Abstract
Aspergillus fumigatus (AF) is a ubiquitous fungus in our environment and causes severe airway disorders. Chronic respiratory diseases (CRDs) are a series of chronic airway and lung diseases. Although both are chronic disorders, however, the relationships between AF and CRDs are still unclear. Therefore, we examined 104 Aspergillus species (spp.) isolated samples in our hospital during three consecutive years to further elucidate the relationships between Aspergillus spp. and CRDs. Based on sample isolates, we then grouped these into two groups, AF and non-AF, to retrospectively analyse the clinical features and to clarify the relationships between AF and CRDs. Importantly, the manifestation of CRD was more frequent in the AF group than in the non-AF group (p = 0.035). Among CRDs, lung fibrosis was more evident in the AF group (p = 0.025). Moreover, diabetes mellitus was tended to be evident in AF group than non-AF group (p = 0.035). In conclusion, CRDs, especially lung fibrosis, were highly prevalent in AF group than non-AF group.
Keywords: Aspergillus fumigatus, chronic respiratory disease, lung fibrosis, COPD, asthma
Introduction
Inhalation of various pathogenic microorganisms, such as bacteria, virus and fungi, can affect our health by damaging airways. Among pathogenic microorganisms, fungi are now known to cause a wide range of disorders from infection to allergy, and more importantly, fungal-related diseases are on the rise.1 In particular, Aspergillus species (spp.) commonly contaminate sputum and laboratory specimens exposed to unfiltered air2 and cause severe clinical symptoms in the upper and lower airways.3 Importantly, airway disorders caused by Aspergillus spp. have been increasing globally in the last several decades.4
Among Aspergillus spp., Aspergillus fumigatus (AF) is the most frequently isolated species5 and infection by AF may result in a fatal disease course.6 It was previously described that AF-activated bronchial epithelial cells induce inflammatory cytokines and chemokines and AF-derived proteases may damage bronchial epithelial cells and other airway structures.7 In addition to AF-induced changes, AF produces various toxins and among these, gliotoxin has been shown to inhibit nuclear factor-kappa B (NF-κB), leading to impaired airway mucosal function as a result of reduced levels of anti-fungal proteins produced by bronchial epithelial cells.8,9 In the clinical settings, Taccone et al. reported that AF was isolated in 92% of critically ill patients who were diagnosed with invasive pulmonary aspergillosis (IPA)6 and Tashiro et al. demonstrated that AF was detected in 54% of chronic necrotizing pulmonary aspergillosis (CNPA) cases.10 Moreover, elevated specific IgE level against AF was an important diagnostic criteria for allergic bronchopulmonary mycosis, suggesting that AF plays a role in various spectrum of airway disorders from infection to allergy.11
Chronic respiratory diseases (CRDs) comprise various upper and lower respiratory disorders ranging from asthma, chronic obstructive pulmonary disorders (COPD), lung fibrosis, allergic rhinitis and others.12 Currently, over 300 million people are diagnosed with asthma and 210 million people with COPD globally.13 In addition, it is thought that there are many subjects who are underdiagnosed for CRDs, because progression of CRD is relatively slow compared to other infectious disorders. As for CRDs, World Health Organization (WHO) predicts that COPD will become the third leading cause of death by 2030.12 Among CRDs, idiopathic pulmonary fibrosis (IPF) is the major cause of lung fibrotic disease.14 The prognosis is poor due to frequent occurrence of acute exacerbation and lung cancer may arise as a comorbidity, also treatment drugs are still limited because of complicated pathogenesis.15
Recently, fungal colonization or infection are thought to play a major role in the initiation and exacerbation of the CRDs.16 In healthy individuals, macrophages and ciliated bronchial epithelial cells in the upper and lower airways efficiently clear fungal conidia. However, these clearance mechanisms could be impaired not only in immunocompromised hosts but also in CRD comorbid patients as well.
We therefore hypothesized that Aspergillus spp., especially AF, may affect CRD conditions. While there were several clinical reports that Aspergillus spp. may initiate or exacerbate upper and lower airway disorders, little was still known about the importance of Aspergillus spp. isolation in the incidence of CRDs. The aim of this study was to survey the impact of Aspergillus spp. isolation among respiratory specimens, such as sputum and bronchoalveolar lavage (BAL) fluid samples, from CRD patients and compared the differences between the AF and non-AF isolated groups.
