Abstract
The purpose of this exploratory research was to describe current referral and practice patterns for behavioral cough suppression therapy (BCST) throughout the United States, and to assess the need for improving the efficiency of BCST referral patterns. In study I, 126 speech-language pathologists, who treat patients with refractory chronic cough (RCC) in the United States, completed a survey about referral patterns, cough duration, and patient frustration level. In study II, 36 adults with RCC referred for BCST completed a four-part survey about cough symptoms and treatment. The survey included the Leicester Cough Questionnaire (LCQ) before and after BCST. Study I revealed significant patient frustration about the treatment process and the wait-time for BCST. Participants in study II reported average cough duration of over 2 years before BCST. Twenty-seven of 31 participants in study II improved by at least 1.3 on the LCQ, indicating a clinically significant improvement in 87% of patients. This study suggests that the current management model for CC may be unduly time-consuming, and expensive for patients with CC who are successfully treated with BCST. Practitioners are encouraged to consider BCST earlier in the treatment process.
Keywords: Chronic cough, refractory chronic cough, behavioral cough suppression therapy (BCST), Leicester Cough Questionnaire, cough hypersensitivity syndrome (CHS), speech-language pathology
Introduction
Chronic cough (CC) is estimated to effect approximately 12% of the population and is one of the most common complaints for which patients seek medical care.1–6 It accounts for over 20 million physician visits per year3 and has a significant impact on quality of life.7,8 After ruling out the most overt causes of cough (i.e. smoking, angiotensin-converting-enzyme (ACE) inhibitor medications, chronic obstructive pulmonary disease (COPD)/chronic bronchitis, and pulmonary lesion), management typically begins with empiric pharmacologic treatment for the most common causes of CC—upper airway cough syndrome (also known as postnasal drip (PND)), cough-variant asthma, eosinophilic bronchitis, and/or gastroesophageal reflux (GERD).9–12 Patients who do not respond to one or a combination of these treatments often face extensive medical testing including pulmonary function tests, chest and/or sinus computed tomography, laryngoscopy, bronchoscopy, allergy testing, and 24-h pH monitoring.11,13–15 Despite extensive testing, approximately 20% of patients have a cough that is refractory to medical treatment.1,16–19
Behavioral treatment has been shown to be effective in up to 85% of patients with refractory CC (RCC).20–24 This therapy, which we term behavioral cough suppression therapy (BCST), is typically provided by speech-language-pathologists (SLPs) in the United States, and has been shown to reduce cough severity and frequency,20,23,25 to improve quality of life20,21,26, and to reduce cough sensitivity.21,22,27 BCST involves four components: (1) education (e.g. physiology of cough and the larynx, lack of benefit and negative side effects of nonproductive coughing, cough (or laryngeal) hypersensitivity as a potential cause of the cough, and safety and rationale for cough suppression); (2) instruction in vocal hygiene including avoidance of laryngeal irritants and known cough triggers; (3) instruction in cough suppression strategies; and (4) psychoeducational counseling (e.g. that control is possible, treatment is hard work).20,22,25 Patients typically experience significant relief of cough symptoms within 1 to 2 weeks.22,28–30 Despite established efficacy, and the noninvasive, efficient, and inexpensive nature of BCST, it is rarely considered as an early treatment option.28,31
The goal of this qualitative exploratory research was to describe current referral and practice patterns of BCST throughout the United States, and to assess patients’ perceptions of these patterns and the need for improving the efficiency of BCST referral patterns. The research commenced in two studies. Study I consisted of a survey of SLPs in the United States who treat patients with RCC. Study II consisted of a survey of patients with RCC referred for BCST.
Methods
Study design
A web-based survey methodology was used for both studies. The University of Montana Institutional Review Board approved each study. Study I data were collected anonymously via Survey Monkey Internet application in the fall of 2014. Study II data were collected via Qualtrics Internet application from July 2015 to January 2016.
Participants
One hundred twenty six American Speech Hearing Association (ASHA) certified SLPs in 36 different states across the United States, who treat patients with RCC, participated in study I. Thirty-six adult participants with RCC referred for BCST at two separate SLP clinics in the northwestern United States, without a history of smoking, diagnosis of COPD, lung cancer, chronic bronchitis, or emphysema, and not currently on an ACE-inhibitor medication, participated in study II.
