Abstract
A better knowledge of factors predicting the development of sepsis in patients hospitalized in intensive care unit (ICU) might help deploy more targeted preventive and therapeutic strategies. In addition to the known clinical and demographic predictors of septic syndromes, in this study, we investigated whether measuring T and B lymphocyte subsets upon admission in the ICU may help individualize the prediction of ensuing sepsis during ICU stay. Between May 2015 and December 2016, we performed a prospective cohort study evaluating peripheral blood lymphocyte T-CD4+ (T-helper cells), T-CD8+ (cytotoxic T-cells), T-CD56 + (natural killer cells), and T-CD19+ (B-lymphocytes), using flow cytometry on blood samples collected 2 days after admission in the ICU. We enrolled 176 patients, 65.3% males, with mean age of 61.1 ± 15.4 years. At univariate analyses, higher percentages of CD19 B-cells were significantly associated with ensuing sepsis (20.5% (15.7–27.7)% vs 16.9% (11.3–22)%, P = 0.0001), whereas median interquartile range (IQR) proportions of CD4 T-cells (41.2% (33.4–50.6)% vs 40% (35–47)%, P = 0.5), CD8 T-cells (21.1% (15.8–28.2)% vs 19.6% (14.6–25.1)%, P = 0.2) and CD56 T-cells (1.7% (0.9–3.1)% vs 1.45% (0.7–2.3)%, P = 0.4) did not reveal any significant association. An unexpected, highly significant inverse correlation of CD8 T-cells and CD19 B-cells proportions, however, was observed, suggesting that patients with lower CD19 and higher CD8 proportions might be somehow protected from ensuing sepsis. We therefore studied the ability of the CD8/CD19 ratio to predict ensuing sepsis in our sample. In final models of multivariate logistic regression, the following independent associations were found: previous antibiotic exposure (odds ratio (OR): 3.8 (95% confidence interval (CI): 1.35–10.87), P = 0.01), isolation of at least one multi-drug resistant organism at any time during ICU stay (OR: 8.4 (95% CI: 3.47–20.6), P < 0.0001), decreasing age (OR: 0.9 (95% CI: 0.93–0.99), P = 0.02) and a CD8/CD19 ratio >2.2 (OR: 10.3 (95% CI: 1.91–55.36), P = 0.007). Our data provide preliminary evidence that immune characterization of critically ill patients on ICU admission may help personalize the prediction of ensuing sepsis during their ICU stay. Further polycentric evaluation of the true potential of this new tool is warranted.
Keywords: critically ill patients, ICU, immune-protective phenotype, lymphocyte subsets, sepsis
Introduction
Severe sepsis has been reported as a major cause of increased morbidity, length of stay, and mortality among patients hospitalized in intensive care settings for any cause.1,2 In previous years, procalcitonin (PCT) has been extensively explored as a possible sensitive and specific biomarker for early detection of ensuing sepsis, and most intensive care unit (ICU) clinicians consider PCT the most reliable tool to identify patients with sepsis.3–6 Recent evidence, however, has been provided that only serial assessment of PCT may be sensitive enough in identifying ensuing sepsis in at-risk patients.5,6 As a consequence, defining the individual risk of sepsis in patients at ICU admission has become a well-recognized priority, as timely diagnosis of ensuing sepsis, as well as early and appropriate treatment may both considerably improve patients’ outcome.7 Furthermore, due to the increasing spread of multi-drug resistant organisms (MDROs), MDRO colonization is a frequent condition among ICU patients, and its management is still a rather controversial issue, as overtreatment of colonized patients may increase the spread of MDRO.8 Knowing which biochemical and/or immunological factors may predict progression to sepsis among colonized patients might help to deploy more individualized treatment strategies. In this study, we investigated whether measuring T and B lymphocyte subsets, a relatively simple monitoring of the immune function of critical patients at their admission in the ICU, may help individualize the prediction of sepsis, especially in those colonized with MDRO and heavily exposed to previous antibiotic pressure before ICU admission.
