Abstract
Chronic obstructive pulmonary disease (COPD) has been described as a systemic disease. Sarcopenia is one of the systemic effects that is related to several adverse outcomes. The objectives of this study were to estimate the prevalence of sarcopenia and to determine the factors associated with sarcopenia in COPD patients in Southeast Asia. This was a cross-sectional study of COPD patients who attended a COPD clinic from May 2015 to December 2016. Baseline characteristics were collected and dual-energy X-ray absorptiometry was used to measure skeletal muscle mass. Handgrip strength was used to assess muscle strength, and as a measurement of physical performance, the 6-min walk distance was used. One hundred and twenty-one participants were recruited. Most of them were men (92.6%). Prevalence of sarcopenia was 24% (29 cases). Independent factors associated with sarcopenia were age ≥ 75 years (adjusted odds ratio (AOR) 13.3, severity of COPD (AOR 19.2 and 13.4 for moderate and severe COPD), Modified Medical Research Council (MMRC) scale (AOD 1.9), and obesity (AOR 0.04). Sarcopenia affects about one-quarter of COPD patients. Age, severity of COPD, MMRC scale, and BMI status were the factors associated with sarcopenia.
Keywords: Airway obstruction, body composition, fat-free mass index, lung disease, skeletal muscle mass
Introduction
Chronic obstructive pulmonary disease (COPD) is defined as a disease characterized by persistent airflow limitation that is usually progressive and associated with an enhanced chronic inflammatory response in the airways and the lung to noxious particles or gases.1 It can lead to substantial morbidity and premature death. It affects about 10% of general population and about 50% of very heavy smokers.2 Aging is an independent factor in the prevalence of COPD with 14% in persons 65 years or over compared with 9.9% of younger persons. A doubling of the prevalence of COPD was observed for every 10-year increment in age.2 Existing reports show that COPD is not considered only as a respiratory disease but also described as a systemic disease including weight loss and nutritional abnormalities, skeletal muscle dysfunction, increased risk of cardiovascular disease, hormonal and metabolic disturbances, osteoporosis, anxiety, and depression.3–5 Therefore, a more holistic approach of COPD is essential rather than focusing only on airflow limitations.2
One of the systemic effects of COPD is sarcopenia. This term has been described as an age-related decline in muscle mass and its function.6,7 This condition is associated with unfavorable health outcomes such as falls, disability, hospitalization, poor quality of life, and mortality.8,9 The etiology of sarcopenia is the result of the physiologic changes in addition to the results of disease-related, nutrition, and activity.10 Sarcopenia can be classified as physical frailty where frailty is associated with adverse health outcomes.11,12 Sarcopenia was found to be associated with worsening lung function in male COPD patients.13 COPD patients also have relative or an absolute increase in fat mass which might contribute to systemic inflammation, loss of fat-free mass, and insulin resistance. Fat-free mass index, not body mass index (BMI), was significantly related to pulmonary function, dyspnea severity, quality of life, and reflected reduced skeletal muscle mass.14,15 Currently, the persons with COPD are recommended for sarcopenia screening and assessment according to the recommendation of the Asian Working Group for Sarcopenia (AWGS).10
The prevalence of sarcopenia in COPD men older than 40 years of age varies from 20% to 40% depending on age of studied population, gender, setting of population, and measurement methods.13,16 The prevalence of sarcopenia from existing reports in Asia is 29.3% and for sarcopenic obesity is 14.2%13; however, the definition of sarcopenia from this study focused only on skeletal muscle mass. In a recent study in the United Kingdom using the European Working Group on Sarcopenia in Older People (EWGSOP) criteria which included either low muscle strength or poor physical performance with low skeletal muscle mass, it was found at 14.5% among stable COPD patients in the outpatient setting.17 Factors associated with sarcopenia in COPD patients in literature review included age, nutritional status, exercise capacity, severity of COPD, functional performance, smoking status, comorbid diseases, and self-reported hospital admission. The relation of severity of COPD and BMI is, however, inconsistent with reports.16,17
Regarding the current criteria for sarcopenia diagnosis, they incorporate muscle function which is muscle strength and/or physical performance.10 The prevalence of sarcopenia in COPD patients according to that definition is limited. Additionally, associated factors of sarcopenia in COPD particularly for the outcomes involving the geriatric syndrome such as falling and disability have not been widely studied. Early detection of sarcopenia could facilitate the implementation of interventions targeted at preventing the progression of sarcopenia and improving quality of life in patients with COPD. Therefore, the primary objective of this study was to estimate the prevalence of sarcopenia in COPD patients and the secondary objective was to determine the factors associated with sarcopenia in COPD patients.