Study I (SLP survey) procedures
Study I participants were recruited via ASHA Special Interest Groups, 13 (Swallowing Disorders) and 3 (Voice Disorders) listserves. These listserves consist of approximately 10,000 and 2200 ASHA certified SLPs, with special interest in swallowing and/or voice disorders, respectively. The listserve postings simply asked SLPs, who regularly treat patients for RCC, to complete a short survey, which was accessible via a hyperlink. The purpose of the survey was not mentioned in the post, to reduce the chance of responder bias. Participating SLPs answered nine questions pertaining to referral and practice patterns, patient frustration, symptoms, and treatment success. The questions were developed by the first author for the purpose of the current study. All questions were multiple-choice with one allowable answer, except questions #2 (typical physician type to refer for BCST) and #9 (common symptoms in patients who respond to BCST), which were “choose all that apply.” Questions and the multiple-choice options are shown in Table 1.
Table 1.
Questions and results of SLP survey.a
| Question | Multiple choice options and results |
|---|---|
| 1. Approximately how many patients with CC do you treat each month? |
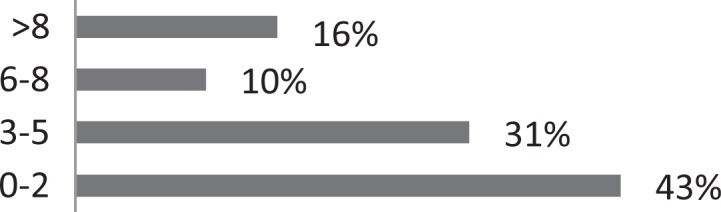
|
| 2. What type of physician typically refers patients with CC to you? (pick all that apply) |
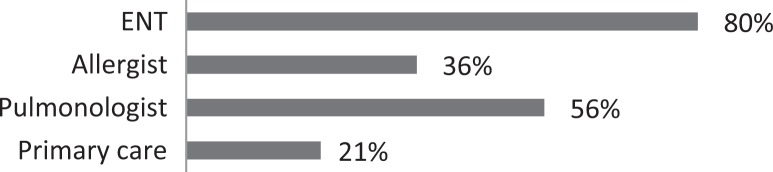
|
| 3. How many physicians have your patients with CC typically seen before they are referred to you? |
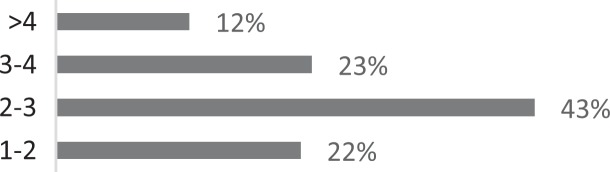
|
| 4. Are your patients frustrated about how many physicians and/or medical tests they have had and how long it took before they came to see you? |
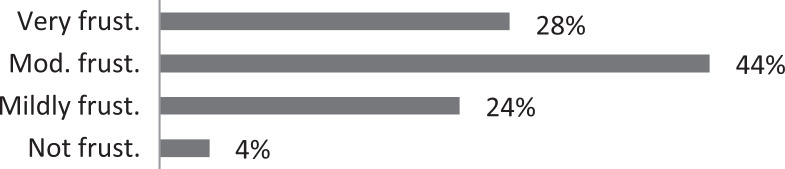
|
| 5. How many months, on average, have your patients been suffering from CC? |
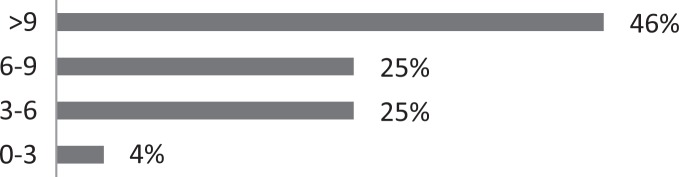
|
| 6. How many of your patients would you estimate respond well to your treatment? |
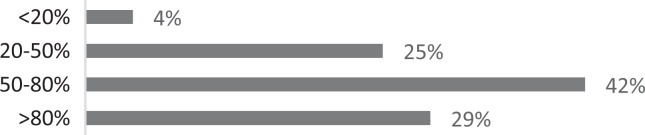
|
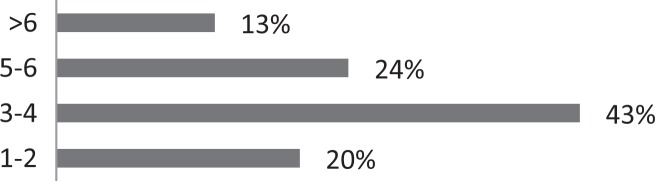
| 7. How many sessions do you typically need to see patients with CC? |
|
| 8. How long does it typically take before patients with CC experience significant relief of their symptoms following onset of treatment? |
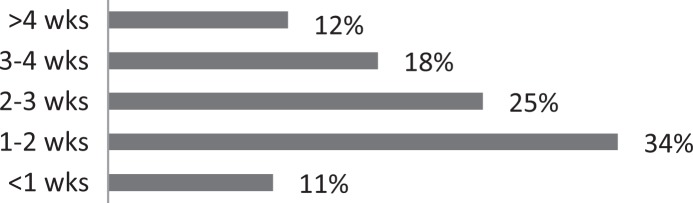
|
| 9. Which of the following symptoms are common in your patients with CC that respond well to treatment? (pick all that apply) |
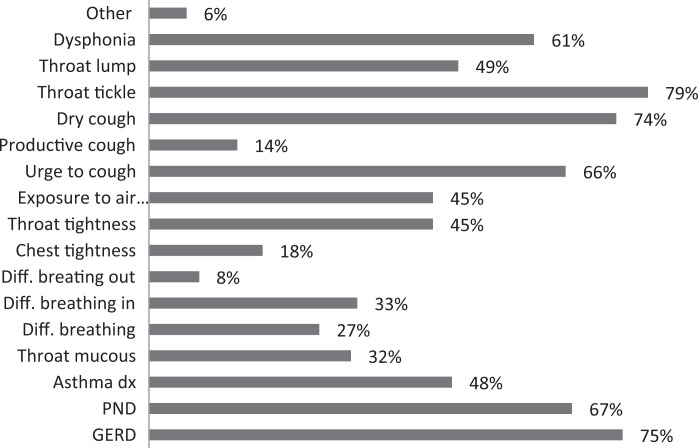
|
SLP: speech-language-pathologists; Diff: difficulty; dx: diagnosis; Exposure to air…: exposure to air pollution; frus: frustrated; GERD: gastroesophageal reflux disease; PND: postnasal drip; CC: chronic cough.
aMultiple-choice options on the x-axis.
Study II (patient survey) procedures
Study II consisted of a four-part survey that was given to patients before and after BCST.
