Abstract
The aim of this study was to evaluate the clinical effects of cognitive impairment in patients with chronic obstructive pulmonary disease (COPD). A total of 91 patients with stable moderate to very severe COPD were included in this study. Cognitive functions of the patients were evaluated using the mini-mental state examination (MMSE) tool and clock-drawing test. The Brody’s Instrumental Activities of Daily Living (IADL) Questionnaire; COPD assessment test (CAT); body mass index, airflow obstruction, dyspnea, and exercise capacity (BODE); and Charlson comorbidity index were assessed. The patients were divided into two groups as those who were diagnosed with cognitive impairment (group 1, n = 16) and those with normal cognitive functions (group 2, n = 75). Group 1 had a lower arterial partial pressure of oxygen , shorter 6-min walking distance, and higher arterial partial pressure of carbon dioxide (PaCO2) than group 2 (p = 0.01, p = 0.024, p = 0.018, respectively). In group 1, the IADL score was lower, and CAT and BODE scores were higher than group 2 (p = 0.002, p = 0.037, p = 0.012, respectively). When we considered all the patients, there was an independent correlation between the IADL score and MMSE score (p = 0.03). This study revealed that COPD patients with cognitive impairment may have more hypoxemia and limited activities of daily living.
Keywords: Chronic obstructive pulmonary disease, cognitive impairment, instrumental activities of daily living
Introduction
Chronic obstructive pulmonary disease (COPD) is widely accepted to be associated with various comorbidities which seem to result from the disease itself and irrespective of other risk factors, such as smoking.1 Recently, neuropsychiatric comorbidities have particularly focused on cognitive dysfunction in COPD. The mechanisms proposed for cognitive impairment in COPD are hypoxia-mediated neuronal damage due to the lung disease or comorbidities, such as vascular disease, which adversely affect the brain.2 Several studies suggest that COPD patients may have a specific pattern of deterioration, compared to Alzheimer’s disease or multi-infarct dementia and healthy controls.3,4 Previous studies demonstrated a relationship between COPD and cognitive impairment5,6 and also in a recent study reporting that COPD is an independent risk factor for cognitive impairment.7 Age, education, physical activity, sleep, smoking, and fatigue are determinant factors of cognitive functions for both general population and COPD patients. However, conditions like smoking, age, and degradation in physical activity may contribute more to cognitive impairment in COPD patients than healthy population. Additionally, advancing airflow limitation, hypoxemia, hypercapnia, and exacerbations may induce further cognitive impairment in COPD patients.6 However, the effects of cognitive dysfunction on COPD have been examined only in a limited number of studies in the literature and demonstrated controversial results.2,8 Cognitive impairment is known to be associated with incorrect use of inhaled drugs and poor adherence to medications, which can adversely affect the management of COPD.9 Therefore, we suggest that there is a need for further studies which investigate the effect of cognitive impairment on the clinical presentation, course, and outcome of COPD.
Patients and methods
This study included a total of 91 consecutive patients with moderate to very severe COPD who were admitted to our outpatient clinic and under follow-up at least for 1 year.
Inclusion criteria were as follows: aged ≥40 years, at least 1-year diagnosis of moderate to very severe COPD according to the Global Initiative for Chronic Obstructive Lung Disease (GOLD) classification with baseline post-bronchodilator forced expiratory volume in 1 s (FEV1) of <80% of predicted value, FEV1/forced vital capacity ratio of ≤0.7,10 and ≥10 pack-year smoking history.
Exclusion criteria were as follows: having COPD exacerbation within the past 6 weeks prior to enrollment, respiratory disease other than COPD, having known neurological or psychiatry diseases, taking any medications which may affect the cognitive tests (lithium, antihistaminics, sedatives, propranolol, and erythromycin), and alcohol or substance addiction.