Methods
This retrospective study was performed in Showa University Hospital (Tokyo, Japan) from January 2012 to December 2014 to explore the relationships between Aspergillus spp. and CRDs. We reviewed 104 samples with Aspergillus spp. isolates cultured from respiratory samples and classified them into AF and non-AF groups. The samples included 92 sputum and 12 BAL fluid samples. Respiratory samples were obtained by either doctors or nurses with disposable gloves. The indications for fungal culture testing either showed abnormal physical findings, serological testing, chest X-ray or CT scanning, or immunocompromised medical history that indicated fungal infection. Taken samples were cultured on Sabouraud’s dextrose agar and incubated at 30°C for at least 5 days. The non-AF group included Aspergillus niger, Aspergillus terreus and unclassified Aspergillus spp. Additionally, we excluded patients who had multiple isolates caused by different Aspergillus spp. for this current study. This study was performed in accordance with the Declaration of Helsinki. This human study was approved by Showa University Ethics Committee – approval number: 1765.
CRDs were defined by chronic diseases of upper and lower airways and other lung structures based on WHO definition,12 specifically asthma, COPD, bronchiectasis, lung cancer, lung fibrosis, sarcoidosis, chronic rhinosinusitis, pneumoconiosis, hypersensitivity pneumonitis, chronic pleural diseases and sleep apnea syndrome (Online Supplemental Table 1, 2, 3). In this study, lung fibrosis contained mainly IPF14 (45% of the patients. Others were connective tissue disease-associated interstitial lung disease and antineutrophil cytoplasmatic autoantibody-associated vasculitis with usual interstitial pneumonia (UIP) pattern on high resolution CT). Definition of pathogenesis was that patients Aspergillus spp. clinically influenced patients who contracted IPA, CNPA or allergic bronchopulmonary aspergillosis.3,17,18 Detection of any Aspergillus spp. but not leading to pathogenic status was defined as colonization.19 Serological tests and respiratory function tests were measured at the time of Aspergillus spp. isolation.
Statistical analysis was performed using JMP version 11 (SAS Institute, Inc, Tokyo, Japan). All data were shown as mean (±SD), median (range) or number (percentage), as required. The differences between categorical variables were analysed by Fisher’s exact test. A p value of less than 0.05 was considered to be statistically significant.
Results
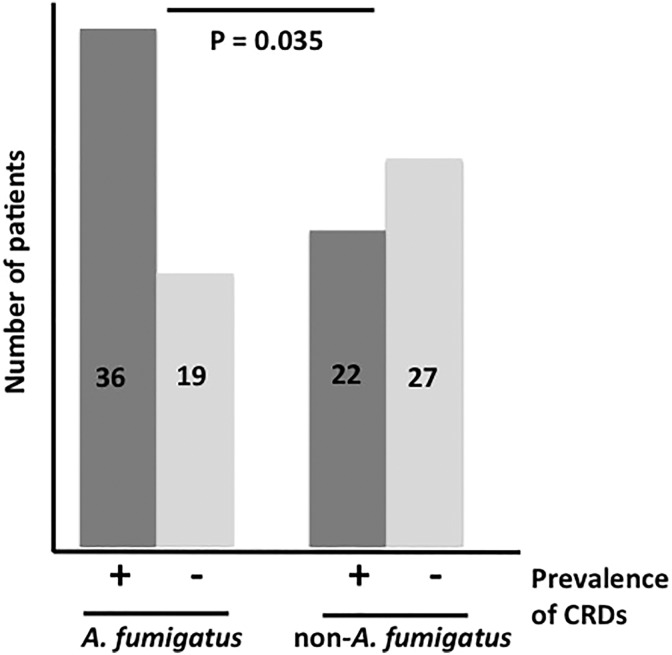
Demographic patients’ characteristics were shown in Table 1. We evaluated 55 AF and 49 non-AF isolated patients. Within the AF group, there was a tendency for more pathogenic/virulrent respiratory disease (p = 0.057). There was no statistical difference in smoking history and blood tests such as β-D glucan assay, Aspergillus galactomannnan antigen, Aspergillus IgG antibody and total IgE. Furthermore, there was no significant difference in administration of inhaled corticosteroid or systemic corticosteroid (Table 1, p = 0.248 and p = 0.817, respectively). In addition, we reviewed the prevalence of comorbidities in each groups. The prevalence of CRDs were significantly higher in the AF group than in the non-AF group (Figure 1, p = 0.035). As comorbidity, diabetes mellitus (DM) was tended to be prevalent in the AF group than the non-AF group (p = 0.071).