The patient survey had four sections. Only data from sections 1 and 3, and two questions from section 4 (i.e. effectiveness of BCST and overall satisfaction with current cough status) were included in the current analysis. Specific questions included in sections 1 and 4 are shown in Appendix A.
Section 1: Demographic questions including name, date of birth, gender, and contact information, as well as one question about cough duration prior to BCST referral.
Section 2: Thirty eight questions designed by an expert panel for a potential future BCST screening tool.
Section 3: Leicester Cough Questionnaire (LCQ; validated survey for assessing cough-related quality of life)7,32
Section 4: Questions pertaining to prior treatment (e.g. medications), compliance to medical and BCST recommendations, effectiveness of treatment, and overall satisfaction with current cough status.
The entire survey was piloted with two unfamiliar, healthy individuals to ensure clarity of the instructions, the questions, and the flow of the survey itself. Minor modifications were made to the survey per the recommendations of these individuals.
Patients enrolled via an iPad or computer at two participating SLP clinics on the first day of BCST. Following enrollment in the study, participants’ care proceeded as determined by the treating SLP. Both SLPs provided the four components of BCST (as described above) in a similar manner and with similar terminology. Treating SLPs encouraged participants to comply with current physician recommendations. When appropriate, treating SLPs also provided education on strategies to reduce PND (e.g. nasal saline rinse) and dietary and behavioral strategies to reduce GERD.
Following discharge from BCST, patients were contacted by phone, text, and/or e-mail to collect posttreatment data. Posttreatment data included sections 3 and 4 of the enrollment survey. Only section 3 (i.e. LCQ data), and two questions from section 4 (i.e. Rate the effectiveness of your behavioral cough strategies during the past week (1 = not at all effective; 7 = completely effective); and, Are you satisfied with your current status regarding your cough? (yes/no) were used for the current analysis. LCQ-change scores were calculated for each participant by subtracting the pre-BCST LCQ total score from the post-BCST LCQ total score. LCQ-change scores were used to determine success of BCST, with a minimum change of 1.3 considered as a clinically significant improvement, as described by Raj et al.33
Data analysis
Statistical analyses were performed using Statistical Package for the Social Sciences software version 23. Study I data were analyzed using descriptive statistics. Study II data were primarily analyzed with descriptive statistics. The Mann Whitney U test was used to analyze differences in age and cough duration between gender groups.
