Abstract
Pea (Pisum sativum) roots were treated with aluminum in a calcium solution, and lipid peroxidation was investigated histochemically and biochemically, as well as other events caused by aluminum exposure. Histochemical stainings were observed to distribute similarly on the entire surface of the root apex for three events (aluminum accumulation, lipid peroxidation, and callose production), but the loss of plasma membrane integrity (detected by Evans blue uptake) was localized exclusively at the periphery of the cracks on the surface of root apex. The enhancement of four events (aluminum accumulation, lipid peroxidation, callose production, and root elongation inhibition) displayed similar aluminum dose dependencies and occurred by 4 h. The loss of membrane integrity, however, was enhanced at lower aluminum concentrations and after longer aluminum exposure (8 h). The addition of butylated hydroxyanisole (a lipophilic antioxidant) during aluminum treatment completely prevented lipid peroxidation and callose production by 40%, but did not prevent or slow the other events. Thus lipid peroxidation is a relatively early symptom induced by the accumulation of aluminum and appears to cause, in part, callose production, but not the root elongation inhibition; by comparison, the loss of plasma membrane integrity is a relatively late symptom caused by cracks in the root due to the inhibition of root elongation.
Aluminum is a major growth-limiting factor for plants in acid soils (for review, see Rengel, 1992, 1996; Horst, 1995; Kochian and Jones, 1997). The primary site of aluminum accumulation and toxicity is the root meristem, suggesting that aluminum interacts with actively dividing and expanding cells, but not with quiescent cells in the basal region. Among various aluminum toxicity symptoms, the most sensitive responses are the inhibition of root elongation and the induction of callose synthesis that appear after short-term exposures to aluminum (Zhang et al., 1994; Horst et al., 1997). The molecular mechanisms of these symptoms, however, have not been elucidated.
Because aluminum ions form electrostatic bonds preferentially with oxygen donor ligands (e.g. carboxylate or phosphate groups), cell wall pectin and the outer surface of the plasma membrane seem to be major targets of aluminum. Most of the aluminum associated with cells actually seems to be localized at apoplast. However, it is not known whether the effects of aluminum are derived entirely from external association with root cells only or if there has been an uptake of aluminum into root cells that is responsible for observed toxic symptoms (Taylor et al., 2000; for review, see Rengel, 1996; Kochian and Jones, 1997).
Aluminum ions strongly interact with lipid components of the plasma membrane (Akeson et al., 1989), depending on phospholipid charge (Jones and Kochian, 1997). The binding of aluminum to the membrane lipids causes the rigidification of the plasma membrane (Deleers et al., 1986), which seems to affect metabolism in the plasma membrane. Several responses of plant cells to aluminum seem related to the alteration of several plasma membrane functions (for review, see Rengel, 1996; Kochian and Jones, 1997). The responses include the blockage of Ca2+ channel, the depolarization of transmembrane electrical potentials (Papernik and Kochian, 1997; Takabatake and Shimmen, 1997), the exudation of organic acids from aluminum-tolerant cultivars of several species (for review, see Delhaize and Ryan, 1995; Ma et al., 1997), the inhibition of the H2O2-stimulated increase of inositol 1, 4, 5-triphosphate (Jones and Kochian, 1995), and the enhancement of Fe(II or III)-mediated peroxidation of lipids (Ono et al., 1995; Yamamoto et al., 1997). The induction of callose production by aluminum also seems related to the alteration of the plasma membrane function, since β-1, 3-glucan synthase (callose synthase) is located at the inner part of the plasma membrane and is activated by an increase in intracellular Ca2+ concentration, which may be caused by an increase in influx of Ca2+ through the damaged plasma membrane (for review, see Kauss, 1987; Delmer and Amor, 1995). Furthermore, it was reported that the alteration of the plasma membrane permeability following temporal contact with aluminum ions was a rapid response (within 0.5 h in whole plant) and seemed to be an indicator of aluminum sensitivity among a variety of plant species (Ishikawa and Wagatsuma, 1998).
The enhancement of the Fe(II)-dependent peroxidation of lipids by aluminum has been reported to occur in phospholipid liposomes (Gutteridge et al., 1985; Oteiza, 1994; Xie and Yokel, 1996). Since aluminum is not a transition metal, it cannot catalyze redox reactions. However, Oteiza (1994) suggested that the increased rigidity of membranes caused by aluminum binding facilitates a Fe-mediated free-radical chain reaction. These in vitro studies were supported by an in vivo study using cultured tobacco cells that were treated with a simple Ca solution containing aluminum (Ikegawa et al., 2000). In the simple Ca solution containing aluminum, aluminum accumulated immediately in tobacco cells. Although the accumulation of aluminum itself did not cause the peroxidation of lipids, the addition of Fe(II) to the aluminum-accumulated tobacco cells resulted in lipid peroxidation immediately and the loss of integrity of the plasma membrane several hours later. These results suggested that the accumulation of aluminum sensitizes the membrane to the Fe(II)-mediated peroxidation of lipids, and that the aluminum-enhanced peroxidation of lipids is a direct cause of cell death. The aluminum-enhanced Fe(II)-dependent peroxidation of lipids was also reported in the root tips of soybean only after prolonged treatment with aluminum in nutrient solution (Cakmak and Horst, 1991; Horst et al., 1992).
In Arabidopsis and cultured tobacco cells, aluminum induces the expression of several genes (e.g. peroxidase, glutathione-S-transferase, and superoxide dismutase) that are induced also by oxidative stress. Thus a possible induction of oxidative stress by aluminum is suggested (Snowden and Gardner, 1993; Ezaki et al., 1995, 1996; Richards et al., 1998). However, biochemical and physiological studies related to the possible role of aluminum induction of oxidative stress in plant roots have not been extensively investigated.
The peroxidation of unsaturated lipids in biological membranes is the most prominent symptom of oxidative stress in animals and plants. Thus in this paper we investigated the peroxidation of lipids as a phenomenon of aluminum-induced oxidative stress in pea (Pisum sativum) roots. The details of this phenomenon and the cause and effect relationship between the peroxidation of lipids and other symptoms caused by aluminum are reported.
