Abstract
Volatile components of seven species of the Bazzanioideae sub-family (Lepidoziaceae) native to New Caledonia, including three endemic species (Bazzania marginata, Acromastigum caledonicum and A. tenax), were analyzed by GC-FID-MS in order to index these plants to known or new chemotypes. Detected volatile constituents in studied species were constituted mainly by sesquiterpene, as well as diterpene compounds. All so-established compositions cannot successfully index some of them to known chemotypes but afforded the discovery of new chemotypes such as cuparane/fusicoccane. The major component of B. francana was isolated and characterized as a new zierane-type sesquiterpene called ziera-12(13),10(14)-dien-5-ol (23). In addition, qualitative intraspecies variations of chemical composition were very important particularly for B. francana which possessed three clearly defined different compositions. We report here also the first phytochemical investigation of Acromastigum species. Moreover, crude diethyl ether extract of B. vitatta afforded a new bis(bibenzyl) called vittatin (51), for which a putative biosynthesis was suggested.
Keywords: liverwort, Bazzania, Acromastigum, sesquiterpene, diterpene, bis(bibenzyl), biosynthesis, zierane, vittatin
1. Introduction
Liverworts are part of Bryoflora (mosses: 14,000 species, liverworts: 6000 species and hornworts: 300 species), considered as the first terrestrial plants and taxonomically indexed between algae and pteridophytes. Bryophytes possess archaic characteristics such as the absence of seeds and vascularized leaves [1]. Morphological traits such as small size of organs or relatively simple structure or high intraspecies variability and fugacity of some microscopic details (which may disappear within the plant dryness such as oil bodies), add difficulties for liverwort’s taxonomic identification. Nevertheless, many liverworts have unique organelles called oil bodies in their cells which are linked to the biosynthesis of original secondary metabolites such as mono-, sesqui- and diterpenes or phenolic compounds that could be cladistic biomarker. Most of liverwort’s sesquiterpenes are enantiomers of those found in higher plants [2].
New Caledonia is an archipelago of 18,600 km2 located in South Pacific region and considered as a hotspot of biodiversity [3]. In New Caledonia, 482 species and infraspecific taxa of liverworts have been described in a recent checklist [4]. The rate of endemism is comprised between 13% and 39% that makes New Caledonia as one of the richest liverwort areas in the world, together with Japan, New Zealand, and Costa Rica [5].
Bazzanioideae is a subfamily of the Lepidoziaceae family including three genera Bazzania, Acromastigum and Mastigopelma, this latter one had never been inventoried in New Caledonia [4]. Through the world, nearly 280 Bazzania species and 40 Acromastigum species are described [6]. In New Caledonia, 20 Bazzania species or varieties including 12 endemics and 16 Acromastigum species or varieties including 10 endemics have been described [4]. Usually, Bazzania species are divided into two chemotypes: albicanyl(drimenyl)-caffeate-cuparane (I) and calamenane (II) [7].
Volatile compounds of 27 specimens belonging to seven different species, including three endemics (marked with an asterisk) were investigated in order to check intra- and inter-variability of molecular contents. Studied Bazzanioideae species belong to the following genera: Acromastigum (A. tenax*, A. caledonicum*) and Bazzania (B. parisii, B. marginata*, B. vittata, B. francana, B. bernieri and B. serrifolia). B. serrifolia is a synonym of B. bernieri according to Kitagawa (1973) [8] but we studied here its putative specific status by separating the samples in two lots of specimens on the basis of their morphological traits.
Volatile components of diethyl ether extracts were analyzed by GC-MS-FID in order to index these plants into known Bazzania chemotypes. In the present work, two new molecules: an oxygenated dimer of lunularic acid (51) and an alcohol zierane-type sesquiterpene (23) are characterized for the first time (Table 1 and Table 2). They were respectively isolated from diethyl ether extract of B. vittata and B. francana. To our best knowledge, this is the first phytochemical investigation of all these species and the first chemical analysis of those belonging to the Acromastigum genus.
Table 1.
NMR data of compound 23 in CDCl3 at 300 K (1H at 500 MHz, 13C at 125 MHz).
| Compound 23 | |||
|---|---|---|---|
| Atom | δH (J in Hz) | δC | HMBC JH→C |
| 1 | 2.56 (t, 8.6, 1H) | 57.6 | 5, 9, 14 |
| 2 | 2.01 (m, 1H) | 27.1 | 4, 5 |
| 1.94 (m, 1H) | |||
| 3 | 2.16 (m, 1H) | 32.1 | 1, 5, 15 |
| 1.47 (m, 1H) | |||
| 4 | 1.90 (m, 1H) | 46.1 | 1, 2, 5, 15 |
| 5 | _ | 87.2 | |
| 6 | 2.30 (brd, 9.8, 1H) | 49.9 | 5, 7, 8, 11, 12, 13 |
| 7 | 2.03 (m, 1H) | 31.4 | 6 |
| 1.52 (m, 1H) | |||
| 8 | 1.94 (m, 1H) | 29.8 | 6 |
| 1.44 (m, 1H) | |||
| 9 | 2.52 (brdt, 13.2, 4.8, 1H) | 40.9 | 1, 7, 8, 10, 14 |
| 2.01 (m, 1H) | |||
| 10 | _ | 151.1 | |
| 11 | _ | 150.1 | |
| 12 | 1.82 (brs, 3H) | 23.7 | 6, 11,13 |
| 13 | 4.85 (brs, 1H) | 112.5 | 6, 11, 12 |
| 4.78 (brs, 1H) | |||
| 14 | 4.93 (brs, 1H) 4.92 (brs, 1H) |
110.2 | 1, 5, 9, 10 |
| 15 | 0.91 (d, 7.1, 3H) | 16.0 | 3, 4, 5 |
s: singlet, d: doublet, t: triplet, m: multiplet, br: broad.
Table 2.
NMR data of compound (51) in DMSO-d6 at 300 K (1H at 600 MHz, 13C at 150 MHz).
| Compound (51) | |||
|---|---|---|---|
| Atom | δH (J in Hz) | δC | HMBC JH→C |
| 1, 1′ | - | 153.0 | - |
| 2, 2′ | - | 128.3 | - |
| 3, 3′ | 7.51 (d, 2.0, 2H) | 128.3 | 1, 2, 5, 7 |
| 4, 4′ | - | 137.8 | - |
| 5, 5′ | 7.17 (dd, 8.2, 2.0, 2H) | 128.8 | 1, 3, 7 |
| 6, 6′ | 7.07 (d, 8.2, 2H) | 120.6 | 1, 2, 4 |
| 7, 7′ | 2.89 (m, 4H) | 36.6 | 3, 5, 9 |
| 8, 8′ | 2.97 (m, 4H) | 36.2 | 4, 10, 14 |
| 9, 9′ | - | 141.0 | - |
| 10, 10′ | - | 119.9 | - |
| 11, 11′ | - | 156.6 | - |
| 12, 12′ | 6.75 (d, 7.9, 2H) | 114.0 | 10, 11, 14, 15 |
| 13, 13′ | 7.20 (t, 7.9, 2H) | 130.9 | 9, 11 |
| 14, 14′ | 6.76 (d, 7.9, 2H) | 120.4 | 8, 10, 12, 15 |
| 15, 15′ | 13.27 (s, 2H) | 170.7 | - |
| 16 | 5.55 (brs, 2H) | 98.9 | 1 |
| OH-11 | 10.43 (brs, 2H) | - | - |
s: singlet, d: doublet, t: triplet, m: multiplet, br: broad.
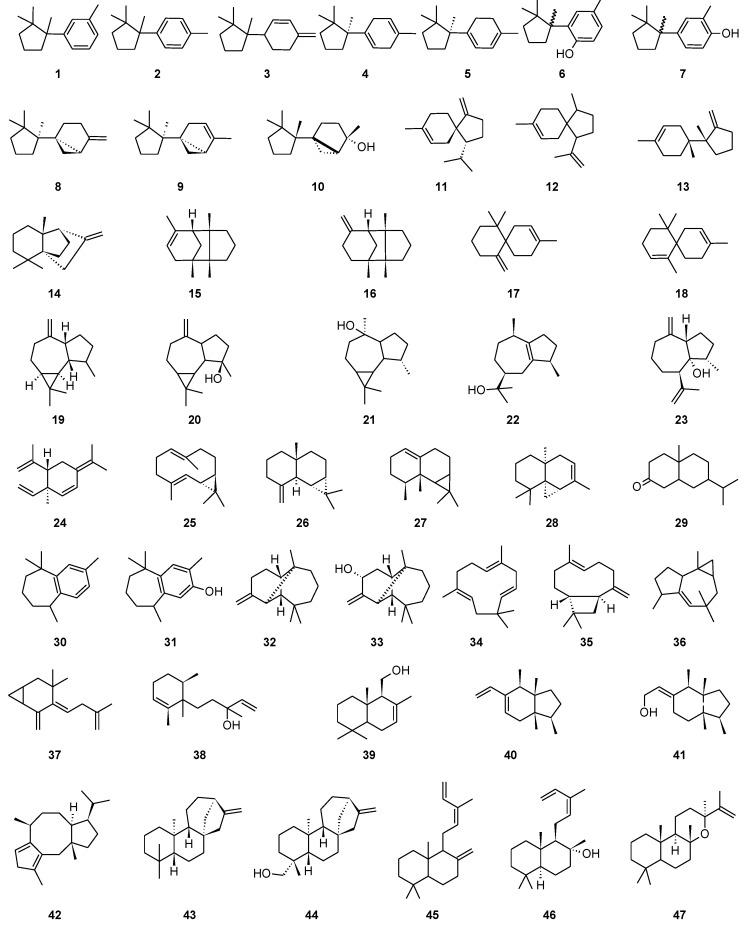
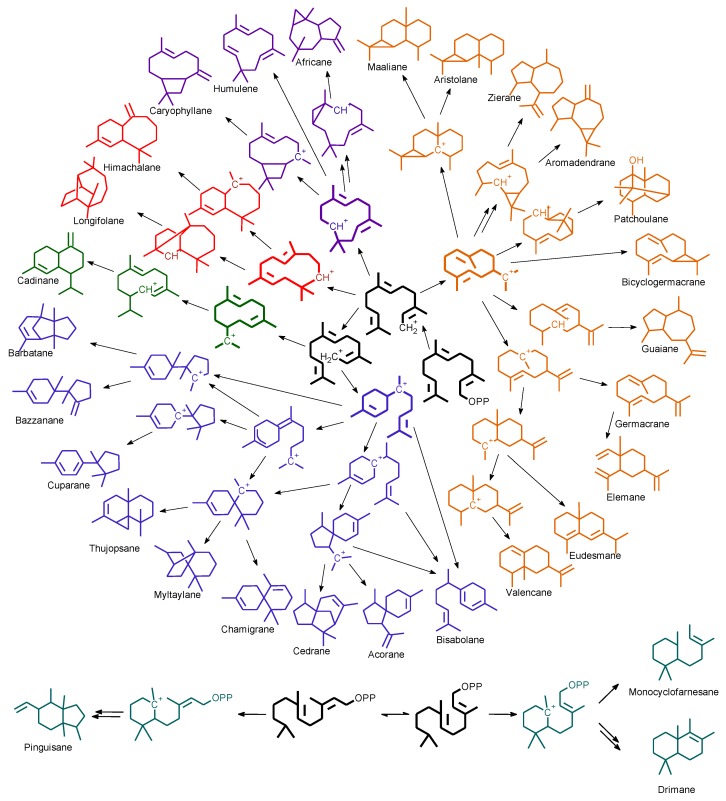
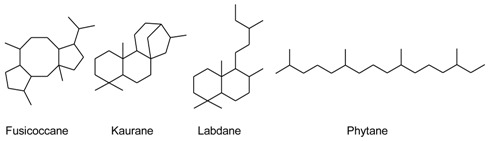
In order to sort the detected volatile compounds in this study, diterpene-type contents are listed (Table 3), listed sesquiterpene molecules were classified following their first cyclization precursors (Figure 1), and their sesquiterpene types (Table 4, Table 5 and Table 6) related to the corresponding biosynthesis pathway scheme from the literature data [9,10,11,12,13,14,15,16,17,18,19]. So, compilation of chemical composition of all studied species are gathered in Table 7. List of identified and unknown compounds is shown in Table 8, Table 9 and Table 10. Most important detected compounds for chemotaxonomy are shown in Figure 2.
Table 3.