Results
Study I: SLP survey
About 26% of respondents to the SLP survey reported treating six or more patients with RCC per month; 31% reported treating 3 to 5 per month. The remaining 43% reported treating 0 to 2 patients per month. SLPs reported receiving referrals commonly from otolaryngologists (80.3%) and pulmonologists (55.7%). About 42% of SLPs reported their patients see, on average, 2 to 3 physicians before receiving an SLP referral; 35% reported their patients see three or more physicians, prior to SLP referral. About 46% of SLPs reported that the majority of their patients wait over 9 months before receiving a referral for BCST; 25% reported an average delay of 6 to 9 months. Approximately 70% reported their patients are moderately or very frustrated about the number of physicians and/or medical tests completed before a referral to the SLP is made. Furthermore, the majority (63%) of SLPs reported typically seeing patients with RCC for no more than four sessions, and 87% reported their patients that respond to BCST are significantly improved within 4 weeks of starting BCST. Over 60% of SLPs reported that the following patient-reported symptoms are common in patients who respond well to BCST: heartburn/reflux, PND, throat mucous, feeling of eminent coughing spell, dry cough, tickle in the throat, and dysphonia. The full results of the SLP survey can be seen in Table 1.
Study II: Patient survey
Thirty females and six males with RCC enrolled in study II. The average age of each gender group was 55 years and 42 years, respectively—a statistically significant difference (U = 39.00, z = −2.10, and p = 0.035). The calculated average cough duration was 28.9 and 37.2 months per gender group, respectively—an insignificant difference (U = 61.5, z = −0.922, and p = 0.357). The actual average cough duration of the sample was likely higher, given that eight participants were unable to recall the exact duration of their cough, and, rather, reported something like, “for years,” or “at least 5 years.” These patients were given a cough duration score of 60 months. All demographic data are shown in Table 2.
Table 2.
Study II participant characteristics.
| Females (N = 30) | Males (N = 6) | ||||||
|---|---|---|---|---|---|---|---|
| Min | Max | Mean (SD) | Min | Max | Mean (SD) | p-Value | |
| Age (years) | 22 | 78 | 55.5 (13.0) | 25 | 65 | 42.0 (14.2) | 0.035 |
| Cough duration (months) | 3.0 | 108 | 28.9 (26.9) | 9.0 | 60 | 37.2 (25.4) | 0.357 |
SD: standard deviation.
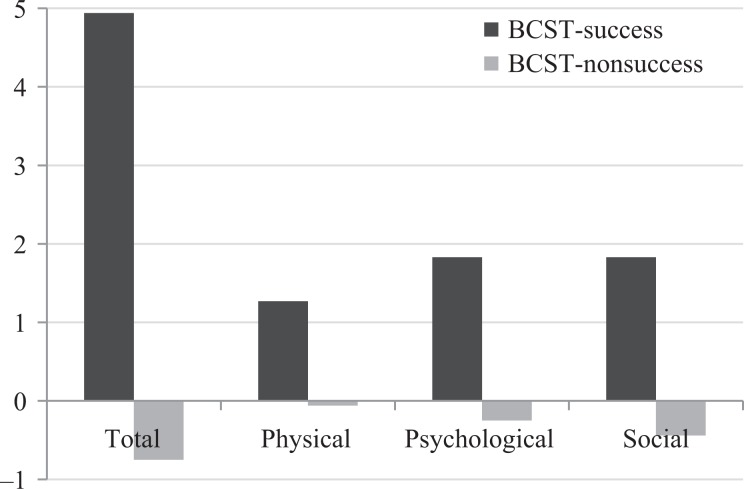
Of the 36 participants with RCC who enrolled in the study II, 31 provided post-BCST data. Of those who provided post-BCST data, 27 improved by at least 1.3 on the LCQ total score, indicating a clinically significant improvement in cough-related quality of life in 87.1% of participants following BCST. The mean change in LCQ total score was 4.94 in the patients with clinically significant improvement, and −0.75 in the four participants without clinically significant improvement. Patients who improved overall also improved on each of the LCQ domain scores, evidenced by mean change scores of 1.27, 1.83, and 1.83, in the physical, psychological, and social domains, respectively. In contrast, mean LCQ domain change scores for the participants who did not improve were −0.06, −0.25, and −0.44, respectively. Of those with a clinically significant improvement, 19(70%) reported they were fully satisfied with their cough status following BCST. The remaining eight participants reported they had improved (evidenced by a score of 5 or 6 on the effectiveness question (i.e. section 4: question #7) but were not fully satisfied. LCQ domain and total change scores are presented in Table 3 and Figure 1.
Table 3.