RESULTS
The Localization of Lipid Peroxidation and Other Events Caused by Aluminum in Pea Roots
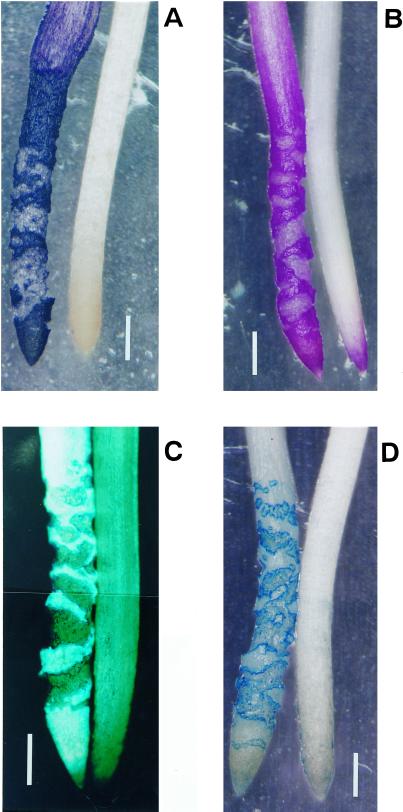
Pea roots were treated with or without 10 μm AlCl3 in 100 μm CaCl2 solution (pH 4.75) for 24 h. Observation of the events caused by aluminum on root surface (aluminum accumulation, lipid peroxidation, callose production, and the loss of plasma membrane integrity) was performed by histochemical staining with hematoxylin, Schiff's reagent, aniline blue, and Evans blue, respectively. The four events were observed exclusively at the root apex (from the tip [0 mm] toward the basal region [approximately 7 mm]; Figure 1). The distribution of the histochemical staining patterns of three events (aluminum accumulation, lipid peroxidation, and callose production) were similar to each other. These three staining patterns were observed on root surface, but not within any cracks in the root (Fig. 1, A–C). The loss of plasma membrane integrity was not observed on the root surface. Instead, the loss of membrane integrity was observed clearly at the periphery of cracks in the roots and weakly seen inside the cracks (Fig. 1D). Cracks in the roots were observed after approximately 8 h of exposure to aluminum (data not shown). These results suggest that the peroxidation of lipids and callose production are caused directly by the interaction of aluminum with the root surface, whereas the loss of plasma membrane integrity is caused by the formation of cracks after prolonged treatment with aluminum.
Figure 1.
Histochemical detection of lipid peroxidation and other events caused by aluminum in pea roots. Pea seedlings were treated with (left) or without (right) 10 μm aluminum in 100 μm CaCl2 (pH 4.75) for 24 h. The roots were stained with either hematoxylin (A, aluminum accumulation), Schiff's reagent (B, lipid peroxidation), aniline blue (C, callose production), or Evans blue (D, the loss of plasma membrane integrity; see “Materials and Methods”). The positive staining of each technique in the photomicrographs shows as bright images in panels (A, B, and D) and as a fluorescent image in panel (C). Bar in each graph indicates 1 mm.
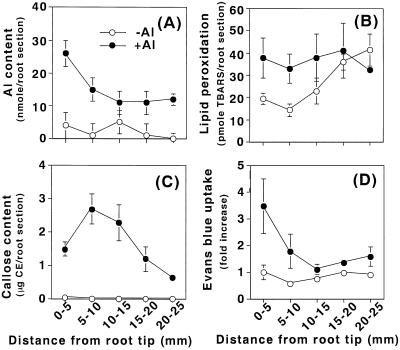
The histochemical observations described above were supported by the quantitative determinations of these events. After aluminum treatment (10 μm AlCl3, 24 h), roots were sectioned in 5-mm intervals from the tip (0 mm) toward basal region (25 mm) and each section was examined for aluminum content, lipid peroxidation, callose content, and the loss of plasma membrane integrity. Compared with control roots treated without aluminum, the values of all four events were higher in aluminum-treated roots, especially at the root apex (Fig. 2). The highest level of aluminum accumulation was detected at the root apical region, 0 to 5 mm, although the accumulation of aluminum was still detected at the 20- to 25-mm region (Fig. 2A). Although lipid peroxidation detected by the thiobarbituric acid (TBA) assay in the control roots was similar to aluminum-treated roots in sections at the basal region (15–20 and 20–25 mm), TBA-reactive substances in the controls were approximately 2-fold lower in sections at the root apex (0–5and 5–10 mm) than in aluminum-treated roots (Fig. 2B). Thus the enhancement of lipid peroxidation by aluminum was significant, but limited at the root apex. There were no significant changes detected for callose production (Fig. 2C) and the loss of plasma membrane integrity (Fig. 2D) in the control roots, but these two events increased significantly and exclusively at the root apex in aluminum-treated roots.
Figure 2.
Biochemical detection of lipid peroxidation and other events caused by aluminum in pea roots. Pea seedlings were treated with (●) or without (○) 10 μm aluminum in 100 μm CaCl2 (pH 4.75) for 24 h. The root apex was sectioned in 5-mm intervals from the tip (0 mm) toward the basal region (25 mm). Each section was analyzed for aluminum content (A), lipid peroxidation (B), callose content (C), or the loss of plasma membrane integrity (D), as described in “Materials and Methods.” All data show the means ± se of a total of three replicates from two independent experiments, each replicate comprising four root sections (A, B, and D), or one root section (C) from identical positions.