Table 1.
Patient characteristics.a
| AF (n = 55) | Non-AF (n = 49) | p Value | |
|---|---|---|---|
| Age (year)b | 73.1 ± 13.6 | 68.3 ± 16.6 | 0.109 |
| Sex, male/ female | 35/20 | 25/24 | 0.194 |
| Inpatient/outpatient | 40/15 | 29/20 | 0.145 |
| Pathogenic/colonization | 16/39 | 6/43 | 0.057 |
| IPA, no. | 6 | 0 | |
| CNPA, no. | 9 | 5 | |
| ABPA, no. | 1 | 1 | |
| Smoking status, pack-yearc | 0 (0–47.0) | 0 (5–40) | 0.490 |
| β-D glucan assay (pg/mL)c | 13.5 (6.6–45.8) | 10.1 (7.55–17.3) | 0.323 |
| Aspergillus galactomannan antigenc | 0.3 (0.1–0.6) | 0.3 (0.1–0.8) | 0.539 |
| Aspergillus antibody (positive/negative) | 7/4 | 0/4 | 0.077 |
| Total IgE (IU/mL)c | 150 (55–328.5) | 106 (8–376) | 0.970 |
| Inhaled corticosteroids, no. (%) | 10 (18.2) | 5 (10.2) | 0.248 |
| Systemic corticosteroids, no. (%) | 18 (32.7) | 15 (40.8) | 0.817 |
| Comorbidities, no. (%) | |||
| Diabetes mellitus | 13 (23.6) | 5 (10.2) | 0.071 |
| Chronic kidney disease | 4 (7.3) | 4 (8.2) | 0.865 |
| Coronary heart disease | 4 (7.3) | 2 (4.1) | 0.486 |
| Hematologic disease | 1 (1.8) | 0 (0) | 0.343 |
| Malignancy (except lung cancer) | 5 (9.0) | 7 (14.3) | 0.408 |
| Autoimmune disease | 1 (1.8) | 2 (4.1) | 0.491 |
| Non-tuberculosis mycobacteriosis | 4 (7.3) | 2 (4.1) | 0.486 |
AF: Aspergillus fumigatus; IPA: invasive pulmonary aspergillosis; CNPA: chronic necrotizing pulmonary aspergillosis; ABPA: Allergic bronchopulmonary aspergillosis.
aAF group was tended to be pathogenic (p = 0.057). In addition, diabetes mellitus was tended to be prevalent in AF group than non-AF group as comorbidity (p = 0.071).
bData are presented as mean ± SD
cData are presented as median (range).
Figure 1.
The prevalence of CRDs in AF and non-AF group. Thirty-six of 57 patients in the AF group had CRDs (63%) while 45% was observed in the non-AF group. The prevalence of CRDs was significantly higher in the AF group than in the non-AF group. CRDs: chronic respiratory diseases; AF: Aspergillus fumigatus.
The distribution of CRDs were different between AF and non-AF groups (Table 2). Among CRDs, lung fibrosis was more prevalent in the AF group than in the non-AF group (p = 0.025). Otherwise, significant differences were not observed in other CRDs comparing between the two groups.
Table 2.
The prevalence of each CRDs in AF and non-AF group.a
| CRDs, no. (%) | AF (n = 55) | Non-AF (n = 49) | p Value |
|---|---|---|---|
| Asthma | 10 (18.2) | 6 (12.2) | 0.402 |
| COPD | 14 (25.5) | 12 (24.5) | 0.908 |
| Bronchiectasis | 4 (7.3) | 3 (6.1) | 0.815 |
| Lung cancer | 5 (9.1) | 3 (6.1) | 0.578 |
| Lung fibrosis | 10 (16.4) | 2 (4.1) | 0.025 |
| Sarcoidosis | 1 (1.8) | 0 (0) | 0.343 |
| Chronic rhinosinusitis | 2 (3.6) | 0 (0) | 0.178 |
| Pneumoconiosis | 1 (1.8) | 0 (0) | 0.343 |
| Hypersensitivity pneumonitis | 0 (0) | 1 (2.0) | 0.287 |
| Chronic pleural diseases | 2 (3.6) | 0 (0) | 0.178 |
| Sleep apnea syndrome | 0 (0) | 0 (0) | 1.000 |
CRDs: chronic respiratory diseases; AF: Aspergillus fumigatus; COPD: chronic obstructive pulmonary disorders.
aLung fibrosis was significantly more prevalent in AF group than in non-AF group (p = 0.025).
