Abstract
Diabetes mellitus is the most common medical condition and with increased awareness of heath and related issues, several patients are getting diagnosed with diabetes. The poor control of sugar and long-standing status of disease affects the autonomic system of body. The autonomic nervous system innervates cardiovascular, gastrointestinal, and genitourinary system, thus affecting important functions of the body. The cardiovascular system involvement can manifest as mild arrhythmias to sudden death. Our search for this review included PubMed, Google Search and End Note X6 version and the key words used for the search were autonomic neuropathy, diabetes, anesthesia, tests and implications. This review aims to highlight the dysfunction of autonomic system due to diabetes and its clinical presentations. The various modalities to diagnose the involvement of different systems are mentioned. An estimated 25% of diabetic patients will require surgery. It has been already established that mortality rates in diabetic patients are higher than in nondiabetic patients. Hence, complete workup is needed prior to any surgery. Diabetic autonomic neuropathy and its implications may sometimes be disastrous and further increase the incidence of in hospital morbidity and mortality. Overall, complete knowledge of diabetes and its varied effects with anaesthetic implications and careful perioperative management is the key guiding factor for a successful outcome.
Key words: Anaesthesia, autonomic neuropathy, assessment, diabetes, implications
INTRODUCTION
With the increasing population and changing life styles, the incidence of diabetes and its complications has significantly increased. More number of such patients present for both elective and emergency surgery. The estimated incidence of diabetic patients requiring surgery was found to be around 25%.[1] The end-organ damage caused by diabetes is responsible for the increased morbidity and mortality compared with nondiabetics.[2,3] Microangiopathic changes include retinopathy, nephropathy, and neuropathy. Macroangiopathy includes mainly atherosclerosis. Both the changes are responsible for the increased risk of complications like cardiac dysfunction and infections, these in turn lead to increased need for surgical interventions.[2,3,4] Because of the increased risk of complications associated with diabetes mellitus, appropriate perioperative assessment and management are crucial and vital to the anaesthesiologist. This requires good understanding of the pathophysiology of diabetes and its effect on different systems. This text will deal with the autonomic disturbances in diabetes, how to assess those changes preoperatively, and application of the overall knowledge for managing and preventing complications for a better outcome. We searched PubMed, Google Search and End Note X6 version, and the key words used for the search were autonomic neuropathy, diabetes, anesthesia, tests and implications.
Why nerves are affected along with the retina and kidney?
All the cells in the body require insulin to gain entry into the cells. Exceptions are nerves, retina, and kidney. These are independent of insulin and glucose entry and exit from these cells is free and does not depend on any action of insulin. Uptake of glucose in these organs depends on concentration gradient mediated by sodium independent glucose transporter GLUT1. That is the reason, insulin deficiency does not deprive these organs of glucose. In hyperglycaemia, these cells get damaged easily due to more intracellular concentration of glucose.
Pathophysiology of autonomic disturbances
The autonomic nervous system innervates the cardiovascular, gastrointestinal (GI), and genitourinary systems. Impaired glucose regulation affects both somatic and autonomic nerves, mainly the small fibers.
Initially, microangiopathic changes include thickening of the capillary basement membrane and hyperplasia of the endothelium. This in turn causes vasoconstriction leading to reduced oxygen tension and hypoxia and neuronal ischaemia.[5] Later, hyperglycaemia will further lead to different pathways causing sorbitol accumulation, increase in diacylglycerol levels and oxidative stress further leading to nerve damage.[6,7] The mechanism of neural damage has been explained in Figure 1. In few cases, immunologically mediated damage has also been suggested.[8]
Figure 1.