Change scores for total LCQ score and physical, psychological, and social LCQ domain scores for participants with clinically significant improvement (i.e. BCST-success) and participants without clinically significant improvement (i.e. BCST-non-success).
| Group | LCQ domain | Min | Max | Mean | SD |
|---|---|---|---|---|---|
| BCST-success (N = 27) | Total | 1.32 | 11.36 | 4.94 | 2.92 |
| Physical | −0.500 | 4.5 | 1.27 | 0.98 | |
| Psychological | −0.43 | 4.57 | 1.83 | 1.15 | |
| Social | 0.00 | 4.5 | 1.83 | 1.27 | |
| BCST-non-success (N = 4) | Total | −1.86 | 0.32 | −0.75 | 1.10 |
| Physical | −0.625 | 0.25 | −0.06 | 0.41 | |
| Psychological | −0.86 | 0.57 | −0.25 | 0.62 | |
| Social | −1.25 | 1.25 | −0.44 | 1.18 |
LCQ: Leicester Cough Questionnaire; BCST: behavioral cough suppression therapy; SD: standard deviation.
Figure 1.
Mean LCQ change scores per group for total score, and physical, psychological, and social domain scores. LCQ: Leicester Cough Questionnaire.
Discussion
This work challenges current management models for CC that recommend extensive medical assessment and treatment before considering behavioral therapy.9,11,14,15 Our survey of SLPs across the United States, who treat patients with CC, as well as the patient survey (and several other studies that confirm high levels of success of BCST), suggest that the current management model is unduly time-consuming, frustrating, and expensive for patients with RCC who are successfully treated with BCST. These patients typically wait months to years before ultimately finding relief from a simple, efficient, and inexpensive behavioral treatment. With CC estimated to effect 12% of the population,6 and, conservatively, 10–20% of those patients having RCC,1,16–19 we are talking about 3 to 7 million patients in the United States per year with RCC. Given the high levels of efficacy of BCST for patients with RCC, and the noninvasive, efficient, and inexpensive nature of BCST, we purport BCST should be considered earlier in the treatment process.
At this point, the most appropriate time to consider BCST in the management algorithm of CC is unclear. Given the potential serious medical conditions that can contribute to CC, and that the majority of patients are successfully treated medically, a minimum level of medical assessment and treatment is clearly essential. Further research is needed to determine the risk-benefit ratio of inserting a trial of BCST at different points in the management algorithm. Additionally, a valid screening tool is likely necessary to assist physicians in determining patients who are appropriate candidates for BCST.
Research geared towards the clinical application of cough hypersensitivity testing may also be helpful. The theoretical mechanistic explanation for BCST is that it works by normalizing cough sensory receptors that were hypersensitized due to a neuropathic process following a cough-inducing illness.16,34–36 Specifically, reduction of laryngeal irritants, avoidance of cough triggers, and suppression of cough over time, result in down-modulation of cough-inducing afferent receptors through a neuroplastic mechanism.22,37,38 Support for this theory is provided by Ryan et al., who found that BCST resulted in an increase in capsaicin-induced cough threshold (i.e. reduced cough sensitivity) in patients with RCC.22 If, in fact, BCST works by changing cough hypersensitivity, we would expect BCST to only work for patients with cough hypersensitization, or what Morice et al. termed cough hypersensitivity syndrome (CHS).31,39,40 Hence, the clinical application of cough hypersensitivity testing may be helpful in determining candidates for BCST. The Newcastle Laryngeal Hypersensitivity Questionnaire (NCHQ)38 discriminates patients with several types of laryngeal dysfunction syndromes (including RCC) from healthy controls, and provides further evidence of CHS in patients with RCC. The NCHQ may prove to be helpful in determining candidates for BCST, but it has not yet been tested for such use. Future research is needed to determine if the NCHQ, or a similar tool, can help discriminate candidates for BCST from patients who would be better treated medically.
Until further work is done in this vein, physicians must use their clinical judgment for determining when and who to consider for BCST. However, there is data to guide physicians in the clinical decision-making process. First, RCC often co-occurs with paradoxical vocal fold movement,41 which has several identifiable symptoms including dyspnea, audible inhalation, inspiratory stridor, tightness in neck muscles, sensation of restriction in the larynx/throat, tendency for more difficulty breathing in than out, and quick or no response to rescue inhalers. Muscle tension dysphonia also often co-occurs with RCC,41 the symptoms of which include strained voice quality, unpredictable voice pattern (e.g. short or long periods of normal voicing without explanation). Patients who present with any of these symptoms may be good candidates for BCST. Finally, physicians may also want to consider the increasingly long wait times to see specialists in the United States when considering BCST. In many areas, patients may be able to complete a trial of BCST before they are able to see a specialist. In these cases, it may be appropriate to refer patients to a speech-language pathologist trained in BCST and a specialist at the same time. If the patient is successfully treated with BCST prior to the specialist appointment, the specialist consultation may be unnecessary.