Material and methods
Study participants and setting
This was a cross-sectional study that was carried out with COPD patients who were 18 years old or over and attended COPD clinic of Srinagarind Hospital, Khon Kaen, Thailand from May 2015 to December 2016. This COPD clinic is a tertiary care referral center of the university hospital which enrolled patients from our internal medicine/family medicine clinic and other community hospitals. Patients were excluded if they had other active medical illnesses such as heart failure functional class III–IV, pneumonia, septicemia, COPD exacerbation within the preceding 4 weeks, patients who were unable to complete study due to physical limitations, patients who were unwilling to participate in this study, and patients who had limitations in performing dual energy X-ray absorptiometry (DXA) or conditions that would affect DXA results; history of barium taking or enemas over the past 2 weeks, patients who had metallic instrumentation such as spinal fixation, patients with history of a radionuclide scan over the past 2 weeks, and patients with a body weight over 200 kg (exceeded the capacity). The study population was similar to the previously published article.18
Definition
COPD
COPD was diagnosed in patients according to the criteria of the Global Initiative for Chronic Obstructive Lung Disease (GOLD) that was characterized by persistent airflow limitation, a post-bronchodilator force expiratory volume in 1 s (FEV1) less than 70% of forced vital capacity. Severity of COPD was classified into three groups: mild (FEV1 > 80% normal), moderate (FEV1 50–79% normal), and severe (FEV1 < 49% normal). For patients with severe COPD in this study, it included patients with severe COPD (FEV1 30–49% normal) and very severe COPD (FEV1 < 30% normal) based on the GOLD staging system.1
Sarcopenia
Criteria for sarcopenia diagnosis were based on the definition of the AWGS which consists of low muscle mass and low muscle strength and/or poor physical performance. Skeletal muscle mass was estimated using DXA and appendicular skeletal muscle mass index calculated as appendicular skeletal mass/height2 (ASMI). Handgrip strength was measured using a handheld dynamometer and measurement of physical performance that used the 6-min walk distance (6-MWD) according to the American Thoracic Society guidelines.19 The following cutoff values were used to identify sarcopenia; ASMI of ≤7.0 kg/m2 for men and ≤5.4 kg/m2 for women, and either handgrip strength of <26 kg for men and <18 kg for women or a gait speed of <0.8 m/s.10 Pre-sarcopenia is defined as presence of low muscle mass without influence on muscle strength or physical performance and sarcopenic obesity is characterized by the presence of sarcopenia according to the definition of the AWGS with BMI > 25kg/m2.20,21
Modified Medical Research Council scale
The Modified Medical Research Council (MMRC) scale was used to evaluate dyspnea in daily living. It included five grades (0–4) of various physiological activities that provoke dyspnea that was self-rated by the patient. The descriptions were as follows: 0, not troubled with breathlessness except with strenuous exercise; 1, troubled by shortness of breath when hurrying on the level or walking up a slight hill; 2, walked slower than people of the same age on the level because of breathlessness or has to stop for breath when walking at own pace on the level; 3, stopped for breath after walking about 100 yards or after a few minutes on the level; and 4, too breathless to leave the house, or breathless when dressing or undressing.22
Instruments
Muscle mass measurement used DXA (General Electric (Lunar-Progidy) model). A grip dynamometer (GRIP-D (T.K.K.5401) model) was used to measure handgrip strength and 6-MWD was used to measure physical performance.