Spirometry was performed using a Sensor Medics model 2400 (Yorba Linda, California, USA) in accordance with the GOLD guidelines, and COPD was classified by their predictive FEV1 values: stage 2 (moderate; 50% ≤ FEV1 < 80%), stage 3 (severe; 30% ≤ FEV1 < 50%), and stage 4 (very severe; FEV1 < 30%).10 All patients were divided into four risk/symptom categories: (A) low risk, fewer symptoms; (B) low risk, more symptoms; (C) high risk, fewer symptoms; and (D) high risk, more symptoms. Based on this categorization, the cutoff points for risks (exacerbations in previous year ≥2 or ≥1 leading to hospital admission) and the cutoff points for symptoms (COPD assessment test (CAT) scores ≥ 10 and/or modified Medical Research Council (mMRC) ≥ 2) were chosen according to the GOLD 2017 classification.11
Arterial blood gas analysis was performed for each patient using a PAPIDLab 348EX system (Siemens Healthcare Diagnostic) while breathing at room air. The 6MWT is a self-paced test of walking capacity. The six minute walk test (6MWT) is a sub-maximal exercise test used to assess aerobic capacity and endurance. Patients are asked to walk as far as possible in 6 minutes along a flat corridor. The distance in metres is recorded (6MWD). Standardised instructions and encouragement are commonly given during the test. The 6MWT was performed twice for each patient and the best measurement was recorded.12
The body mass index (BMI), airflow obstruction, dyspnea, and exercise capacity (BODE) score was determined by the BMI, airflow obstruction (FEV1), grade of dyspnea (mMRC), and exercise capacity as measured 6-min walking distance (6MWD) for all patients. Each component is assigned a specific score and the total score (BODE index) ranges from 0 to 10 points. Higher scores indicate higher severity of disease.13
The Charlson comorbidity index (CCI) is a method of categorizing comorbidities of patients and it has been widely utilized by health researchers to measure burden of disease. Each comorbidity category has an associated weight from 1 to 6, based on the adjusted risk of mortality or resource use and a score of zero indicates that no comorbidities were found. The CCI was calculated in a standard way for each patient.14
Cognitive dysfunction was evaluated using two validated psychometric questionnaires: the mini-mental state examination (MMSE), which assesses orientation, recall and language, short-term memory, attention, and calculation (normal score >24)15,16; and the clock-drawing test (CDT), which assesses memory, attention, and symbolic representation (normal score >3).17,18 The patients were divided into two groups as those who were diagnosed with cognitive impairment, defined as an MMSE score of ≤24 (group 1) and those who were diagnosed with normal cognitive functions, defined as an MMSE score of >24 (group 2). The Beck Depression Inventory (BDI) is a valid and reliable scale developed to determine the risk of depression and severity of depressive symptoms. Depression was defined as a BDI score of ≥17.19
Disability was assessed using the Instrumental Activities of Daily Living (IADL) Questionnaire, which is defined as a self-reported difficulty or inability to perform any of the followings: heavy housework, light housework, shopping, preparing meals, paying bills, or using the phone. It has a scoring ranging from 0 to 24 points. The test results are evaluated as 0–8 points: dependency, 9–16 points: semi-dependency, and 17–24 points: independency.20
An informed consent was taken from each patient. The study protocol was approved by the Ethics Committee of Yedikule Chest Diseases and Thoracic Surgery Training and Research Hospital.
Statistical analysis
Statistical analysis was performed using the IBM SPSS version 23 software (SPSS Inc., Chicago, Illinois, USA). Program descriptive data were expressed in number and percentage for categorical variables and in mean and standard deviation for continuous numerical variables. Independent t-test was used to analyze the significant differences between the categorical variables having two groups, whereas one-way analysis of variance was used to examine the significant differences between the categorical variables having more than two groups. The χ 2 test was used to analyze the correlation between the two categorical variables, while the Pearson correlation coefficients were used to analyze the correlation between the two numerical variables. Multiple linear regression analysis was performed to investigate the effect of the independent variables (6MWD, BODE score, IADL score, arterial partial pressure of oxygen (PaO2)) on the dependent variable (MMSE). A p value of ≤ 0.05 was considered statistically significant.
Results
A total of 91 consecutive and stable COPD patients were included. Of these patients, 86 (94.5%) were males. The mean age was 61.9 ± 7.9 years. Demographic and clinical characteristics of all the patients are given in Table 1. Three patients with exacerbation history within the last 6 weeks, one patient with alcohol abuse, and two patients with neuropsychiatric disease were excluded from the study.
Table 1.
Demographic and clinical features of patients with COPD.