Limitations and future directions
The primary limitation to this study is the qualitative and exploratory nature of the design, which limits the inferences that can be made from the results. The biggest limitation of study I is the lack of objective measures. All data were obtained via recall of SLPs who treat patients with CC, which most certainly contributes to a level of error in the data. Any conclusions drawn from the data, therefore, must be taken with caution. There are three main limitations of study II. First, the sample size is relatively small. Second, the lack of a control group negates the ability to determine the impact of a placebo effect. Lastly, we did not gather repeat data from patients who reported they were satisfied with BCST. We, therefore, do not know if improvements following BCST were long-standing. However, prior studies on behavioral cough treatment, which have looked at sustainability of effect from 8 weeks to 18 weeks following treatment, have found no degradation of treatment effect.21,22,26,27
The data from study II were used to estimate sample size for a study designed to create a valid screening tool for identifying candidates for BCST, which is currently underway. The project includes patients treated with BCST as well as patients successfully treated with medical management alone in order to determine which screening questions are related to success of medical versus behavioral treatment.
Acknowledgements
The authors would like to thank the following experts who assisted with creation of the screening items and with recruitment for the study: Carol Cady, MD, Eric Stern, MD, Jeffrey Haller, MD, and Lynn Harris, MS, CCC-SLP.
Appendix A: Patient Survey
Section 1: Demographic Questions
Please enter your first name.
Please enter your last name.
-
Please choose your gender.
Male
Female
Other
Please enter your date of birth.
Please enter your phone number including area code.
Please enter your email address. We promise we will not send you anything other than information specific to this study. We will not give anyone your email address. If you do not have an email address, leave blank.
-
Which form of communication do you prefer?
Phone
Text message
Email
Any of these forms are fine
-
Do you have access to the internet? (If you do not, we will plan to gather data with you over the phone.)
Yes
No
Please enter how long ago your cough started.
Please enter the professional that referred you to this survey.
Section 2: Potential Screening Items (not included in this analysis)
Section 3: Leicester Cough Questionnaire
Section 4: Prior Treatment, Compliance, and Effectiveness of Treatment
-
1. Are you now or have you within that last 2 weeks taken a prescribed medication for your cough (including medication for post-nasal drip, reflux, sinus problems)?
Yes
No
2. Which of the following medications have you taken in the past 2 weeks and how long have you been taking each? (leave blank if not applicable)
| Medication for post-nasal drip (1) | ○ <1 week | ○ 1-2 weeks | ○ 3-4 weeks | ○ >4 weeks |
| Medication for reflux (2) | ○ <1 week | ○ 1-2 weeks | ○ 3-4 weeks | ○ >4 weeks |
| Medication for asthma (3) | ○ <1 week | ○ 1-2 weeks | ○ 3-4 weeks | ○ >4 weeks |
| Sinus rinse (4) | ○ <1 week | ○ 1-2 weeks | ○ 3-4 weeks | ○ >4 weeks |
| Other medication for cough (please explain): (5) | ○ <1 week | ○ 1-2 weeks | ○ 3-4 weeks | ○ >4 weeks |
3. How compliant have you been with taking your medication as prescribed?
| Not very compliant | Somewhat compliant (50-70% of the time) | Fairly compliant (70-90% of the time) | Very compliant (>90% of the time) | |
|---|---|---|---|---|
| Choose one (1) | ○ | ○ | ○ | ○ |
4. On a scale from 1-7, please rate the effectiveness of the medication in the past week in regards to your cough.
| 1 (not at all effective) | 2 | 3 | 4 | 5 | 6 | 7 (completely effective) | |
| Please rate your answer (1) | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
-
5. Are you now, or have you within the last 2 weeks, used behavioral strategies recommended by a speech-language pathologist to manage your cough?
Yes
No
6. How compliant have you been with following the behavioral strategies as prescribed by your speech-language pathologist?
| Not very compliant | Somewhat compliant (50-70% of the time) | Fairly compliant (70-90% of the time) | Very compliant (>90% of the time) | |
|---|---|---|---|---|
| Choose one (1) | ○ | ○ | ○ | ○ |
7. On a scale from 1-7, please rate the effectiveness of behavioral cough strategies during the past week.
| 1 (not at all effective) | 2 | 3 | 4 | 5 | 6 | 7 (completely effective) | |
|---|---|---|---|---|---|---|---|
| Please rate your answer (1) | ○ | ○ | ○ | ○ | ○ | ○ | ○ |
-
8. Are you satisfied with your current status in regards to your cough?
Yes
No
Footnotes
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: This research was supported by the Mountain West Clinical Translational Research—Infrastructure Network, under a grant from the National Institute of General Medical Sciences.