Procedure
After consent, baseline patient data were collected by trained clinical researchers. The demographic information consisted of age, sex, educational level, smoking status, severity of COPD using the most recent report of pulmonary function tests within the past 6 months (the patients were asked to take the deepest breath they could, and then exhaled into the sensor as hard as possible, preferably at least 6 s), MMRC scale, underlying disease, regular medication uses, history of falls over the past 12 months, self-report baseline basic and instrumental activities of daily living (ADL) using Barthel ADLs index and Chula index, history of nonelective admission over the past 12 months, weight, height, and systolic and diastolic blood pressure at rest. Weight and height were used to calculate BMI, and systolic and diastolic blood pressures were presented as mean arterial blood pressure (MAP). For sarcopenia diagnosis, muscle mass using DXA, handgrip strength using grip dynamometer (it was measured by asking the patients to hold the handgrip dynamometer in one hand, with the arm at right angles and the elbow by the side of the body. When ready, the patients squeezed the dynamometer with maximum isometric effort, which was maintained for about 5 s. Three attempts were tested and the maximal one was recorded),23 and physical performance using the 6-MWD as previously described were performed on all patients in the same period.10,24,25
Sample size calculation
Sample size calculations were based on the primary objective of this study which was the estimated prevalence of sarcopenia in COPD patients. As there was no study regarding the prevalence of sarcopenia in COPD patients according to the definition of the AWGS, the estimated prevalence of 14.5% was derived from an existing study.17 The estimation of a population proportion with a specified absolute precision formula was used to calculate this.26 At least 98 participants were sufficient to achieve the required sample size at the significance level of 0.05.
Statistical analyses
Descriptive statistics for baseline data were presented in percentage, mean, and standard deviation. If the distribution of these data was not a normal distribution, then medians, and interquartile ranges were used instead. Effects of factors associated with sarcopenia were evaluated using univariate and multivariate regressions analysis. For univariate analysis, crude odds ratios (OR) and 95% confidence intervals (CIs) were used to consider the strength of association between factors associated with sarcopenia. Factors with a p < 0.20 or clinical significance in literature review were then entered into a multiple logistic regression model. A value of p < 0.05 was considered to indicate statistically significant differences, and adjusted OR and their 95% CI were reported to consider the strength of association. All the data analyses were carried out using STATA version 10.0 (StataCorp, College Station, Texas, USA).
Ethics approval was provided by the Ethics Committee of the Faculty of Medicine, Khon Kaen University as instituted by the Helsinki Declaration.
Results
Prevalence of sarcopenia among COPD patients
There were 121 participants with stable COPD recruited in this study. Baseline characteristics of the studied population are shown in Table 1. The majority of them were men (112 cases, 92.6%) with an age older than 65 years old (maximum age was 92 and minimum age was 47 years old). Most of them were ex-smokers with a moderate degree in severity of COPD. Hypertension was the most common comorbid disease. The overall ADL were well-preserved. The distribution of low skeletal muscle mass, low handgrip strength, and walking speed is demonstrated in Figure 1. Low skeletal muscle mass was the main component, followed by low handgrip strength. Low gait speed was found in the minority. According to the definition of sarcopenia from the recommendation of the AWGS,10 the prevalence of pre-sarcopenia was about 15.7% (19 cases) and sarcopenia was 24% (29 cases); all of them were men and severe sarcopenia was found in about 2.5% (three cases). The prevalence of sarcopenic obesity was 0.8% (one case).