Patients and methods
We performed a prospective, monocentric cohort study for the immune monitoring of critical patients by assessment of peripheral blood lymphocyte subsets. The study was planned in 2014 and authorization was required by the local ethics committee (EC). The study was authorized from January, 2015, and no specific additional informed consent was required, as measurement of lymphocyte subsets at entrance in the ICU was judged as an established good clinical practice in other settings of Internal Medicine. Data were collected between May 2015 and December 2016 from patients admitted to the ICU of Pescara General Hospital, an urban 650-bedded tertiary hospital of regional reference for adult traumas and acute diseases of neurosurgical interest. Its ICU facility is a single, 11-bed unit, receiving critically ill patients from regional emergency departments, as well as from medical and surgical units in the hospital. To avoid inclusion of patients destined to short-term ICU stays, patients were consecutively enrolled after 48 h of hospitalization in the ICU. At enrollment, lymphocytes were assayed using flow cytometry. Percentages of lymphocyte T-CD4+ (T-helper cells), T-CD8+ (cytotoxic T-cells), T-CD56+ (natural killer cells), and T-CD19+ (B-lymphocytes) were evaluated with AQUIOS CL instrument (Beckman Coulter, Inc., Indianapolis, USA) in the local laboratory.9 Patients were microbiologically characterized with surveillance cultures of nose and rectum at entrance and weekly afterwards.
Demographic and clinical characteristics of patients, past medical history, and other laboratory measurements were collected upon enrollment. During the follow-up period, enrolled patients who died from any cause were classified as non-survivors.
Sepsis and septic shock were diagnosed according to the diagnostic criteria of the Sepsis-2 classification (2003), as established in the 2015 EC-approved study protocol. Criteria for organ dysfunction were: sepsis-induced hypotension; lactate above normal upper limits; urine output <0.5 mL/kg/h for >2 h despite adequate fluid resuscitation or creatinine >2.0 mg/dL (176.8 μmol/L); acute lung injury with PaO2/inspired oxygen fraction (FiO2) <250 mmHg in the absence of pneumonia or acute lung injury with PaO2/FiO2 <200 mmHg in the presence of pneumonia; bilirubin >2.0 mg/dL (34.2 μmol/L); and platelet count <100,000/mm3.
Epidemiological and clinical factors analyzed were age; sex; presence of comorbidity; APACHE II score; previous antibiotic exposure, that is at least a week of either quinolone, beta-lactam, or carbapenem prescribed in the month preceding hospitalization; previous surgery, that is surgery in the month preceding ICU admission; surgery during hospitalization in the ICU; blood counts and other available biomarkers of sepsis; any microbiological isolate including methicillin-resistant Staphylococcus aureus (MRSA) and MDRO(s). MDROs were defined as the presence of at least one of the following alert microorganisms: Klebsiella pneumoniae producing carbapenemase, MRSA, vancomycin-resistant Enterococci (VRE), multi-drug Acinetobacter baumannii, and multi-drug Pseudomonas aeruginosa. The major study outcome was the rate of ensuing of sepsis, severe sepsis, or septic shock during ICU stay. The secondary outcome was overall mortality during ICU stay. Differences in the selected variables were first examined using chi-square test for categorical variables and non-parametric Kruskal–Wallis rank test for continuous variables. Stepwise forward logistic regression was used to examine the independent association between unfavorable outcome and each potential determinant. Relationships between CD8 T-cells and CD19 B-cells were analyzed by Scatter plot distribution. Statistical significance was defined as a two-sided P-value <0.05, and all analyses were performed using Stata package, version 12 (StataCorp., College Station, Texas, USA).