Distribution of fusicoccane-, kaurane-, labdane- and phytane-type diterpenes in studied species.
| Species | Samples (METXXX) | Fusicoccane | Kaurane | Labdane | Phytane | Total |
|---|---|---|---|---|---|---|
| A. caledonicum | MET116 | - | 14.1 | - | 2.8 | 17.0 |
| A. tenax | all | - | 0.0–2.6 | - | 1.2–7.1 | 1.3–7.1 |
| B. bernieri | BB1 | 29.3–53.7 | - | 3.5–7.5 | 0.0–1.3 | 34.6–60.8 |
| MET038 | 63.7 | - | 0.7 | 0.7 | 65.1 | |
| B. francana | MET032 | 1.6 | - | 1.1 | 0.4 | 3.2 |
| MET062,65 | 0.6–1.0 | - | 1.2–1.6 | - | 2.1–2.2 | |
| MET106 | 3.2 | - | 11.0 | 2.2 | 16.4 | |
| B. marginata | MET048 | 0.3 | - | - | 0.3 | 0.6 |
| B. parisii | MET109A | 4.1 | - | 15.0 | - | 19.1 |
| B. serrifolia | BS1 | 16.1–22.4 | - | 2.3–3.4 | 0.0–0.4 | 18.4–26.3 |
| BS2 | 50.4–72.6 | - | 0.8–2.1 | 0.0–0.3 | 50.4–74.7 | |
| B. vittata | all | 8.2–21.0 | - | 0.0–2.2 | 10.2–13 | 20.5–34.0 |
Figure 1.
Scheme of cationic first cyclization precursor for the detected sesquiterpenes (1 ring closure) [19].
Table 4.
Distribution of detected sesquiterpene types affiliated with the (E-E)-germacradienyl cation (relative percentage, %).
| Species | Samples (METXXX) | Maaliane | Aris. | Arom. | BG | Elemane | Germacrane | Eude. | Vala. | Guaiane | Zierane | Patc. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A. tenax | MET116 | - | - | 10.6 | 5.0–1.5 | 11.2 | - | - | - | - | - | - |
| A. caledonicum | all | 0.0–0.3 | - | 1.0–2.3 | 41.0–44.9 | 1.4–5.8 | - | - | - | - | - | - |
| B. bernieri | BB1 | - | - | 0.4–2.3 | - | 0.0-0.8 | - | - | - | - | - | - |
| MET038 | - | - | 1.3 | - | 1.0 | - | - | 1.0 | - | - | - | |
| B. francana | MET032 | - | - | 1.4 | - | - | - | 1.0 | 0.6 | - | - | 1.5 |
| MET062,65 | - | 0.0–0.6 | 1.9–8.7 | - | - | 0.0–1.9 | - | - | 0.0–0.8 | 86.0–90.1 | - | |
| MET106 | 11.9 | 5.0 | 1.8 | - | 0.3 | - | - | 5.0 | - | - | - | |
| B. marginata | MET048 | - | - | 1.0 | - | - | - | - | - | - | - | - |
| B. parisii | MET109A | - | - | 6.4 | - | - | - | - | - | - | - | - |
| B. serrifolia | BS1 | - | - | - | 0.4–0.8 | - | - | - | - | - | - | - |
| BS2 | - | - | 0.0–3.6 | - | - | - | - | - | - | - | - | |
| B. vittata | all | - | - | 12.9–13.4 | - | - | - | 2.9–5.5 | - | 7.4–10.0 | - | - |
Aris.: aristolane-, Arom.: aromadendrane-,BG: bicyclogermacrane-, Eude.: eudesmane-, Patc.: patchoulane- and Vala.: valancane-type.
Table 5.
Distribution of detected sesquiterpene types affiliated with the bisaboyl cation (relative percentage, %).
| Species | Samples (METXXX) | Barbatane | Bazzanane | Cuparane | Thujopsane | Myltaylane | Chamigrane | Cedrane | Acorane | Bisabolane |
|---|---|---|---|---|---|---|---|---|---|---|
| A. tenax | MET116 | - | - | - | - | - | 1.1 | - | - | - |
| A. caledonicum | all | - | - | - | - | - | 6.8–10.2 | - | 1.3–2.0 | 2.4–4.0 |
| B. bernieri | BB1 | 1.1–4.2 | 5.6–14.9 | 3.5–28.6 | - | 0.9–6.3 | 0.9–5.0 | 0.0–1.5 | 0.0–0.6 | 0.6–2.0 |
| MET038 | - | - | 3.2 | - | 1.0 | - | - | - | - | |
| B. francana | MET032 | 0.7 | - | 2.9 | - | 0.8 | - | - | - | 1.0 |
| MET062, 65 | 0.0–1.6 | - | - | - | - | - | - | - | - | |
| MET106 | 8.5 | 0.6 | 40.4 | - | 1.8 | 9.8 | - | - | - | |
| B. marginata | MET048 | - | - | 97.7 | - | - | - | - | - | |
| B. parisii | MET109A | 19.4 | 21.4 | 2.0 | 5.7 | - | 6.4 | - | 1.4 | |
| B. serrifolia | BS1 | 0.0–1.5 | 2.4–3.2 | 9.3–23.0 | - | 0.0–6.3 | 0.9–2.0 | 0.2–0.5 | - | 0.7–0.9 |
| BS2 | 0.0–4.5 | 0.0–1.3 | 0.8–8.2 | - | 0.0–0.4 | - | - | - | - | |
| B. vittata | all | - | - | 0.0–1.3 | - | - | - | - | - | - |
Table 6.
Distribution of detected pinguisane-, monocyclofarnesane-, drimane-type sesquiterpenes and sesquiterpene types belonging with the (Z-E)-humulyl cation, (E-E)-humulyl cation and (Z-E)-germacradienyl cation precursors (relative percentage, %).
| Species | Samples (METXXX) | Cation from First Cyclization Precursor | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| ___________Other___________ | __________(E-E)-Humulyl__________ | (Z-E)-Germacradienyl | __(Z-E)-Humulyl__ | |||||||
| Ping. | Mono. | Drimane | Africane | Humulane | Cary. | Calamenane | Hima. | Longi. | ||
| A. tenax | MET116 | - | - | - | - | - | - | - | - | - |
| A. caledonicum | all | - | - | - | - | 8.0–10.1 | 0.6–1.3 | - | - | 3.4–3.8 |
| B. bernieri | BB1 | - | - | - | 0.0–1.5 | - | 0.0–23.8 | - | - | - |
| MET038 | - | - | - | - | - | - | - | - | 2.8 | |
| B. francana | MET032 | 3.6 | 58.8 | 3.6 | 1.1 | 1.0 | 3.1 | - | - | - |
| MET062, 65 | - | - | - | - | - | - | - | - | - | |
| MET106 | 1.7 | - | - | 0.9 | - | - | - | - | - | |
| B. marginata | MET048 | - | - | - | - | - | - | - | 0.4 | - |
| B. parisii | MET109A | - | - | - | - | - | - | - | 1.0 | - |
| B. serrifolia | BS1 | 0.3–0.4 | - | - | 5.4–6.5 | 0.0–3.5 | 29.6–38.0 | - | 0.0–0.7 | - |
| BS2 | - | - | - | - | - | - | 0.0–2.3 | - | 0.4–1.6 | |
| B. vittata | all | 1.8–10.1 | 13.9–16.0 | - | - | - | - | - | - | - |
Mono.: monocyclofarnesane-, Ping.: pinguisane-, Cary.: caryophyllane-, Hima.: himachalane-, Longi.: Longifolane-type.
Table 7.
Main constituents of studied Bazzanioideae species.
| Species | Samples (METXXX) | Main Detected Compounds by GC-FID-MS | Characteristics | |
|---|---|---|---|---|
| Sesquiterpene | Diterpene | |||
| A. caledonicum | 116 | isolepidozene (25) (41.0–49.0%) α-humulene (34) (8.0–13.4%) |
isolepidozene (25) | |
| A. tenax | all | isolepidozene (25) (51.5%) elema-1,3,7(11),8-tetraene (24) (11.2%) |
kaur-16-en-19-ol (44) (7.5%) kaur-16-ene (43) (6.7%) |
|
| B .bernieri | BB1 | β-bazzanene (13) (5.6–14.9%) δ-cuprenene (4) (1.8–25.2%) |
fusicocca-2,5-diene (42) (29.3–53.7%) | fusicoccane-type diterpene and cuparane-type sesquiterpene |
| 038 | fusicocca-2,5-diene (42) (62.6%) | fusicoccane-type diterpene | ||
| B. francana | 032 | striatol (38) (57.9%) | striatane-type sesquiterpene | |
| 062 065 |
ziera-12(13),10(14)-dien-5-ol (23) (86.0–90.1%) | zierane-type sesquiterpene | ||
| 106 | β-microbiotene (8) (29.0%) γ-maaliene (26) (11.9%) α-chamigrene (18) (9.8%) |
(Z)-biformene (45) (8.9%) | labdane-type diterpene and microbiotane-type sesquiterpene |
|
| B. marginata | 048 | β-herbertenol (6) (95.9%) | cuparane-type sesquiterpene | |
| B. parisii | 109A | β-bazzanene (13) (21.5%) β-barbatene (16) (17.8%) |
(12Z)-abienol (46) (10.7%) | bazzanane- and barbatane-type sesquiterpene |
| B. serrifolia | BS1 | β-caryophyllene (35) (29.6–38.0%) african-1-ene (36) (5.2–6.2%) |
fusicocca-2,5-diene (42) (50.4–72.6%) | fusicoccane-type diterpene |
| BS2 | δ-cuprenene (4) (8.6–21.9%) | fusicocca-2,5-diene (42) (16.1–22.0%) | fusicoccane-type diterpene and cuparane-type sesquiterpene |
|
| B. vittata | all | 4,4-dimethyl-3-(3-methylbut-3-enylidene)-2-methylenebicyclo[4.1.0]heptane (37) (14.0–16.0%) viridiflorol (21) (10.8–13.5%) |
fusicocca-2,5-diene (42) (8.9–21.0%) | fusicoccane-type diterpene and monocyclofarnesane-type sesquiterpene |
Table 8.
Sesquiterpene composition (relative percentage, %) of the analyzed samples.