Table 1.
Baseline characteristics of studied population.a
| Variables | N = 121 | |
|---|---|---|
| Age (years), mean (SD) | 70 | 9.0 |
| Age group (years), n (%) | ||
| <65 | 32 | 26.45 |
| 65–74 | 47 | 38.84 |
| ≥75 | 42 | 43.71 |
| Male sex, n (%) | 112 | 92.6 |
| Educational level, n (%) | ||
| 6 years or lower | 59 | 48.76 |
| 7–12 years | 28 | 23.14 |
| >12 years | 34 | 28.10 |
| Smoking status, n (%) | ||
| Never smoker | 7 | 5.79 |
| Ex-smoker | 104 | 85.95 |
| Current smoker | 10 | 8.26 |
| Severity of COPD, n (%) | ||
| Mild | 31 | 25.62 |
| Moderate | 69 | 57.02 |
| Severe | 21 | 17.36 |
| MMRC scale, mean (SD) | 0.69 | 1.08 |
| Comorbid diseases, n (%) | ||
| Diabetes mellitus | 12 | 9.92 |
| Hypertension | 46 | 38.02 |
| Dyslipidemia | 10 | 8.26 |
| Chronic arthritis | 10 | 8.26 |
| Cancer | 6 | 4.96 |
| Regular medication uses, n (%) | ||
| Inhaled corticosteroid | 113 | 93.39 |
| Systemic steroid | 2 | 1.65 |
| Oral hypoglycemic drugs | 7 | 5.79 |
| Statin | 13 | 10.74 |
| NSAIDs | 1 | 0.83 |
| History of fall at least two times over the past 12 months, n (%) | 3 | 0.89 |
| Barthel scores, mean (SD) | 19.9 | 1 |
| Chula IADLs scores, mean (SD) | 8.98 | 0.18 |
| Nonelective admission over the past 12 months, n (%) | 32 | 26.45 |
| BMI (kg/m2), n (%) | ||
| Underweight (<18.5) | 19 | 15.7 |
| Normal and over weight (18.5–24.9) | 65 | 53.72 |
| Obesity (>25) | 37 | 30.58 |
| MAP (mmHg), mean (SD) | 95.96 | 10.68 |
| 6-MWD (m), mean (SD) | 423.39 | 73.67 |
| Gait speed (m/s), mean (SD) | 1.18 | 0.2 |
| Handgrip strength (kg), mean (SD) | ||
| Male | 26.65 | 6.02 |
| Female | 21.22 | 3.43 |
| Appendicular skeletal mass index (kg/m2), mean (SD) | ||
| Male | 7.1 | 0.89 |
| Female | 6.25 | 0.46 |
| Osteoporosis, n (%) | 45 | 37.19 |
| Sarcopenia, n (%) | 29 | 24 |
COPD: chronic obstructive pulmonary disease; NSAIDs: nonsteroidal anti-inflammatory drugs; IADL: instrumental activities of daily living; BMI: body mass index; MAP: mean arterial blood pressure; SD: standard deviation; osteoporosis: defined when T-score of femoral neck or lumbar spine ≤−2.5 SD; 6-MWD: 6-min walk distance; MMRC scale: Modified Medical Research Council scale (it was available in 116 patients).
aAppendicular skeletal mass was calculated by summing the muscle masses of the four limbs.
Figure 1.
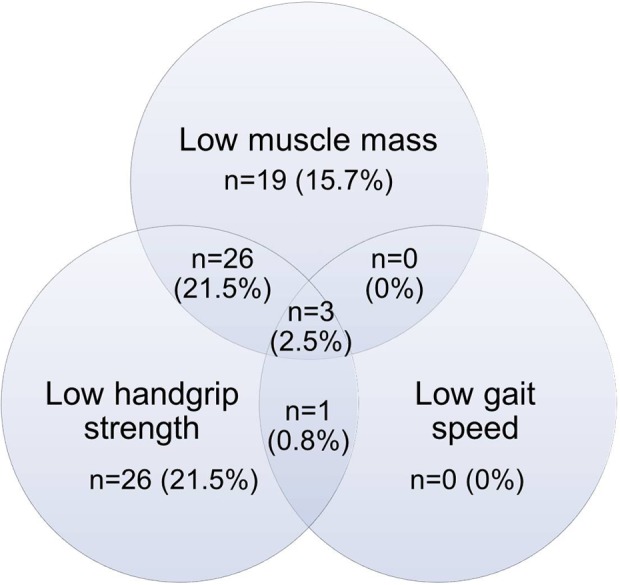
The relationship of low skeletal muscle mass, low handgrip strength, and low gait speed in study populations.
Factors associated with sarcopenia among COPD patients
Comparison of the risk factors between sarcopenic and nonsarcopenic patients with COPD using univariate analysis is shown in Table 2. For multivariate analysis, six factors where p < 0.2 or clinical significance from literature review from univariate analysis were entered in the multiple regression models: age, severity of COPD, MMRC scale, nonelective admission over the past 12 months, BMI, MPA, and presence of osteoporosis (Table 3). After multicollinearity was checked, advanced age (>75 years), greater severity of COPD, MMRC scale, and nonobese patients were the factors associated with sarcopenia in this study.
Table 2.
Factors associated with sarcopenia using univariate analyses.