| n (%) | |
|---|---|
| Patients | 91 (100) |
| Male/female | 86 (94.5)/5 (5.5) |
| Educated/uneducated | 79 (86.8)/12 (13.2) |
| Exacerbations | |
| Frequent (≥2 years) | 18 (%19.8) |
| Unfrequent (<2 years) | 73 (%80.2) |
| Mean ± SD | |
| Age (years; SD) | 61.9 ± 7.9 |
| BMI | 24.9 ± 4.5 |
| 6MWD (m) | 346.1 ± 112.9 |
| FEV1% predicted | 44.0 ± 14.1 |
| mMRC | 1.6 ± 1.1 |
| BODE score | 3.2 ± 2.5 |
| PaO2 (mmHg) | 74.6 ± 17.3 |
| PaCO2 (mmHg) | 40.3 ± 6.5 |
| CAT score | 13.5 ± 8.4 |
| CCI | 2.8 ± 0.8 |
| MMSE score | 26.8 ± 2.5 |
| CDT score | 4.14 ± 1.3 |
| BODE index | 8.1 ± 8.9 |
| IADL score | 21.6 ± 3.5 |
COPD: chronic obstructive pulmonary disease; MMSE: mini-mental state examination; mMRC: modified Medical Research Council; CAT: COPD assessment test; 6MWD: 6-min walk distance; BMI: body mass index; BODE: BMI, obstruction, dyspnea, and exercise capacity; FEV1: forced expiratory volume in 1 s; IADL: Instrumental Activities of Daily Living; PaO2: arterial partial pressure of oxygen; PaCO2: arterial partial pressure of carbon dioxide; CCI: Charlson comorbidity index; CDT: clock-drawing test; SD: standard deviation.
The severity of the disease was classified using the predictive FEV1% values as follows: stage 2 (n = 33, 36.3%), stage 3 (n = 42, 46.2%), and stage 4 (n = 16, 17.6%). According to the GOLD 2017 classification, 20 patients (22%) were in category A, 13 (14.3%) in category B, 12 (13.2%) in category C, and 46 (50.5%) in category D.
Group 1 consisted of a total of 16 patients (17.6%) with cognitive impairment according to the MMSE scoring, whereas group 2 consisted of a total of 75 patients (82.4%) with normal cognitive functions. A comparison of the clinical and laboratory variables of groups 1 and 2 is shown in Table 2.
Table 2.
Comparison of clinical parameters between groups 1 and 2.
| Group 1 (n = 16) | Group 2 (n = 75) | t | p | |||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | |||
| Age (years) | 62.38 | 7.957 | 61.8 | 7.939 | 0.263 | 0.793 |
| Smoking (pack/years) | 50.19 | 34.233 | 53.91 | 28.07 | −0.463 | 0.645 |
| BMI | 23.13 | 4.193 | 25.23 | 4.498 | −1.716 | 0.09 |
| FEV1% predicted | 39.29 | 13.897 | 45.02 | 14.059 | −1.483 | 0.142 |
| mMRC | 2.06 | 1.526 | 1.53 | 1.057 | 1.672 | 0.098 |
| 6MWD (m) | 285.94 | 136.921 | 358.89 | 103.696 | −2.408 | 0.018a |
| BODE score | 4.63 | 2.872 | 2.91 | 2.349 | 2.552 | 0.012a |
| PaO2 (mmHg) | 64.63 | 10.184 | 76.77 | 17.762 | −2.635 | 0.010a |
| PaCO2 (mmHg) | 43.63 | 6.003 | 39.6 | 6.418 | 2.299 | 0.024a |
| CAT score | 17.5 | 11.402 | 12.69 | 7.442 | 2.117 | 0.037a |
| CCI | 2.75 | 0.683 | 2.83 | 0.86 | −0.334 | 0.739 |
| IADL score | 19.19 | 3.936 | 22.13 | 3.138 | −3.255 | 0.002b |
| BDI | 11.38 | 11.983 | 7.41 | 8.091 | 1.622 | 0.108 |
Group 1: patients with cognitive impairment; Group 2: patients with normal cognitive function; FEV1: forced expiratory volume in 1 s; mMRC: modified Medical Research Council; 6MWD: 6-min walk distance; BMI: body mass index; BODE: BMI, obstruction, dyspnea, and exercise capacity; PaO2: arterial partial pressure of oxygen; PaCO2: arterial partial pressure of carbon dioxide; CAT: chronic obstructive pulmonary disease assessment test; CCI: Charlson comorbidity index; IADL: Instrumental Activities of Daily Living; BDI: Beck Depression Inventory; SD: standard deviation.
a p < 0.05.
b p < 0.01.
Patients were grouped according to sex, exacerbation status (exacerbations in previous year ≥2 or ≥1 leading to hospital admission was determined frequent exacerbator group), CAT score (the equivalent cut point is 10 for CAT score), mMRC score (<2: less breathlessness and ≥2: more breathlessness), BDI score (≥17 points was defined depression), and IADL score (0–8 points: dependency; 9–16 points: semi-dependency; and 17–24 points: independency). Groups 1 and 2 were evaluated comparatively by considering this classification. The number of female patients (p = 0.036) and also semi-dependent patients (p = 0.013) were significantly more in group 1 than group 2. There was no statistically significant difference between the groups regarding other parameters (Table 3).
