties.17–20 Distinguishing between the various autocrine, paracrine, and endocrine actions of α-klotho has been challenging due to its cell- or organ-specific actions as well as its effects on a wide range of metabolic pathways.20
β-Klotho augments FGF-19 and FGF-21 signaling; is found in the liver, gall bladder, pancreas, colon, and adipose tissue; and participates in bile acid metabolic pathways.7,8 γ-Klotho is coupled to FGF19 and is found in the eye, adipose, and kidney, although its function is not clearly understood.3 The cleavage of β-klotho, relative contributions of the three proteins to circulating sKl, the degree of overlap, and mutual influence between the three klotho proteins are unclear.20 Nonetheless, higher sKl levels have been associated with improved survival21 and lower levels with increased cardiovascular and all-cause mortality.22
Physical exercise is known to delay the effects of aging and to protect from cardiovascular disease, diabetes, and cancer.23,24 However, the mechanisms through which exercise exerts these beneficial effects are not fully elucidated. It is, therefore, plausible that sKl may mediate some of the benefits of exercise, given the many parallels between physiological effects of exercise and the pleiotropy exhibited by sKl described above. There have been two previous studies demonstrating increased sKl levels in women only, one in response to long-term exercise25 and the other in response to acute, high-intensity exercise.26 We aimed to investigate the effect of an acute high-intensity exercise on serum klotho, glucose, and phosphate in both healthy male and female adults.
Methods
Study population
This single-center study recruited healthy adult volunteers aged >18 years old to participate in a single-intervention study. Exclusion criteria included history of cardiovascular disease, chronic kidney disease, diabetes mellitus, psychological or medical illness precluding informed consent, and physical handicap impairing ability to undertake a treadmill intervention. Male and females were age-matched with equal numbers of males and females recruited. Informed consent was obtained from all individual participants included in the study. This study was approved by the local human research ethics committee (HREC 2015.097) and conducted in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Exercise intervention
All participants were nonsmokers and given routine instructions on how to prepare for a standard exercise test (e.g. instructed to dress with appropriate footwear for the exercise intervention). Participants fasted from midnight, the night prior to the exercise intervention. None of the study participants were on treatment with medications that could affect exercise capacity or exercise responses. A treadmill-based exercise stress test (EST) was performed following a standard Bruce protocol.27 In brief, 12-lead electrocardiogram (ECG) electrodes were placed on the torso with limb leads placed on shoulders and below the level of the umbilicus on respective sides, ensuring good skin contact to reduce skin resistance. All participants started at low workload treadmill speed. A gradual and continuous increase in workload was instituted every 3 min corresponding to the Bruce protocol. An approximately 0.8 mph increase in treadmill speed and 2% ramp incline occurred from stage I (1.7 mph and 10% incline) to stage V (5.0 mph and 18% incline) and a smaller increase in speed occurred for the final stage (VI—5.5 mph and 20% incline). MHR was estimated for each individual participant using the following formula: MHR = [220–age(years)]. A target of 85% of MHR was applied for the EST. Participants were instructed to advise investigators and EST technician if premature termination of the EST was required. All participants were monitored in the resting phase after the completion of the EST for a minimum of 6 min until blood pressure, heart rate, and ECG tracing approximate baseline values. All participants were provided with a standard meal at 60 min post EST. Participants were also instructed to refrain from strenuous exercise or activity 1-week pre and post the intervention.
Data and sample collection
Baseline measurements including blood pressure, heart rate, and 12-lead ECG were performed on all participants in supine and standing resting positions prior to the EST. ECG recording was continued throughout the EST in accordance with the standard protocol, with standing blood pressure measurements recording at 3-min intervals.
Baseline tests for hemoglobin, blood glucose level, serum biochemistry, and klotho were measured immediately prior to exercise intervention (T pre). Blood samples were also collected 1 week prior to EST (T d-7), 0 (T post), 30 (T 30), 60 (T 60), 120 (T 120), 240 (T 240) min; 1 day (T d+1); and 1 week post EST (T d+7) for repeat evaluation of serum phosphate (sPi) and blood glucose levels. sKl was measured at T d-7, T pre, T post, T 30, T 240, and T d+7.