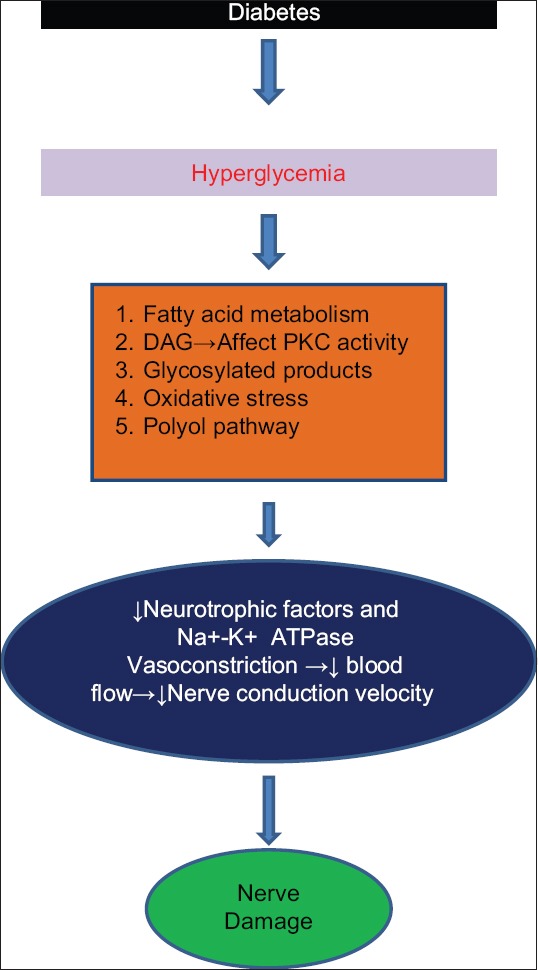
Pathogenesis of diabetic neuropathy. DAG- Diacylglycerol, PKC- Protein kinase C
Autonomic neuropathy and its presentation
Diabetic autonomic neuropathy (AN) is among the least acknowledged and interpreted complications of diabetes despite its substantial negative effect on survival and quality of life in people with diabetes. Clinical symptoms of AN generally are not evident until long after the onset of diabetes. Nevertheless, subclinical autonomic dysfunction can be seen within a year of diagnosis in type 2 diabetic patients and within 2 years in type 1 diabetic patients. Recent evidence has shown that autonomic imbalance may precede the development of the inflammatory cascade in type 2 diabetes.
-
Cardiovascular (CVS) autonomic neuropathy (CAN):[9,10] CAN occurs in ~ 17% of patients with type 1 diabetes and 22% of those with type 2. An additional 9% of type 1 patients and 12% of type 2 patients have borderline dysfunction. CAN may be present at diagnosis, and prevalence increases with age, duration of diabetes, obesity, smoking, and poor glycaemic control.
The usual features include:
- Abnormalities in heart rate control – Unopposed sympathetic activity due to involvement of the vagus nerve results in either resting tachycardia or heart rate variability (HRV). Reduced heart rate variation is earliest indicator of CAN.[11] Fixed heart rate not responding to any manoeuvre is seen in late diabetic patients with almost a denervated heart with further involvement of the sympathetic nervous system.[9,12]
- Abnormalities in central and peripheral vascular dynamics – Impaired sympathetic and parasympathetic responses that normally augment cardiac output and redirect peripheral blood flow to skeletal muscles result in exercise intolerance. This is evident by decreased ejection fraction, systolic dysfunction, and impaired diastolic filling.[13,14] These patients are even prone to arrhythmias due to regional myocardial autonomic denervation. With both altered vascular responsiveness and regional myocardial neuropathy, malignant arrhythmogenesis, and sudden cardiac death are common.[15] Normally a postural change from supine to sitting position results in baroreceptor initiated and centrally mediated sympathetic reflex response leading to increase in peripheral vascular resistance and increase in norepinephrine leading to cardiac acceleration. Impaired reflex response and sympathetic efferent neuropathy usually causes fall in peripheral resistance with change in posture and results in orthostatic hypotension.[16] The presenting features vary from asymptomatic to light-headedness and presyncopal symptoms like weakness, faintness, dizziness, visual impairment, with change of posture from lying down to standing position. Most of them remain asymptomatic despite severe orthostatic hypotension.[17]
- The involvement of coronary arteries – The incidence is high in diabetes. Most of them are asymptomatic due to AN.[9] The neuropathic damage to the myocardial sensory afferent fibres in the autonomic nerve supply reduces the appreciation for ischaemic pain resulting in delayed recognition and intervention. Some patients may present with myocardial pain. Chest pain in diabetic patients should be considered to be of myocardial origin until proven otherwise. The possibility of silent myocardial infarction should not be excluded in patients with symptoms of unexplained fatigue, confusion, tiredness, oedema, haemoptysis, nausea and vomiting, diaphoresis, arrhythmias, cough, or dyspnoea.[18]
Other autonomic neuropathies
-
2. GI AN: GI symptoms are varied and it usually depends on the site and section of the tract affected.
- Oesophageal dysfunction:[19] Most common cause is vagal neuropathy, seen in 40% of cases; presenting symptoms of abnormal peristalsis and impaired lower oesophageal sphincter tone include heartburn and dysphagia for solids.