Results
We enrolled 176 patients, 65.3% males, mean age 61.1 ± 15.4 years. Of them, 77 patients (43.7%) were neurosurgical patients, 38 (21.6%) were hospitalized due to polytrauma, 24 (13.7%) due to respiratory failure, and the same proportion (24 patients, 13.7%) presented a septic syndrome at entrance. At any time during the ICU stay, MDROs were isolated from 60 (34.1%) patients and MRSA from 25 (14.2%). During their ICU stay, 43 patients (24.4%) had positive blood cultures and 51 patients (29.0%) developed sepsis, severe sepsis, or septic shock. A total of 38 (21.6%) patients died during their stay in the ICU. At univariate analyses, ensuing sepsis was tightly linked with MDRO isolation (64.7% vs 21.6%, P < 0.0001). Similarly, other factors linked to ensuing sepsis were MRSA isolation (23.5% vs 10.4%, P = 0.02) and pre-exposure to antibiotic treatments (31.4% vs 16.8%, P = 0.03). All other variables, such as APACHE II score (19.5 ± 6.3 vs 21.2 ± 6.5, P = 0.1), presence of comorbidities (68.8% vs 62.8%, P = 0.4), previous surgery (58.3% vs 41.6%, P = 0.1), or treatment with steroids in the first 2 h of stay in the ICU (16% vs 17.6%, P = 0.23) failed to reveal any association. Among biochemical and hematological variables, higher median concentrations of blood fibrinogen were also associated with ensuing sepsis (341mL/dL (272–555)mL/dL vs 294mL/dL (225–367)mL/dL, P = 0.001). Median interquartile range (IQR) CD4 T-cell proportions (41.2% (33.4–50.6)% vs 40% (35–47)%, P = 0.5) and median (IQR) CD4/CD8 T-cell ratio (2 (1.4–2.7) vs 1.9 (1.5–3), P = 0.7), were our first considered immune subset and ratio as possibly linked with ensuing sepsis.10 They failed, however, to reveal any significant association. At variance, interesting results came from other T- and B-cell subset proportions and ratios. Higher percentages of CD19 B-cells were significantly and directly associated with ensuing sepsis (20.5% (15.7–27.7)% vs 16.9% (11.3–22)%, P = 0.0001). In the analysis of percentage distribution of lymphocyte subsets among different diagnoses at ICU entrance, we found that in patients with respiratory disease median CD8 counts were significantly lower in the sepsis group (57.0cells/µL (27.7–66.5)cells/µL vs 184.0cells/µL (129.54–280.2)cells/µL, P = 0.005). We therefore concentrated on the relationships between CD8 T-cells and CD19 B-cells in our sample, finding a highly significant inverse correlation between CD8 T-cells and CD19 B-cells in the whole sample (Spearman’s rho: –0.47, P < 0.0001, Figure 1). As a consequence, we speculated that a novel absolute ratio between their proportions (CD8%/CD19% ratio) might better predict septic syndromes, possibly identifying a subset of patients with a clear-cut prediction or protection for sepsis. Indeed, we found that patients with ensuing sepsis or sepsis in the ICU had almost invariably a CD8/CD19 ratio ⩽2. As a consequence, we studied the ability of the CD8/19 ratio to predict protection from ensuing sepsis, testing this hypothesis either as a continuous variable or as a derivate dichotomic variable, that we defined immune-protecting phenotype (IPP), given as present (or positive) for a CD8/19 ratio >2.2. Introducing the ratio CD8/CD19 as a continuous variable in the final multivariate models of logistic regression, sepsis was independently predicted by: decreasing age (odds ratio (OR): 0.97 (95% confidence interval (CI): 0.94–1.0), P = 0.03), previous antibiotic exposure (OR: 3.03 (95% CI: 1.1–8.49), P = 0.03), presence of at least one MDRO isolate at any time during ICU stay (OR: 7.64 (95% CI: 3.2–18.4), P < 0.001), and a higher CD8/CD19 (OR: 0.5 for unit increase (95% CI: 0.3–0.85), P = 0.001, Table 1).
Figure 1.
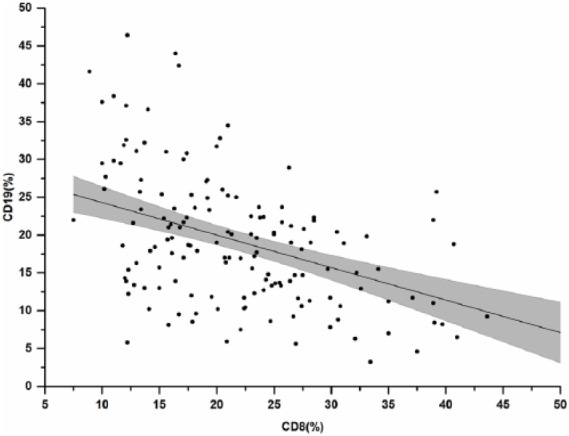
Scatter distribution of CD19 and CD8 B and T-cell proportions in our sample.