The Aluminum Dose Dependency of Lipid Peroxidation and Other Events Caused by Aluminum
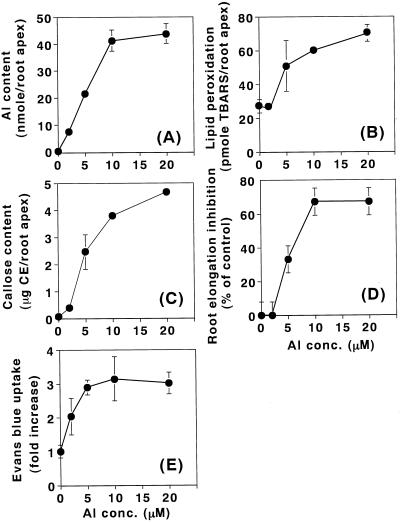
Pea roots were treated with various concentrations of aluminum for 24 h. The accumulation of aluminum in the root apex increased linearly with an increase in aluminum concentration up to 10 μm (Fig. 3A). Dose-dependent curves of lipid peroxidation, callose production, and root elongation inhibition were similar to each other. The degree of the root elongation inhibition (percentage of control) was estimated as: [(A − B)/A] × 100, where A was the root length elongated during treatment without aluminum and B was the root length elongated during treatment with aluminum. Enhancement of these three events were clearly observed at 5 μm aluminum and continued to increase until 20 μm aluminum (lipid peroxidation and callose production) or until 10 μm aluminum (root elongation inhibition; Fig. 3, B–D). However, enhancement of Evans blue uptake was observed at an aluminum concentration of 2 μm and increased up to 5 μm (Fig. 3E). This result indicates that the loss of plasma membrane integrity was induced by aluminum at lower concentrations than the other three events.
Figure 3.
Aluminum dose dependency of lipid peroxidation and other events caused by aluminum in pea roots. Pea seedlings were treated with aluminum at concentrations of 0, 2, 5, 10, and 20 μm in 100 μm CaCl2 (pH 4.75) for 24 h. After the treatment, a 10-mm section of the root apex (from the tip [0 mm] toward basal region [10 mm]) was analyzed for aluminum content (A), lipid peroxidation (B), callose content (C), root elongation inhibition (D), and the loss of plasma membrane integrity (E), as described in “Materials and Methods.” Root elongation inhibition (percentage of control) was calculated as [(A − B)/A] × 100, where A was the root length elongated during treatment without aluminum, and B was the root length elongated during treatment with aluminum. All data show the means ± se of a total of three independent replicates from two independent experiments, each replicate comprising four 10-mm root apices (A–C, and E), or six roots (D).
Time Courses of Lipid Peroxidation and Other Events during Aluminum Treatment
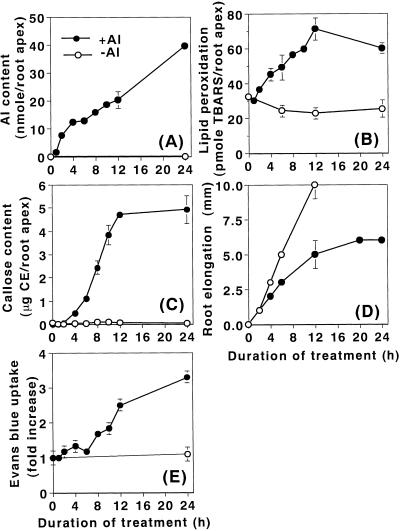
Pea roots were treated in the presence or absence of 10 μm aluminum for up to 24 h. The accumulation of aluminum was immediate and continued to increase for 24 h (Fig. 4A). The kinetic patterns of lipid peroxidation and callose production were similar to each other. Both events increased within 4 h of aluminum exposure and continued to increase until 12 h (Fig. 4, B and C). Root elongation inhibition was also detected within 4 h and reached nearly a maximum level of inhibition at 12 h (Fig. 4D). The loss of the plasma membrane integrity, however, was observed to increase only at 8 h, and then continued to increase until 24 h (Fig. 4E). These results indicate that the three events (aluminum accumulation, lipid peroxidation, callose production, and root growth inhibition) are earlier events relative to the loss of plasma membrane integrity that occurred later.
Figure 4.
Time course of lipid peroxidation and other events during aluminum treatment in pea roots. Pea seedlings were treated with (●) or without (○) 10 μm aluminum in 100 μm CaCl2 (pH 4.75) for up to 24 h. Roots were harvested at selected time points for analyses of aluminum content (A), lipid peroxidation (B), callose content (C), root elongation, (D), or the loss of plasma membrane integrity (E), as described in “Materials and Methods.” Although the elongation data of control roots is shown from 0 to 12 h (E), the control roots continued to elongate until 24 h with an average length of 24 ± 1 mm. All data show the means ± se of a total of three replicates from two independent experiments, each replicate comprising four 10-mm root apices (A, B, and E), one 10-mm root apex (C), or six roots (D).
Cause and Effect Relationship between Lipid Peroxidation and Other Events Caused by Aluminum
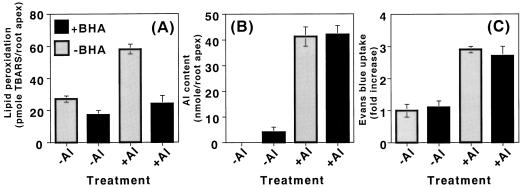
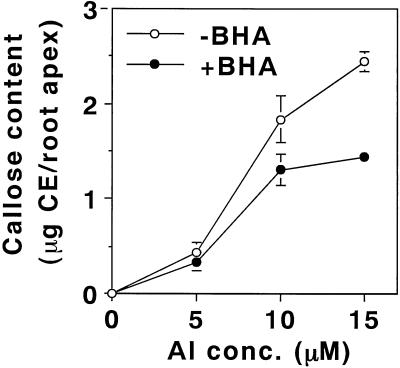
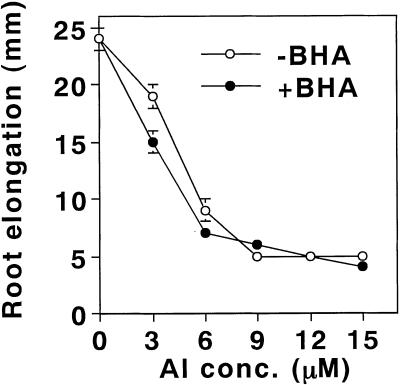
Butylated hydroxyanisole (BHA) is an efficient lipophilic antioxidant. It is incorporated into membrane lipids and protects the membrane from the peroxidation of lipids (Halliwell and Gutteridge, 1989). In fact, the presence of 20 μm BHA during aluminum treatment (10 μm aluminum for 24 h) of pea roots efficiently prevented the enhancement of the peroxidation of lipids. The effect of BHA was clearly seen by the staining procedures with Schiff's reagent (Fig. 5) and with the TBA method (Fig. 6A). Also, BHA significantly decreased the aluminum-induced callose production by 40% (Fig. 7). In contrast, BHA did not affect the degree of aluminum accumulation or the loss of plasma membrane integrity (Fig. 6, B and C). In addition, the presence of BHA with various concentrations of aluminum did not prevent the aluminum-induced inhibition of root elongation (Fig. 8). These results, taken collectively, suggest that a significant part of the aluminum-induced callose production is derived from the aluminum-enhanced peroxidation of lipids, but that the accumulation of aluminum, the loss of the plasma membrane integrity, and root elongation inhibition are not derived directly from the peroxidation of lipids.