- Small bowel dysfunction: Disordered peristalsis may present either as bacterial overgrowth syndrome or diarrhoea. Diabetic diarrhoea manifests as a profuse, watery, typically nocturnal diarrhoea, which can last for hours or days and frequently alternates with constipation.[18] Extrinsic and intrinsic intestinal neuropathy and impaired gastro-colic reflex may present with constipation. This is the most common feature presented, sometimes may alternate with diarrhoea.
- Gall bladder atony and enlargement.
-
3. Genitourinary AN:[22]
- Neurogenic bladder: Cystopathy which may occur due to impaired detrusor functioning and impaired bladder sensation. This may result either in bladder cystopathy, incomplete emptying of the bladder and increased postvoid residual, urinary retention and increased urinary frequency depending upon the fibres affected – afferent or efferent or both, parasympathetic or sympathetic or both. A postvoiding residual volume of more than 150 mL is diagnostic of neurogenic bladder. Neurogenic bladder may put patients at risk for urinary infections.
- Sexual dysfunction, which is more common in diabetes, includes erectile dysfunction (ED), retrograde ejaculation and female sexual dysfunction.[23] ED is the most common form of autonomic disorder and is also found to be a good marker for generalised vascular event. Penile failure is a potent marker for upcoming and preventable CVS events. Female sexual dysfunction includes decreased sexual desire and increased pain during intercourse due to inadequate lubrication.[24]
-
4. Others:
- Hypoglycaemic autonomic failure: Attenuation of epinephrine and other counter regulatory hormones in patients with hypoglycaemia unawareness is defined as hypoglycaemic autonomic failure.[25] Presence of AN further attenuates this response and increases the severity of this incidence. The strict glycaemic control aggravates hypoglycaemic autonomic faillure.
- Impaired microvascular blood flow to the skin: Microvascular insufficiency results in abnormal contraction of the arterioles and arteries of the skin.[26] Laser Doppler flowmetry is a non-invasive method of assessing the changes in microvascular blood flow with mental arithmetic, cold pressor, heating, and handgrip. Dry skin leading to fissures and ulcer development helps in further seedling of infection and gangrene. AN also causes increased osteoclastic activity and reduced bone density.[27]
- Anaemia and AN: Sympathetic AN leading to reduced sympathetic stimulation of erythropoietin production has been previously hypothesised as the cause of anaemia.[28]
- Mortality and major cardiovascular event with AN: AN, in the form of renal failure, cardiac events, such as sudden cardiac death, has been well correlated with 5-year mortality. Mortality rates after a myocardial infarction are also higher for diabetic patients than for nondiabetic patients. This may be due to autonomic insufficiency, increasing the tendency for development of ventricular arrhythmia and cardiovascular event after infarction.[9]
Autonomic assessment
-
GI:
-
Gastric emptying can be assessed by:
- Assessment of glycaemic control
- Medication history, including the use of anticholinergic agents, ganglion blockers, and psychotropic drugs
- Gastroduodenoscopy to exclude pyloric or other mechanical obstruction
- Manometry to detect antral hypomotility and/or pylorospasm
- Double-isotope scintigraphy to measure solid-phase gastric emptying
- Electrogastrography detects abnormalities in GI pacemaking, but its role has not been established in diagnosis or treatment decision making
-
Tests for the diagnosis and assessment of constipation might include the following:
- Anorectal manometry for evaluating sphincter tone and the rectal anal inhibitory reflex. Assessment of colonic segmental transit time. Pelvic examination, with careful bimanual examination for women
- Diarrhoea. Assessment of diarrhoea in patients with diabetes might include detailed history along with examination for enteric pathogens, ova and parasites to rule out different causes of diarrhoea. Further, appropriate tests may be needed to rule out causes like small bowel malabsorptive disorders, steatorrhoea, coeliac disease and bacterial overgrowth.