| Variables | Unadjusted OR | 95% CI | p Value |
|---|---|---|---|
| Age group (years) | |||
| <65 | 1 | — | — |
| 65–74 | 1.2 | (0.3, 4.6) | 0.8 |
| ≥75 | 5.3 | (1.6, 17.7) | <0.05 |
| Severity of COPD | |||
| Mild | 1 | — | — |
| Moderate | 3.3 | (0.9, 12.2) | 0.07 |
| Severe | 5.7 | (1.3, 25.3) | <0.05 |
| MMRC scale | 1.9 | (1.3, 2.8) | <0.05 |
| Comorbid diseases | |||
| Diabetes mellitus | 1.1 | (0.3, 4.2) | 0.9 |
| Hypertension | 1.2 | (0.5, 2.8) | 0.7 |
| Dyslipidemia | 0.8 | (0.2, 3.9) | 0.8 |
| Chronic arthritis | 1 (omitted) | ||
| Cancer | 1.6 | (0.3, 9.4) | 0.6 |
| Regular medication uses | |||
| Inhaled corticosteroid | 0.9 | (0.2, 4.9) | 0.9 |
| Systemic steroid | 3.3 | (0.2, 53.7) | 0.4 |
| Oral hypoglycemic drugs | 0.5 | (0.1, 4.4) | 0.5 |
| Statin | 1.5 | (0.4, 5.2) | 0.6 |
| NSAIDs | 1 (omitted) | ||
| History of falls at least 2 times over the past 12 months | 1.6 | (0.1, 18.4) | 0.7 |
| Barthel scores | 1 (omitted) | ||
| Chula IADLs scores | 1 (omitted) | ||
| Nonelective admission over the past 12 months | 2.0 | (1.1, 3.7) | <0.05 |
| BMI (kg/m2) | |||
| Underweight | 1 | ||
| Normal and over weight | 0.3 | (0.1, 0.8) | <0.05 |
| Obesity | 0.02 | (002, 0.2) | <0.05 |
| MAP (mmHg) | 0.9 | (0.9, 0.9) | <0.05 |
| Osteoporosis | 1.8 | (0.8, 4.3) | 0.2 |
COPD: chronic obstructive pulmonary disease; OR: odds ratio; CI: confidence interval; BMI: body mass index; MAP: mean arterial blood pressure; osteoporosis: defined when T-score of femoral neck or lumbar spine ≤−2.5 standard deviation; MMRC scale: Modified Medical Research Council scale; NSAIDs: nonsteroidal anti-inflammatory drugs; IADL: instrumental activities of daily living.
Table 3.
Factors associated with sarcopenia using multivariate analyses.
| Variables | Adjusted OR | 95% CI | p Value |
|---|---|---|---|
| Age group (years) | |||
| <65 | 1 | — | — |
| 65–74 | 2.0 | (0.4, 10.8) | 0.4 |
| ≥75 | 13.3 | (2.2, 79.9) | <0.05 |
| Severity of COPD | |||
| Mild | 1 | — | — |
| Moderate | 19.2 | (2.2, 166.4) | <0.05 |
| Severe | 13.4 | (1.2, 148.6) | <0.05 |
| MMRC scale | 1.9 | (1.1, 3.3) | <0.05 |
| Nonelective admission over the past 12 months | 3.3 | (0.8, 12.9) | 0.1 |
| BMI (kg/m2) | |||
| Underweight | 1 | — | — |
| Normal and over weight | 1.6 | (0.3, 7.9) | 0.6 |
| Obesity | 0.04 | (0.003, 0.6) | <0.05 |
| MAP (mmHg) | 0.9 | (0.9, 1.0) | 0.1 |
| Osteoporosis | 1.4 | (0.4, 5.1) | 0.6 |
COPD: chronic obstructive pulmonary disease; OR: odds ratio; CI: confidence interval; BMI: body mass index; MAP: mean arterial blood pressure; osteoporosis: defined when T-score of femoral neck or lumbar spine ≤−2.5 standard deviation; MMRC scale: Modified Medical Research Council scale.