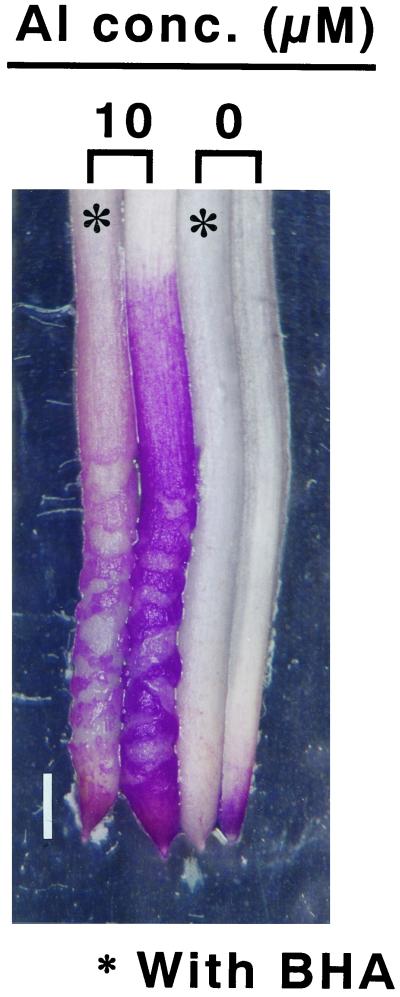
Figure 5.
Histochemical detection of BHA effect on the aluminum-enhanced lipid peroxidation in pea roots. Pea seedlings were treated with or without 10 μm aluminum in the presence (∗) or absence of 20 μm BHA in 100 μm CaCl2 (pH 4.75) for 24 h. The roots were stained with Schiff's reagent for the detection of lipid peroxidation as described in “Materials and Methods.” The positive staining shows a bright image in the photomicrograph. Bar indicates 1 mm.
Figure 6.
Effect of BHA on the aluminum-enhanced lipid peroxidation and other events in pea roots. Pea seedlings were treated with or without 10 μm aluminum in the presence or absence of 20 μm BHA in 100 μm CaCl2 (pH 4.75) for 24 h. Then, a 10-mm section of the root apex was analyzed for lipid peroxidation (A), aluminum content (B), or the loss of plasma membrane integrity (C), as described in “Materials and Methods.” All data show the means ± se of a total of three independent replicates from two independent experiments, each comprising four 10-mm root apices.
Figure 7.
Effect of BHA on the aluminum-induced callose production in pea roots. Pea seedlings were treated with aluminum concentrations of 0, 5, 10, and 15 μm, in the presence or absence of 20 μm BHA in 100 μm CaCl2 (pH 4.75) for 24 h. Then, a 10-mm section of the root apex was analyzed for callose content as described in “Materials and Methods.” All data show the means ± se of a total of four replicates (each replicate comprising two 10-mm root apices) from two independent experiments.
Figure 8.
Effect of BHA on the aluminum-induced inhibition of root elongation in pea roots. Pea seedlings were treated with aluminum concentrations of 0, 3, 6, 9, 12, and 15 μm in the presence or absence of 20 μm BHA in 100 μm CaCl2 (pH 4.75) for 24 h. The roots were harvested for a analysis of root elongation as described in “Materials and Methods.” All data show the means ± se of a total of three replicates (each replicate comprising six roots) from three independent experiments.
DISCUSSION
Although the symptoms of exposure to aluminum are well known, the molecular mechanism(s) of aluminum toxicity remain to be elucidated. Because aluminum in the apoplast region seems to contribute to most of the total aluminum uptake (Taylor et al., 2000; for review, see Rengel, 1996), and aluminum ions have a high affinity for the net negatively charged plasma membrane surface (Akeson et al., 1989), the effects of aluminum are likely to be targeted, in part, to the plasma membrane. In this paper we examined the effects of aluminum exposure on the plasma membrane in pea roots, which focused on two types of membrane damage. These types of damage are the peroxidation of lipids, as a typical symptom under oxidative stress (Halliwell and Gutteridge, 1989), and the loss of integrity of the plasma membrane. The loss of membrane integrity was detected by the uptake of a non-permeable dye (Evans blue) into the root cells, which has been used also as an indicator of cell death (Gaff and Okong'O-Ogola, 1971; Baker and Mock, 1994; Ikegawa et al., 1998).
The Peroxidation of Lipids
It was reported that the aluminum-induced production of callose has a close and positive relation to the aluminum-induced inhibition of root elongation in wheat (Zhang et al., 1994) and in maize (Horst et al., 1997), although a cause and effect relationship between these two symptoms of aluminum exposure has not been demonstrated. This study indicates spatial and temporal positive correlations among the four events investigated in pea roots: aluminum accumulation, lipid peroxidation, callose production, and root elongation inhibition (Figs. 1–4). Lipid peroxidation and callose production are especially strongly correlated as shown by the results in which the increased responses of these events under aluminum treatment have similar distributions in the root regions where aluminum had accumulated, and exhibited the same aluminum dose and time dependencies. Furthermore, the lipophilic antioxidant (BHA) completely prevented the peroxidation of lipids (Fig. 6A) and decreased the production of callose by 40% (Fig. 7), suggesting that a significant part of the aluminum-enhanced callose production is derived from the aluminum-enhanced peroxidation of lipids. In contrast, BHA did not alter the aluminum-induced inhibition of root elongation (Fig. 8), suggesting that lipid peroxidation does not contribute directly to the inhibition of root elongation. However, we could not rule out the possibility that aluminum may enhance oxidative stress not only at the plasma membrane, but also in the symplasmic space that may lead to the inhibition of root elongation.