-
-
Assessing genitourinary autonomic function. ED is routinely assessed by a detailed medical history, sexual function history, medication history, assessment of glycaemic control, hormonal and psychological evaluation. Specific tests related to AN include the following:
- Measurement of nocturnal penile tumescence
- Measurement of penile and brachial blood pressure with Doppler probes and calculation of the penile-brachial pressure index (<0.7 suggests penile vascular disease)
- Sacral outflow (S2, S3, and S4) assessment, which represents the sacral parasympathetic divisions: anal sphincter tone, perianal sensation, anal wink, and bulbo cavernous reflex are clinical features of denervation of the important nerve supply that enable erections to occur
- Intra-cavernosal injection of vasoactive compound (e.g., papaverine and prostaglandin E1) with a response of 65–70% of the time reflecting a predominantly neurogenic cause of ED and compatible with a significant arterial component. Failure of the response suggests venous incompetence
Bladder dysfunction. Evaluation of diabetic bladder dysfunction should be done for any diabetic patient with recurrent urinary tract infection, pyelonephritis, incontinence, or a palpable bladder. The evaluation might include the following: Assessment of renal function, urinary culture, postvoid ultrasound to assess residual volume and upper-urinary tract dilation, cystometry and voiding cystometrogram to measure bladder sensation and volume pressure changes associated with bladder filling with known volumes of water and voiding
Assessing sudomotor function. Testing of the eccrine sweat glands provides a measure of sympathetic cholinergic function. Tests of sudomotor function include the quantitative sudomotor axon reflex test, the sweat imprint, the thermoregulatory sweat test, and the sympathetic skin response.[9] Sudoscan measures electrochemical skin conductance of hands and feet through reverse iontophoresis. This is a promising, sensitive tool to detect neuropathy in patients with diabetes mellitus. This is a very simple, easy-to-perform test that can be done in the clinical setting in 3–5 min.
Assessing peripheral neurovascular responses: These responses are a measure of autonomic microvascular integrity and is markedly depressed in patients with AN. These include orienting response, mental arithmetic, handgrip, and cold pressor response.
Assessing pupillary function. Patients with diabetic AN show delayed or absent reflex response to light reduced resting pupillary diameter.[32]
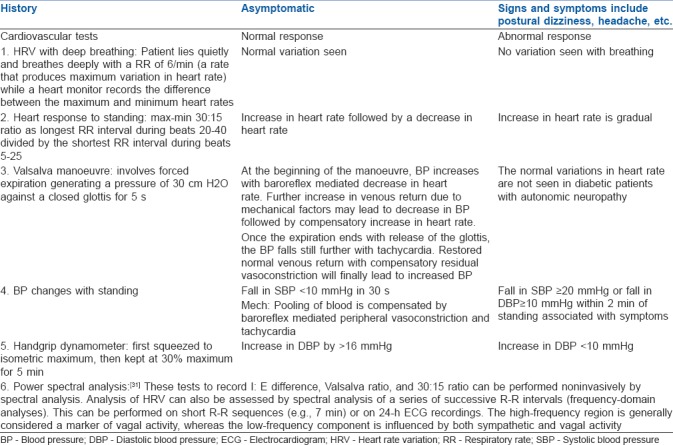
Table 1.
Evaluation of cardiac autonomic neuropathy
The San Antonio Consensus Panel also made several general recommendations regarding the need to fully classify diabetic AN:[9]
Symptoms possibly reflecting AN should not, by themselves, be considered markers for its presence.
Non-invasive validated measures of autonomic neural reflexes should be used as specific markers of AN if end-organ failure is carefully ruled out and other important factors, such as concomitant illness, drug use, and age are taken into account.
An abnormality on more than one test on more than one occasion is desirable to establish the presence of autonomic dysfunction.
Independent tests of both parasympathetic and sympathetic function should be performed.
A battery of quantitative measures of autonomic reflexes should be used to monitor improvement or deterioration of autonomic nerve function.
When used by properly trained individuals, autonomic function tests are a safe and effective diagnostic tool. Non-invasive autonomic tests were found to have a high value-to-risk ratio.
Preventive implications of CAN:
To assist in the establishment (or re-establishment) of tight glycaemic control
To facilitate the decision to initiate treatment for cardiovascular autonomic dysfunction
To emphasise the importance of adherence to diet and exercise interventions.
Anaesthetic implications
The choice of anaesthesia for most of the surgeries is regional anaesthesia in terms of pain relief. For diabetic patients, the regional techniques are best as they blunt the surgical stress response responsible for hyperglycaemia and also block sympathetic efferent signals.[33] In diabetic patients with AN, use of regional anaesthesia can result in deleterious life-threatening hypotension. The same effect may be induced by general anaesthesia. Thus, it is imperative to determine the best choice of anaesthetic technique to facilitate early recovery.[34]
The implications are:
Gastric emptying time is delayed in patients with uncontrolled hyperglycaemia. Strict guidelines for nothing by mouth should be followed to avoid the chances of regurgitation and aspiration. Metoclopramide is administered to decrease the gastric emptying time.[20] Aspiration is likely to get aggravated in diabetes due to stiff joint syndrome and in such patients prokinetics-like cisapride may be ineffective in reducing the volume of gastric content.[35]
Anaemia may be associated patients with AN; this needs to be optimised before undertaking any intervention.[28]
Haemodynamic response to intubation is altered in diabetic patients. Patients with AN may present either with an exaggerated or an attenuated haemodynamic response. Careful monitoring with titrated anaesthetic regimen, minimal airway manipulation, and timely intervention is required to maintain stable dynamics.