| Species | RIexp | B. p | ________B. f________ | ____B. s_____ | ____B. b____ | B. m | B. v | A. t | A. c | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Samples (METXXX) | 109A | 065, 062 | 106 | 032 | BS1 | BS2 | BB1 | 038 | 048 | all | 116 | all | |
| 2,4-patchouladiene | 1370.2 | - | - | - | 1.5 | - | - | - | - | - | - | - | - |
| anastreptene | 1377.9 | - | 0.0–0.5 | - | - | - | - | - | - | - | - | - | - |
| african-1-ene (36) | 1381.8 | - | - | 0.9 | 1.1 | - | 5.2–6.2 | 0.0–1.5 | - | - | - | - | - |
| cyclomyltaylane | 1381.8 | - | - | - | - | - | - | 0.0–2.9 | - | - | - | - | - |
| african-2-ene | 1397.3 | - | - | - | - | - | 0.2–0.2 | - | - | - | - | - | - |
| β-elemene | 1397.3 | - | - | 0.3 | - | - | - | - | - | - | - | - | - |
| aristol-1(2),9(10)-diene | 1426.0 | - | 0.0–0.7 | - | - | - | - | - | - | - | - | - | - |
| (+)-acora-3,7(14)-diene (11) | 1426.0 | - | - | - | - | - | - | - | - | - | - | - | 0.8–1.4 |
| M = 202, 91, 105(90) | 1431.2 | - | - | - | 1.6 | - | - | - | - | - | - | - | - |
| α-barbatene (15) | 1432.6 | 1.6 | - | - | - | - | - | - | - | - | - | - | - |
| α-microbiotene (9) | 1434.3 | - | - | 4.4 | - | - | - | - | - | - | - | - | - |
| (E)-caryophyllene (35) | 1434.3 | - | - | - | 3.1 | - | 29.6–38.0 | 0.0–23.8 | - | - | - | - | 0.6–1.7 |
| γ-maaliene (26) | 1442.6 | - | - | 11.9 | - | - | - | - | - | - | - | - | 0.0-0.3 |
| calarene (27) | 1446.7 | - | - | 5.0 | - | - | - | - | - | - | - | - | - |
| cis-thujopsene (28) | 1450.8 | 5.7 | - | - | - | - | - | - | - | - | - | - | - |
| α-chamigrene (18) | 1450.8 | - | - | 9.8 | - | - | 0.3–0.9 | 0.0–1.8 | - | - | - | 1.1 | 1.2–3.9 |
| α-pinguisene (40) | 1459.1 | - | - | 1.7 | - | - | 0.3–0.5 | - | - | - | 1.8–10.1 | - | - |
| β-barbatene (16) | 1467.4 | 17.8 | 0.0–1.6 | 8.5 | 0.7 | - | 0.0–1.5 | 1.1–4.2 | - | - | - | - | - |
| α-humulene (34) | 1467.4 | - | - | - | - | - | 0.0–3.5 | - | - | - | - | - | 8.0–13.4 |
| myltayl-8,12-ene (14) | 1471.5 | - | - | 1.8 | 0.8 | 0.0–0.4 | 0.0–6.3 | 0.9–3.5 | 1.0 | - | - | - | - |
| allo-aromadendrene (19) | 1475.6 | 3.5 | 1.0–6.8 | - | - | - | - | - | - | - | 0.0–2.1 | - | - |
| striatene | 1488.0 | - | - | - | 0.8 | - | - | - | - | - | - | - | - |
| 4-epi-α-acoradiene (12) | 1488.0 | - | - | - | - | - | - | 0.0–0.6 | - | - | - | - | 0.5–0.6 |
| (+)-β-microbiotene (8) | 1496.3 | - | - | 29.0 | - | - | - | - | - | - | - | - | - |
| herbertene | 1496.3 | - | - | - | - | - | - | - | - | 1.0 | - | - | - |
| β-chamigrene (17) | 1496.3 | 6.5 | - | - | - | - | 0.6–1.1 | 0.9–3.4 | - | - | - | - | 5.6–7.2 |
| M = 234, 161, 203 (70) | 1496.0 | - | |||||||||||
| isolepidozene (25) | 1500.4 | - | - | - | - | - | 0.4–0.8 | - | - | - | - | 51.5 | 41.0–49.0 |
| M = 220, 110, 91(60) | 1503.3 | - | - | - | 0.4 | - | - | - | - | - | - | - | - |
| M = 204, 91, 77 (97) | 1501.9 | - | 0.0–2.9 | - | 0.4–0.5 | - | - | - | - | - | - | - | |
| cis-γ-bisabolene | 1513.5 | - | - | - | - | - | - | - | - | - | - | - | 1.6–2.0 |
| isobarbatene | 1513.5 | - | - | - | - | 0.0–0.6 | - | - | - | - | - | - | - |
| β-bisabolene | 1513.5 | - | - | - | - | - | 0.0–0.2 | 0.0–0.6 | - | - | - | - | - |
| α-cuprenene (7) | 1517.8 | - | - | 1.6 | 2.8 | - | - | - | - | - | - | - | - |
| β-himachalene | 1517.8 | 1.0 | - | - | - | - | 0.0–0.7 | - | - | - | - | - | - |
| β-longipinene (32) | 1522.2 | - | - | - | - | - | - | - | - | - | - | - | 3.4–3.8 |
| cuparene (2) | 1522.2 | 1.4 | - | 1.1 | - | 0.0–0.8 | 0.7–1.1 | 1.7–3.3 | 1.5 | - | - | - | - |
| 1,5,9-trimethyl-1,5,9-cyclododecatriene | 1526.5 | - | - | - | - | - | - | 0.0–0.4 | - | - | - | - | - |
| trans-calamenene | 1530.9 | - | - | - | - | 0.0–0.6 | - | - | - | - | - | - | - |
| germacrene B | 1530.9 | - | 0.0–1.9 | - | - | - | - | - | - | - | - | - | - |
| 1,7-di-epi-β-cedrene | 1530.9 | - | - | - | - | - | 0.2–0.5 | 0.0–1.5 | - | - | - | - | - |
| M = 204, 93, 121 (95) | 1539.0 | - | - | - | - | - | - | - | - | - | - | - | 0.6–0.7 |
| β-bazzanene (13) | 1539.6 | 21.5 | - | 0.6 | - | 0.0–1.3 | 2.4–3.2 | 5.6–14.9 | - | - | - | - | - |
| ar-himachalene (30) | 1548.3 | - | - | - | - | - | - | - | - | 0.2 | - | - | - |
| γ-cuprenene (4) | 1548.3 | - | - | 2.0 | - | - | - | - | - | - | - | - | - |
| khusien-12-al | 1548.3 | - | - | - | 0.6 | - | - | - | 1.0 | - | - | - | - |
| striatol (38) | 1561.3 | - | - | - | 57.9 | - | - | - | - | - | - | - | - |
| δ-cuprenene (3) | 1565.7 | - | - | 1.2 | - | 0.8–7.4 | 8.6–21.9 | 1.8–25.2 | 1.7 | - | 0.0–1.3 | - | - |
| eudesma-4(15),7-dien-1β-ol | 1574.3 | - | - | - | 1.1 | - | - | - | - | - | - | - | - |
| M = 220, 91, 119 (90) | 1575.1 | - | - | - | - | 0.0–1.2 | - | 3.1 | - | - | - | - | |
| vetiselinenol | 1578.7 | - | - | - | - | - | - | - | - | - | 0.0–2.2 | - | - |
| 8α-hydroxy-eudesma-3,11-diene | 1583.0 | - | - | - | - | - | - | - | - | - | 2.9–3.4 | - | - |
| 4,4-dimethyl-3-(3-methylbut-3-enylidene)-2-methylenebicyclo[4.1.0]heptane (37) | 1591.7 | - | - | - | - | - | - | - | - | - | 14.0–16.0 | - | - |
| spathulenol (20) | 1591.7 | - | 0.9–1.4 | - | 1.0 | 0.0–3.6 | - | 0.0.4–2 | 0.5 | 1.0 | - | 10.6 | 0.8–2.3 |
| viridiflorol (21) | 1600.5 | - | - | 1.8 | 0.4 | - | - | 0.0–0.3 | 0.8 | - | 10.8–13.5 | - | - |
| M = 220, 79, 55 (70) | 1596.9 | - | - | - | 0.5 | 0.0–0.2 | - | 0.0–0.4 | 0.8 | - | - | - | - |
| α-guaiol (22) | 1609.5 | - | 0.0–0.8 | - | - | - | - | - | - | - | 7.4–10.0 | - | 0.0–0.4 |
| M ≥ 216, 91, 135 (75) | 1610.3 | - | 0.0–1.1 | - | 1.5 | - | - | - | - | - | - | - | - |
| M = 220, 91, 105 (80) | 1610.3 | - | - | - | - | - | - | 0.0–0.4 | 3.6 | - | - | - | - |
| humulene epoxide I | 1614.0 | - | - | - | 1.0 | - | - | - | - | - | - | - | - |
| M = 218, 137, 95 (95) | 1606.9 | - | - | - | - | 0.2–0.2 | - | - | - | - | - | 0.4 | - |
| M = 220 105, 91 (95) | 1609.8 | - | - | - | - | - | 0.5–1.9 | - | - | - | - | - | - |
| M = 218, 91, 175 (85) | 1620.0 | - | - | 0.9 | 0.8 | - | - | - | - | - | - | - | - |
| M = 220, 159, 145 (80) | 1621.4 | - | - | - | - | - | - | - | - | - | 0.0–6.6 | - | - |
| α-bisabolene | 1627.6 | - | - | - | 1.0 | - | - | 0.0–0.7 | - | - | - | - | 0.3–2.5 |
| M = 232, 145, 91 (90) | 1633.0 | - | - | - | 0.5 | - | - | - | - | - | - | - | - |
| M = 218, 161, 91 (60) | 1640.2 | - | - | - | 0.4 | - | - | - | - | - | - | - | - |
| M = ?, 105, 77 (75) | 1634.9 | - | - | - | - | 0–0.4 | - | - | - | 0.0–6.0 | 4.5 | 0.0–1.0 | |
| microbiotol (10) | 1645.7 | - | - | 1.1 | - | - | - | - | - | - | - | - | - |
| M = 218, 91, 79 (65) | 1648.0 | - | - | - | - | - | - | - | - | - | - | - | 0.4–1.1 |
| gymnomitr-3(15)-ene-4α-ol | 1651.5 | - | - | - | - | 0.0–3.9 | - | - | - | - | - | - | - |
| M = 234, 91, 107 (95) | 1648.9 | - | - | - | 0.9 | - | - | - | - | - | - | - | - |
| ziera-12(13),10(14)-dien-5-ol (23) | 1659.3 | - | 86.0–90.1 | - | - | - | - | - | - | - | - | - | - |
| M = 218, 91, 145 (85) | 1662.4 | - | - | - | 1.6 | - | - | - | - | - | - | - | |
| M = 234, 91, 145 (95) | 1679.3 | - | - | 0.3 | 0.5 | - | - | - | - | - | - | - | - |
| M = ?, 93, 67 (50) | 1682.7 | - | - | - | - | - | - | - | - | - | - | - | 1.4–4.6 |
| M = 236, 112, 91 (80) | 1683.7 | - | - | - | 0.6 | - | 0.0–1.1 | - | 1.6 | - | - | - | - |
| α-bisabolol | 1691.0 | - | - | - | - | - | 0.7–0.7 | 0.4–1.1 | - | - | - | - | - |
| (+)-β-herbertenol (6) | 1709.6 | - | - | - | - | - | - | - | - | 0.3 | - | - | - |
| 7-isopropyl-4α-methyloctahydro-2(1H)-naphthalenone (29) | 1714.4 | - | - | - | - | - | - | - | - | - | - | - | 1.2–2.9 |
| M = 234, 91, 105 (45) | 1711.4 | - | - | - | 1.4 | - | - | - | - | - | - | - | - |
| 5-hydroxycalamenene | 1728.8 | - | - | - | - | 0.0–1.7 | - | - | - | - | - | - | - |
| M = 234, 91, 159 (70) | 1737.0 | - | - | - | 2.5 | - | - | - | - | - | - | - | - |
| α-herbertenol (5) | 1748.1 | 0.7 | - | - | - | - | - | - | - | 0.5 | - | - | - |
| 4-epi-marsupellol (33) | 1748.1 | - | - | - | - | 0.4–1.6 | - | - | 2.8 | - | - | - | - |
| naviculol (39) | 1752.9 | - | - | - | 3.6 | - | - | - | - | - | - | - | - |
| elema-1,3,7(11),8-tetraene (24) | 1752.9 | - | - | - | - | - | - | 0.0–0.8 | 1.0 | - | - | 11.2 | 1.4–5.8 |
| M = 234, 161, 219 (75) | 1764.2 | - | - | - | - | - | - | - | - | - | 0.0–12.0 | - | - |
| γ-curcumen-15-al | 1770.9 | 1.4 | - | - | - | - | - | - | - | - | - | - | - |
| (−)-β-herbertenol | 1781.7 | - | - | - | - | - | - | - | - | 95.9 | - | - | - |
| drimenol (41) | 1786.5 | - | - | - | 3.6 | - | - | - | - | - | - | - | - |
| M = 218, 107, 91(45) | 1788.3 | - | - | - | - | - | 1.5–3.4 | 0.0–0.9 | 4.5 | - | - | - | - |
| M = 220, 91, 105 (70) | 1796.5 | - | - | - | - | - | 0.0–1.1 | - | - | - | - | - | - |
| ar-himachalen-2-ol (31) | 1821.0 | - | - | - | - | - | - | - | - | 0.2 | - | - | - |
| M ≥ 236, 69, 95(90) | 1821.5 | - | - | - | - | - | - | 0.0–1.2 | - | - | - | - | 0.6–1 |
| M = ?, 91, 95 (70) | 1820.9 | - | - | - | - | - | 0.8–1.4 | - | - | - | - | - | - |
| M ≥ 234, 145, 91 (95) | 1831.1 | - | - | - | 0.8 | - | - | - | - | - | - | - | - |
| M = 248, 91, 105 (95) | 1926.1 | - | - | - | - | - | - | - | - | - | - | - | 4.7–7.8 |
| M = 248, 163, 91 (70) | 1935.5 | - | - | - | - | - | - | - | - | - | - | - | 0.6–1.3 |
| M = 236, 69, 55 (85) | 1990.3 | - | - | - | - | - | 0.0–1.3 | - | - | - | - | - | - |
| determined sesquiterpene | 61.0 | 93.9–97.8 | 82.4 | 81.0 | 2.2–10.3 | 62.6–73.9 | 32.3–60.0 | 11.4 | 99.1 | 47.2–48.1 | 74.4 | 74.6–87.0 | |
| undetermined sesquiterpene | 2.5 | 0.0–3.9 | 1.2 | 14.0 | 3.5–8.9 | 0.8–0.8 | 0.3–1.4 | 13.6 | - | 12.0–12.6 | 4.9 | 10.8–16.4 | |
B. p: Bazzania parisii, B. f: B. francana, B. s: B. serrifolia, B. b: B. bernieri, B. m: B. marginata, B. v: B. vittata, A. t: Acromastigum tenax, A. c: A. caledonicum.
Table 9.