Aluminum cannot by itself catalyze the peroxidation reaction, but it enhances the Fe(II or III)-mediated peroxidation of lipids nonenzymatically in phospholipid liposomes and phospholipids (for review see, Gutteridge et al., 1985; Oteiza, 1994; Xie and Yokel, 1996; for review, see Halliwell and Gutteridge, 1989). The aluminum-enhanced Fe-mediated peroxidation of lipids was also reported in biological systems including root tips of soybean (Cakmak and Horst, 1991; Horst et al., 1992) and cultured tobacco cells (Ono et al., 1995; Yamamoto et al., 1997; Yamaguchi et al., 1999; Ikegawa et al., 2000). In cultured tobacco cells, supplemental Fe (II or III) was necessary for aluminum to enhance the peroxidation of lipids (Yamamoto et al., 1997; Ikegawa et al., 2000). However, in this paper aluminum enhances the peroxidation of lipids without added iron ions during aluminum treatment of the pea roots. In pea roots there may be a sufficient endogenous supply of iron to facilitate the aluminum enhancement of lipid peroxidation or the effect of aluminum on lipid peroxidation may occur by another unknown mechanism(s).
The aluminum-enhanced peroxidation of lipids was also reported in root tips of soybean in a nutrient solution without iron, although the enhancement of the peroxidation of lipids was detected after longer treatment with aluminum (>12 h) at concentrations that substantially inhibited root elongation (Horst et al., 1992). Since aluminum enhanced slightly the activities of superoxide dismutase and peroxidase, Cackmak and Horst (1991) hypothesized that modification of membrane structures by aluminum interactions with membrane lipids and proteins may enhance the production of reactive oxygen species, such as O2− and H2O2, and the consequent peroxidation of lipids. O2− arises from several metabolic processes [e.g. respiration and the activation of NAD(P) H oxidase on the plasma membrane], and H2O2 is produced by the spontaneous or enzymatic dismutation of O2−. In the presence of transition metals, Fe(II) for example, a combination of O2− and H2O2, produces highly reactive hydroxyl radical that initiates the free radical chain reaction resulting in the peroxidation of lipids (Halliwell and Gutteridge, 1989). Diphenylene iodonium is a specific inhibitor of plant NAD(P) H oxidase (Levine et al., 1994), and dimethylthiourea is an antioxidant that traps H2O2 (Halliwell and Gutteridge, 1989; Levine et al., 1994). In pea roots, however, the presence of either reagent during aluminum treatment did not affect the level of lipid peroxidation (data not shown). Thus it appears difficult to explain the aluminum-enhanced peroxidation of lipids in pea roots by aluminum-induced de novo production of O2− and H2O2 proposed by Cackmak and Horst (1991). The molecular mechanism of the aluminum-enhanced peroxidation of lipids without additional iron ions in pea roots remains to be elucidated.
In this paper we used a histochemical staining technique for the detection of the peroxidation of lipids on the root surface. The technique is based on the use of Schiff's reagent to detect aldehyde functions that are originated from the peroxidation of membrane lipids and are bound to the membrane protein. This technique was originally established for the investigation of lipid peroxidation in liver tissue of bromobenzene-intoxicated mice (Pompella et al., 1987). We applied this procedure to pea roots and the procedure seems to be reliable for the detection of lipid peroxidation in plants, since the aluminum-induced peroxidation of lipids on root surface detected by this histochemical procedure was similar to that detected by the biochemical procedure with malondialdehyde (MDA) as TBA-reactive substances (see “Materials and Methods;” Figs. 1 and 2). In addition, the presence of BHA (an antioxidant against the peroxidation of lipids) during aluminum treatment prevented the Schiff and TBA reactions (Figs. 5 and 6A). The histochemical procedure has an advantage over the biochemical procedure, because the histochemical procedure reveals the localization of the aluminum-enhanced peroxidation of lipids in situ on root surface with high sensitivity. Positive staining with Schiff's reagent was detected only in aluminum-treated roots and was limited to the root apex (Fig. 1B), whereas TBA-reactive substances were detected even at the basal region of control and aluminum-treated roots (Fig. 2B). These results suggest that the TBA-reactive substances observed in control roots may be MDA located inside roots or several compounds other than MDA that produce chromogens exhibiting absorption spectra similar to that of MDA-TBA complex (e.g. some carbohydrates; Halliwell and Gutteridge, 1989).
The Loss of Integrity of the Plasma Membrane
Several reports suggest that the loss of the plasma membrane integrity during aluminum treatment, monitored by histochemical staining with a non-permeable dye (propidium iodide) or a permeable dye (fluorescein diacetate), seems to be a secondary damage, but not the primary damage related to root elongation inhibition (Koyama et al., 1995; Yokota and Ojima, 1995; Sasaki et al., 1997). Our results confirm the results of these reports. Furthermore, histochemical observation and quantification of the loss of plasma membrane integrity by use of Evans blue suggest that membrane damage induced by aluminum is due to mechanical disruption of cells at the periphery of cracks in the root at the elongation zone (Fig. 1D) after prolonged aluminum exposure (8 h; Fig. 4D). The cracks were not caused by the sloughing off the epidermal layer, since the edges of the cracks are seen to be the compliment edge of one another (see Figs. 1 and 5); also, we did not observe any sloughed epidermal materials in the treatment medium (data not shown). Therefore, it is likely that the cracks are caused by differential cell expansion created by root elongation inhibition (that is, there is an inhibition of surface cell expansion, whereas the expansion of internal cells occurs normally). Since the accumulation of aluminum was observed over the entire surface of the root apex, we conclude that the accumulation of aluminum on the root surface itself does not cause the direct loss of plasma membrane integrity. This conclusion is supported by our previous study with cultured tobacco cells (Ikegawa et al., 2000). When tobacco cells were treated with aluminum in a simple CaCl2 solution, aluminum started to accumulate in the cells immediately, but the loss of integrity of the plasma membrane (monitored by Evans blue uptake) was not detected significantly during aluminum treatment up to 24 h. However, in pea roots and tobacco cells, there remains a possibility that aluminum may cause a minor alteration of the plasma membrane permeability that is not detected by the uptake of Evans blue.