Discussion
In this retrospective study, we analysed the relationships between Aspergillus spp., especially AF and CRDs.
Currently, over one billion people suffer from CRDs, especially in developing countries and CRDs poses serious public problems. Although most of risk factors for CRDs, such as smoking, occupational agents and indoor air pollution, are preventable, diagnosis and management of CRDs are still insufficient even in the well-developed countries.20 Studies have shown that AF was the most isolated pathogen in chronic rhinosinusitis, asthma and COPD patients.21,22 Our study showed that the prevalence of CRDs in the AF group was significantly higher than in the non-AF group (Figure 1) and to the best of our knowledge, the current study is the first report showing relationships between Aspergillus spp. and CRDs.
We showed that prevalence of lung fibrosis in the AF group was significantly higher than in the non-AF group. However, limited data exist showing association between AF and lung fibrosis, but recent data sets support our current data. Kurosaki et al. reported that all 15 patients who were diagnosed with pulmonary aspergillosis had interstitial pneumonia and IPF (UIP) was the main manifestation.23 Fibla et al. reported that two out of 296 surgical lung biopsy specimens from interstitial lung disease patients were infected with AF.24 AF-targeted therapy improved two patients’ outcomes, although the portion was minor in the study.24 A placebo-controlled trial was reported that the use of co-trimoxazole in IPF reduced mortality with unknown mechanism.25 Han et al. suggested that Staphylococcus and Streptococcus spp. within the lung microbiome may associate with disease progression of IPF, representing lung fibrosis, but the fungal community among the microbiome was still unknown.26 Other than AF, several groups have demonstrated that viral infection played a role in pathogenesis of IPF.27, 28 Tang et al. revealed that the DNA of one or more of the four herpesviruses (cytomegalovirus, Esptein-Barr Virus (EBV), human herpes virus 7 (HHV-7) and HHV-8) were detected in lung specimen with IPF when compared to controls.29 Relevancy of lower airway AF infection and lung fibrosis is not still unclear, our findings showed further research is warranted.
While there was no statistical difference of AF isolation among COPD patients in our study, past findings by other groups suggested that AF was associated with COPD. Bulpa et al. reported that IPA in COPD patients showed poor prognosis and AF was the most isolated pathogen in IPA.30 In recent study, prevalence of Aspergillus spp. was higher among stage IV COPD patients, defined by Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines.22 Furthermore, they showed that AF was the most frequently isolated species among Aspergillus spp. from COPD patients and isolation of Aspergillus spp. predicted the length of hospitalization after acute exacerbation of COPD.22 Previously, AF induced inflammatory cytokines and concurrently damaged bronchial epithelial cells using in vitro models.9,24 In particular, AF-produced gliotoxin was known as a major metabolite that directly injures airway epithelial cells. It was reported that clinical and environmental AF produced gliotoxin,8 therefore it was reasonable to assume that exposure of gliotoxin might enhance destruction of airway structure among CRD patients. Severity of COPD within our study was milder than the past reports, this may have influenced our results (Online Supplemental Table 2).
Patients in the isolates from the AF group showed tendency towards pathogenesis rather than colonization. It was reported that treatment of AF-related diseases was more frequently conducted in hospitals, rather than in outpatient clinics, due to high mortality rate.19 Therefore, the factor that showed tendency may be due to being biased by clinical standards and judgment, but our data, at least in part, showed the importance of AF when compared to other Aspergillus spp.
Subjects with DM were the most common immunocompromised host. Aspergillosis was less likely to occur in healthy subjects and was related to hospital-acquired infection. Ohba et al. demonstrated that AF was the most isolated species in chronic pulmonary aspergillosis patients and had higher comorbidity rate of DM compared to the colonization group.31 Past reports suggest that DM may affect the respiratory tract immune system leading to AF colonization or further infection of the airways. Our current data showed that DM was tended to be more prevalent in the AF group than in the non-AF group, which was consistent with previous reports and also suggested to launch large scale study to clarify the relation.