Table 3.
Comparison of clinical features between groups 1 and 2.
| Group 1 (n = 16) | Group 2 (n = 75) | Total | p | |||||
|---|---|---|---|---|---|---|---|---|
| n | % | n | % | n | % | |||
| Gender | Female | 3 | 18.8 | 2 | 2.7 | 5 | 5.5 | 0.036a |
| Male | 13 | 81.3 | 73 | 97.3 | 86 | 94.5 | ||
| GOLD, stage (FEV1) | 2 (50–80%) | 3 | 18.8 | 30 | 40.0 | 33 | 36.3 | 0.262 |
| 3 (30–50%) | 9 | 56.3 | 33 | 44.0 | 42 | 46.2 | ||
| 4 (<30%) | 4 | 25.0 | 12 | 16.0 | 16 | 17.6 | ||
| Exacerbations | Frequent | 6 | 37.5 | 12 | 16.0 | 18 | 19.8 | 0.079 |
| Unfrequent | 10 | 62.5 | 63 | 84.0 | 73 | 80.2 | ||
| CAT | <10 | 5 | 15.2 | 28 | 84.8 | 33 | 100.0 | 0.646 |
| ≥10 | 11 | 19.0 | 47 | 81.0 | 58 | 100.0 | ||
| mMRC | <2 | 7 | 43.8 | 48 | 64.0 | 55 | 60.4 | 0.133 |
| ≥2 | 9 | 56.3 | 27 | 36.0 | 36 | 39.6 | ||
| BDI | Depressive | 4 | 25.0 | 10 | 13.3 | 14 | 15.4 | 0.260 |
| Normal | 12 | 75.0 | 65 | 86.7 | 77 | 84.6 | ||
| IADL | Semi-dependent | 5 | 31.3 | 5 | 6.7 | 10 | 11.0 | 0.013a |
| Independent | 11 | 68.8 | 70 | 93.3 | 81 | 89.0 | ||
Group 1: patients with cognitive impairment; Group 2: patients with normal cognitive function; GOLD: Global Initiative for Chronic Obstructive Pulmonary Disease; FEV1: forced expiratory volume in 1 s; CAT: chronic obstructive pulmonary disease assessment test; mMRC: modified Medical Research Council; BDI: Beck Depression Inventory; IADL: Instrumental Activities of Daily Living.
a p < 0.05.
The correlation between cognitive test scores (MMSE and CDT) and clinical variables was evaluated using the Pearson correlation analysis. Poorer performance on the MMSE was associated with lower PaO2, 6MWD, IADL score, and higher BODE score; however, CDT scores were correlated only with the 6MWD and IADL scores (Table 4).
Table 4.
Correlation between scores of cognitive function tests and clinical parameters.
| MMSE score | CDT | ||
|---|---|---|---|
| mMRC | r | −0.157 | −0.100 |
| p | 0.137 | 0.343 | |
| CAT score | r | −0.155 | 0.017 |
| p | 0.143 | 0.874 | |
| 6MWD (m) | r | 0.294a | 0.258b |
| p | 0.005a | 0.014b | |
| BODE score | r | −0.266b | −0.175 |
| p | 0.011b | 0.097 | |
| FEV1, % predicted | r | 0.183 | 0.140 |
| p | 0.082 | 0.185 | |
| IADL score | r | 0.354a | 0.390a |
| p | 0.001a | 0.000c | |
| PaO2 (mmHg) | r | 0.231b | 0.113 |
| p | 0.028b | 0.291 | |
| PaCO2 (mmHg) | r | −0.172 | −0.117 |
| p | 0.104 | 0.270 | |
MMSE: mini-mental state examination; mMRC: modified Medical Research Council; CAT: chronic obstructive pulmonary disease assessment test; 6MWD: 6-min walk distance; BMI: body mass index; BODE: BMI, obstruction, dyspnea, and exercise capacity; FEV1: forced expiratory volume in 1 s; IADL: Instrumental Activities of Daily Living; PaO2: arterial partial pressure of oxygen; PaCO2: arterial partial pressure of carbon dioxide; CDT: clock-drawing test.
a p < 0.01.
b p < 0.05.
c p < 0.001.