References
- 1. Chung KF, Pavord ID. Prevalence, pathogenesis, and causes of chronic cough. Lancet 2008; 371: 1364–1374. [DOI] [PubMed] [Google Scholar]
- 2. Ford AC, Forman D, Moayyedi P, et al. Cough in the community: a cross sectional survey and the relationship to gastrointestinal symptoms. Thorax 2006; 61: 975–979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Irwin RS, Boulet LP, Cloutier MM, et al. Managing cough as a defense mechanism and as a symptom. A consensus panel report of the American College of Chest Physicians. Chest 1998; 114: 133S–181S. [DOI] [PubMed] [Google Scholar]
- 4. Natt RS, Earis JE, Swift AC. Chronic cough: a multidisciplinary approach. J Laryngol Otol 2012; 126: 441–444. [DOI] [PubMed] [Google Scholar]
- 5. Polverino M, Polverino F, Fasolino M, et al. Anatomy and neuro-pathophysiology of the cough reflex arc. Mulitdiscip Respir Med 2012; 7: 1–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Morice A. Chronic cough: epidemiology. Chron Respir Dis 2008; 5: 43–47. [DOI] [PubMed] [Google Scholar]
- 7. Brignall K, Jayaraman B, Birring SS. Quality of life and psychosocial aspects of cough. Lung 2008; 186 Suppl 1: S55–S58. [DOI] [PubMed] [Google Scholar]
- 8. French CL, Irwin RS, Curley FJ, et al. Impact of chronic cough on quality of life. Arch Intern Med 1998; 158: 1657–1661. [DOI] [PubMed] [Google Scholar]
- 9. Iyer VN, Lim KG. Chronic cough: an update. Mayo Clin Proc 2013; 88: 1115–1126. [DOI] [PubMed] [Google Scholar]
- 10. Lin L, Poh KL, Lim TK. Empirical treatment of chronic cough–a cost-effectiveness analysis. Proc AMIA Symp 2001; 383–387. [PMC free article] [PubMed] [Google Scholar]
- 11. Morice AH, Fontana GA, Sovijarvi AR, et al. The diagnosis and management of chronic cough. Eur Respir J 2004; 24: 481–492. [DOI] [PubMed] [Google Scholar]
- 12. Pratter MR. Unexplained (idiopathic) cough: ACCP evidence-based clinical practice guidelines. Chest 2006; 129: 220S–221S. [DOI] [PubMed] [Google Scholar]
- 13. Kastelik JA, Aziz I, Ojoo JC, et al. Investigation and management of chronic cough using a probability-based algorithm. Eur Respir J 2005; 25: 235–243. [DOI] [PubMed] [Google Scholar]
- 14. Irwin RS, Baumann MH, Bolser DC, et al. Diagnosis and management of cough executive summary: ACCP evidence-based clinical practice guidelines. Chest 2006; 129: 1S–23S. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Pratter MR, Brightling CE, Boulet LP, et al. An empiric integrative approach to the management of cough: ACCP evidence-based clinical practice guidelines. Chest 2006; 129: 222S–231S. [DOI] [PubMed] [Google Scholar]
- 16. Chung KF. Chronic ‘cough hypersensitivity syndrome’: a more precise label for chronic cough. Pulm Pharmacol Ther 2011; 24: 267–271. [DOI] [PubMed] [Google Scholar]
- 17. Haque RA, Usmani OS, Barnes PJ. Chronic idiopathic cough a discrete clinical Entity? Chest 2005; 127: 1710–1713. [DOI] [PubMed] [Google Scholar]
- 18. McGarvey LP, Heaney LG, Lawson JT, et al. Evaluation and outcome of patients with chronic non-productive cough using a comprehensive diagnostic protocol. Thorax 1998; 53: 738–743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Poe RH, Harder RV, Israel RH, et al. Chronic persistent cough experience in diagnosis and outcome using an anatomic diagnostic protocol. Chest 1989; 95: 723–728. [DOI] [PubMed] [Google Scholar]
- 20. Patel AS, Watkin G, Willig B, et al. Improvement in health status following cough-suppression physiotherapy for patients with chronic cough. Chron Respir Dis 2011; 8: 253–258. [DOI] [PubMed] [Google Scholar]
- 21. Ryan NM, Vertigan AE, Gibson PG. Chronic cough and laryngeal dysfunction improve with specific treatment of cough and paradoxical vocal fold movement. Cough 2009; 5: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Ryan NM, Vertigan AE, Bone S, et al. Cough reflex sensitivity improves with speech language pathology management of refractory chronic cough. Cough 2010; 6: 5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Soni R, Ebersole B, Jamal N. Treatment of chronic cough: single-institution experience utilizing behavioral therapy. Otolaryngol Head Neck Surg 2016; 156: 1–6. [DOI] [PubMed] [Google Scholar]