Biochemical analysis
Routine biochemistry was performed at a central laboratory using standard techniques. sPi was measured via the Olympus AU7000 platform (Beckman Coulter, Inc., Brea, California, USA) using molybdate or the chromogen Arsenazo III, respectively. The analytical range for sPi was 0.32–5.00 mmol/L with analytical coefficient of variation (CV) <2.5%.
Blood collected in a serum separator tube (SST™; BD Biosciences, Franklin Lakes, New Jersey, USA) was allowed to stand at room temperature for 30 min prior to centrifugation (10 min, 4°C, 3000 × g) and aliquoted for storage at −80°C until batched analysis. Serum sKl concentrations were measured using the IBL sKl ELISA kit (Immuno-Biological Laboratories Co., Ltd, Gunma, Japan) according to the manufacturer’s protocol. Based on duplicate measurements, the intra-assay and inter-assay CVs for this study were 3.1 and 7.2%, respectively.
Blood glucose levels were tested at the above time points using a calibrated Freestyle Optium H™ (Abbott Diabetes Care, Doncaster, VIC, Australia) blood glucometer and accompanying blood glucose test strips.
Statistical analysis
Continuous variables have been reported using mean and standard deviation or median and interquartile range (IQR) as appropriate. Student’s t-test or Mann–Whitney tests were used first to evaluate differences between male and females as appropriate. Friedman’s test with Dunn’s multiple comparison was used to assess changes in sKl and blood glucose levels across all time points measured. Wilcoxon-signed rank test was used to evaluate individual time points against either T pre or T post. Repeated measures ANOVA was used to assess changes in sPi. All statistical analyses were performed with SPSS Statistics Version 24.0 (IBM Corp., Armonk, New York, USA). All graphics have been created with GraphPad Prism 7 for Macintosh (La Jolla, California, USA). A p-value of <0.05 was considered significant unless otherwise stated.
Results
A total of 10 participants were included in the analysis. All participants were nonsmokers and none were on treatment with medications that could affect exercise capacity or exercise responses (such as beta-blockers or calcium channel blockers). Five participants (50%) were male. Median (IQR) age of participants was 47.5 (44–51) years. Baseline (resting/fasting) characteristics of study participants are presented in Table 1. All participants had baseline blood pressure, heart rate, hemoglobin, glucose, and serum creatinine values that were within the expected range for age and sex. Males exhibited higher resting systolic blood pressures (137 ± 9.1 vs. 125 ± 5 mmHg (females); p = 0.032). Males demonstrated a trend toward lower baseline sKl levels compared with females (423 (415–614) pg/mL in males vs. 669 (448–1095) pg/mL in females; p = 0.117).
Table 1.
Baseline characteristics of study participants (n = 10).a
| Clinical parameter | Total (n = 10) | Males (n = 5) | Females (n = 5) |
|---|---|---|---|
| Age (years) | 49 (44.75–50.75) | 47 (44–50) | 50 (48–51) |
| Gender (male; n, %) | 5/10 (50%) | 5 | 5 |
| Resting heart rate (bpm) | 84.3 ± 10.9 | 86.2 ± 10.1 | 82.4 ± 12.6 |
| Resting systolic blood pressure (mmHg) | 131 ± 9.4 | 137 ± 9.1 | 125 ± 5* |
| Resting diastolic blood pressure (mmHg) | 83 ± 7.1 | 83 ± 4.5 | 83 ± 9.7 |
| Hemoglobin (g/L) | 141.1 ± 16.7 | 154.6 ± 4.8 | 124.3 ± 5.3 |
| Serum calcium (mmol/L) | 2.38 ± 0.05 | 2.38 ± 0.04 | 2.38 ± 0.06 |
| sPi (mmol/L) | 0.98 ± 0.12 | 0.92 ± 0.12 | 1.03 ± 0.11 |
| Serum creatinine (mmol/L) | 71.5 ± 11.1 | 79.0 ± 8.6 | 64.0 ± 8.0 |
| eGFR (mL/min/1.73 m2) | 98.9 ±12.1 | 99.4 ± 11.0 | 98.3 ± 14.4 |
| Serum sKl (pg/mL) | 483 (423–767) | 423 (415–614) | 669 (448–1095) |
| Blood glucose reading (optima glucometer; mmol/L) | 5.07 ± 0.42 | 5.36 ± 0.15 | 4.78 ± 0.41 |
eGFR: estimated glomerular filtration rate; sPi: serum phosphate; sKl: soluble klotho.