Discussion
This is the first study that examines sarcopenia in COPD using AWGS criteria in Southeast Asia. The prevalence of sarcopenia was about 1 in four of all COPD patients. This finding was higher than previously reported in the United Kingdom which was 14.5% but was comparable to the study in South Korea which reported 25%.17,27 The possible explanations are the differences in body composition of different ethnicities and Asian people appear to have a higher prevalence of sarcopenia than other regions.28 Additionally, the variation of techniques to estimate all parameters to diagnose sarcopenia is another reason. The study in the United Kingdom diagnosed COPD using EWGSOP criteria and measured skeletal muscle mass with bioelectrical impedance analysis (BIA) and quadriceps strength to evaluate muscle strength, whereas this study used DXA to measure skeletal muscle mass and handgrip strength to rate muscle strength. Although BIA presents good reliability and correlation to DXA, BIA underestimated muscle mass compared to DXA in prior studies.29,30 The study in Brazil regarding the prevalence of sarcopenia in COPD using DXA was 40%; however, this report diagnosed sarcopenia using only low skeletal muscle mass which was defined as pre-sarcopenia in the current study. These figures were comparable to the report herein (48 cases, about 40%).16 Overall, the prevalence of pre-sarcopenia in this study is consistent with the previous data that reported the prevalence of sarcopenia in COPD using only low skeletal muscle mass that varied from 20% to 40%.13,31 For sarcopenic obesity, the prevalence was very low in this study (0.8%). To the best of authors’ knowledge, there has been no study using similar criteria in COPD patients. The prevalence of sarcopenic obesity in Asia in existing studies is about 15%; however, this current study used only skeletal muscle mass to detect sarcopenia.13,32
There were four factors associated with sarcopenia using multivariate analyses: age, severity of COPD, MMRC scale, and BMI. Advancing age and severity of COPD showed high magnitudes of associations. Commonly, a progressive loss of muscle mass occurs at the age of 40 and is greater after 70 years.21 The decline in gait speed and grip strength was faster than muscle mass especially after the age of 70 years.21,33 The result supports that age is one of the independent factors; however, only age >75 years showed statistical significance. Because the patients in this study were with age over than 40 years old, an age of 65–74 years compared to an age of less than 65 years might not show the differences. For the severity of COPD, it was consistent with the previous reports that skeletal muscle masses were lower in a greater degree of airflow limitation.14,17 This association might be explained by (1) high resting energy expenditure as a result of increased work of breathing and inadequate dietary intake in severe COPD, (2) physical inactivity due to exercise intolerance, (3) excessive apoptosis of skeletal muscle due to increased systemic inflammation, and (4) possible presence of hypoxia and the more frequent use of systemic corticosteroids.34 Conversely, severity of COPD showed no association with some reports; this might be due to the definition of sarcopenia that used low skeletal muscle mass alone in that study while muscle strength and physical performance were additional factors associated with severity of COPD did not measure.16 The MMRC scale increased the risk of sarcopenia. This finding was similar to the prior report.17,27 This scale had been widely studied regarding its correlation with pulmonary function tests. Additionally, it could predict morbidity and mortality in COPD patients.22,35 Higher MMRC scale represented poorer pulmonary function that is also found its association with sarcopenia in this study.22,35 Being underweight increased the risk of sarcopenia compared to obesity, the results support the findings that lower BMI was related to lower lean mass in COPD patients.16 BMI was not, however, a good indicator to predict adverse outcome in COPD patients since several studies showed that BMI was not associated with staging of the disease. The explanation was due to the increased extra fat stores in greater stages of COPD. Consequently, BMI could remain unchanged.14,15 Cigarette smoking did not show the association with sarcopenia in this study. The existing reports show inconsistent results. It was one of the related factors in epidemiologic studies.25 The mechanism was due to the increased protein catabolism.16 In contrast, there was evidence that COPD patients had a high persistent inflammatory state, with increased TNF-α which was related to pathophysiology of sarcopenia, independent of smoking status. Subsequently, cigarette smoking alone did not show a significant association with sarcopenia.16,36 Sarcopenia has been studied as an independent factor for decreased bone mineral density (BMD) due to the systemic consequences of COPD.37 This study could not demonstrate this association. One explanation is that prior studies diagnosed sarcopenia using low skeletal muscle mass alone and analyzed the relationship between BMD and sarcopenia, while this study examined the association of the presence of osteoporosis and sarcopenia.
There were some limitations of this study. Firstly, the overall prevalence of sarcopenia in this study might be under represented as this study estimated gait speed using the 6-MWD. There is evidence that gait speed taken from the 6-MWD was approximately 17% higher than when the 4-m gait speed which was used to measure usual gait speed. Both parameters, however, had a good correlation property (r = 0.7).38 Secondly, the cutoff values of each parameter for detecting sarcopenia were based on the recommendation of AWGS where there might be some differences in individual ethnicities. Therefore, the prevalence might be under or overestimated. Thirdly, the vast majority of patients were men, so the results might not be representative of the population. Fourthly, sarcopenia in previous reports did not measure some associated factors such as exercise capacity and level of daily activity. Finally, this study conducted in tertiary care hospital, and the study populations may have more comorbidities or severity than community hospital setting. Therefore, the prevalence of sarcopenia might not be generalizable in other settings. Further study in diverse study populations is required.