Perioperative cardiovascular lability:[9,36] The normal autonomic response of vasoconstriction and tachycardia did not completely compensate for the vasodilating effects of anaesthesia. This may result in severe degree of hypotension sometimes not responding to vasopressors and inotropes. Assessment of the blood volume status before and during the conduct of anaesthesia will guide in titrating the dose of anaesthesia and in combating hypotension. Monitoring mandates the use of arterial blood pressure for beat-to-beat variation in blood pressure, electrocardiogram to identify rhythm disturbances and ischemic changes and central venous pressure to assess fluid volume status.
These patients are more prone to hypothermia and thus decreased drug metabolism and impaired wound healing, and rarely with hyperthermia. Effective techniques to prevent hypothermia and continuous temperature monitoring should be considered.[36]
Insulin has paradoxical effects on cardiovascular system. At low doses, it has vasoconstrictor effect but at high doses, which are often used for diabetes treatment, it causes vasodilatation. That is the reason, in patients with AN, insulin causes a decrease in supine arterial pressure and exacerbates postural hypotension.[36]
Patients with AN have reduced hypoxic-induced ventilatory drive. Choice of anaesthesia and dosage proper planning of perioperative management is essential to decrease the incidence of complications. Judicious use of opioid should be done to avoid postoperative respiratory depression.[37]
The severity of AN can have adverse consequences in diabetic patients. Patients may have uncontrolled cardiovascular stability, especially with central neuraxial anaesthesia. Proper preoperative assessment and planning is essential for a safe outcome. Nerve blocks preferably ultrasound guided is safe. The presence of peripheral neuropathy must be well documented prior to any regional procedure.
Risk of infection and vascular damage may be increased with the use of regional techniques in diabetic patients, consequently increasing risk of development of epidural abscess.[38]
Evidence also suggests that there is increased risk of neuropathy after peripheral nerve blocks.[39,40]
Strict vigilance, monitoring and control of blood sugars are essential depending upon the type and duration of surgery throughout the perioperative procedure.
Slow controlled positioning is essential to avoid precipitous fall of blood pressure. Padding of vulnerable area is properly done to avoid iatrogenic injuries during positioning.
Orthostatic hypotension may precipitate in the postoperative period. Continuous monitoring of blood pressure, respiration with oxygen supplementation, and adequate analgesia are mandatory at least for 24 h following any intervention.
Pneumoperitoneum in laparoscopic surgeries is again a concern in diabetic patients with AN. This should be used with caution or avoided to negate its ischemic effects on the adjacent viscera and blood vessels. Another major concern is postoperative paralytic ileus, especially following abdominal surgeries. Strict control of blood sugars in the perioperative period also helps in the management of this problem.
Several strategies need to be utilised for enhanced recovery of patients undergoing surgery to promote earlier resumption of normal diet, earlier mobilisation, and reduced length of stay.[41] Adequate postoperative pain management, preferably with ultrasound-guided regional blocks are helpful.
Diabetes patients for emergency surgery needs special attention as risk of diabetic ketoacidosis is present and uncontrolled diabetes with AN can present with various haemodynamic disturbances. It is preferred to operate after stabilisation of patient. The decision to operate the patient carries significant risk of mortality and should be avoided if at all possible.[42]
Though there is large number of tests to assess autonomic dysfunction, we need specific test to answer our question and it is preferred to have one single test for single function of body.
Cardiovascular autonomic reflex tests CART) such as heart rate responses to deep breathing, standing, and Valsalva manoeuvre, as well as blood pressure response to standing are considered as the gold standard in clinical testing for AN.[43] Their applicability in bedside clinical practice is based on their sensitivity, specificity, reproducibility, ease and safety of use, and standardisation.