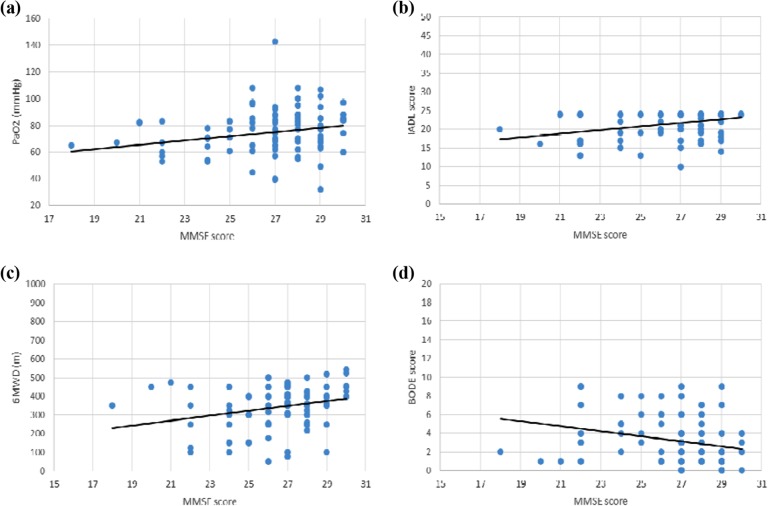
While weak correlation between MMSE score and PaO2, 6MWD, and BODE was determined, correlation between MMSE score and IADL was moderate. The correlation between MMSE score and PaO2, 6MWD, IADL, and BODE score was shown in Figure 1. We found independent association only between the IADL score and MMSE score in multivariate regression analysis. Accordingly, MMSE scores increased by 0.279 units with every unit increase in IADL (p = 0.03; Table 5).
Figure 1.
Relationship between MMSE score and PaO2, IADL score, 6MWD, and BODE score (Pearson correlations). (a) The MMSE score was correlated with the PaO2 (r = 0.23, p = 0.02). (b) The MMSE score was correlated with the IADL score (r = 0.35, p = 0.001). (c) The MMSE score was correlated with the 6MWD (r = 0.29, p = 0.005). (d) The MMSE score was correlated with the BODE score (r = −0.26, p = 0.01). MMSE: mini-mental state examination; PaO2: arterial partial pressure of oxygen; 6MWD: 6-min walk distance; BMI: body mass index; IADL: Instrumental Activities of Daily Living; BODE: BMI, airflow obstruction, dyspnea, and exercise capacity.
Table 5.
Multivariate regression analysis between MMSE score and clinical parameters.
| Unstandardized coefficients | Standardized coefficients | t | p | ||
|---|---|---|---|---|---|
| B | Standard error | Beta | |||
| Fixed | 19.868 | 2.810 | 7.072 | 0.000a | |
| 6MWD (m) | 0.002 | 0.004 | 0.109 | 0.638 | 0.525 |
| BODE score | 0.039 | 0.175 | 0.040 | 0.225 | 0.822 |
| IADL score | 0.200 | 0.090 | 0.279 | 2.214 | 0.030b |
| PaO2 (mmHg) | 0.022 | 0.015 | 0.153 | 1.445 | 0.152 |
| Model coefficients: R 2: 0.152 Adj. R 2: 0.112 F = 3.812 p = 0.007 | |||||
MMSE: mini-mental state examination; 6MWD: 6-min walk distance; BMI: body mass index; BODE: BMI, obstruction, dyspnea, and exercise capacity; IADL: Instrumental Activities of Daily Living; PaO2: arterial partial pressure of oxygen.
ap < 0.001.
bp < 0.05.
Discussion
In recent years, COPD has been considered as a disease not limited only to the lungs but as a complex condition with various systemic components.21 Neuropsychiatric disorders, particularly depression, anxiety, and cognitive dysfunction, are a substantial contributor to COPD-related health.22,23 The incidence of cognitive impairment varies from 10% to 61% in COPD patients, based on the study population and neuropsychological assessment.23,24 In the present study, we found cognitive impairment in 17.6% of the patients with COPD, as assessed using MMSE. The MMSE is an adequate and useful test for the global evaluation of cognitive functions. In a recent study, each test was performed separately for the evaluation of cognitive functions in COPD patients (MMSE, trail making A and B, CDT, forward and backward digit span tests, Brown–Peterson test, and verbal fluency test) and only MMSE was found to be correlated with all clinical COPD variables.25 Aiming to evaluate relationship between clinical characteristics of COPD patients and their global cognitive functions, we decided to utilize MMSE.
Although a number of studies have shown a clear association between hypoxemia and poor cognitive performance, there are controversial results on whether patients with early disease or mild hypoxemia have significantly impaired cognitive functions.8,26 A study conducted using the single-photon emission computed tomography with Tc-99m hexamethylpropyleneamine oxime (HMPAO) supported the hypothesis that cerebral perfusion was significantly altered in patients with COPD and hypoxemic patients showed more impairment in cerebral perfusion and cognitive performance than non-hypoxemic patients.27 In our study, we demonstrated that PaO2 was lower in the patients with cognitive impairment and also indicating a correlation between PaO2 level and MMSE score.