Table 1.
Final multivariate prediction models for septic syndromes among the assisted patients using the two novel prediction variables identified: (a) logistic regression using the CD8%/CD19% ratio as a continuous variable and (b) logistic regression using the immune-protecting phenotype as a dichotomic variable.
| Sepsis | OR | P-value | 95% CI |
|---|---|---|---|
| (a) | |||
| Sex | 0.64 | 0.3 | 0.27–1.57 |
| Age (1-year increase) | 0.97 | 0.03 | 0.94–1.0 |
| APACHE II score | 1.07 | 0.05 | 0.1–1.1 |
| Previous antibiotic exposure | 3.03 | 0.03 | 1.1–8.49 |
| MDRO | 7.64 | <0.0001 | 3.2–18.4 |
| CD8%/CD19% (1-unit increase) | 0.50 | 0.01 | 0.3–0.85 |
| b) | |||
| Sex | 0.6 | 0.27 | 0.25–1.47 |
| Age (1-year increase) | 0.96 | 0.02 | 0.93–0.99 |
| APACHE II score | 1.08 | 0.02 | 1.01–1.15 |
| Previous antibiotic exposure | 3.83 | 0.01 | 1.35–10.87 |
| MDRO | 8.45 | <0.0001 | 3.47–20.6 |
| IPP (CD8%/CD19% ⩽ 2.2) | 10.3 | 0.007 | 1.91–55.36 |
APACHE: Acute Physiology and Chronic Health Evaluation; CI: confidence interval; IPP: immune-protecting phenotype; OR: odds ratio; MDRO: multi-drug resistant organism.
Similarly, introducing the IPP as a dichotomic variable (with cut-off at 2.2) to be used in algorithms to individualize the risk of ensuing sepsis during ICU stay, multivariate models of logistic regression revealed the following independent associations with sepsis: previous antibiotic exposure (OR:3.8 (95% CI: 1.35–10.87), P = 0.03), isolation of at least an MDRO at any time during ICU stay (OR:10.3 (95% CI: 1.91–55.36), P < 0.001), decreasing age (OR: 0.9 for each year increase (95%CI: 0.93–0.99), P = 0.02) and the absence of IPP (i.e. a CD8/CD19 proportion ratio >2.2, OR: 10.3 (95%CI: 1.91–55.36), P = 0.007, Table 1). Overlapping results were obtained on duplicate models, after exclusion of the 24 patients with a diagnosis of sepsis, severe sepsis, or septic shock at the time of admission into the ICU (data not shown). None of the immune predictors evaluated in this study in association with ensuing of sepsis was significantly associated with in ICU mortality (data not shown).
Discussion
Morbidity and mortality of septic patients are known to be influenced by a significant dysregulation of immune response to the infection. While T-lymphopenia and depletion of peripheral blood B lymphocytes in patients with septic shock were found correlated with mortality in ICU patients, very few studies prospectively addressed their role in the prediction of sepsis and sepsis shock.11,12 Our pivotal investigation focused on critical patients at ICU entrance, measuring quantitative proportions of peripheral blood lymphocyte subsets, a well-established routine investigation after the advent of HIV infection, 48 h after ICU admission. We found a significantly higher percentage of B-cell lymphocytes in patients who developed sepsis during their ICU stay and investigated the relationships between CD8 T-cells and CD19 B-cells in our sample, finding a highly significant inverse correlation of CD8 T-cells and CD19 B-cells in the whole sample. Linking these two variables through a novel absolute ratio among their proportions (CD8/CD19 ratio) helped identifying a subset of patients with a very skewed immune protection for ensuing of septic syndromes. This turned out to be the case as patients with ensuing sepsis showed almost invariably a CD8/CD19 ratio ⩽2.2. This derivate immune index, that we defined IPP, hints to a protective immune signature in patients with a ratio >2.2 between the CD8 (protective) and CD19 (responsive) peripheral lymphocyte subsets. In particular, having a positive result of IPP was associated with a 10-fold reduction of the sepsis risk, independent of all other known predictors and may be considered to further development in the context of algorithmic predictions of sepsis together with other currently available predictors of ensuing sepsis.