Diterpene composition (relative percentage, %) of the analyzed samples.
| Species | RIexp | B. p | ________B. f________ | ____B. s_____ | ____B. b____ | B. m | B. v | A. t | A. c | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample (METXXX) | 109A | 065, 062 | 106 | 032 | BS1 | BS2 | BB1 | 038 | 048 | all | 116 | all | |
| M = 272, 121, 229 (70) | 1802.0 | - | - | - | - | 0.4–0.5 | 1.1–1.7 | 2.5 | - | - | - | - | |
| neophytadiene I | 1831.0 | - | - | 2.2 | 0.4 | 0.0–0.3 | 0.0–0.4 | 0.0–0.6 | 0.7 | 0.3 | 3.4–13 | 0.7 | 0.3–1.8 |
| neophytadiene II | 1841.0 | - | - | - | - | - | - | 0.3–0.9 | - | - | 0.0–6.8 | 1.8 | 0.9–5.3 |
| M ≥ 272,135, 122 (45) | 1852.4 | 0.2 | - | - | - | 0.5–0.5 | - | - | - | - | - | - | |
| neophytadiene III | 1866.0 | - | - | - | - | - | - | - | - | - | - | 0.3 | - |
| M = 272, 135, 91 (80) | 1881.7 | - | - | - | - | 0.0–0.5 | - | - | - | - | - | - | |
| labda-7,13,14-triene | 1891.0 | - | - | - | - | 0.0–1.0 | 0.0–0.7 | - | - | - | - | - | - |
| M ≥ 281, 95, 107 (70) | 1976.9 | - | - | - | - | - | 0.0–0.3 | - | - | - | - | - | |
| (Z)-biformene iso1 (45) | 2000.5 | - | 0.0–0.4 | 8.9 | 0.4 | 0.0–1.7 | 2.3–2.8 | 3.5–7.5 | 0.7 | - | 0.0–2.2 | - | - |
| M = 272, 73, 91 (15) | 2009.1 | - | - | - | - | 5.9–6.8 | - | - | - | 0.0–2.3 | - | - | |
| 13-epi-manoyl oxide (47) | 2016.9 | 4.3 | 0.8–1.2 | - | 0.5 | - | - | - | - | - | - | - | - |
| fusicocca-2,5-diene (42) | 2022.4 | 4.1 | 0.6–1.0 | 3.2 | 1.6 | 50.4–72.6 | 16.1–22.3 | 29.3–53.7 | 62.6 | 0.3 | 8.2–21.0 | - | - |
| (Z)-biformene iso 2 | 2027.9 | - | 0.0–0.4 | 2.1 | - | - | - | - | - | - | - | - | - |
| manoyl oxide | 2038.8 | - | - | - | 0.3 | - | - | - | - | - | - | - | - |
| M ≥ 288, 179, 81 (70) | 2044.6 | 0.4 | - | - | - | - | - | - | - | - | - | ||
| fusicocca-3,5-diene | 2060.7 | - | - | - | - | 0.0–0.2 | 0.0–0.4 | 1.1 | - | - | - | - | |
| M ≥ 272, 95, 81 (45) | 2066.8 | - | - | - | - | 0.0–3.2 | - | - | - | - | - | ||
| (−)-kaur-16-ene (43) | 2071.6 | - | - | - | - | - | - | - | - | - | 6.7 | 0.0–1.2 | |
| M ≥ 270, 69, 105 (80) | 2103.1 | - | - | - | - | - | 0.9 | - | - | - | - | ||
| M> = 278, 71, 95 (85) | 2110.5 | - | - | - | 0.7 | 0.0–0.5 | 0.0–1.1 | 0.6 | - | 0.0–8.9 | 0.8 | 0.3–0.8 | |
| (12Z)-abienol (46) | 2196.6 | 10.7 | - | - | - | - | - | - | - | - | - | - | - |
| M ≥ 270, 105, 119 (90) | 2212.0 | - | - | - | - | - | 0.0–2.6 | - | - | - | - | - | - |
| M = 288, 95, 107(80) | 2256.5 | - | - | - | - | - | 0.0–1.6 | - | - | - | - | - | - |
| M ≥ 286, 95, 107 (90) | 2294.0 | - | - | - | - | - | 0.0–4.3 | - | - | - | - | - | - |
| M ≥ 341, 95, 147 (90) | 2294.6 | 1.1 | - | - | - | - | - | - | - | - | - | - | - |
| M ≥ 286, 79, 91 (95) | 2310.2 | - | - | - | - | - | 0.0–5.1 | - | - | - | - | - | - |
| M ≥ 286, 95, 119 (90) | 2321.8 | - | - | - | - | - | 0.0–8.0 | - | - | - | - | - | - |
| M = 286, 81, 95 (85) | 2325.3 | - | - | - | - | 0.0-0.3 | - | - | - | - | - | - | 0.0–0.6 |
| M ≥ 286, 95, 243 (80) | 2339.9 | - | - | - | - | - | 0.0–3.5 | - | - | - | - | 2.5 | 0.0–0.2 |
| M ≥ 355, 83, 286 (90) | 2353.9 | 1.1 | - | - | - | - | - | - | - | - | - | - | - |
| M ≥ 286, 91, 243 (85) | 2355.6 | - | - | - | - | - | 0.0–2.0 | - | - | - | - | - | - |
| kaur-16-en-19-ol (44) | 2390.7 | - | - | - | - | - | - | - | - | - | - | 7.5 | 0.0–1.5 |
| M ≥ 288, 137, 19 (55) | 2402.9 | - | - | - | - | 0.0–0.6 | 3.8–5.5 | - | - | - | - | - | - |
| M ≥ 286, 121, 79 (85) | 2405.3 | 12.9 | - | - | - | - | - | - | - | - | - | - | - |
| M ≥ 286,95, 107 (50) | 2408.7 | - | - | - | - | 0.0–0.5 | - | - | - | - | - | - | - |
| M ≥ 286, 55, 95 (95) | 2413.5 | - | - | - | - | - | 1.7–3.9 | - | - | - | - | - | - |
| M ≥ 286,137, 95 (80) | 2487.2 | - | - | - | - | - | 1.4–6.7 | - | - | - | - | - | - |
| determined diterpene | 19.1 | 2.1–2.2 | 16.4 | 3.2 | 50.4–74.7 | 18.4–26.3 | 34.6–60.8 | 65.1 | 0.6 | 20.5–34 | 17.0 | 1.3–7.1 | |
| undetermined diterpene | 15.5 | - | - | 0.7 | 11.1–29.7 | 6.9–10.4 | 2.7–7.1 | 4.0 | - | 0.0–11.1 | 3.2 | 0.3–1.2 | |
B. p: Bazzania parisii, B. f: B. francana, B. s: B. serrifolia, B. b: B. bernieri, B. m: B. marginata, B. v: B. vittata, A. t: Acromastigum tenax, A. c: A. caledonicum.
Table 10.
Non terpenic constituent composition (relative percentage, %) of the analyzed samples.
| Species | RIexp | B. p | ________B. f________ | ____B. s_____ | ____B. b____ | B. m | B. v | A. t | A. c | ||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample (METXXX) | 109A | 065, 062 | 106 | 032 | BS1 | BS2 | BB1 | 038 | 048 | all | 116 | all | |
| alkane | 1492.1 | - | - | - | - | - | - | - | - | - | 0.0–0.4 | - | - |
| ethyl p-ethoxybenzoate | 1526.5 | 0.5 | - | - | - | - | - | - | - | - | 3.5–6.7 | 0.5 | - |
| 1-(2-benzyloxyethyl) cyclohexene | 1605.0 | - | - | - | - | 0.0–1.7 | - | 0.0–0.8 | 2.9 | - | - | - | - |
| 1,5-diphenyl-1,4-pentadien-3-one | 1641.2 | 0.8 | - | - | - | - | - | - | - | - | - | - | - |
| alkane | 1651.5 | - | - | - | 1.1 | - | - | - | - | 0.1 | - | - | - |
| methyl 4,7-octadecadiynoate | 1686.4 | - | - | - | - | - | - | - | - | - | - | - | 0.7–1.5 |
| (2-methylene-cyclohexyl)-phenyl-methanol | 1767.3 | - | - | - | - | 0.0–1.8 | - | 0.0–0.7 | 3.1 | - | - | - | - |
| aliphatic alcohol | 1995.3 | - | - | - | - | - | - | - | - | 0.2 | - | - | - |
| aliphatic alcohol | 2082.5 | 0.6 | - | - | - | - | - | - | - | - | 0.0–3.8 | - | - |
| total | 2.0 | - | - | 1.1 | 0.6–3.4 | - | 0.3–1.5 | 6.0 | 0.3 | 6.7–7.7 | 0.5 | 0.7–1.5 | |
B. p: Bazzania parisii, B. f: B. francana, B. s: B. serrifolia, B. b: B. bernieri, B. m: B. marginata, B. v: B. vittata, A. t: Acromastigum tenax, A. c: A. caledonicum.
Figure 2.
Selected compound structures detected in the studied species.
2. Results
2.1. Acromastigum tenax, Bicyclogermacrane-Type
A. tenax is characterized by sesquiterpene components following the (E-E)-germacradienyl pathway (Table 4): isolepidozene (25) (51.5%) (previously found in Bazzania tricrenata [15]), elema-1,3,7(11),8-tetraene (24) (11.2%) and spathulenol (20) (10.6%) were so detected.
α-Chamigrene (18) (1.1%) was the only identified sesquiterpene constituent which did not belong to the (E-E)-germacradienyl pathway. Regarding diterpene content, only kaurane-type diterpenes were detected in A. tenax, namely kaur-16-en-19-ol (44) (7.5%) and kaur-16-ene (43) (6.7%). According to the literature, this is the first report of the occurrence of these two diterpene components in Bazzanioideae, although kaurane-type diterpenes had been previously found in Bazzania.
2.2. Acromastigum caledonicum, Bicyclogermacrane-Type
Three specimens of A. caledonicum were studied, their volatile contents were quite similar and characterized by compounds provided by the (E-E)-germacradienyl cation (Table 4). We detected high relative percentage values of isolepidozene (25) (41.0–49.0%) in the three specimens. Elema-1,3,7(11),8-tetraene (24) (1.4–5.8%) and 7-isopropyl-4α-methyloctahydro-2(1H)-naphthalenone (29) (1.2–2.9%) were also detected. The following sesquiterpenes belonging to the bisaboyl cation precursor were detected in the three specimens: β-chamigrene (17) (5.6–7.2%), α-chamigrene (18) (1.2–3.9%), acora-3,7(14)-diene (11) (0.8–1.4%) and 4-epi-α-acoradiene (12) (0.5–0.6%), we noticed that acorane sesquiterpenoids are known to be rare in liverworts [15]. Identified sesquiterpene belonging to the (E-E)-humulyl cation were respectively α-humulene (34) (8.0–13.4%) and β-caryophyllene (35) (0.6–1.7%). The only identified compound belonging to the (Z-E)-humulyl cation was β-longipinene (32) (3.4–3.8%).
2.3 Bazzania francana: MET062 and MET065 Zierane-Type, MET106 Microbiotane-Type and MET032 Striatane-Type
Four specimens of B. francana were investigated and they were found to belong to three different terpene-type compositions. Fusicocca-2,5-diene (42) (0.6–3.2%) was the only common constituent of these four specimens. Fusicoccane-type diterpenes are widely distributed in the genera Plagiochila and Frullania [15].
2.3.1. Microbiotane-Type
Sesquiterpene content of MET106 belongs mainly to the bisaboyl cation pathway dominated by cuparane-type sesquiterpenes (widely distributed in liverworts [15]): β-microbiotene (8) (29.0%), α-microbiotene (9) (4.4%), microbiotol (10) (1.1%), cuparene (2) (1.1%), δ-cuprenene (3) (1.2%), α-cuprenene (7) (1.6%) and γ-cuprenene (4) (2.0%) were detected in this specimen. Other identified constituents following this pathway were mainly α-chamigrene (18) (9.8%) (found in several Bazzania species such as B. trilobata [20] and B. madagassa [21]), β-barbatene (16) (8.5%) (common in liverworts and encountered in the Lepidoziaceae family [15]) and myltayl-8,12-ene (14) (1.8%) (previously found in the Lepidoziaceae: Kurzia trichoclados [15] and Bazzania japonica [22]).
Compounds belonging to the (E-E)-germacradienyl cation precursor were also detected (Table 4): γ-maaliene (26) (11.9%) (detected in Lepidozia fauriana [23]), calarene (27) (5.0%) (also found in Bazzania japonica [22]) and viridiflorol (21) (1.8%), which possess different structure-skeleton but considered to be biosynthetically very close (Figure 3). (Z)-biformene (45) (8.9%), a labdane-type diterpene, was the main detected diterpene constituent.
Figure 3.
Overview of sesquiterpene types detected through the compiled biosynthesis schemes [9,10,11,12,13,14,15,16,17,18].
2.3.2. Striatane-Type
MET032 sample content is characterized by high percentage of striatol (38) (57.9%) whose structure is close to the monocyclofarnesane-type sesquiterpene structure.
Naviculol (39) (3.6%) (previously detected in Bazzania novae-zelandiae [24]), drim-7-en-11-ol (41) (3.6%) (=drimenol, the genera Bazzania and Porella are rich sources of drimane-type sesquiterpenes [15]), β-caryophyllene (35) (3.1%) and α-cuprenene (7) (2.8%) were detected in moderate relative percentages.
2.3.3. Zierane-Type
The specimens MET062 and MET065 produced sesquiterpenes belonging to the (E-E)-germacradienyl cation, mainly ziera-12(13),10(14)-dien-5-ol (23) (86.0–90.1%) (new natural compound, structure is described below) and allo-aromadendrene (19) (1.0–6.8%).
Zierane-type sesquiterpenes are very rare in nature: zierene had been found in four different Plagiochila species [25] and three different zierane-type sesquiterpenes had been found in Saccogyna viticulosa [26], zierane-type sesquiterpene lactone had been found in Chandonanthus hirtellus [27]. This is the first report of zierane-type sesquiterpene regarding Bazzania genus.