In summary, prevalence of sarcopenia in COPD patients based on the recommendation of AWGS was about 25%. Severe sarcopenia was found about 2.5% and only 0.8% had sarcopenic obesity. The major components of sarcopenia were low muscle mass with low handgrip strength. Age, severity of COPD, nonelective admission over the past 12 months, and BMI status were the factors associated with sarcopenia.
Acknowledgements
The authors would like to acknowledge Professor James A Will, University of Wisconsin–Madison, for editing the manuscript via Publication Clinic, Khon Kaen University, Thailand.
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This work was supported by the Neuroscience Research and Development Group, Khon Kaen University, Thailand under grant number 001/2558 and the Thailand Research Fund (number IRG 5780016).
References
- 1. Vestbo J, Hurd SS, Agusti AG, et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med 2013; 187(4): 347–365. [DOI] [PubMed] [Google Scholar]
- 2. Hanania NA, Sharma G, Sharafkhaneh A. COPD in the elderly patient. Semin Respir Crit Care Med 2010; 31(5): 596–606. [DOI] [PubMed] [Google Scholar]
- 3. Wouters EF, Creutzberg EC, Schols AM. Systemic effects in COPD. Chest 2002; 121(5 Suppl): 127S–130S. [DOI] [PubMed] [Google Scholar]
- 4. Yanbaeva DG, Dentener MA, Creutzberg EC, et al. Systemic inflammation in COPD: is genetic susceptibility a key factor? COPD 2006; 3(1): 51–61. [DOI] [PubMed] [Google Scholar]
- 5. Bone AE, Hepgul N, Kon S, et al. Sarcopenia and frailty in chronic respiratory disease. Chron Respir Dis 2017; 14(1): 85–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Baumgartner RN, Waters DL, Gallagher D, et al. Predictors of skeletal muscle mass in elderly men and women. Mech Ageing Dev 1999; 107(2): 123–136. [DOI] [PubMed] [Google Scholar]
- 7. Cooper C, Dere W, Evans W, et al. Frailty and sarcopenia: definitions and outcome parameters. Osteoporos Int 2012; 23(7): 1839–1848. [DOI] [PubMed] [Google Scholar]
- 8. Visser M, Schaap LA. Consequences of sarcopenia. Clin Geriatr Med 2011; 27(3): 387–399. [DOI] [PubMed] [Google Scholar]
- 9. Rizzoli R, Reginster JY, Arnal JF, et al. Quality of life in sarcopenia and frailty. Calcif Tissue Int 2013; 93(2): 101–120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chen LK, Liu LK, Woo J, et al. Sarcopenia in Asia: consensus report of the Asian working group for sarcopenia. J Am Med Dir Assoc 2014; 15(2): 95–101. [DOI] [PubMed] [Google Scholar]
- 11. Cruz-Jentoft AJ, Michelb JP. Sarcopenia: a useful paradigm for physical frailty. Eur Geri Med 2013; 4: 102–105. [Google Scholar]
- 12. Angulo J, El Assar M, Rodriguez-Manas L. Frailty and sarcopenia as the basis for the phenotypic manifestation of chronic diseases in older adults. Mol Aspects Med 2016; 50: 1–32. [DOI] [PubMed] [Google Scholar]
- 13. Koo HK, Park JH, Park HK, et al. Conflicting role of sarcopenia and obesity in male patients with chronic obstructive pulmonary disease: Korean national health and nutrition examination survey. PLoS One 2014; 9(10): e110448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Pothirat C, Chaiwong W, Phetsuk N, et al. The relationship between body composition and clinical parameters in chronic obstructive pulmonary disease. J Med Assoc Thai 2016; 99(4): 386–393. [PubMed] [Google Scholar]
- 15. Wagner PD. Possible mechanisms underlying the development of cachexia in COPD. Eur Respir J 2008; 31(3): 492–501. [DOI] [PubMed] [Google Scholar]
- 16. Costa TM, Costa FM, Moreira CA, et al. Sarcopenia in COPD: relationship with COPD severity and prognosis. J Bras Pneumol 2015; 41(5): 415–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Jones SE, Maddocks M, Kon SS, et al. Sarcopenia in COPD: prevalence, clinical correlates and response to pulmonary rehabilitation. Thorax 2015; 70(3): 213–218. [DOI] [PubMed] [Google Scholar]