a T-test or Mann–Whitney U-test performed for continuous variables between gender groups.
*p < 0.05 compared to males.
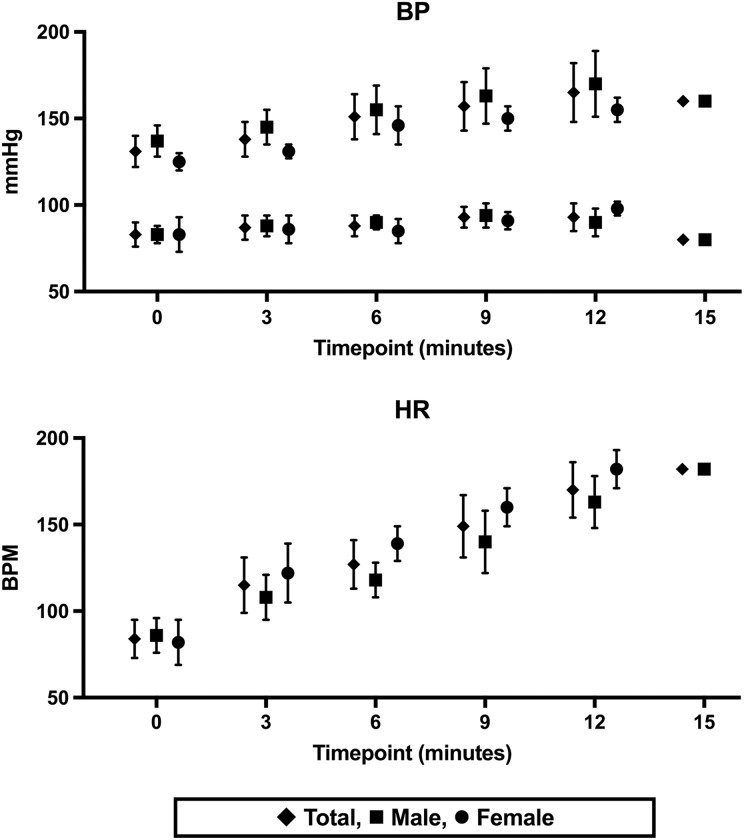
All participants achieved at least 90% estimated MHR, and 9/10 reached 100% MHR, with a mean duration of 12.3 min of exercise. All exercise was terminated by the participants with fatigue, and no ECG changes suggesting ischemia were noted at any time during or at least 6 min into recovery. No adverse events were recorded during or following the EST. Mean heart rate and blood pressure readings throughout the EST are shown in Figure 1.
Figure 1.
Changes in blood pressure and heart rate with EST intervention. EST: exercise stress test.
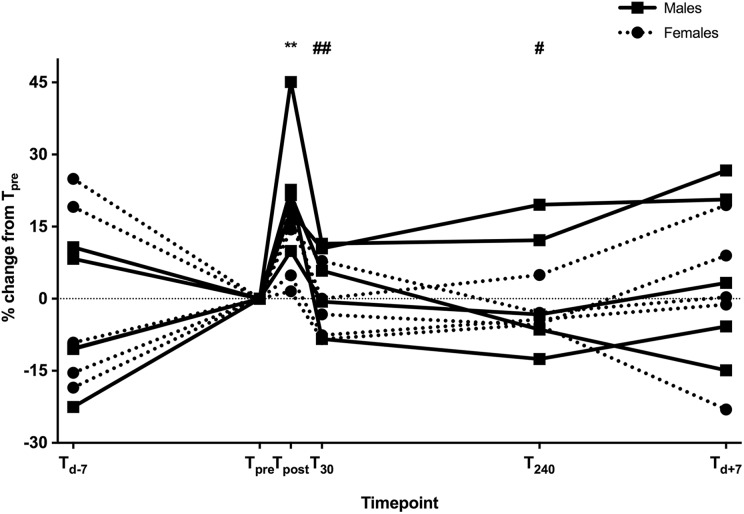
Changes in sKl for study participants throughout the study period are presented in Figure 2 and Table 2. A significant increase after the EST in sKl was seen (T pre median 483 (423–766) pg/mL vs. T post 602 (514–831) pg/mL; p < 0.01) but levels returned to baseline by T 30 (497 (445–746) pg/mL).