Bafadhel et al. reported that patients with AF culture-positive used significant higher doses of inhaled corticosteroid than those with AF culture-negative.32 Also, they concluded neutrophilic airway inflammation was the predictive factor of isolation of AF.32 Similarly, prolonged and high-dose systemic corticosteroid was a risk factor for aspergillosis by promoting growth of AF and further developed clinically severe condition.33 However, our data showed that the ratio of patients with inhaled and systemic corticosteroids use was not significantly higher in the AF group than in the non-AF group and was inconsistent with other previous reports (Table 1). This could be explained by the study population, therefore large population based study is warranted.
Our study has several limitations. First, due to the retrospective nature of our study, all data were obtained from medical records. Therefore, insufficient data and bias may influence our current outcomes. Second, it was possible that other species in non-AF group included AF, since the species were not determined by genotyping. It was also possible that the AF group may have included other spp. Recently, Aspergillus lentulus, Aspergillus udagawae and Aspergillus viridinutans, so called AF-related species, were newly identified and classified.34 All of the newly identified Aspergillus shared most of the features of AF, other than antifungal susceptibility. Due to unstandardized methods of isolation of these newly identified Aspergillus spp., our study was limited to show this results. Third, not all patients underwent respiratory function test since this was retrospective study. Therefore, it may not be representative of the whole population. Fourth, it was possible that Aspergillus spp. including AF was secondary or colonized pathogen. Although, past reports showed that AF were cultured in 43.7% of COPD patients and 68.7% of non-neutropenic and non-transplant patients, but up to date there were no past reports showing differences of contamination rates between AF and non-AF pathogens.35,36
In summary, firstly, this study revealed that CRDs were highly prevalent in AF group than non-AF group. Secondly, prevalence of lung fibrosis was higher in AF group than non-AF group. As we were not able to analyse the clinical outcome of the patients with AF positivity, further research will be required to clarify such outcomes.
Supplemental material
Supplementary Material, Suppelementary_Table_1_(1) for High burden of Aspergillus fumigatus infection among chronic respiratory diseases by Yosuke Fukuda, Tetsuya Homma, Shintaro Suzuki, Takahiro Takuma, Akihiko Tanaka, Takuya Yokoe, Tsukasa Ohnishi, Yoshihito Niki and Hironori Sagara in Chronic Respiratory Disease
Supplemental material
Supplementary Material, Supplementary_Table_2 for High burden of Aspergillus fumigatus infection among chronic respiratory diseases by Yosuke Fukuda, Tetsuya Homma, Shintaro Suzuki, Takahiro Takuma, Akihiko Tanaka, Takuya Yokoe, Tsukasa Ohnishi, Yoshihito Niki and Hironori Sagara in Chronic Respiratory Disease
Supplemental material
Supplementary Material, Supplementary_Table_3 for High burden of Aspergillus fumigatus infection among chronic respiratory diseases by Yosuke Fukuda, Tetsuya Homma, Shintaro Suzuki, Takahiro Takuma, Akihiko Tanaka, Takuya Yokoe, Tsukasa Ohnishi, Yoshihito Niki and Hironori Sagara in Chronic Respiratory Disease
Acknowledgements
The authors gratefully acknowledge the support of the medical technologist of Department of Clinical Laboratory at Showa University Hospital for great assistance and contributions for our study.
Footnotes
Author contributions: YF, TH, SS, TT, YN and HS designed this study. AT, YT and TO conducted the research. YF and SS analysed the data and performed the statistical analyses. YF and TH wrote the manuscript. All authors drafted and reviewed report, and approved the final version for submission of the manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Supplemental material: Supplemental material for this article is available online.