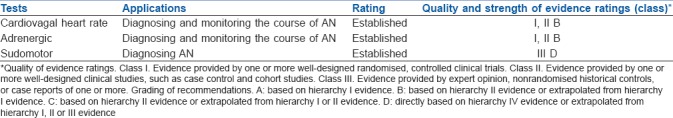
According to the CAN Subcommittee of the Toronto Consensus Panel statement following the 8th international symposium on diabetic neuropathy in 2010,[1] the criteria for diagnosis and staging of CAN are as follows: (1) A single abnormal CART result suffices for the diagnosis of possible or early CAN; (2) The presence of two or three abnormal test among the seven autonomic cardiovascular indices (5 CARTS, time-domain, and frequency-domain heart rate variation tests) are required for the diagnosis of definite or confirmed CAN; and (3) The presence of orthostatic hypotension in addition to the above criteria signifies the presence of severe or advanced CAN. The minimum tests to be done for AN are summarised in Table 2.[44]
Table 2.
Evidence-based minimum mandatory tests for autonomic neuropathy (AN)
SUMMARY
Diabetic AN along with other pathophysiological disturbances plays a role in the perioperative management of patients undergoing surgery. Anaesthesia per se has systemic manifestations. Patients with AN should be properly assessed with battery of tests, optimised with timely interventions for a safe outcome.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Tuttnauer A, Levin PD, BChir MB. Diabetes Mellitus and Anesthesia. Anesthesiol Clin. 2006;24:579–97. doi: 10.1016/j.atc.2006.05.006. [DOI] [PubMed] [Google Scholar]
- 2.Hirsch IB, McGill JB, Cryer PE, White PF. Perioperative management of surgical patients with diabetes mellitus. Anesthesiology. 1991;74:346–59. doi: 10.1097/00000542-199102000-00023. [DOI] [PubMed] [Google Scholar]
- 3.Cade WT. Diabetes-Related Microvascular and Macrovascular Diseases in the Physical Therapy Setting. Phys Ther. 2008;88:1322–35. doi: 10.2522/ptj.20080008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.UK Prospective Diabetes Study (UKPDS) VIII. Study design, progress and performance. Diabetologia. 1991;34:877–90. [PubMed] [Google Scholar]
- 5.Tuck RR, Schmelzer J, Low P. Endoneural blood flow and oxygen tension in the sciatic nerves of rats with experimental diabetic neuropathy. Brain. 1984;107:935–50. doi: 10.1093/brain/107.3.935. [DOI] [PubMed] [Google Scholar]
- 6.Stevens MJ. Nitric oxide as a potential bridge between the metabolic and vascular hypotheses of diabetic neuropathy. Diabetes Med. 1995;12:292–5. doi: 10.1111/j.1464-5491.1995.tb00478.x. [DOI] [PubMed] [Google Scholar]
- 7.Veves A, King GL. Can VEGF reverse diabetic neuropathy in human subjects? J Clin Invest. 2001;107:1215–8. doi: 10.1172/JCI13038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sundkvist G, Lind P, Bergstrom B, Lilja B, Rabinowe SL. Autonomic nerve antibodies and autonomic nerve function in type 1 and type 2 diabetic patients. J Intern Med. 1991;229:505–10. doi: 10.1111/j.1365-2796.1991.tb00386.x. [DOI] [PubMed] [Google Scholar]
- 9.Vinik AI, Maser RE, Mitchell BD, Freeman R. Diabetic autonomic neuropathy. Diabetes Care. 2003;26:1553–79. doi: 10.2337/diacare.26.5.1553. [DOI] [PubMed] [Google Scholar]
- 10.Vinik AI, Erbas T, Casellini CM. Diabetic cardiac autonomic neuropathy, inflammation and cardiovascular disease. J Diabetes Investig. 2013;4:4–18. doi: 10.1111/jdi.12042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ziegler D. Diabetic cardiovascular autonomic neuropathy: Prognosis, diagnosis and treatment. Diabetes Metab Rev. 1994;10:339–83. doi: 10.1002/dmr.5610100403. [DOI] [PubMed] [Google Scholar]
- 12.Schumer MP, Joyner SA, Pfeifer MA. Cardiovascular autonomic neuropathy testing in patients with diabetes. Diabetes Spectrum. 1998;11:227–31. [Google Scholar]