Figure 2.
Change in sKl levels in males (black lines) and females (dotted lines) following EST (percentage change from T pre). ** p < 0.005 versus T pre. # p < 0.05 and ## p < 0.005 versus T post. sKl: soluble klotho; EST: exercise stress test.
Table 2.
Median sKl levels following EST in males (M) and females (F).a
| Time point | T d-7 | T pre | T post | T 30 | T 240 | T d+7 |
|---|---|---|---|---|---|---|
| sKl (n =10; pg/mL) | 484 (442–748) | 483 (423–766) | 602** (514–831) | 497## (445–746) | 485# (449–738) | 517 (419–781) |
| sKl (n = 5 M; pg/mL) | 455 (414–509) | 423 (415–614) | 596* (508–704) | 464# (423–626) | 468 (416– 603) | 507 (416–603) |
| sKl (n = 5 F; pg/mL) | 718 (468–971) | 669 (448–1095) | 765* (527–126) | 722# (440–1006) | 649# (446–1041) | 671 (436–1148) |
sKl: soluble klotho; EST: exercise stress test.
a Friedman’s test with Dunn’s multiple comparison was used to assess changes in sKl across all time points measured. Wilcoxon-signed rank test was used to evaluate individual time points against either T pre or T post.
*p < 0.05 versus T pre.
**p < 0.005 versus T pre.
# p < 0.05 versus T post.
## p < 0.005 versus T post.
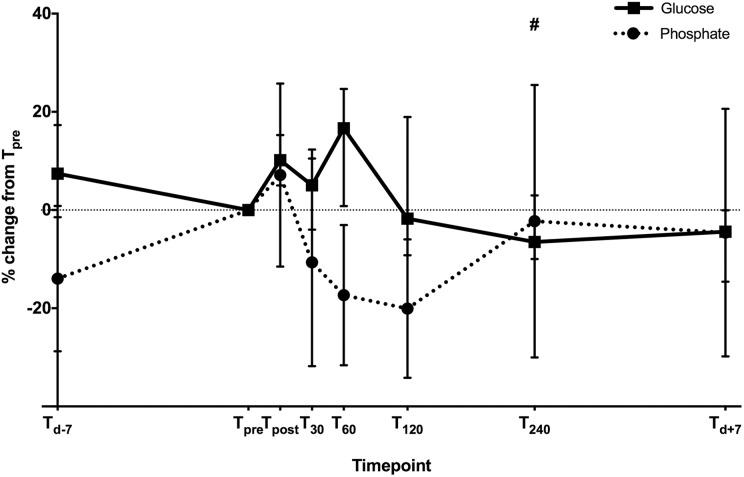
Changes in sPi and blood glucose are presented in Figure 3. A nonsignificant trend toward reduction in sPi post EST was observed, with sPi at T pre compared to T 30 (T pre 0.94 ± 0.12 mmol/L vs. T 30 0.83 ± 0.22 mmol/L; p > 0.05). This reduction was sustained until T 120, 0.77 ± 0.17 mmol/L (Figure 3).
Figure 3.
Changes in median blood glucose level (black line) and mean serum phosphate level (dotted line) following EST (percentage change from T pre). # p < 0.05 compared to T post for blood glucose level. EST: exercise stress test.