2.3.4. Structural Elucidation of Ziera-12(13),10(14)-dien-5-ol (23)
Compound 23 was obtained as a light-orange oil. Its molecular formula was determined to be C15H26O based on the molecular ion peak at m/z 220.1830 [M•]+ (calcd. for C15H26O, 220.1827) as observed in the GC/HR-EI-MS, which corresponds to four degrees of unsaturation. The IR spectrum of 23 showed absorption at 3397.5 cm−1 (hydroxyl), 2985.3, 2923.9, 2854.1 cm−1 (alkane), 1377.4 cm−1 (methyl), 1437.5 cm−1 (methylene), 3086.3, 1636.7 cm−1 (alkene), 1149.8 cm−1 (ter-alcohol). The 13C-NMR (Table 1) and HSQC spectra revealed the presence of 15 carbon resonances including three quaternary carbons, three methine, seven methylene and two methyl groups. Among the three quaternary carbons, one was an oxygenated carbon according to its chemical shift at δC 87.2 and two were exo-methylene carbons according to their chemical shifts at δC 151.1, 150.1. All methine groups are alkane carbons according to their chemical shifts at δC 57.6, 49.9, 46.1. Among the seven methylenes, two of them were assigned as exo-methylene carbons according to their chemical shifts respectively at δC 112.5 and 110.2, then so indicative of the presence of two rings.
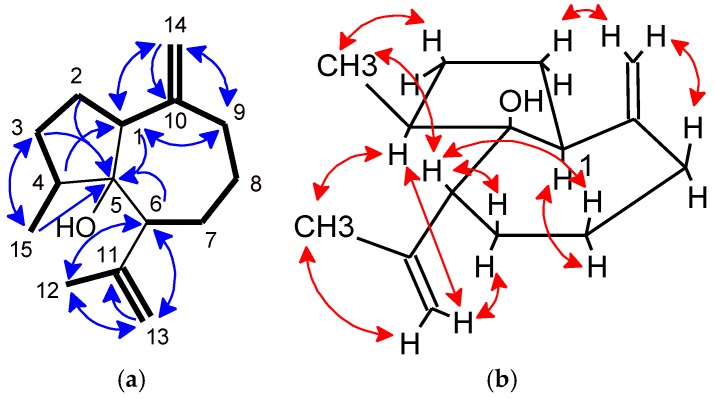
Analysis of 1H-NMR spectrum showed the presence of a secondary methyl at δH 0.91 (3H, d, 7.1, H-15) and two sets of exo-methylene groups resonating at δH 4.85 (1H, brs, H-13a), 4.78 (1H, brs, H-13b) and at δH 4.93 (1H, brs, H-14a), 4.92 (1H, brs, H-14b). The COSY spectra of 23 (Figure 4a) exhibited the presence of two spin systems, first with H-15 at δH 0.91 (d, 7.1, 3H), H-4 at δH 1.90 (m, 1H), H-3 at δH 2.16 (m, 1H) and 1.47 (m, 1H), H-2 at δH 2.01 (m, 1H) and 1.94 (m, 1H) and H-1 at δH 2.56 (t, 8.6, 1H). Then, linkages to a cyclopentane ring was deduced with HMBC correlations between H-3, H-2 and H-1 with C-5 at δC 87.2 (Figure 4a).
Figure 4.
(a) COSY correlations (bold) and HMBC (blue arrows) key correlations of compound (23); (b) Selected NOE (red arrows) correlations of compound (23).
The second spin system with H-9 at δH 2.52 (1H, brdt, 13.2, 4.8) and δH 2.01 (1H, m), H-8 at δH 1.94 (1H, m) and δH 1.44 (1H, m), H-7 at δH 2.03 (1H, m) and δH 1.52 (1H, m) and H-6 at δH 2.30 (1H, brd, 9.8), the HMBC correlation between H-6 and C-5 at δC 87.2, and the correlations between H-14 at δH 4.93 (1H, brs, H-14a) and δH 4.92 (1H, brs, H-14b) with C-1 at δC 57.6, C-9 at 40.9, C-10 at 151.1, led to establish a cycloheptane ring. The presence of HMBC correlations between exo-methylene protons H-13 at δH 4.85 (1H, brs, H-13a) and δH 4.78 (1H, brs, H-13b) with C-6 at δC 49.9, C-11 at δC 150.1, C-12 at δC 23.7 (Figure 4a) afforded to put in evidence the attachment of an isopropenyl moiety at C-6.
The relative configuration was deduced by the presence of NOE correlations between H-1 at δH 2.56 and H-4 at δH 1.90, and between H-6 at δH 2.30 and methyl H-15 at δH 0.91 showing that the protons H-1 and H-4 were in the same plane, H-6 and H-15 were in the other side (Figure 4b). In comparison with precursor zierene [26,28]), the relative configuration of compound (23) was established as rel-(1S, 4S, 5R, 6R) ziera-12(13),10(14)-dien-5-ol.
2.4. Bazzania bernieri, Fusicoccane- and Cuparane-Type
A total of nine specimens of B. bernieri were investigated. Diterpene compositions were very similar. Phytane-, labdane- and fusicoccane-type diterpenes were detected co-occurring in all specimens. Main detected diterpenes were fusicocca-2,5-diene (42) (29.3–62.6%) and (Z)-biformene (45) (0.7–7.5%). Volatile composition of the specimen MET038 is quite different from the other specimens, and so shown apart in the Table 4, Table 5, Table 6, Table 7, Table 8, Table 9 and Table 10. Results of the major specimens of B. bernieri (MET028, 031, 040, 047, 063, 066, 067, 069) were pooled under the appellation BB1.
Most of detected sesquiterpenes belong to the bisaboyl cation precursor: cuparane-, myltaylane- and acorane-type sesquiterpenes were detected in all specimens, mainly δ-cuprenene (3) (1.7–25.2%), myltayl-8,12-ene (14) (0.9–3.5%) and cuparene (2) (1.5–3.3%).
Other sesquiterpene types belonging to the bisaboyl cation such as barbatane- (1.1–4.2%), bazzanane- (5.6–14.9%), chamigrane- (0.9–5.0%) and bisabolane-type sesquiterpenes (0.6–2.0%) were detected in all specimens except in MET038 (Table 5) while 4-epi-marsupellol (33) (2.8%) was detected only in MET038. β-Caryophyllene (35) (0–23.8%) was detected in several specimens.
2.5. Bazzania serrifolia, Fusicoccane- and Cuparane-Type
Six specimens of B. serrifolia were investigated. Volatile compounds content of the specimens MET092 and MET099 were quite different from the others, so these specimens were pooled apart under the appellation BS1 and the other four ones (MET041, 051-053) were pooled under the appellation BS2 as presented in Table 4, Table 5, Table 6, Table 7, Table 8, Table 9 and Table 10.
Fusicocca-2,5-diene (42) was detected as the major volatile component within very variable relative percentages (16.1–72.6%).
We detected in B. serrifolia specimens various sesquiterpene types belonging to the bisaboyl cation pathway but only cuparane-type sesquiterpene was common to all of them, mainly δ-cuprenene (3) (0.8–21.9%). As shown in Table 6, the specimens MET092 and MET099 were different from the others since sesquiterpenes belonging to the (E-E)-humulyl cation were detected, these compounds were β-caryophyllene (35) (29.6–38.0%), african-1-ene (36) (5.2–6.2%) and α-humulene (34) (0.0–3.5%).
2.6. Bazzania vitatta, Bis(bibenzyl)/Aromadendrane-Type
Two specimens of B. vittata were studied. Volatile compounds contents of the two specimens were similar even if 10 constituents were identified for MET060, and 18 ones for MET049. B. vittata was characterized by the presence of aromadendrane-type sesquiterpenes mostly by the co-occurrence in both specimens of viridiflorol (21) (10.8–13.5%) and guaiol (22) (7.4–10.0%). α-Pinguisene (40) (1.8–10.1%) and fusicocca-2,5-diene (42) (8.2–21.0%) were also detected in both B. vittata specimens. Although monocyclofarnesane-type sesquiterpenes are rarely detected in the Jungermanniales class [15], B. vittata contained high relative percentage of 4,4-dimethyl-3-(3-methylbut-3-enylidene)-2-methylene bicyclo[4.1.0] heptane (37) (13.9–16.0%). Observed neophytadiene (10.2–13.0%) could be an artifact from phytol degradation (moiety of esterified side chain of chlorophyll-a) during GC-FID-MS analysis [29].
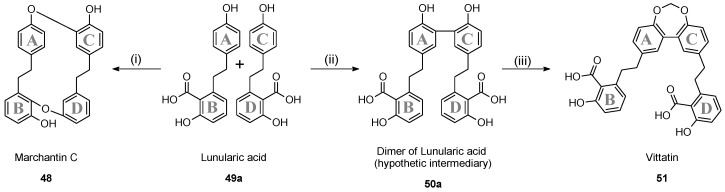
Large amount of a new natural compound called “vittatin”, a dimeric form of lunularic acid (51) was isolated from MET049 (47% of the crude ether extract, structural identification is described below). Lunularic acid (49a) was detected in numerous liverworts and algae but rarely in vascular plants and was known to play a similar biological role than abscisic acid found in vascular plants such as growth inhibitory [30]. Lunularic acid (49a) is known to possess fungicide, algaecide and anti-hyaluronidase activities [31]. Presence of this compound in MET060 was confirmed.
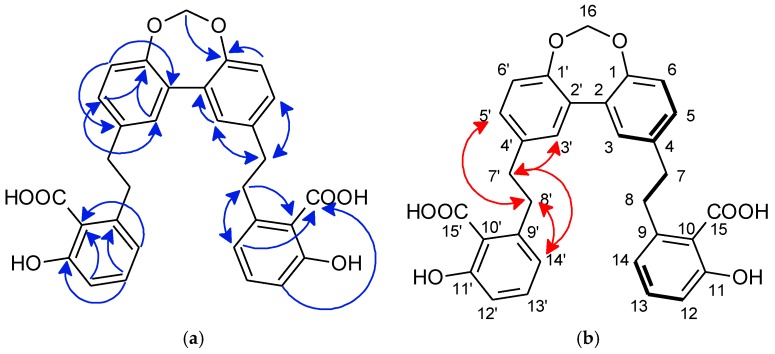
Biaryl meta-meta junction observed for vittatin (51) is a criterion for bis(bibenzyl)s structural classification. The methylenedioxy bond observed between the two bibenzyl units of vittatin (51) is very rare in bis(bibenzyl)s structures [32]. Putative pathway of vittatin (51) is proposed below as well as the role of its hypothetic precursor in the structural biosynthesis scheme of natural bis(bibenzyl)s.
2.6.1. Structural Elucidation of Vittatin (51)
Compound (51) was obtained as a flaky white amorphous powder. Its molecular formula was determined to be C31H26O8 based on the molecular ion peak at m/z 527.1700 [M + H]+ (calcd. for C31H27O8, 527.1700) observed in the HR-ESI-MS, corresponding to nineteen degrees of unsaturation. The IR spectrum of (51) showed absorption at 3414.6 cm−1 (hydroxyl), 2946.7, and 1445.6 cm−1 (methylene), 1608.3, 1575.1, 1496.8 cm−1 (aromatic ring), 1668.6 cm−1 (unsaturated carbonyl), 1250.1 cm−1 (carboxyl), 1205.1 cm−1 (phenol), 1411.2, 921.7 cm−1 (hydroxyl). The 13C-NMR (Table 2) and HSQC spectra revealed the presence of only 16 carbon resonances including seven quaternary carbons, six methine and three methylene groups, suggesting a dimer form. Among the seven quaternary sp2 hybridized carbons, one was attributed to a carbonyl carbon according to its chemical shift at δC 170.7 and six were attributed to aromatic carbons according to their chemical shifts at δC 156.6, 153.0, 141.0, 137.8, 128.3 and 119.9. All methine groups corresponded to aromatic carbons according to their chemical shifts at δC 130.9, 128.8, 128.3, 120.6, 120.4 and 114.0. Among the three methylene groups, one was methylenedioxy carbon according to its chemical shift at δC 98.9. The 1H and COSY correlations of (51) (Figure 5b) exhibited the presence of two aromatic systems, an ABX spin system as observed in the aromatic protons at δH 7.07 (2H, d, 8.2 H-6), 7.17 (2H, dd, 8.2, 2.0, H-5) and 7.51 (2H, d, 2.0, H-3) and a second aromatic system like an AX2 spin system as observed at δH 7.20 (2H, t, 7.9, H-13), 6.75 (2H, d, 7.9, H-12) and 6.76 (2H, d, 7.9, H-14), which indicated the presence of two tri-substituted aromatic rings 1,2,4 and 1,2,3 respectively. The 1H and COSY spectra showed also an ethylene group at δH 2.97 (4H, m, H-8), 2.89 (4H, m, H-7) attached to C-4 and C-9 by the presence of 3J HMBC correlation between H-7 and C-3 at δC 128.3, C-5 at δC 128.8 and between H-8 and C-10 at δC 119.9, C-14 at δC 120.4 (Figure 5a). NOE correlations were observed between H-7 and H-3 and between H-8 and H-14 (Figure 5b). The HMBC spectrum showed two small 4J correlations between H-12 at δH 6.75, H-14 at δH 6.76 and C-15 at δC 170.7 (Figure 5a) which put in evidence the attachment of carboxylic group at C-10. A hydroxyl group was fixed on C-11 within a characteristic 13C chemical shift at δC 156.6 and a 1H chemical shift at δH 10.43 due to an hydrogen bond established with the the carboxylic acid group at C-15. The signal on 1H-NMR spectrum at δH 5.55 (2H, s, H-16) was attributed to a methylenedioxy attached on C-1 determined by the presence of 3J HMBC correlation between H-16 and C-1 at δC 153.3 (Figure 5a). The 13C- and 1H-NMR data of this monomer structure were similar of lunularic acid NMR data [33], the dimer form corresponded to two monomers of lunularic acid attached at C-2 by meta-meta junction within a methylenedioxy bridge at C-1. The structure of this new compound (51) was named vittatin. Spectroscopic data are available in Supplementary Materials.