In summary, the responses of pea roots to aluminum exposure were examined by histochemical and biochemical techniques. The peroxidation of lipids is a relatively early event following aluminum exposure and appears to partly influence the aluminum-induced production of callose, but not the aluminum-induced inhibition of root elongation. By comparison, the loss of plasma membrane integrity is a relatively late event and seems to be a consequence of the cracks in the root formed by the inhibition of root elongation.
MATERIALS AND METHODS
Plant Material and Aluminum Treatment
Pea (Pisum sativum cv Alaska) seeds were sterilized in a solution containing 1% (v/v) sodium hypochlorite and 0.05% (v/v) Tween 20 for 15 min, followed by 70% (v/v) ethanol for 5 s, and then rinsed three times with sterile dH2O. The seeds were germinated on filter paper for 1 d, then transferred to cotton and grown for 1 d in the dark under sterile conditions. Sixty germinated seeds were transferred to 3-L plastic chambers and cultured for 2 d in nutrient medium [one-fifth Hoagland solution without micronutrients except for H3BO3, pH 4.75: 0.2 mm KH2PO4, 1 mm KNO3, 1 mm Ca(NO3)2, 0.4 mm MgSO4, and 0.09 mm H3BO4]. The germinating seeds were transferred again to other 3-L plastic chambers and cultured for 1 d in 100 μm CaCl2 (pH 4.75). During the pre-treatment culture, medium was changed daily. During the pre-treatment and aluminum-treatment (described below), seedlings were cultured in aerated medium at 25°C under fluorescent lamps (60 μE m−2 s−1, 12-h photoperiod; CU-258, Tomy, Tokyo).
Eight seedlings were transferred to 425-mL plastic chambers containing 100 μm CaCl2 (pH 4.75) and AlCl3 was added to final concentrations between 0 to 20 μm. Seedlings were cultured for up to 24 h. After aluminum treatment, roots were washed with 100 μm CaCl2 (pH 4.75) and used for measurements of various events caused by aluminum at root apices as described below. Root elongation during aluminum treatment was determined by measuring the length of whole root before and after aluminum treatment. When the seedlings were treated with aluminum in the presence of BHA (Katayama Chemical Industries, Osaka), BHA was dissolved in methanol and was added to the solution at the start of the treatment, just prior to the addition of aluminum.
Histochemical Analyses
The localization of aluminum was detected with hematoxylin as described previously (Sasaki et al., 1997). In this staining procedure, aluminum acts as a mordant and causes the binding of hematein (oxidized hematoxylin) to constituents of cells with formation of colored complexes (Havas, 1986). Histochemical detection of lipid peroxidation was performed as described by Pompella et al. (1987). In brief, roots were stained with Schiff's reagent for 20 min, which detects aldehydes that originate from lipid peroxides. Schiff's reagent was prepared as described by Jensen (1962). After the reaction with Schiff's reagent, roots were rinsed with a sulfite solution (0.5% [w/v] K2S2O5 in 0.05 m HCl). The stained roots were kept in the sulfite solution to retain the staining color. Callose on root surfaces was stained with a few drops of 0.1% (w/v) aniline blue in 1 m Gly/NaOH buffer (pH 9.5) directly on a microscope slide as described by Kauss (1992). The localization of the loss of plasma membrane integrity was detected by staining four seedlings with 10 mL of an Evans blue solution (0.025% [w/v] Evans blue in 100 μm CaCl2, pH 5.6) for 10 min. Then stained roots were washed three times with 200 mL of 100 μm CaCl2 (pH 5.6), after which the dye no longer eluted from the roots.
The roots stained with Schiff's reagent, hematoxylin, or Evans blue were observed under a light microscope (model SZ60; Olympus, Tokyo) and the roots stained with aniline blue were observed under a fluorescence microscope (Axiotron; Carl Zeiss, Germany) using the Zeiss filter set 5 (excitation 395–440 nm, color splitter 460 nm, secondary filter 470 nm). All stained roots were photographed on color film (ASA 400, Fuji Photo Film, Tokyo).
Determination of Aluminum Content
Each root was cut with a razor blade to yield a 10-mm section from the root tip, unless otherwise indicated. Four root sections from identical positions were placed together and acid-hydrolyzed in a 0.5-mL mixture of 1:1 (v/v) H2SO4 and HNO3, as described previously (Yamamoto, et al., 1994). Aluminum content in the acid-hydrolyzed samples was quantified using a simultaneous multi-element atomic absorption spectrophotometer with a graphite furnace atomizer (model Z-9000; Hitachi, Tokyo).
Determination of Lipid Peroxidation
The peroxidation of lipids in root sections was assessed by TBA method as described (Mihara et al., 1980) with minor modifications, in which the TBA-reactive substance was quantified as MDA that is an end product of lipid peroxidation. Each root was cut to yield a 10-mm section from the root tip, unless otherwise indicated. Four root sections from identical positions were placed together and homogenized in a solution of 0.5 mL of 0.1% (w/v) TCA containing 1 mm butylated hydroxytoluene with a microhomogenizer at 4°C. The homogenate was added to 0.375 mL of a solution containing 2% (v/v) H3PO4 and 0.25 mL of 0.6% (w/v) aqueous TBA. The mixture was incubated at 100°C for 30 min, then cooled to room temperature. The heated mixture was then added to 1 mL of n-butanol and mixed vigorously. The butanol and aqueous phases were separated by centrifugation. Absorbance of the TBA-reactive substance was determined as TBA-MDA complex at 532 nm. The value for non-specific absorption at 520 nm was subtracted and the amount of MDA was calculated (ε = 155,000).