Median glucose levels at T pre, T post, T 30, and T 240 were 6.0, 6.5, 6.3, and 5.7 mmol/L, respectively. An initial nonsignificant rise in blood glucose was seen at T post following EST (p = 0.26 vs. T pre). This was followed by a reduction in blood glucose observed at the earliest at T 120, median glucose level 5.8 mmol/L (p = 0.65 vs. T post). This reduction was sustained at T 240 (p = 0.013 vs. T post; Figure 3).
Discussion
A standardized high-intensity physical exercise in healthy adults causes a transient but immediate increase in sKl levels and a delayed reduction in blood glucose levels. Two other studies have reported an increase in sKl following exercise, and neither of these showed the time course of this rise and were performed in postmenopausal women undergoing a 12-week aerobic exercise program25 or in men and women after a 20-min run.26 In contrast to this study, the latter failed to demonstrate an increase in sKl level in men who were recruited to the same study. However, all participants in this latter study26 were involved in regular physical activity with a minimum of 1-year resistance and aerobic training prior to the study. There are limited studies in this area and the effect of gender has not been widely investigated. It has been suggested though that preexisting fitness level and type of exercise, aerobic versus anaerobic, could impact on the intensity of sKl inflection following the prescribed exercise.28,29
The study presented here included a standardized EST in order to individualize exercise according to age of participants by targeting MHR and included healthy participants who were not allowed to undertake physical activity in the 1 week prior to the intervention. sKl measurements were performed at more than one time point allowing temporal evaluation in sKl change following the intervention. This provides novel additional information compared to the prior two reports,25,26 demonstrating that a single episode of high-intensity exercise is not only associated with an increase in sKl in both men and women but is not sustained.
Multiple mechanistic pathways have been considered in the exercise-induced elevation of circulating sKl levels. Physical exercise has been reported to upregulate PPARγ,30 downregulate angiotensin-II type-1 receptor (AT1 R),31 and oxidative stress32 in animal models. In turn, these pathways have been linked to increased renal klotho mRNA expression and sKl levels,33–35 thereby potentially increasing circulating sKl levels. Notably, the time lag in animal models between physical exercise/activity and biochemical evaluation ranged from 6 to 16 weeks,30–32 while the follow-on in vitro PPARγ and oxidative stress effects on klotho mRNA have been reported ranging between 12 h and 48 h33,34 and in vivo effects of AT1 R blockade on klotho mRNA between 4 days and 14 days. Correspondingly, the clinical study by Matsubara et al. demonstrated an increase in sKl levels following a 12-week exercise intervention,25 where the aforementioned mechanisms are likely to be implicated. We have shown that acute and transient increases in circulating sKl occur suggesting more acute physiological processes may be involved, such as a rapid activation of cleavage enzymes or a release of preformed sKl within seconds to minutes of strenuous exercise.
A trend toward lower sPi levels post exercise was observed in this study, and while lower sPi levels may be in response to the increased sKl levels mediating phosphate excretion,13 the apparent reduction in sPi levels occurs promptly in response to physical activity. Both increase and decrease in sPi levels have been reported previously following physical activity, despite consistently documented increases in parathyroid hormone level.36–38 These discrepancies may reflect the different physical exercise programs that the respective study participants were subjected to including a prolonged (5-h) low-intensity bicycle ergometer exercise, a 7-day high-intensity field exercise, and maximal exercise capacity testing prior to and after a 6-week endurance program.36–38 However, the reduction in sPi may simply reflect extracellular Pi depletion related to uptake into muscle which outstrips resupply. The wide variation in these prescribed interventions limits the ability to compare these results and also alludes to the wide variation and day-to-day fluctuations that occur normally when measuring extracellular sPi. Furthermore, no “phosphate sensor” has ever been reported in humans so to invoke changes in sKl levels due to changes in sPi remains highly speculative and this study provides little further insight into such a mechanism.