References
- 1. Vallabhaneni S, Mody RK, Walker T, et al. The global burden of fungal diseases. Infect Dis Clin North Am 2016; 30: 1–11. [DOI] [PubMed] [Google Scholar]
- 2. Gheith S, Ranque S, Bannour W, et al. Hospital environment fungal contamination and aspergillosis risk in acute leukaemia patients in Sousse (Tunisia). Mycoses 2015; 58: 337–342. [DOI] [PubMed] [Google Scholar]
- 3. Patterson KC, Strek ME. Diagnosis and treatment of pulmonary aspergillosis syndromes. Chest 2014; 146: 1358–1368. [DOI] [PubMed] [Google Scholar]
- 4. Zumla A, Mernish ZA, Maeurer M, et al. Emerging novel and antimicrobial-resistant respiratory tract infections: new drug development and therapeutic options. Lancet Infec Dis 2014; 14: 1136–1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Messer SA, Jones RN, Fritsche TR. International surveillance of Candida spp. and Aspergillus spp.: report from the SENTRY Antimicrobial Surveillance Program (2003). J Clin Microbiol 2006; 44: 1782–1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Taccone FS, Van den Abeele AM, Bulpa P, et al. Epidemiology of invasive aspergillosis in critically ill patients: clinical presentation, underlying conditions, and outcomes. Crit Care 2015; 19: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Dagenais TR, Keller NP. Pathogenesis of Aspergillus fumigatus in invasive Aspergillosis. Clin Microbiol Rev 2009; 22: 447–465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kwon-Chung KJ, Sugui JA. Aspergillus fumigatus – What makes the species a ubiquitous human fungal pathogen? PLoS Pathog 2013; 9(12): e1003743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Homma T, Kato A, Bhushan B, et al. Role of Aspergillus fumigatus in triggering protease-activated receptor-2 in airway epithelial cells and skewing the cells toward a T-helper 2 Bias. Am J Respir Cell Mol Biol 2016; 54: 60–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Tashiro T, Izumikawa K, Tashiro M, et al. Diagnostic significance of Aspergillus species isolated from respiratory samples in an adult pneumology ward. Med Mycol 2011; 49: 581–587. [DOI] [PubMed] [Google Scholar]
- 11. Agawal R, Chakrabarti A, Shah A. Allergic bronchopulmonary aspergillosis: review of literature and proposal of new diagnostic and classification criteria. Clin Exp Allergy 2013; 43: 850–873. [DOI] [PubMed] [Google Scholar]
- 12. World Health Organization.: Chronic respiratory diseases, http://www.who.int/respiratory/en/ (2017, accessed 7 July 2017).
- 13. Bousquet J, Kiley J, Bateman ED, et al. Prioritised research agenda for prevention and control of chronic respiratory diseases. Eur Respir J 2010; 36: 995–1001. [DOI] [PubMed] [Google Scholar]
- 14. Raghu G, Collard HR, Egan JJ, et al. An official ATS/ERS/JRS/ALAT statement: idiopathic pulmonary fibrosis: evidence-based guidelines for diagnosis and management. Am J Respir Crit Care Med 2011; 183: 788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Richeldi L, Du Bois RM, Raghu G, et al. Efficacy and safety of nintedanib in idiopathic pulmonary fibrosis. N Eng J Med 2014; 370: 2071–2082. [DOI] [PubMed] [Google Scholar]
- 16. Kolwijck E, Van De Veerdonk FL. The potential impact of the pulmonary microbiome on immunopathogenesis of Aspergillus-related lung disease. Eur J Imminol 2014; 44: 3156–3165. [DOI] [PubMed] [Google Scholar]
- 17. Chaudhar N, Marr KA. Impact of Aspergillus fumigatus in allergic airway diseases. Clin Transl Allergy 2011; 1: 4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ko SC, Chen KY, Hsueh PR, et al. Fungal empyema thoracis: an emerging clinical entity. Chest 2000; 117: 1672–1678. [DOI] [PubMed] [Google Scholar]
- 19. Delsuc C, Cottereau A, Frealle E, et al. Putative invasive pulmonary aspergillosis in critically ill patients with chronic obstructive pulmonary disease: a matched cohort study. Crit Care 2015; 19: 421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Bousquet J, Dahl R, Khaltaev N. Global alliance against chronic respiratory diseases. Allergy 2007; 62: 216–223. [DOI] [PubMed] [Google Scholar]