Our study showed that arterial partial pressure of carbon dioxide (PaCO2) levels were higher in the patients with cognitive impairment, indicating a negative correlation between MMSE scores and PaCO2. Similarly, previous studies reported that as PaCO2 increased, the cognitive function became more impaired.8,28 In addition, Parekh et al.29 demonstrated that lower PaCO2 levels were significantly correlated with improved cognitive performance in patients who were in the waiting list for lung transplantation.
The relationship between cognitive dysfunction and the severity of airway obstruction still remains to be elucidated.2,22 Several studies showed that reduced FEV1 level was related to poor cognitive functions.8,30 Li et al. found that global cognitive function was worse in severe COPD patients, compared to mild to moderate COPD patients.7 On the other hand, some authors did not demonstrate any relationship of pulmonary function with cognitive impairment in COPD.2,26 Similarly, we found no relationship between FEV1 values and cognitive function. This result may suggest FEV1 is poor relevant marker of cognitive status in COPD patients and airflow limitation don’t reflect the multisystem nature of the disease.
To the best of our knowledge, there is only one study investigating the relationship of the cognitive functions with the BODE index in COPD. Thakur et al. found no relationship between the cognitive functions and the BODE score.2 However, in our study, the BODE index was found to be higher in the patients with cognitive impairment. In addition, we showed that there was a negative correlation between the MMSE score and BODE index. In addition, we found that 6MWD which has a prognostic value in patients with COPD was shorter in patients with cognitive impairment.
Tulek et al. investigated the relationship of cognitive impairment with disease control; no correlation was found between the total cognitive score and the CAT score.25 Similarly, we did not find any correlation between cognitive score and CAT score. However, in our study, CAT score was higher in patients with cognitive impairment. In addition, we didn’t show any relationship between cognitive state and mMRC, consistent with the literature findings.25
The previous studies reported impaired cognitive functions during exacerbation of COPD and the impairment recovered within 6 months after discharge.31,32 Dodd et al. showed that the cognitive functions of patients with COPD were significantly worse during exacerbation, and these patients did not recover 3 months after discharge.33 Another study reported that more frequent and more serious cognitive impairment in COPD patients with a history of frequent exacerbation, compared to those who did not.25 In consistent with these findings, we found no correlation between the cognitive state and history of frequent exacerbation.
The comorbidities associated with COPD, particularly cerebrovascular diseases, are known to have a possible role in the pathogenesis of cognitive impairment.2 However, our study did not show any correlation between cognitive dysfunction and the CCI score. Similarly, there are studies showing no relationship between cognitive impairment and cardiovascular/metabolic comorbidities.8,22
Based on the literature data, cognitive dysfunction was associated with impaired IADL in COPD patients, particularly in activity-limited patients who were dependent on supplemental oxygen.6,34 Cognitive dysfunction and IADL limitation often lead to poor compliance to medications and increase the necessity of care services in COPD. In addition, IADL limitations were also shown to be associated with a high mortality rate in patients with COPD.35,36 Similarly, we demonstrated that the patients with cognitive impairment had lower IADL scores, indicating the positive correlation between the MMSE and IADL scores and it was independent of other factors. To the best of our knowledge, this study is the first to demonstrate the independent relationship between the IADL and MMSE scores in patients with COPD.
Nonetheless, there are some limitations to this study. First, the study was a cross-sectional study. Second, the study did not include a control group. However, many national and international studies have shown that patients with COPD have a higher rate of cognitive impairment than healthy controls.5 In addition, a recent study has demonstrated that COPD is an independent risk factor for cognitive impairment.7 Third, the majority of our patients were males, which can be explained by the fact that the inclusion was made among patients who visited our clinic consecutively and also that the prevalence of COPD in Turkey is lower among females.37 Because of these limitations, our results cannot be generalized.
Conclusion
In conclusion, this study revealed that COPD patients with cognitive impairment were more hypoxemic and had limited activities of daily living. Based on our results, regular screening of cognitive functions by MMSE may be beneficial in selecting the management strategies for COPD patients who have limited activity or hypoxemia. Additional prospective randomized studies are needed to further demonstrate these benefits.
Acknowledgements
The authors would like to gratefully acknowledge the support and generosity of Turkish Respiratory Society in statistical analysis of the present study.