- 13.Kahn J, Zola B, Juni J, Vinik AI. Decreased exercise heart rate in diabetic subjects with cardiac autonomic neuropathy. Diabetes Care. 1986;9:389–94. doi: 10.2337/diacare.9.4.389. [DOI] [PubMed] [Google Scholar]
- 14.Roy TM, Peterson HR, Snider HL, Cyrus J, Broadstone VL, Fell RD, et al. Autonomic influence on cardiovascular performance in diabetic subjects. Am J Med. 1989;87:382–8. doi: 10.1016/s0002-9343(89)80818-6. [DOI] [PubMed] [Google Scholar]
- 15.Serhiyenko VA, Serhiyenko AA. Diabetic cardiac autonomic neuropathy: Do we have any treatment perspectives? World J Diabetes. 2015;6:245–58. doi: 10.4239/wjd.v6.i2.245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hilsted J, Parving HH, Christensen NJ, Benn J, Galbo H. Hemodynamics in diabetic orthostatic hypotension. J Clin Invest. 1981;68:1427–34. doi: 10.1172/JCI110394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Freeman R. Cardiovascular autonomic neuropathy. In: Dyck PJ, Thomas PK, editors. Diabetic Neuropathy. 2nd ed. Philadelphia: WB Saunders; 1999. pp. 541–54. [Google Scholar]
- 18.Vinik AI, Erbas T. Recognizing and treating diabetic autonomic neuropathy. Cleve Clin J Med. 2001;68:928–44. doi: 10.3949/ccjm.68.11.928. [DOI] [PubMed] [Google Scholar]
- 19.Channer KS, Jackson PC, O'Brien I, Corrall RJ, Coles DR, Davies ER, et al. Oesophageal function in diabetes mellitus and its association with autonomic neuropathy. Diabet Med. 1985;2:378–82. doi: 10.1111/j.1464-5491.1985.tb00656.x. [DOI] [PubMed] [Google Scholar]
- 20.Varis K. Diabetic gastroparesis. Scand J Gastroenterol. 1989;24:897–903. doi: 10.3109/00365528909089232. [DOI] [PubMed] [Google Scholar]
- 21.Schiller LR, Santa Ana CA, Schmulen AC, Hendler RS, Harford WV, Fordtran JS. Pathogenesis of fecal incontinence in diabetes mellitus: Evidence for internalanal- sphincter dysfunction. N Engl J Med. 1982;307:1666–71. doi: 10.1056/NEJM198212303072702. [DOI] [PubMed] [Google Scholar]
- 22.Watkins PJ. Clinical observations and experiments in diabetic neuropathy. Diabetologia. 1992;35:2–11. doi: 10.1007/BF00400845. [DOI] [PubMed] [Google Scholar]
- 23.Bacon CG, Hu FB, Giovannucci E, Glasser DB, Mittleman MA, Rimm EB. Association of type and duration of diabetes with erectile dysfunction in a large cohort of men. Diabetes Care. 2002;25:1458–63. doi: 10.2337/diacare.25.8.1458. [DOI] [PubMed] [Google Scholar]
- 24.Enzlin P, Mathieu C, Vanderschueren D, Demyttenaere K. Diabetes mellitus and female sexuality: A review of 25 years' research. Diabetes Med. 1998;15:809–15. doi: 10.1002/(SICI)1096-9136(199810)15:10<809::AID-DIA689>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 25.Cryer PE. Hypoglycemia-associated autonomic failure in diabetes. Am J Physiol Endocrinol Metab. 2001;281:E1115–21. doi: 10.1152/ajpendo.2001.281.6.E1115. [DOI] [PubMed] [Google Scholar]
- 26.Low P, Lagerlund TD, McManis PG. Nerve blood flow and oxygen delivery in normal, diabetic, and ischemic neuropathy. Int Rev Neurobiol. 1989;31:355–438. doi: 10.1016/s0074-7742(08)60283-4. [DOI] [PubMed] [Google Scholar]
- 27.Young MJ, Marshall A, Adams JE, Selby PL, Boulton AJM. Osteopenia, neurological dysfunction, and the development of Charcot neuroarthropathy. Diabetes Care. 1995;18:34–8. doi: 10.2337/diacare.18.1.34. [DOI] [PubMed] [Google Scholar]
- 28.Bosman DR, Osborne CA, Marsden JT, Macdougall IC, Gardner WN, Watkins PJ. Erythropoietin response to hypoxia in patients with diabetic autonomic neuropathy and non-diabetic chronic renal failure. Diabet Med. 2002;19:65–9. doi: 10.1046/j.1464-5491.2002.00634.x. [DOI] [PubMed] [Google Scholar]