Lower glucose levels were also detected at T 240 post EST and this is consistent with well-established evidence showing improvements in insulin sensitivity after a single bout of physical exercise,39,40 although the intensity and duration of such activity to achieve maximal beneficial outcome for the healthy population as well as those with insulin resistance continues to be debated.41 Physical exercise, however, may be one of the key stimulators of skeletal muscle GLUT4 expression.42 Taken together, the data presented here might suggest a number of pathways implicated in beneficial changes of physical activity with a central role for glucose and energy metabolism, PPARγ and sKl.
There are several limitations to this study. The cohort is small in size and, therefore, the findings from this study require validation within a larger cohort. Although there is a lack of representation of patients with chronic disease in this study such as chronic kidney disease, diabetes, and coronary heart disease, the study findings in healthy adults are encouraging. The standardized meal provided to the study participants following the EST in a fasting state was intended to standardize the glucose load, though may have affected the statistical significance of the glucose and phosphate results following T 60. Further studies are required to quantify the long-term effects of standardized cardiovascular fitness and high-intensity exercise programs on sKl, sPi, and blood glucose in healthy individuals. Subsequently, studies are needed to evaluate the same programs in disease cohorts in order to identify beneficial lifestyle modifications that could improve chronic disease management and long-term outcomes.
Conclusion
In summary, this study demonstrated that a single short-duration episode of high-intensity physical exercise is associated with an acute and transient increase in sKl, a delayed glucose reduction, and with sPi levels showing a trend to acute reduction also. Future studies, both larger standardized interventional clinical studies and basic science research, are critical to improve the understanding of the mechanisms involved in benefits of physical exercise and also to enable translation of these concepts to guide clinical practice especially in cohorts with lower sKl or hyperphosphatemia, both of which are associated with impaired glucose tolerance and poorer survival. The relative benefit of exercise in the low baseline fitness group should also be investigated as this may be the group that benefits most from the flow-on effects of sKl rise.
Acknowledgment
The authors would like to thank Ms Sarra Byrns for her technical assistance with conducting EST.
Authors’ note: The contents of this article are solely the views of the individual authors and do not reflect the views of NHMRC or the Jacquot Foundation.
Author contributions: SJT designed and conducted the clinical study, undertook the assays, performed the data analysis, and prepared the manuscript. MMC conducted the clinical study and contributed to manuscript. NDT, MMXC, and SGH aided with the study design and critically appraised the manuscript. TDH provided assistance with the assays and contributed to manuscript.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: SJT has received speaking honoraria from Shire. SGH has received research funding or honoraria from Amgen, Baxter, Gilead, Novartis, and Shire. NDT has received consultancy fees, honoraria and research funding from Amgen, Sanofi, and Shire Pharmaceuticals. MMC, MMXC, and TDH have no conflict of interests to declare.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: SJT is a current recipient of a National Health and Medical Research Council (NHMRC) Postgraduate Research Scholarship. MMXC is a current recipient of a combined NHMRC and Jacquot Foundation Postgraduate Research Scholarship. NDT is supported by a Jacquot Foundation Research Establishment Award.