Figure 5.
(a) HMBC (blue arrows) key correlations of compound (51); (b) COSY (bold) and selected NOE (red arrows) correlations of compound (51).
2.6.2. Putative Biosynthesis of Vittatin (51)
Bis(bibenzyl)s are biosynthesized from lunularin (49b) or its precursor lunularic acid (49a). This assumption was supported by feeding experiments using radioactive and 13C-labelled precursors [34]. Marchantin C synthase (isolated from a cell culture of Marchantia polymorpha) was supposed to be involved in the coupling mechanism of two molecules of lunularic acid (49a) leading to marchantin C (48) (type II) [35].
Due to the functionalization of vittatin (51), hypothesis can be proposed suggesting that this bis(bibenzyl) should be formed by biaryl meta-meta coupling at the aromatic ring A (or C) of lunularic acid (49a) leading to a putative intermediate (50a). It is interesting to note that Momordica charantia peroxidase catalyzes biaryl meta-meta coupling with dihydroresveratrol as substrate [36]. The last step could be the formation of a methylenedioxy junction between the two phenol functions in para position of the aromatic cycle (A and C) leading to vittatin (51) as shown in Figure 6.
Figure 6.
Putative biosynthesis pathway of vittatin (51), (i): marchantin C synthase; (ii): biaryl coupling; (iii): methylenedioxy formation.
2.6.3. Structural Relationships in Natural Bis(bibenzyl)s: Role of Putative Intermediate of Vittatin (51)
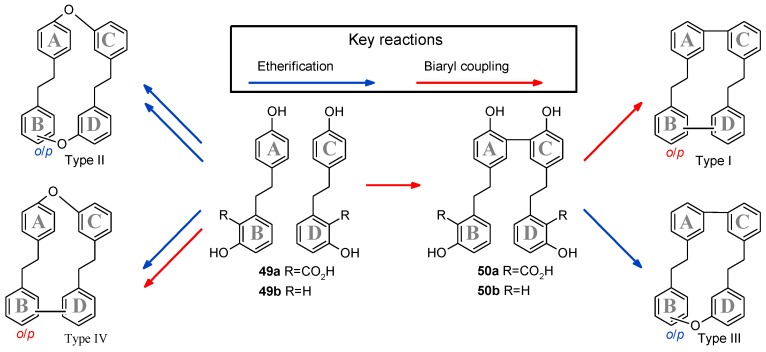
According to the literature, bis(bibenzyl)s are classified into four structural types (I–IV, Figure 7), each structure is composed of two bibenzyl units which differ from linkages between these units [37]. Due to its structure, the putative biosynthetic intermediate of vittatin (50) should be added into a previous global scheme of bis(bibenzyl) biosynthesis pathway [38] (Figure 7). This scheme highlights that the dimer of lunularin (50b) (=isoperrottetin A, isolated from Radula perrottetii [39]) and the dimer of lunularic acid (50a) might play the role of the precursor of the bis(bibenzyl)s of types I and III which encompassed more than 30 compounds [32].
Figure 7.
Structural relationships and interconversion of natural occuring bis(bibenzyl)s.
A survey of the literature showed that bis(bibenzyl)s from liverworts of types I and III were found only in the Jungermanniopsida class. These structures were detected in liverworts from the genera Herbertus, Lepidozia, Mastigophora, Plagiochila and Bazzania, which belong to the Lophocoleineae sub-order (Jungermanniales). Nevertheless, bis(bibenzyl) compounds with a (m-m)-(C-C) bond linkage had been detected in two species which do not belong to the Lophocoleineae sub-order: Jamesoniella colorata (Jungermanniineae) [40] and Radula perrottetii (Radulineae) [39].
2.7. Bazzania parisii, Cuparane-Type
Main sesquiterpenes detected in B. parisii belong to the bisaboyl cation pathway. The barbatane- and bazzanane-type sesquiterpenes (known to share the same precursor, cf. Figure 3) were found to be dominant with β-bazzanene (13) (21.5%), β-barbatene (16) (17.8%) and α-barbatene (15) (1.6%). β-Chamigrene (17) (6.5%) and cis-thujopsene (28) (5.7%) (rare in liverworts and detected in Bazzania trilobata and Lepidozia fauriana [15]) were detected as minor compounds. Diterpene compounds were also detected as main constituents: (12Z)-abienol (46) (10.7%), 13-epi-manoyl oxide (47) (4.3%) and fusicocca-2,5-diene (42) (4.1%).
2.8. Bazzania marginata, Cuparane-Type
We detected high relative percentages of cuparane-type sesquiterpenes for B. marginata with two isomers of β-herbertenol (6) (respectively (95.9%) and (0.3%)), herbertene (1.0%) and α-herbertenol (5) (0.5%). Spathulenol (20) (1.0%), ar-himachalen-2-ol (31) (0.2%) and ar-himachalene (30) (0.2%), were detected as minor compounds.
3. Discussion
Important sesquiterpene-type diversity illustrated by the Figure 3 was observed among the studied samples of New-Caledonian liverworts. The genus Bazzania had been widely investigated in phytochemistry and most of detected sesquiterpene types belong to bazzanane-, cuparane-, barbatane-, aromadendrane-, bicyclogermacrane-, calamenane-, drimane-, chamigrane-, pinguisane-, myltaylane- and cyclomyltaylane-type [41]. Our results are consistent with the literature data except for the bicyclogermacrane-, calamenane- and drimane-type sesquiterpenes which do not seem to be widespread in Bazzania species from New Caledonia.
Drimane-type sesquiterpenes were detected within moderate relative percentages in one chemotype of B. francana. Calamenane-type, which is considered as a valuable chemotype for several Japanese Bazzania species [42], is the only sesquiterpene-type belonging to the (Z,E)-germacradienyl cation that had been detected in the New-Caledonian Bazzania species studied herein: calamenane-type sesquiterpene was detected with a moderate relative percentage in only one specimen of B. serrifolia. Thus, calamenane-type chemotype seemed to be rare in the present studied New-Caledonian Bazzania species: none of the analyzed specimen compositions could be chemically classified into the known chemotype II.
Isolepidozene (25) was detected in two specimens of B. serrifolia as a minor compound, but seemed to be a good biomarker for the Acromastigum genus which had been studied for the first time in this work. The new compound ziera-12(13),10(14)-dien-5-ol (23), belonging to the (E-E) germacradienyl cation sounds to be an important biomarker for two specimens of B. francana (MET062 and MET065).
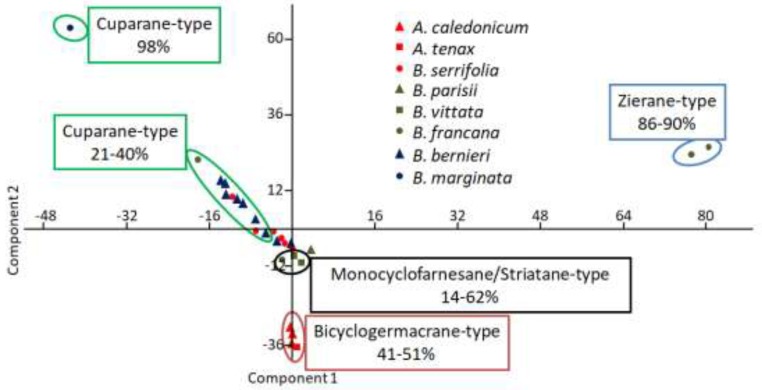
Multivariate PCA analysis of sesquiterpene type distribution (Figure 8) highlights the striatane/monocyclofarnesane-type chemotype for B. vittata and one specimen of B. francana (MET032). This fact is noteworthy since this sesquiterpene-type is very rare in the Jungermanniales order and seemed to be more specific to the Porellales order [15].
Figure 8.
Principal Components Analysis (PCA) plot of sesquiterpene-type distribution of studied New-Caledonian Bazzanioideae order (PC1 = 36.7%; PC2 = 27.8%).
Several studied species contain high percentages of cuparane-type sesquiterpenes (namely cuparane-, herbertane- and microbiotane-type sesquiterpenes). Concerned species are the following ones: B. marginata, B. francana (MET106), B. serrifolia (MET092 and MET099) and B. bernieri (MET028, 063, 066, 067, 069) from which we detected from 9.3 to 97.7% of cuparane-type sesquiterpenes. This fact suggested that a “special” chemotype I based only on the cuparane-type should be more appropriate to characterize Bazzania species from New Caledonia. Moreover, the detection of microbiotane-type (derivative of cuparane-type) sesquiterpene for one specimen of B. francana, is noticeable because this compound is also very rare in liverworts [15].
We have noticed that two samples of B. serrifolia (MET099 and MET092) shared many common characteristics with all specimens of B. bernieri (except MET038) such as the high amount of β-caryophyllene (35) and the presence of chamigrane-, cedrane- and bisabolane-type sesquiterpenes (these four structural-type sesquiterpenes were not detected in the other specimens of B. serrifolia or in the specimen MET038 of B. bernieri). In addition, one specimen of B. bernieri (MET038) shared many characteristics with specimens of B. serrifolia (except MET099 and MET092). These data indicated proximity between B. bernieri and B. serrifolia species. Therefore, we didn’t find any evidence of chemospecific status of B. serrifolia, and so our phytochemical data would add more assumption of its taxonomic synonymy with B. bernieri [8].
Hypothesis regarding a chemotaxonomic proximity between B. parisii and seven Bazzania species from Japan (B. bidentula, B. japonica, B. pompeana, B. tricrenata, B. tridens, B. trilobata and B. yoshinagana) could be proposed as these species are all characterized by barbatane- and bazzanane-type sesquiterpene [42].
The liverwort B. francana comprised at least three chemotypes in New Caledonia: (1) striatane; (2) microbiotane; and (3) zierane chemotype. Numerous chemotypes for specimens belonging to the same species, collected in a restricted area, is a rarely observed fact but sometimes may occur, for example analysis of Lepidozia fauriana (Lepidoziaceae) samples collected in Taiwan, led to split them into three different chemotypes : (1) amorphane; (2) chiloscyphane; and (3) eudesmane chemotypes [15].
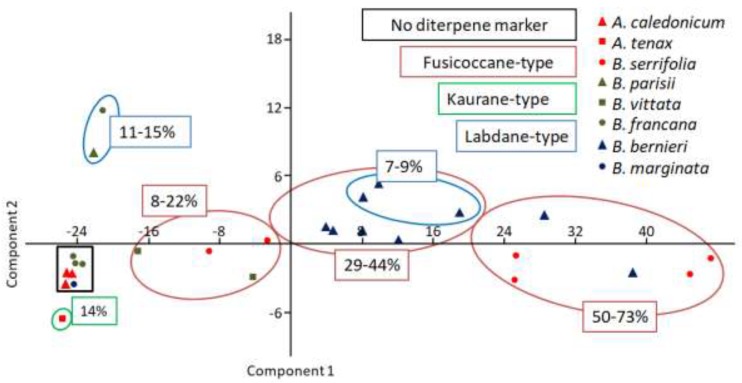
Amongst the Lepidoziaceae family, the literature data reported that fusicoccane-type diterpenes were only found in Bazzania involuta and Lepidozia concinna species [15], so our findings pointed out that fusicoccane-type diterpenes (mainly fusicocca-2,5-diene (42)) seemed to be specific biomarkers to Bazzania species of New Caledonia. As shown in PCA chart of diterpene-type distribution (Figure 9), fusicoccane-type is a characteristic biomarker of B. serrifolia, B. bernieri and B. vittata while labdane-type diterpene is detected mainly in B. bernieri and in one specimen of B. francana (MET106). The labdane-type diterpenes (mainly (Z)-biformene (45)) seemed to be characteristic biomarkers of Bazzania species from New Caledonia because this structural-type compound is very rare in the Lepidoziaceae family. Kaurane-type diterpenes were only detected in two specimens of herein studied Acromastigum species and may be considered as a good biomarker for A. tenax.