Determination of Callose Content
Callose content in the root apex was determined as described by Kauss (1992) with minor modifications. In brief, each root was incubated in ethanol for at least 1 h, then cut to yield a 10-mm section from the root tip, unless otherwise indicated. Each root section was transferred to 500 μL 1 m NaOH and homogenized with a microhomogenizer. The homogenates were heated at 80°C for 15 min, cooled, and centrifuged. The alkaline-extracted callose was quantified with aniline blue (water soluble; Wako Pure Chemical Industries, Osaka) by fluorometry. Curdlan (Wako Pure Chemical Industries) was used as a standard.
Assessment of the Loss of Integrity of the Plasma Membrane
The loss of the plasma membrane integrity was evaluated by a spectrophotometric assay of Evans blue stain retained by cells as described by Baker and Mock (1994) and Ikegawa et al. (1998) with minor modifications. After aluminum treatment, roots were stained with an Evans blue solution and washed as described above in Histochemical Analyses. The stained region (usually, a 0- to 10-mm section from the root tip, unless otherwise indicated) was removed with a razor blade. Four root sections from identical positions were placed together, and the trapped Evans blue was released by homogenizing the root sections in 1 mL of 1% (w/v) aqueous SDS with the microhomogenizer at room temperature. The homogenate was centrifuged at 13,500g for 10 min. The optical density of the supernatant was determined spectrophotometrically at 600 nm.
Statistical Analysis
Each experiment was repeated at least two times with similar results. All values are shown as the means ± se of a total of three replicates obtained from two independent experiments, unless otherwise indicated.
ACKNOWLEDGMENTS
We would like to thank Mrs. Sanae Rikiishi (Okayama University, Kurashiki, Japan) for her technical assistance, and Dr. Larry Zee Morand (University of California, Davis) for his critical reading and editing of our manuscript.
Footnotes
This work was supported by the Program for Promotion of Basic Research Activities for Innovative Biosciences, by a Grant-in-Aid for General Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan, and by the Ohara Foundation for Agricultural Science.
LITERATURE CITED
- Akeson MA, Munns DN, Burau RG. Adsorption of Al3+ to phosphatidylcholine vesicles. Biochim Biophys Acta. 1989;986:33–40. doi: 10.1016/0005-2736(89)90269-1. [DOI] [PubMed] [Google Scholar]
- Baker CJ, Mock NM. An improved method for monitoring cell death in cell suspension and leaf disc assays using Evans blue. Plant Cell Tissue Organ Cult. 1994;39:7–12. [Google Scholar]
- Cackmak I, Horst WJ. Effect of Aluminum on lipid peroxidation, superoxide dismutase, catalase, and peroxidase activities in root tips of soybean (Glycine max) Physiol Plant. 1991;83:463–468. [Google Scholar]
- Deleers M, Servais JP, Wülfert E. Neurotoxic cations induce membrane rigidification and membrane fusion at micromolar concentrations. Biochim Biophys Acta. 1986;855:271–276. doi: 10.1016/0005-2736(86)90174-4. [DOI] [PubMed] [Google Scholar]
- Delhaize E, Ryan PR. Aluminum toxicity and tolerance in plants. Plant Physiol. 1995;107:315–321. doi: 10.1104/pp.107.2.315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delmer DP, Amor Y. Cellulose biosynthesis. Plant Cell. 1995;7:987–1000. doi: 10.1105/tpc.7.7.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ezaki B, Tugita S, Matsumoto H. Expression of a moderately anionic peroxidase is induced by aluminum treatment in tobacco cells: possible involvement of peroxidase isozymes in aluminum ion stress. Physiol Plant. 1996;96:21–28. [Google Scholar]
- Ezaki B, Yamamoto Y, Matsumoto H. Cloning and sequencing of the cDNAs induced by aluminum treatment and Pi starvation in cultured tobacco cells. Physiol Plant. 1995;93:11–18. [Google Scholar]
- Gaff DF, Okong'O-Ogola O. The use of non-permeating pigments for testing the survival of cells. J Exp Bot. 1971;22:756–758. [Google Scholar]
- Gutteridge JMC, Ouinlan J, Clark I, Halliwell B. Aluminum salts accelerate peroxidation of membrane lipids stimulated by iron salts. Biochim Biophys Acta. 1985;835:441–447. doi: 10.1016/0005-2760(85)90113-4. [DOI] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JMC. Free Radicals in Biology and Medicine. Ed 2. Oxford: Clarendon Press; 1989. [Google Scholar]
- Havas M. A hematoxylin staining technique to locate sites of aluminum binding in aquatic plants and animals. Water Air Soil Pollut. 1986;30:735–741. [Google Scholar]
- Horst WJ. The role of the apoplast in aluminum toxicity and resistance of higher plants: a review. Z Pflanzenernäehr Bodenkd. 1995;158:419–428. [Google Scholar]
- Horst WJ, Asher CJ, Cakmak I, Szulkiewica P, Wissemeier AH. Short-term responses of soybean roots to Aluminum. J Plant Physiol. 1992;140:174–178. [Google Scholar]
- Horst WJ, Püschel AK, Schmohl N. Induction of callose formation is a sensitive marker for genotypic aluminum sensitivity in maize. Plant Soil. 1997;192:23–30. [Google Scholar]
- Ikegawa H, Yamamoto Y, Matsumoto H. Cell death caused by a combination of aluminum and iron in cultured tobacco cells. Physiol Plant. 1998;104:474–478. [Google Scholar]
- Ikegawa H, Yamamoto Y, Matsumoto H. Responses to aluminum of suspension-cultured tobacco cells in a simple calcium solution. Soil Sci Plant Nutri. 2000;46:503–514. [Google Scholar]