Ethical approval: All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
References
- 1. Kuro-o M, Matsumura Y, Aizawa H, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature 1997; 390(6655): 45–51. [DOI] [PubMed] [Google Scholar]
- 2. Li SA, Watanabe M, Yamada H, et al. Immunohistochemical localization of Klotho protein in brain, kidney, and reproductive organs of mice. Cell Struct Funct 2004; 29(4): 91–99. [DOI] [PubMed] [Google Scholar]
- 3. Hu MC, Shiizaki K, Kuro-o M, et al. Fibroblast growth factor 23 and klotho: physiology and pathophysiology of an endocrine network of mineral metabolism. Annu Rev Physiol 2013; 75: 503–533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kurosu H, Yamamoto M, Clark JD, et al. Suppression of aging in mice by the hormone klotho. Science 2005; 309(5742): 1829–1833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Kuro-o M. Klotho in health and disease. Curr Opin Nephrol Hypertens 2012; 21(4): 362–368. [DOI] [PubMed] [Google Scholar]
- 6. Kurosu H, Ogawa Y, Miyoshi M, et al. Regulation of fibroblast growth factor-23 signaling by klotho. J Biol Chem 2006; 281(10): 6120–6123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kurosu H, Choi M, Ogawa Y, et al. Tissue-specific expression of beta-Klotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J Biol Chem 2007; 282(37): 26687–26695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Ogawa Y, Kurosu H, Yamamoto M, et al. Beta-Klotho is required for metabolic activity of fibroblast growth factor 21. Proc Natl Acad Scinces USA 2007; 104(18): 7432–7437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Matsumura Y, Aizawa H, Shiraki-Iida T, et al. Identification of the human klotho gene and its two transcripts encoding membrane and secreted klotho protein. Biochem Biophys Res Commun 1998; 242(3): 626–630. [DOI] [PubMed] [Google Scholar]
- 10. Imura A, Iwano A, Tohyama O, et al. Secreted Klotho protein in sera and CSF: implication for post-translational cleavage in release of Klotho protein from cell membrane. FEBS Lett 2004; 565(1-3): 143–147. [DOI] [PubMed] [Google Scholar]
- 11. Chen CD, Podvin S, Gillespie E, et al. Insulin stimulates the cleavage and release of the extracellular domain of klotho by ADAM10 and ADAM17. Proc Natl Acad Sci U S A 2007; 104(50): 19796–19801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Lim K, Groen A, Molostvov G, et al. Alpha-Klotho expression in human tissues. J Clin Endocrinol Metab 2015; 100(10): E1308–E1318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hu MC, Shi M, Zhang J, et al. Klotho: a novel phosphaturic substance acting as an autocrine enzyme in the renal proximal tubule. FASEB J 2010; 24(9): 3438–3450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Tohyama O, Imura A, Iwano A, et al. Klotho is a novel beta-glucuronidase capable of hydrolyzing steroid beta-glucuronides. J Biol Chem 2004; 279(11): 9777–9784. [DOI] [PubMed] [Google Scholar]
- 15. Cha SK, Ortega B, Kurosu H, et al. Removal of sialic acid involving klotho causes cell-surface retention of TRPV5 channel via binding to galectin-1. Proc Natl Acad Sci U S A 2008; 105(28): 9805–9810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Cha SK, Hu MC, Kurosu H, et al. Regulation of renal outer medullary potassium channel and renal K(+) excretion by klotho. Mol Pharmacol 2009; 76(1): 38–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Huang CL. Regulation of ion channels by secreted klotho: mechanisms and implications. Kidney Int 2010; 77(10): 855–860. [DOI] [PubMed] [Google Scholar]
- 18. Kuro-o M. Klotho as a regulator of oxidative stress and senescence. Biol Chem 2008; 389(3): 233–241. [DOI] [PubMed] [Google Scholar]