Figure 9.
Principal Components Analysis (PCA) plot of diterpene types distribution of studied New-Caledonian Bazzanioideae species (PC1 = 96.4%; PC2 = 2.5%).
Vittatin (51), as a dimer of lunularic acid, could be indexed in type I and III in the bis(bibenzyl)s classification. Bis(bibenzyl)s from type I and III possess various interesting biological activities such as bactericidal towards methicillin-resistant strains like Staphylococcus aureus [43], antimitotic agents [44], vasorelaxant [45]. Vittatin (51) possesses interesting chemical functions such as carboxylic acid, phenol and a methylenedioxy moiety, these features allow numerous chemical transformation through hemisynthesis. Due to its occurrence and functionalization, vittatin (51) should be a valuable raw material for the synthesis of interesting bis(bibenzyl)s (type I and type III), which could have promising pharmaceutical potential.
4. Materials and Methods
4.1. General Experimental Procedure
Plant material was air-dried at room temperature and small amount of samples were crushed and extracted with Et2O with mortar and pestle. Extract was then purified through a Pasteur pipette packed with silica gel using Et2O as eluent to retrieve polar compounds. Crude extracts have been analyzed by GC-FID-MS. GC-FID-MS analysis was performed using a gas chromatograph coupled with a mass detector (Clarus® 580, Perkin Elmer Inc, Waltham, MA, USA) and a flame ionization detector (Clarus® 580 , Perkin Elmer Inc, Waltham, MA, USA) using helium at 1 mL/min. Capillary column was a elite-5MS (30 m × 0.25 mm, 0.25 μm) (Perkin Elmer Inc, Akron, OH, USA). Analyses were performed using EI mode. The injection temperature was set at 250 °C. Analyses were carried out using a temperature program starting from 50 °C, with an initial 3 min hold, to 250 °C with a 5 °C/min heating ramp, and keeping the final temperature stable for 15 min. Mass range was set at m/z 40–500. The individual peaks were identified by comparison of mass spectra from libraries as well as the retention indices (RI), which were calculated for all volatile constituents using a homologous series of n-alkanes C8–C32 and were compared with available literature data.
Mass Finder 2.3 library, NIST library (Gaithersburg, MD, USA), Wiley library (Hoboken, NJ, USA) were used for mass spectra comparison and identification. We used mainly NIST MS Search 2.2 software, Pherobase [46] and literature data [47] for retention index comparison to identify constituents of the crude extracts. Relative percentages of constituents were calculated with the area from the FID GC chromatogram corrected with the number of carbon of the corresponding compound (based on the MS identification).
NMR analyses were performed on a Varian (500 MHz) or Bruker AVANCE III 600 (600 MHz) NMR spectrometers (Bruker, Billerica, MA, USA). Chemical shifts are given as δ (ppm) and deuterated solvent peaks as references for 1H- and 13C-NMR spectra. Infra-Red spectra were performed using IR spectrometer (FT-IR spectrometer Frontier, Perkin Elmer Inc, Waltham, MA, USA). Optical rotation was measured with an Atago Polax D polarimeter or Anton Paar MCP200 589 nm polarimeter equipped with a sodium lamp (c in g/mL). TLC analyses were carried out on Si gel plates F254 (Merck, Kenilworth, NJ, USA) with cyclohexane-EtOAc (1:1 and 4:1). Detection was realized with spraying 30% aqueous H2SO4 and then heated. For normal-phase column chromatography, Si gel 60 was used (0.040–0.063, 0.2–0.5 mm, Merck).
UV analyses were measured with HPLC apparatus (Waters 2695 Separation module, Milford, MA, USA) equipped with a diode array detector (Waters 2996 photodiode array detector) on a 250 mm × 4.6 mm i.d., 5 µm, ec 250/4.6 nucleodur 100-5 C18 Ec (Macherey-Nagel). The mobile phase consisted of purified water with 0.1% formic acid (A) and acetonitrile (B) at a flow rate of 0.8 mL/min. Gradient elution was performed as follows: 0 min, 20% B; 3 min, 30% B; 11 min, 35% B; 25 min, 50% B; 37–40 min, 100% B.
HR-ESI-MS analyses were measured with a SYNAPT G2 HDMS mass spectrometer (Waters, Manchester, United Kingdom). Accurate mass measurements were performed in triplicate with two internal calibrations.
4.2. Plant Material
Liverwort species were identified by Mr. Louis Thouvenot. Voucher specimens were deposited at the herbarium of the Institute of Research for Development (IRD), Noumea, New Caledonia (NOU). Sample Collections were realized within scientific authorizations delivered by the South Province (N°2050-2014 and N°1234-2016) and the North Province (60912-2014). Plant material is listed in Table 11.
Table 11.
Sample collection geodata informations.
| Species | Voucher Specimen | Date of Collection | Ecosystem | GPS | Collection Site | |
|---|---|---|---|---|---|---|
| South | East | |||||
| A. caledonicum* | MET113B | 06-2016 | maquis | 22.27 | 166.95 | Plateau de Goro |
| MET107 | 06-2016 | maquis | 22.27 | 166.97 | Plateau de Goro | |
| MET109B | 06-2016 | maquis | 22.27 | 166.95 | Plateau de Goro | |
| A. tenax* | MET116 | 06-2016 | maquis | 22.28 | 166.96 | Plateau de Goro |
| B. bernieri | MET038 | 11-2014 | maquis | 21.91 | 166.34 | Tontouta |
| MET028 | 08-2014 | rain forest | 22.18 | 166.50 | Koghis | |
| MET031 | 08-2014 | rain forest | 22.18 | 166.51 | Koghis | |
| MET040 | 11-2014 | maquis | 21.91 | 166.34 | Tontouta | |
| MET047 | 11-2014 | rain forest | 21.62 | 165.88 | Dogny | |
| MET063 | 06-2015 | rain forest | 22.22 | 166.66 | Mouirange | |
| MET066 | 06-2015 | rain forest | 22.17 | 166.79 | Marais Kiki | |
| MET067 | 06-2015 | rain forest | 22.17 | 166.79 | Marais Kiki | |
| MET069 | 06-2015 | rain forest | 22.17 | 166.79 | Marais Kiki | |
| B. francana | MET062 | 06-2015 | rain forest | 22.22 | 166.66 | Mouirange |
| MET065 | 06-2015 | rain forest | 22.17 | 166.79 | Marais Kiki | |
| MET032 | 08-2014 | rain forest | 22.18 | 166.5 | Koghis | |
| MET106 | 06-2016 | maquis | 22.28 | 166.97 | Goro | |
| B. marginata* | MET048 | 11-2014 | rain forest | 21.62 | 165.88 | Dogny |
| B. parisii | MET109A | 06-2016 | rain forest | 22.27 | 166.95 | Dogny |
| B. serrifolia | MET041 | 09-2014 | maquis | 21.91 | 166.34 | Tontouta |
| MET051 | 11-2014 | rain forest | 21.62 | 165.85 | Dogny | |
| MET052 | 11-2014 | rain forest | 21.62 | 165.85 | Dogny | |
| MET053 | 11-2014 | rain forest | 21.62 | 165.85 | Dogny | |
| MET092 | 04-2014 | sclerophyllous forest | 22.17 | 166.79 | Pindaï | |
| MET099 | 04-2014 | sclerophyllous forest | 22.17 | 166.79 | Pindaï | |
| B. vittata | MET049 | 11-2014 | rain forest | 21.62 | 165.87 | Dogny |
| MET060 | 05-2015 | rain forest | 21.63 | 165.87 | Dogny | |
4.3. Extraction and Isolation
Plant material of Bazzania vittata (90 g) was extracted by maceration with Et2O (three times during one week). The crude extract was obtained as a green pale powder (2.09 g), was then washed through a Büchner funnel with successively: cyclohexane, dichloromethane, methanol and acetonitrile to yield vittatin (51) (980 mg, 47%).
Plant material of Bazzania francana (20.5 g) was extracted by maceration with diethyl ether (three times during one week). The obtained crude extract (610 mg) was subjected to fractionation using open silica gel column chromatography with a stepwise gradient system of cyclohexane/ethyl acetate to yield 17 fractions (FI to FXVII). Fraction II yielded to ziera-12(13),10(14)-dien-5-ol (23) (200 mg, 33%).
4.4. Compound Characterization
Ziera-12(13),10(14)-dien-5-ol (23): transparent light-orange oil (200 mg); [α] = −40.4 (c 6.92, CH2Cl2). UV (CH3CN/H2O, 3:1) λmax: 195, 230 nm; IR (FT-IR) νmax: 3397.5, 3086.3, 2985.3, 2923.9, 2854.1, 1636.7, 1437.5, 1377.4, 1149.8 cm−1; 1H-NMR and 13C-NMR see Table 1; HR-EI-MS: m/z 220.1830 [M•]+ (calcd. for C15H26O, 220.1827).
Vittatin (51): flaky white amorphous powder (980 mg); [α] = +200.0 (c 5.00, pyridine). UV (CH3CN/H2O, 3:1) λmax: 255.78 nm; IR (FT-IR) νmax: 3414.6, 2946.7, 1608.39, 1575.1, 1496.8, 1445.6, 1411.2, 1250.1, 1205.1, 921.7 cm−1; 1H-NMR and 13C-NMR see Table 2; HR-ESI-MS: m/z 527.1700 [M + H]+ (calcd. for C31H27O8, 527.1700)
4.5. Statistical Analysis
In order to investigate intra- and inter specific variability from 27 specimens of the Bazzanioideae, two data sets were included in the multivariate analysis using the software program past 3. Principal Component Analysis (PCA) was performed for variance-covariance.
First data was set up for analysis of the different sesquiterpene-types (28 sesquiterpene-types corresponding to 82 identified sesquiterpenes). Second data was set up for analysis of the different diterpene-types (four diterpene-types corresponding to 12 identified diterpenes).
Acknowledgments
This work was supported by grants from the French Ministry of Research and Higher Education. We are thankful to Nurunajah Ab Ghani and Yoshida for helpful technical assistance and mostly for spectroscopic analysis at the Faculty of Pharmaceutical Sciences (Tokushima Bunri). We thank deeply Cyril Antheaume from the University of New Caledonia for his help in structure determination and providing data base and the LAboratoire des Moyens Analytiques (LAMA) from Institut de la Recherche pour le Developpement (Nouméa) for GC-FID-MS analysis assistance. We are thankful to the South and North Provinces of New Caledonia for delivering collection authorizations.
Supplementary Materials
1D and 2D NMR spectra, UV and IR spectra of compounds 23 and 51 are available in the Supplementary Materials.
Author Contributions
B.M. performed the research, wrote the paper and made structural elucidation. L.T. identified liverwort specimens and was in charge of all botanical aspects of this study. N.L. was involved in study conception, conceived and designed the experiments, analyzed the PCA data and revised critically the manuscript. G.H. analyzed the samples by NMR spectroscopy and performed structural identification. E.H., M.N. and Y.A. revised the manuscript redaction and were involved in study conception. P.R. was involved in the overall project design, revised structure elucidation and the manuscript redaction, is the corresponding author.
Funding
This research was funded by the French Ministry of Research and Higher Education addressed to Université de la Nouvelle-Calédonie, grant number [E1K-E18PROP2].
Conflicts of Interest
The authors declare no conflict of interest.