- Ishikawa S, Wagatsuma T. Plasma membrane permeability of root-tip cells following temporary exposure to Aluminum ions is a rapid measure of Aluminum tolerance among plant species. Plant Cell Physiol. 1998;39:516–525. [Google Scholar]
- Jensen WA. Botanical Histochemistry: Principles and Practice. W. H. San Francisco: Freeman and Company; 1962. [Google Scholar]
- Jones DL, Kochian LV. Aluminum interaction with plasma membrane lipids and enzyme metal binding sites and its potential role in Aluminum cytotoxicity. FEBS Lett. 1995;400:51–57. doi: 10.1016/s0014-5793(96)01319-1. [DOI] [PubMed] [Google Scholar]
- Jones DL, Kochian LV. Aluminum inhibition of the inositol 1, 4, 5-triphosphate signal transduction pathway in wheat roots: a role in aluminum toxicity? Plant Cell. 1997;7:1913–1922. doi: 10.1105/tpc.7.11.1913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kauss H. Some aspects of calcium-dependent regulation in plant metabolism. Annu Rev Plant Physiol. 1987;38:47–72. [Google Scholar]
- Kauss H. Callose and callose synthase. In: Gurr SJ, McPherson MJ, Bowles DJ, editors. Molecular Plant Morphology: A Practical Approach. Vol. 2. New York: Oxford University Press; 1992. pp. 1–8. [Google Scholar]
- Kochian LV, Jones DL. Aluminum toxicity and resistance in plants. In: Yokel RA, Golub MS, editors. Research Issues in Aluminum Toxicity. London: Taylor & Francis Ltd; 1997. pp. 69–89. [Google Scholar]
- Koyama H, Toda T, Yokota S, Dawair Z, Hara T. Effects of aluminum and pH on root growth and cell viability in Arabidopsis thaliana strain Landsberg in hydroponic culture. Plant Cell Physiol. 1995;36:201–205. [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Ma JF, Zheng SJ, Hiradate S, Matsumoto H. Detoxifying aluminum with buck wheat. Nature. 1997;390:569–570. [Google Scholar]
- Mihara M, Uchiyama M, Fukazawa K. Thiobarbituric acid value on fresh homogenate of rat as a parameter of lipid peroxidation in aging, CCl4 intoxication, and vitamin E deficiency. Biochem Med. 1980;23:302–311. doi: 10.1016/0006-2944(80)90040-x. [DOI] [PubMed] [Google Scholar]
- Ono K, Yamamoto Y, Hachiya A, Matsumoto H. Synergistic inhibition of growth by Aluminum and iron of tobacco (Nicotiana tabacum L.) cells in suspension culture. Plant Cell Physiol. 1995;36:115–125. [Google Scholar]
- Oteiza PI. A mechanism for the stimulatory effect of aluminum on iron-induced lipid peroxidation. Arch Biochem Biophys. 1994;308:374–379. doi: 10.1006/abbi.1994.1053. [DOI] [PubMed] [Google Scholar]
- Papernik LA, Kochian LV. Possible involvement of Aluminum-induced electrical signals in Aluminum tolerance in wheat. Plant Physiol. 1997;115:657–667. doi: 10.1104/pp.115.2.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pompella A, Maellaro E, Casini AF, Comporti M. Histochemical detection of lipid peroxidation in the liver of bromobenzene–poisoned mice. Am J Pathol. 1987;129:295–301. [PMC free article] [PubMed] [Google Scholar]
- Rengel Z. Role of calcium in aluminum toxicity. New Phytol. 1992;121:499–513. [Google Scholar]
- Rengel Z. Uptake of aluminum by plant cells. New Phytol. 1996;134:389–406. [Google Scholar]
- Richards KD, Schott EJ, Sharma YK, Devis KR, Gardner RC. Aluminum induces oxidative stress genes in Arabidopsis thaliana. Plant Physiol. 1998;116:409–418. doi: 10.1104/pp.116.1.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasaki M, Yamamoto Y, Ma JF, Matsumoto H. Early events induced by aluminum stress in elongating cells of wheat root. Soil Sci Plant Nutri. 1997;43:1009–1014. [Google Scholar]
- Snowden KC, Gardner RC. Five genes induced by aluminum in wheat (Triticum aestivum) roots. Plant Physiol. 1993;103:855–861. doi: 10.1104/pp.103.3.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takabatake R, Shimmen T. Inhibition of electrogenesis by aluminum in Characean cells. Plant Cell Physiol. 1997;38:1264–1271. [Google Scholar]
- Taylor GJ, McDonald-Stephens JL, Hunter DB, Bertsch PM, Elmore D, Rengel Z, Reid RJ. Direct measurement of aluminum uptake and distribution in single cells of Chara corallina. Plant Physiol. 2000;123:987–996. doi: 10.1104/pp.123.3.987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie CX, Yokel RA. Aluminum facilitation of iron-mediated lipid peroxidation is dependent on substrate, pH, and aluminum and iron concentrations. Arch Biochem Biophys. 1996;327:222–226. doi: 10.1006/abbi.1996.0113. [DOI] [PubMed] [Google Scholar]
- Yamaguchi Y, Yamamoto Y, Matsumoto H. Cell death process initiated by a combination of aluminum and iron in suspension-cultured tobacco cells (Nicotiana tabacum): apoptosis-like cell death medicated by calcium and proteinase. Soil Sci Plant Nutri. 1999;45:647–657. [Google Scholar]
- Yamamoto Y, Hachiya A, Matsumoto H. Oxidative damage to membranes by a combination of aluminum and iron in suspension-cultured tobacco cells. Plant Cell Physiol. 1997;38:1333–1339. [Google Scholar]
- Yamamoto Y, Rikiishi S, Chang YC, Ono K, Kasai M, Matsumoto H. Quantitative estimation of Aluminum toxicity in cultured tobacco cells: correlation between Aluminum uptake and growth inhibition. Plant Cell Physiol. 1994;35:575–583. [Google Scholar]
- Yokota S, Ojima K. Physiological response of root tip of alfalfa to low pH and aluminum stress in water culture. Plant and Soil. 1995;171:163–165. [Google Scholar]
- Zhang G, Hoddinott J, Taylor GJ. Characterization of 1,3-β-d-glucan (callose) synthesis in roots of Triticum aestivum in response to aluminum toxicity. J Plant Physiol. 1994;144:229–234. [Google Scholar]