- 29.Ewing DJ, Martyn CN, Young RJ, Clarke BF. The value of cardiovascular autonomic function tests: 10 years' experience in diabetes. Diabetes Care. 1985;8:491–8. doi: 10.2337/diacare.8.5.491. [DOI] [PubMed] [Google Scholar]
- 30.American Diabetes Association and American Academy of Neurology: Proceedings of a consensus development conference on standardized measures in diabetic neuropathy. Diabetes Care. 1992;15:1080–107. [Google Scholar]
- 31.Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology: Heart rate variability: Standards of measurement, physiological interpretation and clinical use. Circulation. 1996;93:1043–65. [PubMed] [Google Scholar]
- 32.Clarke BF, Ewing DJ, Campbell IW. Diabetic autonomic neuropathy. Diabetologia. 1979;17:195–212. doi: 10.1007/BF01235856. [DOI] [PubMed] [Google Scholar]
- 33.Moraca RJ, Sheldon DG, Thirlby RC. The role of epidural anesthesia and analgesia in surgical practice. Ann Surg. 2003;238:663–73. doi: 10.1097/01.sla.0000094300.36689.ad. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rehman HU, Mohammed K. Perioperative management of diabetic patients. Curr Surg. 2003;60:607–11. doi: 10.1016/j.cursur.2003.07.002. [DOI] [PubMed] [Google Scholar]
- 35.Reissell E, Taskinen MR, Orko R, Lindgren L. Increased volume of gastric contents in diabetic patients undergoing renal transplantation: Lack of effect with cisapride. Acta Anaesthesiol Scand. 1992;36:736–40. doi: 10.1111/j.1399-6576.1992.tb03555.x. [DOI] [PubMed] [Google Scholar]
- 36.McAnulty GR, Robertshaw HJ, Hall GM. Anaesthetic management of patients with diabetes mellitus. Br J Anaesth. 2000;8:80–90. doi: 10.1093/bja/85.1.80. [DOI] [PubMed] [Google Scholar]
- 37.Rose DK, Cohen MM, Wigglesworth DF, DeBoer DP. Critical respiratory events in the postoperative care unit. Anaesthesiology. 1994;81:410–8. doi: 10.1097/00000542-199408000-00020. [DOI] [PubMed] [Google Scholar]
- 38.Kindler CH, Seeberger MD, Staender SE. Epidural abscess complicating epidural anaesthesia and analgesia. An analysis of the literature. Acta Anaesthesiol Scand. 1998;42:614–20. doi: 10.1111/j.1399-6576.1998.tb05291.x. [DOI] [PubMed] [Google Scholar]
- 39.Royal College of Anaesthetists. Major complications of central neuraxial block in the UK. Third National Audit of The Royal College of Anaesthetists. 2009. Jan, [Last accessed on 2015 Aug 11]. Available from: www.rcoa.ac.uk/nap3 .
- 40.Hebl JR, Kopp SL, Schroeder DR, Horlocker TT. Neurologic complications after neuraxial anesthesia or analgesia in patients with pre-existing peripheral sensorimotor neuropathy or diabetic polyneuropathy. Anesth Analg. 2006;103:1294–9. doi: 10.1213/01.ane.0000243384.75713.df. [DOI] [PubMed] [Google Scholar]
- 41.Varadhan KK, Neal KR, Dejong CH, Fearon KC, Ljungqvist O, Lobo DN. The enhanced recovery after surgery (ERAS) pathway for patients undergoing major elective open colorectal surgery: A meta-analysis of randomized controlled trials. Clin Nutr. 2010;29:434–40. doi: 10.1016/j.clnu.2010.01.004. [DOI] [PubMed] [Google Scholar]
- 42.Barker P, Creasey PE, Dhatariya K, Levy N, Lipp A, Nathanson MH, et al. Peri-operative management of the surgical patient with diabetes. Anaesthesia. 2015;70:1427–40. doi: 10.1111/anae.13233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dimitropoulos G, Tahrani AA, Stevens MJ. Cardiac autonomic neuropathy in patients with diabetes mellitus. World J Diabetes. 2014;5:17. doi: 10.4239/wjd.v5.i1.17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.[No authors listed] Assessment: Clinical autonomic testing report of the Therapeutics and Technology Assessment Subcommittee of the American Academy of Neurology. Neurology. 1996;46:873–80. [PubMed] [Google Scholar]