Authors’ note: EEY has helped in design; SA, GG, ACG, SK, and EEY have helped in data acquisition; EEY, PY, and Cem Güzel have helped in statistical analysis. All authors reviewed and commented on the final manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
Reference
- 1. Van Eeden S, Leipsic J, Paul Man S, et al. The relationship between lung inflammation and cardiovascular disease. Am J Respir Crit Care Med 2012; 186(1): 11–16. [DOI] [PubMed] [Google Scholar]
- 2. Thakur N, Blanc PD, Julian LJ, et al. COPD and cognitive impairment: the role of hypoxemia and oxygen therapy. Int J Chron Obstruct Pulmon Dis 2010; 7(5): 263–269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Incalzi RA, Gemma A, Marra C, et al. Chronic obstructive pulmonary disease. An original model of cognitive decline. Am Rev Respir Dis 1993; 148(2): 418–424. [DOI] [PubMed] [Google Scholar]
- 4. Kozora E, Filley CM, Julian LJ, et al. Cognitive functioning in patients with chronic obstructive pulmonary disease and mild hypoxemia compared with patients with mild Alzheimer disease and normal controls. Neuropsychiatry Neuropsychol Behav Neurol 1999; 12(3): 178–183. [PubMed] [Google Scholar]
- 5. Sarınç Ulaşlı S, Oruç S, Günay E, et al. Effects of COPD on cognitive functions: a case control study. Tuberk Toraks 2013; 61(3): 193–199. [DOI] [PubMed] [Google Scholar]
- 6. Hung WW, Wisnivesky JP, Siu AL, et al. Cognitive decline among patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2009; 180(2): 134–137. [DOI] [PubMed] [Google Scholar]
- 7. Li J, Huang Y, Fei GH. The evaluation of cognitive impairment and relevant factors in patients with chronic obstructive pulmonary disease. Respiration 2013; 85(2): 98–105. [DOI] [PubMed] [Google Scholar]
- 8. Dal Negro RW, Bonadiman L, Tognella S, et al. Extent and prevalence of cognitive dysfunction in chronic obstructive pulmonary disease, chronic non-obstructive bronchitis, and in asymptomatic smokers, compared to normal reference values. Int J Chron Obstruct Pulmon Dis 2014; 9: 675–683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Incalzi RA, Gemma A, Marra C, et al. Verbal memory impairment in COPD: its mechanisms and clinical relevance. Chest 1997; 112(6): 1506–1513. [DOI] [PubMed] [Google Scholar]
- 10. Rabe KF, Hurd S, Anzueto A, et al. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am J Respir Crit Care Med. 2007; 176(6): 532–555. [DOI] [PubMed] [Google Scholar]
- 11. Vogelmeier CF, Criner GJ, Martinez FJ, et al. Global Strategy for the Diagnosis, Management and Prevention of Chronic Obstructive Lung Disease 2017 Report: GOLD Executive Summary. Respirology 2017; 22(3): 575–601. [DOI] [PubMed] [Google Scholar]
- 12. Holland AE, Spruit MA, Troosters T, et al. An official European Respiratory Society/American Thoracic Society technical standard: field walking tests in chronic respiratory disease. Eur Respir J 2014; 44(6): 1428–1446. [DOI] [PubMed] [Google Scholar]
- 13. Celli BR, Cote CG, Marin JM, et al. The body-mass index, airflow obstruction, dyspnea, and exercise capacity index in chronic obstructive pulmonary disease. N Engl J Med 2004; 350(10): 1005–1012. [DOI] [PubMed] [Google Scholar]
- 14. Charlson ME, Pompei P, Ales KL, et al. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chron Dis 1987; 40(5): 373–383. [DOI] [PubMed] [Google Scholar]
- 15. Folstein MF, Folstein SE, McHugh PR. Mini-mental state. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12(3): 189–198. [DOI] [PubMed] [Google Scholar]
- 16. Gungen C, Ertan T, Eker E, et al. Reliability and validity of the standardized Mini Mental State Examination in the diagnosis of mild dementia in Turkish population. Turk Psikiyatri Derg 2002; 13(4): 273–281. [PubMed] [Google Scholar]
- 17. Shulman KI. Clock-drawing: Is it the ideal cognitive screening test? Int J Geriatr Psychiatry. 2000; 15(6): 548–561. Review. [DOI] [PubMed] [Google Scholar]
- 18. Cangöz B, Karakoç E, Selekler K. The norm determination and validity-reliability studies of clock drawing test on Turkish adults and elderlys (ages 50 and over). Turkish J Geriatrics 2006: 9; 136–142. [Google Scholar]