- 18. Limpawattana P, Inthasuwan P, Putraveephong S, et al. Frailty syndrome in ambulatory patients with COPD. Int J COPD 2017; 12: 1–6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. ATS committee on proficiency standards for clinical pulmonary function laboratories. ATS statement: guidelines for the six-minute walk test. Am J Respir Crit Care Med 2002; 166(1): 111–117. [DOI] [PubMed] [Google Scholar]
- 20. Wang C, Bai L. Sarcopenia in the elderly: basic and clinical issues. Geriatr Gerontol Int 2012; 12(3): 388–396. [DOI] [PubMed] [Google Scholar]
- 21. Kim TN, Choi KM. Sarcopenia: definition, epidemiology, and pathophysiology. J Bone Metab 2013; 20(1): 1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mahler DA, Wells CK. Evaluation of clinical methods for rating dyspnea. Chest 1988; 93(3): 580–586. [DOI] [PubMed] [Google Scholar]
- 23. Roberts HC, Denison HJ, Martin HJ, et al. A review of the measurement of grip strength in clinical and epidemiological studies: towards a standardised approach. Age Ageing 2011; 40(4): 423–429. [DOI] [PubMed] [Google Scholar]
- 24. Jitapunkul S, Kamolratanakul P, Ebrahim S. The meaning of activities of daily living in a Thai elderly population: development of a new index. Age Ageing 1994; 23(2): 97–101. [DOI] [PubMed] [Google Scholar]
- 25. Limpawattana P, Kotruchin P, Pongchaiyakul C. Sarcopenia in Asia. Osteop Sarcop 2015; 1: 92–97. [Google Scholar]
- 26. Chirawatkul A. Biostatistics for Medical Sciences. Khon Kaen: Klang Na Na Wittaya, 2008. [Google Scholar]
- 27. Byun MK, Cho EN, Chang J, et al. Sarcopenia correlates with systemic inflammation in COPD. Int J Chron Obstruct Pulmon Dis 2017; 12: 669–675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Beaudart C, Rizzoli R, Bruyere O, et al. Sarcopenia: burden and challenges for public health. Arch Public Health 2014; 72(1): 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Lerario MC, Sachs A, Lazaretti-Castro M, et al. Body composition in patients with chronic obstructive pulmonary disease: which method to use in clinical practice? Br J Nutr 2006; 96(1): 86–92. [DOI] [PubMed] [Google Scholar]
- 30. Steiner MC, Barton RL, Singh SJ, et al. Bedside methods versus dual energy X-ray absorptiometry for body composition measurement in COPD. Eur Respir J 2002; 19(4): 626–631. [DOI] [PubMed] [Google Scholar]
- 31. Cesari M, Pedone C, Chiurco D, et al. Physical performance, sarcopenia and respiratory function in older patients with chronic obstructive pulmonary disease. Age Ageing 2012; 41(2): 237–241. [DOI] [PubMed] [Google Scholar]
- 32. Kim TN, Park MS, Yang SJ, et al. Prevalence and determinant factors of sarcopenia in patients with type 2 diabetes: the Korean Sarcopenic Obesity Study (KSOS). Diabetes Care 2010; 33(7): 1497–1499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Auyeung TW, Lee SW, Leung J, et al. Age-associated decline of muscle mass, grip strength and gait speed: a 4-year longitudinal study of 3018 community-dwelling older Chinese. Geriatr Gerontol Int 2014; 14(Suppl 1): 76–84. [DOI] [PubMed] [Google Scholar]
- 34. Ischaki E, Papatheodorou G, Gaki E, et al. Body mass and fat-free mass indices in COPD: relation with variables expressing disease severity. Chest 2007; 132(1): 164–169. [DOI] [PubMed] [Google Scholar]
- 35. Stenton C. The MRC breathlessness scale. Occup Med (Lond) 2008; 58(3): 226–227. [DOI] [PubMed] [Google Scholar]
- 36. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, et al. Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing 2010; 39(4): 412–423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Lee DW, Choi EY. Sarcopenia as an independent risk factor for decreased BMD in COPD patients: Korean national health and nutrition examination surveys iv and V (2008-2011). PLoS One 2016; 11(10): e0164303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. DePew ZS, Karpman C, Novotny PJ, et al. Correlations between gait speed, 6-min walk distance, physical activity, and self-efficacy in patients with severe chronic lung disease. Respir Care 2013; 58(12): 2113–2119. [DOI] [PMC free article] [PubMed] [Google Scholar]