References
- 1.Langdale J.A. Evolution of developmental mechanisms in plants. Curr. Opin. Genet. Dev. 2008;18:368–373. doi: 10.1016/j.gde.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 2.He X., Sun Y., Zhu R.-L. The Oil Bodies of Liverworts: Unique and Important Organelles in Land Plants. Crit. Rev. Plant Sci. 2013;32:293–302. doi: 10.1080/07352689.2013.765765. [DOI] [Google Scholar]
- 3.Sloan S., Jenkins C.N., Joppa L.N., Gaveau D.L.A., Laurance W.F. Remaining natural vegetation in the global biodiversity hotspots. Biol. Conserv. 2014;177:12–24. doi: 10.1016/j.biocon.2014.05.027. [DOI] [Google Scholar]
- 4.Thouvenot L., Gradstein S.R., Hagborg A., Söderström L., Bardat J. Checklist of the Liverworts and Hornworts of New Caledonia. Cryptogam. Bryol. 2011;32:287–390. doi: 10.7872/cryb.v32.iss4.2011.287. [DOI] [Google Scholar]
- 5.Von Konrat M., Hagborg A., Söderström L., Renner M.A.M. Early Land Plants Today: Global patterns of liverwort diversity, distribution, and floristic knowledge. Bryol. New Millenn. 2008:425–438. doi: 10.3158/0015-0746-47.1.91. [DOI] [Google Scholar]
- 6.Söderström L., Hagborg A., von Konrat M., Bartholomew-Began S., Bell D., Briscoe L., Brown E., Cargill D.C., da Costa D.P., Crandall-Stotler B.J., et al. World checklist of hornworts and liverworts. PhytoKeys. 2016;59:1–828. doi: 10.3897/phytokeys.59.6261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ludwiczuk A., Asakawa Y. Chemotaxonomic value of essential oil components in liverwort species. A review: Chemotaxonomic value of essential oils from liverworts. Flavour Fragr. J. 2015;30:189–196. doi: 10.1002/ffj.3236. [DOI] [Google Scholar]
- 8.Kitagawa N. Miscellaneous notes on little-known species of Hepaticae. J. Haltori Bot. Lab. 1973:444–454. [Google Scholar]
- 9.Durán-Peña M.J., Botubol Ares J.M., Hanson J.R., Collado I.G., Hernández-Galán R. Biological activity of natural sesquiterpenoids containing a gem-dimethylcyclopropane unit. Nat. Prod. Rep. 2015;32:1236–1248. doi: 10.1039/C5NP00024F. [DOI] [PubMed] [Google Scholar]
- 10.Kanehisa M., Susumu G. Kyoto Encyclopedia of Genes and Genomes. Nucleic Acids Res. 2000;28:27–30. doi: 10.1093/nar/28.1.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sell C. A Fragrant Introduction to Terpenoid Chemistry. Royal Society of Chemistry; Cambridge, UK: 2003. [Google Scholar]
- 12.Hong Y.J., Tantillo D.J. Branching Out from the Bisabolyl Cation. Unifying Mechanistic Pathways to Barbatene, Bazzanene, Chamigrene, Chamipinene, Cumacrene, Cuprenene, Dunniene, Isobazzanene, Iso-γ-bisabolene, Isochamigrene, Laurene, Microbiotene, Sesquithujene, Sesquisabinene, Thujopsene, Trichodiene, and Widdradiene Sesquiterpenes. J. Am. Chem. Soc. 2014;136:2450–2463. doi: 10.1021/ja4106489. [DOI] [PubMed] [Google Scholar]
- 13.Moore B.S. Biosynthesis of marine natural products: Macroorganisms (Part B) Nat. Prod. Rep. 2006;23:615. doi: 10.1039/b508781n. [DOI] [PubMed] [Google Scholar]
- 14.Schmidt-Dannert C. Biosynthesis of Terpenoid Natural Products in Fungi. In: Schrader J., Bohlmann J., editors. Biotechnology of Isoprenoids. Volume 148. Springer International Publishing; Cham, Switzerland: 2014. pp. 19–61. [Google Scholar]
- 15.Asakawa Y., Ludwiczuk A., Nagashima F. In: Chemical Constituents of Bryophytes. Bio- and Chemical Diversity, Biological Activity, and Chemosystematics. Kinghorn A.D., Falk H., Kobayashi J., editors. Volume 95. Springer; Vienna, Austria: 2013. Progress in the Chemistry of Organic Natural Products. [DOI] [PubMed] [Google Scholar]
- 16.O’Maille P.E., Chappell J., Noel J.P. Biosynthetic potential of sesquiterpene synthases: Alternative products of tobacco 5-epi-aristolochene synthase. Arch. Biochem. Biophys. 2006;448:73–82. doi: 10.1016/j.abb.2005.10.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Agger S., Lopez-Gallego F., Schmidt-Dannert C. Diversity of sesquiterpene synthases in the basidiomycete Coprinus cinereus. Mol. Microbiol. 2009;72:1181–1195. doi: 10.1111/j.1365-2958.2009.06717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dumas F., Kousara M., Chen L., Wei L., Le Bideau F. Studies in Natural Products Chemistry. Volume 52. Elsevier; Amsterdam, The Netherlands: 2017. Nonhalogenated Heterotricyclic Sesquiterpenes From Marine Origin I; pp. 269–302. [Google Scholar]
- 19.Lebouvier N., Lesaffre L., Hnawia E., Goué C., Menut C., Nour M. Chemical Diversity of Podocarpaceae in New Caledonia: Essential Oils from Leaves of Dacrydium, Falcatifolium, and Acmopyle Species. Chem. Biodivers. 2015;12:1848–1861. doi: 10.1002/cbdv.201400445. [DOI] [PubMed] [Google Scholar]
- 20.Warmers U., König W.A. Sesquiterpene constituents of the liverwort Bazzania trilobata. Phytochemistry. 1999;52:99–104. doi: 10.1016/S0031-9422(99)00123-5. [DOI] [Google Scholar]
- 21.Harinantenaina L., Kurata R., Takaoka S., Asakawa Y. Chemical constituents of Malagasy liverworts: Cyclomyltaylanoids from Bazzania madagassa. Phytochemistry. 2006;67:2616–2622. doi: 10.1016/j.phytochem.2006.06.001. [DOI] [PubMed] [Google Scholar]
- 22.Lu R., Paul C., Basar S., König W.A., Hashimoto T., Asakawa Y. Sesquiterpene constituents from the liverwort Bazzania japonica. Phytochemistry. 2003;63:581–587. doi: 10.1016/S0031-9422(03)00188-2. [DOI] [PubMed] [Google Scholar]
- 23.Paul C., König W.A., Wu C.-L. Sesquiterpenoid constituents of the liverworts Lepidozia fauriana and Lepidozia vitrea. Phytochemistry. 2001;58:789–798. doi: 10.1016/S0031-9422(01)00322-3. [DOI] [PubMed] [Google Scholar]
- 24.Burgess E.J., Larsen L., Perry N.B. A Cytotoxic Sesquiterpene Caffeate from the Liverwort Bazzania novae-zelandiae. J. Nat. Prod. 2000;63:537–539. doi: 10.1021/np990492x. [DOI] [PubMed] [Google Scholar]
- 25.Figueiredo A.C., Sim-Sim M., Costa M.M., Barroso J.G., Pedro L.G., Esquível M.G., Gutierres F., Lobo C., Fontinha S. Comparison of the essential oil composition of four Plagiochila species: P. bifaria, P. maderensis, P. retrorsa and P. stricta. Flavour Fragr. J. 2005;20:703–709. doi: 10.1002/ffj.1627. [DOI] [Google Scholar]
- 26.Hackl T., Konig W., Muhle H. Isogermacrene A, a proposed intermediate in sesquiterpene biosynthesis. Phytochemistry. 2004;65:2261–2275. doi: 10.1016/j.phytochem.2004.05.024. [DOI] [PubMed] [Google Scholar]
- 27.Komala I., Ito T., Nagashima F., Yagi Y., Kawahata M., Yamaguchi K., Asakawa Y. Zierane sesquiterpene lactone, cembrane and fusicoccane diterpenoids, from the Tahitian liverwort Chandonanthus hirtellus. Phytochemistry. 2010;71:1387–1394. doi: 10.1016/j.phytochem.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 28.Connolly J.D., Harrison L.J., Rycroft D.S. Two Novel Zierane Sesquiterpenoids from the Liverwort Saccogyna viticulosa. J. Chem. Res. Synop. 1994;7:284–285. [Google Scholar]
- 29.Rontani J.-F., Volkman J.K. Phytol degradation products as biogeochemical tracers in aquatic environments. Org. Geochem. 2003;34:1–35. doi: 10.1016/S0146-6380(02)00185-7. [DOI] [Google Scholar]
- 30.Yoshikawa H., Ichiki Y., Sakakibara K.D., Tamura H., Suiko M. The biological and structural similarity between lunularic acid and abscisic acid. Biosci. Biotechnol. Biochem. 2002;66:840–846. doi: 10.1271/bbb.66.840. [DOI] [PubMed] [Google Scholar]
- 31.Asakawa Y. Biologically active compounds from bryophytes. Pure Appl. Chem. 2007;79:557–580. doi: 10.1351/pac200779040557. [DOI] [Google Scholar]
- 32.Asakawa Y., Toyota M., Toshihiro H., Fumihiro N., Liva H. Marchantiophyta (Liverworts): Rich sources of marcrocyclic bis(bibenzyls) Heterocycles. 2008;76:99–127. doi: 10.3987/REV-08-SR(N)3. [DOI] [Google Scholar]
- 33.Nguyen T.-H., Castanet A.-S., Mortier J. Directed Ortho-Metalation of Unprotected Benzoic Acids. Methodology and Regioselective Synthesis of Useful Contiguously 3- and 6-Substituted 2-Methoxybenzoic Acid Building Blocks. Org. Lett. 2006;8:765–768. doi: 10.1021/ol0530427. [DOI] [PubMed] [Google Scholar]
- 34.Friederich S., Maier U.H., Deus-Neumann B., Asakawa Y., Zenk M.H. Biosynthesis of cyclic bis (bibenzyls) in Marchantia polymorpha. Phytochemistry. 1999;50:589–598. doi: 10.1016/S0031-9422(98)00557-3. [DOI] [Google Scholar]
- 35.Friederich S., Rueffer M., Asakawa Y., Zenk M.H. Cytochromes P-450 catalyze the formation of marchantins A and C in Marchantia polymorpha. Phytochemistry. 1999;52:1195–1202. doi: 10.1016/S0031-9422(99)00340-4. [DOI] [Google Scholar]
- 36.Xie C.-F., Yuan H.-Q., Qu J.-B., Xing J., Lü B.-B., Wang X.-N., Ji M., Lou H.-X. Biocatalytic production of acyclic bis [bibenzyls] from dihydroresveratrol by crude Momordica charantia peroxidase. Chem. Biodivers. 2009;6:1193–1201. doi: 10.1002/cbdv.200800229. [DOI] [PubMed] [Google Scholar]
- 37.Li R.-J., Zhao Y., Tokuda H., Yang X.-M., Wang Y.-H., Shi Q., Morris-Natschke S.L., Lou H.-X., Lee K.-H. Total synthesis of plagiochin G and derivatives as potential cancer chemopreventive agents. Tetrahedron Lett. 2014;55:6500–6503. doi: 10.1016/j.tetlet.2014.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Almalki F.A., Harrowven D.C. A Corey-Seebach Macrocyclisation Strategy for the Synthesis of Riccardin C and an Unnatural Macrocyclic Bis(bibenzyl) Analogue. Eur. J. Org. Chem. 2016;2016:5738–5746. doi: 10.1002/ejoc.201601179. [DOI] [Google Scholar]
- 39.Toyota M., Kinugawa T., Asakawa Y. Bibenzyl cannabinoid and bisbibenzyl derivative from the liverwort Radula perrotteti. Phytochemistry. 1994;37:859–862. doi: 10.1016/S0031-9422(00)90371-6. [DOI] [Google Scholar]
- 40.Hertewich U.M., Zapp J., Becker H. Secondary metabolites from the liverwort Jamesoniella colorata. Phytochemistry. 2003;63:227–233. doi: 10.1016/S0031-9422(03)00049-9. [DOI] [PubMed] [Google Scholar]
- 41.Ludwiczuk A., Asakawa Y. Chapter Five: Distribution of Terpenoids and Aromatic Compounds in Selected Southern Hemispheric Liverworts. Fieldiana Bot. 2008;47:37. doi: 10.3158/0015-0746-47.1.37. [DOI] [Google Scholar]
- 42.Asakawa Y. Chemosystematics of the Hepaticae. Phytochemistry. 2004;65:623–669. doi: 10.1016/j.phytochem.2004.01.003. [DOI] [PubMed] [Google Scholar]
- 43.Fujii K., Morita D., Onoda K., Kuroda T., Miyachi H. Minimum structural requirements for cell membrane leakage-mediated anti-MRSA activity of macrocyclic bis(bibenzyl)s. Bioorg. Med. Chem. Lett. 2016;26:2324–2327. doi: 10.1016/j.bmcl.2016.03.033. [DOI] [PubMed] [Google Scholar]
- 44.Morita H., Tomizawa Y., Tsuchiya T., Hirasawa Y., Hashimoto T., Asakawa Y. Antimitotic activity of two macrocyclic bis(bibenzyls), isoplagiochins A and B from the Liverwort Plagiochila fruticosa. Bioorg. Med. Chem. Lett. 2009;19:493–496. doi: 10.1016/j.bmcl.2008.11.036. [DOI] [PubMed] [Google Scholar]
- 45.Morita H., Zaima K., Koga I., Saito A., Tamamoto H., Okazaki H., Kaneda T., Hashimoto T., Asakawa Y. Vasorelaxant effects of macrocyclic bis(bibenzyls) from liverworts. Bioorg. Med. Chem. 2011;19:4051–4056. doi: 10.1016/j.bmc.2011.05.019. [DOI] [PubMed] [Google Scholar]
- 46.The Pherobase: Database of pheromones and semiochemicals. [(accessed on 29 September 2017)]; Available online: http://www.pherobase.com/
- 47.Babushok V.I., Linstrom P.J., Zenkevich I.G. Retention indices for frequently reported compounds of plant essential oils. J. Phys. Chem. Ref. Data. 2011;40:043101. doi: 10.1063/1.3653552. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.