- 19. Yilmaz N, Gencoz T, Ak M. Psychometric properties of the defense style questionnaire: a reliability and validity study. Turk Psikiyatri Derg 2007; 18(3): 244–253. [PubMed] [Google Scholar]
- 20. Lawton MP, Brody E. Assessment of older people: self-maintaining and instrumental activities of daily living. Gerontologist 1969; 9(3): 179–186. [PubMed] [Google Scholar]
- 21. Van Eeden SF, Sin DD. Chronic obstructive pulmonary disease: a chronic systemic inflammatory disease. Respiration 2008; 75(2): 224–238. [DOI] [PubMed] [Google Scholar]
- 22. Chaudhary SC, Nanda S, Tripathi A, et al. Prevalence of psychiatric comorbidities in chronic obstructive pulmonary disease patients. Lung India. 2016; 33(2): 174–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Dodd JW, Getov SV, Jones PW. Cognitive function in COPD. Eur Respir J 2010; 35(4): 913–922. [DOI] [PubMed] [Google Scholar]
- 24. Grant I, Heaton RK, McSweeny AJ, et al. Neuropsychologic findings in hypoxemic chronic obstructive pulmonary disease. Arch Intern Med 1982; 142(8): 1470–1476. [PubMed] [Google Scholar]
- 25. Tulek B, Atalay NB, Yildirim G, et al. Cognitive function in chronic obstructive pulmonary disease: relationship to global initiative for chronic obstructive lung disease 2011 categories. Respirology 2014; 19(6): 873–880. [DOI] [PubMed] [Google Scholar]
- 26. Liesker JJ, Postma DS, Beukema RJ, et al. Cognitive performance in patients with COPD. Respir Med 2004; 98(4): 351–356. [DOI] [PubMed] [Google Scholar]
- 27. Ortapamuk H, Naldoken S. Brain perfusion abnormalities in chronic obstructive pulmonary disease: comparison with cognitive impairment. Ann Nucl Med 2006; 20(2): 99–106. [DOI] [PubMed] [Google Scholar]
- 28. Crişan AF, Oancea C, Timar B, et al. Cognitive impairment in chronic obstructive pulmonary disease. PLoS One 2014; 9(7): e102468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Parekh PI, Blumenthal JA, Babyak MA, et al. INSPIRE Investigators. Gas exchange and exercise capacity affect neurocognitive performance in patients with lung disease. Psychosom Med 2005; 67(3): 425–432. [DOI] [PubMed] [Google Scholar]
- 30. Sachdev PS, Anstey KJ, Parslow RA, et al. Pulmonary function, cognitive impairment and brain atrophy in a middle-aged community sample. Dement Geriatr Cogn Disord 2006; 21(5–6): 300–308. [DOI] [PubMed] [Google Scholar]
- 31. Ranieri P, Bianchetti A, Margiotta A, et al. Predictors of 6-month mortality in elderly patients with mild chronic obstructive pulmonary disease discharged from a medical ward after acute nonacidotic exacerbation. J Am Geriatr Soc 2008; 56(5): 909–913. [DOI] [PubMed] [Google Scholar]
- 32. Ambrosino N, Bruletti G, Scala V, et al. Cognitive and perceived health status in patient with chronic obstructive pulmonary disease surviving acute on chronic respiratory failure: a controlled study. Intensive Care Med 2002; 28(2): 170–177. [DOI] [PubMed] [Google Scholar]
- 33. Dodd JW, Charlton RA, van den Broek MD, et al. Cognitive dysfunction in patients hospitalized with acute exacerbation of COPD. Chest 2013; 144(1): 119–127. [DOI] [PubMed] [Google Scholar]
- 34. Incalzi RA, Corsonello A, Pedone C, et al. GIFA Investigators. Construct validity of activities of daily living scale: a clue to distinguish the disabling effects of COPD and congestive heart failure. Chest 2005; 127(3): 830–838. [DOI] [PubMed] [Google Scholar]
- 35. Liu Y, Croft JB, Anderson LA, et al. The association of chronic obstructive pulmonary disease, disability, engagement in social activities, and mortality among US adults aged 70 years or older, 1994–2006. Int J COPD 2014: 9; 75–83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Martinez F, Foster G, Curtis JL, et al. Predictors of mortality of patients with emphysema and severe airflow obstruction. Am J Respir Crit Care Med 2006; 173(12): 1326–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Buist AS, Vollmer WM, Sullivan SD, et al. The Burden of Obstructive Lung Disease Initiative (BOLD): rationale and design. COPD 2005; 2: 227–283. [PubMed] [Google Scholar]