- 24. Vertigan AE, Theodoros DG, Winkworth AL, et al. Perceptual voice characteristics in chronic cough and paradoxical vocal fold movement. Folia Phoniatr Logop 2007; 59: 256–267. [DOI] [PubMed] [Google Scholar]
- 25. Vertigan AE, Theodoros DG, Gibson PG, et al. Efficacy of speech pathology management for chronic cough: a randomised placebo controlled trial of treatment efficacy. Thorax 2006; 61: 1065–1069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Chamberlain Mitchell SA, Garrod R, Clark L, et al. Physiotherapy, and speech and language therapy intervention for patients with refractory chronic cough: a multicentre randomised control trial. Thorax 2017; 72: 129–136. [DOI] [PubMed] [Google Scholar]
- 27. Vertigan A, Kapela SL, Ryan NM, et al. Pregabalin and speech pathology combination therapy for refractory chronic cough: A randomized controlled trial. Chest 2016; 149: 639–648. [DOI] [PubMed] [Google Scholar]
- 28. Chamberlain S, Garrod R, Birring SS. Cough suppression therapy: does it work? Pulm Pharmacol Ther 2013; 26: 524–527. [DOI] [PubMed] [Google Scholar]
- 29. Gibson PG, Vertigan AE. Speech pathology for chronic cough: a new approach. Pulm Pharmacol Ther 2009; 22: 159–162. [DOI] [PubMed] [Google Scholar]
- 30. Vertigan AE, Theodoros DG, Gibson PG, et al. Review series: chronic cough: behaviour modification therapies for chronic cough. Chron Respir Dis 2007; 4: 89–97. [DOI] [PubMed] [Google Scholar]
- 31. Morice AH, Faruqi S, Wright CE, et al. Cough hypersensitivity syndrome: a distinct clinical entity. Lung 2011; 189: 73–79. [DOI] [PubMed] [Google Scholar]
- 32. Birring SS, Prudon B, Carr AJ, et al. Development of a symptom specific health status measure for patients with chronic cough: Leicester Cough Questionnaire (LCQ). Thorax 2003; 58: 339–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Raj AA, Pavord DI, Birring SS. Clinical cough IV: what is the minimal important difference for the Leicester Cough Questionnaire? In: Chung KF, Widdicombe J. (eds) Pharmacology and Therapeutics of Cough Handb Exp Pharmacol. Springer, Berlin, Heidelberg, 2009, pp. 311–320. [DOI] [PubMed] [Google Scholar]
- 34. Adcock JJ. TRPV1 receptors in sensitisation of cough and pain reflexes. Pulm Pharmacol Ther 2009; 22: 65–70. [DOI] [PubMed] [Google Scholar]
- 35. Groneberg DA, Niimi A, Dinh QT, et al. Increased expression of transient receptor potential vanilloid-1 in airway nerves of chronic cough. Am J Respir Crit Care Med 2004; 170: 1276–1280. [DOI] [PubMed] [Google Scholar]
- 36. Lee LY, Gu Q. Role of TRPV1 in inflammation-induced airway hypersensitivity. Curr Opin Pharmacol 2009; 9: 243–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Murry T, Branski RC, Yu K, et al. Laryngeal sensory deficits in patients with chronic cough and paradoxical vocal fold movement disorder. Laryngoscope 2010; 120: 1576–1581. [DOI] [PubMed] [Google Scholar]
- 38. Vertigan AE, Bone SL, Gibson PG. Development and validation of the Newcastle Laryngeal Hypersensitivity Questionnaire. Cough 2014; 10: 1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Morice AH. The cough hypersensitivity syndrome: a novel paradigm for understanding cough. Lung 2010; 188 Suppl 1: S87–S90. [DOI] [PubMed] [Google Scholar]
- 40. Morice AH. Chronic cough hypersensitivity syndrome. Cough 2013; 9: 14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Vertigan AE, Theodoros DG, Gibson PG, et al. Voice and upper airway symptoms in people with chronic cough and paradoxical vocal fold movement. J Voice 2007; 21: 361–383. [DOI] [PubMed] [Google Scholar]