- 19. Sun H, Gao Y, Lu K, et al. Overexpression of klotho suppresses liver cancer progression and induces cell apoptosis by negatively regulating wnt/beta-catenin signaling pathway. World J Surg Oncol 2015; 13: 307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Razzaque MS. The role of klotho in energy metabolism. Nat Rev Endocrinol 2012; 8(10): 579–587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Sakan H, Nakatani K, Asai O, et al. Reduced renal alpha-klotho expression in CKD patients and its effect on renal phosphate handling and vitamin D metabolism. PloS One 2014; 9(1): e86301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Otani-Takei N, Masuda T, Akimoto T, et al. Association between serum soluble klotho levels and mortality in chronic hemodialysis patients. Int J Endocrinol 2015; 2015: 406269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Castillo-Garzon MJ, Ruiz JR, Ortega FB, et al. Anti-aging therapy through fitness enhancement. Clin Interv Aging 2006; 1(3): 213–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Warburton DE, Nicol CW, Bredin SS. Health benefits of physical activity: the evidence. CMAJ 2006; 174(6): 801–809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Matsubara T, Miyaki A, Akazawa N, et al. Aerobic exercise training increases plasma klotho levels and reduces arterial stiffness in postmenopausal women. Am J Physiol Heart Circ Physiol 2014; 306(3): H348–H355. [DOI] [PubMed] [Google Scholar]
- 26. Santos-Dias A, MacKenzie B, Oliveira-Junior MC, et al. Longevity protein klotho is induced by a single bout of exercise. Br J Sports Med 2017; 51(6): 549–550. [DOI] [PubMed] [Google Scholar]
- 27. Fletcher GF, Ades PA, Kligfield P, et al. Exercise standards for testing and training: a scientific statement from the American Heart Association. Circulation 2013; 128(8): 873–934. [DOI] [PubMed] [Google Scholar]
- 28. Saghiv MS, Sira DB, Goldhammer E, et al. The effects of aerobic and anaerobic exercises on circulating soluble-klotho and IGF-I in young and elderly adults and in CAD patients. J Circ Biomark 2017; 6: 1849454417733388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Avin KG, Coen PM, Huang W, et al. Skeletal muscle as a regulator of the longevity protein, Klotho. Front Physiol 2014; 5: 189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kawamura T, Yoshida K, Sugawara A, et al. Regulation of skeletal muscle peroxisome proliferator-activated receptor gamma expression by exercise and angiotensin-converting enzyme inhibition in fructose-fed hypertensive rats. Hypertens Res 2004; 27(1): 61–70. [DOI] [PubMed] [Google Scholar]
- 31. Ciampone S, Borges R, de Lima IP, et al. Long-term exercise attenuates blood pressure responsiveness and modulates kidney angiotensin II signalling and urinary sodium excretion in SHR. J Renin Angiotensin Aldosterone Syst 2011; 12(4): 394–403. [DOI] [PubMed] [Google Scholar]
- 32. Asghar M, George L, Lokhandwala MF. Exercise decreases oxidative stress and inflammation and restores renal dopamine D1 receptor function in old rats. Am J Physiol Renal Physiol 2007; 293(3): F914–F919. [DOI] [PubMed] [Google Scholar]
- 33. Zhang H, Li Y, Fan Y, et al. Klotho is a target gene of PPAR-gamma. Kidney Int 2008; 74(6): 732–739. [DOI] [PubMed] [Google Scholar]
- 34. Mitobe M, Yoshida T, Sugiura H, et al. Oxidative stress decreases klotho expression in a mouse kidney cell line. Nephron Exp Nephrol 2005; 101(2): e67–e74. [DOI] [PubMed] [Google Scholar]
- 35. Mitani H, Ishizaka N, Aizawa T, et al. In vivo klotho gene transfer ameliorates angiotensin II-induced renal damage. Hypertension 2002; 39(4): 838–843. [DOI] [PubMed] [Google Scholar]
- 36. Zerath E, Holy X, Douce P, et al. Effect of endurance training on postexercise parathyroid hormone levels in elderly men. Med Sci Sports Exerc 1997; 29(9): 1139–1145. [DOI] [PubMed] [Google Scholar]
- 37. Ljunghall S, Joborn H, Roxin LE, et al. Prolonged low-intensity exercise raises the serum parathyroid hormone levels. Clin Endocrinol (Oxf) 1986; 25(5): 535–542. [DOI] [PubMed] [Google Scholar]
- 38. Ljunghall S, Joborn H, Roxin LE, et al. Increase in serum parathyroid hormone levels after prolonged physical exercise. Med Sci sports Exerc 1988; 20(2): 122–125. [DOI] [PubMed] [Google Scholar]
- 39. Devlin JT, Horton ES. Effects of prior high-intensity exercise on glucose metabolism in normal and insulin-resistant men. Diabetes 1985; 34(10): 973–979. [DOI] [PubMed] [Google Scholar]
- 40. Mikines KJ, Sonne B, Farrell PA, et al. Effect of physical exercise on sensitivity and responsiveness to insulin in humans. Am J Physiol 1988; 254(3 Pt 1): E248–E259. [DOI] [PubMed] [Google Scholar]
- 41. Adams OP. The impact of brief high-intensity exercise on blood glucose levels. Diabetes Metab Syndr Obes 2013; 6: 113–122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Richter EA, Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev 2013; 93(3): 993–1017. [DOI] [PubMed] [Google Scholar]