- 21. Agbetile J, Fairs A, Desai D, et al. Isolation of filamentous fungi sputum in asthma is associated with reduced post-bronchodilator FEV1. Clin Exp Allergy 2012; 42: 782–791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Huerta A, Soler N, Esperatti M. Importance of Aspergillus spp. isolation in acute exacerbations of severe COPD: prevalence, factors and follow-up: the FUNGI-COPD study. Respir Res 2014; 15: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kurosaki F, Bando M, Nakayama M. Clinical features of pulmonary aspergillosis associated with interstitial pneumonia. Intern Med 2014; 53: 1299–1306. [DOI] [PubMed] [Google Scholar]
- 24. Fibla JJ, Brunelli A, Allen MS, et al. Microbiology specimens obtained at the time of surgical lung biopsy for interstitial lung disease: clinical yield and cost analysis. Eur J Cardiothorac Surg 2012; 41: 36–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shulgina L, Cahn AP, Chilvers ER, et al. Treating idiopathic pulmonary fibrosis with the addition of co-trimoxazole: a randomised controlled trial. Thorax 2013; 68: 155–162. [DOI] [PubMed] [Google Scholar]
- 26. Han MK, Zhou Y, Murray S, et al. Lung microbiome and disease progression in idiopathic pulmonary fibrosis: an analysis of the COMET study. Lancet Respir Med 2014; 2: 548–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Molyneaux PL, Maher TM. The role of infection in the pathogenesis of idiopathic pulmonary fibrosis. Eur Respir Rev 2013; 22: 376–381 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Wootton SC, Kim DS, Kondoh Y, et al. Viral infection in acute exacerbation of idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2011; 183: 1698–1702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tang YW, Johnson JE, Browning PJ, et al. Herpesvirus DNA is consistently detected in lungs of patients with idiopathic pulmonary fibrosis. J Clin Microbiol 2003; 41: 2633–2640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Bulpa P, Dive A, Sibille Y. Invasive pulmonary aspergillosis in patients with chronic obstructive pulmonary disease. Eur Respir J 2007; 30: 782–800. [DOI] [PubMed] [Google Scholar]
- 31. Ohba H, Miwa S, Shirai M. Clinical characteristics and prognosis of chronic pulmonary aspergillosis. Respir Med 2012; 106: 724–729. [DOI] [PubMed] [Google Scholar]
- 32. Bafadhel M, McKenna S, Agbetile J, et al. Aspergillus fumigatus during stable state and excerbations of COPD. Eur Respir J 2014; 43: 64–71. [DOI] [PubMed] [Google Scholar]
- 33. Ng TT, Robson GD, Denning DW. Hydrocortisone-enhanced growth of Aspergillus spp.: implications for pathogenesis. Microbiology 1994; 140: 2475–2479. [DOI] [PubMed] [Google Scholar]
- 34. Lamoth F. Aspergillus fumigatus-related species in clinical practice. Front Microbiol 2016; 7: 683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Shahi M, Ayatollahi Mousavi SA, Nabili M, et al. Aspergillus colonization in patients with chronic obstructive pulmonary disease. Curr Med Mycol 2015; 1; 45–51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Barberán J, García-Pérez FJ, Villena V, et al. Development of Aspergillosis in a cohort of non-neutropenic, non-transplant patients colonised by Aspergillus spp. BMC Infec Dis 2017; 17: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Material, Suppelementary_Table_1_(1) for High burden of Aspergillus fumigatus infection among chronic respiratory diseases by Yosuke Fukuda, Tetsuya Homma, Shintaro Suzuki, Takahiro Takuma, Akihiko Tanaka, Takuya Yokoe, Tsukasa Ohnishi, Yoshihito Niki and Hironori Sagara in Chronic Respiratory Disease
Supplementary Material, Supplementary_Table_2 for High burden of Aspergillus fumigatus infection among chronic respiratory diseases by Yosuke Fukuda, Tetsuya Homma, Shintaro Suzuki, Takahiro Takuma, Akihiko Tanaka, Takuya Yokoe, Tsukasa Ohnishi, Yoshihito Niki and Hironori Sagara in Chronic Respiratory Disease
Supplementary Material, Supplementary_Table_3 for High burden of Aspergillus fumigatus infection among chronic respiratory diseases by Yosuke Fukuda, Tetsuya Homma, Shintaro Suzuki, Takahiro Takuma, Akihiko Tanaka, Takuya Yokoe, Tsukasa Ohnishi, Yoshihito Niki and Hironori Sagara in Chronic Respiratory Disease