This study has several limitations: the sample size, the monocentric nature of the study, the lack of possibility to perform functional assays of B-lymphocytes such as expression of CD40 and CD69, as well as the ability to produce immunoglobulins in specific settings of patients. Our novel findings, however, may be amenable to further development in the context of algorithmic predictions of sepsis together with other currently available predictors of ensuing sepsis.4,5
Further evaluation of the IPP by a multicentric investigation will pinpoint the potential benefit of introducing the measurement of lymphocyte subsets in the routine laboratory evaluation of ICU patients.
Acknowledgments
The authors are grateful to all the nurses and the physicians in the ICU unit in Pescara General Hospital, who helped with the observed patients in deploying care and the study design. They are also indebted with the Fondazione Camillo de Lellis per l’Innovazione e la Ricerca in Medicina, based in Pescara, Italy, who funded E.P. and T.U. during their period of stay as Research Fellows at Pescara General Hospital with a Research Grant. They are also indebted with Elena Ricci, MD, who assisted them with expert revision of their statistical analyses. The study was totally independent in its design and deployment, as no grant of support was received from any commercial promoter for either study design, data collection, or final database analyses.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Shapiro N, Howell MD, Bates DW, et al. (2006) The association of sepsis syndrome and organ dysfunction with mortality in emergency department patients with suspected infection. Annals of Emergency Medicine 48: 583–590, 590.e1. [DOI] [PubMed] [Google Scholar]
- 2. Dellinger RP, Levy MM, Rhodes A, et al. (2013) Surviving sepsis campaign: International guidelines for management of severe sepsis and septic shock, 2012. Intensive Care Medicine 39: 165–228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Faix JD. (2013) Biomarkers of sepsis. Critical Reviews in Clinical Laboratory Sciences 50: 23–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Kibe S, Adams K, Barlow G. (2011) Diagnostic and prognostic biomarkers of sepsis in critical care. The Journal of Antimicrobial Chemotherapy 66(Suppl 2): ii33–ii40. [DOI] [PubMed] [Google Scholar]
- 5. Shehabi Y, Sterba M, Garrett PM, et al. (2014) Procalcitonin algorithm in critically ill adults with undifferentiated infection or suspected sepsis. A randomized controlled trial. American Journal of Respiratory and Critical Care Medicine 190: 1102–1110. [DOI] [PubMed] [Google Scholar]
- 6. Huang DT, Clermont G, Dremsizov TT, et al. (2007) Implementation of early goal-directed therapy for severe sepsis and septic shock: A decision analysis. Critical Care Medicine 35: 2090–2100. [DOI] [PubMed] [Google Scholar]
- 7. Marik PE. (2014) Don’t miss the diagnosis of sepsis! Critical Care 18: 529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tenover FC, Canton R, Kop J, et al. (2013) Detection of colonization by carbapenemase-producing Gram-negative Bacilli in patients by use of the Xpert MDRO assay. Journal of Clinical Microbiology 51: 3780–3787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gossez M, Malcus C, Demaret J, et al. (2017) Evaluation of a novel automated volumetric flow cytometer for absolute CD4+ T lymphocyte quantitation. Cytometry. Part B, Clinical Cytometry 92: 456–464. [DOI] [PubMed] [Google Scholar]
- 10. De Pablo R, Monserrat J, Torrijos C, et al. (2012) The predictive role of early activation of natural killer cells in septic shock. Critical Care 16: 413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Monserrat J, De Pablo R, Diaz-Martin D, et al. (2013) Early alterations of B cells in patients with septic shock. Critical Care 17: R105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. De Pablo R, Monserrat J, Prieto A, et al. (2014) Role of circulating lymphocytes in patients with sepsis. BioMed Research International 2014: 671087. [DOI] [PMC free article] [PubMed] [Google Scholar]


